Open Access Article
Open Access ArticleSuperhydrophobic cotton fabric membrane prepared by fluoropolymers and modified nano-SiO2 used for oil/water separation
Chengmin Hou * and
Congjun Cao
* and
Congjun Cao
Faculty of Printing, Packaging Engineering and Digital Media Technology, Xi'an University of Technology, Xi'an 710048, Shaanxi Province, People's Republic of China. E-mail: 1042067175@qq.com
First published on 24th September 2021
Abstract
At present, the preparation methods of oil–water separation membranes include chemical vapor deposition, electrospinning, atom transfer radical polymerization, etc. Basically, they all have issues of low recycling rate and incontinuous use. In this paper, the epoxy polymer P(GMA-r-MMA) obtained by traditional radical polymerization of glycidyl methacrylate (GMA) monomer and methacrylic acid (MMA) monomer, and pentafluoropropionic acid (PFPA) is used to modify polymer P(GMA-r-MMA) to obtain fluorine-containing epoxy polymer P(GMA-r-MMA)-g-PFPA. Secondly, fluorine-containing epoxy polymer P(GMA-r-MMA)-g-PFPA and amino-modified nano SiO2 is blended, and the cotton fabric is dip-coated to obtain a superhydrophobic surface, thereby preparing an oil–water separation membrane. By controlling the solution concentration, dipping time, drying time and other conditions, the superhydrophobic performance of the separation membrane was characterized, and the best construction conditions for the superhydrophobic surface were obtained: 0.3 mg mL−1 polymer concentration, immersion time 6 h, drying temperature 120°, and drying time 4 h, and the maximum water contact angle can reach to 150° ± 2°. Finally, the cotton fabric was modified under the best dipping conditions, and used as an oil–water separation membrane to study the oil–water separation performance of n-hexane, n-octane, kerosene, chloroform and water mixtures in batch operation and continuous operation. In batch operations, the separation efficiency can reach 99% and can achieve 5 consecutive high-efficiency separations without intermittent drying. In continuous flow operation, oil–water separation can last for more than 12 hours and the separation efficiency can reach 98%. It also has stable oil–water separation performance for oil–water emulsion.
1. Introduction
With the development of the national economy, a large number of oil–water mixed pollutants are produced in industry and life, which seriously threaten human health and the ecological environment. For example, industrial wastewater pollutes rivers, oily wastewater evaporates and pollutes air, and frequent marine crude oil spills.1 The oil–water separation technology can treat oil–water mixtures. At present, common oil–water separation technologies include physical, chemical, biological, and membrane separation methods.2,3 Among them, the membrane separation method has mild conditions, simple process, high efficiency and no pollution,4,5 which is the main research and development direction at present. Oil–water separation membranes are also widely used in various fields such as medical and pharmaceutical fields,6 industrial fields,7,8 and daily life.9According to the separation mechanism, oil–water separation membranes can be divided into superhydrophobic–lipophilic membranes and superhydrophilic–oleophobic membranes. For the former membranes, oil can penetrate the membrane but water cannot penetrate the membrane. But for the latter membranes, water can penetrate the membrane but the oil cannot penetrate the membrane. Two kinds of membranes can realize the oil–water separation, and are suitable for different separation requirements. At present, the preparation methods of superhydrophobic films mainly include dip coating,10 spray coating,11 spin coating,12 sol–gel,13 layer-by-layer,14 vapor pressure deficit (VPD),15 chemical vapor deposition (CVD),16 electrodeposition,17, electrospinning,18 acid–base treatment,19 grafting,20 thermal,21 plasma,22 ion beam irradiation,23 and femtosecond laser.24 These methods still have the problems of harsh synthesis conditions, cumbersome preparation process, high cost, instability and easy shedding of super-hydrophobic coatings, unrecyclable and continuous use, etc., which are difficult to be applied in the industrial field.
In this article, aiming at the above-mentioned problems, a simple preparation process is used to prepare a superhydrophobic cotton cloth surface that can be recycled and continuously operated for the separation of oil and water. First, the epoxy polymer P(GMA-r-MMA) is obtained by radical polymerization of glycidyl methacrylate (GMA) monomer and methyl methacrylate (MMA) monomer. The polymer contains epoxy groups and has excellent adhesion on various interfaces, which is conducive to adhesion on the surface of the substrate, and has low shrinkage during curing, good film-forming properties, and good light transmittance. It is non-toxic and environmentally friendly, and has good mechanical strength. Secondly, P(GMA-r-MMA) is fluorinated modified by pentafluoropropionic acid (PFPA) to obtain a fluorine-containing epoxy polymer P(GMA-r-MMA)-g-PFPA (Scheme 1). By introducing fluorine element, the polymer has low surface energy, which is conducive to the realization of hydrophobicity. KH550 was used to modify nano-SiO2 to provide a nano-rough structure on the surface of the substrate. The modified nano-SiO2 and the fluorine-containing epoxy polymer P(GMA-r-MMA)-g-PFPA form a hybrid solution, and the cotton fabric is dipped to construct a superhydrophobic coating on its surface. By controlling the hybrid solution concentration, dipping time, drying time and other conditions, the best superhydrophobic surface construction conditions were explored. Finally, the wetting performance of the obtained oil–water separation membrane for different liquids, the selective adsorption performance of the oil–water mixture, the separation performance of the intermittent operation and the continuous operation to separate the oil–water mixture are studied.
