A mini-review on recent developments in SAPO-34 zeolite membranes and membrane reactors
Jeff
Xu
 ,
Kok-Giap
Haw
,
Kok-Giap
Haw
 ,
Zhan
Li
,
Subhasis
Pati
,
Zhigang
Wang
,
Zhan
Li
,
Subhasis
Pati
,
Zhigang
Wang
 * and
Sibudjing
Kawi
* and
Sibudjing
Kawi
 *
*
Department of Chemical and Biomolecular Engineering, National University of Singapore, 117576, Singapore. E-mail: wangzhigang01@u.nus.edu; chekawis@nus.edu.sg
First published on 2nd November 2020
Abstract
Silicoaluminophosphate (SAPO) zeolite SAPO-34 membranes with a uniform pore size of 0.38 nm and unique adsorption properties have been used in natural gas purification, H2 purification, Kr/Xe separation and other gas separations. Additionally, owing to their high thermal and chemical stability, SAPO-34 membranes have been used as catalytic membrane reactors to enhance the conversion and selectivity of high temperature reactions such as propane dehydrogenation (PDH) and urea methanolysis to dimethyl carbonate (UM-to-DMC) reactions. This review focuses on the state-of-the-art applications of SAPO-34 membranes in gas separation and catalytic membrane reactors. Their synthesis conditions, permeation mechanism, and performance advantages are examined. Finally, current challenges and future trends of SAPO-34 membranes for industrial application are discussed. We expect this review to be a comprehensive resource on the fabrication of high quality SAPO-34 membranes, and thus help stimulate the application of SAPO-34 membranes in different industrial applications.
1. Introduction
SAPO-34 is a silicoaluminophosphate (SAPO) zeolite exhibiting the chabazite (CHA)-type structure with 8-membered pores having an average pore size of 3.8 Å and the composition of SixAlyPzO2, where x + y + z = 1 and x + z = y. Formed by substitution of some silicon with phosphorus in an aluminophosphate material (AlPO4), SAPO-34 is an excellent membrane material for light gas separation under harsh conditions owing to its uniform and small pore size coupled with high thermal and chemical stability.1–3 These features, coupled with preferential adsorption towards some gases, have led to increasing interest in using SAPO-34 membranes for gas separation.One such important gas is natural gas, which often contains CO2 and N2 as impurities. Both CO2 and N2 decrease the heat value of gas, while CO2 at low temperatures can form corrosive compounds that can damage pipelines. This coupled with pipeline standards limiting inert gas ratios necessitates their removal from natural gas.3 Presently, the main methods used for CO2/CH4 and N2/CH4 separation in industry are cryogenic distillation and pressure swing adsorption, but these methods have drawbacks of high energy costs and high design complexity. On the other hand, separation using membrane technology is often simpler, more space efficient, and more energy efficient. Polymeric membranes are commercially used for CO2/CH4 separation, but they face limitations: high pressure CO2 can plasticize polymeric membranes and decrease their permeance, and the intrinsic performance of polymeric membranes is limited by the Robeson upper bound. By contrast, SAPO-34 membranes can overcome this limitation and offer CO2/CH4 separation performances far superior to those of polymeric membranes as shown in Fig. 1.4 Besides being extensively studied in CO2/CH4 and N2/CH4 separations, SAPO-34 membranes have also been investigated for H2/CH4, H2/N2, H2/C3Hx, H2/C4Hx, and Xe/Kr gas separations.3,5–10
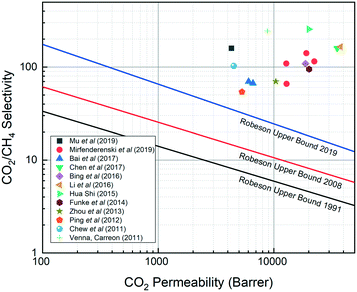 | ||
| Fig. 1 SAPO-34 membranes vs. polymeric membranes for CO2/CH4 separation (polymeric membrane performance falls at or under the upper bound).11–13 | ||
Furthermore, SAPO-34 zeolite membranes with excellent chemical and thermal stability can be used for gas separation under conditions unsuitable for polymeric membranes. For example, SAPO-34 has been used in membrane reactors for some high temperature reactions (200–600 °C). SAPO-34 was studied for H2 permeable membrane reactors in the propane dehydrogenation (PDH) reaction at 600 °C.9 Running PDH reactions using a membrane reactor not only reduces downstream H2 separation requirements, but also increases the conversion of the reaction by overcoming the thermodynamic equilibrium through simultaneous separation of hydrogen from the reaction.14–17
Gas permeation through zeolite membranes involves three major mechanisms: adsorption, diffusion and molecular sieving. The importance of each depends on temperature, pressure, and kinetic diameter of the gases.18 For light gases with a kinetic diameter smaller than the pore size of SAPO-34, such as H2 of 2.89 Å, CO2 of 3.30 Å, or N2 of 3.64 Å, permeation through SAPO-34 membranes is determined by adsorption and diffusion.19 For single gas permeation, adsorption is temperature and pressure dependent, while diffusion is temperature dependent. On the other hand, for heavier gases with kinetic diameters larger than the pore size of SAPO-34 (e.g. larger hydrocarbons such as C3Hx or C4Hx), molecular sieving is the predominant separation mechanism.8 However, the presence of non-zeolitic micropores or defects on synthesized membranes (which are difficult to avoid) will allow for gas permeance through pathways such as Knudsen diffusion, molecular diffusion, and for larger defects, even viscous flow, which decreases membrane selectivity.20
SAPO-34 zeolite membranes are predominantly synthesized via a hydrothermal process using phosphoric acid and various Al and Si precursors by in situ synthesis or secondary growth methods. Most groups strive for the growth of the thinnest uniform zeolite membrane on a support whilst minimising defects and impurity phases to obtain membranes with high selectivity and permeance. Towards this end, different groups have explored various ways to optimise membrane synthesis such as more uniform membrane seeding methods, improved hydrothermal heating methods, and using higher permeance membrane supports.21–25 However, the factors affecting the growth of SAPO-34 zeolite membranes and their application in membrane reactors are rarely reviewed. The aim of this review is to summarise recent developments in SAPO-34 membranes and membrane reactors, including the effect of synthesis conditions on the quality of SAPO-34 zeolite membranes, the application of SAPO-34 membranes in gas separations, and the application of SAPO-34 membrane reactors for various reactions. Finally, perspectives for future research on SAPO-34 membranes and membrane reactors will be discussed.
2. Factors influencing the quality of SAPO-34 zeolite membranes
Most SAPO-34 membranes are synthesized by a seeded secondary growth method. Supports (usually alumina) are first coated with seeds through dip coating or rub coating before being placed into an autoclave with synthesis gel, which contains inorganic precursors (silica, alumina, and phosphorus source) and organic structure directing agents (SDA). The autoclave is then heated to 180–210 °C for several hours to allow for the growth of a uniform SAPO-34 layer on the substrate. After hydrothermal synthesis, the SDA is then removed from the membrane through high-temperature calcination.Several factors during membrane synthesis influence the final quality of synthesized SAPO-34 membranes and can be broadly generalized as the hydrothermal conditions, synthesis gel composition, seeding method, organic template selection, template removal method, and substrate properties. The following sections will briefly describe how these factors can contribute significantly to the gas separation performance of SAPO-34 membranes, as well as methods different groups have used to improve membrane synthesis.
