Open Access Article
Open Access ArticleA TiO2 composite with graphitic carbon nitride as a photocatalyst for biodiesel production from waste cooking oil
Mahrukh Khana,
Humera Farah a,
Naseem Iqbal
a,
Naseem Iqbal *b,
Tayyaba Noor
*b,
Tayyaba Noor c,
M. Zain Bin Amjad
c,
M. Zain Bin Amjad b and
Syeda Sidrah Ejaz Bukharia
b and
Syeda Sidrah Ejaz Bukharia
aEarth and Environmental Sciences, Bahria University, Islamabad 44000, Pakistan. E-mail: naseem@uspcase.nust.edu.pk; Tel: +925190855281
bU.S.-Pakistan Center for Advanced Studies in Energy (USPCAS–E), National University of Sciences and Technology (NUST), Islamabad 44000, Pakistan
cSchool of Chemical and Materials Engineering (SCME), National University of Sciences and Technology, Islamabad, Pakistan
First published on 23rd November 2021
Abstract
Semiconductor-based photocatalysts have attracted a lot of interest due to their environmental friendliness and high stability. Waste cooking oil can be converted to biodiesel by the process of transesterification. A TiO2/g-C3N4 combination was prepared by using a wet impregnation process. The photocatalyst was analyzed by X-Ray Diffraction (XRD), Scanning Electron Microscopy (SEM), Energy Dispersive X-Ray spectroscopy (EDX), Thermogravimetric analysis (TGA), and Ultraviolet-visible spectroscopy (UV-vis). For effective transesterification, WCO was collected and acid-esterified to reduce the FFA concentration (below 3%). For the transesterification reaction, esterified WCO was used and the reactions were carried out under solar irradiation at 60 °C with an oil to methanol ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and stirred for 1 hour, using different TiO2/g-C3N4 catalysts (10, 20 & 30%) with different catalyst concentrations of 1%, 2% and 3%. The results showed that TiO2/20% g-C3N4 with 2% catalyst concentration gives the highest yield of biodiesel production (89.5%) as compared to other catalyst concentrations used. In addition to (FTIR), additional fuel characteristics such as density, viscosity, flashpoint, acid value, and pH were tested to determine the quality of the generated biodiesel and were found to comply with fuel standards. With high stability and good catalytic activity, the synthesized composite TiO2/g-C3N4 is a viable option for producing biodiesel from WCO.
9 and stirred for 1 hour, using different TiO2/g-C3N4 catalysts (10, 20 & 30%) with different catalyst concentrations of 1%, 2% and 3%. The results showed that TiO2/20% g-C3N4 with 2% catalyst concentration gives the highest yield of biodiesel production (89.5%) as compared to other catalyst concentrations used. In addition to (FTIR), additional fuel characteristics such as density, viscosity, flashpoint, acid value, and pH were tested to determine the quality of the generated biodiesel and were found to comply with fuel standards. With high stability and good catalytic activity, the synthesized composite TiO2/g-C3N4 is a viable option for producing biodiesel from WCO.
1. Introduction
In the current energy scenario, fossil-based fuels are being used to meet the majority of energy demand. Due to their environmental impact, these energy supplies will cause an energy crisis in the next several decades if they are used excessively.1,2 Furthermore, the scarcity of petroleum sources and rising oil costs have increased the demand to find a clean, environmentally friendly alternative fuel.3,4 Biodiesel is gaining more acceptance in the energy sector as a unique fuel due to its availability, eco-friendly, non-toxicity, and most importantly its production from renewable feedstocks.5,6 Biodiesel is composed of fatty acid methyl ester that is formed when oils are transesterified with alcohol in the presence of a catalyst.7 Several food oils can be used to produce biodiesel (castor oil, olive oil, and mustard oil) as well as non-consumable feedstock oils (Jatropha oil, Jojoba oil).8–10 Consumption of edibles oils has a food supply problem whereas crops that produce non-edible oils need additional farmland, limiting their utilization on a big scale. Other raw material sources must be identified to manufacture low-cost biodiesel.11When we cook meals, we consume a lot of vegetable oil, which may be used to produce biodiesel.12 As a result of its lower solubility in water, disposing of WCO in open areas will harm the environment. Hence the renewable energy research targets this oil used for biodiesel production being a cheap feedstock.13,14 A transesterification reaction was used to produce biodiesel in the presence of a catalyst (alkaline, acidic, and photocatalyst). WCO requires pretreatment before transesterification, constant exposure to heat during frying causes oxidation as well as a rise in the free fatty acid (FFA) concentration which increases the time and energy required for transesterification.15 For this reason, WCO for biodiesel production needs to be purified to minimize the amount of FFA. The WCO was collected from restaurants and the waste oil was firstly esterified before transesterification. Sulphuric acid was able to catalyze esterification in the presence of alcohol and WCO and after that, the esterified oil is then reacted with a catalyst to produce fatty acid methyl ester (FAME).16 There is a growing interest in semiconductor photocatalysis because of its possible applications in fields such as nature, solar energy, and renewable energy, as well as medicine. A variety of semiconductors, including ZnO, CdS, WO3, and TiO2, have been utilized as photocatalysts in the production of biodiesel from a wide range of oils.17–19 TiO2 nanoparticles have been a center of attraction for photocatalyst applications due to its non-toxicity, chemical stability, large surface area and less production cost. Graphitic carbon nitrides have also emerged as carbon nano materials for wide range of applications including photocatalyst due to their low-cost synthesis, high stability and good optical properties.
