Implementation of self-regulatory instruction to promote students’ achievement and learning strategies in the high school chemistry classroom†
Cansel
Kadioglu-Akbulut
 *a and
Esen
Uzuntiryaki-Kondakci
*a and
Esen
Uzuntiryaki-Kondakci
 b
b
aTokat Gaziosmanpasa University, Faculty of Education, Department of Mathematics and Science Education, Tokat, Turkey. E-mail: canselkadioglu@gmail.com
bMiddle East Technical University, Faculty of Education, Department of Mathematics and Science Education, Ankara, Turkey
First published on 28th July 2020
Abstract
The current study investigated the effectiveness of self-regulatory instruction developed based on guided inquiry on 11th grade students’ use of learning strategies and achievement in chemistry, compared to traditionally designed chemistry instruction. Additionally, the self-regulatory processes in which students engaged and the development of these processes over the course of the study were examined. For this purpose, mixed-method design was employed. In total, 78 students participated in the study: 38 students in the experimental group and 40 students in the control group. Additionally, four students from each classroom were selected as focal students, using the maximum variation sampling method. Quantitative data were collected using Chemistry Achievement Test and Cognitive and Metacognitive Strategies Scale; qualitative data were obtained through journals and think-aloud protocols. Five dependent variables were studied: achievement in chemistry, and the four learning strategies of rehearsal, elaboration, organization, and metacognitive self-regulation. A mixed multivariate analyses of variance was run to analyze the quantitative data and the deductive method was used to analyze the qualitative data. Quantitative results indicated a slight improvement in student achievement in the experimental group and no significant difference regarding learning strategies. However, analyses of think-aloud protocols revealed that students in the experimental group used metacognitive strategies more often, which were associated with self-regulated learning, and in turn achieved more—gave more correct responses and/or provided more complete scientific explanations—compared to the students in the control group.
Introduction
“When planning for a year, plant corn. When planning for a decade, plant trees. When planning for life, train and educate people.” Chinese proverb, Guanzi (c. 645 BC)As the Chinese proverb foresaw centuries ago, education does not end with graduation. Individuals need new skills after school, and learning continues the whole life span. Learning how to learn should be an important goal of education so that individuals can adapt their skills to new conditions and accomplish their learning needs throughout their lives (Bandura, 1986; Zimmerman, 2000). Therefore, curriculum should be designed to support development of learning skills as well as content knowledge. Accordingly, the aim of this study was to investigate the effectiveness of self-regulatory instruction on 11th grade students’ use of learning strategies and their achievement in chemistry class, compared to traditionally designed chemistry instruction.
Researchers have proposed different theories to describe how individuals become independent learners, i.e., masters of their own learning. Most of those investigations are conducted based on the social cognitive theory proposed by Bandura (1986). This theory emphasizes “the agency of learner,” which means that individuals have control over their thoughts, feelings, and actions as a result of their self-beliefs. With respect to this view, learners make their own choices and continue their learning in order to achieve their goals. From self-regulatory perspective, “learning is not something that happens ![[t with combining low line]](https://www.rsc.org/images/entities/char_0074_0332.gif)
![[o with combining low line]](https://www.rsc.org/images/entities/char_006f_0332.gif) students; it is something that happens
students; it is something that happens ![[b with combining low line]](https://www.rsc.org/images/entities/char_0062_0332.gif)
![[y with combining low line]](https://www.rsc.org/images/entities/char_0079_0332.gif) students. …for learning to occur, students must become proactively engaged at both a covert and an overt level” (Zimmerman, 2001; p. 33, underlined in original).
students. …for learning to occur, students must become proactively engaged at both a covert and an overt level” (Zimmerman, 2001; p. 33, underlined in original).
The social cognitive theory (Bandura, 1986) guides a great body of self-regulatory research, including this study. Self-regulated learning (SRL) is defined as “self-generated thoughts, feelings, and actions that are planned and cyclically adopted to the attainment of personal goals” (Zimmerman, 2000; p. 14). SRL is composed of three main dimensions: motivation, strategy use, and metacognition. Zimmerman (2000) explains SRL processes in three cyclic phases: forethought, performance, and self-reflection. The forethought phase includes the processes that students use to get ready for learning. Highly self-regulated learners initially determine their learning goals and spend time on strategic planning. In order to employ the required strategies effectively, motivational factors such as the students’ self-efficacy beliefs also play an important role. Next, in the performance phase, students employ these strategies to achieve their goals, considering the demands of the task and the facilities in the learning environment; they make necessary changes if required. Finally, in the self-reflection phase, which occurs after a study period, the learner judges the effectiveness of her/his learning process and sets new goals for further learning.
Self-regulatory processes activate students’ learning in several ways. Students determine their learning goals, give importance to mastery of the task, are aware of their strengths and weaknesses in learning, select the most appropriate strategies, are responsible for applications of these strategies, observe their progress, accept teacher guidance when necessary, evaluate whether they achieve their goals or not, monitor the learning process, and make necessary changes (Zimmerman, 2000; Paris and Paris, 2001; Schraw et al., 2006). In general, highly self-regulated learners are aware of the processes that improve their academic performance; they monitor those processes considering various factors—such as previous learning experiences—and motivate themselves to learn.
The first wave of research on Zimmerman's SRL model utilized interventions that were separate from the curriculum. For instance, Hofer et al. (1998) designed a preparatory strategy instruction course, named “Learning to Learn,” for first-year undergraduate students to support their learning. Later, SRL intervention programs were employed within curricular activities in diverse disciplines. The effects of these intervention programs on learning outcomes were summarized and discussed in three meta-analyses (Hattie et al., 1996; Dignath and Buttner, 2008; Donker et al., 2014). In the first meta-analysis, Hattie et al. (1996) suggest that strategy instruction works better in the actual classroom context, and that specific attention should be given to active student participation and metacognitive awareness. Next, Dignath and Buttner (2008) search for the effect of intervention programs on three outcomes associated with SRL, namely academic performance, strategy use, and motivation. They found that intervention programs contribute mostly to academic performance, next to students’ motivation, and then strategy use. Additionally, intervention programs that are directed by the researcher and extend over longer periods of time work better. The recent study conducted by Donker et al. (2014) revealed that among metacognitive strategies, planning and monitoring strategies, and cognitive strategies, elaboration makes the highest contribution to academic performance. Metacognitive strategies are important in being aware of the effective strategies considering task demands and monitoring/changing these strategies with respect to accomplishment of learning goals. As for cognitive strategies, elaboration strategy is useful to connect recently learned information to existed knowledge to improve duration of the information. Moreover, all three meta-analyses showed that studies conducted in the domain of science education were limited in number. Therefore, there seems to be a need for an intervention program that has been tested in a high school chemistry class.
In chemistry classrooms, research findings have indicated that inquiry-oriented teaching strategies are effective in encouraging students to generate scientific questions, practice inquiry skills, associate their observations to related concepts, and improve their performance (e.g., NRC, 2000; Hofstein et al., 2004; Mutlu and Acar-Şeşen, 2018). Inquiry is commonly used as a strategy for teaching and learning; students generate research questions, select important variables, design the process, collect data, and come to a conclusion. During this inquiry, they need SRL strategies, such as planning and monitoring learning processes and evaluating their efficiency. Schraw et al. (2006) link inquiry as an instructional strategy to SRL. They also suggest metacognition as a key element in SRL. Additionally, students also need cognitive strategies to acquire information. Accordingly, both cognitive and metacognitive strategies were selected as a focus in the present study.
SRL skills develop through social influences. As control moves to self, as opposed to instructors, students are expected to have more control over the learning process and become more independent learners in time (Zimmerman, 2000). The shift of control from teachers to students also follows a parallel sequence in guided inquiry. Colburn (2004) classifies inquiry-oriented instruction as either structured, guided, or open inquiry based on who is making decisions on the question to be investigated, the procedures to be followed, and the data to be collected and analyzed. In structured inquiry, the teacher provides students with step-by-step instruction. Students form the data table, however, and select important data with respect to their observations. On the other hand, students make almost all the decisions in open inquiry; they identify the question to investigate, design the procedure, and collect and interpret the data. Guided inquiry exists in the spectrum between structured and open inquiry. The teacher introduces the research question to the students, and the students design the experiment, collect relevant data, and interpret them. Because guided inquiry provides an opportunity to employ scientific reasoning and shift the control of learning to students, in this study the self-regulatory instruction was designed based on guided inquiry. The guided inquiry strategy may be related to the Process Oriented Guided Inquiry Learning (POGIL) Project (https://pogil.org). POGIL is an instructional strategy which merges learning cycle and cooperative learning pedagogies together in the guided inquiry activities (Moog and Spencer, 2008) and has been successfully implemented in graduate and high school chemistry courses for nearly two decades. However, in the present study guided inquiry referred to a general term defining the roles of the teacher and students, i.e., who makes decisions during the inquiry process. Although POGIL and the instruction implemented in the present study share mutual pedagogical issues in terms of using guided inquiry approach (chemistry as a research context, students working in small groups, active participation or giving responsibility of learning to students, defining teacher's role as a facilitator and focusing on process skills as well as content knowledge), we did not explore the features of students’ collaborative learning or social interactions that emerged among students. We accepted group work as a social learning environment in which students develop self-regulatory skills. However, in POGIL group roles are well defined, feedback is provided students in terms of group performance and group performance was assessed as well as individual learning outcomes. Additionally, while developing learning tasks POGIL activities were centered around learning cycle pedagogy giving more emphasis on the content; while we utilized Zimmerman's SRL model (i.e., journals) paying attention to students’ learning experiences as well as the content. Moreover, POGIL pedagogy emphasizes the development of process skills such as teamwork, problem solving, critical thinking, management, information processing and assessment (self-assessment and metacognition). Although we acknowledge the importance of all these skills, we focused more on metacognitive skills. For these reasons POGIL was not employed in the present study.
