Open Access Article
Open Access ArticleTemperature sensing in Tb3+/Eu3+-based tetranuclear silsesquioxane cages with tunable emission†
Karina Nigoghossian *a,
Alena N. Kulakovabc,
Gautier Félix
*a,
Alena N. Kulakovabc,
Gautier Félix a,
Victor N. Khrustalevcd,
Elena S. Shubina
a,
Victor N. Khrustalevcd,
Elena S. Shubina b,
Jérôme Long
b,
Jérôme Long a,
Yannick Guari
a,
Yannick Guari a,
Saad Senea,
Luís D. Carlos
a,
Saad Senea,
Luís D. Carlos e,
Alexey N. Bilyachenko
e,
Alexey N. Bilyachenko *bc and
Joulia Larionova
*bc and
Joulia Larionova *a
*a
aICGM, Univ. Montpellier, CNRS, ENSCM, Montpellier, France. E-mail: karina.nigoghossian@umontpellier.fr; joulia.larionova@umontpellier.fr
bNesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Vavilova str., 28, Moscow, 119991, Russia. E-mail: bilyachenko@ineos.ac.ru
cPeoples' Friendship University of Russia (RUDN), Miklukho-Maklay Str., 6, Moscow, 117198, Russia
dZelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Leninsky Prospect 47, Moscow 119991, Russia
ePhantom-g, Physics Department and CICECO – Aveiro Institute of Materials, University of Aveiro, Aveiro, 3810-193, Portugal
First published on 27th October 2021
Abstract
New luminescent cage-like tetranuclear silsesquioxanes [NEt4][(Ph4Si4O8)2(Tb3Eu)(NO3)4(OH)(EtOH)3(H2O)]·4(EtOH) (1) and [NEt4]2[(Ph4Si4O8)2(Tb2Eu2)(NO3)6(EtOH)2(MeCN)2]·4(MeCN) (2) present a tunable thermosensitive Tb3+-to-Eu3+ energy transfer driven by Tb3+ and Eu3+ emission and may be used as temperature sensors operating in the range 41–100 °C with excellent linearity (R2 = 0.9990) and repeatability (>95%). The thermometer performance was evidenced by the maximum relative sensitivity of 0.63% °C−1 achieved at 68 °C.
Introduction
Cage-like metallasilsesquioxanes (CLMSs) are an exciting family of molecule-based architectures of kaleidoscopic structural diversity built from metal ions or lanthanides and silsesquioxane ligands.1,2 Their design benefits from both coordination and sol–gel chemistries, and for this reason these compounds are also described as hybrid organic–inorganic materials.1,3 Indeed, the presence of silsesquioxanes, (RSiO1.5)n (where n = 6, 8, 10, 12, …), permits construction of inorganic Si–O–Si skeletons as a basic structural unit realizing cyclic and polycyclic types of matrixes, which offer to these architectures chemical stability, mechanical robustness, thermal stability, and the possibility to form a cage-like topology providing porosity and host–guest properties.4 Moreover, these ligands can provide several modes of metal ion coordination, which explains the important diversity of structural arrangements of polyhedral cages, which can be designed in various shapes including adamantane, cooling tower, birdcage, cube, drum, sandwich-like, Asian lantern, and others.1,2 On the other hand, the presence of various metal ions (for instance Mg2+, Ca2+, Al3+, Sn4+, etc.), transition metal ions (such as Pt2+/4+, Cr3+, Mn2+, Ti3+, Fe3+, Ni2+, Cu2+ or their combinations) or lanthanides (such as Yb3+, Nd3+, Er3+, Ce3+, Eu3+, Sm3+ or Pr3+) usually situated in the core of CLMSs not only ensures the assembly of the whole architecture but also brings functional physical and chemical properties.5 Among those, CLMSs have been widely investigated as molecule-based models for catalysis6 or as a particular family of molecule-based magnets.7 Unexpectedly, the optical properties of CLMSs have been investigated only scarcely. We can cite only one work reporting on luminescence in a mononuclear Eu3+ based CLMS, but its crystal structure has not been clarified.8 In this line of thought, we recently reported on the first series of luminescent tetranuclear lanthanide ions containing CLMSs presenting Ln3+ characteristic emission and interesting magnetic properties.9,10 However, the employment of CLMSs as temperature emissive sensors has never been reported up to now.Indeed, accurate temperature measurements are an important issue for innovative technologies in a wide range of fields, including electronics, photonics, and biology. The conventional thermometers require close contact between the probe and the specimen, which hampers temperature sensing with high spatial resolution. Luminescent thermometry enables remote sensing by monitoring the emission of a probe.11 Several types of luminescent materials can be used for temperature sensing, including Ln3+-based complexes, semiconductor nanocrystals (quantum dots), and organic dyes.11 The former allows designing thermometry self-referencing methods based on the luminescence intensity ratio (LIR) of two distinct Ln3+ transitions,12 which are not affected by variations in probe concentration, excitation power, detection system, and others.11 Among them, materials based on a solid solution of Eu3+/Tb3+ materials have widely been proposed as temperature sensors13–16 using the LIR between Tb3+ green and Eu3+ red emissions (5D4 → 7F5 and 5D0 → 7F2 transitions, respectively). The nature of the host lattice and ligands highly affects the temperature dependence of both emissions. The ratiometric Eu3+/Tb3+ luminescent thermometers are designed from a simple physical mixture of mononuclear complexes of both Ln3+ (ref. 13–16) or as a solid solution of mono or polynuclear complexes containing both Ln3+ in the same compound.17–19 In the latter case, the proximity of Ln3+ emitting centers enables energy transfer (ET) among them, which affects the green-to-red LIR. Therefore, the thermometric parameter (LIR) can be adjusted by varying the Ln3+ composition, and serve as an interesting strategy to optimize the thermometer performance.19
In this work, we report on tunable emission of tetranuclear CLMSs, linked to the Tb3+-to-Eu3+ energy transfer (ET), which may be used for temperature sensing. The investigation on the sensing properties reveals great stability and repeatability after multiple heating/cooling cycles. This important stability to photobleaching and a relatively high working temperature (to 100 °C) are ensured by the presence of a siloxane matrix, which plays a protective role.
Results and discussion
The synthesis of [NEt4] [(Ph4Si4O8)2(Tb3Eu)(NO3)4(OH)(EtOH)3(H2O)]·4(EtOH) 1 and [NEt4]2[(Ph4Si4O8)2(Tb2Eu2)(NO3)6(EtOH)2(MeCN)2]·4(MeCN) 2 compounds has been performed by adapting the previously described procedure consisting in a two-step reaction involving, first: the in situ formation of phenylsiloxanolate [PhSi(O)ONa]x species with their following self-assembling reaction with Et4NCl and the corresponding Tb3+ and Eu3+ salts with the Tb3+/Eu3+ ratio equal to 3/1 for 1 and 2/2 for 2, respectively. X-ray diffraction analysis performed on single crystals of 1 and 2 indicates that these compounds crystalize in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (Table S1, ESI†). The crystal structure of 2 is similar to those previously published pure Eu and Tb analogues.9
space group (Table S1, ESI†). The crystal structure of 2 is similar to those previously published pure Eu and Tb analogues.9
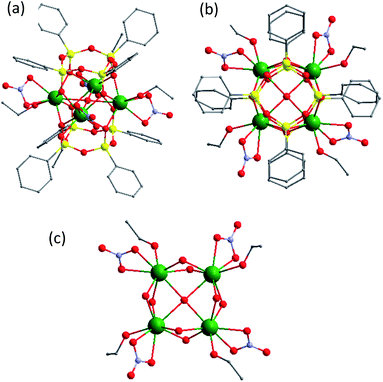
Briefly, the molecular structure may be described as a (Tb1−xEuxO2)4 core (where x = 0.25 for 1 and 0.50 for 2) caught between two tetraphenylcyclotetrasiloxanolates and assembled in a prism-like polyhedron (Fig. 1, S1, S3 and S4, ESI†). The cores are constituted by four statistically distributed Tb/Eu ions linked through oxygen atoms forming a distorted square. In both compounds, there are two different eight coordinated Tb/Eu sites, which adopt a distorted square antiprism geometry. Each lanthanide ion in 1 is coordinated by four bridging oxygens and three oxygens from terminal nitrate and ethanol. The tetranuclear cycle encapsulates also an hydroxyl group situated in the centre. In 2, one lanthanide site is coordinated by four bridging oxygen atoms and four oxygen atoms from two terminal nitrate ligands, while another is linked to four bridging oxygens, three oxygens from one terminal nitrate, and one ethanol and nitrogen from acetonitrile. The main distances and angles are given in ESI (Table S2†). The anionic CLMS molecules are aligned along the b axis and alternated with Et4N+ cations (Fig. S2 and S5, ESI†). The atomic Tb/Eu ratio determined by SEM-EDX is equal to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 1 and 2, respectively, confirming the expected Tb/Eu ratio in the core of CLMSs (Table S3, ESI†). The thermogravimetric analysis indicates that the complexes 1 and 2 start to decompose from 240 °C (Fig. S6, ESI†).
1 for 1 and 2, respectively, confirming the expected Tb/Eu ratio in the core of CLMSs (Table S3, ESI†). The thermogravimetric analysis indicates that the complexes 1 and 2 start to decompose from 240 °C (Fig. S6, ESI†).
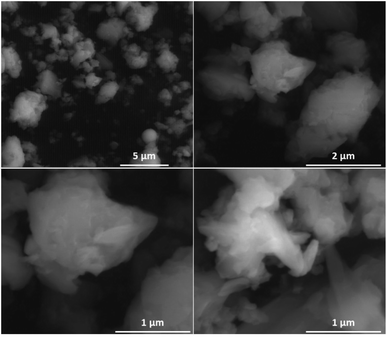
The morphological characterisations have been performed by Scanning Electronic Microscopy (SEM), which indicates the presence of microparticles in samples (Fig. 2 and S7, ESI†). The profile curve permitting to probe the chemical analysis indicates the homogeneity of sample.
The magnetic measurements performed for both compounds are perfectly coherent with the presence of 75 and 50% occupation of Tb3+ ions (7F6, S = 3, L = 3, g = 3/2, χT = 11.82 cm3 K mol−1) for 1 and 2, respectively, since Eu3+ is diamagnetic (Fig. S8, ESI†).
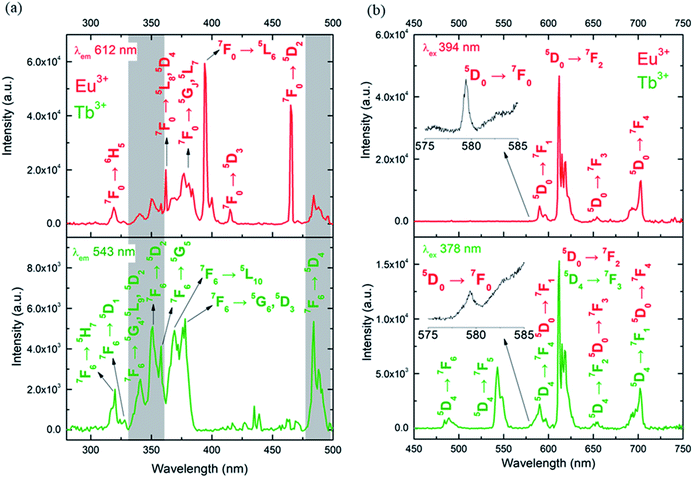
The photoluminescence of CLMSs in the solid-state was first investigated at room temperature. The excitation spectra of 1 were recorded by monitoring the main emissions of Eu3+ at 612 nm (5D0 → 7F2) and Tb3+ at 543 nm (5D4 → 7F5) (Fig. 3a). The diverse observed lines are assigned to intra-4f transitions of Eu3+ (ref. 20) and Tb3+.21 Its luminescence properties are similar to those of previously published single Eu3+ or Tb3+ containing CLMSs.9 The maximum intensity of excitation for Eu3+ red emission is located at 394 nm, which corresponds to the Eu3+ transition 7F0 → 5L6. As for Tb3+ green emission, the highest excitation intensity is observed at 378 nm, which is attributed to the transition 7F6 → 5G6,5D3. The Tb3+-to-Eu3+ ET is evidenced by the presence of the Tb3+ transition lines in the excitation spectra monitored in the Eu3+ emission (at 612 nm).
The emission spectra of 1 were measured under excitation at 394 nm and 378 nm (Fig. 3b). Upon 394 nm irradiation, the Eu3+ ion is selectively excited to the 5L6 energy level, and, subsequently, non-radiative relaxations lead to the population of the 5D0 emitting state. The Eu3+ transitions are then observed from 5D0 to 7FJ (J = 0–4) manifold. The excitation at a higher energy state, under 378 nm irradiation, leads to the emissions of Tb3+ from 5D4 to 7FJ (J = 6–2) manifold, as well as Eu3+ transitions (5D0 → 7FJ, J = 0–4). This result indicates an intramolecular Tb3+-to-Eu3+ ET considering the relatively short distances between lanthanides in the CLMS core (the shortest direct Ln3+–Ln3+ distance in 1 is 3.5087 Å and the shortest Ln3+–Ln3+ distance through the bridging oxygen atom is 4.6616 Å, Table S2 and Fig. S1, ESI†).22 Furthermore, the observation of the very weak transition 5D0 → 7F0 at 579 nm visible in both emission spectra (insets of Fig. 3b), indicates low symmetry of the Eu3+ coordination environment. The presence of one sharp peak at this region suggests rather a single chemical environment.23
The excitation and emission spectra of 2 show some differences in comparison with 1 (Fig. S9, ESI†). In particular, the emission spectrum under excitation at 378 nm exhibits lower green-to-red (543 to 612 nm) LIR in comparison with 1. The relative higher red emission at lower Eu3+ amount for 1 also suggests that the intramolecular Tb3+-to-Eu3+ ET occurs to the detriment of Tb3+ emission 5D4 → 7F5 (543 nm), which is in agreement with the literature.17,19
The Tb3+-to-Eu3+ ET has previously been reported in inorganic24,25 and molecular luminescent materials.17,26–28 Its occurrence and its efficiency highly depends on the crystal structure, the presence of relatively short intra or intermolecular Tb3+…Eu3+ distances and/or the nature of ligands.27 Indeed, the efficient Tb3+-to-Eu3+ ET occurred under direct excitation of Tb3+ (ref. 26) or through an antenna effect in Eu3+/Tb3+ coordination polymers having relatively short intramolecular distances between lanthanide ions.28 Alternatively, in coordination polymers built from Tb3+ and Eu3+ β-diketone complexes linked through a bridging phosphine oxide the presence of organic bridging ligands allowed the ET to occur despite the long distances between the lanthanides (13.6 Å). In fact, the Tb3+-to-Eu3+ ET rates depends on the operative multipolar and exchange mechanisms that are a function of the distance between the two ions (RL). While for the former, the electric dipole-electric dipole, electric dipole-electric quadrupole, and electric quadrupole-electric quadrupole rates depend on RL−6, RL−8 and RL−10, respectively, the exchange transfer rate decreases exponentially with increasing RL. In this later case, the exchange mechanism becomes significant for Tb3+-to-Eu3+ separations lower than 4 Å.22
In order to confirm the presence of an intramolecular Tb3+-to-Eu3+ ET in 1 and 2, the luminescence of a physical 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of powders of two previously published (Et4N)2[(PhSiO2)8Tb4(NO3)6(EtOH)2(MeCN)2] and (Et4N)2[(PhSiO2)8Eu4(NO3)6(EtOH)2(MeCN)2]9 was investigated (ratio Tb
1 mixture of powders of two previously published (Et4N)2[(PhSiO2)8Tb4(NO3)6(EtOH)2(MeCN)2] and (Et4N)2[(PhSiO2)8Eu4(NO3)6(EtOH)2(MeCN)2]9 was investigated (ratio Tb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Eu = 3
Eu = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
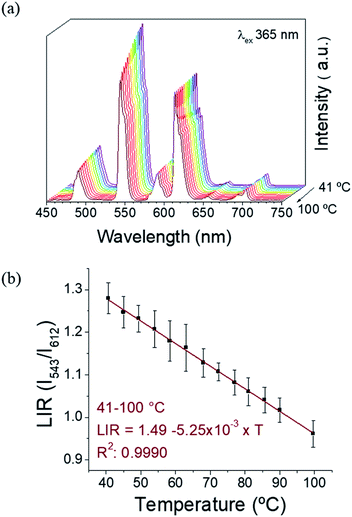
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as confirmed by SEM-EDX, Table S3, ESI†). The excitation spectra (Fig. S10a, ESI†) monitored for Eu3+ red emission (612 nm) and Tb3+ green emission (at 543 nm) have a similar profile as observed for 1. The emission spectra under 394 and 378 nm are presented on Fig. S10b, ESI.† Under 394 nm-excitation, the expected Eu3+ transitions are observed (5D0 → 7F0-4). The excitation at 378 nm leads mainly to Tb3+ emissions, while some low intensity Eu3+ emission bands can also be detected due to the less-efficient intermolecular ET. This fact reinforces the evidence of an intramolecular ET in 1 and 2 occurring due to the short distances between the donor (Tb3+) and the acceptor (Eu3+) ions.22 Therefore, the emissive levels of Tb3+ and Eu3+ (5D4 and 5D0, respectively) are close enough in energy, thus permitting this Tb3+-to-Eu3+ ET. The observation of Eu3+ red emission (at ∼612 nm) via Tb3+ excitation (at ∼350–380 nm), where the Eu3+ emission occurs to the detriment of the Tb3+ radiative decay, clearly proves this fact. In order to investigate the possibility to use our CLMSs as emissive thermometers, the luminescence has been investigated at different temperatures. Fig. 4 shows the spectra of 1 measured under 365 nm excitation from 40 to 100 °C, and the corresponding LIR between Tb3+ and Eu3+ emissions (at 543 nm and 612 nm, respectively). The wavelength ranges used to compute the integrated areas are 530–565 nm (Tb3+: 5D4 → 7F5) and 603–637 nm (Eu3+: 5D0 → 7F2). The error bars represent the standard deviation of average values obtained upon three consecutive temperature cycles (Fig. S11, ESI†). The temperature-dependent variation of the parameter LIR (I543/I612) shows a linear correlation, indicative of a self-referencing temperature sensor. The calibration parameters are presented in Table S4 (ESI†) along with several metric parameters that provide the thermometric performance in the operating temperature range. The regression coefficient (R2) revealed an excellent calibration linearity (R2 = 0.9990) in the temperature range 41–100 °C. We also verified the repeatability, which corresponds to the variability among the measurements, which is indicated by the relative standard deviation (RSD). Therefore, satisfactory repeatability was observed as the maximum RSD among measurements values was lower than 5%. The relative thermal sensitivity (Sr) is the parameter that allows the comparison of thermometric performance among different types of thermometers.29,30 The Sr represents the variation of the experimental parameter (LIR in the present case) per degree of temperature, expressed as:
1, as confirmed by SEM-EDX, Table S3, ESI†). The excitation spectra (Fig. S10a, ESI†) monitored for Eu3+ red emission (612 nm) and Tb3+ green emission (at 543 nm) have a similar profile as observed for 1. The emission spectra under 394 and 378 nm are presented on Fig. S10b, ESI.† Under 394 nm-excitation, the expected Eu3+ transitions are observed (5D0 → 7F0-4). The excitation at 378 nm leads mainly to Tb3+ emissions, while some low intensity Eu3+ emission bands can also be detected due to the less-efficient intermolecular ET. This fact reinforces the evidence of an intramolecular ET in 1 and 2 occurring due to the short distances between the donor (Tb3+) and the acceptor (Eu3+) ions.22 Therefore, the emissive levels of Tb3+ and Eu3+ (5D4 and 5D0, respectively) are close enough in energy, thus permitting this Tb3+-to-Eu3+ ET. The observation of Eu3+ red emission (at ∼612 nm) via Tb3+ excitation (at ∼350–380 nm), where the Eu3+ emission occurs to the detriment of the Tb3+ radiative decay, clearly proves this fact. In order to investigate the possibility to use our CLMSs as emissive thermometers, the luminescence has been investigated at different temperatures. Fig. 4 shows the spectra of 1 measured under 365 nm excitation from 40 to 100 °C, and the corresponding LIR between Tb3+ and Eu3+ emissions (at 543 nm and 612 nm, respectively). The wavelength ranges used to compute the integrated areas are 530–565 nm (Tb3+: 5D4 → 7F5) and 603–637 nm (Eu3+: 5D0 → 7F2). The error bars represent the standard deviation of average values obtained upon three consecutive temperature cycles (Fig. S11, ESI†). The temperature-dependent variation of the parameter LIR (I543/I612) shows a linear correlation, indicative of a self-referencing temperature sensor. The calibration parameters are presented in Table S4 (ESI†) along with several metric parameters that provide the thermometric performance in the operating temperature range. The regression coefficient (R2) revealed an excellent calibration linearity (R2 = 0.9990) in the temperature range 41–100 °C. We also verified the repeatability, which corresponds to the variability among the measurements, which is indicated by the relative standard deviation (RSD). Therefore, satisfactory repeatability was observed as the maximum RSD among measurements values was lower than 5%. The relative thermal sensitivity (Sr) is the parameter that allows the comparison of thermometric performance among different types of thermometers.29,30 The Sr represents the variation of the experimental parameter (LIR in the present case) per degree of temperature, expressed as:
| Sr(T) = |∂LIR(T)/∂T|/LIR(T). |
The maximum Sr value estimated from the calibration data was found to be 0.63% °C−1 at 68 °C, which is close to a frequently considered high relative thermal sensitivity (∼1% °C−1),31 and in proximity with Sr values reported for mixed Eu3+/Tb3+ compounds.19 Temperature uncertainty (or thermal resolution, δT) is the smallest temperature change that can be detected.31 This value is related to Sr as follows:
| δT = |δLIR(T)/LIR(T)|/Sr(T), |
Compound 2 also shows the features making it interesting as self-referencing temperature sensor (Fig. S12, ESI†), but with lower sensitivity (Table S4, ESI†).
The thermal behavior of the emission spectra of CLMSs containing only Eu3+ ((Et4N)2[(PhSiO2)8Eu4(NO3)6(EtOH)2(MeCN)2]) or Tb3+ ((Et4N)2[(PhSiO2)8Tb4(NO3)6(EtOH)2(MeCN)2]) in the same temperature range is shown in Fig. S13 and S14 (ESI†), respectively. The slight decrease in Tb3+ emission intensity with temperature (Fig. S14†) is related to the increase of nonradiative energy transfer from the Tb3+ ion to the ligands to the detriment of the radiative emission. The thermal effect on Tb3+ emission observed for 1 in the presence of Eu3+ suggests an additional energy transfer pathway among emitting centers probably arising due to the presence of the hydroxyl bridge in the center of the cage 1 and also due to the shorter intermolecular Tb/Eu⋯Tb/Eu distances (see Fig. S1 and S4, ESI†). As the thermal effect on Tb3+ emission is more relevant in the presence of Eu3+, we consider that the ET Tb3+ → Eu3+ has improved the thermal sensitivity of Tb3+ emission, and therefore enabled the application of the heteronuclear Tb3+/Eu3+ CLMSs for luminescent thermometry.19
Conclusions
In summary, two tetranuclear Tb3+/Eu3+ based silsesquioxane cages with a prism-like topology have been synthesized and characterized. The morphological characterisations performed by means of SEM indicate the presence of microparticles in samples as well as their homogeneity. The compounds exhibit the terbium and europium characteristic green and red emissions. The relatively short intramolecular distances between the lanthanide atoms in the cores of CLMSs have enabled the intramolecular Tb3+-to-Eu3+ energy transfer, as evidenced by the europium emissions observed under excitation in the terbium-excited levels. The tetranuclear structure has enabled the design of the compounds with well-defined Eu3+ and Tb3+ molar ratio, and therefore the control of the intrametallic interactions. The relative intensity of the red Eu3+ emission (5D0 → 7F2) increases along with the relative amount of the Eu3+ with respect to the green Tb3+ one (5D4 → 7F5) because of the increased ET effect. In this sense, a better thermal sensitivity in 1 in comparison with 2 can be attributed to its lower relative intensity of Eu3+ red emission. On the other hand, the thermal sensitivity of Tb3+ emission increased in the presence of Eu3+ in the heteronuclear CLMSs in comparison with the homonuclear Tb3+-based one, because of the occurrence of an additional nonradiative ET pathway. The Tb3+/Eu3+-based CLMSs were then proposed as self-referenced temperature sensors based on the LIR of Tb3+and Eu3+ transitions (5D4 → 7F5 and 5D0 → 7F2). The sensor 1 showed an excellent linearity in the temperature range 41–100 °C with the maximum relative thermal sensitivity of 0.63% °C−1 at 68 °C, which indicates its successful performance. Its important stability to photobleaching at a relatively high working temperature (100 °C) is certainly due to the presence of the siloxane matrix, which plays a protective role. This work opens great perspectives for the design of new class of CLMSs-based temperature sensors.Experimental procedures
Materials
Phenyltrimethoxysilane (98%), Et4NCl (≥98%), Eu(NO3)3·6H2O (99.9% trace metals basis), Tb(NO3)3·6H2O (99.9% trace metals basis), ethanol and acetonitrile were purchased from Merck and used as received.Synthesis
The synthesis of compounds 1 and 2 has been performed in a similar way. A mixture of PhSi(OMe)3 and NaOH was dissolved in 30 mL of ethanol. The resulting solution was heated to reflux for 1.0 h. Afterwards the mixture of Tb(NO3)3·6H2O and Eu(NO3)3·6H2O in proportions 3/1 for 1 and 2/2 for 2 and Et4NCl dissolved in 30 mL of CH3CN were added at once. The resulted mixture was heated to reflux for 3 h. Filtration of the mixture from the insoluble part gave a non-colored solution. Slow evaporation of solvents (ethanol/CH3CN) gave in a period of 5–10 days a bunch of crystalline material. The single crystals suitable for a single crystal X-ray diffraction were collected. The crystal products were dried in vacuum to perform elemental analysis and to calculate the yield.Anal. calcd for C62H81N5O33Si8Tb3Eu: % C 32.69, % H 3.58, % N 3.07. Found: % C 29.68, % H 3.39, % N 3.01. IR in KBr pellets (cm−1): 3448 (w), 3073 (s), 3050 (s), 3005 (s), 1624 (w), 1593 (s), 1500 (w), 1430 (s), 1384 (s), 1314 (w), 1129 (s), 1027 (m), 997 (s), 945 (w), 813 (s), 785 (s), 747 (s), 705 (s), 678 (s), 646 (s), 576 (s), 548 (s), 499 (s), 467 (s).
Scanning Electronic Microscopy (SEM) image is shown in Fig. S14 (ESI†).
Anal. calcd for C72H98N10O36Si8Tb2Eu2: % C 34.23, % H 3.91, % N 5.54. Found: % C 33.72, % H 3.83, % N 5.29. IR in KBr pellets (cm−1): 3437 (w), 3484 (w), 3074 (s), 1617 (w), 1593 (s), 1500 (w), 1430 (s), 1384 (s), 1316 (s), 1129 (s), 1058 (m), 997 (s), 950 (w), 746 (s), 704 (s), 678 (s), 576 (s), 497 (s), 467 (s).
Characterizations
Morphological characterizations and quantifications of Eu, Tb and Si elements were performed by using Scanning electron microscope and Energy Dispersive X-ray Analysis (SEM-EDX) on a FEI Quanta FEG 200 instrument. The powders were deposited on an adhesive carbon film and analyzed under vacuum. The quantification of the heavy elements was carried out with the INCA software, with a dwell time of 3 μs. IR spectra (KBr pellets) were recorded using PerkinElmer Spectrum Two FT-IR Spectrometer. The N, C, H elemental analyses were carried out in the microanalytical laboratory of the IOC RAS (Moscow) by means of a Carlo Erba Model 1106 elemental analyzer with an accepted tolerance of 0.4 unit on carbon (C), hydrogen (H), and nitrogen (N).The emission and excitation spectra were at first evaluated at room (298 K) and low (77 K) temperatures using a spectrofluorimeter Edinburgh FLS-920. The excitation source was a 450 W Xe arc lamp. The spectra were corrected for detection and optical spectral response of the spectrofluorimeter. In the second step, the emission spectra were measured as a function of temperature. The temperature setup included a thermal element (Heidolph, MR Hei-Tec (EU), 825 W, plate diameter 145 mm), a thermal camera (Optris PI 450i, accuracy ± 0.01 °C), an excitation source and a detector. The powder sample was placed on a cover glass (14 mm dia.) at the center of the heating source. The thermal camera was positioned at an angle of 30° relative to the sample to work as a temperature standard controller. A UV LED operating at 365 nm (ThorLabs M365L2) was used to excite the samples (I = 0.7 A) by irradiating at a distance of 15 mm from the sample surface. The spectrometric detector and the excitation source were coupled by using a multimode fiber. The fiber excitation output and detector were located at the top of sample. A long pass filter (in-line fiber optic filter mount, ThorLabs FOFMS/M, 450 nm, 20 μm) was placed in light path between sample and detector to avoid artefacts arising from excitation source. The emission spectra were recorded in the temperature range from 20 to 110 °C. At each temperature step, a period of 10 min was given to allow the temperature to stabilize, and then 10 emission spectra were recorded from an average of 10 consecutive spectra with an integration time of 100 ms.
UV-visible-NIR absorption spectrum was measured using a spectrophotometer Specord 210 Plus (Analytik Jena AG, Germany).
Magnetic susceptibility data were collected with a Quantum Design MPMS-XL SQUID magnetometer working between 1.8–350 K with the magnetic field up to 7 Tesla. The sample was prepared in an ambient condition. The data were corrected for the sample holder and the diamagnetic contributions calculated from the Pascal's constants.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the RFBR (project 19-53-15008). J. La., K. N., G. F., Y. G. and J. Lo. thank the University of Montpellier and CNRS for financial support (project PRC2287 Premium 2019–2021), as well as for the project MAGCELL which was co-financed by the European Union (European Regional Development Fund) as part of the support of interdisciplinary or innovative research projects in S3 fields of the Occitanie region. The work was also developed within the scope of the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020 & UIDP/50011/2020, financed by Portuguese funds through the FCT/MEC and, when appropriate, co-financed by FEDER under the PT2020 Partnership Agreement. Authors are grateful to Platform of Analysis and Characterization (PAC) of ICGM for magnetic and X-Ray diffraction measurements. J. La. and A. N. K. are grateful for Vernadski program (Embassy of France in Russian Federation). Elemental analyses were performed with the financial support from the Ministry of Science and Higher Education of the Russian Federation and using the equipment of the Center for Molecular Composition Studies of INEOS RAS. This paper was supported in part by the RUDN University Strategic Academic Leadership Program (A. N. K., A. N. B. and V. N. K.).References
- H. W. Roesky, G. Anantharaman, V. Chandrasekhar, V. Jancik and S. Singh, Chem.-Eur J., 2004, 10, 4106 CrossRef CAS PubMed.
- V. Lorenz and F. T. Edelmann, Adv. Organomet. Chem., 2005, 53, 101 CrossRef CAS; M. M. Levitsky, B. G. Zavin and A. N. Bilyachenko, Russ. Chem. Rev., 2007, 76, 847 CrossRef; M. M. Levitsky and A. N. Bilyachenko, Coord. Chem. Rev., 2016, 306, 235 CrossRef.
- R. Murugavel, A. Voigt, M. G. Walawalkar and H. W. Roesky, Chem. Rev., 1996, 96, 2205–2236 CrossRef CAS PubMed.
- R. Duchateau, Chem. Rev., 2002, 102, 3525 CrossRef CAS PubMed; M. M. Levitsky, Y. V. Zubavichus, A. A. Korlyukov, V. N. Khrustalev, E. S. Shubina and A. N. Bilyachenko, J. Clust. Sci., 2019, 30, 1283 CrossRef.
- F. Edelmann, P. Jutzi and U. Schubert, Silicon Chemistry: From the Atom to Extended Systems, ed. P. Jutzi and U. Schubert, 2007, p. 383 CrossRef CAS; K. Sheng, Y.-N. Liu, R. K. Gupta, M. Kurmoo and D. Sun, Sci. China: Chem., 2021, 64, 419 CrossRef CAS.
- M. Levitskii, V. Smirnov, B. Zavin, A. Bilyachenko and A. Y. Rabkina, Kinet. Catal., 2009, 50, 490 CrossRef CAS; E. A. Quadrelli and J.-M. Basset, Coord. Chem. Rev., 2010, 254, 707 CrossRef; K. Wada and T.-A. Mitsudo, Catal. Surv. Asia, 2005, 9, 229 CrossRef; M. M. Levitsky, A. N. Bilyachenko and G. B. Shul’pin, J. Organomet. Chem., 2017, 849–850, 201 CrossRef; M. M. Levitsky, A. I. Yalymov, A. N. Kulakova, A. A. Petrov and A. N. Bilyachenko, J. Mol. Catal. A: Chem., 2017, 426, 297 CrossRef.
- M. M. Levitsky, A. N. Bilyachenko, E. S. Shubina, J. Long, Y. Guari and J. Larionova, Coord. Chem. Rev., 2019, 398, 213015 CrossRef CAS.
- S. Marchesi, F. Carniato and E. Boccaleri, New J. Chem., 2014, 38, 2480 RSC.
- A. N. Kulakova, A. N. Bilyachenko, M. M. Levitsky, V. N. Khrustalev, E. S. Shubina, G. Félix, E. Mamontova, J. Long, Y. Guari and J. Larionova, Chem.-Eur. J., 2020, 26, 16594 CrossRef CAS PubMed.
- A. N. Kulakova, K. Nigoghossian, G. Félix, V. N. Khrustalev, E. S. Shubina, J. Long, Y. Guari, L. D. Carlos, A. N. Bilyachenko and J. Larionova, Eur. J. Inorg. Chem., 2021,(27), 2696–2701 CrossRef CAS.
- C. D. S. Brites, P. P. Lima, N. J. O. Silva, A. Millán, V. S. Amaral, F. Palacio and L. D. Carlos, Nanoscale, 2012, 4, 4799 RSC.
- C. D. S. Brites, S. Balabhadra and L. D. Carlos, Adv. Opt. Mater., 2019, 7, 1801239 CrossRef.
- R. Piñol, C. D. S. Brites, R. Bustamante, A. Martínez, N. J. O. Silva, J. L. Murillo, R. Cases, J. Carrey, C. Estepa, C. Sosa, F. Palacio, L. D. Carlos and A. Millán, ACS Nano, 2015, 9, 3134 CrossRef CAS PubMed; A. M. Kaczmarek, D. Esquivel, B. Laforce, L. Vincze, P. Van Der Voort, F. J. Romero-Salguero and R. Van Deun, Luminescence, 2018, 33, 567 CrossRef PubMed.
- L. B. Guimarães, A. M. P. Botas, M. C. F. C. Felinto, R. A. S. Ferreira, L. D. Carlos, O. L. Malta and H. F. Brito, Materials Advances, 2020, 1, 1988 RSC.
- C. D. S. Brites, P. P. Lima, N. J. O. Silva, A. Millán, V. S. Amaral, F. Palacio and L. D. Carlos, Adv. Mater., 2010, 22, 4499 CrossRef CAS PubMed.
- C. J. Salas-Juárez, R. E. Navarro, A. Pérez-Rodríguez, U. Orozco-Valencia and R. Acevesm, Sens. Actuators, A, 2020, 315, 112293 CrossRef.
- M. Hatanaka, Y. Hirai, Y. Kitagawa, T. Nakanishi, Y. Hasegawa and K. Morokuma, Chem. Sci., 2017, 8, 423 RSC.
- Y. Cui, H. Xu, Y. Yue, Z. Guo, J. Yu, Z. Chen, J. Gao, Y. Yang, G. Qian and B. Chen, J. Am. Chem. Soc., 2012, 134(9), 3979 CrossRef CAS PubMed.
- V. Trannoy, A. N. Carneiro Neto, C. D. S. Brites, L. D. Carlos and H. Serier-Brault, Adv. Optical Mater., 2021, 9, 2001938 CrossRef CAS.
- W. T. Carnall, P. R. Fields and K. Rajnak, J. Chem. Phys., 1968, 49, 4450 CrossRef CAS.
- W. T. Carnall, P. R. Fields and K. Rajnak, J. Chem. Phys., 1968, 49, 4447 CrossRef CAS.
- O. L. Malta, J. Non-Cryst. Solids, 2008, 354, 4770 CrossRef CAS; A. N. Carneiro Neto, R. T. Moura, A. Shyichuk, V. Paterlini, F. Piccinelli, M. Bettinelli and O. L. Malta, J. Phys. Chem. C, 2020, 124, 10105 CrossRef; P. A. Tanner, L. Zhou, C. Duan and K.-L. Wong, Chem. Soc. Rev., 2018, 47, 5234 RSC; D. L. Dexter and J. H. Schulman, J. Chem. Phys., 1954, 22, 1063 CrossRef; G. Blasse and B. C. Grabmaier. Luminescent materials, Springer, Berlin, 1994 Search PubMed.
- J. Kai, D. F. Parra and H. F. Brito, J. Mater. Chem., 2008, 18, 4549 RSC.
- V. Khudoleeva, L. Tcelykh, A. Kovalenko, A. Kalyakina, A. Goloveshkin, L. Lepnev and V. Utochnikova, J. Lumin., 2018, 201, 500 CrossRef CAS.
- T. Li, P. Li, Z. Wang, S. Xu, Q. Bai and Z. Yang, Dalton Trans., 2015, 44, 16840 RSC; M. Jiao, N. Guo, W. Lü, Y. Jia, W. Lv, Q. Zhao, B. Shao and H. You, Dalton Trans., 2013, 42, 12395 RSC; T. K. Anh, T. Ngoc, P. T. Nga, V. T. Bitch, P. Long and W. Stręk, J. Lumin., 1988, 39, 215 CrossRef CAS.
- E. Chelebaeva, J. Long, J. Larionova, R. A. S. Ferreira, L. D. Carlos, F. A. Almeida Paz, J. B. R. Gomes, A. Trifonov, C. Guérin and Y. Guari, Inorg. Chem., 2012, 51, 9005 CrossRef CAS PubMed.
- C. Piguet, J. C. G. Bünzli, G. Bernardinelli, G. Hopfgartner and A. F. Williams, J. Am. Chem. Soc., 1993, 115, 8197 CrossRef CAS.
- A. R. Ramya, D. Sharma, S. Natarajan and M. L. P. Reddy, Inorg. Chem., 2012, 51, 8818 CrossRef CAS PubMed.
- Thermometry at the Nanoscale, ed. L. D. Carlos and F. Palacio, Nanoscience & Nanotechnology Series, 2015 Search PubMed.
- A. Bednarkiewicz, L. Marciniak, L. D. Carlos and D. Jaque, Nanoscale, 2020, 12, 14405 RSC.
- C. D. S. Brites, A. Millán, and L. D. Carlos, In Handbook on the Physics and Chemistry of Rare Earths, ed. J. C. G. Bünzli and V. K. Pecharsky, Elsevier B.V., Amsterdam, 2016, vol. 49, p. 339 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2094637 and 2002352. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra06755a |
| This journal is © The Royal Society of Chemistry 2021 |