Here in this study, novel improved semiconductor-based photocatalyst TiO2/g-C3N4 composite was synthesized and characterized for their structural, morphological, and optical properties and it was utilized to carry out the transesterification reaction of WCO, which resulted in the cleaner production of biodiesel. Optimized reaction parameters were chosen for transesterification reaction under the photocatalytic condition. FTIR was used to characterize the synthesized biodiesel, and other fuel characteristics such as density, viscosity, flashpoint, acid value, and pH were also evaluated and compared with fuel standards. The improved reaction catalyst resulted in an 89.5% yield, and other fuel characteristics were determined to comply with ASTM requirements, indicating that TiO2/g-C3N4 is a promising candidate for biodiesel synthesis. This work shows the potential of cheap semiconductor-based photo-catalyst for biodiesel production using waste cooking oil as a cheaper feedstock for clean energy production.
2. Experimental
2.1. Materials and chemical reagents
Waste cooking oil was gathered from small eateries in nearby areas. Precursors utilized in the synthesis of TiO2 and g-C3N4 are titanium(IV) oxide (Sigma Aldrich, ≥99%, CAS number 12188-41-9) and melamine (Sigma Aldrich, ≥99%, CAS number 108-78-1). Methanol (analytical grade, 99%), ethanol (analytical grade, 99%), concentrated sulfuric acid (analytical grade, 99%), and deionized water (DI) are used as solvents for synthesis, esterification, and transesterification processes.2.2. Photocatalyst synthesis
2.3. Photocatalyst characterization
The photocatalyst crystal structure was investigated by using the X-ray diffraction (Bruker D8 Advance (Cu-kα)) the morphology and elemental composition were revealed using scanning electron microscopy (VEGA 3LMU, TESCAN), for thermogravimetric analysis (DTG-60/60H) is used to analyze thermal degradations of the synthesized photocatalyst. To analyze the optical properties of synthesized photocatalyst is observed under UV-visible light spectrum by PerkinElmer Lambda 900 Ultraviolet Spectrophotometer.2.4. Biodiesel production
To generate biodiesel, a two-step method, comprising esterification and transesterification, was employed. Esterification was employed before transesterification to reduce the high FFA concentration in WCO which might promote unwanted saponification during transesterification.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, reaction time 60 min, reaction temperature 60 °C. The reactions were carried out under solar irradiation at the U.S. Pakistan center for advanced studies in energy, National University of Science and Technology, NUST within the longitudes and latitudes 33.642319°E, 72.98451°N in Islamabad Capital Territory, Pakistan with the reaction mixture being exposed to sunlight between 11:00–13:00 Hrs of the day.
9, reaction time 60 min, reaction temperature 60 °C. The reactions were carried out under solar irradiation at the U.S. Pakistan center for advanced studies in energy, National University of Science and Technology, NUST within the longitudes and latitudes 33.642319°E, 72.98451°N in Islamabad Capital Territory, Pakistan with the reaction mixture being exposed to sunlight between 11:00–13:00 Hrs of the day.
 | (1) |
3. Results and discussion
XRD analysis of titanium dioxide nanoparticles, g-C3N4, and the composite of TiO2/g-C3N4 (10, 20 and 30%) was performed to determine the crystalline phase of the synthesized photocatalysts. Fig. 1(a) reveals the XRD patterns of TiO2 nanoparticles and pure g-C3N4, main diffraction peaks of TiO2 nanoparticles that appears at 2θ = 25.304°, 36.945°, 37.804°, 38.501°, 48.054°, 53.067°, 55.133°, 62.171°, 62.724°, 68.78°, 70.314°, 74.035°, 75.41°, and (76.104°) which corresponds to (101), (103), (004), (112), (200), (105), (211), (204), (116), (220), (107), (215), and (301) diffraction planes of tetragonal anatase TiO2 (JCPDS card no. 21-1272) respectively. Two main characteristics peaks were found in pure g-C3N4 at about 2θ = 13.14° and 27.57° which corresponds to (100) and (002) diffraction planes of hexagonal graphitic carbon nitride (JCPDS # 87-1526). Fig. 1(b) shows typical diffraction peaks of titanium dioxide and graphitic carbon nitride in TiO2/10% g-C3N4, TiO2/20% g-C3N4, and TiO2/30% g-C3N4 composite. Fig. 1(c) shows the increasing trend of the composition of g-C3N4 in composites. The diffraction peaks of g-C3N4 increased dramatically with the addition of 30% of g-C3N4 in the composite. As a result of the XRD data, several TiO2/g-C3N4 composites were effectively produced. TiO2 nanoparticles and pure g-C3N4 have average crystalline sizes of 28.4 nm and 5.05 nm respectively.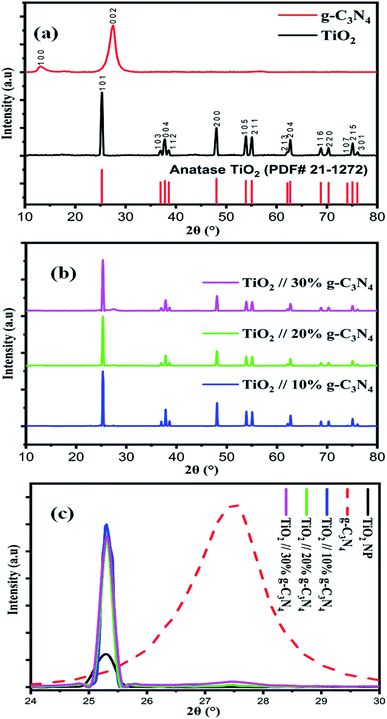 | ||
| Fig. 1 XRD spectrum of (a) TiO2 nanoparticles and g-C3N4 (b) composite TiO2/g-C3N4 (10, 20 and 30%), (c) trend of g-C3N4 in composites. | ||
The morphology of the synthesized material was revealed by using scanning electron microscopy. Fig. 2 shows SEM images of TiO2 nanoparticles, pure g-C3N4, and composite TiO2/g-C3N4 (10, 20, and 30%). The SEM images of TiO2 nanoparticles (a and b) and g-C3N4 (c) revealed an agglomeration of small spherical-like nanoparticles and stacked sheet-like structures. Similarly, the SEM image of composite (d–f) TiO2/g-C3N4 (10, 20 and 30%) showed that agglomerate TiO2 nanoparticles were attached on a sheet-like structure, the composite shows larger porosity indicates the higher number of catalytic sites. The elemental composition of the produced sample was determined using EDS. Table 1 shows the elemental composition of TiO2, g-C3N4, and their respective composites.
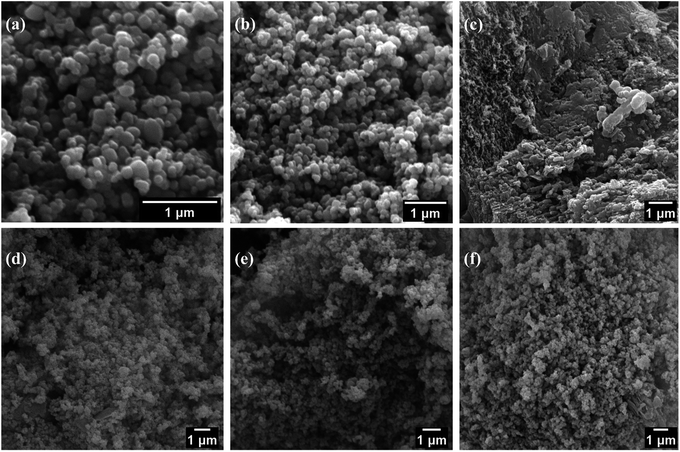 | ||
| Fig. 2 SEM images of (a and b) TiO2 nanoparticles (c) pure g-C3N4, (d) TiO2/10% g-C3N4, (e) TiO2/20% g-C3N4, and (f) TiO2/30% g-C3N4 composites. | ||
| TiO2 | GCN | TiO2/g-C3N4 (10%) | TiO2/g-C3N4 (20%) | TiO2/g-C3N4 (30%) | |
|---|---|---|---|---|---|
| Weight% | Weight% | Weight% | Weight% | Weight% | |
| Ti | 63.15 | 37.29 | 37.41 | 17.25 | |
| O | 36.85 | 53.49 | 43.99 | 40.29 | |
| C | 63.4 | 7.11 | 15.28 | 35.99 | |
| N | 36.6 | 2.11 | 3.32 | 6.47 |
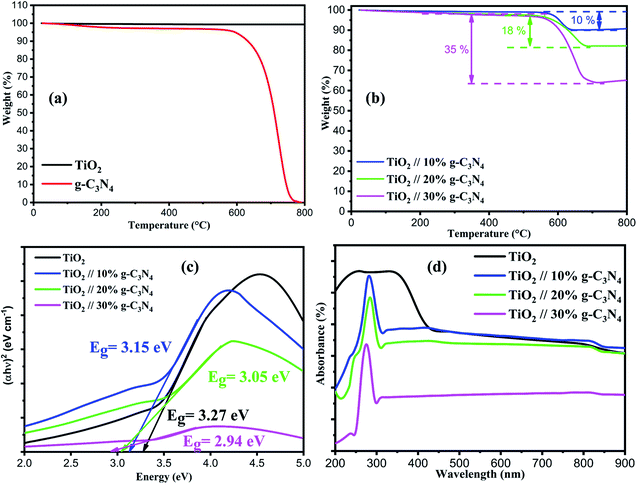
The thermal stability of the produced photocatalyst was investigated using TGA. Fig. 3(a and b) shows the TGA curves of TiO2 nanoparticles, g-C3N4, and composite TiO2/g-C3N4 (10, 20, and 30%). Throughout the whole temperature range (0–800 °C), the TGA curve of TiO2 nanoparticles shows no weight change. Similarly, at roughly 200 °C, the TGA curve of g-C3N4 loses 5 wt% weight, which has been attributed to the elimination of absorbed water. Following that, a rapid mass loss from 600 to 800 °C indicated the loss of tri-s-triazine-based units, which were decomposed at around 800 °C. TGA curve of composite TiO2/g-C3N4 (10, 20 and 30%) shows the sharp weight loses at 600 to 800 °C, and the mass loss was 10%, 18%, and 36% respectively, this is due to the decomposition of g-C3N4 at these temperatures, which also corresponds to the weight percentage of g-C3N in the corresponding composite material.