The effectiveness of self-regulatory instruction based on guided inquiry (SRI-GI) on 11th grade students’ use of learning strategies and achievement in chemistry class, compared to traditionally designed chemistry instruction, was investigated in this mixed-method study. This study is significant in several ways: Firstly, chemistry education is one of the less frequently studied contexts in SRL literature. This study has the potential to fill a gap in the SRL literature by incorporating theoretical approaches on SRL practices into high school chemistry classrooms. Secondly, SRI-GI provides students with a learning environment that encourages them to attend discussions, question the trustworthiness of information coming from different sources, and come up with conclusions. In this way, it supports development of scientifically literate individuals. Lastly, most of the earlier studies on this topic were conducted based on questionnaires and single instance data collection. Conversely, this study was designed based on mixed-method inquiry and compared results from both qualitative and quantitative sources. Thus, this study enables the exploration and comparison of different aspects of SRL. The following research questions guided this study:
1. What is the effect of SRI-GI on students’ use of learning strategies and their achievement in the topics Solubility Equilibrium and Acids and Bases, in an 11th grade chemistry course?
2. How does students’ use of self-regulatory strategies change over the course of the study?
Methodology
Research design: mixed-method design
SRL is a multi-dimensional construct including several components. Winne and Perry (2000) suggest using different instruments to cover the different aspects of SRL. In line with their suggestion, to provide better understanding of students’ self-regulatory processes, a mixed-method design incorporating both quantitative and qualitative approaches was used in this study. Specifically, the complementary strengths stance—which suggests using quantitative and qualitative methods with nonoverlapping weaknesses and complementary strengths together—was utilized (Greene, 2007). Quantitative and qualitative data were collected and analyzed separately; then, results coming from different sources were compared to draw inferences and conclusions. Thus, the data collection and analysis processes are described separately under the quantitative and qualitative approaches sections. However, results coming from both sources are compared and contrasted in the discussion section in order to make more comprehensive inferences. Fig. 1 displays the research process.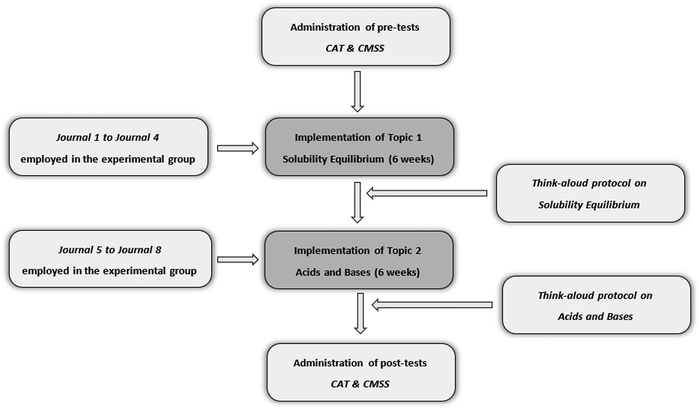 | ||
| Fig. 1 Implementation of instruction and data collection procedure. * CAT: chemistry achievement test; CMSS: cognitive and metacognitive strategies scale. | ||
Research context
High schools in Turkey provide a four-year education after middle school. They follow the chemistry curriculum offered by the Ministry of National Education. Students must take a nationwide University Entrance Examination after high school in order to attend a university program. Therefore, they must choose a major considering the undergraduate program they want to follow at the end of grade 9. At the time of the current study, all students are required to take a chemistry course, which covers basic chemistry concepts such as nature of matter, solutions, and atomic structure at grade 9. Only “Science and Mathematics” majors take the chemistry course that includes more advanced topics, like solubility equilibrium and electrochemistry, at upper grade levels. Therefore, grade 11 was chosen as a research context in this study. The 11th grade chemistry course took place over three 40-minute sessions each week.The instructional materials, think-aloud protocols, and interviews in the present study were originally in Turkish and translated into English. Taber (2018) highlighted how reporting an academic work in another language is a part of research and requires cautious effort. In line with his suggestions, the following steps were carried out to ensure quality of translation. Initially, the samples (e.g., instructional materials, students’ responses to think aloud protocols, etc.) selected to be shared in this paper were translated by the authors, who have a good command of the English language. Next, two experts who had a doctorate degree in chemistry education from an English-speaking university were asked to check these translations to ensure that they reflected the original material in the best possible way. Finally, experts in the English language checked the translations and gave final recommendations.
Ethical issues
During the study, ethical principles were employed to protect participants’ rights. First, because this study was conducted in a public high school, necessary permissions were taken from the University Ethical Board and the Ministry of National Education. Second, the school administration was informed about the purpose of the study and the research process in order to obtain their permission for the study. Third, the cooperative teacher was enlightened about the research process and her role throughout the study. She voluntarily agreed to participate in the study. Fourth, since the participants were below 18 years of age, consent forms were sent to students’ parents; they were informed about the research process and their permission was asked. Finally, students were asked to give their permission for volunteer participation. They were informed that they could leave the study whenever they wanted. All students agreed to participate and all remained involved until the end of the study. The participants were guaranteed that the data would be kept confidential. Students’ real names were not used; rather, the data from different instruments and different times were matched using their ID numbers and reported using pseudonyms. Additionally, both groups took safety guidance training for their laboratory work before entering the laboratory.Quantitative approach: quasi experimental design
Quasi experimental design was used for the purpose of exploring the effect of SRI-GI on 11th grade students’ learning strategies and achievement in chemistry over time. Dependent variables were achievement and learning strategies (rehearsal, elaboration, organization, and metacognitive self-regulation), while independent variables were intervention group (experimental and control groups) and testing period (Time I and Time II).The CAT, developed by the researchers, was used as a measure of the students’ achievement in chemistry regarding the topics of Solubility Equilibrium and Acids and Base Units. Initially, an item pool with 45 multiple-choice questions (one correct response and four distractors) was prepared considering objectives in the national curriculum and related literature. Afterwards, for content validity, the table of specifications and test items were sent to three experts in chemistry education and one expert in chemistry. After necessary revisions were made based on these expert opinions, the 45-item test was piloted with 154 grade 11 students from two different high schools. Item analysis via the ITEMAN program was performed to select items with varying difficulty levels, discriminating well, that covered the content of both units. As a result, we decided to exclude 15 items from the 45-item test considering the following factors: First, we examined the item discrimination index. Six poorly discriminating items with indices below 0.19 (Crocker and Algina, 1986) were removed from the test. Second, we checked item difficulty levels: values around 0.50 maximize total score variance and reliability (Crocker and Algina, 1986). Accordingly, 15 items with moderate difficulty levels were selected; 8 items had high difficulty, and the remaining 7 items were labeled as easy difficulty level. To maintain percentage of item difficulties, four of the easy items and one of the difficult items were deleted. The subject matter of those items was covered in other items with the desired difficulty level and strong discrimination indices. Third, the researchers evaluated the questions for subject matter. If the same subject matter was covered in two different items, the item with the better discrimination index and difficulty level was selected. Consequently, four more items (which had subject matter covered in remaining items with better item discrimination indices) were removed. Of the remaining items, seven with item discrimination indices ranging between 0.20 and 0.29 (Crocker and Algina, 1986) were revised considering expert opinions; 23 items were used without any revision. As a result, the final form of the CAT included 30 items. A sample item (item difficulty = 0.519; item discrimination index = 0.433) from the test is given in Table 1.
| Sample | pH | Color |
|---|---|---|
What will be the color of the 0.4 M HX solution when some red onion juice is added? Ka (HX) = 2.5 × 10−4. *![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (A) Pink (B) Pale pink (C) Pale green (D) Green (E) Yellow. * (A) Pink (B) Pale pink (C) Pale green (D) Green (E) Yellow. *![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Correct response. Correct response. |
||
| Digestive fluid | 1.9 | Pink |
| Vinegar | 3.4 | Pale pink |
| Milk | 6.4 | Pale green |
| Baking powder | 8 | Green |
| Detergent | 10 | Yellow |
A researcher measures the pH values of the solutions given in the table below. S/he adds some red onion juice and observes the color changes as given below:
The 30-item test was used as pre-test and post-test to measure participants’ chemistry achievement in given topics. In the present study, the KR-20 reliability coefficient was found to be 0.76 for the pre-CAT and 0.82 for the post-CAT, which pointed to internally consistent test scores for both administrations.
The CMSS was used to determine students’ use of different cognitive and metacognitive strategies. The rehearsal, elaboration, organization, and metacognitive self-regulation subscales of the Motivated Strategies for Learning Questionnaire, developed by Pintrich et al. (1991) and translated into Turkish by Sungur (2004), were selected for this purpose. The CMSS is composed of 26 items. Students rated themselves on a seven-point rating scale (1 for “not at all true for me” and 7 for “very true of me”). A pilot study was conducted with 236 students taking a grade 11 chemistry course. Satisfactory fit indices (χ2/df (1616.499/424) = 3.81, RMSEA = 0.049, SRMR = 0.049, CFI = 0.89, and NNFI = 0.87) were obtained. Although CFI and NNFI were slightly lower than the criteria, RMSEA and SRMR values indicated that the model provided a good fit to the data (Kline, 2005). Cronbach's alpha reliability coefficients ranged between 0.76 and 0.85 for pre-CMSS and 0.62 and 0.85 for post-CMSS scores.
Qualitative approach: case study
The case study method as a qualitive approach was employed to explore how students employed the self-regulatory processes while learning chemistry concepts and how these processes developed over time. Yin (2009) defines the case study method as a practice of inquiry that focuses on in-depth understanding of a phenomenon in a real-life context. According to social cognitive theory, all learners are assumed to use self-regulatory learning processes to some degree; therefore, concepts like un-self-regulated learners or lack of self-regulation are not acknowledged (Winne, 1997). The degree of students’ self-regulation is based on the degree to which students are metacognitively, motivationally, and behaviorally active in the learning process. Because students in the control group also utilized self-regulatory processes to some degree while studying for the chemistry course, each classroom was accepted as a case, in other words, as a unit of analysis. Accordingly, two cases were explored in the present study: self-regulatory practice in the experimental group and self-regulatory practice in the control group. Four students were selected from each class as representative instances of their cases.| Student | Gender | Group | Achievement | SRL skills |
|---|---|---|---|---|
| Ex.L | Male | Experimental | Low | Medium |
| Ex.M1 | Female | Experimental | Medium | Medium |
| Ex.M2 | Male | Experimental | Medium | Medium |
| Ex.H | Female | Experimental | High | High |
| Co.L | Male | Control | Low | Medium |
| Co.M1 | Female | Control | Medium | Medium |
| Co.M2 | Male | Control | Medium | Medium |
| Co.H | Male | Control | High | High |
Additionally, a pre-interview was conducted with focal students from both groups before the intervention to determine their initial self-regulatory skills. Students were asked to explain their “typical” behavior while they were studying for their chemistry course. The interview schedule, covering three cyclic phases of Zimmerman's SRL model (2000), was developed by the authors and used in an earlier study (Kadioglu et al., 2006). Results of the pre-interview revealed similar study patterns for six of the eight focal students. None of them used strategic planning; instead, they studied occasionally to pass the exams or to prepare for the university entrance examination. In other words, they displayed performance-approach type goals. Students possessed a limited number of strategies and generally employed task-based strategies, such as rehearsal, highlighting, attention focusing, reviewing classroom material, and solving extra questions from different resources. To conclude, although the participants were not studying in line with a study plan prior to the interventions, they exhibited some adaptive learning strategies. Therefore, their self-regulatory skills were evaluated at medium level.
On the other hand, two students (Ex.H and Co.H) reported that they studied daily without the use of a study plan. In addition to working to maintain their grades, they studied course material to satisfy their curiosity. They stated that while working at home they review the notes they take during class, resolve the questions the teacher solved in class, note important points, solve additional questions, and ask for help with questions that they cannot solve. Both students employed more strategic study habits compared to their classmates; their self-regulatory skills were evaluated at a higher level accordingly.
Journals were primarily designed as instructional materials to guide the students in the experimental group through the three phases of Zimmerman's model. They provided data about the SRL processes that the students used during the forethought, performance, and self-reflection phases. The resulting data were highly important to track the development of SRL processes in the experimental group over the course of the study. Students would need to choose the necessary cognitive and metacognitive strategies during the forethought phase, implement them in the performance phase, and evaluate their efficiency in the self-reflection phase. Collecting data from the four focal students in the experimental group, eight journals were evaluated considering three self-regulatory processes. Students took notes during class while working on given tasks in groups. After class hours, they were asked to individually write down their learning experiences based on their notes to evaluate and reflect on the processes under three SRL phases. As a result, although journals were primarily written individually at home, they reflected the group performance to some degree. More detailed information about the journals is provided under the section “Intervention in the experimental group.”
| Categories | Examples |
|---|---|
| Rehearsal | Memorizing key words |
| Elaboration | Summarizing main points |
| Organization | Grouping the items based on shared properties |
| Metacognitive self-regulation | Metacognitive awareness (awareness of everyday applications of a topic) |
| Metacognitive monitoring (monitoring change in ideas) | |
| Metacognitive evaluation (evaluation of existing ideas) |
Students’ responses to think-aloud protocols were analyzed in terms of chemistry achievement as well. Seven achievement categories were determined regarding students’ responses and explanations: (1) wrong response, (2) wrong response with poor/insufficient scientific explanation, (3) partially correct response, (4) correct response without scientific explanation, (5) correct response with irrelevant explanation, (6) correct response with poor/insufficient scientific explanation, and (7) correct response with sufficient scientific explanation. For example, when explaining why the barium sulfate solution was harmless to patients in radiological examinations, one student came to the conclusion that barium sulfate was slightly soluble. Accordingly, he gave a correct response with a scientific explanation and his achievement level was coded as a 7.
All think-aloud protocol data for the focal students from both groups were coded by the first researcher. Two think-aloud protocol transcripts belonging to the same student were then coded by an expert in chemistry education. In case of disagreement between the researcher and the expert, consensus was reached through face-to-face discussion. The inter-rater reliability was found to be 0.73, which was a satisfactory level (Miles and Huberman, 1994).
To analyze the journals, the deductive method was used (as in the think-aloud protocols). Each phase of Zimmerman's model was coded as categories and the processes that fall into those categories were coded as subcategories (see Table 4).
| Categories | Subcategories | Examples |
|---|---|---|
| Forethought | 1. Planning activity | Describing what to do |
| 2. Planning data recording | Preparing a table to fill | |
| 3. Predictions | Reporting expected outcomes | |
| Performance | 1. Procedure | Stating the steps |
| 2. Observation Data | Reporting observations | |
| 3. Inference | Making conclusions | |
| Self-reflection | 1. Unexpected outcomes | Comparing observations with predictions |
| 2. Assessing learned material | Summarizing the learned concepts | |
| 3. Experienced difficulties | Reporting any problems or questions | |
| 4. Evaluation/Elaboration | Applying what they learned in new situations | |
| 5. Assessing the activity | Assessing how challenging or helpful a task was | |
The subcategories were evaluated considering four criteria: non-existent, not satisfactory, satisfactory, and not applicable. When students did not report anything or the reported information was irrelevant, it was coded as non-existent. If students reported the related process with deficiencies, it was coded as not satisfactory. On the other hand, if they explained their reasoning in terms of their observations, it was accepted as satisfactory. Finally, if an action or process was not relevant considering the activity, it was marked as not applicable (NA). For example, the conclusions made by students based on their observations were coded under the inference subcategory. When the students summarized their observations rather than writing their conclusions, it was marked as non-existent. If the students came up with the right conclusion but did not explain their reasoning, it was coded as not satisfactory inference. On the other hand, if they explained their reasoning with respect to their observations, it was accepted as a satisfactory inference.
All of the journals for the focal students from the experimental group were coded by the first researcher. Additionally, eight journals of one student were coded by the same expert who coded think-aloud protocols for reliability. Likewise, consensus was reached through face-to-face discussion. The inter-rater reliability was found to be 0.85, indicating a high degree of agreement between the two raters (Miles and Huberman, 1994).
Intervention
SRI-GI was employed in the experimental group, while traditional teaching methods were used in the control group. During the intervention, both groups covered two units of the grade 11 national chemistry curriculum. The chemistry course was scheduled for three 40-minute sessions each week and the intervention took 12 weeks in total.However, in order to minimize threats to internal validity, some arrangements were made in the control group and it was ensured that the arrangements were consistent with the teacher's regular practice. To control novelty effect and attitudes of subjects as a threat to internal validity of the study, five laboratory activities covering the same topics were prepared for the control group as well as the experimental group. Students spent nearly an hour on each activity and six hours in total in the chemistry laboratory. Scheduling was arranged such that both groups entered the laboratory during the same week to investigate the same topics. The teacher continued to follow a traditional teaching approach and the activities were precisely structured. The teacher made all decisions; students only followed directions during laboratory sessions in the control group.
The journals were designed considering Zimmerman's three SRL phases (see Table 4). In total, eight journals were prepared covering the national curricular objectives on solubility equilibrium (Journals 1–4) and acids and bases (Journals 5–8). The title and purpose of each journal is presented in Table 5. All eight journals required students to use the same self-regulatory processes to optimize their performance before, during, and after the task. Students followed the processes given in the journals to regulate their work in line with Zimmerman's cyclic model. Journals started with the processes under forethought phase. To begin with, each journal included an introductory section in which students’ prior knowledge was prompted and new concepts essential to achieve the related task were introduced. While introducing the concepts that students have not encountered earlier, the teacher mostly discussed the key features of the topic and gave real life examples. Meanwhile, the students asked unclear points. Next, the purpose of that task was stated explicitly, and the equipment and chemicals that students could use were listed. Then, time was given to students to plan what they would do, write down their predictions, and prepare a table to report the collected data. Meanwhile, the teacher guided students through open-ended questions when they needed help. In the performance phase, the technicians gathered the necessary materials and prepared the setting. While the groups were completing tasks, the teacher walked around the class, observing groups’ performances and giving feedback if necessary. Meanwhile, the reporter wrote down their observations. After completing the task, in the self-reflection phase, students made inferences based on their observations, discussed any unexpected outcomes, assessed what they had learned, talked over the difficulties they had experienced, extended the concepts and principles in the new tasks, and assessed the activity itself. After the groups finished their work, a whole-class discussion was conducted to share the experiences of different groups. In all tasks, students were given some degree of choice: groups could design the experiments their own way, work with various chemicals or household substances, and report results in their own way. Each of the eight journals were also designed to assist the students to improve their metacognitive thinking: Students reflected on what they had already known about a given task, what they were doing and why, and assessed the effectiveness of their learning process.
| Journal title | Purpose of the task |
|---|---|
| 1. Solutions | To help students get laboratory experience before the actual intervention started and help them get accustomed to SRI-GI. |
| 2. The effect of temperature on solubility | To study the solubility concept and the effect of temperature on solubility. |
| 3. Let's examine solubility at a microlevel | To examine solutions and solubility concepts at a microlevel using the “Salts and Solubility” simulation. |
| 4. Does it precipitate? | To investigate whether precipitate occurs when two salt solutions are mixed together. |
| 5. Acid or base? | To explore whether a given liquid could be used as an acid–base indicator and identify whether a substance had acidic or basic properties. |
| 6. How acidic or how basic? | To compare the strength of different acid and base solutions. |
| 7. Acid–base reactions with metals | To explore whether any reaction occurred between different metals and different acid/base solutions. |
| 8. Acid–base titration | To make experiment on titration of a strong acid and a strong base and discuss related concepts. |
Since the timeline of regular curriculum was followed in the present study, students worked in small groups consisting of four members to use time efficiently. For effective group discussions, students were suggested to respect others’ ideas, acknowledge contribution of each group member to the discussions, make clear and understandable statements, evaluate others’ ideas in terms of accuracy and rationality and ask for additional evidence, and propose alternative explanations. Throughout the laboratory activities, students shared their opinions with group members and came up with a decision. They planned and conducted the experiments according to the group's agreement. The reporter took notes during class hour and students individually wrote down their learning experiences to the journals at home. Accordingly, each journal reflected group performance to some degree.
Solving algorithmic questions was the most significant part of the teacher's traditional teaching in general. In an attempt to control internal validity, some of the algorithmic questions used in the control group were also solved in the experimental group. However, they were organized to prompt students’ inquiry skills. Initially, the students thought about the questions individually; then they discussed the case as a class, considering their laboratory experiences. Next, students did any required calculations and finally the class argued whether the results were reasonable or not.
The following strategies were followed to ensure SRI-GI was employed as it was planned. A guide for the new teaching method was prepared for the teacher. The lesson plans were distributed to the teacher two weeks earlier than the implementation and before the intervention weekly meetings were done to discuss the journals face to face. She was also informed about the basic differences between the two teaching methods repeatedly. All chemistry classes of both groups were observed by the researcher throughout the study capturing approximately four-month period. After each class, the implementation was reviewed with the teacher, she assessed the difficulties she experienced and the necessary modifications for future classes were done.
Results
Results of the quantitative and qualitative analyses are presented separately but interpreted together in the discussion section.Results of quantitative analysis
| Dependent variables | Range | Experimental group (N = 38) | Control group (N = 40) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time I | Time II | Time I | Time II | ||||||
| M | SD | M | SD | M | SD | M | SD | ||
| *M: mean; SD: standard deviation. | |||||||||
| Rehearsal | 1–7 | 5.17 | 1.40 | 5.37 | 1.03 | 5.43 | 1.42 | 5.28 | 1.42 |
| Elaboration | 1–7 | 5.01 | 1.02 | 5.31 | 0.90 | 4.93 | 1.24 | 5.05 | 1.26 |
| Organization | 1–7 | 5.20 | 0.99 | 5.45 | 5.29 | 5.11 | 1.24 | 5.29 | 1.53 |
| Metacognitive self-regulation | 1–7 | 5.12 | 0.83 | 5.36 | 0.80 | 5.06 | 1.14 | 5.16 | 1.27 |
| Achievement | 1–30 | 4.70 | 2.22 | 15.71 | 3.62 | 3.47 | 2.54 | 12.84 | 4.72 |
In order to understand which dependent variables contributed to the overall main effects, univariate tests were done. A Bonferroni adjustment was done to control Type 1 error; the adjusted alpha was 0.01 (0.05/5). In terms of the time effect, only the achievement variable (F(1,74) = 279.97, p < 0.01, η2 = 0.80, r = 0.89) changed significantly from Time I to Time II. As for the intervention effect, again only the mean of achievement was significantly different among groups (F(1,74) = 15.72, p < 0.01, η2 = 0.17, r = 0.42).
Results of qualitative analyses
To explain the data regarding student self-regulatory practices, analyses of think-aloud protocols (for focal students from both groups) and the journals from the experimental group are reported in the following section.The following excerpt (Example 1) from a think-aloud protocol indicated how Ex.M2 used rehearsal in the barium sulfate case. Initially, he explained the case in terms of neutralization reaction because the acids and bases unit had already begun. Then, after the researcher prompted him, he recalled the formula used to calculate solubility product constant, or Ksp (rehearsal). In this case, using the rehearsal strategy also triggered metacognitive monitoring. He calculated the solubility of the salt and monitored the consistency between the new idea (the dissolution of a sparingly soluble salt) and his existing idea (neutralization of the toxic substance) and evaluated whether the result was reasonable (metacognitive evaluation).
EXAMPLE 1:
Ex.M2: Barium sulfate … but it says only that barium ion is very dangerous. Sulfate came next to it. Because sulfate ion comes next to it, might it make the toxic substance neutralize? Make the toxic substance harmless for the patient?
Researcher (R): Well, Ksp value is given there.
Ex.M2: Yes.
R: Why do you think it is given?
Ex.M2: K sp value? 10 −10… K sp value, is it because it is strong or week, is that the reason? [Make calculation] Yes, x is equal to 10 −5 .
R: What does 10−5 mean?
Ex.M2: Isn’t that a very small number? Is that the reason why its effect decreases?
R: So?
Ex.M2: Very small number … Probably, it has not got any effect because solubility is very small … It won’t dissolve when it goes through the stomach path, because its solubility is so small.
When students began working on the acids and bases unit, we observed an increase in the use of metacognitive strategies to solve the tasks. For instance, Ex.L, who refused to even think during some tasks in the first unit, used metacognitive thinking strategies on a few occasions in the second unit. While explaining the reason for the deterioration of the marble sculpture, he gave the example of the deterioration of a marble sink in the kitchen after a lemon was left on it (awareness of everyday applications). Similarly, Ex.M1 also started to use metacognitive processes in the acids and bases unit. For example, in Example 2 below, she describes how the pH value would change when the same amount of water was added to a detergent with a pH value of 10. She was aware of the processes she had employed in the laboratory (metacognitive awareness) and linked it to the given case (elaboration).
EXAMPLE 2:
Ex.M1: Starting Ph value is 10 … at first when water is added to the detergent, pH gets close to neutral.
Researcher (R): Why?
Ex.M1: That is because water will dilute the pH value of detergent. For example, the pH is given as 10 here, however, it might go down to 9 or 8 as it will be in a diluted state. Because we did the same.
R: What did you do?
Ex.M1: When we plunged litmus paper into the bleach, the paper got white. The teacher told us that if we had added more water, it would have diluted it and we might get better results.
R: Yes.
Ex.M1: Based on that, pH value gets close to neutral, such as 8, however it will not become neutral.
In addition, the accuracy of responses given during the think-aloud protocols was used as an indicator of participant achievement. In the solubility equilibrium unit, Ex.L and Ex.M1 gave incorrect responses to all tasks, sometimes with insufficient explanations. On the other hand, Ex.M2 and Ex.H gave partially correct or correct responses at about the same rate as each other, indicating higher achievement. In the second unit, Ex.L and Ex.M1 showed an increase in achievement. Even so, Ex.M2 and Ex.H still performed better than their classmates in both units. These findings suggest that improved use of learning strategies resulted in an increase in achievement over time.
The analyses of the journals also supported these findings. Although students had difficulty with the first three activities, they showed a noticeable development in the use of self-regulatory processes in all three SRL phases for the remaining five journals. When the journals of the experimental group (Appendix B) were examined, initially, students showed improvement in the processes falling under the performance phase, such as reporting their observations. Later, they began to use forethought phase processes, such as making predictions. For example, when reporting what he would do (under the “planning the activity” section in Journal 5, Acid or Base?), Ex.M2 stated that “I want to investigate basic and acidic matter [and] their effect on litmus paper. To learn what the medium would be when I mixed HCl acid and distilled water … which matter shows which property and acidic properties.” In this excerpt, he explains the purpose of the activity and the process he would follow. However, because the information included irrelevant data and an incomplete explanation of the process, it was coded as “not satisfactory.” In the self-reflection phase students showed less progress compared to other SRL phases. For example, several students experienced difficulties in reporting unexpected outcomes. Although Ex.L showed improvement in the forethought and performance phases, he did not use or report any self-reflection processes in any of the eight journals. In Journal 4 (Does it precipitate?), while reporting the unexpected results he stated that “The colors were interesting, different colors when two liquids mixed together.” This statement did not include any reflection of the unexpected result; however, it could be used as evidence for his increased motivation.
Conversely, Ex.M1 started to use self-reflection processes in Journal 4. In the following excerpt from Journal 6 (How acidic or how basic?), she satisfactorily explained the processes under self-reflection phase: “I was expecting that the household materials that I brought would show acidic properties. However, the drain opener and mineral water showed basic properties. I learned that they are not acidic. I learned which household substances possess acidic or basic properties.” She explained the unexpected outcomes she observed and stated what she learned as a result.
EXAMPLE 3:
Co.L: Picture below … (continued mumbling) … So, it shows the latter condition of the marble sculpture which stayed here for a long time. So, deterioration is happening.
Researcher (R): How can you explain this?
Co.L: This [is] calcium carbonate, so, as the day goes by, its vitality disappears.
Researcher (R): What could the reason be?
Co.L: The reason is due to staying too long, it has deteriorated. How about the pieces falling? What could be reason? What else? Nothing.
Another noticeable finding was that there was an increase in Co.M2's performance and a decrease in Co.M1 and Co.H's performance in terms of metacognitive strategy use during the acids and bases unit. In Question 6 in the second unit, a table for the titration of a weak acid with a strong base was given to students. They were asked to define the equivalence point, which was 8.73 in the given case. In the following except (Example 4), Co.H initially gave the definition of equivalence point, having memorized the material presented in class (rehearsal). Next, he checked the given table, but could not find the pH value (7). At that point he was confused. With the help of a prompt from the researcher, he recalled the formula (rehearsal) and employed it to the given case (elaboration). Because he explained why he employed that formula and he was aware of what he learned, this indicated metacognitive awareness. He evaluated whether his response (the point when 50 mL NaOH was added, pH = 8.73) was reasonable or not (metacognitive evaluation), to reach a decision. He monitored the consistency between the definition he gave (pH = 7) and the result of his calculation (pH = 8.73) (metacognitive monitoring). As a result of this monitoring, he reached the conclusion that equivalence point should be the point when the 50 mL base is added.
EXAMPLE 4:
Co.H: Equivalence point. Oh, isn’t it the point where it is exactly neutralized? It is 7.
Researcher (R): By looking at this information, where is the equivalence point? How do we find it?
Co.H: There was something like M. V. NE [Molarity x Volume x Number of Equivalents], we were using formulas; we were writing acids on one side and base on the other. Then we equalize them when we make the m equal. So you want me to make calculations? Umm, one of them is 0.1–50 and the other one is 0.1–50 and their things are 1. So 50 mL. But … here again, that is the equivalence point. (He marks 50 mL on paper.)
R: Why do we write this equation? What was it used for?
Co.H: To equate the mole number, the number of the hydrogen and the oxygen of the acid and base. … That's the reason we write mole numbers…
R: Okay. You have just said you expected the equivalence point to be 7, [but] marked it as 8.73 on the paper. Why did it happen like this?
Co.H: We might have added more base.
R: 50 mL was added.
Co.H: Therefore, I really don’t know.
R: Then what do you think now?
Co.H: I’m not sure, but if 7 is in the alternatives I would say it may happen, but there is no 7. 50 mL doesn’t fit either. I don’t think so. But [50 mL] is the most logical one.
As for the accuracy of students’ responses, most of Co.L's responses were totally wrong or included inaccurate explanations. Co.M1 did slightly better in the solubility equilibrium unit than the acids and bases unit. Co.M2 did not give any correct responses in the first unit; however, he showed better performance in the second unit. Finally, although Co.H performed better than his classmates in both units, the accuracy of his responses decreased in the second unit. As seen in Example 4, although Co.H decided that the point when the 50 mL base is added should be the equivalence point, he could not provide a full explanation and his response was coded as a correct response with poor/insufficient (scientific) explanation.
Discussion
In this study, the effectiveness of SRI-GI on 11th grade students’ learning strategies and achievement in two chemistry units (solubility equilibrium and acids and bases) was investigated. Moreover, how students employed self-regulatory processes while learning chemistry concepts and how these processes developed over the course of the study was explored. To fulfill these purposes, two research strands were employed simultaneously; the quantitative and qualitative methodologies were merged to get a better understanding of the SRL phenomenon. One of the most frequently used questionnaires in SRL literature (MSLQ) was triangulated with a less frequently used instrument (think-aloud protocol). The questionnaire, which was measuring SRL as an aptitude, was helpful in collecting data from the whole sample and identifying students’ own perceptions. Because students are active agents in SRL (Bandura, 1986), their perceptions play a key role in shaping their learning processes. To observe students’ actual behaviors, the self-report measure was triangulated with the think-aloud protocol, which measured SRL as an event. This provided an opportunity to gain access to students’ working memory (Ericsson, 2003) and to understand how students approached the cases.With respect to students’ learning strategies, although the results did not demonstrate any significant difference among groups for the questionnaire data, mean scores slightly increased from pre-test to post-test for both groups. Likewise, Capanzana and Avilla (2017) did not find any significant effect of the reciprocal teaching approach with self-regulated learning (RT-SRL) on 9th grade chemistry students’ SRL skills. On the contrary, Cleary et al. (2008) and Labuhn et al. (2008) found that students improved their SRL skills over time as a result of a self-regulation empowerment program (SREP) and SRL classroom intervention, respectively. In the present study, the students primarily followed the SRL processes embedded in the journals. The teacher for the experimental group also guided them through modeling. Because the Turkish chemistry curriculum covers a significant amount of content in a brief time, the researchers chose to use implicit strategy instruction instead of explaining SRL and its properties in classroom time. Based on Cleary et al. (2008) and Labuhn et al. (2008) and their approaches, our SRI-GI might have been enhanced through the use of explicit/direct strategy instruction to help students think more about their learning process.
Another explanation for the non-significant results from the quantitative data might be students’ tendency to overestimate their actual SRL skills in self-report questionnaires, which has also been observed in earlier studies (e.g., Boekaerts and Corno, 2005; Cromley and Azevedo, 2006; Heirweg et al., 2019). Similarly, we also obtained non-significant results in the quantitative analyses and observed differences among groups in the qualitative analyses, which also confirms this claim. This is a serious problem in SRL development; students who overrate their SRL skills believe that they employ these skills successfully and do not change their strategies even when it might be necessary to do so.
On the other hand, findings from the think-aloud protocols revealed that in the second unit, students in the experimental group used diverse cognitive and metacognitive strategies regardless of achievement level. Students in the control group, however, did not show much change in strategy use over time. The improvement in metacognitive thinking found in these results—such as using evaluation or monitoring strategies—is quite promising. Science educators such as Schraw et al. (2006) highlighted that metacognition plays a key role in the development of SRL in science education. Similarly, Heirweg et al. (2019) also observed that highly regulated learners (i.e., active learners) used metacognitive strategies like evaluation and monitoring more often in think-aloud protocols. Eilam and Reiter (2014) also observed that a self-regulating class outperformed the teacher-controlled class in terms of strategy use according to their data.
The divergent results regarding learning strategies could be attributed to the fact that the participants did not use all of the self-regulatory phases in Zimmerman's model. Although the journals used in this study were designed to help students plan their learning according to Zimmerman's model, it was found that the focal students’ reports included less information about self-reflection processes compared to forethought and performance. This might have occurred because of a lack of conscious engagement in the learning process, difficulty in reading comprehension, or unwillingness to write on these topics. We accepted each of these possible reasons as an indicator of low level of self-regulation. Although the SRI-GI in the present study revealed some gains in terms of SRL processes, students may require more practice to become independent learners. Furthermore, in the think-aloud protocols students employed metacognitive evaluation less frequently than other strategies. Earlier studies have also suggested that more attention should be given to students’ evaluation of their learning process and learning outcomes (Eilam and Reiter, 2014; Heirweg et al., 2019). Teachers, at this point, may want to pay more attention to the implementation of evaluation strategies compared to the other self-regulatory strategies.
The higher use of learning strategies in the experimental group may have been affected by various contextual factors. First, as earlier studies have highlighted, “task challenge”—in which students need to use various learning strategies to accomplish given tasks—is an important requirement for self-regulatory intervention programs (Paris and Paris, 2001; Pintrich and Schunk, 2002; Schraw et al., 2006). As several researchers have stressed, students experience challenges understanding triplet nature of chemistry concepts: macroscopic, submicroscopic, and symbolic representations (Johnstone, 1991; Kozma et al., 2000; Taber, 2013). This perspective was necessary for the completion of certain tasks in the units covered during this study. Second, the guided inquiry approach provided students with several opportunities for metacognitive strategy use. For example, students discussed each step of their inquiry—designing the experiments, reporting their observations, making inferences based on their observations, etc.—with their group members. Furthermore, students made revisions to their inquiry based on feedback from their teacher; this also required monitoring strategies. Third, tasks in the SRI-GI classroom also required the use of various resources and the management of information coming from these resources. These factors may have encouraged and reinforced the use of various cognitive and metacognitive strategies.
Although students’ self-regulatory strategies from individualistic perspective were explored, social forms of regulation might have been occurred naturally during collaborative learning tasks as found out in earlier studies (Hadwin et al., 2011; Perry and Winne, 2013; Ucan and Webb, 2015). For example, Ucan and Webb (2015) found that more capable students assisted less capable group members when they need help (co-regulation) or multiple group members regulated the learning process collaboratively to achieve a shared goal (shared-regulation) in the study they explored social forms of regulation in a natural elementary science class. Overall, we anticipate that group members might also use social regulatory processes in addition to self-regulatory processes while they were planning the tasks, negotiating their ideas, seeking for consensus or explaining lack of understanding.
In terms of students’ achievement, although the results of the mixed-MANOVA did not indicate a significant interaction effect, there was an improvement in both groups over time. The degree of improvement was slightly higher in the experimental group. Therefore, the results do not conclusively show that SRI-GI was as influential as was predicted. On the other hand, think-aloud protocols showed that students in the experimental group performed considerably better than students in the control group regarding achievement. They improved their scientific explanations over time, even in cases where they could not find the correct solution. When these findings are interpreted together, this indicates that objective tests may not be able to reflect students’ knowledge. Think-aloud protocols and journals seemed to be more sensitive to the improvements in students’ SRL skills and learning. As Wilcox and Pollock (2015) explored in the context of physics, specifically electrostatics, paper-and-pencil tests can be informative to identify common errors; however, think-aloud protocols are more informative about the nature of those difficulties. Similarly, the think-aloud protocols in this study identified how students approached the tasks when experiencing difficulties. In addition to providing more data, think-aloud protocols were helpful for students in terms of making sense of the given cases, increasing awareness of their thinking, evaluating their progress, and monitoring their learning process, which are critical strategies in SRL and which in turn improved their explanations. These findings provide valuable evidence for progress in the experimental group's academic achievement, parallel to earlier studies in science education (Capanzana and Avilla, 2017; Cleary et al., 2008; Eilam and Reiter, 2014; Heirweg et al., 2019; Labuhn et al., 2008). There is an alternative explanation: seeing the benefits of SRI-GI may take longer than 12 weeks, which was illustrated by pre-post-retention test data in Labuhn et al. (2008). They found no difference between the experimental and control groups in the post-test; however, the experimental group did better in the retention test.
In conclusion, think-aloud protocols showed that the more students employed metacognitive processes, the more they were able to produce satisfactory explanations. Although think-aloud protocols were primarily used for measuring SRL, they produced implications for teaching that exceed the aim of the present study. This study showed that think-aloud protocols help students make sense of problems by increasing awareness of their thinking and encouraging them to evaluate their progress and monitor their learning process, which are critical strategies in SRL. Similarly, different researchers in SRL literature have employed think-aloud protocols for the purpose of assessing students’ learning difficulties and identifying how students deal with these difficulties (Wilcox and Pollock, 2015). However, researchers have also employed think-aloud protocols as a teaching strategy to encourage their students to work on a task and reflect on what they have done. For example, Felder and Brent (2009) and Millis (2012) suggested a “thinking-aloud pair problem solving” approach in which students work in pairs in the roles of explainer and questioner. First, the explainer describes how they approached a given task and the questioner listens, asks questions to clarify unclear points or gives hints if necessary. Then, they change their roles until they have reached a satisfactory solution.
The research on SRL in science education is limited. As a result, more research is required to compare our findings to those in other learning contexts. Overall, however, this study fills a gap in SRL literature and improves its ecologic validity by employing SRL principles in a less frequently studied context: a high school chemistry classroom.
Implications for practice
This study provides considerable information about classroom tasks. First, this study may encourage and guide teachers to prepare lessons based on the SRL model. Second, guided inquiry can be utilized to help students actively participate in their learning process and gain control of it gradually. Teachers may employ the discussion technique in chemistry/science classrooms and give time for students to think about, discuss, and reflect on their learning. Third, employing processes under three SRL phases (forethought, performance, and self-reflection) during chemistry tasks may help students be aware of what they are doing in the laboratory and give meaning to their observations. In this way, laboratory tasks will be more than hands-on practices, becoming minds-on activities. Self-evaluation of learning processes and learning outcomes can particularly increase students’ cognitive and metacognitive thinking. Lastly, SRI-GI requires the use of various learning strategies that have been proven effective in reaching learning goals. Teachers, thus, may benefit from referring to this study when designing tasks to support the use of different learning strategies.More specifically, since laboratory tasks are the most important part of chemistry education, the present findings provide a learning framework for students’ inquiry in the laboratory to support their meta/cognitive engagement in the given tasks. To do so, teachers can prepare journals/reflection papers covering all three SRL phases to encourage their students think on their learning experiences as well as the content itself. Teachers should pay specific attention to the self-reflection phase. They should encourage their students evaluate their ideas in terms of accuracy, rationality or consistency with evidence and judge the effectiveness of their work. This way students can notice when it is required to change their strategies and when to employ metacognitive monitoring.
In addition, algorithmic questions are indispensable part of both chemistry topics. Teachers should connect algorithmic questions to the students’ laboratory experiences. For example, in the same way as our study initially students can investigate the solubility construct conceptually, next solutions at a microlevel using a simulation and then the conditions for the formation of a precipitate. After completing these tasks in the laboratory, teachers can apply algorithmic questions regarding precipitate formation and discuss related concepts considering students’ laboratory experiences. Likewise, regarding second unit, teachers can employ algorithmic questions after discussing the concepts regarding titration in the laboratory. This way teachers can support students’ understanding of triplet nature of chemistry concepts (macroscopic, submicroscopic, and symbolic representations) and promote their students’ metacognitive engagement during algorithmic questions.
Compared to paper-and-pencil tests, teachers can use think-aloud protocols as a teaching strategy to identify students’ difficulties and as an assessment technique to evaluate students’ reasoning behind their answers (i.e., students can give a correct response with a wrong scientific reasoning or they can give a wrong response even though they have correct reasoning). Additionally, think-aloud protocols can provide more information about students’ understanding of triplet nature of chemistry compared to classical classroom tests.
Suggestions for further research
The present study also has implications for further research. This study utilizes a mixed-method design to explore how students use self-regulatory processes during the learning of chemistry concepts. Future studies can also employ mixed-method design applying different quantitative and qualitative instruments together to obtain deeper information about the SRL phenomena.In addition, in the present study, think-aloud protocols were employed after the implementation of the topic. However, researchers could benefit from using think-aloud protocols as a teaching tool while students are learning the topic. Future studies could also investigate different chemistry topics at different grade levels. For example, in the present study, Journal 1 was designed as an introductory activity to help students get accustomed to using journals and reflecting on their learning experiences. However, results revealed that students experienced challenges related to using journals in the next two tasks as well. The teacher gave constant feedback on journal writing and students became more confident by Journal 4. Therefore, researchers should consider that it might take time for students to develop the skills to explain their learning experiences in journals. The introductory activities/journals might take more time than was allotted in this study to be as successful as possible. Moreover, researchers could employ additional curricular activities, especially for the students with low self-regulation. Researchers can also train teachers in SRL and develop classroom tasks in cooperation with them. Finally, social forms of SRL (i.e., co-regulation and shared regulation) can be explored in addition to self-regulation in the further research.
Limitations of the study
The study is limited to two 11th grade topics (“Solubility Equilibrium” and “Acids and Bases” units) lasting for 12-week period. Although SRL is defined as an umbrella term under which variables that influence students’ learning are explained following Zimmerman's (2000) framework and claim that self-regulation does not depend on a domain or a topic, rather learners take strategic actions and employ necessary strategies depending on task requirements, authors are aware that some topics might be more adequate to promote certain self-regulatory skills. For example, students may have more chances to use metacognitive monitoring while applying the concepts related to solubility to a real problem compared to studying the definition of the “acid” concept. Although, students’ metacognitive thinking is emphasized in all tasks equally, this may not ensure that it could be blocked completely.An important limitation of this study is that students’ use of SRL processes are explored from the self-perspective (in other words, as an individual trait influenced by social context). Since the cooperative teacher did not agree on videotaping the class hours, students’ social forms of regulation (shared or co-regulation processes) could not be observed. From this perspective, although authors acknowledge the journals reflect the group work to some degree; unfortunately, whether the students were influenced by their peers and whether the journals completely reflect their actual thoughts could not have been captured.
Finally, this study involved only two chemistry classes and their chemistry teacher. Considering the requirements of the intervention, this study could be carried out with more participants in order to have more extensive and generalizable results.
Conflicts of interest
There are no conflicts of interest to declare.Appendix A. The analyses of think-aloud protocols for the focal students in the experimental group
| Solubility equilibrium | Acids and bases | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | |
| Note. ✓: The process is existent. *Q1: Question 1; Q2: Question 2; Q3: Question 3; Q4: Question 4; Q5: Question 5; Q6: Question 6.* 1: Wrong response; 2: Wrong response with poor/insufficient explanation; 3: Partially correct response; 4: Correct response without explanation; 5: Correct response with irrelevant explanation; 6: Correct response with poor/insufficient explanation; 7: Correct response with explanation. | ||||||||||||
| Ex.L | ||||||||||||
| Rehearsal | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Elaboration | ✓ | |||||||||||
| Organization | ||||||||||||
| Metacognitive awareness | ✓ | |||||||||||
| Metacognitive monitoring | ✓ | ✓ | ||||||||||
| Metacognitive evaluation | ||||||||||||
| Achievement | 1 | (A) 1 | (A) 1 | (A) 2 | 1 | 1 | 3 | (A) 1 | 3 | 7 | (A) 1 | (A) 2 |
| (B) 1 | (B) 1 | (B) 2 | (B) 1 | (B) 1 | (B) 3 | |||||||
| (C) 2 | ||||||||||||
| (D) 3 | ||||||||||||
| (E) 1 | ||||||||||||
| (F) 1 | ||||||||||||
| Ex.M1 | ||||||||||||
| Rehearsal | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Elaboration | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Organization | ||||||||||||
| Metacognitive awareness | ✓ | ✓ | ✓ | |||||||||
| Metacognitive monitoring | ✓ | ✓ | ||||||||||
| Metacognitive evaluation | ||||||||||||
| Achievement | 1 | (A) 2 | (A) 1 | (A) 1 | 1 | 2 | 7 | (A) 1 | 2 | 7 | (A) 3 | (A) 2 |
| (B) 1 | (B) 1 | (B) 1 | (B) 1 | (B) 1 | (B) 3 | |||||||
| (C) 3 | ||||||||||||
| (D) 3 | ||||||||||||
| (E) 2 | ||||||||||||
| (F) 2 | ||||||||||||
| Ex.M2 | ||||||||||||
| Rehearsal | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Elaboration | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Organization | ||||||||||||
| Metacognitive awareness | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Metacognitive monitoring | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Metacognitive evaluation | ✓ | ✓ | ✓ | ✓ | ||||||||
| Achievement | 7 | (A) 1 | (A) 7 | (A) 2 | 7 | 3 | 2 | (A) 7 | 2 | 7 | (A) 7 | (A) 2 |
| (B) 1 | (B) 7 | (B) 6 | (B) 1 | (B) 1 | (B) 3 | |||||||
| (C) 2 | ||||||||||||
| (D) 3 | ||||||||||||
| (E) 7 | ||||||||||||
| (F) 7 | ||||||||||||
| Ex.H | ||||||||||||
| Rehearsal | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Elaboration | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Organization | ||||||||||||
| Metacognitive awareness | ✓ | ✓ | ✓ | |||||||||
| Metacognitive monitoring | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Metacognitive evaluation | ✓ | |||||||||||
| Achievement | 6 | (A) 3 | (A) 7 | (A) 3 | 6 | 3 | 1 | (A) 2 | 2 | 7 | (A) 7 | (A) 7 |
| (B) 2 | (B) 7 | (B) 3 | (B) 1 | (B) 7 | (B) 3 | |||||||
| (C) 2 | ||||||||||||
| (D) 3 | ||||||||||||
| (E) 2 | ||||||||||||
| (F) 5 | ||||||||||||
Appendix B. The analyses of journals for the focal students in the experimental group
| Journal 1 | Journal 2 | Journal 3 | Journal 4 | Journal 5 | Journal 6 | Journal 7 | Journal 8 | |
|---|---|---|---|---|---|---|---|---|
| Note. Empty cells: non-existent, ✓: not satisfactory, ✓✓: satisfactory, NA: not applicable. | ||||||||
| Ex.L | ||||||||
| Planning activity | ✓ ✓ | ✓✓ | NA | NA | ||||
| Planning data recording | ✓ | ✓✓ | ✓✓ | ✓✓ | NA | ✓✓ | ||
| Predictions | ✓✓ | ✓ | ||||||
| Procedure | ✓ | ✓✓ | ✓✓ | ✓✓ | NA | ✓✓ | ||
| Observation data | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓ | ✓✓ | ||
| Inference | ✓✓ | ✓✓ | ✓ | ✓✓ | ✓✓ | |||
| Unexpected outcomes | ||||||||
| Assessing learned material | ||||||||
| Experienced difficulties | ||||||||
| Evaluation/elaboration | ||||||||
| Assessing the activity | ✓ | |||||||
| Ex.M1 | ||||||||
| Planning activity | ✓✓ | ✓✓ | NA | NA | ||||
| Planning data recording | ✓✓ | ✓✓ | ✓✓ | NA | ✓✓ | |||
| Predictions | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓ | |||
| Procedure | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | NA | ✓✓ | |
| Observation data | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | |
| Inference | ✓✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | |||
| Unexpected outcomes | ✓✓ | |||||||
| Assessing learned material | ✓✓ | ✓✓ | ||||||
| Experienced difficulties | ✓✓ | ✓ | ||||||
| Evaluation/elaboration | ✓✓ | ✓✓ | ✓✓ | ✓ | ✓✓ | |||
| Assessing the activity | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | |||
| Ex.M2 | ||||||||
| Planning activity | ✓ | ✓ | ✓ | NA | NA | |||
| Planning data recording | ✓✓ | ✓✓ | ✓✓ | ✓✓ | NA | ✓✓ | ||
| Predictions | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ||
| Procedure | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | NA | ✓✓ | |
| Observation data | ✓✓ | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ |
| Inference | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ||
| Unexpected outcomes | ✓✓ | |||||||
| Assessing learned material | ✓ | ✓✓ | ✓✓ | |||||
| Experienced difficulties | ✓✓ | ✓ | ✓✓ | ✓✓ | ||||
| Evaluation/elaboration | ✓ | ✓ | ✓ | |||||
| Assessing the activity | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ||
| Ex.L | ||||||||
| Ex.H | ||||||||
| Planning activity | ✓✓ | ✓ | ✓ | NA | NA | |||
| Planning data recording | ✓✓ | ✓✓ | ✓✓ | NA | ✓✓ | |||
| Predictions | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓ | |||
| Procedure | ✓ | ✓✓ | ✓✓ | ✓✓ | NA | ✓✓ | ||
| Observation data | ✓✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | |
| Inference | ✓✓ | ✓✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ||
| Unexpected outcomes | ✓ | ✓✓ | ✓ | ✓ | ||||
| Assessing learned material | ✓✓ | ✓✓ | ||||||
| Experienced difficulties | ✓✓ | |||||||
| Evaluation/elaboration | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | |||
| Assessing the activity | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ||||
Appendix C. The analyses of think-aloud protocols for the focal students in the control group
| Solubility equilibrium | Acids and bases | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | |
| Note. ✓: The process is existent. *Q1: Question 1; Q2: Question 2; Q3: Question 3; Q4: Question 4; Q5: Question 5; Q6: Question 6.*1: Wrong response; 2: Wrong response with poor/insufficient explanation; 3: Partially correct response; 4: Correct response without explanation; 5: Correct response with irrelevant explanation; 6: Correct response with poor/insufficient explanation; 7: Correct response with explanation. | ||||||||||||
| Co.L | ||||||||||||
| Rehearsal | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Elaboration | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Organization | ||||||||||||
| Metacognitive awareness | ||||||||||||
| Metacognitive monitoring | ||||||||||||
| Metacognitive evaluation | ||||||||||||
| Achievement | 2 | (A) 1 | (A) 7 | (A) 2 | 3 | 3 | 1 | (A) 1 | 1 | 1 | (A) 1 | (A) 2 |
| (B) 1 | (B) 1 | (B) 1 | (B) 1 | (B) 1 | (B) 2 | |||||||
| (C) 2 | ||||||||||||
| (D) 2 | ||||||||||||
| (E) 2 | ||||||||||||
| (F) 6 | ||||||||||||
| Co.M1 | ||||||||||||
| Rehearsal | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Elaboration | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Organization | ||||||||||||
| Metacognitive awareness | ||||||||||||
| Metacognitive monitoring | ✓ | ✓ | ||||||||||
| Metacognitive evaluation | ||||||||||||
| Achievement | 7 | (A) 2 | (A) 7 | (A) 3 | 2 | 1 | 7 | (A) 1 | 2 | 1 | (A) 1 | (A) 2 |
| (B) 1 | (B) 7 | (B) 3 | (B) 1 | (B) 1 | (B) 2 | |||||||
| (C) 3 | ||||||||||||
| (D) 2 | ||||||||||||
| (E) 2 | ||||||||||||
| (F) 6 | ||||||||||||
| Co.M2 | ||||||||||||
| Rehearsal | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Elaboration | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Organization | ||||||||||||
| Metacognitive awareness | ✓ | |||||||||||
| Metacognitive monitoring | ✓ | ✓ | ||||||||||
| Metacognitive evaluation | ✓ | ✓ | ||||||||||
| Achievement | 1 | (A) 1 | (A) 1 | (A) 1 | 2 | 1 | 6 | (A) 1 | 2 | 6 | (A) 1 | (A) 2 |
| (B) 1 | (B) 1 | (B) 1 | (B) 1 | (B) 1 | (B) 2 | |||||||
| (C) 6 | ||||||||||||
| (D) 2 | ||||||||||||
| (E) 2 | ||||||||||||
| (F) 6 | ||||||||||||
| Co.H | ||||||||||||
| Rehearsal | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Elaboration | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Organization | ||||||||||||
| Metacognitive awareness | ✓ | |||||||||||
| Metacognitive monitoring | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Metacognitive evaluation | ✓ | ✓ | ✓ | |||||||||
| Achievement | 7 | (A) 7 | (A) 7 | (A) 7 | 7 | 3 | 7 | (A) 1 | 2 | 1 | (A) 2 | (A) 2 |
| (B) 1 | (B) 7 | (B) 6 | (B) 1 | (B) 1 | (B) 3 | |||||||
| (C) 6 | ||||||||||||
| (D) 3 | ||||||||||||
| (E) 1 | ||||||||||||
| (F) 6 | ||||||||||||
References
- Bandura A., (1986), Social foundations of thought and action: A social cognitive theory, Englewood Cliffs, NJ: Prentice Hall.
- Boekaerts M., and Corno L., (2005), Self-regulation in the classroom: A perspective on assessment and intervention, Appl. Psychol., 54(2), 199–231.
- Capanzana C. O. and Avilla R. A., (2017), Reciprocal teaching approach with self-regulated learning (RT-SRL): Effects on students’ reading comprehension, achievement and self-regulation in Chemistry, Normal Lights, 11(2), 31–59.
- Cleary T. J., Platten P. and Nelson A. C., (2008), Effectiveness of the self-regulation empowerment program, J. Adv. Acad., 20, 70–107.
- Colburn A., (2004), Inquiring scientists want to know, Educ. Leadership, 62, 63–66.
- Crocker L. and Algina J., (1986), Introduction to Classical and Modern Test Theory, Fort Worth, TX: Harcourt Brace Jovanovich College Publishers.
- Cromley J. G. and Azevedo R., (2006), Self-report of reading comprehension strategies: What are we measuring? Metacogn. Learn., 1(3), 229–247.
- Dignath C. and Buttner G., (2008), Components of fostering self-regulated learning among students. A meta-analysis on intervention studies at primary and secondary school level, Metacogn. Learn., 3, 231–264.
- Donker A. S., de Boer H., Kostons D., Dignath van Ewijk C. C. and van der Werf M. P. C., (2014), Effectiveness of learning strategy instruction on academic performance: A Meta-Analysis, Educ. Res. Rev., 11, 1–26.
- Eilam B. and Reiter S., (2014), Long-term self-regulation of biology learning using standard junior high school science curriculum, Sci. Educ., 98(4), 705–737.
- Ericsson K. A. (2003) Valid and non-reactive verbalization of thoughts during performance of tasks, J. Conscious. Stud., 10(9–10), 1–18.
- Felder R. M. and Brent R., (2009), Active learning: An introduction, ASQ Higher Educ. Brief, 2(4), 1–5.
- Field A., (2009), Discovering Statistics Using SPSS, 3rd edn, London: Sage.
- Fraenkel J., Wallen N. and Hyun H. H., (2012), How to design and evaluate research in education, 8th edn, Boston: McGraw Hill.
- Greene J. C., (2007), Mixed methods in social inquiry, San Francisco: Jossey-Bass.
- Hadwin A., Järvelä S. and Miller M., (2011), Self-regulated, co-regulated, and socially shared regulation of learning, in Zimmerman B. and Schunk D. (ed.), Handbook of self-regulation of learning and performance, New York, NY: Routledge, pp. 65–84.
- Hattie J. A., Biggs J. and Purdie N., (1996), Effects of learning skills interventions on student learning: a meta-analysis, Rev. Educ. Res., 66, 99–136.
- Heirweg S., De Smul M., Devos G. and Van Keer H., (2019), Profiling upper primary school students’ self-regulated learning through self-report questionnaires and think-aloud protocol analysis, Learn. Individ. Differ., 70, 155–168.
- Hofer B., Yu S. and Pintrich P. R., (1998), Teaching college students to be self-regulated learners, in Schunk D. H. and Zimmerman B. J. (ed.), Self-regulated learning: From teaching to self-reflective practice, New York: Guilford, pp. 57–85.
- Hofstein A., Shore R. and Kipnis M., (2004), Providing high school chemistry students with opportunities to develop learning skills in an inquiry-type laboratory: a case study, Int. J. Sci. Educ., 26(1), 47 62.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comput. Assisted Learn., 7, 75–83.
- Kadioglu C., Uzuntiryaki E. and Capa-Aydin Y., (2006), A qualitative study about 10th grade students' self-regulatory skills, Poster presented at the 7th National Science and Mathematics Education Congress, Ankara, Tükiye.
- Kline R. B., (2005), Principles and practice of structural equation modeling, 2nd edn, New York: Guilford Press.
- Kozma R., Chin E., Russell J. and Marx N., (2000), The roles of representations and tools in the chemistry laboratory and their implications for chemistry learning, J. Learn. Sci., 9(2), 105–143.
- Labuhn A. S., Bögeholz S. and Hasselhorn M., (2008), Lernförderung durch Anregung der Selbstregulation im naturwissenschaftlichen, [Fostering learning through stimulation of self-regulation in science lessons], Unterr. Z. Pädagogische Psychol., 22, 2008, 13–24 DOI:10.1024/1010-0652.22.1.13.
- Miles M. B. and Huberman A. M., (1994), Qualitative data analysis: an expanded sourcebook, 2nd edn, Thousand Oaks, CA: Sage.
- Millis B., (2012), Active learning strategies in face-to-face courses, Manhattan, NY: IDEA.
- Moog R. S. and Spencer J. N., (2008), POGIL: an overview, in Moog et al. (ed.), Process Oriented Guided Inquiry Learning (POGIL), Washington DC: American Chemical Society, pp. 1–13.
- Mutlu A. and Acar-Şeşen B., (2018), Pre-service science teachers’ understanding of chemistry: A factorial design study, Eurasia J. Math., Sci. Technol. Educ., 14(7), 2817–2837.
- National Research Council (NRC), (2000), Inquiry and National Science Educational Standards, Washington, D.C.: National Academy Press.
- Paris S. G. and Paris A. H., (2001), Classroom applications of research on self-regulated learning, Educ. Psychol., 35, 89–101.
- Patton M. Q., (2002), Qualitative research & evaluation methods, 3rd edn, Thousand Oaks, CA: Sage.
- Perry N. E. and Winne P. H., (2013), Tracing students’ regulation of learning in complex collaborative tasks, in Volet S. and Vauras M. (ed.), Interpersonal regulation of learning and motivation: Methodological advances, London: Routledge, pp. 45–66.
- Pintrich P. R. and Schunk D., (2002), Motivation in education: Theory, research, and applications, Upper Saddle River, NJ: Merrill Prentice Hall.
- Pintrich P. R., Smith D. A. F., Garcia T. and McKeachie W. J., (1991), A Manuel for the use of Motivated Strategies for Learning Questionnaire (MSLQ), Ann Arbor, MI: National Center for Research to Improve Postsecondary Teaching and Learning, The University of Michigan.
- Schraw G., Crippen K. J. and Hartley K., (2006), Promoting self-regulation in science education: Metacognition as part of a broader perspective on learning, Res. Sci. Educ., 36, 111–139.
- Sungur S., (2004), An implementation of problem based learning in high school biology courses, Unpublished doctoral dissertation, The Middle East Technical University.
- Taber K. S., (2013), Revisiting the chemistry triplet: Drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Pract., 14(2), 156–168.
- Taber K. S., (2018), Lost and found in translation: guidelines for reporting research data in an ‘other’ language, Chem. Educ. Res. Pract., 19(3), 646–652.
- Ucan S. and Webb M., (2015), Social regulation of learning during collaborative inquiry learning in science: How does it emerge and what are its functions? Int. J. Sci. Educ., 37(15), 2503–2532.
- Weinstein C. E. and Mayer R. E., (1986), The teaching of learning strategies, in Wittrock M. (ed.), Handbook of research on teaching, New York: Macmillan, pp. 315–327.
- Wilcox B. R. and Pollock S. J., (2015), Upper-division student difficulties with separation of variables, Phys. Rev. Spec. Top.-Phys. Educ. Res., 11(2), 20–31.
- Winne P. H., (1997), Experimenting to bootstrap self-regulated learning, J. Educ. Psychol., 88, 397–410.
- Winne P. H. and Perry N. E., (2000), Measuring self-regulated learning, in Boekaerts M., Pintrich P. and Ziedner M. (ed.), Handbook of self-regulation, Orlando, FL: Academic, pp. 531–566.
- Yin R. K., (2009), Case study research, Design and methods, 4th edn, Thousand Oaks, California: Sage.
- Yuruk N., Beeth M. E. and Andersen C., (2009), Analyzing the effect of metaconceptual teaching practices on students’ understanding of force and motion concepts, Res. Sci. Educ., 39, 449–475.
- Zimmerman B. J., (2000), Attaining self-regulation: A social cognitive perspective, in Boekaerts M., Pintrich P. and Ziedner M. (ed.), Handbook of self-regulation, Orlando, FL: Academic Press, pp. 13–39.
- Zimmerman B. J., (2001), Theories of self-regulated learning and academic achievement: an overview and analysis, in Zimmerman B. J. and Schunk D. H. (ed.), Self-regulated learning and academic achievement: Theoretical perspectives, 2nd edn, Lawrence Erlbaum Associates, pp. 1–37.
Footnote |
| † This study is a part of Cansel Kadioglu-Akbulut's dissertation under the supervision of Esen Uzuntiryaki-Kondakci. |
| This journal is © The Royal Society of Chemistry 2021 |
