Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of tertiary alkyl fluorides and chlorides by site-selective nucleophilic ring-opening reaction of α-aryl azetidinium salts†
Eiji Tayama *a and
Kohei Kawaib
*a and
Kohei Kawaib
aDepartment of Chemistry, Faculty of Science, Niigata University, Niigata, 950-2181, Japan. E-mail: tayama@chem.sc.niigata-u.ac.jp
bGraduate School of Science and Technology, Niigata University, Niigata, 950-2181, Japan
First published on 13th December 2021
Abstract
Site-selective nucleophilic ring-opening reactions of 2-arylazetidine-2-carboxylic acid ester-derived tetraalkyl ammonium salts 2 with tetrabutylammonium halides (Bu4NX) to give tertiary alkyl halides are successfully demonstrated. For example, a nucleophilic ring-opening reaction of 2-(o-tolyl) derivative 2a with 1.2 equivalents of tetrabutylammonium fluoride (Bu4NF) in THF at 60 °C preferentially proceeded at a more substituted carbon atom (2-position) compared to a less-substituted carbon atom (4-position) and afforded tert-butyl 4-(dimethylamino)-2-fluoro-2-(o-tolyl)butanoate 3aa in 71% yield as the corresponding tertiary alkyl fluoride. This result was applied to synthesize optically active organofluorine compounds starting from commercially available (R)-1-phenylethylamine.
Introduction
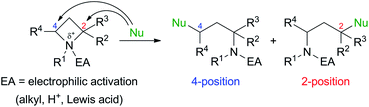
Ring-strained four-membered N-heterocycle azetidines are valuable building blocks in organic synthesis. Although they are chemically stable without any additives, nucleophilic ring-opening reactions proceed to give various types of functionalized nitrogen-containing compounds by electrophilic activation of the nitrogen atom by N-quaternization,1,2 or addition of Brønsted acid (H+)3 or Lewis acids4 (Scheme 1).5 These transformations are applicable for the synthesis of amino acids, alkaloids, and biologically active drugs.The initial studies of this ring-opening reaction were mainly performed by Couty's group using tetraalkylazetidinium salts as substrates.1 One point to consider in this reaction is site-selectivity at the 2- and 4-positions, which reacts with a nucleophile (Nu). In many cases, a less-substituted and/or electron-deficient carbon atom is attacked by a nucleophile because of the SN2 process. For example, a reaction of a substrate with a nucleophile in Scheme 1 proceeded at the 4-position preferentially to afford the corresponding product. However, some nucleophiles do not act according to this tendency, and the reaction occurs at the 2-position, which is a much-substituted carbon atom. Although these phenomena are currently difficult to explain, the site selectivity at the 2- and 4-positions can be determined based on the properties of nucleophiles, substituents at the 2- and 4-positions, and reaction conditions.1d,g,h Previously, our group reported that the site-selective nucleophilic ring-opening reaction of α-arylazetidine-2-carboxylic acid ester-derived tetraalkylammonium salt (S)-2b prepared from 95% ee of (S)-1b (Scheme 2, Our previous work).6 Cesium acetate (AcOCs) and dimethylamine (Me2NH) as nucleophiles reacted at the 4-position. In contrast, sodium azide (NaN3) reacted at the 2-position with inversion of the configuration. This result shows that the SN2 substitution at the tertiary carbon atom (2-position) proceeded.7 With the results, our group started to further investigate the scope of this reaction, since some nucleophiles such as fluoride (F−) provide valuable compounds. Furthermore, previous examples of the ring-opening reaction of azetidine derivatives with F− to give organofluorine compounds are rare2a,8 compared to the reaction of three-membered N-heterocycle aziridine derivatives.9 Herein, we wish to report the site-selective nucleophilic ring-opening reaction of α-aryl azetidinium salts 2 with halides to afford α-aryl-α-halo-carboxylic acid esters 3 (Scheme 2, this work). Further synthetic applications of the resulting products 3, e.g., asymmetric synthesis of organofluorine compounds, are also demonstrated.
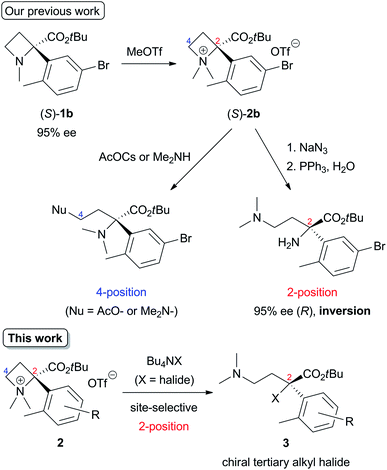 | ||
| Scheme 2 Nucleophilic ring-opening of α-arylazetidine-2-carboxylic acid ester-derived tetraalkylammonium salts 2. | ||
Results and discussion
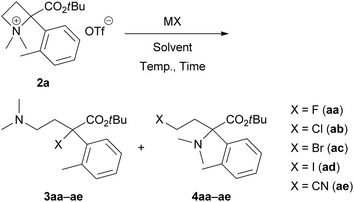
We started investigating the nucleophilic ring-opening reaction of 2a with a halide source (Table 1). First, the reaction of 2a with sodium fluoride (NaF) as an F− source in DMF at room temperature for 2 h was examined to obtain the corresponding organofluorine compounds 3aa and 4aa; however, no products were obtained (entry 1). Although a reaction with potassium fluoride (KF) gave the same result (entry 2), the use of cesium fluoride (CsF) afforded 3aa in 13% yield (entry 3). We expected that tetrabutylammonium fluoride (Bu4NF) might be more reactive, and its solubility in organic solvents would improve the yields of 3aa and 4aa. In addition, Ghorai et al. reported the Lewis acid-promoted nucleophilic ring-opening reaction of N-tosylazetidines with tetrabutylammonium chloride (Bu4NCl) and bromide (Bu4NBr).10 Thus, we attempted a reaction with a THF solution of Bu4NF, and the desired 3aa was obtained in 33% yield with trace amounts of 4aa (<4% yield) (entry 4). The use of THF as a solvent and other F− sources, such as Bu4NF·3H2O, did not show any improvements (entries 5 and 6). We found that the yield of 3aa could be improved to 71% with minimization of the formation of 4aa (7% yield) when the reaction was performed at 60 °C (entry 7).| Entry | MX (equiv.) | Solvent | Temp., time | 3a (%) | 4a (%) |
|---|---|---|---|---|---|
| a Isolated yield. | |||||
| 1 | NaF (5) | DMF | rt, 2 h | 0 | 0 |
| 2 | KF (5) | DMF | rt, 2 h | 0 | 0 |
| 3 | CsF (5) | DMF | rt, 2 h | 13 | 0 |
| 4 | Bu4NF in THF (1.2) | DMF | rt, 2 h | 33 | <4 |
| 5 | Bu4NF in THF (1.2) | THF | rt, 2 h | 35 | 2 |
| 6 | Bu4NF·3H2O (1.2) | THF | rt, 2 h | 41 | <3 |
| 7 | Bu4NF in THF (1.2) | THF | 60 °C, 1 h | 71 | 7 |
| 8 | Bu4NCl (1.2) | DMF | rt, 2 h | 74 | 14 |
| 9 | Bu4NCl (1.2) | THF | rt, 2 h | 76 | 23 |
| 10 | Bu4NCl (1.2) | CH2Cl2 | rt, 2 h | 69 | 14 |
| 11 | Bu4NCl (1.2) | THF | 0 °C, 2 h | 34 | 10 |
| 12 | Bu4NCl (1.2) | THF | 60 °C, 2 h | 70 | 27 |
| 13 | Bu4NBr (1.2) | THF | rt, 1 h | 61 | 21 |
| 14 | Bu4NI (1.2) | THF | rt, 1 h | 0 | 0 |
| 15 | KCN (5) | DMF | rt, 2 h | 42 | 55 |
| 16 | Bu4NCN (1.2) | THF | rt, 2 h | 38 | 62 |
Next, we examined the same reaction with other tetrabutylammonium salts (Bu4NX) to define the scope of this site-selective ring-opening reaction. Reactions with Bu4NCl in THF, DMF and CH2Cl2 proceeded even at room temperature, and similar yields of 3ab (69–76% yields) and 4ab (14–23% yields) were observed (entries 8–10). At 0 °C, the yields of 3ab (34% yield) and 4ab (10% yield) decreased (entry 11). When the reaction was performed at 60 °C, the yield of undesired 4ab was slightly improved (27% yield) (entry 12). The use of Bu4NBr is also applicable; however, the selectivity between 3ac (61% yield) and 4ac (21% yield) was insufficient (entry 13). Additionally, the resulting isolated bromo products 3ac and 4ac were unstable because of the self-N-quaternization. Therefore, a reaction with tetrabutylammonium iodide (Bu4NI) did not give 3ad and 4ad (entry 14). Finally, we applied this reaction for pseudohalogen salts (MCN) to provide α-cyano derivative 3ae with an all-carbon quaternary stereocentre (entries 15 and 16). Unfortunately, both reactions with potassium cyanide (KCN) and tetrabutylammonium cyanide (Bu4NCN) gave similar results to provide 3ae and 4ae without selectivities.11
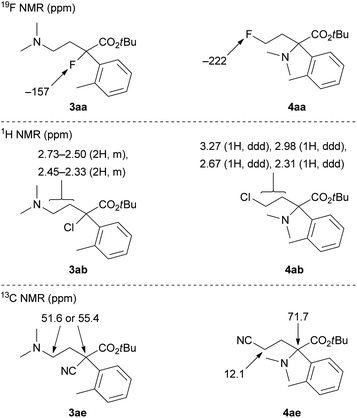
The ring-opening products 3 and 4 in Table 1 were assigned by NMR analyses, and their representative results are shown in Fig. 1. Fluorine derivatives 3aa and 4aa were clearly identified by the 19F NMR analysis. Tertiary alkyl fluoride 3aa showed a chemical shift of −157 ppm. Primary alkyl fluoride 4aa showed a chemical shift of −222 ppm. These values are reasonable for the corresponding alkyl fluorides. In contrast, chlorine derivatives 3ab and 4ab did not show clear differences in 1H and 13C NMR analyses. Consequently, we assigned these by comparison of 1H NMR chemical shifts of methylene protons. Primary alkyl chloride 4ab had low-field chemical shifts due to an electron-withdrawing effect of chloride. One of the two products (3ab or 4ab) with chemical shifts of 3.27 and 2.98 ppm was assigned to 4ab. Another product was assigned to tertiary alkyl chloride 3ab, which showed chemical shifts of 2.73–2.33 ppm. Bromine derivatives 3ac and 4ac were assigned by analogy to 3ab and 4ab. Meanwhile, nitrile derivatives 3ae and 4ae could be clearly identified by 13C NMR analysis. 4ae showed a chemical shift of 12.1 ppm, which is a reasonable value as a primary nitrile.1d
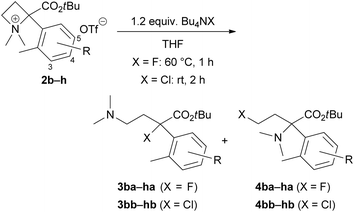
To define the scope and limitations of this site-selective ring-opening reaction to produce tertiary alkyl halides 3, we prepared various azetidinium salts 2b–h and examined their reactions with Bu4NF or Bu4NCl under identical conditions (Table 2). First, we attempted the reactions of 5-substituted aryl derivatives 2a–e with Bu4NF and obtained the corresponding organofluorine compounds 3ba–ea in moderate yields (entries 1–4). The minor products 4 were not isolated (N.D.), although their formations were observed by TLC analysis. The pure products of these organofluorine 4 for spectroscopic characterizations were difficult to isolate because of small amounts (ca. 5% yield). Electron-withdrawing substituents on the α-aryl substituent, such as bromo (2b) and trifluoromethyl (2c), might be desirable to yield 3 (entries 1 and 2, approximately 75%). Reactions of methyl (2d) and methoxy (2e) derivatives resulted in lower yields of 3 (entries 3 and 4, approximately 60%). Thus, we next examined the reactions of 4-bromo (2f) and 4-trifluoromethyl (2g) derivatives and obtained 3fa–ga in approximately 70% yields (entries 5 and 6). However, the reaction of 3-bromo derivative 2h was resulted in a 58% yield of 3ha (entry 7). The use of Bu4NCl for the reactions of 2b, 2d, 2f, and 2h provided the corresponding organochlorine compounds 3bb–hb (entries 8–11) with a similar tendency to the reaction with Bu4NF. In these cases, the minor products 4bb–hb could be isolated as a pure form to perform their spectroscopic characterizations.
| Entry | X | R | 3a (%) | 4a,b (%) | |
|---|---|---|---|---|---|
| a Isolated yields.b N.D. = not determined. | |||||
| 1 | F | 5-Br | 2b | 77 (3ba) | N.D. |
| 2 | F | 5-CF3 | 2c | 75 (3ca) | N.D. |
| 3 | F | 5-Me | 2d | 59 (3da) | N.D. |
| 4 | F | 5-OMe | 2e | 61 (3ea) | N.D. |
| 5 | F | 4-Br | 2f | 72 (3fa) | N.D. |
| 6 | F | 4-CF3 | 2g | 68 (3ga) | N.D. |
| 7 | F | 3-Br | 2h | 58 (3ha) | N.D. |
| 8 | Cl | 5-Br | 2b | 82 (3bb) | 17 (4bb) |
| 9 | Cl | 5-Me | 2d | 53 (3db) | 19 (4db) |
| 10 | Cl | 4-Br | 2f | 83 (3fb) | 17 (4fb) |
| 11 | Cl | 3-Br | 2h | 65 (3hb) | 33 (4hb) |
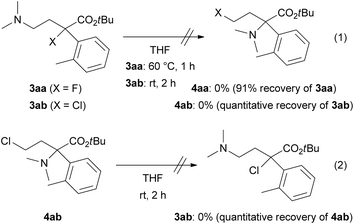
We confirmed the chemical stability of products 3 and 4 (Scheme 3) because a transformation between 3 and 4 might proceed via the formation of ammonium salts generated from the alkyl halides and dimethylamino substituents as in the products (self-N-quaternization). A THF solution of tertiary alkyl halides 3aa (X = F) or 3ab (X = Cl) was subjected to the reaction temperature depicted in Table 1. The removal of THF by evaporation and 1H NMR analysis of the residue did not show any formation of 4aa or 4ab, respectively (eqn (1)). Similarly, a stirring at room temperature of a THF solution of primary alkyl chloride 4ab did not afford 3ab (eqn (2)).
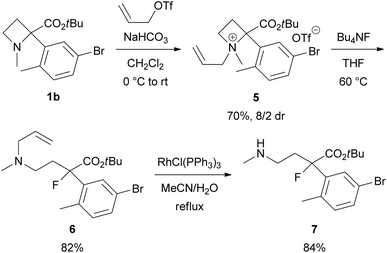
The N,N-dimethylamino substituent, as in product 3, is not synthetically valuable because of the impossibility of removing the N-methyl substituents. One N-methyl substituent could be changed into an N-allyl, which would be removable via Rh-catalysed isomerization, by N-quaternization of 1 with allyl triflate12 (Scheme 4). For example, azetidinium salt 5 was prepared from 1b in 70% yield as an 8/2 mixture of diastereomers followed by ring-opening with Bu4NF to provide N-allyl derivative 6 in 82% yield. Rh-catalyzed deallylation of 6 gave secondary amine 7 in 84% yield.
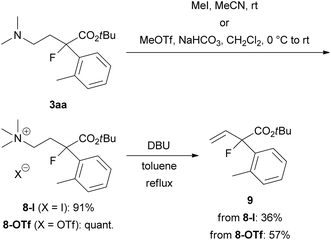
To demonstrate the utility of this ring-opening reaction, we attempted further synthetic transformations of organofluorine product 3aa. First, Hofmann elimination of 3aa to produce α-aryl-α-fluoro-α-vinylacetic acid ester 9 was examined (Scheme 5). N-Quaternization with iodomethane (MeI) or methyl trifluoromethanesulfonate (MeOTf) gave 8-I or 8-OTf in good yields (8-I: 91% yield, 8-OTf: quant.). Treatment of iodide salt 8-I with 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in refluxing toluene for 1 day gave desired 9 in 36% yield. We expected that the iodide ion in the reaction mixture might cause undesirable side reactions such as nucleophilic substitutions, and the reaction resulted in a low yield. Thus, we examined the same reaction using triflate salt 8-OTf. As expected, the yield of 9 was improved to 57%.
Next, the synthesis of optically active tertiary organofluorine compounds from chiral (R)-1-phenylethylamine, which is one of the least expensive chiral sources, was examined (Scheme 6). 93% ee of (S)-1a was prepared according to our previous work.6,13 N-Quaternization of (S)-1a with MeOTf to prepare (S)-2a (quant.) followed by the ring-opening reaction with Bu4NF under the conditions in Table 1 afforded (R)-3aa (68% yield). The ee of the obtained 3aa was determined after conversion into (R)-11 because of the low sensitivity of 3aa towards a UV/vis detector in chiral HPLC analysis. Reduction of (R)-3aa with LiAlH4 to amino alcohol (R)-10 (73% yield) followed by O-benzoylation gave benzoate (R)-11 (95% yield). The ee of (R)-11 was determined to be 93% ee by the chiral HPLC analysis. No lack of the ee was confirmed during the transformations from (S)-1a into (R)-11. This result indicates that the Bu4NF-promoted ring-opening reaction of (S)-2a affording (R)-3aa proceeds by inverting the tertiary carbon configuration (SN2) in the same manner as the reaction of (S)-2b with NaN3, which was previously reported by our group.6 Therefore, the absolute configuration of 3aa was determined to be (R).
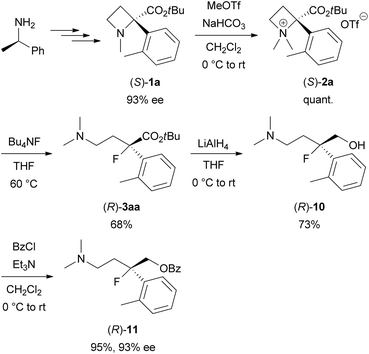 | ||
| Scheme 6 Synthesis of optically active organofluorine compound (R)-3aa starting from (R)-1-phenylethylamine. | ||
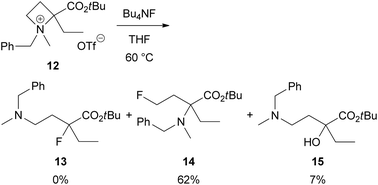
To clarify that the α-aryl substituent as in 2 is necessary for this site-selective ring-opening reaction to produce 3, we investigated a reaction α-ethyl derivative 12 with Bu4NF (Scheme 7). As expected, the reaction proceeded at 4-position preferentially to give γ-fluoro product 14 in 62% yield. Identifiable amount of the corresponding α-fluoro product 13 was not obtained. Instead, α-hydroxy derivative 15, which might be derived from 13, was isolated in 7% yield.
Couty's group described in the previous literature1d that the nucleophilic ring-opening of α,α-disubstituted azetidinium ions at the quaternary α-carbon (2-position) is intrinsically favoured. Steric repulsions generated by substituents as in the azetidine ring affect the site-selectivity. The highly nucleophilic azide anion (N3−) reacts at 2-position, the less nucleophilic cyanide anion (CN−) reacts at 2- and 4-positions, and the poor nucleophilic acetate anion (AcO−) reacts at 4-position. The exact reason of the site-selective ring-opening reaction to produce 3 demonstrated by our group are difficult to explain at present, a size of the nucleophiles might affect the site-selectivity. F− and Cl− are small and enable to react at the quaternary α-carbon (2-position) although they are poor nucleophilic anion. Further experimental studies are needed to discuss.
Conclusions
In conclusion, we described that the site-selective nucleophilic ring-opening reaction of 2-arylazetidine-2-carboxylic acid ester-derived ammonium salts 2 with Bu4NF or Bu4NCl proceeded at a much-substituted 2-position preferentially over a less-substituted 4-position and produced the corresponding tertiary alkyl fluorides and chlorides 3. Our result is a rare successful example of the fluoride ion-promoted ring-opening reaction of azetidine derivatives that yields organofluorine compounds. Further synthetic transformations of the product 3 were also successfully demonstrated. Our protocol enables the production of optically active organofluorine compound (R)-3aa starting from commercially available chiral (R)-1-phenylethylamine, which is an inexpensive chiral compound.Experimental
General
Specific rotations were recorded on a JASCO polarimeter P-1010. Normal phase chiral HPLC analyses were performed using a JASCO HPLC pump (PU-2089) and a UV/vis detector (UV-2075). Infrared spectra (IR) were recorded on a JASCO FT/IR-4600 spectrometer. 1H, 13C and 19F NMR spectra were measured on a Varian (1H: 400 MHz, 13C: 101 MHz, 19F: 376 MHz) or a Bruker (1H: 400 MHz, 13C: 101 MHz) spectrometer. 19F NMR analysis were performed for representative products. As an internal standard in CDCl3, Me4Si (δ 0 ppm) for 1H NMR and CDCl3 (δ 77.00 ppm) for 13C NMR were used. As an internal standard in acetone-d6, the residual protons (δ 2.05 ppm) for 1H NMR and acetone-d6 (δ 29.92 ppm) for 13C NMR were used. In 19F NMR, hexafluorobenzene (C6F6) was used as an internal standard (δ −162.9 ppm). In 1H and 13C NMR, the splitting patterns are denoted as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; and br, broad peak. In 19F NMR, the splitting patterns are not denoted. High-resolution mass spectra (ESI) were measured on a Thermo Fisher Scientific LC/FT-MS spectrometer. Reactions involving air- or moisture-sensitive compounds were conducted in appropriate round-bottomed flasks with a magnetic stirring bar under an argon (Ar) atmosphere. A 1 M tetrabutylammonium fluoride (Bu4NF) THF solution was purchased from Tokyo Chemical Industry Co., Ltd. (TCI). Anhydrous tetrahydrofuran (THF) was purchased from KANTO Chemical Co., Inc. For the thin layer chromatography (TLC) analysis throughout this work, Silicagel 70 TLC Plate-Wako purchased from FUJIFILM Wako Chemical Corporation was used. The products were purified by column chromatography on silica gel (Wakosil 60, 64–210 μm) purchased from FUJIFILM Wako Chemical Corporation. For strong basic compound such as (S)-10, NH TLC plates and amino-functionalized silica gel (Chromatorex NH-DM1020) purchased from Fuji Silysia Chemical Ltd. (Japan) were used.Representative procedure for ring-opening of 2a with Bu4NF in THF to afford 3aa and 4aa (Table 1, entry 7)
A solution of 2-(tert-butoxycarbonyl)-1,1-dimethyl-2-(o-tolyl)azetidin-1-ium trifluoromethanesulfonate (2a) (62.3 mg, 0.146 mmol) in THF (0.55 mL) was stirred at 60 °C under an Ar atmosphere and treated with a 1 M Bu4NF THF solution (175 μL, 0.175 mmol). After stirring for 1 h at 60 °C, the resulting mixture was cooled to room temperature and diluted with H2O. The mixture was extracted with cyclohexane and the combined extracts were washed with H2O. The organic solution was dried over Na2SO4 and concentrated by evaporation. The residue was purified by chromatography on silica gel (CH2Cl2/MeOH = 100/0 to 10/1 as the eluent, Rf: 3aa < 4aa) to obtain tert-butyl 4-(dimethylamino)-2-fluoro-2-(o-tolyl)butanoate (3aa) (30.7 mg, 71% yield) as a colourless oil and tert-butyl 2-(dimethylamino)-4-fluoro-2-(o-tolyl)butanoate (4aa) (3.1 mg, 7% yield) as a colourless oil. 3aa: IR (ATR) νmax/cm−1 3063, 2976, 2938, 2862, 2818, 2766, 1748, 1731, 1459, 1392, 1368, 1281, 1250, 1148, 1091, 1064, 1042, 988, 965, 939, 844, 749; 1H NMR (400 MHz, CDCl3) δ 7.39 (1H, ddd, J = 7.6, 1.4, 1.4 Hz, ArH), 7.26–7.12 (3H, m, ArH), 2.68–2.37 (4H, m, CH2), 2.43 (3H, d, 5JFH = 3.6 Hz, ArCH3), 2.28 (6H, s, N(CH3)2), 1.42 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 169.2 (d, 2JFC = 27 Hz), 136.8 (d, 3JFC = 2 Hz), 136.1 (d, 2JFC = 21 Hz), 132.1 (d, JFC = 1 Hz), 128.5, 126.1 (d, 3JFC = 7 Hz), 125.6, 96.5 (d, 1JFC = 188 Hz), 82.6, 53.7 (d, 3JFC = 5 Hz), 45.5, 34.7 (d, 2JFC = 22 Hz), 27.7, 20.7 (d, 4JFC = 7 Hz); 19F NMR (376 MHz, CDCl3) δ −157; HRMS (ESI): calcd for C17H27FNO2 [M + H]+ 296.2020, found 296.2016. 4aa: IR (ATR) νmax/cm−1 3061, 2978, 2930, 2874, 2837, 2796, 1713, 1474, 1456, 1392, 1366, 1305, 1289, 1240, 1207, 1153, 1130, 1080, 1049, 1011, 986, 950, 883, 844, 814, 782, 749; 1H NMR (400 MHz, CDCl3) δ 7.43–7.37 (1H, m, ArH), 7.18–7.08 (3H, m, ArH), 4.24 (1H, dddd, 2JFH = 47.2 Hz, J = 9.4, 9.2, 5.4 Hz, 4H), 4.06 (1H, dddd, 2JFH = 46.8 Hz, J = 9.6, 9.2, 5.6 Hz, 4H), 2.66 (1H, dddd, 3JFH = 15.3 Hz, J = 14.2, 9.6, 5.4 Hz, 3H), 2.42–2.24 (1H, m, 3H), 2.36 (6H, s, N(CH3)2), 2.35 (3H, s, ArCH3), 1.54 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 168.5, 137.8, 136.2, 132.2, 128.1, 127.0, 124.9, 81.9, 81.5 (d, 1JFC = 161 Hz), 71.4 (d, 3JFC = 11 Hz), 40.0, 34.5 (d, 2JFC = 21 Hz), 28.5, 21.1; 19F NMR (376 MHz, CDCl3) δ −222; HRMS (ESI): calcd for C17H27FNO2 [M + H]+ 296.2020, found 296.2013.Representative procedure for ring-opening of 2a with Bu4NCl in THF to afford 3ab and 4ab (Table 1, entry 9)
Bu4NCl (230 mg, 0.828 mmol) was added to a solution of 2a (293 mg, 0.689 mmol) in THF (3.6 mL) at room temperature and the mixture was degassed under reduced pressure and filled with an Ar. After stirring for 2 h, the resulting mixture was diluted with H2O. The mixture was extracted with n-hexane/EtOAc = 3/1 mixed solvent and the combined extracts were washed with H2O. The organic solution was dried over Na2SO4 and concentrated by evaporation. The residue was purified by chromatography on silica gel (CH2Cl2/MeOH = 100/0 to 30/1 as the eluent, Rf: 3ab < 4ab) to obtain tert-butyl 2-chloro-4-(dimethylamino)-2-(o-tolyl)butanoate (3ab) (163 mg, 76% yield) as a pale yellow oil and tert-butyl 4-chloro-2-(dimethylamino)-2-(o-tolyl)butanoate (4ab) (49.8 mg, 23% yield) as a colourless oil. 3ab: IR (ATR) νmax/cm−1 3062, 2976, 2939, 2861, 2818, 2766, 1732, 1458, 1392, 1368, 1254, 1145, 1080, 1028, 967, 934, 898, 843, 752, 722, 694; 1H NMR (400 MHz, CDCl3) δ 7.60 (1H, dd, J = 7.0, 1.8 Hz, ArH), 7.26–7.13 (3H, m, ArH), 2.73–2.50 (2H, m, CH2), 2.45–2.33 (2H, m, CH2), 2.35 (3H, s, ArCH3), 2.24 (6H, s, N(CH3)2), 1.43 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 169.2, 138.1, 135.9, 132.0, 128.1, 126.2, 125.7, 83.0, 73.7, 55.1, 45.7, 38.0, 27.6, 20.5; HRMS (ESI): calcd for C17H27ClNO2 [M + H]+ 312.1725, found 312.1715. 4ab: IR (ATR) νmax/cm−1 3060, 2977, 2931, 2872, 2836, 2795, 1712, 1479, 1454, 1392, 1366, 1336, 1294, 1230, 1151, 1081, 1046, 981, 961, 893, 843, 821, 774, 755, 725; 1H NMR (400 MHz, CDCl3) δ 7.41 (1H, d, J = 6.8 Hz, ArH), 7.19–7.09 (3H, m, ArH), 3.27 (1H, ddd, J = 12.0, 10.9, 4.3 Hz, 4H), 2.98 (1H, ddd, J = 12.1, 10.9, 5.2 Hz, 4H), 2.67 (1H, ddd, J = 13.9, 12.1, 4.3 Hz, 3H), 2.35 (6H, s, N(CH3)2), 2.33 (3H, S, ArCH3), 2.31 (1H, ddd, J = 13.9, 12.0, 5.2 Hz, 3H), 1.54 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 168.3, 137.4, 136.1, 132.3, 128.3, 127.0, 124.9, 82.0, 72.3, 40.8, 40.0, 37.7, 28.5, 21.1; HRMS (ESI): calcd for C17H27ClNO2 [M + H]+ 312.1725, found 312.1719.Ring-opening of 2a with Bu4NBr in THF to afford 3ac and 4ac (Table 1, entry 13)
The procedure was similar to the synthesis of 3ab and 4ab. The reaction was performed at room temperature for 1 h using 2a (67.2 mg, 0.158 mmol) as a substrate. Purification by chromatography on silica gel (CH2Cl2/MeOH = 100/0 to 30/1 as the eluent, Rf: 3ac < 4ac) gave tert-butyl 2-bromo-4-(dimethylamino)-2-(o-tolyl)butanoate (3ac) (34.1 mg, 61% yield) as a colourless oil and tert-butyl 4-bromo-2-(dimethylamino)-2-(o-tolyl)butanoate (4ac) (12.1 mg, 21% yield) as a colourless oil. 3ac: IR (ATR) νmax/cm−1 3062, 2975, 2938, 2860, 2818, 2765, 1726, 1681, 1457, 1392, 1367, 1252, 1144, 1078, 1040, 1028, 965, 889, 843, 791, 751, 721, 689; 1H NMR (400 MHz, CDCl3) δ 7.68–7.61 (1H, m, ArH), 7.24–7.10 (3H, m, ArH), 2.72 (1H, ddd, J = 14.0, 11.0, 4.8 Hz, CH2), 2.64 (1H, ddd, J = 14.0, 10.8, 4.8 Hz, CH2), 2.44 (1H, ddd, J = 12.0, 10.8, 4.8 Hz, CH2), 2.38–2.28 (1H, m, CH2), 2.33 (3H, s, ArCH3), 2.25 (6H, s, N(CH3)2), 1.45 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 169.3, 138.0, 135.4, 131.9, 128.2, 127.8, 125.8, 83.0, 69.2, 56.2, 45.6, 38.7, 27.5, 20.7; HRMS (ESI): calcd for C17H27BrNO2 [M + H]+ 356.1220, found 356.1218. 4ac: IR (ATR) νmax/cm−1 3060, 2977, 2931, 2872, 2835, 2795, 1712, 1476, 1455, 1392, 1367, 1328, 1292, 1249, 1238, 1212, 1151, 1108, 1077, 1045, 1007, 979, 957, 892, 843, 819, 754; 1H NMR (400 MHz, CDCl3) δ 7.41 (1H, d, J = 7.2 Hz, ArH), 7.19–7.09 (3H, m, ArH), 3.11 (1H, ddd, J = 12.7, 9.0, 3.6 Hz, 4H), 2.84 (1H, ddd, J = 13.1, 9.0, 4.1 Hz, 4H), 2.75 (1H, ddd, J = 13.1, 12.8, 3.6 Hz, 3H), 2.39 (1H, ddd, J = 12.8, 12.7, 4.1 Hz, 3H), 2.35 (6H, s, N(CH3)2), 2.33 (3H, s, ArCH3), 1.54 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 168.2, 137.3, 136.1, 132.3, 128.5, 127.0, 124.9, 82.0, 73.2, 40.0, 38.2, 28.9, 28.4, 21.1; HRMS (ESI): calcd for C17H27BrNO2 [M + H]+ 356.1220, found 356.1217.Ring-opening of 2a with KCN in DMF to afford 3ae and 4ae (Table 1, entry 15)
KCN (50.5 mg, 0.775 mmol) was added to a solution of 2a (67.0 mg, 0.157 mmol) in DMF (0.8 mL) at room temperature. The mixture was degassed under reduced pressure and filled with Ar. After stirring for 2 h under an Ar atmosphere, the resulting mixture was diluted with H2O and extracted with n-hexane/EtOAc = 3/1 mixed solvent. The combined extracts were washed with brine, dried over Na2SO4, and concentrated by evaporation. Purification of the residue by chromatography on silica gel (CH2Cl2/MeOH = 100/0 to 10/1 as the eluent, Rf: 3ae < 4ae) to obtain tert-butyl 2-cyano-4-(dimethylamino)-2-(o-tolyl)butanoate (3ae) (20.1 mg, 42% yield) as pale yellow crystals and tert-butyl 4-cyano-2-(dimethylamino)-2-(o-tolyl)butanoate (4ae) (26.3 mg, 55% yield) as a colourless crystals. 3ae: mp 29–31 °C; IR (ATR) νmax/cm−1 3065, 2977, 2941, 2863, 2820, 2769, 2240, 1734, 1459, 1393, 1369, 1244, 1147, 1098, 1041, 969, 936, 838, 753, 730; 1H NMR (400 MHz, CDCl3) δ 7.40 (1H, dd, J = 7.8, 1.8 Hz, ArH), 7.30–7.19 (3H, m, ArH), 2.65–2.50 (3H, m, CH2), 2.48 (3H, s, ArCH3), 2.40 (1H, ddd, J = 11.4, 8.6, 4.4 Hz, CH2), 1.47 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 166.7, 136.4, 133.1, 132.3, 128.5, 126.5, 126.4, 118.5, 84.2, 55.4, 51.6, 45.5, 33.9, 27.5, 20.3; HRMS (ESI): calcd for C18H27N2O2 [M + H]+ 303.2067, found 303.2057. 4ae: mp 31–33 °C; IR (ATR) νmax/cm−1 3061, 2977, 2933, 2875, 2837, 2796, 2246, 1708, 1475, 1455, 1441, 1392, 1367, 1234, 1151, 1083, 1041, 1028, 987, 968, 915, 873, 842, 813, 756; 1H NMR (400 MHz, CDCl3) δ 7.42–7.36 (1H, m, ArH), 7.21–7.11 (3H, m, ArH), 2.64–2.53 (1H, m, CH2), 2.34 (6H, s, N(CH3)2), 2.32 (3H, s, ArCH3), 2.20–2.07 (2H, m, CH2), 1.92–1.75 (1H, m, CH2), 1.54 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 168.0, 136.6, 135.9, 132.5, 128.6, 127.4, 125.0, 120.1, 82.3, 71.7, 40.0, 30.3, 28.4, 21.0, 12.1; HRMS (ESI): calcd for C18H27N2O2 [M + H]+ 303.2067, found 303.2058.tert-Butyl 2-(5-bromo-2-methylphenyl)-4-(dimethylamino)-2-fluorobutanoate (3ba) (Table 2, entry 1)
Obtained from 2b (76.0 mg, 0.151 mmol) by the same procedure with 3aa. Purification by chromatography on silica gel (CH2Cl2/MeOH = 30/1 to 20/1 as the eluent) gave 3ba (43.3 mg, 77% yield) as a pale yellow oil. IR (ATR) νmax/cm−1 2976, 2938, 2861, 2818, 2767, 1749, 1731, 1592, 1565, 1481, 1459, 1391, 1368, 1283, 1251, 1218, 1149, 1114, 1092, 1041, 989, 969, 944, 876, 842, 809, 769, 738, 704; 1H NMR (400 MHz, CDCl3) δ 7.53 (1H, dd, J = 1.8, 1.8 Hz, ArH), 7.35 (1H, dd, J = 8.0, 1.8 Hz, ArH), 7.03 (1H, d, J = 8.0 Hz, ArH), 2.73–2.30 (4H, m, CH2), 2.37 (3H, d, 5JFH = 3.6 Hz, ArCH3), 2.27 (6H, s, N(CH3)2), 1.43 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 168.4 (d, 2JFC = 27 Hz), 138.3 (d, 2JFC = 21 Hz), 135.5 (d, 3JFC = 2 Hz), 133.7, 131.4, 129.1 (d, 3JFC = 9 Hz), 119.3, 95.8 (d, 1JFC = 190 Hz), 83.1, 53.4 (d, 3JFC = 5 Hz), 45.4, 34.4 (d, 2JFC = 22 Hz), 27.7, 20.2 (d, 4JFC = 7 Hz); HRMS (ESI): calcd for C17H26BrFNO2 [M + H]+ 374.1125, found 374.1124.tert-Butyl 4-(dimethylamino)-2-fluoro-2-(2-methyl-5-(trifluoromethyl)phenyl)butanoate (3ca) (Table 2, entry 2)
Obtained from 2c (167 mg, 0.338 mmol) by the same procedure with 3aa. Purification by chromatography on silica gel (CH2Cl2/MeOH = 30/1 to 20/1 as the eluent) gave 3ca (92.2 mg, 75% yield) as a pale yellow oil. IR (ATR) νmax/cm−1 2979, 2942, 2864, 2821, 2769, 1752, 1732, 1621, 1460, 1393, 1370, 1331, 1286, 1252, 1151, 1120, 1091, 1040, 1006, 990, 976, 948, 896, 842, 829, 771, 748, 721; 1H NMR (400 MHz, CDCl3) δ 7.66 (1H, s, ArH), 7.49 (1H, d, J = 8.0 Hz, ArH), 7.29 (1H, d, J = 8.0 Hz, ArH), 2.78–2.31 (4H, m, CH2), 2.49 (3H, d, 5JFH = 3.2 Hz, ArCH3), 2.28 (6H, s, N(CH3)2), 1.43 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 168.4 (d, 2JFC = 27 Hz), 140.9 (d, 3JFC = 2 Hz), 137.2 (d, 2JFC = 22 Hz), 132.6, 128.1 (q, 2JFC = 33 Hz), 125.3–125.0 (m), 124.1 (q, 1JFC = 273 Hz), 123.2 (dq, 3JFC, 3JFC = 8, 4 Hz), 96.0 (d, 1JFC = 190 Hz), 83.1, 53.4 (d, 3JFC = 5 Hz), 45.4, 34.7 (d, 2JFC = 22 Hz), 27.6, 20.7 (d, 4JFC = 8 Hz); HRMS (ESI): calcd for C18H26F4NO2 [M + H]+ 364.1894, found 364.1876.tert-Butyl 4-(dimethylamino)-2-(2,5-dimethylphenyl)-2-fluorobutanoate (3da) (Table 2, entry 3)
Obtained from 2d (105 mg, 0.239 mmol) by the same procedure with 3aa. Purification by chromatography on silica gel (CH2Cl2/MeOH = 30/1 to 20/1 as the eluent) gave 3da (43.8 mg, 59% yield) as a pale yellow oil. IR (ATR) νmax/cm−1 2976, 2936, 2862, 2818, 2766, 1749, 1731, 1499, 1459, 1392, 1368, 1285, 1250, 1149, 1090, 1040, 990, 952, 844, 811, 771, 748, 707; 1H NMR (400 MHz, CDCl3) δ 7.19 (1H, s, ArH), 7.07–6.99 (2H, m, ArH), 2.67–2.39 (4H, m, CH2), 2.38 (3H, d, 5JFH = 4.0 Hz, ArCH3), 2.32 (3H, s, ArCH3), 2.29 (6H, s, N(CH3)2), 1.43 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 169.2 (d, 2JFC = 26 Hz), 135.8 (d, 2JFC = 21 Hz), 134.9, 133.4 (d, 3JFC = 2 Hz), 132.0 (d, JFC = 2 Hz), 129.1, 126.8 (d, 3JFC = 8 Hz), 96.5 (d, 1JFC = 187 Hz), 82.6, 53.6 (d, 3JFC = 5 Hz), 45.4, 34.6 (d, 2JFC = 22 Hz), 27.7, 21.1, 20.2 (d, 4JFC = 8 Hz); HRMS (ESI): calcd for C18H29FNO2 [M + H]+ 310.2177, found 310.2163.tert-Butyl 4-(dimethylamino)-2-fluoro-2-(5-methoxy-2-methylphenyl)butanoate (3ea) (Table 2, entry 4)
Obtained from 2e (127 mg, 0.279 mmol) by the same procedure with 3aa. Purification by chromatography on silica gel (CH2Cl2/MeOH = 30/1 to 20/1 as the eluent) gave 3ea (55.4 mg, 61% yield) as a pale yellow oil. IR (ATR) νmax/cm−1 2976, 2938, 2862, 2818, 2766, 1748, 1731, 1612, 1577, 1498, 1459, 1392, 1368, 1290, 1249, 1149, 1090, 1078, 1041, 978, 957, 863, 844, 810, 771, 746, 735, 708; 1H NMR (400 MHz, CDCl3) δ 7.07 (1H, d, J = 8.4 Hz, ArH), 6.97 (1H, dd, J = 2.4, 1.2 Hz, ArH), 6.77 (1H, dd, J = 8.4, 2.4 Hz, ArH), 3.79 (3H, s, OCH3), 2.64–2.37 (4H, m, CH2), 2.35 (3H, d, 5JFH = 3.6 Hz, ArCH3), 2.27 (6H, s, N(CH3)2), 1.43 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 168.9 (d, 2JFC = 26 Hz), 157.4 (d, 4JFC = 2 Hz), 137.2 (d, 2JFC = 21 Hz), 132.9, 128.3 (d, 3JFC = 2 Hz), 113.1 (d, 5JFC = 2 Hz), 112.6 (d, 3JFC = 9 Hz), 96.3 (d, 1JFC = 189 Hz), 82.6, 55.3, 53.6 (d, 3JFC = 5 Hz), 45.4, 34.6 (d, 2JFC = 22 Hz), 27.7, 19.7 (d, 4JFC = 7 Hz); HRMS (ESI): calcd for C18H29FNO3 [M + H]+ 326.2126, found 326.2115.tert-Butyl 2-(4-bromo-2-methylphenyl)-4-(dimethylamino)-2-fluorobutanoate (3fa) (Table 2, entry 5)
Obtained from 2f (318 mg, 0.631 mmol) by the same procedure with 3aa. Purification by chromatography on silica gel (CH2Cl2/MeOH = 30/1 to 20/1 as the eluent) gave 3fa (170 mg, 72% yield) as a pale yellow oil. IR (ATR) νmax/cm−1 2976, 2938, 2862, 2818, 2766, 1749, 1731, 1590, 1561, 1480, 1459, 1391, 1368, 1275, 1250, 1216, 1148, 1092, 1042, 988, 960, 940, 900, 844, 811, 768, 747, 704; 1H NMR (400 MHz, CDCl3) δ 7.35–7.29 (2H, m, ArH), 7.29–7.23 (1H, m, ArH), 2.66–2.30 (4H, m, CH2), 2.40 (3H, d, 5JFH = 3.6 Hz, ArCH3), 2.26 (6H, s, N(CH3)2), 1.42 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 168.6 (d, 2JFC = 27 Hz), 139.0 (d, 3JFC = 2 Hz), 135.3 (d, 2JFC = 21 Hz), 134.7, 128.6, 127.8 (d, 3JFC = 8 Hz), 122.4 (d, JFC = 2 Hz), 96.0 (d, 1JFC = 189 Hz), 82.8, 53.4 (d, 3JFC = 5 Hz), 45.4, 34.6 (d, 2JFC = 22 Hz), 27.6, 20.4 (d, 4JFC = 8 Hz); HRMS (ESI): calcd for C17H26BrFNO2 [M + H]+ 374.1125, found 374.1110.tert-Butyl 4-(dimethylamino)-2-fluoro-2-(2-methyl-4-(trifluoromethyl)phenyl)butanoate (3ga) (Table 2, entry 6)
Obtained from 2g (74.8 mg, 0.152 mmol) by the same procedure with 3aa. Purification by chromatography on silica gel (CH2Cl2/MeOH = 30/1 to 20/1 as the eluent) gave 3ga (37.4 mg, 68% yield) as a colourless oil. IR (ATR) νmax/cm−1 2979, 2940, 2864, 2821, 2769, 1750, 1732, 1619, 1460, 1411, 1394, 1370, 1333, 1285, 1252, 1217, 1150, 1122, 1090, 1042, 1008, 990, 965, 944, 890, 840, 814, 774, 741, 711; 1H NMR (400 MHz, CDCl3) δ 7.52 (1H, d, J = 8.6 Hz, ArH), 7.46 (1H, d, J = 8.6 Hz, ArH), 7.42 (1H, s, ArH), 2.71–2.35 (4H, m, CH2), 2.49 (3H, d, 5JFH = 4.0 Hz, ArCH3), 2.28 (6H, s, N(CH3)2), 1.42 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 168.4 (d, 2JFC = 26 Hz), 139.9 (d, 2JFC = 21 Hz), 137.6 (d, 3JFC = 1 Hz), 130.5 (qd, 2JFC, 5JFC = 33, 2 Hz), 128.8 (q, 3JFC = 4 Hz), 126.7 (d, 3JFC = 9 Hz), 123.9 (q, 1JFC = 273 Hz), 122.5 (q, 3JFC = 4 Hz), 96.1 (d, 1JFC = 189 Hz), 83.2, 53.4 (d, 3JFC = 5 Hz), 45.4, 34.6 (d, 2JFC = 22 Hz), 27.7, 20.8 (d, 4JFC = 8 Hz); HRMS (ESI): calcd for C18H26F4NO2 [M + H]+ 364.1894, found 364.1882.tert-Butyl 2-(3-bromo-2-methylphenyl)-4-(dimethylamino)-2-fluorobutanoate (3ha) (Table 2, entry 7)
Obtained from 2h (155 mg, 0.307 mmol) by the same procedure with 3aa. Purification by chromatography on silica gel (CH2Cl2/MeOH = 30/1 to 20/1 as the eluent) gave 3ha (66.7 mg, 58% yield) as a pale yellow oil. IR (ATR) νmax/cm−1 2976, 2938, 2861, 2818, 2766, 1748, 1732, 1562, 1459, 1432, 1392, 1368, 1283, 1250, 1148, 1092, 1076, 1033, 1005, 965, 944, 903, 842, 783, 763, 742, 715; 1H NMR (400 MHz, CDCl3) δ 7.57 (1H, d, J = 8.0 Hz, ArH), 7.38 (1H, d, J = 8.0 Hz, ArH), 7.06 (1H, ddq, J = 8.0, 8.0, 0.5 Hz, ArH), 2.66–2.34 (4H, m, CH2), 2.47 (3H, d, 5JFH = 2.8 Hz, ArCH3), 2.26 (6H, s, N(CH3)2), 1.43 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 169.0 (d, 2JFC = 26 Hz), 138.4 (d, 2JFC = 21 Hz), 136.4, 133.1 (d, 3JFC = 2 Hz), 127.7 (d, JFC = 2 Hz), 126.6, 125.5 (d, 3JFC = 8 Hz), 96.0 (d, 1JFC = 190 Hz), 83.0, 53.5 (d, 3JFC = 5 Hz), 45.5, 34.9 (d, 2JFC = 22 Hz), 27.7, 20.3 (d, 4JFC = 7 Hz); HRMS (ESI): calcd for C17H26BrFNO2 [M + H]+ 374.1125, found 374.1120.tert-Butyl 2-(5-bromo-2-methylphenyl)-2-chloro-4-(dimethylamino)butanoate (3bb) and tert-butyl 2-(5-bromo-2-methylphenyl)-4-chloro-2-(dimethylamino)butanoate (4bb) (Table 2, entry 8)
Obtained from 2b (135 mg, 0.268 mmol) by the same procedure with 3ab and 4ab. Purification by chromatography on silica gel (CH2Cl2/MeOH = 100/0 to 30/1 as the eluent Rf: 3bb < 4bb) gave 3bb (85.5 mg, 82% yield) as colourless crystals and 4bb (18.0 mg, 17% yield) as a colourless oil. 3bb: mp 57–59 °C; IR (ATR) νmax/cm−1 2998, 2975, 2939, 2857, 2813, 2759, 1739, 1593, 1566, 1481, 1459, 1393, 1366, 1288, 1263, 1232, 1179, 1143, 1100, 1080, 1063, 1041, 1029, 920, 875, 849, 811, 796, 768, 755, 722; 1H NMR (400 MHz, CDCl3) δ 7.74 (1H, d, J = 2.0 Hz, ArH), 7.35 (1H, dd, J = 8.2, 2.0 Hz, ArH), 7.03 (1H, d, J = 8.2 Hz, ArH), 2.63 (1H, ddd, J = 13.5, 10.1, 5.6 Hz, CH2), 2.52 (1H, ddd, J = 13.5, 9.6, 5.8 Hz, CH2), 2.44–2.31 (2H, m, CH2), 2.29 (3H, s, ArCH3), 2.25 (6H, s, N(CH3)2), 1.44 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 168.6, 140.1, 134.8, 133.5, 131.1, 129.5, 119.4, 83.4, 72.8, 54.9, 45.6, 37.8, 27.6, 20.0; HRMS (ESI): calcd for C17H26BrClNO2 [M + H]+ 390.0830, found 390.0822. 4bb: IR (ATR) νmax/cm−1 2977, 2931, 2872, 2837, 2797, 1712, 1589, 1563, 1476, 1455, 1391, 1367, 1336, 1294, 1231, 1151, 1119, 1100, 1082, 1049, 1030, 984, 967, 912, 876, 843, 803, 772, 734; 1H NMR (400 MHz, CDCl3) δ 7.61 (1H, d, J = 2.0 Hz, ArH), 7.29 (1H, dd, J = 8.2, 2.0 Hz, ArH), 7.01 (1H, d, J = 8.2 Hz, ArH), 3.33 (1H, ddd, J = 12.0, 10.8, 4.0 Hz, CH2), 2.93 (1H, ddd, J = 12.2, 10.8, 5.5 Hz, CH2), 2.68 (1H, ddd, J = 14.4, 12.2, 4.0 Hz, CH2), 2.34 (6H, s, N(CH3)2), 2.31–2.16 (1H, m, CH2), 2.25 (3H, s, ArCH3), 1.53 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 167.3, 139.9, 135.0, 133.9, 131.2, 130.0, 119.1, 82.4, 71.8, 40.3, 40.0, 37.2, 28.4, 20.7; HRMS (ESI): calcd for C17H26BrClNO2 [M + H]+ 390.0830, found 390.0826.tert-Butyl 2-chloro-4-(dimethylamino)-2-(2,5-dimethylphenyl)butanoate (3db) and tert-butyl 4-chloro-2-(dimethylamino)-2-(2,5-dimethylphenyl)butanoate (4db) (Table 2, entry 9)
Obtained from 2d (85.8 mg, 0.195 mmol) by the same procedure with 3ab and 4ab. Purification by chromatography on silica gel (CH2Cl2/MeOH = 100/0 to 30/1 as the eluent Rf: 3db < 4db) gave 3db (33.9 mg, 53% yield) as colourless crystals and 4db (12.3 mg, 19% yield) as a colourless oil. 3db: mp 41–42 °C; IR (ATR) νmax/cm−1 2976, 2938, 2861, 2818, 2765, 1732, 1615, 1497, 1458, 1392, 1367, 1252, 1146, 1080, 1039, 992, 969, 928, 911, 846, 809, 771, 745, 718, 700; 1H NMR (400 MHz, CDCl3) δ 7.40 (1H, s, ArH), 7.07–6.99 (2H, m, ArH), 2.71–2.52 (2H, m, CH2), 2.47–2.35 (2H, m, CH2), 2.34 (3H, s, ArCH3), 2.30 (3H, s, ArCH3), 2.26 (6H, s, N(CH3)2), 1.44 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 169.3, 137.8, 135.0, 132.6, 131.9, 128.8, 127.0, 82.9, 73.8, 55.1, 45.6, 37.9, 27.6, 21.2, 20.0; HRMS (ESI): calcd for C18H29ClNO2 [M + H]+ 326.1881, found 326.1873. 4db: IR (ATR) νmax/cm−1 2977, 2929, 2871, 2836, 2795, 1712, 1613, 1497, 1455, 1392, 1366, 1336, 1297, 1234, 1151, 1081, 1055, 1035, 978, 967, 898, 846, 810, 773, 757, 725; 1H NMR (400 MHz, CDCl3) δ 7.17 (1H, br s, ArH), 7.01 (1H, d, J = 7.7 Hz, ArH), 6.96 (1H, dd, J = 7.7, 1.8 Hz, ArH), 3.22 (1H, ddd, J = 12.0, 10.8, 4.5 Hz, CH2), 3.03 (1H, ddd, J = 12.4, 10.8, 5.1 Hz, CH2), 2.63 (1H, ddd, J = 13.8, 12.4, 4.5 Hz, CH2), 2.37–2.27 (1H, m, CH2), 2.35 (6H, s, N(CH3)2), 2.294 (3H, s, ArCH3), 2.288 (3H, s, ArCH3), 1.54 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 168.7, 137.1, 134.2, 132.9, 132.2, 129.0, 127.7, 81.9, 72.5, 41.0, 40.0, 37.9, 28.5, 21.2, 20.6; HRMS (ESI): calcd for C18H29ClNO2 [M + H]+ 326.1881, found 326.1874.tert-Butyl 2-(4-bromo-2-methylphenyl)-2-chloro-4-(dimethylamino)butanoate (3fb) and tert-butyl 2-(4-bromo-2-methylphenyl)-4-chloro-2-(dimethylamino)butanoate (4fb) (Table 2, entry 10)
Obtained from 2f (95.6 mg, 0.190 mmol) by the same procedure with 3ab and 4ab. Purification by chromatography on silica gel (CH2Cl2/MeOH = 100/0 to 30/1 as the eluent Rf: 3fb < 4fb) gave 3fb (61.4 mg, 83% yield) as a pale yellow oil and 4fb (12.9 mg, 17% yield) as colourless crystals. 3fb: IR (ATR) νmax/cm−1 2975, 2939, 2861, 2818, 2766, 1734, 1589, 1560, 1458, 1391, 1368, 1252, 1145, 1079, 1029, 967, 934, 899, 871, 843, 803, 770, 739, 722, 699; 1H NMR (400 MHz, CDCl3) δ 7.47 (1H, dd, J = 7.8, 1.0 Hz, ArH), 7.35–7.30 (2H, m, ArH), 2.63 (1H, ddd, J = 13.6, 10.1, 5.7 Hz, CH2), 2.52 (1H, ddd, J = 13.6, 9.3, 5.9 Hz, CH2), 2.43–2.31 (2H, m, CH2), 2.33 (3H, s, ArCH3), 2.24 (6H, s, N(CH3)2), 1.43 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 168.7, 138.2, 137.3, 134.6, 128.7, 128.0, 122.1, 83.3, 73.1, 54.9, 45.6, 37.8, 27.5, 20.3; HRMS (ESI): calcd for C17H26BrClNO2 [M + H]+ 390.0830, found 390.0820. 4fb: mp 132–134 °C; IR (ATR) νmax/cm−1 3001, 2974, 2943, 2927, 2869, 2850, 2839, 2800, 1714, 1587, 1560, 1473, 1453, 1389, 1367, 1338, 1300, 1261, 1229, 1177, 1152, 1083, 1049, 1027, 984, 961, 871, 831, 814, 771, 756, 739, 724; 1H NMR (400 MHz, CDCl3) δ 7.36–7.23 (3H, m, ArH), 3.29 (1H, ddd, J = 12.3, 10.8, 4.3 Hz, CH2), 2.93 (1H, ddd, J = 12.1, 10.8, 5.3 Hz, CH2), 2.67 (1H, ddd, J = 14.3, 12.1, 4.3 Hz, CH2), 2.33 (6H, s, N(CH3)2), 2.29 (3H, s, ArCH3), 2.24 (1H, ddd, J = 14.3, 12.3, 5.3 Hz, CH2), 1.53 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 167.6, 138.4, 136.8, 134.9, 130.2, 128.0, 120.9, 82.4, 71.9, 40.3, 40.0, 37.3, 28.4, 20.9; HRMS (ESI): calcd for C17H26BrClNO2 [M + H]+ 390.0830, found 390.0824.tert-Butyl 2-(3-bromo-2-methylphenyl)-2-chloro-4-(dimethylamino)butanoate (3hb) and tert-butyl 2-(3-bromo-2-methylphenyl)-4-chloro-2-(dimethylamino)butanoate (4hb) (Table 2, entry 11)
Obtained from 2h (149 mg, 0.295 mmol) by the same procedure with 3ab and 4ab. Purification by chromatography on silica gel (CH2Cl2/MeOH = 100/0 to 30/1 as the eluent Rf: 3hb < 4hb) gave 3hb (74.7 mg, 65% yield) as colourless crystals and 4hb (37.5 mg, 33% yield) as a colourless oil. 3hb: mp 45–47 °C; IR (ATR) νmax/cm−1 2976, 2940, 2861, 2818, 2766, 1733, 1561, 1459, 1429, 1392, 1368, 1304, 1251, 1146, 1078, 1031, 997, 967, 939, 912, 841, 783, 765, 742, 714; 1H NMR (400 MHz, CDCl3) δ 7.59 (1H, dd, J = 8.1, 1.0 Hz, ArH), 7.55 (1H, dd, J = 8.0, 1.0 Hz, ArH), 7.07 (1H, dd, J = 8.1, 8.0 Hz, ArH), 2.66 (1H, ddd, J = 13.4, 11.1, 4.8 Hz, CH2), 2.52 (1H, ddd, J = 13.4, 10.4, 4.8 Hz, CH2), 2.41 (1H, ddd, J = 12.0, 10.4, 4.8 Hz, CH2), 2.38 (3H, s, ArCH3), 2.34 (1H, ddd, J = 12.0, 11.1, 4.8 Hz, CH2), 2.24 (6H, s, N(CH3)2), 1.44 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 169.1, 140.1, 135.6, 132.7, 127.5, 126.6, 125.7, 83.4, 73.5, 54.9, 45.6, 38.3, 27.5, 21.1; HRMS (ESI): calcd for C17H26BrClNO2 [M + H]+ 390.0830, found 390.0823. 4hb: IR (ATR) νmax/cm−1 2976, 2930, 2872, 2837, 2797, 1713, 1560, 1455, 1428, 1392, 1366, 1336, 1295, 1233, 1151, 1098, 1081, 1048, 986, 962, 875, 842, 821, 789, 736, 719; 1H NMR (400 MHz, CDCl3) δ 7.50 (1H, dd, J = 7.9, 1.0 Hz, ArH), 7.46 (1H, br d, J = 8.0 Hz, ArH), 7.01 (1H, ddd, J = 8.0, 7.9, 0.4 Hz, ArH), 3.33 (1H, ddd, J = 11.6, 10.8, 3.7 Hz, CH2), 2.92 (1H, ddd, J = 12.1, 10.8, 5.2 Hz, CH2), 2.71 (1H, ddd, J = 14.2, 12.1, 3.7 Hz, CH2), 2.40–2.15 (1H, m, CH2), 2.35 (3H, s, ArCH3), 2.33 (6H, s, N(CH3)2), 1.53 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 167.4, 139.8, 135.6, 131.7, 127.8, 127.6, 126.0, 82.4, 72.4, 40.3, 40.1, 37.5, 28.4, 21.8; HRMS (ESI): calcd for C17H26BrClNO2 [M + H]+ 390.0830, found 390.0826.1-Allyl-2-(5-bromo-2-methylphenyl)-2-(tert-butoxycarbonyl)-1-methylazetidin-1-ium trifluoromethanesulfonate (5)
A solution of allyl alcohol (50 μL, 0.74 mmol) and pyridine (55 μL, 0.68 mmol) in CCl4 (1.7 mL) was treated with trifluoromethanesulfonic anhydride (0.11 mL, 0.65 mmol) at 0 °C. The mixture was stirred for 20 min at the same temperature to precipitate a pale-brown solid. The generated allyl triflate in CCl4 (ref. 12) was added by decantation to a mixture of 1b6 (156 mg, 0.458 mmol) and NaHCO3 (122 mg, 1.45 mmol) in CH2Cl2 (2.3 mL) at 0 °C. After stirring for 1 h at room temperature, the resulting mixture was purified by chromatography on silica gel (CH2Cl2/MeOH = 20/1 to 10/1 as the eluent) to obtain 5 (170 mg, 70% yield, 8/2 mixture of diastereomers) as a colourless gum. IR (ATR) νmax/cm−1 2980, 2936, 1731, 1484, 1459, 1427, 1397, 1372, 1252, 1223, 1149, 1139, 1029, 952, 911, 831, 808, 790, 755, 728; 1H NMR (400 MHz, acetone-d6) δ 7.81 (0.8H, d, J = 2.0 Hz, ArH), 7.76 (0.2H, br, ArH), 7.70–7.61 (0.2H, m, ArH), 7.65 (0.8H, dd, J = 8.2, 2.0 Hz, ArH), 7.36 (1H, d, J = 8.2 Hz, ArH), 6.19 (0.2H, ddt, J = 17.0, 9.6, 7.2 Hz, CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 6.05 (0.8H, ddt, J = 17.1, 10.1, 7.2 Hz, CH2CH
CH2), 6.05 (0.8H, ddt, J = 17.1, 10.1, 7.2 Hz, CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.86 (0.2H, ddt, J = 17.0, 1.2, 1.2 Hz, CH2CH
CH2), 5.86 (0.2H, ddt, J = 17.0, 1.2, 1.2 Hz, CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.76–5.70 (0.2H, m, CH2CH
CH2), 5.76–5.70 (0.2H, m, CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.74 (0.8H, ddt, J = 17.1, 1.4, 1.2 Hz, CH2CH
CH2), 5.74 (0.8H, ddt, J = 17.1, 1.4, 1.2 Hz, CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.65 (0.8H, ddt, J = 10.1, 1.4, 0.8 Hz, CH2CH
CH2), 5.65 (0.8H, ddt, J = 10.1, 1.4, 0.8 Hz, CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.90 (0.2H, ddd, J = 9.6, 9.6, 9.6 Hz, 4H), 4.61 (0.8H, dddd, J = 10.6, 10.6, 9.2, 1.6 Hz, 4H), 4.48–4.32 (0.2H, br, CH2), 4.38 (0.8H, ddd, J = 10.3, 9.2, 3.2 Hz, 4H), 4.24–4.09 (1.8H, m, CH2), 4.09–4.00 (0.2H, br m, CH2), 3.82–3.66 (0.4H, br m, CH2), 3.71 (3H, s, NCH3), 3.24–3.04 (1.6H, br m, CH2), 2.42 (2.4H, s, ArCH3), 2.41 (0.6H, s, ArCH3), 1.473 (1.8H, s, tBu), 1.466 (7.2H, s, tBu); 13C{1H} NMR (101 MHz, acetone-d6) δ 167.2, 137.8, 137.4 (minor), 135.2, 134.5, 133.2, 133.0, 129.8, 128.9 (minor), 126.3 (minor), 125.9, 122.7 (q, J = 324 Hz), 120.6, 87.8, 87.7 (minor), 87.5, 64.0, 59.3, 48.2 (minor), 47.9, 27.8, 27.64 (minor), 27.63, 21.1; HRMS (ESI): calcd for C19H27BrNO2 [M + H]+ 380.1220, found 380.1207.
CH2), 4.90 (0.2H, ddd, J = 9.6, 9.6, 9.6 Hz, 4H), 4.61 (0.8H, dddd, J = 10.6, 10.6, 9.2, 1.6 Hz, 4H), 4.48–4.32 (0.2H, br, CH2), 4.38 (0.8H, ddd, J = 10.3, 9.2, 3.2 Hz, 4H), 4.24–4.09 (1.8H, m, CH2), 4.09–4.00 (0.2H, br m, CH2), 3.82–3.66 (0.4H, br m, CH2), 3.71 (3H, s, NCH3), 3.24–3.04 (1.6H, br m, CH2), 2.42 (2.4H, s, ArCH3), 2.41 (0.6H, s, ArCH3), 1.473 (1.8H, s, tBu), 1.466 (7.2H, s, tBu); 13C{1H} NMR (101 MHz, acetone-d6) δ 167.2, 137.8, 137.4 (minor), 135.2, 134.5, 133.2, 133.0, 129.8, 128.9 (minor), 126.3 (minor), 125.9, 122.7 (q, J = 324 Hz), 120.6, 87.8, 87.7 (minor), 87.5, 64.0, 59.3, 48.2 (minor), 47.9, 27.8, 27.64 (minor), 27.63, 21.1; HRMS (ESI): calcd for C19H27BrNO2 [M + H]+ 380.1220, found 380.1207.
tert-Butyl 4-(allyl(methyl)amino)-2-(5-bromo-2-methylphenyl)-2-fluorobutanoate (6)
Performed by the same procedure with 3aa using 5 (97.5 mg, 0.184 mmol) as a substrate. Purification by chromatography on silica gel (CH2Cl2/MeOH = 30/1 to 20/1 as the eluent) gave 6 (60.1 mg, 82% yield) as a pale yellow oil. IR (ATR) νmax/cm−1 3076, 2977, 2934, 2874, 2846, 2780, 1749, 1731, 1644, 1592, 1564, 1481, 1456, 1392, 1368, 1282, 1251, 1142, 1054, 1033, 995, 920, 875, 841, 810, 771, 748, 705; 1H NMR (400 MHz, CDCl3) δ 7.54 (1H, dd, J = 1.9, 1.9 Hz, ArH), 7.34 (1H, dd, J = 8.1, 1.9 Hz, ArH), 7.03 (1H, d, J = 8.1 Hz, ArH), 5.86 (1H, ddt, J = 17.2, 10.0, 6.6 Hz, CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.22–5.12 (2H, m, CH2CH
CH2), 5.22–5.12 (2H, m, CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 3.04 (2H, ddd, J = 6.6, 1.1, 1.1 Hz, CH2CH
CH2), 3.04 (2H, ddd, J = 6.6, 1.1, 1.1 Hz, CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 2.66–2.44 (3H, m, CH2), 2.44–2.28 (1H, m, CH2), 2.36 (3H, d, 5JFH = 3.6 Hz, ArCH3), 2.27 (3H, s, NCH3), 1.43 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 168.5 (d, 2JFC = 27 Hz), 138.3 (d, 2JFC = 22 Hz), 135.6 (d, 3JFC = 2 Hz), 135.2, 133.6, 131.4 (d, JFC = 2 Hz), 129.2 (d, 3JFC = 8 Hz), 119.3, 117.9, 95.8 (d, 1JFC = 190 Hz), 83.0, 61.0, 50.8 (d, 3JFC = 5 Hz), 42.1, 34.2 (d, 2JFC = 22 Hz), 27.7, 20.2 (d, 4JFC = 8 Hz); HRMS (ESI): calcd for C19H28BrFNO2 [M + H]+ 400.1282, found 400.1273.
CH2), 2.66–2.44 (3H, m, CH2), 2.44–2.28 (1H, m, CH2), 2.36 (3H, d, 5JFH = 3.6 Hz, ArCH3), 2.27 (3H, s, NCH3), 1.43 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 168.5 (d, 2JFC = 27 Hz), 138.3 (d, 2JFC = 22 Hz), 135.6 (d, 3JFC = 2 Hz), 135.2, 133.6, 131.4 (d, JFC = 2 Hz), 129.2 (d, 3JFC = 8 Hz), 119.3, 117.9, 95.8 (d, 1JFC = 190 Hz), 83.0, 61.0, 50.8 (d, 3JFC = 5 Hz), 42.1, 34.2 (d, 2JFC = 22 Hz), 27.7, 20.2 (d, 4JFC = 8 Hz); HRMS (ESI): calcd for C19H28BrFNO2 [M + H]+ 400.1282, found 400.1273.
tert-Butyl 2-(5-bromo-2-methylphenyl)-2-fluoro-4-(methylamino)butanoate (7)
A mixture of 6 (103 mg, 0.257 mmol) and RhCl(PPh3)3 (5 mg, 0.005 mmol) in MeCN (2.2 mL) and H2O (0.4 mL) was refluxed for 3 h under an Ar atmosphere. The resulting mixture was cooled to room temperature and treated with saturated aqueous NaHCO3. The mixture was extracted with EtOAc and the combined extracts were washed with brine. The organic solution was dried over Na2SO4 and concentrated by evaporation. Purification of the residue by chromatography on silica gel (CH2Cl2/MeOH = 15/1 to 5/1 as the eluent) gave 7 (77.7 mg, 84% yield) as pale yellow crystals, mp 38–42 °C. IR (ATR) νmax/cm−1 3040, 2981, 2942, 2887, 1733, 1499, 1461, 1425, 1397, 1372, 1357, 1327, 1254, 1223, 1141, 1078, 1029, 958, 930, 877, 837, 802, 768, 754, 736, 706; 1H NMR (400 MHz, CDCl3) δ 7.53 (1H, dd, J = 1.9, 1.9 Hz, ArH), 7.35 (1H, dd, J = 8.1, 1.9 Hz, ArH), 7.04 (1H, d, J = 8.1 Hz, ArH), 2.78 (1H, ddd, J = 12.0, 9.2, 5.8 Hz, CH2), 2.72 (1H, ddd, J = 12.0, 9.2, 5.8 Hz, CH2), 2.64–2.33 (2H, m, CH2), 2.45 (3H, s, NCH3), 2.36 (3H, d, 5JFH = 3.6 Hz, ArCH3), 1.59 (1H, br, NH), 1.44 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 168.6 (d, 2JFC = 27 Hz), 138.3 (d, 2JFC = 21 Hz), 135.5 (d, 3JFC = 2 Hz), 133.7, 131.4 (d, JFC = 2 Hz), 129.1 (d, 3JFC = 9 Hz), 119.3, 96.2 (d, 1JFC = 189 Hz), 83.2, 46.2 (d, 3JFC = 5 Hz), 36.43 (d, 2JFC = 22 Hz), 36.36, 27.7, 20.2 (d, 4JFC = 7 Hz); HRMS (ESI): calcd for C16H24BrFNO2 [M + H]+ 360.0969, found 360.0964.4-(tert-Butoxy)-3-fluoro-N,N,N-trimethyl-4-oxo-3-(o-tolyl)butan-1-aminium iodide (8-I)
A mixture of 3aa (67.0 mg, 0.227 mmol) and MeI (21 μL, 0.34 mmol) in MeCN (1.1 mL) was stirred for 2 h at room temperature. The resulting mixture was concentrated by evaporation and the residue was purified by chromatography on silica gel (CH2Cl2/MeOH = 20/1 to 10/1 as the eluent) to obtain 8-I (90.3 mg, 91% yield) as a yellow solid, mp 162–164 °C. IR (ATR) νmax/cm−1 3002, 2978, 2932, 1738, 1484, 1455, 1418, 1394, 1368, 1335, 1294, 1259, 1243, 1215, 1145, 1089, 1062, 1009, 992, 975, 934, 912, 838, 777, 748; 1H NMR (400 MHz, CDCl3) δ 7.51–7.44 (1H, m, ArH), 7.32–7.23 (2H, m, ArH), 7.22–7.15 (1H, m, ArH), 3.70 (1H, ddd, J = 13.1, 8.7, 7.3 Hz, CH2), 3.60–3.40 (1H, m, CH2), 3.52 (9H, s, N(CH3)3), 2.93–2.76 (2H, m, CH2), 2.41 (3H, d, 5JFH = 3.6 Hz, ArCH3), 1.43 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 167.7 (d, 2JFC = 25 Hz), 135.7 (d, 3JFC = 2 Hz), 133.8 (d, 2JFC = 21 Hz), 132.5, 129.2 (d, JFC = 2 Hz), 126.4 (d, JFC = 2 Hz), 126.1 (d, 3JFC = 9 Hz), 96.1 (d, 1JFC = 190 Hz), 84.4, 62.7–62.4 (m), 54.0, 30.3 (d, 2JFC = 22 Hz), 27.8, 20.7 (d, 4JFC = 7 Hz); 19F NMR (376 MHz, CDCl3) δ −157; HRMS (ESI): calcd for C18H29FNO2 [M − I]+ 310.2177, found 310.2166.4-(tert-Butoxy)-3-fluoro-N,N,N-trimethyl-4-oxo-3-(o-tolyl)butan-1-aminium trifluoromethanesulfonate (8-OTf)
A mixture of 3aa (91.3 mg, 0.309 mmol) and NaHCO3 (82 mg, 0.98 mmol) in CH2Cl2 (1.5 mL) was treated with methyl trifluoromethanesulfonate (52 μL, 0.46 mmol) at 0 °C and stirred for 2 h at room temperature. The resulting mixture was purified by chromatography on silica gel (CH2Cl2/MeOH = 20/1 to 10/1 as the eluent) to obtain 8-OTf (143 mg, quant.) as a white solid, mp 140–142 °C. IR (ATR) νmax/cm−1 3039, 2979, 2936, 1760, 1733, 1484, 1459, 1420, 1395, 1371, 1257, 1227, 1155, 1084, 1030, 1008, 976, 933, 910, 841, 793, 746; 1H NMR (400 MHz, CDCl3) δ 7.42–7.35 (1H, m, ArH), 7.30–7.21 (2H, m, ArH), 7.21–7.13 (1H, m, ArH), 3.55–3.43 (1H, m, CH2), 3.41–3.27 (1H, m, CH2), 3.21 (9H, s, N(CH3)3), 2.86–2.69 (2H, m, CH2), 2.38 (3H, d, 5JFH = 3.6 Hz, ArCH3), 1.41 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 167.8 (d, 2JFC = 26 Hz), 135.9 (d, 3JFC = 2 Hz), 134.0 (d, 2JFC = 21 Hz), 132.4, 129.2, 126.3, 125.9 (d, 3JFC = 9 Hz), 120.5 (q, 1JFC = 321 Hz), 95.9 (d, 1JFC = 190 Hz), 84.3, 62.1 (d, 3JFC = 5 Hz), 53.3, 30.0 (d, 2JFC = 22 Hz), 27.6, 20.5 (d, 4JFC = 8 Hz); 19F NMR (376 MHz, CDCl3) δ −80, −157; HRMS (ESI): calcd for C18H29FNO2 [M − OTf]+ 310.2177, found 310.2171.tert-Butyl 2-fluoro-2-(o-tolyl)but-3-enoate (9)
A mixture of 8-OTf (67.9 mg, 0.148 mmol) and DBU (66 μL, 0.44 mmol) in toluene (1.5 mL) was refluxed for 1 day under an Ar atmosphere. The resulting mixture was cooled to room temperature and treated with aqueous saturated NH4Cl. The mixture was extracted with EtOAc and the combined extracts were washed with saturated aqueous NaHCO3 followed by brine. The solution was dried over Na2SO4 and concentrated by evaporation. The residue was purified by chromatography on silica gel (n-hexane/EtOAc = 30/1 to 20/1 as the eluent) to obtain 9 (21.0 mg, 57% yield) as a colourless oil. IR (ATR) νmax/cm−1 2979, 2934, 1751, 1731, 1476, 1457, 1406, 1394, 1369, 1282, 1253, 1223, 1146, 1119, 1061, 1042, 1024, 992, 973, 935, 841, 828, 800, 769, 747; 1H NMR (400 MHz, CDCl3) δ 7.40–7.35 (1H, m, ArH), 7.29–7.22 (1H, m, ArH), 7.21–7.14 (2H, m, ArH), 6.37 (1H, ddd, 3JFH = 17.6, J = 17.4, 11.1 Hz, 3H), 5.60 (1H, ddd, 4JFH = 0.8 Hz, J = 17.4, 0.8 Hz, 4H), 5.51 (1H, ddd, 4JFH = 1.6 Hz, J = 11.1, 0.8 Hz, 4H), 2.40 (3H, d, 5JFH = 2.8 Hz, ArCH3), 1.46 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 168.6 (d, 2JFC = 26 Hz), 137.2, 136.1 (d, 2JFC = 22 Hz), 134.7 (d, 2JFC = 23 Hz), 131.8, 129.0, 127.9 (d, 3JFC = 6 Hz), 125.5, 117.7 (d, 3JFC = 12 Hz), 96.1 (d, 1JFC = 190 Hz), 83.0, 27.8, 20.1 (d, 4JFC = 5 Hz); 19F NMR (376 MHz, CDCl3) δ −153; HRMS (ESI): calcd for C15H19FO2Na [M + Na]+ 273.1261, found 273.1260.(S)-2-(tert-Butoxycarbonyl)-1,1-dimethyl-2-(o-tolyl)azetidin-1-ium trifluoromethanesulfoante [(S)-2a]
A mixture of (S)-1a (171 mg, 0.654 mmol, 93% ee) and NaHCO3 (168 mg, 2.00 mmol) in CH2Cl2 (3.3 mL) was treated with methyl trifluoromethanesulfonate (148 μL, 1.31 mmol) at 0 °C and stirred for 2.5 h at room temperature. The resulting mixture was evaporated to ca. 1/2 volume and purified by chromatography on silica gel (CH2Cl2/MeOH = 15/1 to 6/1 as the eluent) to obtain (S)-2a (277 mg, quant.) as colourless crystals, mp 110–112 °C. [α]24589 +34.6 (c 1.0 in CHCl3); IR (ATR) νmax/cm−1 3076, 3041, 2979, 2940, 1728, 1459, 1395, 1371, 1302, 1254, 1225, 1145, 1105, 1078, 1030, 995, 969, 946, 856, 832, 770, 753, 728, 988, 965, 939, 844, 749; 1H NMR (400 MHz, CDCl3) δ 7.45–7.33 (3H, m, ArH), 7.29–7.23 (1H, m, ArH), 4.52 (1H, ddd, J = 10.6, 10.4, 9.4 Hz, 4H), 4.27 (1H, ddd, J = 9.6, 9.4, 2.4 Hz, 4H), 3.90 (1H, ddd, J = 12.4, 10.6, 9.6 Hz, 3H), 3.63 (3H, s, NCH3), 3.02–2.84 (1H, br m, 3H), 2.97 (3H, s, NCH3), 2.33 (3H, s, ArCH3), 1.39 (9H, s, tBu); 13C{1H} NMR (101 MHz, CDCl3) δ 166.4, 135.9, 132.5, 130.6, 129.7, 128.5, 126.9, 120.6 (q, J = 322 Hz), 86.8, 86.3, 62.7, 50.9, 50.7, 27.4, 27.1, 20.7; HRMS (ESI): calcd for C17H26NO2 [M − OTf]+ 276.1958, found 276.1955.(R)-tert-Butyl 4-(dimethylamino)-2-fluoro-2-(o-tolyl)butanoate [(R)-3aa]
Obtained from (S)-2a (277 mg, 0.651 mmol) by the same procedure with 3aa. Purification by chromatography on silica gel (CH2Cl2/MeOH = 20/1 to 10/1 as the eluent) gave (R)-3aa (131 mg, 68% yield) as a colourless oil. [α]24589 −5.2 (c 1.0 in EtOH).(R)-4-(Dimethylamino)-2-fluoro-2-(o-tolyl)butan-1-ol [(R)-10]
A solution of (R)-3aa (131 mg, 0.443 mmol) in THF (2.2 mL) was added to a suspension of LiAlH4 (35 mg, 0.92 mmol) in THF (2.2 mL) at 0 °C under an Ar atmosphere. After stirring for 4 h at room temperature, the resulting mixture was cooled at 0 °C and diluted with Et2O (4 mL). The mixture was quenched at 0 °C by addition of H2O (35 μL), 15 wt% NaOH·H2O solution (35 μL), and H2O (105 μL). The suspension was diluted with EtOH (4 mL) and stirred for 12 h at room temperature. The resulting mixture was filtered through a pad of Celite and the filtrate was concentrated by evaporation. The residue was purified by chromatography on amino-functionalized silica gel (Chromatorex NH-DM1020, n-hexane/EtOAc = 2/1 to 1/1 as the eluent) to obtain (R)-10 (73.0 mg, 73% yield) as a colourless oil. [α]24589 +9.9 (c 1.0 in EtOH); IR (ATR) νmax/cm−1 3339, 3061, 3020, 2947, 2863, 2825, 2778, 1462, 1385, 1312, 1290, 1258, 1217, 1180, 1163, 1095, 1057, 1038, 1000, 944, 878, 846, 800, 757, 726; 1H NMR (400 MHz, CDCl3) δ 7.47–7.40 (1H, m, ArH), 7.23–7.10 (3H, m, ArH), 3.92 (1H, dd, 3JFH = 26.8 Hz, J = 12.8 Hz, 1H), 3.88 (1H, dd, 3JFH = 20.2 Hz, J = 12.8 Hz, 1H), 2.87–2.76 (1H, m, OH), 2.48 (3H, d, 5JFH = 4.4 Hz, ArCH3), 2.45–2.13 (4H, m, CH2), 2.31 (6H, s, N(CH3)2); 13C{1H} NMR (101 MHz, CDCl3) δ 139.9 (d, 2JFC = 20 Hz), 135.2 (d, 3JFC = 2 Hz), 132.5, 127.7, 125.7 (d, JFC = 2 Hz), 125.5 (d, 3JFC = 11 Hz), 99.4 (d, 1JFC = 179 Hz), 67.6 (d, 2JFC = 28 Hz), 53.5 (d, 3JFC = 6 Hz), 44.9, 35.2 (d, 2JFC = 25 Hz), 21.8 (d, 4JFC = 9 Hz); 19F NMR (376 MHz, CDCl3) δ −161; HRMS (ESI): calcd for C13H21FNO [M + H]+ 226.1602, found 226.1600.(R)-4-(Dimethylamino)-2-fluoro-2-(o-tolyl)butyl benzoate [(R)-11]
A solution of (R)-10 (73.0 mg, 0.324 mmol) and Et3N (135 μL, 0.969 mmol) in CH2Cl2 (3.2 mL) was treated with benzoyl chloride (45 μL, 0.39 mmol) at 0 °C and stirred for 2 h at room temperature. The resulting mixture was diluted with H2O and extracted with EtOAc. The combined extracts were washed with H2O, dried over Na2SO4, and concentrated by evaporation. Purification by chromatography on silica gel (CH2Cl2/MeOH = 20/1 to 10/1 as the eluent) gave (R)-11 (101 mg, 95% yield) as a colourless oil. 93% ee [determined by HPLC analysis: Daicel Chiralcel AD-H column (25 cm), n-hexane/EtOH/Et2NH = 100/2/0.1 as the eluent, flow rate: 0.50 mL min−1, tR = 14.7 min for (R)-11 (96.5%) and 19.1 min for (S)-11 (3.5%)]. [α]23589 +6.6 (c 1.0 in EtOH); IR (ATR) νmax/cm−1 3062, 3022, 2970, 2944, 2861, 2818, 2765, 1719, 1602, 1584, 1491, 1450, 1377, 1314, 1265, 1176, 1156, 1111, 1095, 1068, 1042, 1026, 936, 893, 849, 802, 760, 727, 708; 1H NMR (400 MHz, CDCl3) δ 7.98–7.93 (2H, m, ArH), 7.54 (1H, tt, J = 7.6, 1.3 Hz, ArH), 7.43–7.35 (3H, m, ArH), 7.25–7.15 (3H, m, ArH), 4.73 (1H, dd, 3JFH = 17.4 Hz, J = 12.6 Hz, 1H), 4.67 (1H, dd, 3JFH = 15.8 Hz, J = 12.6 Hz, 1H), 2.50 (3H, d, 5JFH = 3.6 Hz, ArCH3), 2.50–2.33 (3H, m, CH2), 2.25–2.09 (1H, m, CH2), 2.20 (6H, s, N(CH3)2); 13C{1H} NMR (101 MHz, CDCl3) δ 166.1, 136.5 (d, 2JFC = 21 Hz), 134.8 (d, 3JFC = 2 Hz), 133.1, 132.6, 129.7, 129.6, 128.3, 128.1, 126.1 (d, 3JFC = 14 Hz), 125.9 (d, JFC = 2 Hz), 98.8 (d, 1JFC = 180 Hz), 68.4 (d, 2JFC = 25 Hz), 53.7 (d, 3JFC = 3 Hz), 45.5, 33.7 (d, 2JFC = 24 Hz), 21.8 (d, 4JFC = 8 Hz); 19F NMR (376 MHz, CDCl3) δ −157; HRMS (ESI): calcd for C20H25FNO2 [M + H]+ 330.1864, found 330.1859.tert-Butyl 2-(benzyl(methyl)amino)-2-ethyl-4-fluorobutanoate (14) and tert-butyl 4-(benzyl(methyl)amino)-2-ethyl-2-hydroxybutanoate (15)
Obtained from 12 (141 mg, 0.321 mmol) by the same procedure with 3aa and 4aa. Purification by chromatography on silica gel (CH2Cl2/MeOH = 100/0 to 30/1 as the eluent Rf: 14 > 15) gave 14 (62.0 mg, 62% yield) as a colourless oil and 15 (7.2 mg, 7% yield) as a colourless oil. 14: IR (ATR) νmax/cm−1 3086, 3062, 3027, 2975, 2934, 2882, 2801, 1715, 1604, 1495, 1454, 1391, 1366, 1238, 1165, 1124, 1064, 1029, 999, 961, 940, 888, 847, 831, 773, 732, 697; 1H NMR (400 MHz, CDCl3) δ 7.34–7.27 (4H, m, Ph), 7.26–7.18 (1H, m, Ph), 4.66 (1H, dddd, 2JFH = 47.2 Hz, J = 9.2, 7.2, 7.2 Hz, 4H), 4.62 (1H, dddd, 2JFH = 47.2 Hz, J = 9.2, 7.2, 5.6 Hz, 4H), 3.72 (1H, d, J = 14.4 Hz, CH2Ph), 3.65 (1H, d, J = 14.4 Hz, CH2Ph), 2.38–2.11 (2H, m, 3H), 2.20 (3H, s, NCH3), 1.97 (1H, dq, J = 14.0, 7.4 Hz, CH2CH3), 1.72 (1H, dq, J = 14.0, 7.4 Hz, CH2CH3), 1.52 (9H, s, tBu), 0.95 (3H, dd, J = 7.4, 7.4 Hz, CH2CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 172.6, 140.8, 128.2, 128.0, 126.6, 81.2 (d, 1JFC = 163 Hz), 81.1, 67.4 (d, 3JFC = 6 Hz), 55.3, 35.3, 31.4 (d, 2JFC = 20 Hz), 28.3, 25.9, 8.7; 19F NMR (376 MHz, CDCl3) δ −221; HRMS (ESI): calcd for C18H29FNO2 [M + H]+ 310.2177, found 310.2167. 15: IR (ATR) νmax/cm−1 3408, 3087, 3063, 3028, 2973, 2930, 2880, 2801, 1715, 1603, 1495, 1454, 1391, 1366, 1298, 1244, 1162, 1134, 1101, 1069, 1022, 951, 909, 892, 845, 829, 776, 729, 697; 1H NMR (400 MHz, CDCl3) δ 7.35–7.27 (4H, m, Ph), 7.27–7.21 (1H, m, Ph), 5.28 (1H, br, OH), 3.92 (1H, ddd, J = 11.3, 11.2, 3.2 Hz, CH2), 3.784 (1H, ddd, J = 11.2, 5.2, 3.2 Hz, CH2), 3.775 (2H, s, CH2Ph), 2.35 (1H, dddd, J = 15.6, 11.3, 5.2, 1.2 Hz, CH2), 2.27–2.15 (1H, m, CH2CH3), 2.23 (3H, s, NCH3), 1.90 (1H, dq, J = 13.4, 7.4 Hz, CH2CH3), 1.84 (1H, ddd, J = 15.6, 3.2, 3.2 Hz, CH2), 1.54 (9H, s, tBu), 0.89 (3H, dd, J = 7.4, 7.4 Hz, CH2CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 172.2, 139.1, 128.9, 128.5, 127.2, 81.5, 69.8, 59.5, 55.6, 35.2, 29.7, 28.4, 25.7, 9.4; HRMS (ESI): calcd for C18H30NO3 [M + H]+ 308.2220, found 308.2214.Author contributions
E. T. was supervisor of this project and conducted all area of this work, idea, development of the methodology, a part of experiments and writing the manuscript. K. K. performed the main experiments and compounds analyses.Conflicts of interest
There are no conflicts to declare.Notes and references
- Representative examples: (a) K. Wright, B. Drouillat, L. Menguy, J. Marrot and F. Couty, Eur. J. Org. Chem., 2017, 7195 CrossRef CAS; (b) B. Drouillat, I. V. Dorogan, M. Kletskii, O. N. Burov and F. Couty, J. Org. Chem., 2016, 81, 6677 CrossRef CAS PubMed; (c) L. Menguy, B. Drouillat and F. Couty, Tetrahedron Lett., 2015, 56, 6625 CrossRef CAS; (d) B. Drouillat, K. Wright, O. David and F. Couty, Eur. J. Org. Chem., 2012, 6005 CrossRef CAS; (e) F. Couty, O. David and B. Drouillat, Tetrahedron Lett., 2007, 48, 9180 CrossRef CAS; (f) F. Couty, O. David and F. Durrat, Tetrahedron Lett., 2007, 48, 1027 CrossRef CAS; (g) M. Vargas-Sanchez, S. Lakhdar, F. Couty and G. Evano, Org. Lett., 2006, 8, 5501 CrossRef CAS PubMed; (h) F. Couty, O. David, F. Durrat, G. Evano, S. Lakhdar, J. Marrot and M. Vargas-Sanchez, Eur. J. Org. Chem., 2006, 3479 CrossRef CAS.
- Representative examples: (a) G. Roagna, D. M. H. Ascough, F. Ibba, A. C. Vicini, A. Fontana, K. E. Christensen, A. Peschiulli, D. Oehlrich, A. Misale, A. A. Trabanco, R. S. Paton, G. Pupo and V. Gouverneur, J. Am. Chem. Soc., 2020, 142, 14045 CrossRef CAS PubMed; (b) D. Qian, M. Chen, A. C. Bissember and J. Sun, Angew. Chem., Int. Ed., 2018, 57, 3763 CrossRef CAS PubMed; (c) A. Feula, S. S. Dhillon, R. Byravan, M. Sangha, R. Ebanks, M. A. H. Salih, N. Spencer, L. Male, I. Magyary, W.-P. Deng, F. Müller and J. S. Fossey, Org. Biomol. Chem., 2013, 11, 5083 RSC; (d) S. Kenis, M. D’hooghe, G. Verniest, T. A. D. Thi, C. P. The, T. V. Nguyen and N. D. Kimpe, J. Org. Chem., 2012, 77, 5982 CrossRef CAS PubMed; (e) D.-H. Leng, D.-X. Wang, J. Pan, Z.-T. Huang and M.-X. Wang, J. Org. Chem., 2009, 74, 6077 CrossRef CAS PubMed; (f) S. Ma, D. H. Yoon, H.-J. Ha and W. K. Lee, Tetrahedron Lett., 2007, 48, 269 CrossRef CAS.
- Recent examples: (a) X. Zhou, B. Mao and Z. Zhang, Molecules, 2021, 26, 857 CrossRef CAS; (b) A. J. Boddy, C. J. Cordier, K. Goldberg, A. Madin, A. C. Spivey and J. A. Bull, Org. Lett., 2019, 21, 1818 CrossRef CAS PubMed; (c) M. L. Sarazen and C. W. Jones, Macromolecules, 2017, 50, 9135 CrossRef CAS; (d) Z. Wang, F. K. Sheong, H. H. Y. Sung, I. D. Williams, Z. Lin and J. Sun, J. Am. Chem. Soc., 2015, 137, 5895 CrossRef CAS PubMed.
- Recent examples: (a) G. Goswami, N. Chauhan, A. Mal, S. Das, M. Das and M. K. Ghorai, ACS Omega, 2018, 3, 17562 CrossRef CAS; (b) T. N. Nguyen and J. A. May, Org. Lett., 2018, 20, 3618 CrossRef CAS PubMed; (c) J. Luginina, J. Uzulena, D. Posevins and M. Turks, Eur. J. Org. Chem., 2016, 1760 CrossRef CAS , see also ref. 3a..
- For reviews on ring-opening of azetidines: (a) H. Mughal and M. Szostak, Org. Biomol. Chem., 2021, 19, 3274 RSC; (b) G. Masson, D. G. Pardo and J. Cossy, Chirality, 2021, 33, 5 CrossRef CAS PubMed; (c) G. S. Singh, Adv. Heterocycl. Chem., 2020, 130, 1 CrossRef CAS; (d) V. Mehra, I. Lumb, A. Anand and V. Kumar, RSC Adv., 2017, 7, 45763 RSC; (e) D. Antermite, L. Degennaro and R. Luisi, Org. Biomol. Chem., 2017, 15, 34 RSC; (f) F. Couty, B. Drouillat, G. Evano and O. David, Eur. J. Org. Chem., 2013, 2045 CrossRef CAS.
- E. Tayama, K. Watanabe and Y. Matano, Eur. J. Org. Chem., 2016, 3631 CrossRef CAS.
- A review: X. Zhang and C.-H. Tan, Chem, 2021, 7, 1451 CAS.
- Previous examples of ring-opening reaction of azetidine derivatives with fluoride (F−): L. Zhu, J. Xiong, J. Ah, N. Chen, J. Xue and X. Jiang, Org. Biomol. Chem., 2019, 17, 3797 RSC , see also ref. 2a..
- Ring-opening of three-membered N-heterocycle aziridine derivatives with fluoride (F−) were well known and applied in in various areas. For a representative review: (a) A. M. Remete and L. Kiss, Eur. J. Org. Chem., 2019, 5574 CrossRef CAS; (b) Representative recent examples: H. Wang, J. Li, Z. Li, B. Liu, K. Chen, Z. Zhang, Y. Hu, F. Zhou, Y. Li and K. Guo, Eur. Polym. J., 2020, 140, 109999 CrossRef CAS; (c) M. Morlot, F. Gourand and C. Perrio, Eur. J. Org. Chem., 2019, 3751 CrossRef CAS , see also ref. 8..
- M. K. Ghorai, A. Kumar and D. P. Tiwari, J. Org. Chem., 2010, 75, 137 CrossRef CAS PubMed.
- Previous examples of ring-opening of azetidinium salts with cyanide (CN−): see also ref. 1a, 1b, 1d, 1e, 2d and 2e.
- Preparation of allyl triflate: L. Jordi, S. Ricart, J. M. Viñas and J. M. Moretó, Organometallics, 1997, 16, 2808 CrossRef CAS.
- The experimental details were shown in the ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H, 13C, and 19F NMR spectra of substrates and products, preparation of substrates, and copies of chiral HPLC chromatogram of chiral compounds. See DOI: 10.1039/d1ra08706a |
| This journal is © The Royal Society of Chemistry 2021 |