Open Access Article
Open Access ArticleSynaptic transistors based on a tyrosine-rich peptide for neuromorphic computing†
Min-Kyu Songa,
Young-Woong Song a,
Taehoon Sunga,
Seok Daniel Namgungbd,
Jeong Hyun Yoona,
Yoon-Sik Leec,
Ki Tae Nam
a,
Taehoon Sunga,
Seok Daniel Namgungbd,
Jeong Hyun Yoona,
Yoon-Sik Leec,
Ki Tae Nam bd and
Jang-Yeon Kwon
bd and
Jang-Yeon Kwon *a
*a
aSchool of Integrated Technology, Yonsei University, Incheon 21983, Republic of Korea. E-mail: jangyeon@yonsei.ac.kr
bDepartment of Materials Science and Engineering, Seoul National University, Seoul 08826, Republic of Korea
cSchool of Chemical and Biological Engineering, Seoul National University, Seoul 08826, Republic of Korea
dSoft Foundry, Seoul National University, Seoul 08826, Republic of Korea
First published on 13th December 2021
Abstract
In this article, we propose an artificial synaptic device based on a proton-conducting peptide material. By using the redox-active property of tyrosine, the Tyr–Tyr–Ala–Cys–Ala–Tyr–Tyr peptide film was utilized as a gate insulator that shows synaptic plasticity owing to the formation of proton electric double layers. The ion gating effects on the transfer characteristics and temporal current responses are shown. Further, timing-dependent responses, including paired-pulse facilitation, synaptic potentiation, and transition from short-term plasticity to long-term plasticity, have been demonstrated for the electrical emulation of biological synapses in the human brain. Herein, we provide a novel material platform that is bio-inspired and biocompatible for use in brain-mimetic electronic devices.
Introduction
The recent emergence of artificial intelligence has impacted a wide range of research areas owing to its superior performance in cognitive computing including inference, decision, and prediction, which has been regarded as a human role.1 The large amount of data and repetitive processing for training have driven increasing demands for the development of high-performance processors. However, conventional processors have parallel processing limits for cognitive computing owing to their von Neumann architecture, which has separate memory and processing units. Transferring data between them induces a considerable waste of time and power, called the von Neumann bottleneck. Neuromorphic devices have emerged to emulate human brain functions to overcome the von Neumann bottleneck.2–6 In contrast to man-made computers, the biological brain is composed of ∼1011 neurons and ∼1015 synapses that process data locally and in parallel, thus affording extreme efficiency in terms of time and energy.7 Synaptic devices that mimic the fundamental function of synapses have been proposed as building blocks for brain-like processors.8–10 Several researchers have developed artificial synapses for spiking neural networks by using the timing-dependent electrical characteristics of ion-based devices to realize synaptic plasticity.11,12 Among the various approaches, synaptic transistors are regarded as promising candidates for artificial synapses owing to their functional similarity to synapses, low power consumption, and controllability of synaptic performance.13–16Peptide materials, which are short chains of amino acids, offer significant advantages for use in electronic devices as their chemical and electrical properties can be programmed by designing amino acid sequences, controlling their folding, and inducing assembly.17–20 More importantly, they play key roles in ion transfer in biological signaling systems, implying their potential role for ion-controlling in electronics.21 Recently, we designed a certain tyrosine-rich peptide (TRP) to show proton-conducting characteristics using tyrosine.22–24 Introducing repeating tyrosine units at both ends of the peptide sequences enables high proton-conducting and redox-active insulating properties in thin films. In this regard, we explored the possibility of TRP materials for use in biomedical devices by utilizing their proton-mediated redox reaction as well as their biocompatibility and biodegradability, and further applicability for neuromorphic devices.25–27
In this letter, we report a proton-gated synaptic transistor using an In–Ga–Zn–O (IGZO) semiconducting film on a Tyr–Tyr–Ala–Cys–Ala–Tyr–Tyr (YYACAYY, Y7C) peptide thin film. The proton-conducting property of the TRP film not only enabled excellent performance with an on/off ratio of around 107 but also large hysteresis of the transfer curves owing to the formation of electric double layers of protons. Thus, the timing-dependent synapse-mimetic responses of the drain current modulated by gate voltage spikes are presented. Treating the device as an artificial synapse, we explored its synaptic plasticity including paired-pulse facilitation (PPF), short-term to long-term transition, and potentiation.
Experimental section
Fabrication of the Y7C peptide synaptic transistor
4 wt% Y7C peptide powder (scipeptide, 97%) was dissolved in trifluoroacetic acid (Daejung, 99.0%). The Y7C solution was sonicated for 30 min and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 min. Before the spin-coating process, highly doped Si substrates with a resistivity of 0.001–0.005 Ω cm were cleaned via sonication in acetone, isopropyl alcohol, and water for 5 min per cleaner. The solution was then spin-coated onto the P+ Si substrates at 4000 rpm for 60 s. The IGZO channel layer was patterned using a shadow mask. The lateral size of the IGZO was 400 × 400 μm. The 50 nm-thick IGZO was deposited by RF sputtering under a working pressure of 7 mTorr and an RF power of 100 W, and the gas flow rates of O2 and Ar were 30 sccm and 0.5 sccm, respectively. A 100 nm-thick Mo source/drain layer patterned with a shadow mask was deposited by DC sputtering under a power of 200 W and an Ar gas flow rate of 30 sccm. The lateral size of the Mo electrodes was 200 × 200 μm. The channel length and width were both 200 μm. The rear side of the substrate was connected to copper tape on the glass with silver paste for the gate electrode, followed by the native oxide etching of Si.
000 rpm for 1 min. Before the spin-coating process, highly doped Si substrates with a resistivity of 0.001–0.005 Ω cm were cleaned via sonication in acetone, isopropyl alcohol, and water for 5 min per cleaner. The solution was then spin-coated onto the P+ Si substrates at 4000 rpm for 60 s. The IGZO channel layer was patterned using a shadow mask. The lateral size of the IGZO was 400 × 400 μm. The 50 nm-thick IGZO was deposited by RF sputtering under a working pressure of 7 mTorr and an RF power of 100 W, and the gas flow rates of O2 and Ar were 30 sccm and 0.5 sccm, respectively. A 100 nm-thick Mo source/drain layer patterned with a shadow mask was deposited by DC sputtering under a power of 200 W and an Ar gas flow rate of 30 sccm. The lateral size of the Mo electrodes was 200 × 200 μm. The channel length and width were both 200 μm. The rear side of the substrate was connected to copper tape on the glass with silver paste for the gate electrode, followed by the native oxide etching of Si.
Film characterization of the Y7C peptide
The thickness of the Y7C peptide film as a function of concentration was measured using an atomic force microscope (AFM, XE-100, Park systems). A semiconductor parameter analyzer (Keithley SCS 4200) was used for the electrical characterization. The humidity and temperature conditions were maintained at 40 ± 5% RH and 22 ± 2 °C during the measurements.Results and discussion
Tyrosine is a redox-active amino acid known to play a key role in various enzymatic reactions in biological systems such as photosynthesis II.21 Tyrosine transfers protons and electrons simultaneously via an inherent deprotonation of its phenolic hydroxyl group, known as proton-coupled electron transfer (PCET).21,28 This phenomenon can also be observed in the TRP thin film, where protons are transported via phenolic hydroxyl groups as hopping sites.24,27 Therefore, the Y7C peptide thin film exhibits high proton conductivity, in contrast to low electron conductivity. This suggests its potential for use in proton-based synaptic devices for neuromorphic applications. Thus, we fabricated an IGZO thin-film transistor with a Y7C peptide film as a gate insulator. The Y7C peptide solution was spin-coated onto a highly doped p-type Si wafer. The thickness of the Y7C film was measured by the AFM (see Fig. S1†). Fig. S2† shows the I–V characteristics of the insulating Y7C film. The IGZO active layer and Mo source/drain metal layers were deposited using RF sputtering and DC sputtering, respectively.Fig. 1 shows the schematics of the Y7C peptide synaptic transistor and the corresponding biological synapse between the two neurons. In biological synapses, presynaptic stimuli from pre-neurons induce the emission of neurotransmitters such as Na+, K+, and dopamine to the synaptic cleft, as shown in Fig. 1a. Subsequently, neurotransmitters activate receptors on post-neurons, resulting in postsynaptic responses.29,30 Changes in synaptic connectivity under stimulus construct memory activity in the brain. Similarly, in the Y7C peptide synaptic transistor, which is shown in Fig. 1b, voltage spikes on the bottom gate corresponding to the presynaptic spikes facilitate current responses through the channel corresponding to the excitatory postsynaptic current (EPSC). Therefore, the Y7C peptide thin film acts as a synapse between the two neurons.
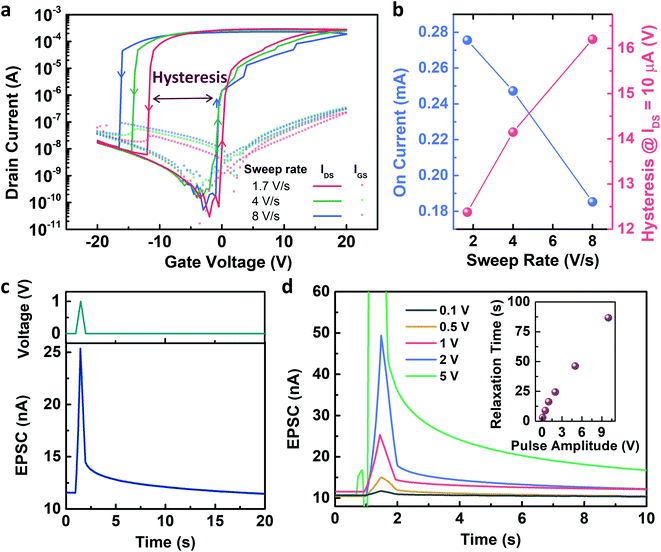
Fig. 2 shows the electrical characteristics of the Y7C peptide synaptic transistor. The gate voltage sweep induced current modulation, as shown in the transfer curves. Fig. 2a shows the changes in the on-current at a gate voltage of 20 V, and voltage hysteresis at a drain current of 1 μA as a function of the gate voltage sweep rate is shown in Fig. 2b. The on-current values are 27.56 μA, 24.72 μA, and 18.32 μA at sweep rates of 1.7, 4, and 8 V s−1, respectively. A slower voltage sweep was induced, and a higher current modulation was observed. Thus, the on/off current ratio reached 9.4 ×106 at the slowest sweep rate. In addition, the voltage hysteresis values, i.e., the voltage differences between the forward and reverse sweeps at which the drain current equals 10 μA, are 12.38 V, 14.15 V, and 16.2 V at sweep rates of 1.7 V s−1, 4 V s−1, and 8 V s−1, respectively. A large hysteresis, which increases as the sweep rate increases, indicates that ionic movement in the peptide film is the origin of the gating effect.31 This result corresponds to our previous results from the electrochemical impedance analysis of the peptide-insulating layer.32 The response of the transfer curves to repeated gate sweeps is shown in Fig. S3.† Therefore, a presynaptic spike induced a decay curve of the EPSC, as shown in Fig. 2c. This decaying property corresponds to the history-dependent output, resulting in the emulation of synaptic plasticity. As the amplitude of the presynaptic spikes increased, the EPSC decay slowed (Fig. 2d). Thus, the relaxation time after the stimulus increased almost linearly with the spike amplitudes. This indicates that the short-term plasticity of the device changes to long-term plasticity depending on the amplitude of the spikes. Thus, the memory property of the device can be modulated by controlling the amplitude of the stimuli, which is an important characteristic of spiking neural networks.
Several reports have shown that the accumulation of protons under the channel region induces electrostatic effects, enabling carrier generation in the channel.31,33–35 The Y7C peptides exhibit high proton conductivity, compared to the other peptides, owing to the phenolic OH groups in tyrosine that act as hopping sites for protons and their structural stability from disulfide bonding.22,27 Fig. 3 shows the mechanism of proton-induced gating phenomenon in the Y7C peptide film. When a positive gate bias is applied to the bottom electrode, protons (i.e. positively charged hydrogen atoms) move upward through the Y7C peptide layer. Protons accumulate at the interface between the Y7C layer and the IGZO channel layer, thereby forming an electric double layer (EDL). Thus, carrier generation in the IGZO channel is induced, resulting in the current modulation of the device. This mechanism is analogous to the residual effects of Ca2+ dynamics in biological synapses.
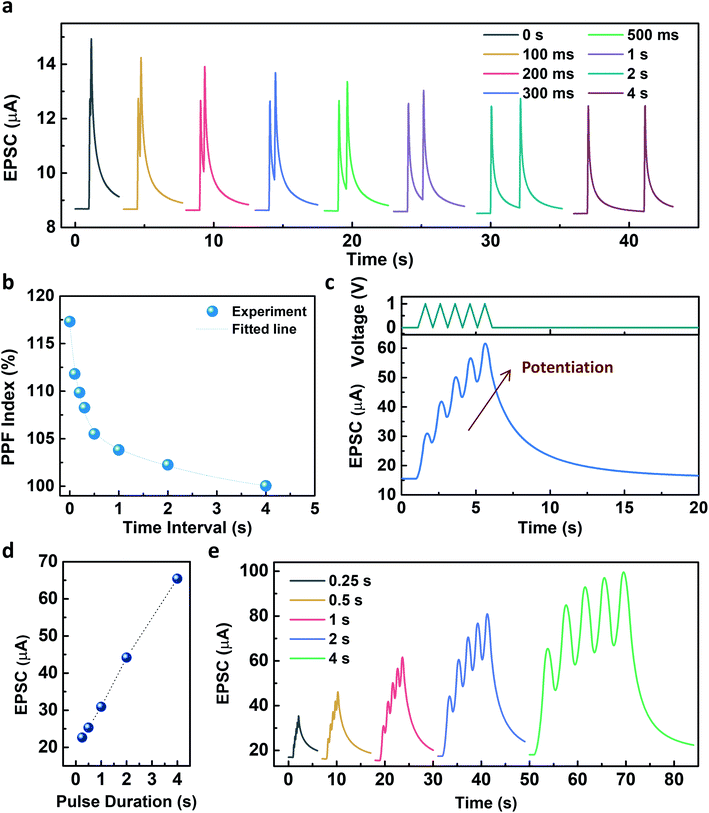
Fig. 4 describes the EPSC responses triggered by consecutive presynaptic stimuli representing the emulation of synaptic plasticity. PPF, which denotes the amplitude ratio of the second EPSC response to the first response facilitated by two successive spikes (1 V, 100 ms), was investigated at various time intervals, as shown in Fig. 4a. It was observed that the PPF gradually decreased as the time interval increased. The PPF index, defined as A2/A1 (%), as a function of the time interval, was fitted by using a double exponential decay curve:
The pulse duration dependency on the facilitation of the EPSC was further investigated with a voltage amplitude of 1 V. As shown in Fig. 4d, the facilitation of EPSC increased as the pulse duration increased from 0.25 s to 4 s. Five consecutive presynaptic pulses (1 V) with various pulse durations from 0.25 s to 4 s were applied to the Y7C peptide synaptic transistor (Fig. 4e). As the number of stimuli increased, the increase of the EPSC was facilitated independent of the pulse duration. The fabricated Y7C peptide synaptic transistors have analogous synaptic responses to a biological neuron. This makes the implementation of synaptic functions in an artificial device to mimic the processing system of an actual neural network.
Conclusion
In summary, our study demonstrates the brain-mimetic performance of three-terminal artificial synapses based on tyrosine-rich peptides. The Y7C peptide, designed to realize the coupling effect between protons and electrons, enabled synaptic plasticity owing to high proton conduction, in addition to low power operation. In an effort to enable its use in artificial spiking neural networks for neuromorphic computing, we explored various aspects of synaptic plasticity, including the PPF, transition from STP to LTP, and synaptic potentiation. Our findings highlight the material potential of the peptide for novel processors and also provide a new strategy for designing proton-based electronics by using tyrosine as a design motif.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A2C2004864).References
- Y. LeCun, Y. Bengio and G. Hinton, Nature, 2015, 521, 436–444 CrossRef CAS PubMed.
- Y. He, L. Zhu, Y. Zhu, C. Chen, S. Jiang, R. Liu, Y. Shi and Q. Wan, Adv. Intell. Syst., 2021, 2000210 CrossRef.
- S. W. Cho, S. M. Kwon, Y.-H. Kim and S. K. Park, Adv. Intell. Syst., 2021, 2000162 CrossRef.
- Z. Wang, H. Wu, G. W. Burr, C. S. Hwang, K. L. Wang, Q. Xia and J. J. Yang, Nat. Rev. Mater., 2020, 5, 173–195 CrossRef CAS.
- M. A. Zidan, J. P. Strachan and W. D. Lu, Nat. Electron., 2018, 1, 22–29 CrossRef.
- Q. Xia and J. J. Yang, Nat. Mater., 2019, 18, 309–323 CrossRef CAS PubMed.
- S. Yu, Proc. – IEEE, 2018, 106, 260–285 CAS.
- Y. van De Burgt, A. Melianas, S. T. Keene, G. Malliaras and A. Salleo, Nat. Electron., 2018, 1, 386–397 CrossRef.
- E. J. Fuller, S. T. Keene, A. Melianas, Z. Wang, S. Agarwal, Y. Li, Y. Tuchman, C. D. James, M. J. Marinella and J. J. Yang, Science, 2019, 364, 570–574 CrossRef CAS PubMed.
- E. J. Fuller, F. E. Gabaly, F. Léonard, S. Agarwal, S. J. Plimpton, R. B. Jacobs-Gedrim, C. D. James, M. J. Marinella and A. A. Talin, Adv. Mater., 2017, 29, 1604310 CrossRef PubMed.
- H. Kim, S. Hwang, J. Park and B.-G. Park, Nanotechnology, 2017, 28, 405202 CrossRef PubMed.
- H. Kim, S. Hwang, J. Park, S. Yun, J.-H. Lee and B.-G. Park, IEEE Electron Device Lett., 2018, 39, 630–633 CAS.
- L. Q. Zhu, C. J. Wan, L. Q. Guo, Y. Shi and Q. Wan, Nat. Commun., 2014, 5, 1–7 Search PubMed.
- S. Wang, C. Chen, Z. Yu, Y. He, X. Chen, Q. Wan, Y. Shi, D. W. Zhang, H. Zhou and X. Wang, Adv. Mater., 2019, 31, 1806227 CrossRef PubMed.
- J. Shi, S. D. Ha, Y. Zhou, F. Schoofs and S. Ramanathan, Nat. Commun., 2013, 4, 1–9 Search PubMed.
- S. Kim, J. Yoon, H.-D. Kim and S.-J. Choi, ACS Appl. Mater. Interfaces, 2015, 7, 25479–25486 CrossRef CAS PubMed.
- X. Daura, K. Gademann, B. Jaun, D. Seebach, W. F. Van Gunsteren and A. E. Mark, Angew. Chem., Int. Ed., 1999, 38, 236–240 CrossRef CAS.
- K. Tao, P. Makam, R. Aizen and E. Gazit, Science, 2017, 358, eaam9756 CrossRef PubMed.
- N. Ashkenasy, W. S. Horne and M. R. Ghadiri, Small, 2006, 2, 99–102 CrossRef CAS PubMed.
- J. Lee, M. Ju, O. H. Cho, Y. Kim and K. T. Nam, Adv. Sci., 2019, 6, 1801255 CrossRef PubMed.
- L. Hammarström and S. Styring, Energy Environ. Sci., 2011, 4, 2379–2388 RSC.
- J. Lee, I. R. Choe, Y. O. Kim, S. D. Namgung, K. Jin, H. Y. Ahn, T. Sung, J. Y. Kwon, Y. S. Lee and K. T. Nam, Adv. Funct. Mater., 2017, 27, 1702185 CrossRef.
- H.-S. Jang, J.-H. Lee, Y.-S. Park, Y.-O. Kim, J. Park, T.-Y. Yang, K. Jin, J. Lee, S. Park and J. M. You, Nat. Commun., 2014, 5, 1–11 Search PubMed.
- M. Ju, O. H. Cho, J. Lee, S. D. Namgung, M.-K. Song, M. Balamurugan, J.-Y. Kwon and K. T. Nam, Phys. Chem. Chem. Phys., 2020, 22, 7537–7545 RSC.
- M.-K. Song, S. D. Namgung, T. Sung, A.-J. Cho, J. Lee, M. Ju, K. T. Nam, Y.-S. Lee and J.-Y. Kwon, ACS Appl. Mater. Interfaces, 2018, 10, 42630–42636 CrossRef CAS PubMed.
- S. D. Namgung, M. K. Song, T. Sung, O. H. Cho, M. Ju, H. Kim, Y. S. Lee, K. T. Nam and J. Y. Kwon, Adv. Mater. Technol., 2020, 5, 2000516 CrossRef CAS.
- M.-K. Song, S. D. Namgung, D. Choi, H. Kim, H. Seo, M. Ju, Y. H. Lee, T. Sung, Y.-S. Lee and K. T. Nam, Nat. Commun., 2020, 11, 1–8 Search PubMed.
- M. Sjödin, S. Styring, H. Wolpher, Y. Xu, L. Sun and L. Hammarström, J. Am. Chem. Soc., 2005, 127, 3855–3863 CrossRef PubMed.
- R. S. Zucker and W. G. Regehr, Annu. Rev. Physiol., 2002, 64, 355–405 CrossRef CAS PubMed.
- G.-q. Bi and M.-m. Poo, J. Neurosci., 1998, 18, 10464–10472 CrossRef CAS PubMed.
- Y. H. Liu, L. Qiang Zhu, Y. Shi and Q. Wan, Appl. Phys. Lett., 2014, 104, 133504 CrossRef.
- T. Sung, S. D. Namgung, J. Lee, I. R. Choe, K. T. Nam and J.-Y. Kwon, RSC Adv., 2018, 8, 34047–34055 RSC.
- L. Q. Zhu, C. J. Wan, P. Q. Gao, Y. H. Liu, H. Xiao, J. C. Ye and Q. Wan, ACS Appl. Mater. Interfaces, 2016, 8, 21770–21775 CrossRef CAS PubMed.
- G. Wu, J. Zhang, X. Wan, Y. Yang and S. Jiang, J. Mater. Chem. C, 2014, 2, 6249–6255 RSC.
- L. Q. Guo, J. Wen, L. Q. Zhu, Y. M. Fu and H. Xiao, IEEE Electron Device Lett., 2017, 38, 1248–1251 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra06492d |
| This journal is © The Royal Society of Chemistry 2021 |