UV-vis was used to investigate the optical properties of the materials; absorption spectra were obtained and the bandgap of prepared photocatalysts was calculated by using Tauc plot. It can be observed in Fig. 3(d) that the TiO2 nanoparticles absorb ultraviolet strongly from 200 nm to 400 nm with less absorbance of visible light, UV-vis Spectra of the synthesized composite materials shows the sharp peak ranging in between the 270–285 nm can be associated with π → π* transition with the C and N centers of g-C3N4 linked as a composite material with TiO2, where N atoms are treated as an n-dopant type in g-C3N4, thus the in composite material this band is most likely to related to the appearance of free electron conduction in g-C3N4 unit and within TiO2 nanoparticles and 2D sheets of g-C3N4. The UV-vis in between 200 nm to 400 nm of pristine TiO2 and composite material is showing typical semiconductor absorption behavior which can be ascribed to the charge transfer response of TiO2 and g-C3N4 from valence to the conduction band. Accordingly, the (Tauc plot) of (αhv) 2 versus energy (eV) are shown in Fig. 3(c), corresponding bandgap energies of TiO2 nanoparticles and composites TiO2/g-C3N4 (10%, 20% and 30%) were estimated to be 3.27, 3.15 3.05 and 2.94 eV respectively. The resulting narrow bandgap of the composite TiO2/g-C3N4 shows that the band gap of TiO2 was found to be lowered by the addition respective wt% of g-C3N4. The conduction band edge of graphitic carbon nitride (g-C3N4) is higher than that of TiO2, which prevents the photogenerated electron–hole pairs from recombination during photocatalytic processes. Therefore, graphitic carbon nitride was used as an efficient co-catalyst photocatalyst to improve the photocatalytic activity of the TiO2.23
The waste cooking oil collected from eateries was firstly filtered to remove any particulate matter and then was heated to 80 °C to remove any water content present in the oil, then it is characterized based on certain physical properties including FFA content, density, and viscosity of the oil. Table 2 shows the physical properties of collected waste cooking oil before its conversion to biodiesel are mentioned. Transesterification reaction occurs when oil FFA content is less than 3%, thus WCO oil is acid esterified and the FFA level is reduced down to less than 3 percent, then transesterification is performed with varied catalyst concentrations.
| Properties | Waste cooking oil | Bio-diesel produced (using TiO2/g-C3N4 20% (2%)) | Standard limit |
|---|---|---|---|
| FFA content (wt% of oil) | 10 | <3 for transesterification | |
| pH | 6.2 | 7.1 | 7–9 |
| Flash point (°C) | 110 | 93 (ASTM D93) | |
| Viscosity (mm2 s−1) | 3.38 | 4.65 | 1.9–6.0 (ASTM D445) |
| Acid value (mg KOH−1 g−1) | 0.5 | 0.5 (ASTM D664) | |
| Density (kg m−3) | 911 | 890 | 860–900 (EN ISO 3675) |
The amount of biodiesel produced as compared with the amount of feedstock used mainly depends on the catalyst used. Therefore, the effect of three different catalyst concentration TiO2/10% g-C3N4 (1, 2 and 3%), TiO2/20% g-C3N4 (1, 2 and 3%) and TiO2/30% g-C3N4 (1, 2 and 3%) on the yield of biodiesel was studied. Out of them the composite TiO2/g-C3N4 (30%) gives no or very less yield (i.e., <10%) because of the congealing temperature is lower than conventional biodiesel, which is why it becomes a jelly-like substance within a fraction of minutes. The obtained results for TiO2/10% g-C3N4 (1, 2 and 3%) and TiO2/20% g-C3N4 (1, 2 and 3%) catalysts show the biodiesel yield of 65%, 70%, 74%, 78%, 89.5% and 68% respectively. It can be seen that product yield varies depending on catalyst concentration. According to the data, the biodiesel yield of 65% was found to be lowest at a catalyst concentration of TiO2/10% g-C3N4 (1% w/w) of oil. The highest biodiesel production of 89.5% was found at a catalyst concentration of TiO2/20% g-C3N4 (2% w/w), which was related to an increase in catalytic sites participating in WCO transesterification processes, and afterward, the yield declined.
The surface functional group of the produced biodiesel by WCO using synthesized TiO2 and its composites used in different wt% ratio and conventional diesel were identified via FTIR was shown in Fig. 4(a and b). In the region of 650 cm−1 to 4000 cm−1, the FTIR spectra were examined. Biodiesel's absorption peak was discovered at 3466.83 cm−1, which corresponds to the bending of O–H bonds in alkyl esters. The alkane cluster C–H is stretched unevenly in the strong band at 2850.87 cm−1. At 1740.71 cm−1, the oxygen functional group in biodiesel was identified as symmetrical carbonyl group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The aromatic cluster C–H stretching vibrations are assigned to the band at 1455.57 cm−1. The presence of oxygen components in biodiesel provides cleaner and complete combustion, unlike conventional diesel. A mixture of bending and stretching aromatic compounds C–H is responsible for the remaining peaks at 1023.73 and 851.30 cm−1. The absorption peak at 1651.90, 1363.49, and 720.39 cm−1 confirms the presence of long-chain fatty acids in biodiesel. The regular diesel's spectrum resembled that of biodiesel in parts, particularly in the 3000–2850 cm−1 range. A little C
O. The aromatic cluster C–H stretching vibrations are assigned to the band at 1455.57 cm−1. The presence of oxygen components in biodiesel provides cleaner and complete combustion, unlike conventional diesel. A mixture of bending and stretching aromatic compounds C–H is responsible for the remaining peaks at 1023.73 and 851.30 cm−1. The absorption peak at 1651.90, 1363.49, and 720.39 cm−1 confirms the presence of long-chain fatty acids in biodiesel. The regular diesel's spectrum resembled that of biodiesel in parts, particularly in the 3000–2850 cm−1 range. A little C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration was also identified at 1740.42 cm−1. The peak in the region from 1740–1750 cm−1 is for diesel.
O vibration was also identified at 1740.42 cm−1. The peak in the region from 1740–1750 cm−1 is for diesel.
 | ||
| Fig. 4 FTIR spectrum of (a) biodiesel produced by different catalysts by using different catalyst concentrations and (b) WCO biodiesel and conventional diesel. | ||
The fuel properties of produced biodiesel such as pH, flash point, kinematic viscosity, relative density, acid number were compared with standards represented in Table 2. The pH value of biodiesel must be taken into consideration. The pH of waste cooking oil biodiesel is neither acidic nor basic. The pH value of biodiesel produced by WCO using TiO2/20% g-C3N4 is 7.1 which falls within the ASTM standard limits. The standard value of flashpoint for biodiesel should be 93 °C or above. The higher flashpoint reduces the chance of unexpected fire hazards. The flashpoint of biodiesel produced by WCO using TiO2/20% g-C3N4 having a value of 110 °C that falls within the ASTM standard limits.
The average viscosity of WCO biodiesel was found to be 4.65 mm2 s−1 using TiO2/20% g-C3N4 when used in 2% concentration. WCO biodiesel has a viscosity that is within ASTM standard limits (1.9–6.0 mm2 s−1) and characteristics that are substantially equivalent to petro-diesel (1.9–4.1 mm2 s−1). The amount of free fatty acids is measured by acid numbers. Oil hydrolysis produces considerable free fatty acids when the acid level is high. The higher the acid number, the lower the oil quality. The acid value of biodiesel produced by WCO using TiO2/20% g-C3N4 was found to be 0.50 mg KOH−1 g−1. The average density of biodiesel produced by WCO using TiO2/20% g-C3N4 was found to be 890 kg m−3. Our findings support the findings of,22 which found that the density of waste oil (850 kg m−3) was more than petro diesel.
The photocatalyst TiO2/g-C3N4 composite was successfully synthesized for biodiesel production from WCO. The detailed characterization of the prepared photocatalyst (TiO2/10% g-C3N4 and TiO2/20% g-C3N4) was carried out, in which XRD indicates the formation of anatase TiO2 nanoparticles and g-C3N4 with crystal sizes of 28.4 nm and 5.05 nm respectively, SEM images of the composite TiO2/g-C3N4 shows larger porosity, which indicates the higher number of catalytic sites, the thermal stability of the composites TiO2/g-C3N4 (10, 20 and 30%) shows that have great stability against high temperature. UV-vis analysis shows the decreasing bandgaps of 3.27 eV, 3.15 eV, 3.05 eV, and 2.94 eV of TiO2 nanoparticles with the addition of g-C3N4, in 10%, 20%, and 30% concentration. The synthesized photocatalyst was then employed for biodiesel production from WCO. For transesterification with different catalysts TiO2/g-C3N4 (10, 20 & 30%) with different catalysts concentrations (1, 2 and 3%) was experimented. The highest FAME yield (89.5%) was achieved when TiO2/20% g-C3N4, 2% catalyst concentration was used as compared to other catalyst concentrations. The FTIR analysis of WCO biodiesel confirms the presence of specific characteristic peaks as compared with regular petro-diesel. Other fuel properties of WCO biodiesel such as density, viscosity, flashpoint, acid value, and pH, were tested, their values are found in good agreement with certain fuel standards. Hence the outcomes of this study reveal that the photocatalytic efficiency of composite TiO2/g-C3N4 for the synthesis of biodiesel from waste cooking oil is greatly improved. Fig. 5 and Table 3 represent a study comparison of this study with recent work done in the domain of TiO2 based photocatalyst for the transesterification process, which ultimately shows that the TiO2/g-C3N4 (20%) when used in 2% concentration for transesterification yields the biodiesel up to 89.5% has a certain advantage over the certain parameters including oil to methanol ratio, reaction time and temperature when compared with recent work done using similar catalysts, which indicated the higher photocatalytic activity of TiO2/g-C3N4 (20%) due to which the transesterification reaction is faster and requires less reaction temperature and use of methanol is in moderate quantity which indicates that the catalyst is performing well under photocatalytic conditions to convert the maximum quantity of oil to biodiesel. The composite TiO2/g-C3N4 can be used to produce low-cost biodiesel from low-cost feedstocks (WCO) for sustainable energy.
 | ||
| Fig. 5 Represent the recent catalyst yields of similar catalysts used for biodiesel production for transesterification. | ||
| Feed stock oil | Catalyst | Oil to methanol ratio | Temperature (°C) | Time (min) | Yield (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| 1 | WCO | TiO2/g-C3N4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 09 09 |
60 | 60 | 84.9 | This work |
| 2 | Waste olive oil | TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
120 | 240 | 91.2 | 24 |
| 3 | Kemiri sunan oil | TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
60 | 90 | 84 | 25 |
| 4 | Waste cooking olive oil | TiO2 NT | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 08 08 |
65 | 180 | 91.2 | 26 |
| 5 | WCO | TiO2/RGO | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
65 | 180 | 98 | 27 |
| 6 | WCO | TiO2/RGO nano composite | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 08 08 |
65 | 60 | 89 | 28 |
| 7 | Palm oil | TiO2-GO | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 09 09 |
70 | 40 | 96.73 | 29 |
| 8 | Canola oil | Li/TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
55 | 90 | 98 | 30 |
| 9 | Palm oil | Cu/TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
45 | 45 | 90.93 | 31 |
| 10 | Microalgae biomass | CaO/TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 08 08 |
55 | 120 | 51.6 | 32 |
| 11 | Jatropha curcas L. seed oil | SO42−/TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 08 08 |
65 | 60 | 93.7 | 33 |
| 12 | Canola oil | g-C3N4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
60 | 90 | 88 | 34 |
| 13 | Canola oil | g-C3N4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
150 | 180 | 90 | 35 |
| 14 | WCO | La3+/ZnO–TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
65 | 180 | 87 | 35 |
| 15 | WFO | Fe3O4@ZIF-8/TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
50 | 65 | 80 | 2 |
4. Conclusions
The photocatalyst TiO2/g-C3N4 composite was synthesized and employed in the transesterification reaction of waste cooking oil into biodiesel. Under optimum reaction conditions of 60 °C using oil to methanol ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, and 1 hour of reaction under sunlight, maximum biodiesel conversion of 89.5% using the catalyst concentration of TiO2/20% g-C3N4, 2 wt% was achieved. Biodiesel produced was characterized by the FTIR technique and its fuel properties were found to be within standards. Biodiesel may be made from cheap feedstocks using semiconductor based photocatalyst TiO2/g-C3N4 (20%) photocatalysts, lowering the cost of the fuel and reducing environmental issues.
9, and 1 hour of reaction under sunlight, maximum biodiesel conversion of 89.5% using the catalyst concentration of TiO2/20% g-C3N4, 2 wt% was achieved. Biodiesel produced was characterized by the FTIR technique and its fuel properties were found to be within standards. Biodiesel may be made from cheap feedstocks using semiconductor based photocatalyst TiO2/g-C3N4 (20%) photocatalysts, lowering the cost of the fuel and reducing environmental issues.
Author contributions
M. K. (MS Student) conducted the research and investigation process and wrote the original draft, H. F. (Senior Associate Professor) supervised the student, N. I. (Professor) supervised the student and revised the manuscript, T. N. (Associate Professor) supervised the student, M. Z. B. A. (MS Student) assisted in completing characterization results and experimentation, S. S. E. B. (MS Student) participated across the entire investigation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to U.S-Pakistan Center for Advance Studies in Energy, NUST, Islamabad and Bahria University, Islamabad for providing all the necessities and financial aid to complete the research project.References
- R. A. Meena, J. Banu, R. Kannah, K. Yogalakshmi and J. Kumar, Biohythane production from food processing wastes – Challenges and perspectives, Bioresour. Technol., 2020, 298, 122–449 CrossRef.
- A. Sabzevar, M. Ghahramaninezhad and M. Shahrak, Enhanced biodiesel production from oleic acid using TiO2-decorated magnetic ZIF-8 nanocomposite catalyst and its utilization for used frying oil conversion to valuable product, Fuel, 2020, 288, 119–586 Search PubMed.
- M. Hossain, M. Bhuyan, A. Alam and Y. Seo, Biodiesel from hydrolyzed waste cooking oil using a S-ZrO2/SBA-15 super acid catalyst under sub-critical conditions, Energies, 2018, 11, 212–323 CrossRef.
- M. Borah, A. Das, V. Das, N. Bhuyan and D. Deka, Transesterification of waste cooking oil for biodiesel production catalyzed by Zn substituted waste egg shell derived CaO nanocatalyst, Fuel, 2019, 242, 345–354 CrossRef CAS.
- J. Gardy, A. Osatiashtiani, O. Cespedes, A. Hassanpour, X. Lai, A. Lee, K. Wilson and M. Rehan, A magnetically separable SO4/Fe–Al–TiO2 solid acid catalyst for biodiesel production from waste cooking oil, Appl. Catal., B, 2018, 234, 268–278 CrossRef CAS.
- S. Bhatia, R. Gurav, T. Choi, Y. Han, Y. Park, H. Jung, S. Yang, H. Song and Y. Yang, A clean and green approach for odd chain fatty acids production in Rhodococcus sp. YHY01 by medium engineering, Bioresour. Technol., 2019, 286, 121383 CrossRef CAS.
- M. Harabi, S. Bouguerra, F. Marrakchi, L. Chrysikou, S. Bezergianni and M. Bouaziz, Biodiesel and crude glycerol from waste frying oil: production, characterization and evaluation of biodiesel oxidative stability with diesel blends, Sustainable, 2019, 11, 1937 CrossRef CAS.
- G. Corro, U. Pal and N. Tellez, Biodiesel production from Jatropha curcas crude oil using ZnO/SiO2 photocatalyst for free fatty acids esterification, Appl. Catal., B, 2013, 129, 39–47 CrossRef CAS.
- I. Thushari and S. Babel, Sustainable utilization of waste palm oil and sulfonated carbon catalyst derived from coconut meal residue for biodiesel production, Bioresour. Technol., 2018, 248, 199–203 CrossRef CAS.
- L. Buchori, D. Ubay and K. Syahidah, Biodiesel production from waste cooking oil purified with activated charcoal of Salak peel, Reaktor, 2018, 18, 149–154 CrossRef.
- S. Bhatia, R. Gurav, T. Choi, Y. Han, Y. Park, H. Jung, S. Yang, H. Song and Y. Yang, Effect of synthetic and food waste-derived volatile fatty acids on lipid accumulation in Rhodococcus sp. YHY01 and the properties of produced biodiesel, Energy Convers. Manage., 2019, 192, 385–395 CrossRef.
- M. Loizides, X. Loizidou, D. Orthodoxou and D. Petsa, Circular bioeconomy in action: Collection and recycling of domestic used cooking oil through a social, reverse logistics system, Recycling, 2019, 4, 16 CrossRef.
- S. Keera, S. Sabagh and A. Taman, Transesterification of vegetable oil to biodiesel fuel using alkaline catalyst, Fuel, 2011, 1, 42–47 CrossRef.
- D. Ackbarali, R. Maharaj, N. Mohamed and V. Harry, Potential of used frying oil in paving material: solution to environmental pollution problem, Environ. Sci. Pollut. Res., 2017, 24, 12220–12226 CrossRef PubMed.
- D. Thoai, P. Hang and D. Lan, Pre-treatment of waste cooking oil with high free fatty acids content for biodiesel production: an optimization study via response surface methodology, Vietnam J. Chem., 2019, 57, 568–573 CrossRef.
- M. Li, Y. Zheng, Y. Chen and X. Zhu, Biodiesel production from waste cooking oil using a heterogeneous catalyst from pyrolyzed rice husk, Bioresour. Technol., 2014, 154, 345–348 CrossRef CAS.
- M. Rahman, Fundamentals of Semiconductor Photocatalysis, Concepts Semicond. Photocatal., 2019, 1–7 Search PubMed.
- G. Corro, N. Sánchez, U. Pal, S. Cebada, J. Luis and G. Fierro, Solar-irradiation driven biodiesel production using Cr/SiO2 photocatalyst exploiting cooperative interaction between Cr6+ and Cr3+ moieties, Appl. Catal., B, 2016, 203, 43–52 CrossRef.
- A. Olivo, D. Zanardo, E. Ghedini, F. Menegazzo and M. Signoretto, Solar fuels by heterogeneous photocatalysis: from understanding chemical bases to process development, Chem. Eng., 2018, 3, 32 Search PubMed.
- H. Zhu, D. Chen, D. Yue, Z. Wang and H. Ding, In situ synthesis of g-C3N4-P25 TiO2 composite with enhanced visible light photoactivity, J. Nanopart. Res., 2014, 10, 1–10 Search PubMed.
- H. Liu, Z. Zhang, H. He, X. Wang, J. Zhang, Q. Zhang, Y. Tong, H. Liu, S. Ramakrishna, S. Yan and Y. Long, One-step synthesis heterostructured g-C3N4/TiO2 composite for rapid degradation of pollutants in utilizing visible light, Nanomaterials, 2018, 8, 1–15 Search PubMed.
- J. Marchetti and A. s. Errazu, Esterification of free fatty acids using sulfuric acid as catalyst in the presence of triglycerides, Biomass Bioenergy, 2008, 32, 892–895 CrossRef.
- T. Isimjan, S. Rasul, M. Alouf, M. Khan, I. Alhowaish and T. Ahmed, Rational design of Pd-TiO2/g-C3N4 heterojunction with enhanced photocatalytic activity through interfacial charge transfer, Clean Energy, 2019, 3(1), 59–68 CrossRef.
- T. Mihankhah, M. Delnavaz and N. Khaligh, Application of TiO2 nanoparticles for eco-friendly biodiesel production from waste olive oil, Int. J. Green Energy, 2018, 15, 69–75 CrossRef CAS.
- A. Redjeki and S. Sukirno, Photocatalytic esterification process for methyl ester synthesis from kemiri sunan oil: a novel approach, AIP Conf. Proc., 2019, 2085, 020058 CrossRef.
- N. Khaligh, T. Mihankhah, Z. Shahnavaz, L. Zaharania and M. John, Solar energy and TiO2 nanotubes: biodiesel production from waste cooking olive oil, Environ. Prog. Sustainable Energy, 2021, 40, e13537 CrossRef.
- M. Borah, A. Devi, R. Saikia and D. Deka, Biodiesel production from waste cooking oil catalyzed by in situ decorated TiO2 on reduced graphene oxide nanocomposite, Energy, 2018, 158, 881–889 CrossRef.
- S. Soltani, N. Khanian, T. Choong, N. Asim and Y. Zhao, Microwave-assisted hydrothermal synthesis of sulfonated TiO2-GO core–shell solid spheres as heterogeneous esterification mesoporous catalyst for biodiesel production, Energy Convers. Manage., 2021, 238, 114165 CrossRef.
- A. Alsharifi, H. Znad, S. Hena and M. Ang, Biodiesel production from canola oil using novel Li/TiO2 as a heterogeneous catalyst prepared via impregnation method, Renewable Energy, 2017, 114, 1077–1089 CrossRef.
- A. De and S. Boxi, Application of Cu impregnated TiO2 as a heterogeneous nanocatalyst for the production of biodiesel from palm oil, Fuel, 2020, 265, 117019 CrossRef.
- M. Aghilinategh, M. Barati and M. Hamadanian, Supercritical methanol for one put biodiesel production from chlorella vulgaris microalgae in the presence of CaO/TiO2 nano-photocatalyst and subcritical water, Biomass Bioenergy, 2019, 123, 34–40 CrossRef.
- C. Chen, L. Cai, X. Shangguan, L. Li, Y. Hong and G. Wu, Heterogeneous and efficient transesterification of Jatropha curcas L. seed oil to produce biodiesel catalysed by nano-sized SO42−/TiO2, R. Soc. Open Sci., 2018, 5, 30564419 Search PubMed.
- M. Devi, M. Barbhuiya and B. Das, Modified mesoporous graphitic carbon nitride: a novel high-performance heterogeneous base catalyst for transesterification reaction, Sustainable Energy Fuels, 2020, 4, 3537–3545 RSC.
- T. deMedeiros, A. Macina and R. Naccache, Graphitic carbon nitrides: efficient heterogeneous catalysts for biodiesel production, Nano Energy, 2020, 78, 105306 CrossRef.
- M. Guo, W. Jiang, C. Chen, S. Qu, W. Yi and J. Ding, Process optimization of biodiesel production from waste cooking oil by esterification of free fatty acids using La3+/ZnO-TiO2 photocatalyst, Energy Convers. Manage., 2020, 229, 113745 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |