Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Ultrafast conversion of carcinogenic 4-nitrophenol into 4-aminophenol in the dark catalyzed by surface interaction on BiPO4/g-C3N4 nanostructures in the presence of NaBH4
Ahmed B. Azzam*a,
Ridha Djellabib,
Sheta M. Shetac and
S. M. El-Sheikhd
aFaculty of Science, Chemistry Department, Helwan University, Ain Helwan, Cairo 11795, Egypt. E-mail: ahmed_azzam2000@hotmail.com; Tel: +201285259709
bUniversità degli Studi di Milano, Dip. Chimica and INSTM-UdR Milano, Via Golgi, 19, 20133 Milano, Italy
cDepartment of Inorganic Chemistry, National Research Centre, 33, El-Behouth St., Dokki, Giza 12622, Egypt
dNanomaterials and Nanotechnology Department, Advanced Materials Division, Central Metallurgical R & D Institute (CMRDI), P. O. Box, 87 Helwan, 11421 Cairo, Egypt
First published on 15th September 2021
Abstract
Correction for ‘Ultrafast conversion of carcinogenic 4-nitrophenol into 4-aminophenol in the dark catalyzed by surface interaction on BiPO4/g-C3N4 nanostructures in the presence of NaBH4’ by Ahmed B. Azzam et al., RSC Adv., 2021, 11, 18797–18808. DOI: 10.1039/D1RA02852A.
The authors regret that some misleading statements were included in section 3.2.1 ‘Effect of initial concentration on 4-NP’. The corrected version of section 3.2.1 is presented below. There are no changes to Fig. 8 or its caption.
3.2.1 Effect of initial concentration of 4-NP
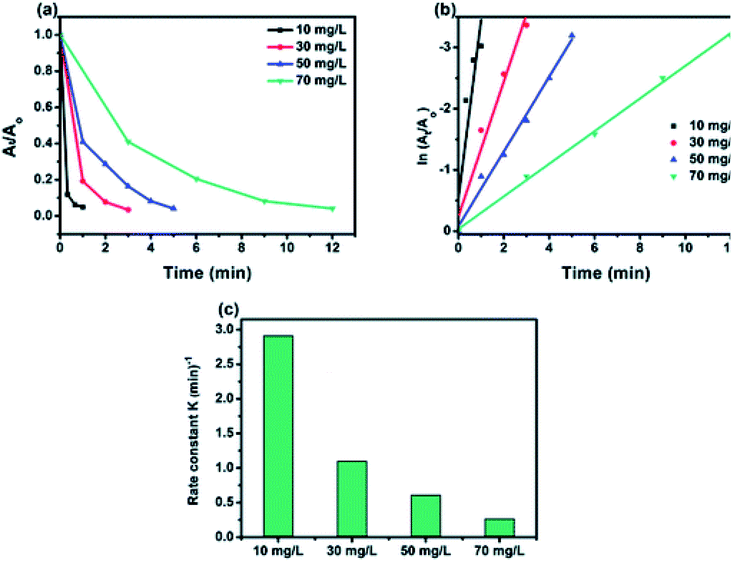
The effect of the initial concentration of 4-NP on the catalytic efficiency rate using 50% BiPO4/g-C3N4 catalyst was carried out by varying the concentration from 10 to 70 mg L−1, and the obtained results are shown in Fig. 8a. Interestingly, 50% BiPO4/g-C3N4 was able to reduce all 4-NP solutions at concentrations from 10 to 70 mg L−1, reflecting the high efficiency of such a catalyst towards this 4-NP reduction. At lower concentrations, a superior constant rate was recorded due to the availability of a large number of catalytic sites per given amount of 4-NP moles. And vice versa, the higher the concentration, the lower the rate constant (Fig. 8b), due to the high competition of 4-NP molecules on the limited sites. In addition, the number of molecules adsorbed at the surface of the BiPO4/g-C3N4 heterojunction increases with the increase in concentration of 4-nitrophenol and hence, the surface becomes saturated by 4-nitrophenol molecules. This leads to a decrease in concentration of BH4− ions approaching the surface of the BiPO4/g-C3N4 heterojunction, hence lowering the rate of hydrogen transfer from BH4− ion to the 4-nitrophenol molecule.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2021 |