Investigating first-year undergraduate chemistry students’ reasoning with reaction coordinate diagrams when choosing among particulate-level reaction mechanisms
Molly B.
Atkinson
 a,
Michael
Croisant
b and
Stacey Lowery
Bretz
a,
Michael
Croisant
b and
Stacey Lowery
Bretz
 *b
*b
aUniversity of North Texas, Department of Chemistry, Denton, TX, USA
bMiami University, Department of Chemistry & Biochemistry, Oxford, OH, USA. E-mail: bretzsl@miamioh.edu
First published on 23rd October 2020
Abstract
Reaction coordinate diagrams (RCDs) are an important tool used to visualize the energetics of a chemical reaction. RCDs provide information about the kinetics of the reaction, the mechanism by which the reaction occurs, and the relative thermodynamic stability of the molecules in a reaction. Previous research studies have characterized student thinking about chemical kinetics, including their confusion in distinguishing between kinetics and thermodynamics. Semi-structured interviews were conducted with 44 students enrolled in a second-semester, first-year undergraduate chemistry course to elicit students’ ideas about surface features of RCDs and to examine how students connect those surface features to features of particulate-level reaction mechanisms. Students were provided both a gas-phase reaction and its accompanying RCD, and then they were asked to choose the particulate-level reaction mechanism that best corresponded to both the reaction and the RCD from among several possible particulate-level reaction mechanisms. Students were asked to explain their reasoning throughout the interview. Findings include students who chose the correct mechanism with appropriate reasoning, as well as students who chose the correct mechanism yet still expressed inaccurate ideas related to the surface features of RCDs and the concepts encoded within them. Students struggled to explain and reason with surface features such as peaks, valleys, and peak height. Moreover, students frequently found it difficult to identify meaningful connections between these surface features, the stoichiometry of the reaction, and the steps in a reaction mechanism. In addition, many students failed to mention important features of RCDs when describing their reasoning about the connections between particulate-level reaction mechanisms and RCDs. The implications for incorporating these research findings into teaching practices in first-year undergraduate chemistry contexts are discussed.
Introduction
Chemical kinetics and thermodynamics have been identified as anchoring concepts and core topics in the undergraduate chemistry curriculum (Justi, 2006; Holme and Murphy, 2012; Murphy et al., 2012; Holme et al., 2015). Recent literature reviews (Bain et al., 2014; Bain and Towns, 2016) have highlighted that undergraduate chemistry students hold a broad range of misconceptions related to chemical kinetics and thermodynamics, including the differences between kinetics and thermodynamics and concepts related to reaction mechanisms (Cakmakci, 2010; Çalik et al., 2010; Taştan et al., 2010; Popova and Bretz, 2018b, 2018c). These recent reviews have called for investigations into students’ understanding of external representations in the context of chemical kinetics and thermodynamics (Bain et al., 2014; Bain and Towns, 2016).Several previous studies have explored students’ reasoning about kinetics and thermodynamics concepts when viewing graphical and mathematical models. Becker and colleagues found that first-year undergraduate chemistry students have difficulty when reasoning with the kinetic information encoded in mathematical models involving rate laws and that these students also struggle when analyzing and interpreting rate and concentration data when constructing rate laws (Becker et al., 2017; Brandriet et al., 2018). Several studies have also investigated students’ blended reasoning with chemistry and mathematics when solving problems related to chemical kinetics (Bain et al., 2018; Rodriguez et al., 2018). Bain and co-workers found that students often rely heavily upon mathematical reasoning while lacking particulate-level chemical understanding, while results from a study conducted by Rodriguez and colleagues showed that students must be able to reason both symbolically and graphically in order to blend chemistry and mathematics concepts. Additionally, Rodriquez and co-workers explored students’ use and attention to relevant features of chemical kinetics problems and found that productive problem solving requires students to conceptually reason with important kinetic features of the task, as well as to metacognitively reflect on their problem-solving process during that task (Rodriguez et al., 2019).
Reaction coordinate diagrams (RCDs) are an important external representation used throughout both the first-year undergraduate chemistry and organic chemistry curricula, as they allow students to visualize the energetic changes of a reaction and reason about the mechanistic steps of a reaction. However, RCDs are complex representations in that both the kinetic and thermodynamic information of the corresponding reaction mechanism is encoded (Allinger, 1963; Meek et al., 2016). For example, the representation conveys thermodynamic information about the relative stability of different molecular species throughout the reaction, while differences in the height of energy barriers convey kinetic information about the relative speed of mechanistic steps in the reaction (Meek et al., 2016). Previous qualitative research studies by Popova and Bretz have shown that organic chemistry students struggle with not only understanding the meanings encoded in the surface features of RCDs, but also appropriately connecting RCD features to reaction mechanisms, conflating intermediates and transition states, and misinterpreting reaction progress to indicate time or speed of the reaction (Popova and Bretz, 2018a, 2018b, 2018c). While student thinking is well characterized when reasoning about RCDs and organic chemistry mechanisms for substitution and elimination reaction mechanisms (Popova and Bretz, 2018a, 2018b, 2018c), the reasoning of chemistry students in the first-year undergraduate course, where RCDs are typically introduced, has not previously been reported. The Reaction Coordinate Diagram Inventory (Atkinson et al., 2020) is a 19-item assessment to measure not only the misconceptions reported by Popova and Bretz for organic chemistry students, but also misconceptions held by first year university students regarding RCDs. The RCDI development paper presented evidence for the validity and reliability of the data generated by the inventory, but did not present rich descriptions (Geertz, 1973) of the students’ thinking as elicited in the extensive interviews used to develop the RCDI. The research reported herein shares some of the rich qualitative data generated to create the RCDI. Specifically, this manuscript details the methods and findings of an investigation into first-year undergraduate chemistry students’ reasoning with kinetics and thermodynamics concepts as they used RCDs to choose among possible reaction mechanisms depicted using particulate-level representations for gas-phase reactions.
Research question
The purpose of this study was to investigate first-year undergraduate chemistry students’ ideas about and reasoning with the kinetic and thermodynamic information encoded in the surface features of RCDs. Specifically, the goal of this research reported herein was to gain a better understanding into how students connect their knowledge of RCDs and the concepts encoded within them to the features of particulate-level reaction mechanisms. The research question guiding this study was how do students interpret and reason with the kinetic and thermodynamic information encoded in RCDs to choose among particulate-level reaction mechanisms?Theoretical frameworks
Methods
Participants
The students in this research study were enrolled in second-semester, first-year undergraduate chemistry course at a medium-sized, public, liberal arts university in the midwestern United States. Institutional Review Board (IRB) approval was obtained prior to the collection of all data to ensure the protection of participants’ rights and confidentiality. Students were provided a description of the research study during the accompanying second-semester, first-year undergraduate chemistry laboratory section and were invited to fill out a brief online survey in which they could volunteer to participate in the study and provide demographic information, including gender, race/ethnicity, course enrollment, year of study, major of study, and prior chemistry courses. This survey was administered to all students enrolled in second-semester first-year undergraduate chemistry majors and non-majors laboratory sections.From among the students who volunteered to participate, 44 students were purposefully selected for the research study to ensure that the sample captured the variety in the demographic information provided (Patton, 2002; Bretz, 2008).
This sample included 27 students who identified as female and 16 students who identified as male. One participant did not report any additional demographic information. The majority of students were Caucasian/White (n = 36), and the remaining 7 students were African American/Black, Asian/Pacific Islander, or Hispanic. Thirty-two students were in their first year of undergraduate study, 8 were sophomores in their second year of study, and 3 were juniors in their third year of study. Thirty-six of the students interviewed were science (non-chemistry) majors, 6 students were chemical engineering majors, and one student was a biochemistry major. This sample was representative of the enrollment in the first-year undergraduate chemistry course as well as university enrollment. Pseudonyms were assigned to all 44 participants to protect their identity and confidentiality.
Data collection and analysis
Semi-structured interviews were conducted using a think-aloud protocol to elicit students’ ideas regarding RCDs, including follow-up questions to deeply probe students’ understandings (Drever, 1995). Students were informed of their rights as human subjects and provided signature consent prior to participating in interviews. Students were interviewed after they had been taught and tested on all relevant kinetic and thermodynamic material in their second-semester, first-year undergraduate chemistry lecture course (Treagust, 1988; Novak, 1998; Bretz, 2001, 2008). Of the 44 students interviewed, 12 were interviewed in the spring 2017 semester and 32 were interviewed in the spring 2018 semester. Each student was interviewed during the semester they were enrolled, after they had been taught and tested upon the material. Each student was compensated for their time with a $20 gift card.Interviews were conducted individually by the second author (M.C.) and were audio- and video-recorded, lasting 52 minutes on average. All RCDs and reaction mechanisms used in the interviews were printed on LivescribeTM dot paper, and students were provided a LivescribeTM Smartpen to digitally record their verbal words, written words, and drawings on the dot paper (Linenberger and Bretz, 2012). Students were also provided a periodic table to use, if they wished to do so, during each interview.
Each interview was transcribed verbatim, and transcripts were annotated with all drawings generated and all nonverbal gestures that occurred during each interview. All qualitative data contained within the transcripts was managed using the software program NVivo12 (NVivo Qualitative Data Analysis Software, 2020). The data was both inductively and deductively coded for students’ ideas regarding RCDs and connecting reaction mechanisms to RCDs, using the constant comparative method of analysis to form themes to combine similar codes (Strauss and Corbin, 1998; Fram, 2013). A modified version of a scheme of RCD surface features and their meanings, originally generated by Popova and Bretz (2018c), was used to deductively code students’ reasoning with the kinetic and thermodynamic information found within RCDs. To provide trustworthiness for the codes both applied (deductively) and generated (inductively), the authors held weekly meetings and debriefing sessions to discuss, revise, and come to consensus on codes throughout the coding process (Lincoln and Guba, 1985; Creswell, 2003).
Description of interview guide
Each interview consisted of four phases. Phase I consisted of questions to investigate students’ prior knowledge concerning terms related to kinetics, thermodynamics, and reaction mechanisms. These ideas were elicited through a card sorting activity (Herrington et al., 2011; Krieter et al., 2016; Galloway et al., 2019) in which students were provided 15 cards, each containing a single term related to kinetics, thermodynamics, and/or reaction mechanisms. The 15 terms were temperature, enthalpy, activation energy, collisions, reaction rate, mechanism, kinetics, thermodynamics, reaction time, equilibrium, product yield, rate-determining step, intermediate, transition state, and reaction coordinate diagram. Students were asked to sort these 15 cards into as many stacks or groups as they wished, placing terms they thought to be related to each other in the same stack. Students were also asked to assign a name to each stack of cards.Each student examined one RCD in Phase II and another RCD in Phase III of the interview. If a student was assigned an exothermic RCD in Phase II, they were assigned an endothermic RCD in Phase III (and vice versa). This was done to ensure students were asked to reason about RCDs for both endothermic and exothermic reactions within the interview.
In Phase II, students were provided a one-step RCD for either an endothermic or an exothermic, gas-phase reaction. Students were asked to describe and explain the surface features of the RCD including: the labels of reaction progress (x-axis) and energy (y-axis), the starting and ending points, the minima and maxima, the processes occurring between points along the curve, and the effect, if any, upon the RCD of heating and cooling the reaction. The final portion of Phase II allowed students to revisit their card sort from Phase I and describe any changes they would make to that initial sort.
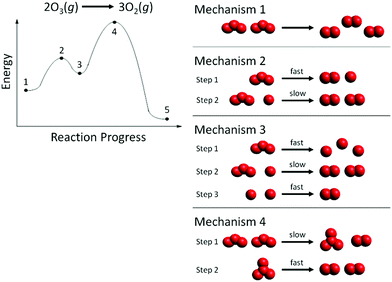
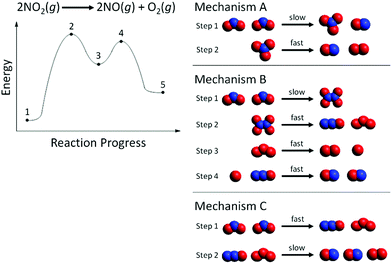
Phase III focused on gas-phase reactions with two elementary steps. Gas phase reactions with two elementary steps are typically taught in first-year undergraduate chemistry textbooks (Silberberg, 2012; Gilbert et al., 2017). In Phase III, students were provided one of two additional gas phase reactions, a two-step RCD, and multiple, possible particulate-level mechanisms as shown in Fig. 1 (exothermic reaction) and Fig. 2 (endothermic reaction). The space-filling representations were generated using the freeware Avogadro (Avogadro, 2020). The set of particulate mechanisms included one correct reaction mechanism and several incorrect mechanisms that did not correspond to the RCD. Students were asked to identify which mechanism correctly matched both the provided reaction and the provided RCD. Students were also asked to explain their reasoning for selecting that mechanism and for rejecting each of the others.
After the sixth student was interviewed in the pilot study during spring 2017, an additional task was added to Phase III to better elicit students’ reasoning about connecting particulate-level reaction mechanisms to RCDs. For the final 38 interviews, students were provided paper cut-outs of the particles represented in the reaction mechanism that they chose to best correspond to the RCD in the first activity described in Phase III. These students were asked to place these particle cut-outs along the curve of the RCD to explain how they thought the reaction occurred at the particulate level. Students were not told if the reaction mechanism they chose was correct or incorrect, and therefore they were provided the particle cut-outs that corresponded to the reaction mechanism they chose, regardless of correctness of that choice.
In Phase IV, students were offered a final opportunity to revisit their card sort and make any changes that they deemed necessary. They were also asked questions to reflect on the interview and provide any final information regarding kinetics, thermodynamics, and RCDs. Analyses of the card sort activity and revisions are beyond the scope of answering the research question reported herein. The data presented in this manuscript focuses on the results from interview Phase III.
Results and discussion
In Phase III of the interviews, 22 students were assigned the exothermic reaction in Fig. 1, and 22 students were assigned the endothermic reaction in Fig. 2. A summary of which mechanism was selected by the students to be correct is listed in Table 1.| Exothermic RCD (n = 22) | Endothermic RCD (n = 22) | ||
|---|---|---|---|
| Mechanism | n | Mechanism | n |
| 1 | 1 | A* | 12 |
| 2* | 16 | B | 5 |
| 3 | 0 | C | 5 |
| 4 | 5 | ||
Although the majority of students chose the appropriate particulate-level reaction mechanism corresponding to the provided RCD (n = 16/22 for Fig. 1, n = 12/22 for Fig. 2), analyses of the transcripts using Fig. 3 and Table 2 indicated that many students demonstrated inaccurate reasoning regarding the kinetic and thermodynamic information encoded within the RCD, even though they selected the correct mechanism. Similarly, some students demonstrated accurate reasoning when thinking about certain components of the RCD, even though they did not select the correct reaction mechanism. Four distinct patterns in the students’ reasoning emerged from the data, as summarized in Table 2. Groups I and II chose the correct mechanism corresponding to the provided RCD, with Group I reasoning accurately and Group II reasoning inaccurately. Groups III and IV chose an incorrect mechanism, even though Group III reasoned accurately. Group IV reasoned inaccurately.
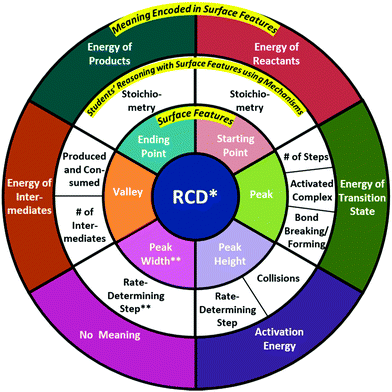 | ||
| Fig. 3 Modified version of the RCD scheme generated by Popova and Bretz (2018c), originally containing the inner and outer rings of Surface Features and Meaning Encoded in Surface Features. An additional middle ring was added to include Students’ Reasoning with Surface Features using Mechanisms. *Catalysts were not examined in the interview. **This category contains no accurate reasoning. | ||
| Reasoning | Mechanism choice | |
|---|---|---|
| Correct | Incorrect | |
| Accurate | Group I | Group III |
| Inaccurate | Group II | Group IV |
The transcripts were analyzed to better understand how students explained their choice of mechanism and the reasoning for why students connected or did not connect each particulate-level reaction mechanism to the provided RCD. A modified version of the scheme of RCD surface features and their meanings (Fig. 3), originally generated by Popova and Bretz (2018c), was created to deductively code students’ reasoning. The original RCD scheme consisted of two distinct regions: (1) an inner ring of Surface Features and (2) an outer ring of Meaning Encoded in Surface Features. Based on the findings of this research study, the scheme was expanded through an additional ring to reflect Students’ Reasoning with Surface Features using Mechanisms (Fig. 3). This additional region was intentionally placed between the two original rings to best reflect that students first recognized one or more structural surface features of the RCD, then reasoned with the surface feature(s) using the provided reaction mechanisms, and then made connections between the surface feature(s) and the underlying meaning encoded within the feature(s). The modified RCD scheme now consists of three distinct, but related, regions: (1) the inner ring, denoting the surface features of an RCD, (2) the middle ring, indicating reasoning with each RCD surface feature using particulate-level reaction mechanisms, and (3) the outer ring, indicating the meaning encoded within each surface feature.
Ten categories were created in the middle ring to reflect distinct elements of students’ reasoning with each RCD surface feature while using particulate-level reaction mechanisms: stoichiometry of reactants, number of steps, activated complex, bond breaking/forming, rate-determining step related to peak height, rate-determining step related to peak width, number of intermediates, produced and consumed, and stoichiometry of products. Descriptions of each of these ten categories are summarized in Table 3.
| Surface feature | Reasoning category | Reasoning elements |
|---|---|---|
| Reactants | • Stoichiometry | • Stoichiometry of reactants, or number of atoms/molecules in the initial reactants of reaction mechanism |
| Transition state | • Bond breaking/formation | • Particulate reasoning of bond breaking and bond formation along the curve of RCD |
| • Activated complex | • Nature of transition state(s) as a transient/dynamic state, bonds are in the process of breaking and forming | |
| • Number of Steps | • Identification of number of steps from RCD to determine corresponding reaction mechanism | |
| Activation energy | • Collisions | • Ideas of collision theory when leading up to energy maxima, frequency of collisions with sufficient energy and proper orientation |
| • Rate-determining step | • Height of peaks on RCD to define the rate-determining step of the reaction mechanism | |
| Peak width | • Rate-determining step** | • Width of peaks on RCD to define the rate-determining step of the reaction mechanism |
| Intermediates | • Number of intermediates | • Identification of intermediate(s) from particulate mechanism and on RCD |
| • Produced and consumed | • Nature of intermediate(s) as produced and then consumed in the reaction, products of the first step and reactants of the second step in the mechanism | |
| Products | • Stoichiometry | • Stoichiometry of products, or proportion of atoms/molecules in the final products of reaction mechanism |
To better understand students’ reasoning within each of the ten categories, a data reduction method was needed to distinguish among accurate reasoning, inaccurate reasoning, and no attention at all for each surface feature. The data were visualized using plots similar to those created by Pratt and Yezierski (2018) for depicting accurate and inaccurate ideas used to explain popular chemistry demonstrations. Plots were created for each mechanism choice (Fig. 4) to depict the accuracy of reasoning with regard to each of the ten categories for the new middle ring in Fig. 3. Green indicates the number of students with accurate reasoning regarding the RCD and corresponding particulate-level reaction mechanism, while red indicates the number of students with inaccurate reasoning. Gray dots represent the number of students who did not mention this particular component. Data from students who selected Mechanisms 1 and 3 in Fig. 1 are not displayed because only one student chose Mechanism 1 and no students chose Mechanism 3 (Table 1).
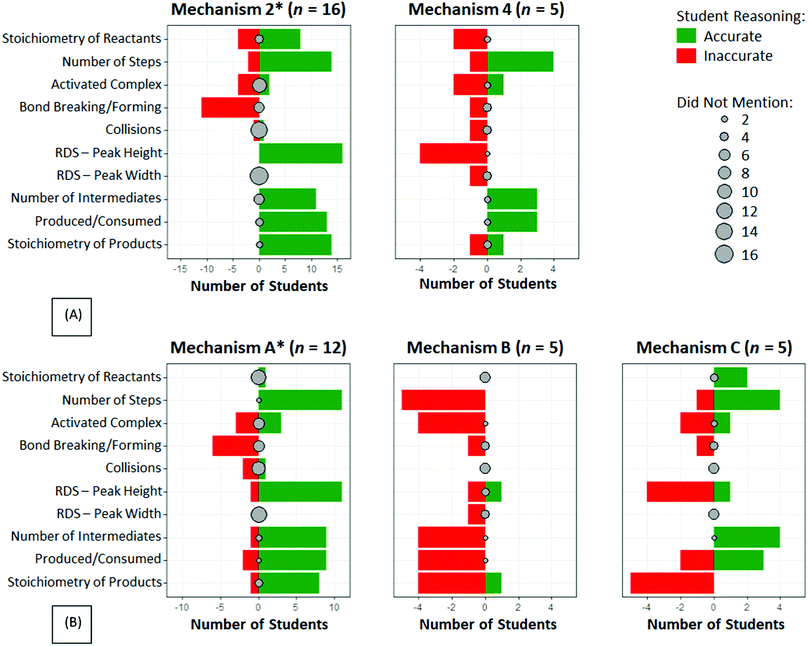 | ||
| Fig. 4 Analysis of student reasoning by mechanism choice for (A) the exothermic mechanism in Fig. 1 and (B) the endothermic mechanism in Fig. 2 when connecting particle-level mechanistic features to RCDs for each category of the modified RCD scheme middle ring in Fig. 3. Green represents accurate reasoning, red represents inaccurate reasoning, and gray represents components not mentioned by students during their reasoning. *Correct mechanism choice. | ||
The accuracy of students’ reasoning varied by RCD (exothermic reaction in Fig. 1 or endothermic reaction in Fig. 2), by mechanism choice (response options 1–4 in Fig. 1 and response options A–C in Fig. 2), and by the reasoning features in the middle ring of the modified RCD scheme (Fig. 3). That is to say, the number of students in Groups I–IV in Table 2 vary by RCD, mechanism choice, and reasoning feature. Furthermore, one student can be in different groups for different reasoning features, depending on the accuracy of their reasoning within each category. Therefore, the total number of students plotted in each part of Fig. 4 varies. A discussion of the insights gained into students’ reasoning, organized by reasoning feature from the middle ring of Fig. 3, follows.
Stoichiometry of reactants and products
Students who reasoned accurately and chose the correct mechanisms (Group I, Table 2) were distributed across accurately reasoning about the reactants (n = 18) and/or accurately reasoning about the stoichiometry of the final products (n = 10). An additional n = 5 students accurately discussed the initial reactant stoichiometry and n = 2 students accurately discussed final product stoichiometry, but did not select the correct mechanism (Group III, Table 2). A large number of students mentioned neither the stoichiometry of reactants (n = 12) nor of the products (n = 22) when reasoning with their mechanism choice.Students in Groups II (correct mechanism choice, n = 4) and IV (incorrect mechanism choice, n = 5) faced challenges related to the stoichiometry of starting reactants when selecting a reaction mechanism to correspond with the provided RCD. Likewise, 10 students (n = 10, Group II; n = 0, Group IV) struggled with inaccurate reasoning related to the stoichiometry of the final products.
These challenges became particularly apparent when students viewed the starting reactants in Mechanisms 2 and 3 (Fig. 1) where the first step in each of these two mechanisms has a single reactant of one ozone molecule. Eugene (first-year psychology major) ruled out Mechanisms 2 and 3 and instead incorrectly selected Mechanism 1:
“None of them have the right amount of- not- um…dots like, amount of molecules for the same ratio… None of them have the right amount of stuff on both sides except [Mechanism] 1.”
Students like Annie (first-year biology major) inaccurately reasoned with the stoichiometry of both the starting reactants and the final products, showing a lack of understanding on how to determine the overall reaction across the sum of elementary steps for particulate-level mechanisms:
“Okay well this one [Mechanism 2] is for sure like, I'd mark it off right away, because it started… so it doesn't start with the right number of like reactants and it doesn't end with the right number.”
Annie rejected Mechanism 2 because the products in the second step consisted of only two O2 molecules, not three as given in the balanced equation above the RCD.
For the endothermic reaction in Fig. 2, students raised concerns with the final products of Mechanisms A and B. Mary (third-year biology major) incorrectly chose Mechanism C after failing to recognize that one molecule of NO was formed as a product in the first elementary step of Mechanism A:
Mary: “[I choose] the last one [Mechanism C]. Um, so you start with 2NO2, and then you finish with two NO and one O2…These would be my reactants [pointing to the 2NO2 molecules in the first elementary step of Mechanism C] and these would be my products [pointing to the 2NO molecules and one O2 molecule as the products of the second elementary step in Mechanism C].”
Interviewer: “Why this one [Mechanism C] over this one [Mechanism A]?”
Mary: “Um, because of the end products, so we have 2NO [in the products of the overall reaction given above the RCD]. And here we only have 1NO [products of Mechanism A], and here we have 2NO [products of Mechanism C] which is what we should end up with.”
Number of steps
A majority of students selected the appropriate reaction mechanism while accurately reasoning about the number of steps in the reaction based on the number of peaks on the RCD (n = 25, Group I). Eight students (Group III) accurately reasoned about the number of elementary steps in the mechanism given the number of peaks in the RCD, but they ultimately chose an incorrect mechanism based on their reasoning with other ideas in the middle ring of the RCD scheme in Fig. 3. Only one student did not mention the number of steps when describing their reasoning, and this student chose the correct mechanism.Ten students (n = 2, Group II; n = 8, Group IV) reasoned incorrectly about the number of steps in the mechanism due to how they interpreted the progress of the reaction along the curve. For example, Natalie (second-year biology major) claimed that the RCD in Fig. 1 indicated that there were 4 steps in the reaction mechanism:
“This is going to kind of go against what I thought, but like… this [Point 1 to Point 2] would be step one, and then step two [Point 2 to Point 3] and then step three… [Point] 3 to 4, and [step four] [Point] 4 to 5.”
Likewise, Jade (second-year biology and environmental science major) offered similar reasoning about the RCD in Fig. 2:
Jade: “I kind of think of this one [Mechanism B] as like the…representative of like what's actually happening on the graph [RCD in Fig. 2]… I would say like this is a step [Point 1 to 2], this is a step [Point 2 to 3], and [Point] 3 to 4, and [Point] and 4 to 5.”
Interviewer: “So why does the energy go up for some steps and then down for other ones?”
Jade: “Because it takes a different amount of energy to form different molecules.”
Jade's reasoning offers evidence for the face validity of the 4-step reaction mechanism as a distractor in Fig. 2. Students who did not know how to accurately decode an RCD to determine the number of steps in a mechanism instead adopted an incorrect strategy of mapping the reactant and product particles from each mechanism step onto the RCD. For example, Jade supported her choice of Mechanism B by mapping the particle cut-outs from this mechanism as shown in Fig. 5. Step 1 in Mechanism B (2 NO2 react to form N2O4) happens from point 1 to point 2 in Fig. 2. Step 2 in Mechanism B (N2O4 reacts to form N2O and O3) happens from point 2 to point 3 in Fig. 2, etc. To represent the first step in Mechanism B, Jade placed 2 molecules of NO2 at point 1 and then 1 molecule of N2O4 at point 2. Then to represent step 2 of Mechanism B, she placed an additional molecule of N2O4 at point 2 and 1 molecule of N2O and 1 molecule of O3 at point 3. (So now there were 2 molecules of N2O4 at point 2 on her RCD.) Then to represent step 3, she placed an additional molecule of O3 at point 3 and then one molecule of O2 and two oxygen atoms at point 4. (So now there were 2 molecules of O3 at point 3 on her RCD). Finally to represent step 4 of Mechanism B, she placed one molecule of N2O at point 4 and then 2 molecules of NO at point 5. This mapping of particles strategy has also been reported in a research study of organic chemistry students who used what they termed a “counting parts strategy,” which involves mapping each elementary step from the reaction mechanism onto a different surface feature of the RCD (Popova and Bretz, 2018b). The use of particle representations in this current study, however, elicited a new finding that students were ‘duplicating’ particles along the RCD rather than recognizing that the product of one step in the mechanism would function as the reactant of a subsequent step in the mechanism.
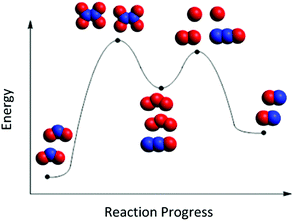 | ||
| Fig. 5 Jade's mapping of particle cut-outs onto the RCD from Mechanism B in Fig. 2. | ||
Activated complex
The number of first-year undergraduate chemistry students who discussed transition states was smaller than the number who reasoned about intermediates. Nearly half of the students in the study (n = 21) did not mention any ideas related to the nature of the transition state or activated complex when reasoning about the RCD and possible choices for the reaction mechanism.Only seven students reasoned accurately about the activated complex. Five chose the correct mechanism for their assigned RCD (Group I), and two did not (Group III). This excerpt from Edna's (first-year chemical engineering major) interview about Fig. 2 is representative:
Edna: “It's um, when the reactants…are becoming products. So it's like, the one specific point where there's just like, complete chaos, and…it's happening up here [Point 2]. And it's just like, the highest energy of the reaction.”
Interviewer: “I see. So when you said it's like total chaos…what did you mean by that?”
Edna: “There's just a lot of energy and um, the particles are moving around all over. And like some of them are half-formed, some of them are half-not, like it's just…that's the state of unstableness.”
Interviewer: “You mentioned…an activated complex, do you have an idea of like, what an activated complex might look like? … What is an activated complex?”
Edna: “Um well, you can’t really see it…when the bonds are starting to break from your reactants and they’re starting to form with your products, that's the state when it's… You have half the reactants are still reactants, half are broken up, some are already products.”
Sixteen students (n = 7, Group II; n = 9, Group IV) reasoned inaccurately about the transition state, including Corrine (first-year chemical engineering major) who described the transition state as simply a mixture composed of reactants and products:
“Here [Point 2 in Fig. 1] we have I guess a mix of… reactants and products. And then when you get to Point 3, it would just be the products of the first step.”
Meanwhile, Ricardo (first-year biology major) explained that transition states are all molecules being broken apart into separate atoms at the top of the peaks on the RCD:
Ricardo: “Here [Point 2 in Fig. 2] the individual atoms [of the reactants] would separate and then recombine… I don't have a bunch of individual stuff [atoms] to put up there [Point 2]. But if I did, that's where I would put them… They’ll recombine here [at Point 3 to form products of step one].”
Interviewer: “With it happening, like separating and recombining… Why do we not note that in the steps, why do the steps just like, show this going directly from here [reactants of step one] to here [products of step one]?”
Ricardo: “I would guess for the sake of brevity… It seems like something that would be commonly understood. Like how for some Lewis diagrams you don't draw the hydrogen atoms everywhere. So I guess it's sort of just like shorthand.”
Other students had difficulty distinguishing between a transition state and an intermediate. Carol (second-year psychology major) eventually concluded that the RCD does not contain information about transition states:
Carol: “Point 3 [in Fig. 2] would be a transition state so it's- once all of the products have broken down, there's going to be a phase where you would have to add more energy again to get to the final desired products.”
Interviewer: “What's the difference between like what happens at Point 2 and 4 and what happens at Point 3?”
Carol: “[Point] 2 and 4 are the amount of activation that you would need to go to the next phase, but [Point] 3 is like…more so intermediate, where it's in between the phases of the reaction.”
Interviewer: “So this [Point 3] is like the intermediate and it's also like the transition state?”
Carol: “It's more so the intermediate.”
Interviewer: “Is it [Point 3] still also a transition state or is [a transition state] something else? “
Carol: “That's [a transition state is] something else. I don't think that that's information you can get from this [RCD].”
Interviewer: “Where would you get that information from?”
Carol: “Um, not really sure.”
First-year undergraduate students’ confusion between transition states and intermediates is not surprising, given that both terms have a connotation of an ‘in between’ state or a ‘period of change’ into another state. This conflation, confusion, and interchangeable use of the two terms has also been reported to be a prevalent misconception of organic chemistry students (Popova and Bretz, 2018c).
Bond breaking and bond formation
One startling feature of the data visualization in Fig. 4 is that no student reasoned accurately about bond breaking and bond formation as dynamic processes occurring along the RCD curve. Furthermore, more than half of the students (n = 24) did not discuss the breaking or forming of bonds when discussing how the particulate mechanism steps related to the RCD.Twenty students (n = 17, Group II; n = 3, Group IV) reasoned about specific RCD features as indicative of bond breaking and/or bond formation, but they did so inaccurately. Eleven students, including Ricardo (first-year biology major), reported that bond breaking and formation occurs only at the peaks and valleys. Ricardo explained that bond breaking in Mechanism C occurs at the top of the peaks of the RCD in Fig. 2, with molecules separating completely into individual atoms, while bond formation occurs in the valley:
“Starting out we have two NO 2 molecules [at Point 1], and they’re- it's all gaseous, so it kind of looks like, um, as they go up to here [at Point 2] the individual atoms would separate and then recombine like this [N2O and O3 molecules at Point 3]… They’ll recombine here [to form the molecules N2O and O3 at Point 3] and then separate again here [separate into individual atoms at Point 4] and then recombine like that [forming two NO molecules and an O2 molecule at Point 5].”
Other students (n = 5) reasoned that bond breaking and forming occurred only between the peaks and valleys of the RCD curve. Ellie (first-year medical laboratory science major) described bond breaking in Mechanism 2 as occurring on the left halves of the peaks of the RCD in Fig. 1 and bond formation occurring on the right halves of those peaks:
“As you're going along this curve [between Point 1 and Point 2],… one [one O3 molecule] is going to like break apart [into one O2 molecule and one oxygen atom]… This is when they're breaking apart [between Point 1 and Point 2]. So then maybe some of them reform [the O2 molecule and the oxygen atom recombine to form one O3 molecule, between Point 2 and Point 3]… More of these [O3] can be breaking apart [between Point 3 and Point 4]… So then we have these [two oxygen atoms], and then these [two oxygen atoms] would be coming together [between Point 4 and Point 5] forming these [final products in the second step of Mechanism 2].
The remaining 3 students specified certain points where bond breaking and formation occur. Susan (first-year biology major) explained that bond formation in Mechanism B occurs at the tops of the peaks in Fig. 2, while bond breaking occurs between the peaks/valleys:
Susan: “So then going along, you have two NO2 [at Point 1]. Those bond together [at Point 2] to create this N2O4. The N2O4breaks apart [between Point 2 and Point 3] into the N2O and the O3, and then you just, using the O3to break apart [between Point 3 and Point 4] into one of the products which is the O2and then just a single oxygen. And then that single oxygen reacts with another N2O [at Point 4]…and creating the products [two NO molecules] at the end [Point 5].”
Interviewer: “What's going on here [between Point 4 and Point 5]?”
Susan: “The same with this [between Point 1 and Point 2], like there is nothing because they’re in this area [between Point 4 and Point 5]. There can be a single nitrogen because it's breaking apart from the N2O [at Point 4], but then it immediately bonds to the oxygen, so it's just creating these two at the end [two NO molecules at Point 5].”
Interviewer: “[the third step of Mechanism B] shows like, the single oxygen breaking off of the O3… Why does it [the fourth step of Mechanism B] not show, like, a single nitrogen breaking off?”
Susan: “Um…because that's like, not a significant reaction in itself [referring to the nitrogen atom breaking off of the N2O molecule at Point 4].”
Interviewer: “Why would that not be a like significant reaction but then this is [referring to the third step of Mechanism B]?”
Susan: “Um, because this one [third step in Mechanism B] is creating one of the products [one O2 molecule]. And then what's left over from that reaction [one oxygen atom] is being used to create the rest of the products [two NO molecules in the fourth step of Mechanism B].”
This assignment of different processes to different portions of the RCD curve was similarly reported in an earlier study of substitution and elimination reactions with organic chemistry students who often described that the hill preceding a peak on an RCD merely showed the “acquisition of the necessary conditions for the process of bond breaking to start” (Popova and Bretz, 2018b).
Collisions
Only seven students mentioned collisions when reasoning with the RCD and the possible particulate level mechanisms, and only two students (Group I) reasoned accurately about the importance of molecules colliding with both sufficient energy and proper orientation. Jay (first-year biochemistry major) described collisions in the second step of Mechanism 2 when reasoning about the RCD in Fig. 1:“Not every collision reacts- uh causes a reaction. If it doesn't have the right amount of energy. So when you heat it up you give the molecules more energy, more likely that they're going to react or collide… A collision occurs between these [O3 and O molecules between Point 3 and Point 4], and- with sufficient energy and proper orientation. And that begins to form this transition state [at Point 4].”
No students were in Group III (accurate reasoning, incorrect mechanism choice) mentioned collisions. Five students (n = 3, Group II; n = 2, Group IV) reasoned inaccurately about collisions when identifying connections between their assigned RCD and the possible particulate-level reaction mechanisms. Reed (first-year biology major) initially described collisions, but ultimately decided against the idea based on the particulate-level reaction mechanism steps when reasoning about Fig. 2:
Reed: “A bunch of these products which are intermediates [2NO3 molecules in Mechanism A at Point 3] will keep on colliding as more energy is then reinvested into the collision of these [2NO3, drags them up from Point 3 toward Point 4]…These [2NO3] will eventually uh, form these two end products [NO and O2] at the end [Point 5].”
Interviewer: “If there's like two of these [2NO3] coming together and hitting each other… Why does this [step 2 of Mechanism A] only show one [NO3] instead of two [2NO3]?”
Reed: “Uh, just be- hmm, actually [long pause]. I would actually maybe reverse my claim. Um, it could also be more likely that um, the more energy that you invest into- or like, as the energy is increasing, one of these molecules [NO3], then um, the bonds can break and then they form into a more stable…forms [NO and O2 products at Point 5]. And they're like, less- less likely that they’re [the two NO3 molecules] like, colliding with each other.”
Rate-determining step
Most students reasoned accurately about the rate-determining step of the reaction mechanism by examining the surface feature of peak height and chose the correct mechanism (n = 27, Group I).Only 3 students comprised Groups II (n = 1) and III (n = 2). Those in Group II inaccurately reasoned about peak height but selected the correct mechanism due to other RCD surface features. Group II consisted of one student, Will (first-year kinesiology major), who decided that the reaction rate was unrelated to activation energy:
“… one's slow [Step 1 of Mechanism A in Fig. 2]. And so it's going to take more activation energy, but I don’t think that actually really matters. Because I don’t think it [activation energy] has to do with how fast the reaction moves, I think it's just the energy needed for the reaction to happen.”
Four students did not mention peak height when describing their mechanism choices (and none of these students selected the correct mechanism). Ten students reasoned inaccurately with the RCD about the rate-determining step, chose the wrong mechanism (Group IV), and in doing so, expressed a variety of inaccurate ideas. Ricardo (first-year biology major) did not explicitly mention the ‘rate-determining step,’ but he reasoned:
“[Mechanism C] goes fast and then slow, [in Fig. 2] that's higher [first peak] then lower [second peak], which I think should go with [Mechanism C]. I think so because if [the peak is] higher up, it requires more energy which I think should mean it should happen quickly… I’m not as confident about the higher ones [peaks] meaning it's fast or slow.”
Maggie (first-year biology major) inaccurately described the first peak in Fig. 1 as the rate-limiting step and chose Mechanism 4:
“because that's- it's slow and then it goes fast…Because you don't have a lot of energy [gestures to the first peak], so since you don't have a lot of energy it takes a longer time for the molecules to collide and make the product.”
Jude (first-year chemical engineering major) reasoned that the fast/slow indicators on the particulate-level reaction mechanisms in Fig. 2 did not relate to the RCD:
“I don’t really know if they relate to this [Fig. 2]. Um, I mean you can kind of see the reaction starts up slow… I would just say that it doesn’t really pertain a lot to this diagram just because it's a slow reaction, but it's still occurring.”
Two students discussed the rate-determining step in terms of the peak width, ultimately choosing an incorrect mechanism. Annie (first-year biology major) commented on not only the width of the peak, but also the slope of each peak:
“First I just looked at the like, steepness of the slope, and this one looks steeper [second peak in Fig. 1] and then this is like the time of the reaction [gestures to reaction progress on x-axis], and just looked at like it- like the intervals between here [Points 3 and 4] were closer together which means it was less time between them than these two [Points 1 and 2]- so this [first peak] would be slower and that's faster [second peak].”
The idea that reaction progress on the x-axis of RCDs indicates a measure of time or the speed of a reaction is a prevalent student misconception that has been observed in previous research studies in organic chemistry contexts (Popova and Bretz, 2018b, 2018c) but not previously reported in first-year university chemistry courses. Students incorrectly attach a unit of time or speed to the x-axis on an RCD, given that the overall progress of the reaction occurs over a period of time, and the word progress can be defined as moving forward in space or time.
Number of intermediates
Eleven students did not mention the number of intermediates related to the RCD, but most students (n = 20) did accurately reason about the number of intermediates in the reaction mechanism and correctly chose a mechanism (Group I). Although students in Group III (n = 7) were able to accurately discern the number of intermediates present in a mechanism, they were unable to accurately decode the number of intermediates present on the RCD. For example, Chelsea (first-year biology major) chose Mechanism C in Fig. 2 containing two intermediates:“So as this reaction happens, I think that um these are going to get used up [the two O3 molecules]… And these get used up as well [the two N2O molecules]… These [O3 and N2O] are kind of like those intermediates [places them at the peaks of the RCD in Fig. 2]… Like as this reaction uh, takes place, these [O3 and N2O] kind of get used up or kind of absorbed… And they create this [final products at Point 5].”
Six students (n = 1, Group II; n = 5, Group IV) struggled with connecting the number of intermediates in the reaction mechanism to the RCD, even though they had correctly identified the intermediate as represented by the surface feature of the valley on the RCD. Rita (first-year biology major), who incorrectly chose Mechanism B in Fig. 2, described her thinking when placing the molecules in the reaction mechanism along the curve of the RCD:
“I want to just put [the particles] with the steps [of Mechanism B], but also I just really associate this [Point 3 in Fig. 2] with intermediates… I just kind of in my mind I just want to put them [the intermediates] there [Point 3].”
Produced and consumed
Nearly two-thirds of the students described intermediates as being first produced and then consumed in the mechanism (n = 22, Group I; n = 6, Group III), although students in Group III chose the incorrect mechanism due to other features of the RCD. Seven students (Exothermic, n = 5; Endothermic, n = 2) did not mention the transient nature of the intermediate when discussing the steps of the reaction mechanism.Nine students reasoned inaccurately about the existence of intermediate(s) along the RCD (n = 2, Group II; n = 7, Group IV), including Rita (first-year biology major) who seemed to undervalue the role of intermediates when reasoning with multiple possible reaction mechanisms:
“Everything else just that's kind of in the middle, I associate with intermediates, so the stuff that kind of takes place in between the transition states… they [intermediates] don't exist in the end [at Point 5 in Fig. 2] so I kind of just see the stuff as filler.”
Lily (second-year neuroscience major) was also challenged to connect her reasoning to intermediates in Fig. 2:
Interviewer: “Ok, so when you say those [intermediates] cancel, what does that mean?”
Lily: “They are present in the reaction. They- they’re just not…relevant… They do come along [the RCD curve] for the ride.”
Interviewer: “So are they- are they still present at the end [of the RCD curve] … what are your thoughts there?”
Lily: “Um…maybe they’re not present at the end because they just go here and then- like they're not the products. Um, maybe they… just are formed in the reaction and then they kind of make new prod- or like…I don’t know, I mean they just kind of exist in the reaction but then they're not at the end [at Point 5 on the RCD]. I don't really understand the intermediates that well, but like, I know that they’re present in the reaction and then they cancel at the end, is kind of all I really need to know for the exam. Like, we don’t go into so much depth, where they are [on the RCD curve].”
Particle cut-outs
The final activity in Phase III of the interviews provided each student with paper cut-outs of all the space-filling particle representations in the reaction mechanism that they chose as best corresponding with the RCD. Students were asked to place these particle cut-outs along the curve of the RCD. Analysis of students’ choices and explanations during this part of the interview led to two significant insights about students who reasoned inaccurately during this task that required them to meaningfully integrate their understanding of the surface features of an RCD with their prior knowledge about intermediates, transition states, and the elementary steps and overall reaction in a reaction mechanism.The first insight was that some students attended less to the dynamic nature of the reaction, and more to distributing the particles along the curve with attention to the surface features of the RCD. These students wanted each surface feature of the RCD (peaks and valleys, in particular) to have a one-to-one correspondence to some aspect of the particulate mechanism. For example, many students who chose Mechanism B in Fig. 2 placed the particles in the reaction mechanism along the curve, as Jade (second-year biology and environmental science major) – who incorrectly chose Mechanism B as the correct mechanism due to 4 steps – did in Fig. 5.
This reasoning was further challenged by students’ conflation of intermediates and transition states. In Fig. 6, Chelsea (first-year biology major) placed the intermediates in Mechanism C at the top of the peaks. Students were confused when they realized they did not have enough particles to assign to each surface feature of the RCD, e.g., the valley (intermediate) in Fig. 6. When placing the particles from Mechanism A along the RCD in Fig. 2, Judith (second-year kinesiology major) said.
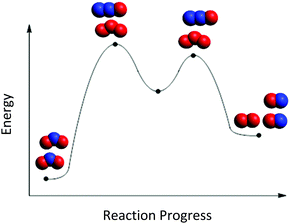 | ||
| Fig. 6 Chelsea's mapping of particle cut-outs from Mechanism C onto the RCD in Fig. 2. | ||
“Hmm…[long pause]… Maybe that would be there [places two NO2 molecules at Point 1, one NO3 molecule at Point 2, one NO molecule at Point 3, one NO3 molecule between Point 3 and Point 4, one NO molecule and one O2 molecule at Point 5]. Or yeah, maybe it looks something like that [moves NO3 molecule from between Points 3 and 4 to Point 4, moves NO molecule from Point 3 to between Points 2 and 3]. I think I was thinking…[long pause]… Actually, I think these would both be down here [moves both NO3 molecules and the NO molecule down to Point 3]… Yeah I guess I don’t know.”
Students were confused that they were not provided particles to represent transition state(s). As Edna (first-year chemical engineering major) placed particle cut-outs of Mechanism C along the RCD in Fig. 2, she commented:
“And then…up here…up here [at Point 2] is like… I don't have a picture to put.”
The second insight was that some students wanted to simplify the reaction mechanism steps, rather than attend to each RCD surface feature. Eugene (first-year psychology major) placed only the reactants at the beginning of the curve and the products at the end of the curve (Fig. 7). He placed no particles at any other point along the RCD because the RCD “just show[ed] like the beginning and the end” of the reaction. When asked if any particles could be found along the curve, he stated:
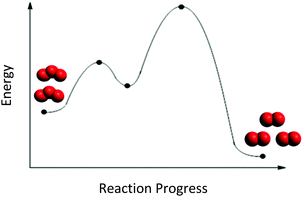 | ||
| Fig. 7 Eugene's mapping of particle cut-outs from Mechanism 1 onto the RCD in Fig. 1. | ||
“I feel like this [Point 2] would be maybe it's like the molecules broken apart… I feel like this [Point 3] would just be like a different ratio of how many- molecules are separated and uh, together… Like some are together [O3molecules], some broken up [O atoms], there's like a random- random order you know… And then like they start to break apart more up here [at Point 4].”
Conclusions and implications
This study investigated how students interpret and reason with both the kinetic and thermodynamic information encoded in RCDs when choosing among possible particulate-level reaction mechanisms. Findings include that many students who chose the correct reaction mechanism that corresponded to the given RCD nonetheless still held inaccurate ideas related to the kinetics and thermodynamics concepts encoded in that diagram and the mechanistic steps of the reaction. As an interesting counterpoint, students who chose an incorrect reaction mechanism as corresponding to an RCD could still hold some accurate ideas about some features of the diagram and reaction mechanism.Novak and Ausubel's meaningful learning theory speaks to the important of meaningful connections between concepts. In the absence of these meaningful connections, students can resort to memorizing fragments of information, in this case, the surface features of RCDs and mechanisms, without developing a deep conceptual understanding of the nature of the relationship between these ideas. Students’ representational competence in translating and connecting multiple representations (RCD, particulate mechanism) were not equally developed, nor well connected. In particular, they struggled to incorporate the spacefilling particulate representations of molecules and atoms into their understanding of the symbolic representations of the RCD and the overall balanced equation given in Fig. 1 or 2. The prevalence of students’ reasoning with these ideas has been investigated and published for undergraduates in first-year chemistry, organic chemistry, and physical chemistry (Atkinson et al., 2020).
Ten categories of students’ reasoning with surface features of RCDs with reaction mechanisms were identified from the data analyses in this study. Many students did not mention transition state(s) or describe the nature of a transition state as an activated complex, and they had more difficulty with describing transition states than intermediates, particularly when attempting to reason with the particle cut-outs. Additionally, students were unsure how to meaningfully connect ideas about bond breaking and bond formation from mechanistic reaction steps to RCDs. Results from this study show that students generally understand the nature of intermediates, in that they are produced and then consumed in the reaction. However, they are often unable to connect this idea to the RCD, and they often confuse intermediates with transition states on the diagrams. Many students also did not mention certain critical RCD surface features when explaining their reasoning for connecting a mechanism to that diagram, with a surprisingly low frequency of students evoking ideas about collision theory.
While it was encouraging that only a few of the students interviewed mentioned the width of the peak when thinking about the rate-determining step of the reaction mechanism, a high number of students (n = 10) described inaccurate ideas related to peak height and chose the incorrect mechanism. In addition, 4 students did not mention peak height when describing their reasoning behind their mechanism choice. If students have inaccurate reasoning about peak height on an RCD and how it relates to mechanistic reaction steps or fail to reason with important surface features of RCDs like peak height, they may attach their own incorrect meaning to the x-axis on the diagram. Previous studies have shown that when confronted with using peak height or peak width to determine the rate-determining step on an RCD, students at the first-year undergraduate chemistry level often choose to use the width of the peak, or reaction progress, instead of correctly using the activation energy (Atkinson et al., 2020). The misconception that reaction progress indicates time has also been seen in studies conducted with undergraduates studying organic chemistry (Popova and Bretz, 2018c).
The implications for implementing the findings of this research study into evidence-based teaching practices include eliciting students’ prior knowledge about the kinetics and thermodynamics concepts that are encoded in RCDs. Much of what experts might expect novice students to reason about RCDs, to reason about reaction mechanisms, and then to connect these multiple representations is, as evidenced by the findings reported herein, a very challenging task that requires careful scaffolding to develop meaningful connections and not just memorize pieces of information. When we do not provide students with opportunities to develop an understanding of each of the underlying concepts encoded within complex representations like RCDs and then to build connections between them, students may attempt to attach their own underlying meaning to the structural features of that representation or just memorize discrete ideas and not recognize the importance of forming connections among them.
Additionally, it is critical to provide first-year undergraduate chemistry students with the opportunity to interpret both symbolic- and particulate-level reaction mechanisms when asking them to reason with RCDs. The challenges that students faced in mapping particle cut-outs to the surface features of RCDs reveal gaps in their understanding about transition states and when bond breaking and bond making happen throughout a reaction mechanism. Students must be able to visualize and reason with events at the molecular level so that they can make meaningful connections between the complex underlying concepts of related multiple representations - RCDs and the elementary steps of reaction mechanisms.
Finally, it is important to reflect upon the implications of our findings for assessment. The distractor options we provide our students on assessments must be thoughtfully chosen with students’ own thinking in mind. The 3-step distractor reaction mechanism included in the exothermic example was not chosen by any students, while the 4-step distractor mechanism in the endothermic example was chosen by 5 students. Based on findings in this study, it may be more helpful for future studies and assessments to focus on 1-, 2-, and 4-step mechanisms when students are given a 2-step RCD, as no students chose the distractor 3-step reaction mechanism. Given that most students could count the number of steps and reason about fast/slow, we must challenge ourselves to also assess students on their reasoning. We must ask why and how in addition to what. If we do not ask students to explain their reasoning with the underlying concepts encoded in features of representations, we may assume that they understand the concepts because they choose the correct answer. This study provides further evidence that this assumption is not always correct, and that we must ask students to explain and reason in conversations in the classroom as well as on assessments (Cooper, 2015).
This research study investigated students’ thinking with RCDs using particulate representations of mechanisms. The findings reported herein suggest that future chemistry education research studies ought to be designed to intentionally investigate additional elements of how first-year university students reason about reaction mechanisms. Particulate-level representations of molecules could be useful tools to characterize students’ thinking about mechanisms which are typically represented using the symbolic domain of Johnstone's triangle through balanced chemical equations. In particular, the description of transition states and/or the activated complex as a collection of individual atoms created by breaking all the bonds in the reactants before then forming any bonds in the products harkens back to a mathematical procedure typically taught when introducing thermochemistry. That is, future research should explore the connections and misconceptions that students have about bond dissociation energy as a thermodynamic concept that they may be erroneously mapping onto understanding reaction mechanisms.
While several findings from this data set of first-year university students resonate with those of the data set for organic chemistry students (Popova and Bretz, 2018a; Popova and Bretz, 2018b; Popova and Bretz, 2018c), there are some key differences. The interview protocols for first-year students used gas phase reactions with space-filling representations of the reaction mechanisms. The organic chemistry interview protocol used substitution and elimination reactions with line structures and Lewis structures. Neither Table 2 nor Fig. 4 were used to analyze the organic chemistry data set as these analytic tools were developed for this study. Research that uses an interview protocol with Lewis structures in first-year chemistry and/or particle representations in organic chemistry would afford an opportunity to conduct a cross-sectional, longitudinal analysis for commonalities in reasoning among students who have differing prior knowledge bases.
Research studies could also be designed in order to investigate the effect of different general chemistry curricula, e.g., traditional vs. atoms-focused, upon students’ meaningful learning (the intentional formation of substantive connections between concepts) and representational competence with connecting multiple representations across Johnstone's symbolic and particulate domains.
Limitations
This study explored students’ reasoning about reaction coordinate diagrams, but did not provide any experimental data or experimentally determined rate laws for students to consider when choosing among possible particulate-level mechanisms. This study also did not directly investigate the presence of catalysts in reactions, and thus, this important category was excluded from the additional middle ring in Fig. 3. Catalysts may impact students’ reasoning about many of the surface features and the meanings encoded in those surface features for experts. However, where catalysts fit on the modified RCD scheme in Fig. 3 for students at the first-year undergraduate chemistry level is beyond the scope of this research and should be further explored in future studies. The study was conducted at a single institution, and additional research studies should include students from multiple institutions to reflect a broader array of students’ ideas and reasoning with RCDs.Although the textbook used in the first-year undergraduate chemistry sequence (Gilbert et al., 2017) featured particulate representations in its pedagogy and accompanying assessment tools, the data collection for this research did not include observation of classroom instruction nor analysis of artifacts such as course exams. Therefore, commenting upon the extent to which particulate representations were included in instruction is beyond the scope of this research.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Grant No. 1432466 from the National Science Foundation. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. We thank the students and instructors of the first-year undergraduate chemistry courses who volunteered to participate in this study.References
- Allinger N. L., (1963), Energy relations in teaching organic chemistry. J. Chem. Educ., 40(4), 201–202.
- Atkinson, M.B., Popova, M., Croisant, M., Reed, D., Bretz, S.L., (2020), Development of the reaction coordinate diagram inventory: Measuring student thinking and confidence. J. Chem. Educ., 97(7), 1841–1851.
- Avogadro Software, (2020), https://avodagdro.cc, last accessed October 26, 2020.
- Bain K. and Towns M. H., (2016), A review of research on the teaching and learning of chemical kinetics. Chem. Educ. Res. Pract., 17(2), 246–262.
- Bain K., Moon A., Mack M. R., and Towns M. H., (2014), A review of research on the teaching and learning of thermodynamics at the university level. Chem. Educ. Res. Pract., 15(3), 320–335.
- Bain K., Rodriguez J. M. G., and Towns M. H., (2018), Zero-Order Chemical Kinetics as a Context to Investigate Student Understanding of Catalysts and Half-Life. J. Chem. Educ., 95(5), 716–725.
- Becker N. M., Rupp C. A., and Brandriet A., (2017), Engaging students in analyzing and interpreting data to construct mathematical models: An analysis of students’ reasoning in a method of initial rates task. Chem. Educ. Res. Pract., 18(4), 798–810.
- Bodner G. M., (1986), Constructivism: A theory of knowledge. J. Chem. Educ., 63(10), 873–878.
- Brandriet A., Rupp C. A., Lazenby K., and Becker N. M., (2018), Evaluating students’ abilities to construct mathematical models from data using latent class analysis. Chem. Educ. Res. Pract., 19(1), 375–391.
- Bretz S. L., (2001), Novak's theory of education: Human constructivism and meaningful learning. J. Chem. Educ., 78(8), 1107.
- Bretz S. L., (2008), Qualitative research designs in chemistry education research, in Bunce D. M. and Cole R. S. (ed.), Nuts and Bolts of Chemical Education Research, ACS Symposium Series, pp. 79–99.
- Cakmakci G., (2010), Identifying alternative conceptions of chemical kinetics among secondary school and undergraduate students in Turkey. J. Chem. Educ., 87(4), 449–455.
- Çalik M., Kolomuç A., and Karagölge Z., (2010), The Effect of Conceptual Change Pedagogy on Students’ Conceptions of Rate of Reaction. J. Sci. Educ. Technol., 19(5), 422–433.
- Chi M. T. H., Feltovich P. J., and Glaser R., (1981), Categorization and representation of physics problems by experts and novices. Cognit. Sci., 5(2), 121–152.
- Cooper M. M., (2015), Why Ask Why? J. Chem. Educ., 92(8), 1273–1279.
- Creswell J. W., (2003), in Laughton C. D. (ed.), Research Design: Qualitative, Quantitative, and Mixed Methods Approaches, 2nd edn, Sage Publications, Inc.
- Davidowitz B. and Chittleborough G., (2009), Linking the macroscopic and sub-microscopic levels: Diagrams, in Gilbert J. K. and Treagust D. F. (ed.), Multiple Representations in Chemical Education, Springer, pp. 169–191.
- Drever E., (1995), Using semi-structured interviews in small-scale research: A teacher's guide, The SCRE Centre.
- Fram S. M., (2013), The constant comparative analysis method outside of grounded theory. Qual. Rep., 18(1), 1–25.
- Galloway K. R., Leung M. W., and Flynn A. B., (2019), Patterns of reactions: a card sort task to investigate students’ organization of organic chemistry reactions. Chem. Educ. Res. Pract., 20(1), 30–52.
- Geertz, C. (1973). Thick description: Towards an interpretive theory of culture. Basic Books: New York.
- Gilbert T. R., Kirss R. V., Foster N., Bretz S. L., and Davies G., (2017), Chemistry: The Science in Context, 5th edn, W. W. Norton & Company.
- Herrington D. G., Yezierski E. J., Luxford K. M., and Luxford C. J., (2011), Target inquiry: Changing chemistry high school teachers’ classroom practices and knowledge and beliefs about inquiry instruction. Chem. Educ. Res. Pract., 12(1), 74–84.
- Holme T. and Murphy K., (2012), The ACS exams institute undergraduate chemistry anchoring concepts content map I: General chemistry. J. Chem. Educ., 89(6), 721–723.
- Holme T., Luxford C., and Murphy K., (2015), Updating the General Chemistry Anchoring Concepts Content Map. J. Chem. Educ., 92(6), 1115–1116.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem. J. Comput. Assist. Learn., 7(2), 75–83.
- Johnstone A. H., (2010), You can’t get there from here. J. Chem. Educ., 87(1), 22–29.
- Justi R., (2006), Teaching and Learning Chemical Kinetics, in Chemical Education: Towards Research-based Practice, Kluwer Academic Publishers, pp. 293–315.
- Kozma R. B. and Russell J., (1997), Multimedia and Understanding: Expert and Novice Responses to Different Representations of Chemical Phenomena. J. Res. Sci. Teach., 34(9), 949–968.
- Kozma R. and Russell J., (2005), Students becoming chemists: Developing representational competence, in Gilbert J. K. (ed.), Visualization in Science Education, Springer, pp. 121–146.
- Krieter F. E., Julius R. W., Tanner K. D., Bush S. D., and Scott G. E., (2016), Thinking Like a Chemist: Development of a Chemistry Card-Sorting Task to Probe Conceptual Expertise. J. Chem. Educ., 93(5), 811–820.
- Lincoln Y. S. and Guba E. G., (1985), Naturalistic Inquiry, Sage Publications, Inc.
- Linenberger K. J. and Bretz S. L., (2012), A Novel Technology to Investigate Students’ Understandings of Enzyme Representations. J. Coll. Sci. Teach., 42(1), 45–49.
- Meek S. J., Pitman C. L., and Miller A. J. M., (2016), Deducing Reaction Mechanism: A Guide for Students, Researchers, and Instructors. J. Chem. Educ., 93(2), 275–286.
- Murphy K., Holme T., Zenisky A., Caruthers H., and Knaus K., (2012), Building the ACS exams anchoring concept content map for undergraduate chemistry. J. Chem. Educ., 89(6), 715–720.
- Nakhleh M. B., (1993), Are our students conceptual thinkers or algorithmic problem solvers? Identifying conceptual students in general chemistry. J. Chem. Educ., 70(1), 52–55.
- Nakhleh M. B. and Mitchell R. C., (1993), Concept learning versus problem solving: There is a difference. J. Chem. Educ., 70(3), 190–192.
- Novak J. D., (1977), A Theory of Education, Cornell University Press.
- Novak J. D., (1998), Learning, Creating, and Using Knowledge, Lawrence Erlbaum Associates.
- Nurrenbem S. C. and Pickering M., (1987), Concept learning versus problem solving: Is there a difference? J. Chem. Educ., 64(6), 508–510.
- NVivo Qualitative Data Analysis Software, 2020.
- Patton M. Q., (2002), Qualitative Research & Evaluation Methods, 2nd edn, Sage Publications, Inc.
- Pickering M., (1990), Further studies on concept learning versus problem solving. J. Chem. Educ., 67(3), 254–255.
- Popova M. and Bretz S. L., (2018a), “It's only the Major Product That We Care about in Organic Chemistry”: An Analysis of Students’ Annotations of Reaction Coordinate Diagrams. J. Chem. Educ., 95(7), 1086–1093.
- Popova M. and Bretz S. L., (2018b), Organic chemistry students’ challenges with coherence formation between reactions and reaction coordinate diagrams. Chem. Educ. Res. Pract., 19(3), 732–745.
- Popova M. and Bretz S. L., (2018c), Organic chemistry students’ interpretations of the surface features of reaction coordinate diagrams. Chem. Educ. Res. Pract., 19(3), 919–931.
- Pratt J. M. and Yezierski E. J., (2018), College students teaching chemistry through outreach: Conceptual understanding of the elephant toothpaste reaction and making liquid nitrogen ice cream, J. Chem. Educ., 95(12), 2091–2102.
- Prins G. T., (2010), Teaching and Learning of Modelling in Chemistry Education Authentic Practices as Contexts for Learning, Leersum.
- Rodriguez J. M. G., Santos-Diaz S., Bain K., and Towns M. H., (2018), Using Symbolic and Graphical Forms to Analyze Students’ Mathematical Reasoning in Chemical Kinetics. J. Chem. Educ., 95(12), 2114–2125.
- Rodriguez J. M. G., Bain K., Hux N. P., and Towns M. H., (2019), Productive features of problem solving in chemical kinetics: More than just algorithmic manipulation of variables. Chem. Educ. Res. Pract., 20(1), 175–186.
- Rouse W. B. and Morris N. M., (1986), On looking into the black box: Prospects and limits in the search for mental models. Psychol. Bull., 100(3), 349–363.
- Sawrey B. A., (1990), Concept learning versus problem solving: Revisited. J. Chem. Educ., 67(3), 253–254.
- Silberberg M., (2012), Principles of General Chemistry, 3rd edn, McGraw Hil Education.
- Strauss A. and Corbin J., (1998), Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory, Sage Publications, Inc.
- Taber K. S., (2013), Revisiting the chemistry triplet: Drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education. Chem. Educ. Res. Pract., 14(2), 156–168.
- Talanquer V., (2011), Macro, submicro, and symbolic: The many faces of the chemistry “triplet.” Int. J. Sci. Educ., 33(2), 179–195.
- Taştan Ö., Yalçinkaya E., and Boz Y., (2010), Pre-service chemistry teachers’ ideas about reaction mechanism. J. Turkish Sci. Educ., 7(1), 47–60.
- Treagust D. F., (1988), Development and use of diagnostic tests to evaluate students’ misconceptions in science. Int. J. Sci. Educ., 10(2), 159–169.
| This journal is © The Royal Society of Chemistry 2021 |