Exploring the use of a writing-to-learn activity embedded with multiple modes using ‘Popplet’ on pre-university students’ alternative conceptions on transition metals
Nilavathi
Balasundram
 and
Mageswary
Karpudewan
and
Mageswary
Karpudewan
 *
*
School of Educational Studies, Universiti Sains Malaysia, Penang, Malaysia. E-mail: bnilavathi@gmail.com; kmageswary@usm.my; mageswary_karpudewan@yahoo.com
First published on 23rd November 2020
Abstract
A study of transition metals includes studying the physical and chemical properties of first-row d block metals in the Periodic Table. The curriculum on transition metals emphasizes learning the physical and chemical properties and is a lecture-based strategy that is predominately employed by teachers to transfer the knowledge on properties of the metals. As the teacher-centred strategy is unable to promote conceptual change, students frequently retain incorrect alternative conceptions about the metals. Literature indicates that multiple modes of representation embedded within the writing to learn activity facilitated learning and improved understanding of several scientific concepts. Literature also indicates that using a digital platform to create the writing encourages embedding of multiple modes. Modes such as diagrams, chemical equations, graphs, chemical formulae, and tables are often employed to illustrate the properties of transition metals. Hence, in this study, an attempt was made to encourage embedding modes commonly associated with transition metals into a writing-to-learn activity using the ‘Popplet’ application communicating the information on the properties of metals to their peers. The study explored the effect of embedding multiple modes of representation within a writing-to-learn activity using ‘Popplet’ in reducing the alternative conceptions on transition metals. For this purpose, 81 pre-university students responded to a diagnostic test administered as a pre-test before the writing-to-learn activity and post-test after the activity. From the pre-test, the alternative conceptions held by the students were identified. The post-test results showed a smaller number of students with the alternative conceptions identified in the pre-test. The paired sample t-test shows that the difference between the pre and post-test means is significant. The qualitative interview responses provided insights into how embedding the modes within the writing activity using ‘Popplet’ reduces the alternative conceptions. The findings of the study inform the teachers of an alternative student-centred approach to teaching the lessons on transition metals. This study introduces multiple modes of representation embedded within a writing-to-learn activity using ‘Popplet’ as an approach that promotes conceptual change.
Introduction
Studies on understanding of chemistry concepts across secondary to tertiary levels have revealed that students predominately conceptualise them differently from the scientific community (Taber, 2002). Such incorrect conceptions are known as misconceptions (Taber, 2002), alternative conceptions (Driver and Easley, 1978), alternative frameworks (Driver, 1981), pre-conceptions (Gauchon and Méheut, 2007), naive conceptions (Howard et al., 2013), erroneous ideas (Sanders, 1993) or misperceptions (Kim et al., 2019). In this study, the term alternative conceptions (ACs) describes the mistaken ideas that the students constructed from their personal experiences interacting with nature before formal instruction (Driver and Easley, 1978). Quite often, the ACs are held tenaciously by students, are resistant to change, and hinder learning (Sendur and Toprak, 2013).Since the 1980's many studies have extensively focused on the ACs on fundamental chemistry concepts such as chemical bonds (Peterson et al., 1989), acids and bases (Nakhleh, 1994), electrochemistry (Garnett and Treagust, 1992), heat and temperature (Harrison et al., 1999), chemical equilibrium (Banerjee, 1991; Voska and Heikkinen, 2000), atoms and molecules (Gabel et al., 1987), stoichiometry (Huddle and Pillay, 1996), and thermodynamics (Granville, 1985). More recent studies have revealed the ACs on chemical properties Kekulé resonance representation (Kim et al., 2019), reaction coordinate diagrams (Lamichhane et al., 2018), net ionic reactions (Ye et al., 2019), solution chemistry (Lutter et al., 2019), chemical bonding (Tsaparlis et al., 2018) and reaction kinetics (Yan and Subramaniam, 2018), and coordination number (Sreenivasulu and Subramaniam, 2019). However, ACs on transition metals (TMs), a curriculum that constitutes the pre-university level inorganic chemistry course, has not been studied (Sreenivasulu and Subramaniam, 2014).
Conventional teaching, which entails minimal student participation, dominates the lessons on TM (Sreenivasulu and Subramaniam, 2014; 2019). The teacher-centred approach has failed to promote any significant conceptual change. Consequently, the ACs from the prior knowledge are retained (Sreenivasulu and Subramaniam, 2014). A similar teacher-centred approach is used commonly during the lessons on TMs at the pre-university level in Malaysia (MEC, 2012). The lesson usually starts with the teacher explaining the metals’ properties and characteristics, followed by a discussion of answers for the questions from the textbook or past year examinations. Students passively received the information, memorized it, and reproduce the learned information on the exam. Conventional teaching accounts for the ACs on TMs harboured by Malaysian pre-university students (Karpudewan and Balasundram, 2019).
Previous studies on conceptual understanding demonstrate that writing activities are of paramount importance for understanding science concepts (Hand et al., 2001; Knipper and Duggan, 2006). The advocates of writing-to-learn (WTL) activity connote that participating in writing leads to discovery when the writer consciously restructures the ideas to find new knowledge rather than directly retrieve the ideas from memory and transfer it to a text (Baaijen and Galbraith, 2018). In a different study Galbraith (1999) indicated that, during restructuring, the writer tends to form a new network of knowledge connecting the interrelated sub-concepts. Galbraith further said that new knowledge is synthesised by constraint satisfaction within the interrelated sub-concepts. The new network connecting the sub-concepts represents new knowledge (Galbraith, 1999). The WTL activity also promoted conceptual change as the students reflected on previous ideas, and the experience with the new conceptions changes the former ideas (Mason and Boscolo, 2000). However, to this end, no studies have been reported on how WTL activity accomplishes the four conditions for conceptual change to remediate the ACs as suggested by Posner et al. (1982).
Besides writing, McDermott (2010) viewed multiple modes of presentations (MMR) as a powerful tool for conceptual understanding. For embedding the MMR in the WTL activities effectively, the writers need to identify the modes appropriate to the topic, create links between the modes and links between the modes and writing, place the modes near to the text and refer to the modes in the text (Gunel et al., 2016). In producing intelligible writing that suits the target audience, the writer consistently reflected on the MMR, considering the strategies mentioned above to embed them effectively.
Continuously reflecting on the strategies and MMR contributes to higher cognitive activity. This subsequently results in a richer understanding of the topic (Pineda and Garza, 2000; Gunel et al., 2006). The enhanced cognitive activities from embedding MMR in WTL activities supported students’ learning of science concepts (Hand et al., 2009; Atila et al., 2010). However, limited studies have researched the use of WTL activities embedded with MMR on understanding chemistry concepts. A few studies have investigated an understanding of general chemistry (McDermott and Hand, 2013) and electrochemistry (Gunel et al., 2016) using a WTL activity embedded with MMR.
Lessons on TMs require students to learn the properties of ten metals from the first row of TMs (MEC, 2012). Learning the properties of ten metals, including the formation of complex ions, the ionization energy of TMs, formation of colours in TM ions, and reactivity of TMs necessitates processing a large volume of segmented facts related to metals stored in episodic and semantic memory (Galbraith, 1999). The trouble in understanding the properties arises from lacking capacity to process the information stored in memory. A WTL activity embedded with MMR engages the students in preparing writing to explain the metals’ properties to communicate the knowledge to their peers.
Participating in a WTL activity prompted them to search, retrieve, evaluate and reflect on the information from the episodic and semantic memories and translate the information into text (Scardamalia and Bereiter, 1999; Galbraith, 1999; Klein, 1999). Galbraith (1999) further asserted that content results from the dialectical disposition between the writer and the text. New ideas are synthesized by constraint satisfaction within the interconnected sub-concepts during the dialectical dispositions. The writer constructs knowledge of the topic while understanding how to effectively embed the MMR in writing to communicate the information effectively to the audience (Gunel et al., 2016). MMRs such as chemical equations, notations, diagrams, and graphs are instrumental for understanding the properties of TMs. Additionally, according to schema theory, using a graphic organizer helps students to connect the new knowledge about TMs with prior knowledge. The act of creating writing (graphic organizer) combined with MMR modifies the schema as the new knowledge is connected to existing knowledge in the memory. Students understanding improved when new and existing knowledge connected in a meaningful manner. The improved understanding implies the modified schema (McDermott, 2010). The knowledge construction and cognitive activities that occur from participating in the WTL activity accomplish the four conditions that promote conceptual change to reduce the ACs (Posner et al., 1982).
McDermott and Hand (2016) recommended using a technology (digital) platform to perform the WTL activity embedded with MMR. This is because writing using a digital tool facilitates embedding MMR. The ‘Popplet’ app is a freely available application (Popplet, 2020). The application emphasizes brainstorming ideas and developing visual links between the ideas for a meaningful organization and presentation (Popplet, 2020). Our study revealed that ‘Popplet’ encouraged embedding MMR in WTL activities during the lessons on TM (Balasundram and Karpudewan, 2020). The characteristics of the application correspond with translation in the WTL activity and embedding MMR. The application requires students to retrieve, reflect, and evaluate while organizing the information (Popplet, 2020). At the same time, the application prompts students to evaluate thinking about linking the modes and the texts to communicate the ideas (Popplet, 2020). The direct proportional characteristics of ‘Popplet’ to WTL and MMR prompted the researchers to employ it as a digital platform for students to generate writing activity in this study.
Background of the study
The alternative conceptions on transition metals
The curriculum on Transition metals (TMs) aims to teach the uses of first-row TMs and their compounds, physical and chemical properties, nomenclature, and bonding of the complexes (MEC, 2012). The lessons are mainly teacher governed and guided by textbooks and notes. Predominately, teachers deliver the lessons by explaining the concepts in the classroom. After teaching, students work in groups discussing the answers to the questions in the textbooks, activity books, and past years examination questions. Students passively receive information, take notes when the teacher explains, and usually reproduce the memorised information in the examination (MEC, 2012). The literature on AC suggests that teacher-centred lessons frequently fail to promote conceptual change (Duit et al., 2008). Since conceptual change does not occur, students retain the ACs that constitute prior knowledge. The formation of complex ions, oxidation states of the metals, ionisation energy, and formation of coloured compounds are some common ACs related to TMs reported by Sreenivasulu and Subramaniam (2014) in their study involving pre-service teachers. An earlier study conducted with pre-university students of a different cohort from Malaysia revealed that this group of students held similar ACs (Karpudewan and Balasundram, 2019).Karpudewan and Balasundram (2019) identified that students held the AC that metals do not form complex ions when in a zero oxidation state because the metal is unable to attract ligands. The AC differs from the correct understanding that the metal has empty orbitals in its valence shell that allows it to accept lone pairs of electrons from the ligands. The authors reiterated that students developed the AC because both electrons and orbitals are invisible to the naked eye. For this reason, they were unable to realise that the electrons occupy the orbitals. The same study revealed that merely imagining the arrangement of electrons resulted in the students having the wrong conception that the tendency to involve all the 3d electrons in bonding increases when electrons are added to the 3d5 configuration of TMs. According to Hund's Rule, the electrons start pairing after the d5 electronic configuration. The study also indicated that students incorrectly understood that TMs are poor reducing agents when in fact TMs, such as Cr2+, Ti2+, and V2+, are good reducing agents because the metals can reduce other substances, and they have negative electrode potential values, E°. Students also identified with the incorrect AC that the reactivity of TM's increases from left to right across a period. Moving across the period from left to right, the number of electrons increases, the availability of empty orbitals reduces, and the tendency of TMs to accept electrons decreases. Additionally, students assumed that the ionisation energy of the alkali metals and the TMs are similar. For instance, copper and potassium were thought to have the same ionisation energy because they are in the same period. Since the screening effect of the inner electrons for copper and potassium are not identical, the ionisation energies of these metals are quite different. The majority of students perceived a Cu+ ion as a coloured ion. However, a Cu+ ion is colourless because the ion has a completely filled 3d orbitals. As such d–d transitions do not occur to form colour.
WTL activity is an appropriate strategy for students to comprehend the large volume of factual knowledge of metals’ properties (Hand et al., 2007). The synthesis of conceptually coherent ideas occurs from the dialectic between the network of interconnected concepts and the text aimed at communicating the properties of metals to the audience effectively (Galbraith, 1999). Multiple representations such as chemical equations, notations, electronic arrangements, graphs, and diagrams are inevitable in comprehending the properties of TMs. Gunel et al. (2006) asserted that cognitively processing information connecting modes and writing leads to new knowledge synthesis. Pineda and Garza (2000) named the process of relating modes and writing as ‘translation’.
Writing-to-learn (WTL) activity embedded with multiple modes of representation (MMR)
Various models and theories have been used to describe the cognition process that occurs during writing activities. The knowledge transforming model describes cognition as a two-way interaction between ‘content space’ and ‘rhetorical space.’ A deeper understanding of the topic results when students are continuously engaged in reflecting the content knowledge and the use of knowledge on the act of writing in producing text to meet the specified rhetoric goals (Bereiter and Scardamalia, 1987). The model presumes that knowledge is independent ideas linked together and stored uniformly in the memory system. In producing the text, the writer retrieves and searches for the ideas to organise them.Galbraith (1999) presented a dual process-model comprised of knowledge retrieval and knowledge constituting processes describing cognition during writing. The knowledge retrieval process is about searching and retrieving already formed ideas from memory and translating those ideas as separate units to satisfy the rhetorical goals. As such, knowledge construction does not occur during the retrieval process. Instead, the retrieved knowledge is structured and organised. Knowledge development occurs during knowledge constituting processes as a consequence of constraint satisfaction within the interconnected sub-concepts. The constraints imposed on the network activates the sub-concepts for forming new links that represent the novel idea. Galbraith named the activation of the network as constraint satisfaction of the network. The constraint satisfaction from the dialectical dispositions between the writer and the external text contributed to knowledge construction.
Klein (1999) proposed four hypotheses to explain how writing affects cognition. The first hypothesis implies that knowledge is generated ‘at the point of utterance’ spontaneously with planning. At this juncture, conceptual change does not occur; instead, the writer assimilates new experiences into existing concepts. The second hypothesis, known as ‘forward search’, postulates that the writer produces the text, makes inferences after re-reading the text, and engages in generating, organising, evaluating, and revising operations. The third genre-related hypothesis suggests using structures to link the ideas to organise them in the text. The fourth hypothesis, ‘backward search,’ suggests setting rhetorical and content goals, and then planning and structuring the writing to reach the goals. In the backward search strategy, the writer again performs planning, evaluating, and revising operations in producing the writing. Klein (1999) recommended the educators to design writing tasks using increasingly sophisticated strategies from spontaneous knowledge generation strategies to forward and backward strategies.
Considering the theories that explain cognition during writing, Prain and Hand (1996) recommended a model with five elements as a guide for the teachers in implementing writing tasks that can lead to knowledge transformation and generation instead of merely reflecting understanding. The elements include (a) topic, (b) type of writing, (c) purpose of the writing, (d) audience who will evaluate the written products, and (e) method of text production. The studies researched the effectiveness of the WTL activities in improving understanding of scientific concepts, and provided empirical evidence on the appropriateness of the theories that explain cognition that occurs when writing tasks. A meta-analysis of six research projects which have used WTL activities guided by the four hypotheses from Klein (1999) reveals that writing tasks demanded actively moving between content and rhetorical knowledge in translating the scientific language into everyday language. The translations required students to immerse themselves in the scientific language, fully engage in identifying rhetorical elements necessary to present the text in the form of writing and display the key concepts using alternative words appropriate to the audience (Gunel et al., 2007). In a separate study it was found that engaging in WTL activities improved understanding of biology concepts mainly because the translation of knowledge to the audience had placed high cognitive demands on the students as the task involves backwards and forward search strategies (Gunel et al., 2009).
Improvement in understanding stoichiometry concepts observed among 11th-grade students results from the constant use of backward and forward search strategies during WTL activity. The backward strategy required the students to understand the subject matter and use language the audience could understand in writing a business letter. The forward strategy occurred when the students re-read and edited the writing. Consistent use of forward and backward search strategies created the cognitive demands in preparing a writing task to translate the knowledge to the audience (Hand et al., 2007).
Research on conceptual understanding in science reveals that MMR is instrumental in ensuring a complete understanding of the idea (diSessa, 2004) as different representations make abstract concepts intelligible and progress towards understanding the concept (Yore and Treagust, 2006). Knowledge construction occurs when students cognitively process information moving from one mode to another to establish the connections between the modes and modes and writing (Gunel et al., 2006). Establishing relationships between the modes and modes and writing is referred to as “Translation” (Pineda and Garza, 2000). The characteristics of translation in MMR parallels translations reflected in the knowledge constitution model in producing content as a consequence of dialectical disposition between the writer and the text (Galbraith, 1999). However, McDermott and Hand (2013) pointed out that translation only occurs when embedding MMR instead of merely placing the modes in the text. The modes are linked to each other and presented in connection with the text.
Several studies that have embedded MMR in WTL activity documented positive outcomes. Students’ who embedded mathematical representations in the text obtained higher scores in the physics concept test than those who only used text (Hand et al., 2009). The same study revealed students who have embedded mathematical representations followed by graphical representations had a higher score than those who had the text only or embedded graphical representation. Chen et al. (2013) indicated that having students embed MMR in writing guided students in building evidence supporting claims and scientific arguments. Gunel et al. (2006) reported that students who used MMR in PowerPoint presentations outperformed the students using summary writing about physics concepts.
WTL activity embedded with MMR using “Popplet”
Visualisation is integral to science teaching and learning as it shapes the formation of scientific knowledge. Visual representation is a mode of communication to convey meaning, and students construct meaning from reflecting the representation. Visual presentation of meaningfully connected ideas in a structured and organised manner is called a graphic organiser (Nakiboglu, 2017). When meaningful structuring and organising knowledge makes abstract and difficult concepts intelligible it designates that the graphic organiser is a useful learning tool. Graphic organiser, when used in writing activities, encourages students to produce quality writing connecting the ideas to clarify concepts (Kress, 2010). Reed et al. (2019) employed an electronic graphic organiser to teach scientific vocabulary because the electronic organiser supports embedding MMR in arranging the ideas that subsequently support learning.The schema theory explains the learning students’ experiences from using a graphic organiser. According to schema theory, learning occurs when students link the existing facts and information stored in semantic memory with new ideas. In creating the links and relationships, knowledge is coded and retrieved. This results in modifying the existing schema and a new schema emerges. A graphic organiser facilitates modifying and producing the new schema. A new schema emerges as a consequence of dialectic between writer and ideas in the semantic memory. Variation in the translation determines the kind of schema that will be produced.
Among the different types of graphic organisers available in the market, a digital organiser is popular among the students as the organiser allows handling and manipulating the writing using a digital interface. Performing the digital interface activities encourages the user to think and evaluate using the available features in organising and structuring the ideas for effective communication. The ‘Popplet’ application is an electronic graphic organiser available at Applestore (Popplet, 2020). Compare to other digital organisers, ‘Popplet’ encourages students to think and learn visually by brainstorming. The brainstorming demanded students to think and reflect on their knowledge and evaluate how to organise and logically present the ideas for the readers to easily understand (Sessions et al., 2016; Lapp and Ariza, 2018). During the systematic arrangement of the units of knowledge in ‘Popplet’, the mind interprets and structures the units of knowledge in a suitable flow. The application provides options for using various colours, types, and font sizes to stress the key points. The experiences encountered by using ‘Popplet’ authenticates the description of learning using schema. The characteristics of ‘Popplet’ signify that the application is an appropriate tool to stage the WTL activity embedded with MMR to learn about the properties of TMs.
WTL activity embedded with MMR using ‘Popplet’ and conceptual change
Conceptual change has been a significant domain of educational research since the 1970s. Analysis of research trends in science education from 2003 to 2007 (Lee et al., 2009); 2008 to 2012 (Lin et al., 2014), and 2013 to 2017 (Lin et al., 2019) has documented that conceptual change is one of the topics researched to a significant extent. Studies deliberated that students do not come to the science classroom with no knowledge (Duit and Treagust, 2003). Instead, students’ are occupied with pre-instruction knowledge, which usually is not in line with the conceptions accepted by the scientific community. Learning best occurs when students experience changes in the conceptions from the pre instructional stage towards intended science concepts (Duit and Treagust, 2003). The literature on conceptual change research specifies Jean Piaget's contribution to the immense body of knowledge in this area (diSessa, 2014). Piaget introduced the idea of constructivism, explaining the formation of new ideas from the old ones. Piaget also described the conceptual change from the perspective of assimilation and accommodation processes. Conceptual change occurs during the assimilation process when the new idea fits into the existing knowledge. In contrast, when new ideas fail to fit into the existing knowledge framework, restructuring or reorganisation of concepts occurs during accommodation.Posner et al. (1982) proposed four conditions for the accommodation process that foster conceptual change during science lessons. According to Posner et al. (1982), for conceptual change to occur, firstly, the learner must be dissatisfied with the currently held idea. Secondly, new concepts must be intelligible. Thirdly, the new concepts must be plausible, and fourthly the new concept must be fruitful. The four conditions put forth by Posner et al. (1982) correspond to the description of cognition provided by the knowledge-constitution model, the four hypotheses on writing (Klein, 1999) and schema and cognitive load theory for the WTL activity embedded with MMR using ‘Popplet.’ The first hypothesis of the writer which assimilates new experiences to existing concepts ‘at the point of utterance’ helps the learner to become aware of the inadequacies of the current conception and create a sense of dissatisfaction. In producing a text that fulfils the rhetorical and content goals, the writer consistently engages in evaluating their implicit disposition and the text. Moving between the spaces engages in planning, evaluating, and revising operations as backwards and forward search strategies make the concept intelligible. The translation that determines the extent of dialectic between the writer and text (knowledge-constituting model) established from linking the modes and linking the modes with the text informs the plausibility and fruitfulness of the concepts. Additionally, staging the WTL activity on the ‘Popplet’ platform, according to schema theory, encourages students to structure and organise information to integrate the new knowledge with prior knowledge. The plausibility and fruitfulness of the concepts are reflected when the link between new and existing knowledge is formed.
Research aim
A review of the literature reveals that very few studies have researched the ACs on TMs (Sreenivasulu and Subramaniam, 2014). Literature has explicitly documented the effectiveness of WTL activity and WTL activity embedded with MMR in improving the conceptual understanding of science concepts (Hand et al., 2009; Gunel et al., 2006), and only two studies reported on improving chemistry concepts (Gunel et al., 2016; McDermott and Hand, 2013). To this end, information on using the WTL activity embedded with MMR on reducing AC is infantile. The literature also calls for research performing the WTL activities on a computer-assisted digital platform (McDermott and Hand, 2016). In responding to the above call and to bridge the gaps, this study was performed to explore outcomes of using WTL activity embedded with MMR using ‘Popplet’ in the area of AC on TMs.Methods
Research design
The concurrent embedded mixed-method research design was employed to explore the outcomes of using WTL activity embedded with MMR using ‘Popplet’ in addressing the ACs on TMs among pre-university students. Both quantitative and qualitative data were collected concurrently. The Transition Metal Diagnostic Test (TMDT) was administered before and after the treatment to collect quantitative data. The responses obtained from the first interview before the treatment and the second interview after the treatment constitute the qualitative data. The data were distinctively analysed, and both findings were merged (Teddlie and Tashakkori, 2009).Participants
The study population comprises pre-university students enrolled in a pre-university program in one of the Malaysian government schools. The pre-university program is a three-semester program that prepares students for tertiary education. The program is also known as the ‘High School Certificate Program.’ Chemistry is a compulsory subject for pre-university students who intend to enrol in medical, engineering, and chemical-related studies at the tertiary level. The schools throughout the country that execute the pre-university program follow the curriculum specification prepared by the Ministry of Education. The curriculum specification covers the depth of the content and suggests practical strategies to deliver the content. The notion that teaching and learning are strictly guided by the curriculum specification implies that the schools throughout the country implement the same curriculum.For this reason, a conveniently located school participated in this study. At the time of the study, there were 81 students, 18 to 19 years old, in semester II of the pre-university program. The study was conducted with semester II students because the curriculum on TMs was incorporated in Semester II of the pre-university program. As the researchers did not possess the authority to eliminate any students, the study employed an intact group sampling strategy to ensure that all 81 students from two classes participated in the research. The students’ usual chemistry teachers agreed to participate in the research and conducted the lessons on TMs. Both teachers hold a Masters in Science Education degree from a local university. The teachers have ten years of experience teaching TMs for pre-university students. For the qualitative study, nine students were purposively identified based on their previous school examination performance. A combination of weak, mediocre, and excellent performers participated in the interview. The excellent performers are students who obtained more than 80% scores in the examination. Mediocre students’ scores fall in the range of 50% to 79% and week students’ scores are less than 50%.
To ensure the ethical consideration of chemistry education research, the researchers engaged in embracing measures as proposed by Taber (2014) while conducting the research. One of the measures is informing the participating students and teachers before the study commences that their participation is solely voluntary and that they are free to withdraw from the study at any time. The researchers informed the students and teachers that participating in this study is worthwhile because they will help in exploring the lessons on TMs in turn, suggesting a strategy that could facilitate the learning of TMs. To ensure the students stay motivated throughout the study, the researchers informed the students that there is no one correct answer for the WTL activity that they would be generating, and the answers would not affect their school grades. The participants granted permission for the researchers to use their responses for publication purposes.
Instruments
From the pilot test conducted with 30 students from a different school, the researchers discovered that 20 minutes was needed to answer the TMDT. The Kuder Richardson Reliability Coefficient (KR-20) of 0.71 denotes that the items in the TMDT are reliable (Fraenkel and Wallen, 2009). Chemistry teachers who conducted the treatment and a chemistry educator from a university checked the validity of TMDT. The questions were modified based on the experts’ feedback, and some questions from the original version were eliminated to tailor to the Malaysian pre-university syllabus requirement. Selection of nine 2 tier items was regarded as sufficient (Nakiboglu, 2003).
The questions for the semi-structured interviews were formulated based on the four concepts covered in the curriculum. The questions in TMDT guided in formulating the interview questions. During the pilot study, the interview questions were validated to ensure that the questions denote the four concepts of TMs. Based on recommendations from the teachers, the researchers rephrased some of the questions for clarity. For example, the question, “Does the tendency for transition metals to involve all 3d electrons in bonding decrease once the d5configuration is exceeded?” was rephrased to “Why do transition metal ions in zero oxidation state not attract ligands to form complex ions?”. The interviewer prompted further asking “why” and “how” to ensure students provide complete answers. The pre-interview responses elucidated the students’ existing ACs on TMs prior to the study. The post-interview responses revealed how the ACs changed after the WTL activity embedded with MMR using ‘Popplet.’ Table 1 presents the interview questions.
| No. | Interview questions | Concepts |
|---|---|---|
| 1. | Why do transition metal ions in zero oxidation state not attract ligands to form complex ions? Explain. | The formation of complex ions |
| 2. | Do transition metals involve all 3d electrons to form the bonds? Explain why? | |
| 3. | Describe the ionisation energy of copper and potassium. Justify your explanation. | The ionisation energy of transition metals |
| 4. | Do the transition metals and alkali metals exhibit similar trends of ionisation energy descending the groups? Explain. | |
| 5. | Do you think only transition metal ions are coloured? Why? | Formation of coloured compounds by transition metal ions |
| 6. | Why is Cu+ colourless? | |
| 7. | Why does the reactivity of transition metals decrease across the period from left to right? | Reactivity of transition metals |
| 8. | Can transition metals be good reducing agents? Why? |
Research procedure
The research was performed over five weeks, starting with measuring students’ existing knowledge on TMs in week 1 using TMDT. In the first week itself, after responding to TMDT, nine students were purposively identified and interviewed. In the second week, both teachers and students participated in training on using ‘Popplet’ to generate writing. In creating the writing using ‘Popplet,’ the participants learned how to embed MMR linking the modes and modes with text effectively. The training session started with both teachers and students downloading and installing the ‘Popplet’ app. Upon completion of the installation, the participants analysed two different texts. The students analysed the first text about the properties of first row metals to recommend strategies to communicate the content to the readers effectively. The participants suggested using modes such as graphs, diagrams, and equations for making the content more intelligible. The second text also described the properties of TMs. However, the second text had various modes such as notation, diagrams, equations, formulas, and graphs embedded within the text. The participants listed the modes and identified strategies used to link the modes and link the modes with the text. Linking the modes, linking the modes and the text, placing the modes near the text that refers them, having captions to the modes, and generating their modes to reflect the originality rather than obtaining them from elsewhere were some of the strategies used to embed MMR in the text. The participants also discussed the appropriateness of the strategies. After identifying the modes and agreeing on the strategies used to embed the modes, participants generated their own writing embedding the MMR using ‘Popplet.’ The generated writing was again analysed and discussed to better understand using the ‘Popplet’ application to create writing with MMR embedded.Upon completion of the training, lessons on the formation of complex ions, the ionisation energy of TMs, the formation of colours, the reactivity of TMs, and TMs as catalysts were conducted in weeks 3 and 4. The lessons were executed in three phases: introduction, activity, and sharing the writing with the designated audience. In the introduction phase, the main and sub-concepts introduced using appropriate examples or phenomena. During the activity phase, students explored and investigated the examples provided in the introduction phase. The investigation required students to work in a group to develop the WTL activity embedded with MMR using ‘Popplet’ explaining the concepts. Then, students shared the writing generated from the activity phase with the audience. In this study, the audience is their peers. The peers read through the writing provided to them, asked questions for a better understanding of the text, and provided feedback to improve the writing. The entire three phases connote the WTL activity with five elements (Hand and Prain, 1996). A detailed illustration of the three phases was further explained using the lesson on the formation of a complex ion.