2.1 Hydrothermal conditions
Hydrothermal conditions, i.e. temperature and duration, can affect the thickness, defect ratio, and phase purity of SAPO-34 membranes. Longer synthesis times form thicker membranes with fewer defects and impurities, offering higher selectivities at the cost of lower permeance. On the other hand, shorter synthesis times form thinner membranes with more defects due to a poor intergrowth of the zeolite crystals. These defects typically manifest as non-zeolitic micropores at the boundaries between crystal domains,26 and negatively affect selectivity as they allow for the passage of heavier gases through Knudsen diffusion.Temperature is also an important factor in membrane synthesis. Low hydrothermal temperature (160 °C) and short synthesis times (2.5 h) will form amorphous or SAPO-18 phases (AEI structure) as impurities, while high temperature (200 °C) and long synthesis times (>12 h) will form SAPO-5 (AFI phase).27 Because of the simultaneous effect of both temperature and pressure on membrane performance, researchers seeking to grow high performance SAPO-34 membranes need to find a balance between membrane selectivity (purity and quality) and membrane permeance (thickness) through careful selection of hydrothermal conditions. The optimum conditions for most syntheses of SAPO-34 membranes are 5–10 h of hydrothermal synthesis at 180–210 °C.7,28–31
Several recent studies have also investigated alternative heating methods to reduce synthesis times and improve membrane quality. Microwave heating can increase the heating rate and temperature uniformity of the synthesis gel, and has been reported to produce high quality membranes at shorter synthesis times (1–2 hours).22,23 Likewise, alternatives to convection ovens such as heated oil baths or stainless-steel autoclaves without a Teflon insert have been investigated as a means of increasing the heating rate of the synthesis gel.21,24 Furthermore, alternatives to hydrothermal synthesis such as dry-gel conversion and steam-assisted vapour–solid conversion have also been investigated.32,33
2.2 Synthesis gel and seeding
The synthesis gel composition is another major factor in determining the quality of SAPO-34 membranes. Notably, the Si/Al ratio of the synthesis gel is extremely important in zeolite membrane formation, with high Si/Al ratio gels forming membranes with increased hydrothermal and chemical stability and fewer defects.18 Low Si/Al ratio gels also lead to impurity phases such as SAPO-5 (AFI type). This zeolite consists of cylindrical channels formed by 12-membered rings with a diameter of 0.74 nm, and thus reduces the selectivity of SAPO-34 membranes by allowing the passage of heavier gases.6 Additionally, the Si/Al gel ratio can also affect the adsorption of gases into zeolite crystals (elaborated later). Generally, synthesis gels for the growth of SAPO-34 membranes have a Si/Al gel ratio of 0.15 to 0.3.Besides varying the Si/Al ratio of the synthesis gels, the water content of the gels can also be varied to control the membrane thickness. Using dilute synthesis gels at higher hydrothermal temperatures appears to be an effective way to increase membrane permeances.10,34,35
Most papers investigating SAPO-34 ceramic membranes use a secondary seeded-growth method where the substrate is first coated with a layer of seed crystals. The secondary growth method, while more time consuming than in situ growth, reduces the formation of other zeolite (impurity) phases and reduces synthesis time, leading to thinner, more uniform membranes.18,36 Seeding is generally done using dip coating or rub coating. Dip coating reliably gives uniform seeding layers, but the support may need several coatings to reach the desired density of seeds. Rub coating allows for application of more seeds at once, although this process is less consistent as the seeding layer is less uniform. Recently, novel seeding methods such as dry rolling and steam-assisted conversion were also developed.37,38 Seeding quality is extremely important for the final membrane quality, and a thin, uniform seed layer is seen as essential to the formation of a high quality, thin intergrown membrane layer. In fact, thin, high aspect-ratio seeds were shown to reduce both the membrane thickness and defect ratio of synthesized membranes.39 The addition of seeds onto the synthesis gel can also increase the rate of membrane growth, leading to improved membrane performance, albeit at the cost of much increased seed use.31,40
2.3 The effect of template selection and template removal
Among the templates or structure directing agents (SDAs) used in SAPO-34 membrane synthesis, tetramethylammonium hydroxide (TEAOH) is the most commonly used, although it is sometimes used with an additional SDA such as dipropylamine (DPA) and morpholine (Mor).41 Template selection has a major impact on membrane and seed quality since it affects the crystal size, phase purity, relative crystallinity and physicochemical properties of the final zeolite as well as the cost of membrane synthesis.42 Several studies have explored the effects of SDA selection on the crystal size, acidity, and crystallinity of SAPO-34 crystals, albeit with a focus towards growing crystals for methanol-to-olefin (MTO) applications.43–45 When using a single template, it was found that TEAOH tends to give small particle sizes and increased crystallinity and phase purity when compared with other SDAs. TEAOH also forms zeolites with the highest Al content. In the case of membrane synthesis, it was found that the addition of DPA can increase alkalinity and thus increase nucleation rate, which encourages the formation of even smaller crystals.41 This favourable growth characteristic leads to thinner and more intergrown membranes, increasing membrane selectivity. TEAOH and TEAOH-DPA are the most common organic SDAs used in SAPO-34 membrane synthesis in the literature.9,31,32,46Unfortunately, the high cost of TEAOH greatly increases the cost of SAPO-34 synthesis, and as such, template-free membrane synthesis has also been investigated, with promising results for CO2/CH4 separation. In template-free synthesis, K+ cations are required and function as a structure-directing agent (SDA), replacing the TEA+ cations to balance the negative charge of the SAPO-34 molecular sieve framework. The hydrothermal reaction was promoted by heating with a microwave oven for fast synthesis (1–2 hours). The resulting SAPO-34 membrane achieved similar CO2 permeance and selectivity to those of the membranes synthesized using traditional synthesis methods.47
Template removal is likewise an important process that affects the quality of SAPO-34 membranes. To decompose the organic template and unblock zeolitic micropores, synthesized membranes are usually calcined at around 400–600 °C in air or in a vacuum. Prolonged calcination (6–10 h) at lower temperatures (<500 °C) with slow heating (<1 °C min−1) and cooling (<1 °C min−1) rates is the most popular strategy for template removal in SAPO-34 zeolite membranes.48 A slow heating rate is essential as high temperature ramping rates can lead to decreased membrane performance. For instance, Poshusta et al. reported that calcination of a SAPO-34 membrane with slow temperature cycles, even under exposure to water vapor, had no permanent effect on the membrane performance. However, a temperature change of approximately 30 K min−1 can decrease the membrane's effectiveness.7
Higher temperatures are preferred in calcination as it leads to the more complete removal of the template. Furthermore, Chang et al. also showed that rapid thermal pretreatment of 1 min at 700 °C followed by conventional calcination (400 °C) can help increase the bonding between neighboring zeolite crystals and thus increase selectivity.49 However, Zhang et al. (2010) reported an increased risk of crack formation at higher calcination temperatures due to the strong negative thermal expansion of SAPO-34.50
In comparing the medium for template removal, Zhang et al. (2010) also reported that template removal in flowing N2 or under vacuum resulted in the more complete removal of templates, leading to almost twice the CO2 permeance and an 8% lower CO2 selectivity compared to template removal in flowing air.50 More than 90% of the template was removed at 773 K during temperature-programmed desorption (TPD) in helium with ethylene as the main decomposition product, while only 70% of the template was removed at 773 K under air or oxygen. In the presence of oxygen, the template could be oxidized to more stable species that remain in the SAPO-34 pores, leading to a decrease in gas permeation.50
Besides calcination at high temperatures, mild template removal has also been studied. Wet ozone treatment at under 200 °C can be used as a facile template removal method to reduce thermal stress on the membranes, although this method requires a longer time for template removal.51
2.4 The choice of substrate
Besides the quality of membrane seeding, the support or substrate also plays an important role in the membrane quality of SAPO-34 ceramic membranes. Not only do substrates need to have high stability under hydrothermal conditions and sufficient chemical, thermal, and mechanical stability for industry use, they also need to have matching thermal expansion coefficients with the zeolite material, as mismatch of thermal expansion coefficients between the substrate and the grown zeolite layer can lead to the zeolite layer peeling off from the substrate with temperature change.18 Substrate smoothness and texture are very important to the growth of uniform SAPO-34 films. The substrate surface should be smooth with a small and uniform pore size distribution to ensure a high quality inter-grown zeolite layer, but a smoother surface can lead to poorer adhesion and a greater likelihood of membrane layer cracking or peeling from the substrate upon high temperature template removal.52 The substrate should also have low mass transfer resistance to enhance the overall permeance through the zeolite membrane.18 Therefore, the optimal substrate for zeolite membrane growth would be a thin, highly porous substrate with a reasonably smooth surface that can be seeded to form a uniform seeding layer. Asymmetric alumina substrates are thus a great choice to grow high performance SAPO-34 membranes. For example, inorganic hollow fiber substrates with a highly asymmetric structure and ultra-thin wall could provide extremely low resistance to mass transfer while maintaining a smooth exterior surface as shown in Fig. 2. Furthermore, the packing density of a hollow fiber membrane module (membrane area per volume) is higher than that of other configurations for membrane modules (e.g. disk or tubular configurations), which could provide more efficiency and less costly separation.53–64 However, hollow fiber supports are also rather challenging to work on due to their high curvature. Despite this, several groups have embraced the challenge of growing SAPO-34 membranes on such substrates and have reported superior performance due to higher substrate permeance.16,32,46,65 Additionally, substrate modification using polymer coatings or surface modification have also been investigated as a means of increasing substrate attraction to seed crystals, leading to highly oriented seed monolayers upon seeding.66,67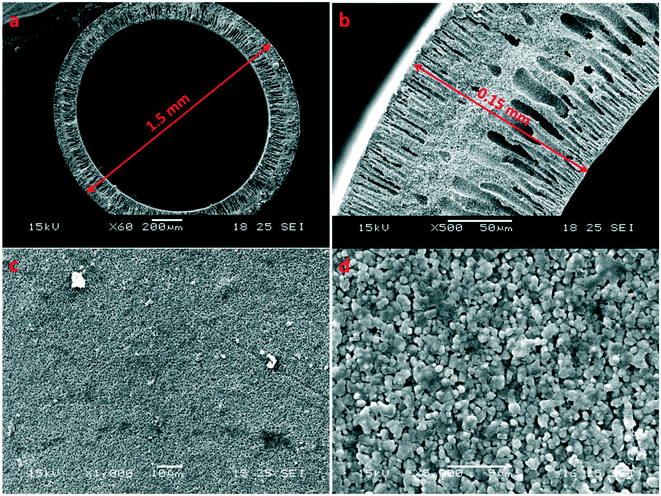 | ||
| Fig. 2 SEM images of an Al2O3 hollow fiber substrate; (a) and (b): cross section; (c) and (d): external surface16 (reprinted with permission from Wiley). | ||
3. The application of SAPO-34 membranes in gas permeation
3.1 CO2 and N2 separation for natural gas purification
Separation of CO2 and/or N2 from CH4 is a problem faced in many different industries. For instance, in natural gas production, wellhead natural gas can contain over 40% inert gases such as N2 or CO2.68 Likewise, biogas (CH4 and CO2) from anaerobic digestion contains about 25–50% CO2.69 Such high concentrations of inert gases decrease the heating value of natural gas or biogas, and thus, they need to be removed. Gas separation using membrane technology is increasingly attractive due to high efficiency and low energy consumption.70–72 Owing to the strong adsorption of CO2 and a small pore size, SAPO-34 membranes are widely studied for CO2 and N2 separation from CH4.Single-gas permeation of H2, CO2, N2, and CH4 through SAPO-34 membranes at different temperatures and pressures was firstly studied by Poshusta et al. as shown in Fig. 3. It can be seen from Fig. 3(a) that CO2 permeation trended differently from other gas permeations with increasing temperature. The CO2 permeance continuously decreases as temperature increases, suggesting that permeation is mainly controlled by adsorption which decreases with increasing temperatures. It also can be seen that the CH4 and N2 permeances are almost one order of magnitude lower than the CO2 permeance at low temperature (∼300 K), which shows the potential of SAPO-34 membranes in CO2/CH4 and CO2/N2 separations.73
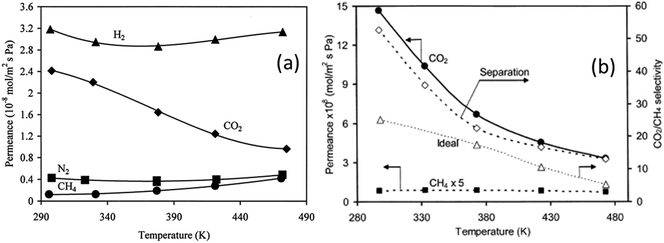 | ||
| Fig. 3 (a) Single gas permeance73 (reprinted with permission from ACS) and (b) mixture gas permeance across a SAPO-34 membrane as a function of temperature at a 222 kPa feed and 128 kPa pressure drop28 (reprinted with permission from Elsevier). | ||
Due to the rapid decrease of CO2 permeance with increasing temperature, both CO2 selectivity and permeance decrease with increasing temperature for CO2/CH4 mixture separations as shown in Fig. 3(b). It can also be seen that the mixture selectivity is higher than the ideal selectivity since the presence of a strongly adsorbing gas such as CO2 will take up adsorption sites in the zeolite and inhibit the adsorption of other gases.74–77
The effect of pressure on gas permeation through SAPO-34 membranes was also investigated which indicated that single-gas CO2 permeance decreased with increasing pressure drop while single-gas CH4 permeance did not show significant changes (Fig. 4(a)). Because of this, both ideal and mixture CO2 selectivity dropped with increasing pressure drop (Fig. 4(b)). This is mainly due to the strong adsorption of CO2 onto SAPO-34. At lower temperature, SAPO-34 saturates with CO2 easily and we can see that the adsorption isotherm starts to plateau with increasing pressure (Fig. 5(a)). However, the adsorption isotherm for CH4 remains rather linear over the same pressure range (Fig. 5(b)). Because of this, we expect the CO2 permeance to fall with increasing pressure (and pressure drop), while the CH4 permeance remains somewhat constant (Fig. 4).
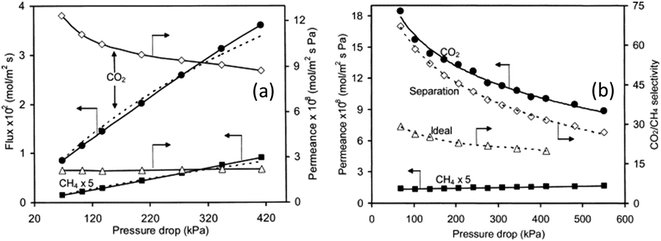 | ||
| Fig. 4 CO2/CH4 separation vs. pressure drop; (a) single gas permeance at 297 K and (b) mixture gas permeance at 297 K (ref. 28) (reprinted with permission from Elsevier). | ||
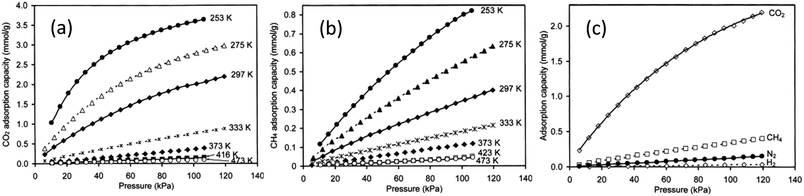 | ||
| Fig. 5 Adsorption isotherms of different gases on SAPO-34: (a) adsorption of CO2 at various temperatures, (b) adsorption of CH4 at various temperatures, and (c) adsorption of various gases at 297 K (ref. 28) (reprinted with permission from Elsevier). | ||
Overall, the separation performance of SAPO-34 membranes in CO2/CH4 separations is largely influenced by the strong adsorption of CO2 on SAPO-34, and the CO2/CH4 selectivity is enhanced at lower temperatures and pressures.
N2 permeation and CH4 permeation through SAPO-34 zeolite membranes are based on adsorption–diffusion mechanisms, but are mainly controlled by diffusion. CH4 adsorbs more strongly than N2 on SAPO-34 zeolite (Fig. 5(c)), but single gas permeation studies have shown that N2 with a smaller molecular size diffuses faster than CH4 with a larger molecular size (Fig. 3(a)). Thus, N2 has an overall higher permeance and can be separated from CH4. Permeances and selectivities of N2/CH4 mixture gases for a SAPO-34 membrane at different temperatures are shown in Fig. 6(a). The N2 permeance decreased with temperature while the CH4 permeance was independent of temperature over the temperature ranges tested. The N2/CH4 separation selectivity thus decreased with increasing temperature. The mixture selectivity is slightly lower than the ideal selectivity as SAPO-34 adsorbs CH4 more strongly, in contrast to CO2/CH4 separation where SAPO-34 adsorbs CO2 more strongly. The effect of pressure on N2/CH4 permeation is shown in Fig. 6(b).78 The N2 permeance and selectivity decreased with increasing pressure drop due to the stronger adsorption of CH4 on SAPO-34.
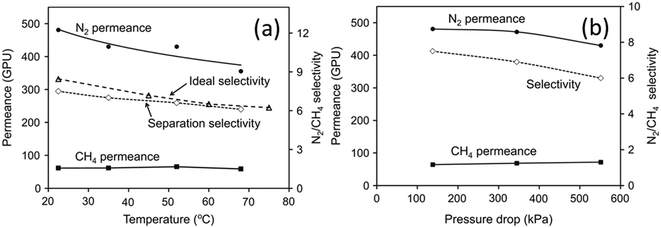 | ||
| Fig. 6 (a) Permeance vs. temperature and (b) permeance vs. pressure drop in N2/CH4 separation78 (reprinted with permission from Elsevier). | ||
As CO2 separation via the SAPO-34 membrane is largely driven by the strong adsorption of CO2 molecules by SAPO-34, most studies try to enhance CO2 flux and selectivity by increasing CO2 adsorption on SAPO-34. Previous work by Li et al. (2008) has attempted to improve CO2 adsorption capacity and thus improve both selectivity and permeance by increasing the Al content (reducing the Si/Al ratio in the gel) of the zeolite.6 Additionally, Ba2+ exchanged ions have been shown to increase the CO2 separation efficiency of SAPO-34 membranes.23,79
Even though SAPO-34 has a 3-dimensional uniform pore size of 0.38 nm, only the a-axis direction [100] has straight channels. Therefore, a SAPO-34 membrane highly oriented in the [100] direction could provide high permeation due to the decrease in mass transfer resistance through the straight channel. Bing et al. (2016) reported a preferentially oriented SAPO-34 membrane with a high CO2 permeance of 15.7 × 10−7 mol m−2 s−1 Pa−1 and CO2/CH4 selectivity of 109.22
Besides improving performance using oriented membranes, Bing et al. (2016) and Hua Shi (2015) have shown that synthesis under microwave heating conditions was effective in synthesis of SAPO-34 membranes with good CO2 permeance and excellent CO2/CH4 selectivity.22,47 Likewise, Bai et al. (2017) also investigated the use of rapid heating in an oil bath for short duration (1 h) synthesis, resulting in thin (0.8 μm) membranes with high flux (27.6 × 10−7 mol m−2 s−1 Pa−1) and reasonable CO2/CH4 selectivity.21 These heating methods heat the synthesis gel more evenly and quickly, leading to an increased rate of growth versus conventional hydrothermal synthesis, allowing for thinner intergrown membranes with greater permeance at shorter synthesis times.
Perhaps the best performing CO2/CH4 separation membranes reported to date were those synthesized by Li et al. (2016) via a novel-two step method where a layer of low-crystallinity zeolite is first grown on a seeded support followed by dry gel conversion; excellent membranes with high CO2/CH4 selectivity (165) and excellent permeance (63 × 10−7 mol m−2 s−1 Pa−1) were obtained.33 More CO2 separation performances of SAPO-34 membranes are summarised in Table 1.
| Gas pair | Substrate | Test conditions | Selectivity | CO2 permeance (× 10−7 mol m−2 s−1 Pa−1) | Thickness (μm) | Ref. |
|---|---|---|---|---|---|---|
| CO2/N2 | Tubular alumina support | 25 °C, equimolar mixture, 100 kPa pressure drop | CO2/N2 = 32.9 (mixed gas) | 18.2 | 7.5 | Liu et al., 2020 (ref. 80) |
| CO2/N2 | Tubular silica support | 27 °C, single gas permeation only, 100 kPa pressure drop | CO2/N2 = 53 (ideal) | 20.1 | 1.7 | Makertihartha et al., 2020 (ref. 31) |
| CO2/N2 | α-Alumina disc | 30 °C, 5% CO2 in CO2/N2 mixture, 100 kPa pressure drop | CO2/N2 = 78 (mixed gas) | 17.5 | 4 | Chew et al., 2019 (ref. 79) |
| CO2/CH4 | Tubular α-alumina | 25 °C, equimolar mixture, 200 kPa pressure drop | CO2/CH4 = 159 (mixed gas) | 2.4 | 6 | Mu et al., 2019 (ref. 81) |
| CO2/CH4 | Tubular α-alumina | 25 °C, equimolar mixture, 100 kPa pressure drop | CO2/CH4 = 141 (mixed gas) | 32 | <2 | Mirfendereski et el., 2019 (ref. 82) |
| CO2/H2 | Asymmetric porous α-alumina tubes | 25 °C, equimolar mixture, 200 kPa pressure drop | CO2/H2 = 17.6 (mixed gas) | 40.3 | 6.0 | Mei et al., 2018 (ref. 25) |
| CO2/CH4 | Tubular α-alumina | 25 °C, equimolar mixture, 200 kPa pressure drop | CO2/CH4 = 67 (mixed gas) | 27.6 | ∼0.8 | Bai et al., 2017 (ref. 21) |
| CO2/CH4 | α-Alumina 4-channel hollow fibers | 25 °C, equimolar mixture, 200 kPa feed, 100 kPa pressure drop | CO2/CH4 = 160 (mixed gas) | 11.8 | 10 | Chen et al., 2017 (ref. 46) |
| CO2/N2 | Asymmetrical α-alumina tubes | 27 °C, single gas permeation only, 200 kPa pressure drop | CO2/N2 = 7.9 (ideal) | 2.44 | 73.2 (effective thickness) | Kgaphola et al., 2017 (ref. 83) |
| CO2/CH4 | α-Alumina disc | 29 °C, equimolar mixture, 138 kPa pressure drop | CO2/CH4 = 109 (mixed gas) | 15.7 | 4 | Bing et al., 2016 (ref. 22) |
| CO2/CH4 | Tubular α-alumina | 29 °C, equimolar mixture, 200 kPa pressure drop | CO2/CH4 = 165 (mixed gas) | 63 | 2 | Li et al., 2016 (ref. 33) |
| CO2/CH4 | Porous α-alumina discs | 22 °C, equimolar mixture, 140 kPa pressure drop | CO2/CH4 = 256 (mixed gas) | 16.8 | 4 | Hua Shi, 2015 (ref. 47) |
| CO2/CH4 | Tubular alumina supports | 22 °C, equimolar mixture, 200 kPa feed pressure | CO2/CH4 ≈ 95 | ∼13.5 | 5 | Funke et al., 2014 (ref. 8) |
| CO2/CH4 | Porous alumina supports | 22 °C, equimolar mixture, 4.6 MPa pressure drop | CO2/CH4 = 70 | 11.6 | 3 | Zhou et al., 2013 (ref. 84) |
| CO2/CH4 | Seven-channel monolith alumina support | 22 °C, equimolar mixture, 4.6 MPa pressure drop | CO2/CH4 = 54 | 7.1 | Outer channels: 3 | Ping et al., 2012 (ref. 40) |
| Inner channels: 2 | ||||||
| CO2/CH4 | α-Alumina discs | 30 °C, equimolar mixture, 100 kPa pressure drop | CO2/CH4 = 103 (mixed gas) | 3.76 | 4 | Chew et al., 2011 (ref. 23) |
| CO2/CH4 | Tubular porous stainless steel | 22 °C, equimolar mixture, 138 kPa pressure drop | CO2/CH4 = 245 (mixed gas) | 4.9 | 6 | Venna, Carreon, 2011 (ref. 85) |
| CO2/N2 | CO2/N2 = 39 (mixed gas) | 2.1 |
For N2/CH4 separation, Huang et al., (2015) used high aspect ratio seeds and a high silicon concentration in the synthesis gel to create a thin but highly uniform membrane with fewer defects displaying both great selectivity (N2/CH4 = 11.3) and permeance (4.02 × 10−7 mol m−2 s−1 Pa−1), demonstrating the importance of seeding quality.39 Also notable is the work of Zong et al. (2017) on creating thin membranes with excellent permeance (8.71 × 10−7 mol m−2 s−1 Pa−1) using high heat-transfer stainless steel autoclaves and a short hydrothermal duration, coupled with a high permeance asymmetric porous α-alumina support.24 More N2 separation performances of SAPO-34 membranes are shown in Table 2.
| Gas pair | Substrate | Test conditions | Selectivity | N2 permeance (× 10−7 mol m−2 s−1 Pa−1) | Thickness (μm) | Ref. |
|---|---|---|---|---|---|---|
| N2/CH4 | Tubular α-alumina supports | 40 °C, equimolar 300 kPa feed, 100 kPa pressure drop | N2/CH4 = 4.4 (mixed gas) | 1.34 | 5 | Alam et al., 2020 (ref. 37) |
| N2/CH4 | Asymmetric porous α-alumina tubes | 25 °C, equimolar 223 kPa feed, 138 kPa pressure drop | N2/CH4 = 7.4 (mixed gas) | 8.71 | 1.9 | Zong et al., 2017 (ref. 24) |
| N2/CH4 | Porous alumina tubes | 23 °C, equimolar 223 kPa feed, 1385 kPa pressure drop | N2/CH4 = 7.2 (mixed gas) | 2.95 | 3 | Zong et al., 2016 (ref. 34) |
| N2/CH4 | Porous α-alumina tubes | 22 °C, equimolar 275 kPa feed, 175 kPa pressure drop | N2/CH4 = 11.3 (mixed gas) | 4.02 | 1.8 | Huang et al., 2015 (ref. 39) |
| N2/CH4 | Porous alumina tubes | 24 °C, equimolar 223 kPa feed, 138 kPa pressure drop | N2/CH4 = 8 (mixed gas) | 1.68 | 6.2 | Li et al., 2015 (ref. 78) |
3.2 H2 separation
H2 purification by membrane separation consumes less energy compared to traditional methods (i.e. cryogenic distillation or PSA) and can help meet the increasing demand for H2.86,87 SAPO-34 membranes can be used for H2 separation owing to their small pore size. In addition, the high thermal and chemical stability of SAPO-34 membranes makes them useful in membrane reactors for various hydrogen-producing reactions. H2 permeation through SAPO-34 membranes is mainly influenced by diffusivity. In the case of H2/CO2 separation, even though hydrogen has a higher diffusivity than CO2, it is adsorbed less strongly than CO2 at low temperatures (Fig. 5(c)). Hence, SAPO-34 membranes are CO2 selective at low temperature. However, the CO2 permeance decreases with increasing temperatures because CO2 adsorption decreases with increasing temperatures (Fig. 5(a)).29 Eventually, SAPO-34 membranes become H2 selective at higher temperatures (Fig. 7(b)). In the case of H2/CH4 and H2/N2 separation, all gases showed weak adsorption on SAPO-34 (Fig. 5(c)). Hence their separation via SAPO-34 membranes is mainly driven by differences in diffusivity, and SAPO-34 membranes show good H2 selectivity in such separations.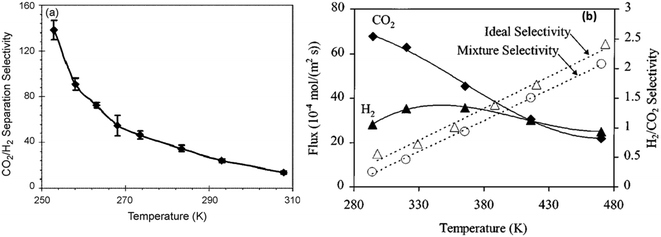 | ||
| Fig. 7 (a) CO2/H2 separation selectivity vs. temperature29 (reprinted with permission from Elsevier); (b) H2/CO2 flux and selectivity vs. temperature7 (reprinted with permission from Wiley). | ||
Recently, Mei et al. (2018) improved CO2/H2 and H2/CH4 separation performance via modifying SAPO-34 membrane synthesis conditions, including the seed concentration and the calcination conditions, and achieved a high CO2 permeance of 40.3 × 10−7 mol m−2 s−1 Pa−1 with a CO2/H2 selectivity of 17.6 and a high H2 permeance of 14.5 × 10−7 mol m−2 s−1 Pa−1 with a H2/CH4 selectivity of 42.2 at 298 K.25,46 Regarding high temperature H2/CO2 and H2/N2 separation, Yu et al. (2011) reported an Al2O3/SAPO-34 zeolite composite membrane prepared by depositing aluminium alkoxide on and between a SAPO-34 zeolite membrane via the molecular layer deposition (MLD) method, showing a remarkable separation performance with a H2/N2 selectivity of 750 and a H2/CO2 selectivity of 20 at 473 K.88
Additionally, H2/C3Hx and H2/C4Hx separations were tested on SAPO-34 membranes. These separations are mainly attributed to molecular sieving, as the kinetic diameters of C3Hx and C4Hx are much larger than SAPO-34's pore size. Notably, Wang et al. (2020) developed a highly hydrogen permeable SAPO-34 membrane for high temperature (up to 600 °C) H2/C3Hx separations, which achieved a remarkable performance with a H2 permeance of 3.1 × 10−7 mol m−2 s−1 Pa−1 and a H2/C3H8 selectivity of 41 at 600 °C (M1) as shown in Fig. 8.16 This study provided very useful information for the application of SAPO-34 membrane reactors in propane dehydrogenation. More H2 separation studies using SAPO-34 membranes are summarised in Table 3.
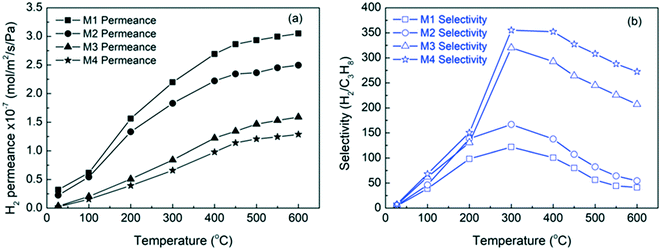 | ||
| Fig. 8 H2 permeance (a) and selectivity (b) at different temperatures through SAPO-34 hollow fiber membranes16 (reprinted with permission from Wiley). | ||
| Gas pair | Substrate | Test conditions | Selectivity | H2 permeance (× 10−7 mol m−2 s−1 Pa−1) | Thickness (μm) | Ref. |
|---|---|---|---|---|---|---|
| H2/C3H8 | α-Alumina hollow fiber | 600 °C, equimolar mixture | H2/C3H8 = 41 (mixed gas) | 3.1 | 2 | Wang et al., 2020 (ref. 16) |
| H2/CH4 | Asymmetric porous α-alumina tubes | 25 °C, equimolar mixture, 200 kPa pressure drop | H2/CH4 = 42.2 (mixed gas) | 14.5 | 6.0 | Mei et al., 2018 (ref. 25) |
| H2/C3H8 | Tubular α-alumina supports | 650 °C, equimolar mixture | H2/C3H8 = 27 (mixed gas) | 2.3 | 1.1 | Kim et al., 2016 (ref. 9) |
| H2/N2 | Tubular alumina supports | 25 °C, equimolar 350 kPa feed, 265 kPa pressure drop | H2/N2 = 4.5 (mixed gas) | 17 | 3.0 | Chisholm et al., 2015 (ref. 74) |
| H2/CO2H2/N2H2/CH4 | Macroporous α-alumina tubes | 25 °C, single gas permeation only, 100 kPa pressure drop | H2/CO2 = 1.83 (ideal)H2/N2 = 7.58 (ideal) H2/CH4 = 14.80 (ideal) |
69.9 (single gas) | 4 | Zhou et al., 2014 (ref. 38) |
| H2/CH4 | Tubular alumina supports | 20 °C, equimolar 275 kPa feed | H2/CH4 ≈ 45 (mixed gas) | ∼7.5 | 5 | Funke et al., 2014 (ref. 8) |
3.3 Other separations
Xe separation, particularly Kr/Xe separation, is another potential application of SAPO-34 membranes. High-purity Xe has been used in commercial lighting, medical imaging, anesthesia, and neuroprotection. Kr/Xe separation is also a critical step in nuclear fuel treatment. Currently, xenon is separated using cryogenic distillation from a Xe/Kr rich stream left over from the distillation of air. Using membranes for this step would result in huge cost savings from the lowered energy costs and simplicity of the separation system. The pore size of SAPO-34 membranes, being 3.8 Å, lies between the kinetic diameters of Kr (3.69 Å) and Xe (4.10 Å), making them an ideal candidate for the separation of Kr from Xe. Molecular simulations have also shown CHA-type zeolites and SAPO-34 to be a promising material for Kr/Xe separation over a range of temperatures.89,90 Feng et al. (2016) achieved a high Kr permeance of 1.2 × 10−7 mol m−2 s−1 Pa−1 with a Kr/Xe selectivity of 31 by using a dilute synthesis gel to grow thin SAPO-34 membranes.10 Xe separation processes are driven by the inherently poor diffusivity of the large Xe atoms in the zeolite framework, and as such, efforts have been made to improve Kr/Xe selectivity by reducing the diffusivity of Xe through the SAPO framework. Kwon et al. (2018) has recently demonstrated the substitution of H-SAPO-34 with alkali metal ions to decrease the pore size and thus reduce Xe diffusivity, leading to an increased membrane selectivity.32 More Kr/Xe separation studies via SAPO-34 membranes are summarised in Table 4.| Gas pair | Substrate | Test conditions | Selectivity | Permeance (× 10−7 mol m−2 s−1 Pa−1) | Thickness (μm) | Ref. |
|---|---|---|---|---|---|---|
| Air/Xe | Porous tubular α-alumina supports | 25 °C, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 air/Xe 223 kPa feed, 138 kPa pressure drop 1 air/Xe 223 kPa feed, 138 kPa pressure drop |
Air/Xe = 14.1 | Air: 2.31 | 6.4 | Wu et al., 2019 (ref. 95) |
| Kr/Xe | Porous α-alumina disc | 25 °C, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Kr/Xe feed 1 Kr/Xe feed |
Kr/Xe = 37 | Kr: 0.063 | 3.2 | Kwon et al., 2018 (ref. 32) |
| Kr/Xe | Porous tubular α-alumina supports | 25 °C, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Kr/Xe feed, 138 kPa pressure drop 1 Kr/Xe feed, 138 kPa pressure drop |
Kr/Xe = 31 | Kr: 1.2 | 3 | Feng et al., 2016 (ref. 10) |
Additionally, SAPO-34 membranes have also been applied for other gas separation applications such as separation of He/CH4, methanol/dimethyl carbonate, and propylene/propane, and NF3 enrichment.35,65,91–94 All these studies demonstrate the wide applications of SAPO-34 membranes.
4. The applications of SAPO-34 membrane reactors
With their high thermal and chemical stability, SAPO-34 membranes can also be applied in membrane reactors at moderate to high temperatures. Particularly for reactions which are limited by thermodynamic equilibrium, SAPO-34 membrane reactors can help increase reaction yield by separating light gas products and thus drive the reaction forward. Furthermore, downstream separation costs are also lowered since the reaction products are pre-separated in the membrane reactor.4.1 PDH reaction
One promising application of SAPO-34 membrane-reactors is dehydrogenation reactions. Because of its small pore size of 0.38 nm and high permeability to hydrogen (kinetic diameter 0.289 nm), SAPO-34 is an ideal candidate for membrane reactors for dehydrogenation reactions. High temperature dehydrogenation reactions include paraffin to olefin reactions such as propane dehydrogenation (PDH) as shown in eqn (1). Propylene (C3H6) is an essential chemical intermediate for the production of other chemical products (mainly polypropylene).96,97 However, the PDH reaction is strongly limited by thermodynamic equilibrium, and the separation of hydrogen in a membrane reactor can thus help drive the reaction forward. Membrane reactors, therefore, have a great potential for process intensification through increased yields and reduced separation costs.| C3H8 ↔ C3H6 + H2, ΔH(298K) = + 124 kJ mol−1 | (1) |
Besides modelling work, Kim et al. (2016) have also demonstrated a catalytic SAPO-34 membrane reactor for the PDH reaction, resulting in high propane conversions of 65–75% with a WHSV of 0.1–0.5 h−1 and a high selectivity of >80% at 600 °C due to simultaneous separation of H2 from the reaction system as shown in Fig. 9. By optimizing the synthesis conditions, they decreased the thickness of the SAPO-34 membrane layer to ~1 μm and achieved a high H2 permeance of >2 × 10−7 mol m−2 s−1 Pa−1 with a H2/C3H8 permselectivity of ∼15 at 600 °C.9 Recently, our research group investigated a highly H2 permeable SAPO-34 membrane for high temperature PDH reactions, and achieved a high H2 permeance of 3.1 × 10−7 mol m−2 s−1 Pa−1 with a H2/C3H8 permselectivity of 41 at 600 °C. These results have shown the potential to further improve the performance of SAPO-34 membrane reactors for the PDH reaction.
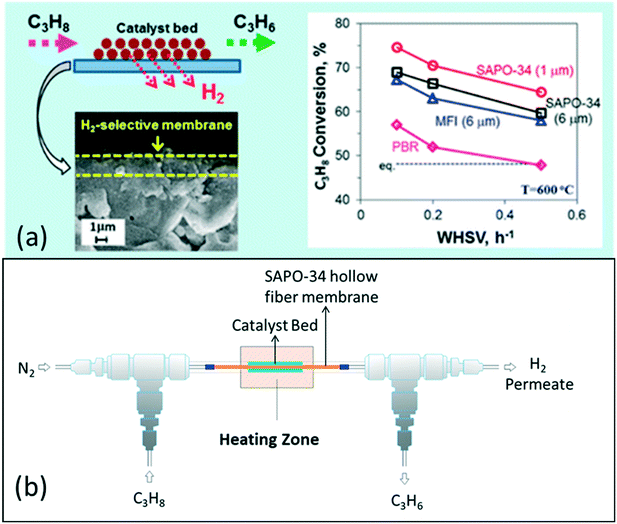 | ||
| Fig. 9 (a) Improvement in reaction conversion using a SAPO-34 membrane reactor9 (reprinted with permission from ACS); (b) general scheme for a membrane reactor for the PDH reaction16 (reprinted with permission from Wiley). | ||
4.2 Urea methanolysis to dimethyl carbonate (UM-to-DMC)
Dimethyl carbonate is widely used as a solvent and intermediate for industrial applications. Catalytic urea methanolysis to dimethyl carbonate (UM-to-DMC) is a promising way to produce DMC. In this reaction, the amino groups of urea are replaced by methoxy groups from methanol to form DMC and NH3 as shown in eqn (2) and (3), respectively. However, this reaction is limited by the thermodynamic equilibrium, which leads to low urea conversion. The application of a catalytic membrane reactor in the reaction can allow for greater conversion if the membrane can effectively and simultaneously separate the side product (NH3). The kinetic diameters of NH3, methanol, urea, methyl carbamate (MC) and DMC is 0.29 nm, 0.36 nm, 0.42 nm, 0.52 nm and 0.60 nm, respectively. To separate NH3 and retain other species, the pore size of the membrane must be sufficiently small. A SAPO-34 membrane with a pore size of 0.38 nm can be modified for effective NH3 separation. Meanwhile, the membrane also requires high thermal and chemical stability under the harsh conditions of the UM-to-DMC reaction which is generally operated at 160–220 °C. Zeng et al. (2019) recently demonstrated modified SAPO-34 membranes with a significantly reduced pore size and acidity via post-synthesis with zinc ion-exchange (Zn-SAPO-34) and thermal NH3–methanol treatments for NH3 separation from the UM-to-DMC reaction as shown in Fig. 10.100 The membrane reactor demonstrated a remarkable performance with 139% higher DMC yield than that of a traditional reactor due to simultaneous separation of NH3 from the reaction system. Moreover, the membrane reactor also illustrated a stable performance for over 2000 hours, which has shown the potential applications of SAPO-34 membrane reactors in industrial NH3 production processes. Additionally, this work also demonstrated that a regular SAPO-34 membrane (without modification) showed a remarkable performance in methanol/DMC separation with methanol permeance and selectivity 1–2 orders higher than those of polymeric membranes. As the mixture of methanol and DMC is an azeotrope, the traditional distillation method to separate methanol and DMC is energy intensive. Membrane separation of methanol/DMC could thus significantly decrease the energy costs of separation.| H2NCONH2 + CH3OH → CH3OCONH2 + NH3 | (2) |
| CH3OCONH2 + CH3OH → CH3OCOOCH3 + NH3 | (3) |
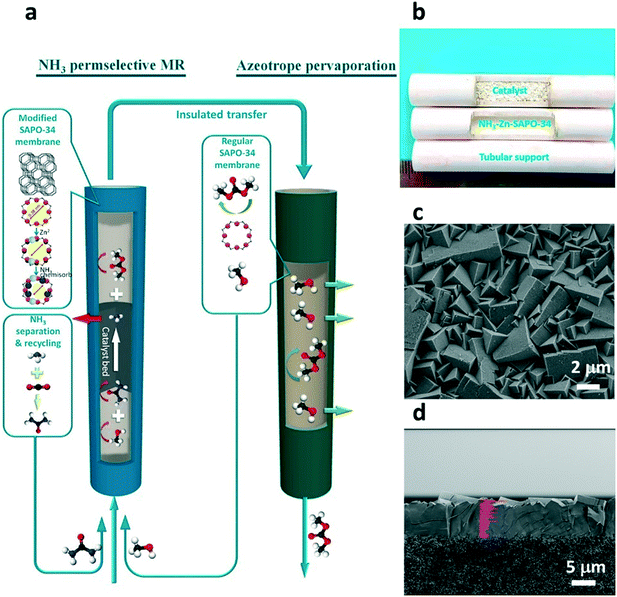 | ||
| Fig. 10 SAPO-34 membrane reactor for the UM-to-DMC reaction;101 (a) the schematic diagram of SAPO-34 membrane reactor for UM-to-DMC reaction; (b) image of the SAPO-34 membrane with catalyst; SEM images of (c) the surface and (d) cross-section of the SAPO-34 membrane (reprinted with permission from ACS). | ||
5. Challenges and future perspectives
According to the literature, SAPO-34 membranes are mainly investigated for potential applications in natural gas purification and hydrogen separation, and catalytic membrane reactors for high temperature reactions. However, several areas must be investigated and improved to further the development of SAPO-34 membranes for industrial applications.5.1 Moisture sensitivity of SAPO-34 membranes
Water adsorption of SAPO-34 membranes at room temperature has been shown to degrade membrane performance upon storage. Adsorbed water molecules block the SAPO pores and thus reduce gas permeance, but can also react with the zeolite framework itself, leading to rapid and irreversible degradation through the formation of non-SAPO pores that are larger than SAPO-34 pores. The rate of this degradation appeared to accelerate after months of exposure to a humid atmosphere.102–104 In some applications such as natural gas separation, the feed gas may contain significant amounts of moisture, and SAPO-34 membranes might partially degenerate during natural gas purification over long term use (several years). As such, there is an urgent need to improve the moisture resistance of SAPO-34 membranes.To resolve this problem, some groups have explored using organosilica surface modification as a hydrophobic barrier to improve the separation performance and stability of SAPO-34 membranes under wet conditions while at the same time plugging defects of the zeolite membrane.81,105,106 More work of this nature is needed in SAPO-34 membrane research, and other methods of improving membrane stability and performance under high humidity conditions should be investigated.
5.2 Defect reduction in membranes
Secondly, defects between SAPO-34 zeolite crystals are currently difficult to avoid. Longer synthesis times can be used to ensure the intergrowth of zeolite crystals and reduce defects, but this also leads to a thicker membrane with lower permeance, as well as longer membrane fabrication times. Post-synthesis defect-sealing has also been investigated using different methods, but such methods add to the fabrication cost of the membranes and may not be suitable for all applications.81,107,108 As such, a solution towards minimizing defects in membranes in a reproducible way would greatly aid the adoption of SAPO-34 in industrial applications.5.3 Scalability of membrane production
Most studies on SAPO-34 membranes use short tubular supports to synthesize membranes with a small surface area. In large-scale SAPO-34 membrane production, modified synthesis conditions might be required. For example, some groups have investigated membrane growth on longer tubular supports (up to 25 cm), and have observed that different conditions are required for synthesis of these longer membranes which necessitate taller autoclaves.16,109 More work on fabricating longer SAPO-34 membranes on a larger scale would thus be needed before they can be successfully commercialized.5.4 Cost
The cost of zeolite membrane modules is a key factor limiting their widespread use in industry. Currently, zeolite membrane modules are prohibitively expensive for industrial applications. For example, Lin and Duke (2013) estimated the cost of tubular MFI membranes to be around $2700 per m2, which is around two orders of magnitude higher than that of polymeric membranes.110 The main cost of SAPO-34 membranes comes from the template, Al/Si sources and support. As discussed before, template-free synthesis and the use of cheaper alumina sources (for instance Al(OH)3 is cheaper than Al(O-i-Pr)3) have been studied; however, such studies are very limited. Further research is required to seek cheaper synthesis methods for SAPO-34 membranes.Ceramic hollow fiber supports can drastically decrease the cost of support as the thin fibers require less raw material per unit area. However, the durability of ceramic hollow fibers must be improved to meet the requirements of industrial applications. Interestingly, Chen et al. (2017) reported a SAPO-34 membrane supported on a durable multi-channel hollow fiber substrate as shown in Fig. 11. Further improvements on the performance of this kind of substrate would greatly accelerate industrial applications of SAPO-34 membranes.
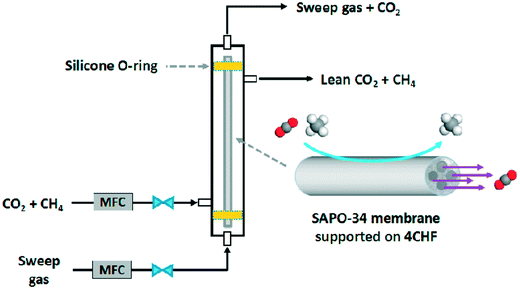 | ||
| Fig. 11 Gas separation with a 4-channel hollow fiber46 (reprinted with permission from Elsevier). | ||
Conclusion
SAPO-34 zeolite, with its high thermal and chemical stability, is a promising material for the fabrication of ceramic membranes for light gas separations at different temperatures. Numerous groups in the past decade have attempted different strategies to improve the selectivity and permeance of SAPO-34 membranes for different gas separations under different conditions using better seeding techniques, improved synthesis methods, or better substrates. Many have shown that SAPO-34 membranes can achieve excellent gas separation performance compared to other zeolites or polymer membranes, particularly in natural gas purification. Furthermore, SAPO-34 membranes also have great potential in membrane reactors for process intensification.While most groups seek to synthesize thin membranes with few defects to obtain high gas separation performance, industrial applications, however, would require high quality membranes which can be synthesized reliably, at a large scale, and at a low cost. As such, future work on SAPO-34 membranes towards industrial applications should focus on the development of scalable, economical ways of membrane fabrication, as well as focus towards better understanding of membrane stability and synthesis conditions to aid in rational membrane design.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors generously thank financial support from the Singapore Agency for Science, Technology and Research (A*STAR) AME IRG grant (No. A1783c0016), the National Environment Agency (NEA) of Singapore (WTE-CRP 1501-103) and a MOE Tier 2 grant (WBS: R279-000-544-112).References
- R. Szostak, Molecular Sieves – Principles of Synthesis and Identification, Van Nostrand Reinhold, New York, 1989 Search PubMed.
- Y. Watanabe, A. Koiwai, H. Takeuchi, S. A. Hyodo and S. Noda, J. Catal., 1993, 143, 430–436 CrossRef CAS.
- M. A. Carreon, J. Mater. Res., 2018, 33, 32–43 CrossRef CAS.
- W. J. Koros and R. Mahajan, J. Membr. Sci., 2001, 181, 141 CrossRef CAS.
- S. Li, J. L. Falconer and R. D. Noble, Adv. Mater., 2006, 18, 2601–2603 CrossRef CAS.
- S. Li, J. L. Falconer and R. D. Noble, Microporous Mesoporous Mater., 2008, 110, 310–317 CrossRef CAS.
- J. C. Poshusta, V. A. Tuan, E. A. Pape, R. D. Noble and J. L. Falconer, AIChE J., 2000, 46, 779–789 CrossRef CAS.
- H. H. Funke, M. Z. Chen, A. N. Prakash, J. L. Falconer and R. D. Noble, J. Membr. Sci., 2014, 456, 185–191 CrossRef CAS.
- S. J. Kim, Y. Liu, J. S. Moore, R. S. Dixit, J. G. Pendergast, D. Sholl, C. W. Jones and S. Nair, Chem. Mater., 2016, 28, 4397–4402 CrossRef CAS.
- X. Feng, Z. Zong, S. K. Elsaidi, J. B. Jasinski, R. Krishna, P. K. Thallapally and M. A. Carreon, J. Am. Chem. Soc., 2016, 138, 9791–9794 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 1991, 62, 165–185 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
- B. Comesaña-Gándara, J. Chen, C. G. Bezzu, M. Carta, I. Rose, M. C. Ferrari, E. Esposito, A. Fuoco, J. C. Jansen and N. B. McKeown, Energy Environ. Sci., 2019, 12, 2733–2740 RSC.
- S. Pati, J. Ashok, N. Dewangan, T. Chen and S. Kawi, J. Membr. Sci., 2020, 595, 117496 CrossRef.
- S. Pati, A. Jangam, Z. Wang, N. Dewangan, M. H. Wai and S. Kawi, Chem. Eng. J., 2019, 362, 116–125 CrossRef CAS.
- Z. Wang, J. Xu, S. Pati, T. Chen, Y. Deng, L. Meng, J. Y. S. Lin and S. Kawi, AIChE J., 2020, 66, e16278 CAS.
- T. Maneerung, K. Hidajat and S. Kawi, J. Membr. Sci., 2016, 514, 1–14 CrossRef CAS.
- N. Kosinov, J. Gascon, F. Kapteijn and E. J. M. Hensen, J. Membr. Sci., 2016, 499, 65–79 CrossRef CAS.
- B. E. Poling, J. M. Prausnitz and J. P. O'connell, The properties of gases and liquids, Mcgraw-hill, 5th edn, 2001 Search PubMed.
- F. Jareman, J. Hedlund, D. Creaser and J. Sterte, J. Membr. Sci., 2004, 236, 81–89 CrossRef CAS.
- L. Bai, N. Chang, M. Li, Y. Wang, G. Nan, Y. Zhang, D. Hu, G. Zeng and W. Wei, Microporous Mesoporous Mater., 2017, 241, 392–399 CrossRef CAS.
- L. Bing, G. Wang, F. Wang, X. Liu and B. Zhang, RSC Adv., 2016, 6, 56170–56173 RSC.
- T. L. Chew, A. L. Ahmad and S. Bhatia, Chem. Eng. J., 2011, 171, 1053–1059 CrossRef CAS.
- Z. Zong and M. A. Carreon, J. Membr. Sci., 2017, 524, 117–123 CrossRef CAS.
- W. Mei, Y. Du, T. Wu, F. Gao, B. Wang, J. Duan, J. Zhou and R. Zhou, J. Membr. Sci., 2018, 565, 358–369 CrossRef CAS.
- X. Ren and Y. Li, in Current Trends and Future Developments on (Bio-) Membranes: Microporous Membranes and Membrane Reactors, Elsevier Inc., 2019, pp. 157–183 Search PubMed.
- S. Askari, A. Bashardoust Siahmard, R. Halladj and S. Miar Alipour, Powder Technol., 2016, 301, 268–287 CrossRef CAS.
- S. Li, J. L. Falconer and R. D. Noble, J. Membr. Sci., 2004, 241, 121–135 CrossRef CAS.
- M. Hong, S. Li, J. L. Falconer and R. D. Noble, J. Membr. Sci., 2008, 307, 277–283 CrossRef CAS.
- Y. Huang, L. Wang, Z. Song, S. Li and M. Yu, Am. Ethnol., 2015, 127, 10993–10997 Search PubMed.
- I. G. B. N. Makertihartha, K. S. Kencana, T. R. Dwiputra, K. Khoiruddin, R. R. Mukti and I. G. Wenten, Microporous Mesoporous Mater., 2020, 298, 110068 CrossRef CAS.
- Y. H. Kwon, B. Min, S. Yang, D. Y. Koh, R. R. Bhave and S. Nair, ACS Appl. Mater. Interfaces, 2018, 10, 6361–6368 CrossRef CAS.
- M. Li, J. Zhang, X. Liu, Y. Wang, C. Liu, D. Hu, G. Zeng, Y. Zhang, W. Wei and Y. Sun, Microporous Mesoporous Mater., 2016, 225, 261–271 CrossRef CAS.
- Z. Zong, X. Feng, Y. Huang, Z. Song, R. Zhou, S. J. Zhou, M. A. Carreon, M. Yu and S. Li, Microporous Mesoporous Mater., 2016, 224, 36–42 CrossRef CAS.
- S. Denning, J. Lucero, C. A. Koh and M. A. Carreon, ACS Mater. Lett., 2019, 1, 655–659 CrossRef CAS.
- C. S. Cundy and P. A. Cox, Microporous Mesoporous Mater., 2005, 82, 1–78 CrossRef CAS.
- S. F. Alam, M. Z. Kim, Y. J. Kim, A. ur Rehman, A. Devipriyanka, P. Sharma, J. G. Yeo, J. S. Lee, H. Kim and C. H. Cho, J. Membr. Sci., 2020, 602, 117825 CrossRef CAS.
- L. Zhou, J. Yang, G. Li, J. Wang, Y. Zhang, J. Lu and D. Yin, Int. J. Hydrogen Energy, 2014, 39, 14949–14954 CrossRef CAS.
- Y. Huang, L. Wang, Z. Song, S. Li and M. Yu, Angew. Chem., Int. Ed., 2015, 54, 10843–10847 CrossRef CAS.
- E. W. Ping, R. Zhou, H. H. Funke, J. L. Falconer and R. D. Noble, J. Membr. Sci., 2012, 415–416, 770–775 CrossRef CAS.
- M. A. Carreon, S. Li, J. L. Falconer and R. D. Noble, Adv. Mater., 2008, 20, 729–732 CrossRef CAS.
- Q. Sun, Z. Xie and J. Yu, Natl. Sci. Rev., 2018, 5, 542–558 CrossRef CAS.
- T. Doan, K. Nguyen, P. Dam, T. H. Vuong, M. T. Le and H. P. Thanh, J. Chem., 2019, 2019, 1–10 CrossRef.
- N. Najafi, S. Askari and R. Halladj, Powder Technol., 2014, 254, 324–330 CrossRef CAS.
- T. Álvaro-Muñoz, C. Márquez-Álvarez and E. Sastre, Catal. Today, 2012, 179, 27–34 CrossRef.
- Y. Chen, Y. Zhang, C. Zhang, J. Jiang and X. Gu, J. CO2 Util., 2017, 18, 30–40 CrossRef CAS.
- H. Shi, RSC Adv., 2015, 5, 38330–38333 RSC.
- S. Li and C. Q. Fan, Ind. Eng. Chem. Res., 2010, 49, 4399–4404 CrossRef CAS.
- N. Chang, H. Tang, L. Bai, Y. Zhang and G. Zeng, J. Membr. Sci., 2018, 552, 13–21 CrossRef CAS.
- Y. Zhang, B. Tokay, H. H. Funke, J. L. Falconer and R. D. Noble, J. Membr. Sci., 2010, 363, 29–35 CrossRef CAS.
- Y. Zhang, M. Wang, S. Liu, H. Qiu, M. Wang, N. Xu, L. Gao and Y. Zhang, Sep. Purif. Technol., 2019, 228, 115758 CrossRef CAS.
- N. Kosinov, C. Auffret, V. G. P. Sripathi, C. Gücüyener, J. Gascon, F. Kapteijn and E. J. M. Hensen, Microporous Mesoporous Mater., 2014, 197, 268–277 CrossRef CAS.
- Z. Wang, N. Dewangan, S. Das, M. H. Wai and S. Kawi, Sep. Purif. Technol., 2018, 201, 30–40 CrossRef CAS.
- Z. Wang, J. Ashok, Z. Pu and S. Kawi, Chem. Eng. J., 2017, 315, 315–323 CrossRef CAS.
- Z. Wang, U. Oemar, M. L. Ang and S. Kawi, J. Membr. Sci., 2016, 510, 417–425 CrossRef CAS.
- Z. Wang, Y. Kathiraser, M. L. Ang and S. Kawi, Ind. Eng. Chem. Res., 2015, 54, 6371–6377 CrossRef CAS.
- Z. Wang, Y. Kathiraser, T. Soh and S. Kawi, J. Membr. Sci., 2014, 465, 151–158 CrossRef CAS.
- N. Yang, Y. Kathiraser and S. Kawi, Int. J. Hydrogen Energy, 2013, 38, 4483–4491 CrossRef CAS.
- N. Yang, Y. Kathiraser and S. Kawi, J. Membr. Sci., 2013, 428, 78–85 CrossRef CAS.
- Y. Kathiraser, Z. Wang, N. Yang, S. Zahid and S. Kawi, J. Membr. Sci., 2013, 427, 240–249 CrossRef CAS.
- Y. Kathiraser, Z. Wang and S. Kawi, Environ. Sci. Technol., 2013, 47, 14510–14517 CrossRef CAS.
- Y. Kathiraser and S. Kawi, AIChE J., 2013, 59, 3874–3885 CrossRef CAS.
- Z. Wang, Z. Li, Y. Cui, T. Chen, J. Hu and S. Kawi, Environ. Sci. Technol., 2019, 53, 9937–9946 CrossRef CAS.
- Z. Wang, Z. Bian, N. Dewangan, J. Xu and S. Kawi, J. Membr. Sci., 2019, 578, 36–42 CrossRef CAS.
- P. S. Lee, M. S. Lim, A. Park, H. Park, S. E. Nam and Y. I. Park, J. Membr. Sci., 2017, 542, 123–132 CrossRef CAS.
- J. K. Das, N. Das and S. Bandyopadhyay, Int. J. Hydrogen Energy, 2012, 37, 10354–10364 CrossRef CAS.
- J. K. Das, N. Das and S. Bandyopadhyay, J. Mater. Chem. A, 2013, 1, 4966–4973 RSC.
- B. Shimekit and H. Mukhtar, in Advances in Natural Gas Technology, 2012, pp. 235–270 Search PubMed.
- E. Favre, R. Bounaceur and D. Roizard, J. Membr. Sci., 2009, 328, 11–14 CrossRef CAS.
- T. Chen, Z. Wang, L. Liu, S. Pati, M. H. Wai and S. Kawi, Chem. Eng. J., 2020, 379, 122182 CrossRef CAS.
- T. Chen, Z. Wang, J. Hu, M. Hui, S. Kawi and Y. S. Lin, J. Membr. Sci., 2020, 597, 117770 CrossRef CAS.
- T. Chen, Z. Wang, S. Das, L. Liu, Y. Li, S. Kawi and Y. S. Lin, J. Membr. Sci., 2019, 581, 72–81 CrossRef CAS.
- J. C. Poshusta, V. A. Tuan, J. L. Falconer and R. D. Noble, Ind. Eng. Chem. Res., 1998, 37, 3924–3929 CrossRef CAS.
- N. O. Chisholm, G. C. Anderson, J. F. McNally, H. H. Funke, R. D. Noble and J. L. Falconer, J. Membr. Sci., 2015, 496, 118–124 CrossRef CAS.
- T. Wu, M. C. Diaz, Y. Zheng, R. Zhou, H. H. Funke, J. L. Falconer and R. D. Noble, J. Membr. Sci., 2015, 473, 201–209 CrossRef CAS.
- N. O. Chisholm, H. H. Funke, R. D. Noble and J. L. Falconer, J. Membr. Sci., 2018, 560, 108–114 CrossRef CAS.
- P. F. Zito, A. Brunetti, A. Caravella, E. Drioli and G. Barbieri, J. Membr. Sci., 2020, 595, 117534 CrossRef CAS.
- S. Li, Z. Zong, S. J. Zhou, Y. Huang, Z. Song, X. Feng, R. Zhou, H. S. Meyer, M. Yu and M. A. Carreon, J. Membr. Sci., 2015, 487, 141–151 CrossRef CAS.
- T. L. Chew, Y. F. Yeong, C. D. Ho and A. L. Ahmad, Ind. Eng. Chem. Res., 2019, 58, 729–735 CrossRef CAS.
- B. Liu, C. Tang, X. Li, B. Wang and R. Zhou, Microporous Mesoporous Mater., 2020, 292, 109712 CrossRef CAS.
- Y. Mu, H. Chen, H. Xiang, L. Lan, Y. Shao, X. Fan and C. Hardacre, J. Membr. Sci., 2019, 575, 80–88 CrossRef CAS.
- S. M. Mirfendereski, Chem. Eng. Sci., 2019, 208, 115157 CrossRef CAS.
- K. Kgaphola, I. Sigalas and M. O. Daramola, Asia-Pac. J. Chem. Eng., 2017, 12, 894–904 CrossRef CAS.
- R. Zhou, E. W. Ping, H. H. Funke, J. L. Falconer and R. D. Noble, J. Membr. Sci., 2013, 444, 384–393 CrossRef CAS.
- S. R. Venna and M. A. Carreon, Langmuir, 2011, 27, 2888–2894 CrossRef CAS.
- T. Maneerung, K. Hidajat and S. Kawi, J. Membr. Sci., 2014, 452, 127–142 CrossRef CAS.
- G. B. Sun, K. Hidajat and S. Kawi, J. Membr. Sci., 2006, 284, 110–119 CrossRef CAS.
- M. Yu, H. H. Funke, R. D. Noble and J. L. Falconer, J. Am. Chem. Soc., 2011, 133, 1748–1750 CrossRef CAS.
- A. Hasanzadeh, J. Azamat, S. Pakdel, H. Erfan-Niya and A. Khataee, Chem. Pap., 2020, 74, 3057–3065 CrossRef CAS.
- R. Anderson, B. Schweitzer, T. Wu, M. A. Carreon and D. A. Gómez-Gualdrón, ACS Appl. Mater. Interfaces, 2018, 10, 582–592 CrossRef CAS.
- M. Wang, M. Li, N. Chang, L. Gao, M. Wang and Y. Zhang, J. Membr. Sci., 2018, 565, 311–321 CrossRef CAS.
- H. Maghsoudi, Polyolefins J., 2017, 5, 1–14 Search PubMed.
- K. Agarwal, M. John, S. Pai, B. L. Newalkar, R. Bhargava and N. V. Choudary, Microporous Mesoporous Mater., 2010, 132, 311–318 CrossRef CAS.
- H. S. Kim, E. Y. Kim and P. S. Lee, Sep. Purif. Technol., 2019, 220, 1–7 CrossRef CAS.
- T. Wu, J. Lucero, J. M. Crawford, M. A. Sinnwell, P. K. Thallapally and M. A. Carreon, J. Membr. Sci., 2019, 573, 288–292 CrossRef CAS.
- N. Dewangan, A. Jangam, M. Sethia, S. Pati, H. Kus and S. Kawi, ChemCatChem, 2019, 11, 4923–4934 CrossRef CAS.
- S. Rimaz, C. Luwei, S. Kawi and A. Borgna, Appl. Catal., A, 2019, 588, 117266 CrossRef CAS.
- S. Choi, D. S. Sholl, S. Nair, J. S. Moore, Y. Liu, R. S. Dixit and J. G. Pendergast, AIChE J., 2017, 63, 4519–4531 CrossRef CAS.
- S. Choi, C. W. Jones, S. Nair, D. S. Sholl, J. S. Moore, Y. Liu, R. S. Dixit and J. G. Pendergast, AIChE J., 2014, 00, 1–14 Search PubMed.
- G. Zeng, Y. Wang, D. Gong, Y. Zhang, P. Wu and Y. Sun, ACS Cent. Sci., 2019, 5, 1834–1843 CrossRef CAS.
- G. Zeng, Y. Wang, D. Gong, Y. Zhang, P. Wu and Y. Sun, ACS Cent. Sci., 2019, 5, 1834–1843 CrossRef CAS.
- J. C. Poshusta, R. D. Noble and J. L. Falconer, J. Membr. Sci., 2001, 186, 25–40 CrossRef CAS.
- M. H. Simonot-Grange, A. Waldeck, D. Barthomeuf and G. Weber, Thermochim. Acta, 1999, 329, 77–82 CrossRef CAS.
- M. Romero, J. C. Navarro, L. F. Bobadilla, M. I. Domínguez, S. Ivanova, F. Romero-Sarria, M. A. Centeno and J. A. Odriozola, Microporous Mesoporous Mater., 2020, 298, 110071 CrossRef CAS.
- R. U. Rehman, Q. Song, L. Peng, Y. Chen and X. Gu, Chin. J. Chem. Eng., 2019, 27, 2397–2406 CrossRef CAS.
- R. U. Rehman, Q. Song, L. Peng, Z. Wu and X. Gu, Chem. Eng. Res. Des., 2020, 153, 37–48 CrossRef CAS.
- H. Maghsoudi, Sep. Purif. Rev., 2016, 45, 169–192 CrossRef CAS.
- Y. Zhang, A. M. Avila, B. Tokay, H. H. Funke, J. L. Falconer and R. D. Noble, J. Membr. Sci., 2010, 358, 7–12 CrossRef CAS.
- S. Li, M. A. Carreon, Y. Zhang, H. H. Funke, R. D. Noble and J. L. Falconer, J. Membr. Sci., 2010, 352, 7–13 CrossRef CAS.
- Y. Lin and M. C. Duke, Curr. Opin. Chem. Eng., 2013, 2, 209–216 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |