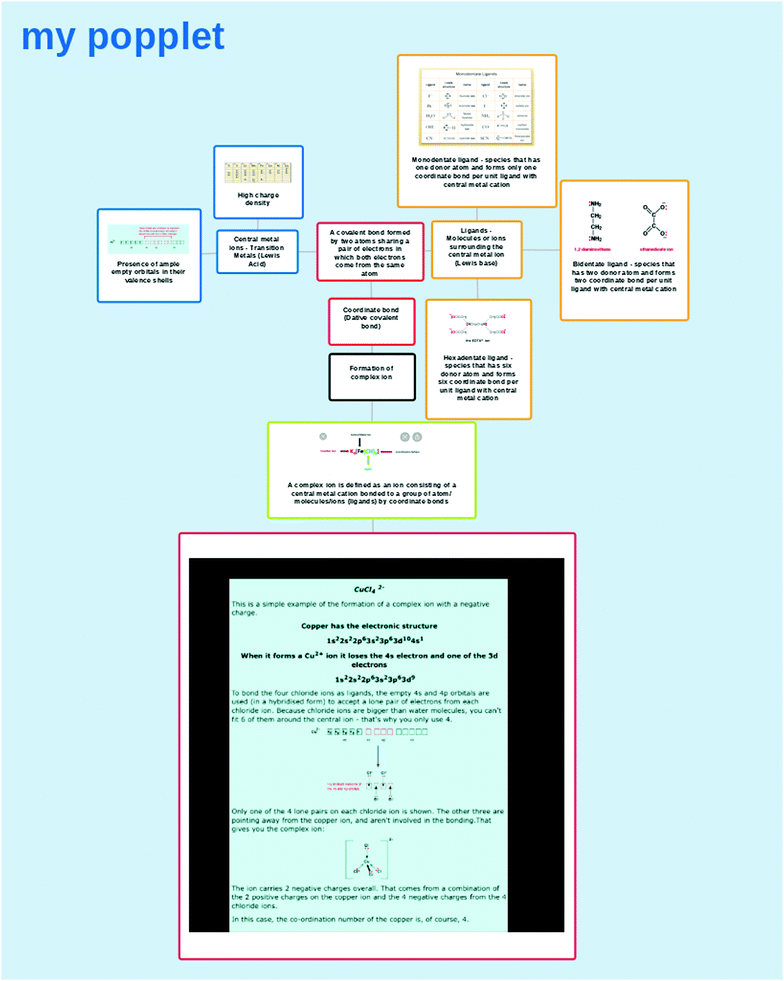
The teachers introduced complex ions such as dichlorocuprate(I), [CuCl2]− and hexacyanoferrate(III), [Fe(CN)6]3−, and explained that TM ions possess the ability to form complex ions because the lone pair electrons of the ligands are attracted to the metals. The metals with high charge density and empty orbitals in the valence shells attract the lone pair electron on the ligands. During the activity, students worked in a group of four to search for answers to the questions posed by the teacher: (i) What is a central metal ion? (ii) What is a ligand? (iii) How does the coordinate bond formed between the central metal ions and the ligands? (iv) Give an example illustrating the formation of a complex ion. Students accessed various websites and reference books to find answers. The answers for the questions were structured and presented in the form of a WTL activity embedded with MMR using ‘Popplet.’ The writing was later shared with peers communicating the explanation on the formation of complex ions. Fig. 1 is an example of writing prepared by the students.
From the WTL activity, there is evidence of the hypothesised cognitive activity in the dual-processing model taking place. The evidence is notable when students engaged in processing information about the formation of complex ions (from the introduction phase) stored in episodic and semantic memory to develop the writing activity embedded with MMR, as in Fig. 1. In order to organise the ideas in a meaningful way connecting the main concept formation of a complex ion with the sub-concepts such as coordinate bond, central metal ion, and ligand text boxes allowed students to consistently organise, evaluate, and reorganise the information stored in memory. During planning and linking, constraints imposed activate the network of sub-concepts. The outcome from constraint satisfaction informs the students on the information to be included in the text box so that information in one box can be linked to other boxes. This information reflects the new knowledge constructed. The knowledge construction at this point accentuated when students identified an appropriate mode, understand how to use the mode, and embed the mode using a correct strategy. Additionally, situating the writing on ‘Popplet,’ according to schema theory, allowed integrating new knowledge to the prior knowledge (Anderson, 2000).
Fig. 1 is divided into four separate segments to describe how students generated the writing with MMR embedded using ‘Popplet.’ The writing began by placing the main idea in the centre, which is the first segment. Text box 1 was created at the centre, stating that the main idea of the lesson is the formation of a complex ion. Text box 1 was extended to text box 2 and text box 3, explaining the formation of a complex ion. In text box 2, the formation of a complex ion was defined as a coordinate bond that holds the central metal ions and ligands. The definition was made explicit by embedding the chemical formula of a complex ion beside the text. Having the ligand and central metal ion labelled in the formula allowed linking the formula with the text. ‘Coordinate bond’ was stated distinctively in text box 3 to stress the importance of the bond in the formation of the complex ion. The definition for formation of complex ions is expanded by placing the essential points in separate boxes which is synthesized by constraint satisfaction of the networks of interrelated concepts and backward search strategy. Embedding chemical formula (placing near to the text and linking to the text) in text box 2 reduces the gap in the dispositional dialectic between the writer and the text in translating to communicate the idea effectively.
Segment 1 was expanded to segment 2, explaining the coordinate bond using text in text box 4. The two essential points of the coordinate bond, central metal ion, and ligands were defined separately in text box 5 and text box 6. The characteristics of central metals as high charge density metals and empty orbitals in the metals were presented separately in text boxes 7 and 8. The charges on the ion are directly proportional to the charge density of metals and indicates which TMs have high charge density. An orbital diagram was used to show the availability of empty orbitals available to accept electrons. In explaining the coordinate bond concerning ligands, segment 3 began from text box 6. Students introduced three types of ligands using different boxes: monodentate ligands in text box 9, bidentate ligands in text box 10, and hexadentate ligands in text box 11. Different types of monodentate ligands were listed using a table describing the ligands with chemical symbols, formulae, and Lewis structures in text box 10 and 11. The constraint satisfaction, and backward and forward hypothesis explains the writer's engagement in communicating the idea. The writer has a set of content goals. In this case, the content goals are linking the coordinate bonds, central metal ions and ligands to explain the formation of complex ions. In reaching the goals, the writer consistently evaluated and revised the writing. New ideas were synthesised by constraint satisfaction within the network of concepts (coordinate bond, ligands, different types of ligands, and characteristics of ligand) stored in semantic memory.
After illustrating the definition of formation of complex ions using the sub-concepts in three segments, the formation of complex ions was made explicit using copper ion, Cu2+, and chloride ion, Cl− in segment 4. For this, another text box, text box 11, was created. The electronic configuration and the orbital diagram indicate the presence of empty orbitals in copper ion, Cu2+. A labelled diagram shows the arrow from the lone pairs of electrons in the chloride ion, Cl− moving towards the empty orbitals in the copper ion, Cu2+ indicating the formation of a coordinate bond. A 3d diagram ends the explanation of the formation of complex ion, [CuCl4]2−. The 3d diagram and the chemical symbol, [CuCl4]2− show the complex ion using different modes. Both constraint satisfaction and the forward and backward strategy explain the writing in communicating the ideas.
The WTL activity embedded with MMR on ‘Popplet’ was shared and presented to the peers. The peers tried to understand the writing presented to them. In understanding the content of the writing, they posed questions such as “what is high charge density?; why are transition metals said to have high charge density?”; “can a chloride ion be considered as a bidentate ligand because it has more than 2 electrons surrounding it?” and “do metals from group 1 and 2 in the Periodic Table form complex ions?”. Students also suggested linking the coordinate bond text box to show more examples of complex ions. The feedback also served to fine tune the explanation on high charge density. The majority of the students, included more sub-concepts in addition to central metal ions and ligands, and extended the writing, while some others included another example of the formation of a complex ion.
In week 5, TMDT and interviews were conducted as a post-test to explore the outcomes of WTL activity embedded with MMR using ‘Popplet’ in addressing students’ ACs on TMs.
| Codes | Categories | Theme | ACs |
|---|---|---|---|
| Existence of zero oxidation state | Oxidation number | Nature of TM ions to form complex ions | TM does not exist in zero oxidation state because the metals are stable, thus cannot attract ligands. |
| The non-existence of zero oxidation state | |||
| Existence of various oxidation state | |||
| Number of bonds increases after d5 configuration exceeded | Bonding in complex ions | Number of bonds increases because electrons are elevated to a higher energy level or number of bonds decreases because TMs donate electrons to form an ionic bond. | |
| The number of bonds decreases after d5 configuration exceeded | |||
| Copper and potassium have the same ionisation energy. | Comparison of ionisation energy between copper and potassium | Comparison of ionisation energy between alkali metals and TMs | The ionisation energy is the same because both are metals with the same oxidation number and involve removing one electron. |
| Copper and potassium have different ionisation energy. | |||
| The trend of the ionisation energy of TMs down a group in the periodic table is similar to alkali metals. | Comparison of the trend in ionisation energy between alkali metals and TM | Both metals exhibit a similar decrease in ionisation energy trends because they are metals that donate electrons from the 4s orbitals. | |
| The trend of the ionisation energy of TMs down a group in the periodic table is different from alkali metals. | |||
| Metals other than TM ions are coloured | Comparisons of colour between TM ions and other ions | Colours of TM ions | Only TM ions are coloured because they undergo a d-d transition. |
| Only TM ions are coloured. | |||
| Cu+ ion is coloured | Some TM ions are colourless. | Cu+ ion is coloured | |
| Cu+ ion is not coloured. | |||
| The trend of the reactivity across the period | Reactivity trend of TM across the period | Chemical reactions of TM | Reactivity increases because the ions are more electronegative due to the rise in the number of protons |
| TM are good reducing agents. | Strength of the TMs as reducing agents | TMs are good oxidising agents because the metals do not accept electrons. | |
| TMs are not good reducing agent. |
In phase 5, a further analysis was performed to show that the themes fit the study and correspond to the research questions. In phase 6, a report was prepared to document the analysis process. The authors randomly selected three interview responses (33%) and scored the answers independently to check the inter-rater reliability. An inter-rater score of 88% implies that the analysis is reliable (Cohen, 1960).
Results
Quantitative findings
Table 3 below shows the percentage of students who provided correct responses for tier 1 and 2 for all the nine items in the TMDT in the pre and post-tests. In responding to tier 1, students needed to state whether the given statement is true or false. In tier 2, students provided reasons for the choice made in tier 1. In the pre-test, for tier 1, some students were able to give a correct answer. However, in tier 2, only a few provided the right reason for the choice made in tier 1. In the post-test, more than 80% of the students were able to correctly identify whether the statement is true or false. Similarly, for all questions, more than 80% of the students were able to state the correct reason for their choice in tier 2.| Items | Pre-test | Post-test | ||
|---|---|---|---|---|
| Tier 1 | Tier 2 | Tier 1 | Tier 2 | |
| 1 | 10.5 | 0 | 89.1 | 84.6 |
| 2 | 23.7 | 2.7 | 93.5 | 86.3 |
| 3 | 22.3 | 1.8 | 91.4 | 88.5 |
| 4 | 36.4 | 1.7 | 88.7 | 81.1 |
| 5 | 27.9 | 0 | 81.3 | 90.7 |
| 6 | 38.3 | 0 | 91.5 | 86.3 |
| 7 | 15.2 | 4.4 | 90.4 | 85.6 |
| 8 | 19.5 | 5.7 | 89.1 | 83.2 |
| 9 | 30.7 | 9.6 | 94.3 | 93.4 |
The paired sample t-test revealed significant differences (t(80) = −34.36, p = 0.00) between the pre-test mean score (M = 2.35; SD = 1.60) and post-test (M = 7.81; SD = 0.95) mean score. Reasons in tier 2 of the pre-test were analysed further to detect whether ACs exist. The analysis was repeated with post-test responses to explore the changes in the ACs. Table 4 presents the results of the analysis of tier 2 responses.
| Constructs | Questions | List of ACs | Percentage of students with ACs in the pre-test | Percentage of students with ACs in the post-test |
|---|---|---|---|---|
| Formation of complex ions | 1 | 1. TMs do not have a zero oxidation state. | 34.5 | 0 |
| 2. TMs with a zero oxidation state is stable and not able to attract ligands. | 21.0 | 0 | ||
| 2 | 3. When d5 configuration exceeds, TMs need a high amount of energy to donate the electrons. Thus it does not involve all the electrons to form bonding. | 23.5 | 4.9 | |
| 4. TMs are stable after reaching d5 configuration. | 22.2 | 2.5 | ||
| The ionisation energy of TMs | 4 | 5. The first ionisation energy is the same for both Cu and K. This is because the metals are in the same period with the same valence electrons. | 16.0 | 1.2 |
| 6. Removal of electrons from the same 4s orbital. | 25.9 | 1.2 | ||
| 6 | 7. Ionisation energy both groups decrease going down the group because atoms’ size increases. | 16.0 | 1.2 | |
| Colours of TM ions | 3 | 8. Copper is a TM. | 34.6 | 0 |
| 9. The d–d transition happens in Cu+ ion. | 18.5 | 1.2 | ||
| 7 | 10. Only TMs compounds are coloured because they undergo the d–d transition. | 24.7 | 2.5 | |
| Reactivity of TMs | 5 | 11. Across the period from left to right, the elements are more electronegative. | 23.5 | 3.7 |
| 8 | 12. TMs are good oxidising agents. | 21.0 | 2.5 | |
| 13. Metals only donate electrons. | 24.7 | 2.5 | ||
| 9 | 14. TMs easily receive and donate electrons. | 30.9 | 1.2 |
According to Peterson (1986), the ACs are significant when at least 10% of the participating samples held the ACs. Many studies employed Peterson's recommendation to ascertain the existence of ACs in their sample (Tan et al., 2005; Tan et al., 2019 and Yan and Subramaniam, 2018). As presented in Table 4, a total of 14 significant and common ACs were identified in the pre-test. This is because more than 10% of the participating students provided similar incorrect answers. The fact that Sreenivasulu and Subramaniam (2014) in their study categorised similar incorrect responses as ACs further establishes the idea that the mistakes listed in Table 4 are ACs and not because students guessed the answer or lacked knowledge. The teachers’ note that similar mistakes were also prevalent among students in the earlier cohorts (Karpudewan and Balasundram, 2019) heightens the fact that the incorrect answers in the post-test are mainly related to ACs. A decrease in the percentage of incorrect answers in the post-test implies that the treatment has been instrumental in reducing the AC.
Qualitative result
Students also held ACs about the bonding formed between the central metal ion and ligands during the formation of complex ions. The ACs include stating that TMs use all the electrons from 3d orbitals to form bonds after exceeding d5 configuration and because the metals are electropositive and reactive, the metals easily form bonds. Additionally, the majority were unsure how all the 3d electrons were involved in forming the bond. Some students said that bonding does not occur because TMs are unable to donate all the electrons since a high amount of energy is required to release the electrons. In the post-interview, students were able to explain that when the d5 configuration was exceeded the electrons start to form pairs. A student responded, “from the electronic configuration of the metals, after the d5configuration has been exceeded, according to Hund's rule, the electrons start pairing and are not available for bonding”. The notation and electronic configuration that the students included in the ‘Popplet’ enables them to view that pairing of the electrons reduces the formation of bonds with ligands. Another student claimed, “when I compare the orbital diagram of TMs, I can see the pairing of the electrons that occurs in the 3d orbitals. When the pairing happens, the particular orbitals are filled, and chemical bonds cannot form”. The orbital diagram helped the student to understand that when the d-orbital is half-filled, the electrons start to form pairs. The pairing subsequently reduces the formation of bonds. Representing electronic configuration of the TM ions and orbital diagram in ‘Popplet’ (as shown in Fig. 3) allows the students to view that pairing of the electrons reduces the formation of bonds with ligands.
The translation occurs during constraint satisfaction between networks of sub-concepts and translation in embedding MMRs in writing, and allowed students to link the sub-concepts ligands, central metal ion, and formation of bonding in explaining the correct understanding on the formation of a complex ion. The course of translation while embedding MMR connecting the modes, and modes and the writing results in a clear explanation of the formation of a complex ion.
The dialectic between the writing process and the text to reach the content goals resulted in the translation of information connecting the sub-concepts such as electron configuration and arrangement of electrons during the WTL activity. Embedding MMR within the writing using an appropriate strategy enabled linking the modes with the writing. The translation, both during the writing and embedding MMR, enabled students to correctly explain the differences in the ionisation energy connecting the sub-concepts referring to the modes simultaneously.
Students also exhibited an AC when comparing the trend of ionisation energy between TMs and alkaline metals, descending the group in the Periodic Table. The majority of students viewed both metals displaying a similar trend of ionisation energy going down the group. In the post-interview, this AC reduced drastically. The students indicated that the ionisation energy for TMs and alkaline metals increases going down the group, but the increase is not similar. Students explained that the trend of ionisation energy for TMs and alkaline metals differs because of the presence of inner electrons from 3d orbitals for TMs. On the other hand, the alkali metals do not have inner electrons from 3d orbitals. In other words, the ionisation energy for TMs is affected by the screening effect of the inner electrons from 3d orbitals, and alkali metals were not affected by the screening effect. Answers such as the screening effect of inner electrons and electrons removed from 4s orbitals, which are shielded from the nucleus by 3d orbitals served to explain the increase in the energy of TMs descending the group. Some students were unable to provide details for the differences in the increase in energy. However, they grasped the idea that the differences existed due to the presence or absence of 3d electrons in the metals. A student stated, “The ionisation energy is different for alkali metals and transition elements. The existence of 3d inner orbitals makes the ionisation energy for transition elements different,” and another student gave a brief answer, “Not the same because of the electronic arrangement.” The number of students with this AC decreased in the post-interview because students could visualize the inner electrons of the 3d orbitals for TMs when they embedded the diagram as shown in Fig. 3 and write the notation representing the electronic configuration in the graphic organiser using ‘Popplet.’ The electronic configuration of alkali metals clearly shows the absence of the inner electron of the 3d orbitals. This made it possible for the students to understand the differences of ionisation energy for the s block and d block metals.
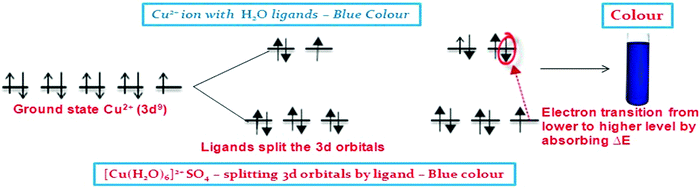
The d–d transition was clearly explained and presented through embedding MMR in the writing activity in ‘Popplet.’ Fig. 5 illustrates how students employed MMR, such as the energy level diagram with labels and symbols in the graphic organiser generated using “Popplet” describing the d–d transition for explaining the colour formation in the Cu2+ ion. The symbols such as Cu2+ representing copper ions, 3d9 representing the orbitals, and ΔE indicating the difference in energy were included in the writing to produce a complete energy level diagram.
In the post-interview, the AC was cleared as the students were able to grasp the idea that for Cu+ ions, the d–d transition does not take place as the 3d orbitals are completely filled with electrons. During the post-interview, a student responded, “Cu+cannot undergo d–d transition. There will be no electrons promoted to a higher energy level to absorb a certain amount of wavelength to reflect the colours. That is why it is colourless”. The labelled energy level diagram enabled the students to understand that the Cu+ ion in the free gaseous state has completely filled 3d orbitals. That there is no available space in 3d orbitals of the higher energy level to accommodate the promoted electron from the 3d orbitals of the lower energy level is clearly seen from the diagram. Therefore, electrons from 3d orbitals of lower energy levels cannot move to the 3d orbitals of higher energy levels, and the d–d transition does not occur. For this reason, the Cu+ ion is colourless. Constraint satisfaction between the networks of sub-concepts, simultaneously embedding multiple modes to link the networks, determines the extent of dispositional dialectic that results in students proposing new ideas on colour formation.
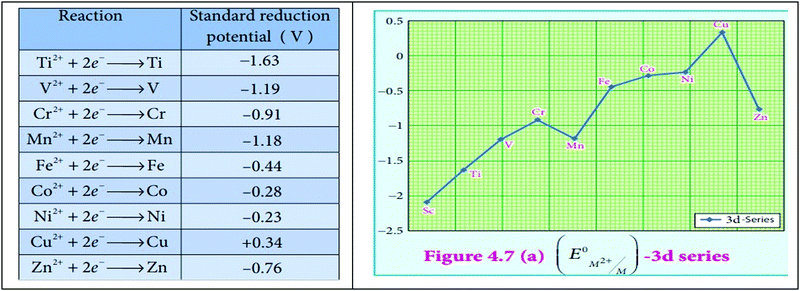
In the pre-interview, students held the AC that TMs cannot be a good reducing agent. Students were unable to elaborate on the efficacy of the TMs as reducing agents in the pre-interview. In the post interview, a student responded “Yes it is a good reducing agent because they are able to form positive ions easily, able to donate electrons. So… they have lower values of standard electrode potential”. In the answer provided in the post-interview students compared the strength of the reducing agents using the standard electrode potential value, E°. This is because in WTL activity students used a graph with the y-axis and x-axis labelled as standard electrode potential value, E° and TM ions to illustrate the reducing and oxidising strength of the metals. Engaging in WTL activity necessitates cognitive processing of information linking the networks of sub-concepts using various modes. The cognitive process during WTL activity explains the ability of the students to connect the sub-concepts effectively using MMR in explaining the reactivity of TMs.
Discussion
One of the primary concerns in chemistry teaching is to identify the ACs and employ an effective strategy to address them (Tümay, 2016). ACs in chemistry have been widely researched (Taber, 2002). Previous studies have listed the common ACs in chemistry (Kim et al., 2019; Lamichhane et al., 2018; Ye et al., 2019; Lutter et al., 2019; Tsaparlis et al., 2018; Yan and Subramaniam, 2018; Sreenivasulu and Subramaniam, 2019). However, studies on ACs related to TMs taught in an inorganic chemistry course at the pre-university level are limited. To this end, only two studies have reported the ACs on TMs (Sreenivasulu and Subramaniam, 2014; Karpudewan and Balasundram, 2019).The current study revealed that the ACs identified in the pre-test among pre-university students are similar to the ACs reported in the earlier studies performed with pre-service teachers (Sreenivasulu and Subramaniam, 2014) and pre-university students of an earlier cohort (Karpudewan and Balasundram, 2019). The higher post-test mean score compared to the pre-test mean score, with the paired sample t-test showing a statistically significant difference between mean scores, denotes that participating in WTL embedded with MMR using ‘Popplet’ reduces the ACs. The qualitative interview findings provided further insights into the quantitative results. The students grasped the correct understanding that TMs can exist in zero oxidation state because the metals are able to attract ligands and form metal complexes. Additionally they comprehended that the tendency for transition metals to involve all 3d electrons in bonding decreases once the d5 configuration is exceeded because the electrons form pairs and are not available for bonding. The Cu+ ion has unpaired d electrons that cannot take part in d–d transitions. Cu and K do not have the same ionisation energy for the loss of their 4s electrons because they do not experience the same screening effect of their inner electrons. The reactivity of transition metals decreases from left to right across a period because electrons progressively fill d orbitals. The ionization energy of TMs down a group in the Periodic Table is not similar to that of the alkali metals, and TM ions are the only ones that are coloured. The qualitative interview responses explicitly showed that the model with five elements (Prain and Hand, 1996) embraced in the WTL activity embedded with MMR using ‘Popplet’ facilitated the students in understanding the concepts correctly.
Both the knowledge-constitution model (Galbraith, 1999) and the forward and backward strategies (Klein, 1999) explain the knowledge construction that occurs while engaged in the WTL activity embedded with MMR using ‘Popplet.’ The new knowledge is stored in episodic and semantic memories developed from constraint satisfaction connecting the networks of sub-concepts. In the process of connecting the networks, students consistently retrieved, evaluated, and reflected on the stored information. Knowledge was also gained from the dispositional dialectic between students’ implicit disposition, and the text. The dispositional dialectic allows translation of the information in memory to new knowledge. The consistent retrieval of information, evaluating, and reflecting on the information mirrors forward and backward strategies. In the process of translating information from memory, MMR was embedded within the writing to effectively communicate the information (Pineda and Garza, 2000). Knowledge acquisition occurs when students cognitively process the information on how to use strategies such as placing modes near the text, referring to the modes in text, linking the modes, having a caption to the modes, and proposing new modes to reflect originality (McDermott and Hand, 2013).
The cognitive activities that result in knowledge construction as explained using the knowledge-constitution model, the four hypotheses (Klein, 1999) and embedding of MMR (Pineda and Garza, 2000) and the schema theory that illustrates the use of ‘Popplet’ confirms the four phases for promoting conceptual change (Posner et al., 1982). Students experienced the first phase dissatisfaction when they assimilated prior knowledge with the new experiences at the beginning of the writing activity. Assimilating new experiences occurs in two ways while engaging in the WTL activity embedded with MMR using ‘Popplet.’ The use of a graphic organizer, in this case, ‘Popplet,’ based on schema theory, allowed integration of new knowledge to prior knowledge. The integration was possible because students were dissatisfied with their prior knowledge. The first hypothesis, ‘at the point of utterance’ at the starting of the writing activity students noted the inadequacy of the existing knowledge. The constraint satisfaction connecting the networks of sub-concepts and the backward and forward strategies of retrieving, evaluating, and reflecting in producing new ideas makes the concept intelligible. During dispositional dialectic, students consistently reflected on the plausibility and fruitfulness of the concepts.
The findings of this study echo other studies that have investigated the effectiveness of using WTL integrated with MMR to improve understanding. The result obtained is coherent with a study conducted by Gunel et al. (2009) that investigated the effects of embedding MMR in writing tasks on learning science among elementary students. The study reveals that embedding MMR in writing tasks improves elementary students’ understanding of the force concept. McDermott (2009) investigated the impact of embedding MMR to represent scientific concepts on students’ conceptual understanding and found a significant difference between the group of students who embedded MMR and the group that did not embed MMR.
In another study, McDermott and Hand (2013) reported that the experimental group students who embedded MMR in writing outperformed the control group students in the understanding test. Furthermore, Gunel et al. (2016) supported the claim that using MMR in writing task enhances students’ conceptual understanding. Nam and Cho (2016) performed a similar study examining the impacts of multimodal instruction in embedding MMR on students’ conceptual understanding. They claimed the ability to use and link the modes appeared to have a strong relationship with conceptual understanding.
The finding of the current study is consistent with several other studies that have used a graphic organizer to facilitate learning (Nakiboglu, 2017; Casteleyn and Mottart, 2012). Notably, the nature of the ‘Popplet” app explains the positive learning experiences encountered by students while performing the WTL activity embedded with MMR. ‘Popplet” encouraged students to brainstorm information about the properties of TMs. While brainstorming, students frequently reflected on the main concepts, connecting the sub-concepts to explain the central idea. This is similar to the findings of a study conducted by Nesbit and Adesope (2006). The authors found that ‘Popplet” helped the students to arrange the data in a systematic manner which subsequently helped students to produce logical arguments.
Conclusions and implications
The mixed-method study reports that WTL activity embedded with MMR using ‘Popplet’ enabled connecting and logically restructuring the information. The representation used allowed visualizing the orbitals, the arrangement of electrons, and the reactions. The concrete view of the arrangement of electrons and reactions and rational organization of the information resulted in reducing the ACs on the formation of complex ions, ionization energy, the formation of coloured compounds, and reactivity of TMs. The study responded to the call for research on teaching, and learning about TMs is essential as few studies have focused on students’ understanding of TMs (Sreenivasulu and Subramaniam, 2014). To date, no study has recommended a suitable strategy to teach the topic of TMs.The suggested strategy offers an alternative approach to the conventional teacher-centred lecturing method, which is prevalent in schools. Teachers frequently do not favour changing the pedagogical strategies as shifting from the usual routine incurs time and energy. The pre-university level education mainly rested on preparing the students for the Higher Schools Certificate examination. The examination qualifies them to enrol in undergraduate courses. For this reason, completing the syllabus on time is the main agenda for the teacher. Generating MMR integrated with graphic organizers using the ‘Popplet’ app as WTL activities does not require additional time to be taken out of the regular school hours. The strategy is feasible for teaching the lessons on TMs during the formal schooling hours.
The current notion is that learning about TMs is to know the properties of the metals; students memorized the characteristic of the metals and reproduced the information while answering the questions. WTL activity embedded with MMR using ‘Popplet’ made them realize that knowing about the metals is not the same as merely memorizing the properties of the metals. Rather it is about understanding the properties of metals through establishing the connections between the concepts of formation of complex ions, ionization energy, the formation of coloured compounds, and reactivity of TMs. The WTL activity embedded with MMR such as diagrams, graphs, mathematical and chemical equations, chemical symbols, and text using a graphic organizer denotes learning with understanding. Learning with understanding commences as the writing activity necessitates recalling and clarifying information to craft the ideas in a way that it makes meaning (Hand et al., 2001).
Many studies have depicted the significance of embracing technology in classroom teaching (Regan et al., 2018; Lapp and Ariza, 2018). Similarly, in this study, using a mobile phone to create the graphic organizer attracted the students’ attention to the learning. The students effortlessly familiarized themselves with the application. Being technology savvy, the students explored the application and generated the graphic organizer with various designs, using fonts with different colours, types and sizes to differentiate the title, explanation and text boxes.
Despite the rigorous measures employed by the researchers to control the threat to validity, the study exhibits several limitations. One of the prominent limitations is the absence of a control group. Comparing the findings of the experimental and control groups ascertains the effectiveness of any treatment (Shadish et al., 2002). The mixed-method design that comprises both quantitative and qualitative approaches employed in this study overcomes the limitations of having only one approach (Creswell, 2014). The qualitative section of the research provided insights into the quantitative findings, and clear evidence illustrating that treatment causes changes in the ACs.
Conflicts of interest
There are no conflicts to declare.Appendix 1
Transition metals diagnostic test (TMDT)You are required to answer all nine questions in the test. A statement was provided for each item. Based on your understanding of the properties of transition metals, indicate whether the given statement is true or false. Then explain the reasons why the statement is true or false.
| No. | Question |
|---|---|
| 1 | A transition metal in zero oxidation state does not attract ligands and hence does not form complexes. |
| A. True B. False | |
| Reason: | |
| 2. | The tendency for transition metals to involve all the 3d electrons to form the bonds increases once the d5 configuration has been exceeded. |
| A. True B. False | |
| Reason: | |
| 3. | Cu and K are expected to have same ionisation energy because of losing 4s electrons. |
| A. True B. False | |
| Reason: | |
| 4. | The ionisation energy of transition metals descending the group in the Periodic Table is similar to those of the alkali metals. |
| A. True B. False | |
| Reason: | |
| 5. | Cu+ has unpaired d electrons which can take part in d–d transitions. |
| A. True B. False | |
| Reason: | |
| 6. | Only transition metal ions are coloured. |
| A. True B. False | |
| Reason: | |
| 7. | The reactivity of transition metals decreases across the period from left to right. |
| A. True B. False | |
| Reason: | |
| 8. | Transition metals are good reducing agents. |
| A. True B. False | |
| Reason: | |
| 9. | Transition metals are good catalysts. |
| A. True B. False | |
| Reason: | |
Acknowledgements
We are grateful to Professor Ram Mohan of Illinois Wesleyan University, Bloomington, IL USA for his insightful comments that significantly improved the clarity of the manuscript.References
- Anderson J. R., (2000), Learning and memory: An integrated approach, John Wiley & Sons Inc.
- Atila M. E., Günel M. and Büyükkasap E., (2010), The effect of using different multi modal representations within writing to learn activities on learning force and motion unit at the middle school setting. J. Turk. Sci. Educ., 7(4), 113–127.
- Baaijen V. M. and Galbraith D., (2018), Discovery through writing: Relationships with writing processes and text quality, Cognition and Instruction, 36(3), 199–223.
- Balasundram N. and Karpudewan M., (2020), Embedding Multiple Modes of Representations in Open-ended Tests on Learning Transition Elements, in Teo T. W., Tan A. L. and Ong Y. S. (ed.), Science Education in the 21st Century: Re-searching Issues that Matters from Different Lenses, Singapore: Springer, pp. 113–136.
- Banerjee A. C., (1991), Misconceptions of students and teachers in chemical equilibrium. Int. J. Sci. Educ., 13 (4), 487–494.
- Bereiter C. and Scardamalia M., (1987), An attainable version of high literacy: Approaches to teaching higher-order skills in reading and writing. Curric. Inquiry, 17(1), 9–30.
- Braun V. and Clarke V., (2006), Using thematic analysis in psychology. Qual. Res. Psychol., 3(2), 77–101.
- Casteleyn J. and Mottart A., (2012), Presenting material via graphic organizers in science classes in secondary education. Proc. Soc. Behav. Sci., 69, 458–466.
- Chen Y. C., Hand B. and McDowell L. E. A. H., (2013), The effects of writing-to-learn activities on elementary students’ conceptual understanding: Learning about force and motion through writing to older peers. Sci. Educ., 97(5), 745–771.
- Creswell J. W., (2014), Research Design Qualitative, Quantitative, and Mixed Methods Approaches, 4th edn, Thousand Oaks, CA: SAGE Publications, p. 304.
- Cohen J., (1960), A coefficient of agreement for nominal scales. Educ. Psychol. Meas., 20(1), 37–46.
- Disessa A. A., (2004), Metarepresentation: Native competence and targets for instruction. Cognit. Instr., 22(3), 293–331.
- Disessa A. A., (2014), The construction of causal schemes: Learning mechanisms at the knowledge level. Cognit. Sci., 38(5), 795–850.
- Duit R. and Treagust D. F., (2003), Conceptual change: A powerful framework for improving science teaching and learning. Int. J. Sci. Educ., 25(6), 671–688.
- Duit R., Treagust D., and Widodo A., (2008), Teaching science for conceptual change: Theory and practice, in Vosniadou S. (ed.), International Handbook of Research on Conceptual Change, New York, USA: Routledge, pp. 629–646.
- Driver R., (1981), Pupils’ alternative frameworks in science. Eur. J. Sci. Educ., 3(1), 93–101.
- Driver R., and Easley J., (1978), Pupils and Paradigms: a Review of Literature Related to Concept Development in Adolescent Science Students. Stud. Sci. Educ., 5(1), 61–84.
- Fraenkel J. R. and Wallen N. E., (2009), How to design and evaluate research in education, 7th edn, New York: McGraw-Hill Companies, Retrieved in December 2019 from URL: https://archive.org/details/methodology-alobatnic-libraries.
- Gabel D. L., Samuel K. V., and Hunn D., (1987), Understanding the particulate nature of matter. J. Chem. Educ., 64(8), 695.
- Galbraith D., (1999), Writing as a Knowledge Constituting Process, in Rijlaarsdam G., Espéret E., Torrance M. and Galbraith D. (ed.), Studies in Writing: Vol. 4. Knowing What to Write: Conceptual Processes in Text Production, Amsterdam: Amsterdam University Press, pp. 139–160.
- Garnett P. J. and Treagust D. F., (1992), Conceptual difficulties experienced by senior high school students of electrochemistry: Electrochemical (galvanic) and electrolytic cells. J. Res. Sci. Teach., 29(10), 1079–1099.
- Gauchon L., and Méheut M., (2007), Learning about stoichiometry: from students’ preconceptions to the concept of limiting reactant. Chem. Educ. Res. Pract., 8(4), 362–375.
- Granville M. F., (1985), Student misconceptions in thermodynamics. J. Chem. Educ., 62(10), 847.
- Gunel M., Hand B. and Gunduz S., (2006), Comparing student understanding of quantum physics when embedding multimodal representations into two different writing formats: Presentation format versus summary report format. Sci. Educ., 90(6), 1092–1112.
- Gunel M., Hand B. and Prain V., (2007), Writing for learning in science: A secondary analysis of six studies. Int. J. Sci. Math. Educ., 5(4), 615–637.
- Gunel M., Hand B. and McDermott M., (2009), Writing for different audiences: Effects on high school students’ conceptual understanding of biology. Learn. Instr., 19(4), 354–367.
- Gunel M., Kingir S. and Aydemir N., (2016), The effect of embedding multimodal representation in non-traditional writing task on students’ learning in electrochemistry, in Hand B., McDermott M. and Prain V. (ed.), Using multimodal representations to support learning in the science classroom, Switzerland: Springer, pp. 59–75.
- Hand B. M., Prain V. and Yore L., (2001), Sequential writing tasks’ influence on science learning, in Tynjala P., Mason L. and Lonka K. (ed.), Writing as a learning tool: Integrating theory and practice, Dordrecht: Kluwer Academic Publishers, pp. 105–129.
- Hand B., Yang O. E. M. and Bruxvoort C., (2007), Using writing-to-learn science strategies to improve year 11 students’ understandings of stoichiometry. Int. J. Sci. Math. Educ., 5(1), 125–143.
- Hand B. M., Gunel M. and Ulu C., (2009), Sequencing embedded multimodal representations in a writing to learn approach to the teaching of electricity. J. Res. Sci. Teach., 46(3), 225–247.
- Harrison A. G., Grayson D. J. and Treagust D. F., (1999), Investigating a grade 11 student's evolving conceptions of heat and temperature. J. Res. Sci. Teach., 36(1), 55–87.
- Howard K. E., Brown S. A., Chung S. H., Jobson B. T., and VanReken T. M., (2013), College students’ understanding of atmospheric ozone formation. Chem. Educ. Res. Pract., 14(1), 51–61.
- Huddle P. A. and Pillay A. E., (1996), An in-depth study of misconceptions in stoichiometry and chemical equilibrium at a South African university. J. Res. Sci. Teach., 33(1), 65–77.
- Karpudewan M. and Balasundram N., (2019), Addressing Alternative Conceptions about Transition Metals among Form Six Students using Information and Communication Technology based Instruction. EURASIA Journal of Mathematics, Sci. Technol. Educ., 15(7), em1731.
- Knipper K. J. and Duggan T. J., (2006), Writing to learn across the curriculum: Tools for comprehension in content area classes. Reading Teach., 59(5), 462–470.
- Kim T., Wright L. K. and Miller K., (2019), An examination of students’ perceptions of the Kekulé resonance representation using a perceptual learning theory lens. Chem. Educ. Res. Pract., 20(4), 659–666.
- Klein P., (1999), Reopening inquiry into cognitive processes in writing-to-learn. Educ. Psychol. Rev., 11(3), 203–270.
- Kress G. R., (2010), Multimodality: A social semiotic approach to contemporary communication, London: Routledge.
- Lamichhane R., Reck C. and Maltese A. V., (2018), Undergraduate chemistry students’ misconceptions about reaction coordinate diagrams. Chem. Educ. Res. Pract., 19(3), 834–845.
- Lapp S. and Ariza E. N. W., (2018), Technology and ELLs in Middle School. The TESOL Encyclopedia of English Language Teaching, 1–6 DOI:10.1002/9781118784235.eelt0672.
- Lee M. H., Wu Y. T. and Tsai C. C., (2009), Trends in science education from 2003 to 2007: A content analysis of publications in selected journals. Int. J. Sci. Educ., 31(15), 1999–2020 DOI:10.1080/09500690802314876.
- Lin T. C., Lin T. J. and Tsai C. C., (2014), Research Trends in Science Education from 2008 to 2012: A systematic content analysis of publications in selected journals. Int. J. Sci. Educ., 36(8), 1346–1372 DOI:10.1080/09500693.2013.864428.
- Lin T. J., Lin T. C., Potvin P. and Tsai C. C., (2019), Research trends in science education from 2013 to 2017: A systematic content analysis of publications in selected journals. Int. J. Sci. Educ., 41(3), 367–387.
- Lutter J. C., Hale L. V. and Shultz G. V., (2019), Unpacking graduate students’ knowledge for teaching solution chemistry concepts. Chem. Educ. Res. Pract., 20(1), 258–269.
- Mason L. and Boscolo P., (2000), Writing and conceptual change. What changes? Instr. Sci., 28(3), 199–226.
- McDermott A. M., (2009), The impact of embedding multiple modes of representation on student construction of chemistry knowledge, Doctoral dissertation, USA: College of The University of Iowa.
- McDermott M., (2010), More than writing-to-learn: Using multimodal writing tasks in the science classroom. Sci. Teach., 77(1), 32–36.
- McDermott M. and Hand B., (2013), The impact of embedding multiple modes of representation within writing tasks on high school students’ chemistry understanding. Instr. Sci., 41(1), 217–246.
- McDermott M. and Hand B., (2016), Modelling Scientific Communication with Multimodal Writing Tasks: Impact on Students at Different Grade Levels, in Hand B., McDermott M. and Prain V. (ed.), Using multimodal representations to support learning in the science classroom, Switzerland: Springer, pp. 183–221.
- Malaysian Examination Council, (2012), S2−: decolourisation, yellow precipitate. Retrieved December 2019 from http://portal.mpm.edu.my/documents/10156/f76a4328-c3e0-4703-9785-70f4e8fffee6.
- Malaysian Examination Council, (2012), Syllabus and specimen paper, Retrieved December 2019 from http://webmpm.mpm.edu.my/bi/main.php?Content=sections&SubSectionID=40&SectionID=39.
- Nakhleh M. B., (1994), Chemical education research in the laboratory environment: How can research uncover what students are learning? J. Chem. Educ., 71(3), 201.
- Nakiboglu C., (2003), Instructional misconceptions of Turkish prospective chemistry teachers about atomic orbitals and hybridization. Chem. Educ. Res. Pract., 4(2), 171–188.
- Nakiboglu C., (2017), Use of Graphic Organizers in Secondary Chemistry Lessons, Eurasia Proc. Educ. Soc. Sci., 7, 72–75.
- Nam J. and Cho H., (2016), Examining the impact of multimodal representation instruction on students’ learning of science, in Hand B., McDermott M. and Prain V. (ed.), Using multimodal representations to support learning in the science classroom, Switzerland: Springer, pp. 117–133.
- Nesbit J. C. and Adesope O. O., (2006), Learning with concept and knowledge maps: A meta-analysis. Rev. Educ. Res., 76(3), 413–448.
- Peterson R. F., (1986), The development, validation and application of a diagnostic test measuring Year 11 and 12 students’ understanding of covalent bonding and structure, Unpublished Master's thesis, Western Australia: Curtin University of Technology.
- Peterson R. F., Treagust D. F. and Garnett P., (1989), Development and application of a diagnostic instrument to evaluate grade-11 and-12 students’ concepts of covalent bonding and structure following a course of instruction. J. Res. Sci. Teach., 26(4), 301–314.
- Pineda L., and Garza G., (2000), A model for multimodal reference resolution. Comput. Linguist., 26(2), 139–193.
- Popplet, (2020), Retrieved December 2019 from http://www.popplet.com/.
- Posner G. J., Strike K. A., Hewson P. W. and Gertzog W. A., (1982), Accommodation of a scientific conception: toward a theory of conceptual change. Sci. Educ., 66(2), 211–227.
- Prain V. and Hand B., (1996), Writing and learning in secondary science: rethinking practices. Teach. Teach. Educ., 12(6), 609–626.
- Reed D. K., Jemison E., Sidler-Folsom J. and Weber A., (2019), Electronic graphic organizers for learning science vocabulary and concepts: the effects of online synchronous discussion. J. Exp. Educ., 87(4), 552–574.
- Regan K., Evmenova A. S., Good K., Legget A., Ahn S. Y., Gafurov B. and Mastropieri M., (2018), Persuasive writing with mobile-based graphic organizers in inclusive classrooms across the curriculum. J. Spec. Educ. Technol., 33(1), 3–14.
- Sanders M., (1993), Erroneous ideas about respiration: The teacher factor. J. Res. Sci. Teach., 30(8), 919–934.
- Scardamalia M. and Bereiter C., (1999), Schools as knowledge building organizations, in Keating D. and Hertzman C. (ed.), Today's children, tomorrow's society: The developmental health and wealth of nations, New York: Guilford, pp. 274–289.
- Sendur G. and Toprak M., (2013), The role of conceptual change texts to improve students’ understanding of alkenes. Chem. Educ. Res. Pract., 14(4), 431–449.
- Sessions L., Kang M. O. and Womack S., (2016), The neglected “R”: improving writing instruction through iPad apps. Tech. Trends, 60(3), 218–225.
- Shadish W. R., Cook T. D. and Campbell D. T., (2002), Experimental and quasi-experimental designs for generalized causal inference, Boston: Houghton, Mifflin and Company.
- Sreenivasulu B. and Subramaniam R., (2014), Exploring undergraduates’ understanding of transition metals chemistry with the use of cognitive and confidence measures. Res. Sci. Educ., 44(6), 801–828.
- Sreenivasulu B., and Subramaniam R., (2019), Mapping the conceptual space formed by students’ understanding of coordination number of a transition metal complex: an exploratory study. Chem. Educ. Res. Pract., 20(3), 468–483.
- Taber K. S., (2002), Alternative Conceptions in Chemistry: Prevention, Diagnosis and Cure? London: The Royal Society of Chemistry.
- Taber K. S., (2014), Ethical consideration of chemistry education research involving ‘human subject’. Chem. Educ. Res. Pract., 15(2), 109–113.
- Tan K. C. D., Taber K. S., Goh N. K., and Chia L. S., (2005), The ionisation energy diagnostic instrument: a two-tier multiple-choice instrument to determine high school students’ understanding of ionisation energy. Chem. Educ. Res. Pract., 6(4), 180–197.
- Tan K. C. D., Taber K. S., Liew Y. Q., and Teo K. L. A., (2019), A web-based ionisation energy diagnostic instrument: exploiting the affordances of technology. Chem. Educ. Res. Pract., 20, 412–427.
- Teddlie C. and Tashakkori A., (2009), Foundations of mixed methods research: Integrating quantitative and qualitative approaches in the social and behavioral sciences, Thousand Oaks, CA: Sage.
- Tsaparlis G., Pappa E. T. and Byers B., (2018), Teaching and learning chemical bonding: research-based evidence for misconceptions and conceptual difficulties experienced by students in upper secondary schools and the effect of an enriched text. Chem. Educ. Res. Pract., 19(4), 1253–1269.
- Tümay H., (2016), Reconsidering learning difficulties and misconceptions in chemistry: emergence in chemistry and its implications for chemical education. Chem. Educ. Res. Pract., 17(2), 229–245.
- Voska K. W. and Heikkinen H. W., (2000), Identification and analysis of student conceptions used to solve chemical equilibrium problems. J. Res. Sci. Teach., 37(2), 160–176.
- Yan Y. K. and Subramaniam R., (2018), Using a multi-tier diagnostic test to explore the nature of students’ alternative conceptions on reaction kinetics. Chem. Educ. Res. Pract., 19(1), 213–226.
- Ye J., Lu S. and Bi H., (2019), The effects of microcomputer-based laboratories on students’ macro, micro, and symbolic representations when learning about net ionic reactions. Chem. Educ. Res. Pract., 20(1), 288–301.
- Yore L. D. and Treagust D. F., (2006), Current realities and future possibilities: Language and science literacy—empowering research and informing instruction. Int. J. Sci. Educ., 28(2–3), 291–314.
| This journal is © The Royal Society of Chemistry 2021 |