2 Experimental part
2.1. Experimental reagents and instruments
Azobisisobutyronitrile (AIBN), aluminum oxide (Al2O3), potassium bromide, glycidyl methacrylate (GMA), methyl methacrylate (MMA), pentafluoropropionic acid (PFPA), cetyl trimethyl ammonium bromide (CTAB), tetrahydrofuran (THF), triethanolamine (TEOA), γ-aminopropyltriethoxysilane (KH550) and nano silica (SiO2) were purchased from Aladdin Technology Limited company. Ethanol (EtOH), triethylamine (TEA), n-hexane, n-octane, chloroform, kerosene, copper sulfate, Sudan IV and sodium dodecyl sulfonate were purchased from Tianjin Damao Chemical Reagent Factory.FTIR Fourier Transform Infrared Spectroscopy (FTIR-8400S) was produced by Japan Shimadzu Co., Ltd; infrared Tablet Press (HY-12) was produced by Tianjin Tianjiang Optical Instrument Co., Ltd; Zeiss Gemini SEM500 (Zeiss GeminiSEM500) Zeiss Optical Instruments (Shanghai) was produced by International Trade Co., Ltd.
2.2. Synthesis of fluorine-containing epoxy polymer P(GMA-r-MMA)-g-PFPA
Basic alumina was used to remove the polymerization inhibitor in GMA monomer and MMA monomer. In a 10 mL single-necked flask, 0.527 g GMA (3.7 mmol) and 1 g MMA (10 mmol) were added respectively, and the amount ratio of the two substances was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Then 3 mL of tetrahydrofuran (THF) and 0.05 g of initiator azobisisobutyronitrile (AIBN, 0.31 mmol) were added sequentially, and the amount of AIBN was 3% of the total amount of the reacting monomers GMA and MMA. The mouth of the bottle was sealed, and the bottle was filled with nitrogen and deoxygenated for 30 minutes. The mixture was reacted at 60 °C for 3 hours. The liquid in the flask became paste-like. The mouth of the bottle was opened to complete the radical polymerization reaction to obtain epoxy polymer P(GMA-r-MMA).
3. Then 3 mL of tetrahydrofuran (THF) and 0.05 g of initiator azobisisobutyronitrile (AIBN, 0.31 mmol) were added sequentially, and the amount of AIBN was 3% of the total amount of the reacting monomers GMA and MMA. The mouth of the bottle was sealed, and the bottle was filled with nitrogen and deoxygenated for 30 minutes. The mixture was reacted at 60 °C for 3 hours. The liquid in the flask became paste-like. The mouth of the bottle was opened to complete the radical polymerization reaction to obtain epoxy polymer P(GMA-r-MMA).
0.184 g of pentafluoropropionic acid (PFPA, 1.12 mmol), 0.02 g of triethanolamine (TEOA, 0.13 mmol) and 0.048 g of cetyl trimethylammonium bromide (CTAB, 0.13 mmol) were added, which are 40%, 5% and 5% of the amount the GMA substances. The mixture was magnetic stirring for 3 hours at 40 °C to complete the fluorination modification of the polymer. The polymer was precipitated in n-hexane and purified by centrifugation using a benchtop centrifuge to obtain the fluoropolymer P(GMA-r-MMA)-g-PFPA, which was dried in an oven at 50 °C for 10 hours. The polymer was grind into powder and put it in a bottle for later use.
2.3. KH550 modified nano-SiO2
0.6 g of nano-SiO2 was weighed and placed in an oven at 130 °C for 10 hours to dry. 22 mL of ethanol was added into a 50 mL single-necked flask, and 3 mL of water and 0.5 g of nano-SiO2 were added to the ethanol solution. The mixture was dispersed with an ultrasonic cleaner to sonicate for 30 min to disperse the nano-SiO2 in the solution. Triethylamine was added dropwise to adjust the pH of the solution to 9, showing an alkaline state. The flask was placed in a heat-collecting oil bath magnetic stirrer at 80 °C, add 1 g KH550 was added dropwise, and a condensing reflux device was installed. The mixture was back-flowed with magnetic stirring for 5 hours to complete the ammoniating modification. The reacted turbid liquid was purified by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 minutes for three times in a benchtop centrifuge to obtain modified nano-silica. The product was placed in an oven at 40 °C for 10 hours, and ground into powder to obtain ammoniated modified nano-SiO2 for use.
000 rpm for 15 minutes for three times in a benchtop centrifuge to obtain modified nano-silica. The product was placed in an oven at 40 °C for 10 hours, and ground into powder to obtain ammoniated modified nano-SiO2 for use.
2.4. Preparation of superhydrophobic cotton cloth with hybrid solution of fluoropolymer and modified SiO2
3 mg, 10 mg, or 20 mg P(GMA-r-MMA)-g-PFPA were weighed and dissolved in 10 mL THF to prepare polymer solutions with concentrations of 0.3 mg mL−1, 1 mg mL−1, or 2 mg mL−1. After stirring uniformly, 2 mg of modified nano-SiO2 and 0.2 g of pentafluoropropionic acid were added respectively, and the mixture was magnetically stirred for 10 hours at room temperature to obtain a hybrid solution of fluorine-containing epoxy polymer and amino-modified SiO2.Cotton fabric was used as the substrate material, soaked in absolute ethanol overnight, rinsed with clean water, dried at 120 °C, and cut into a square base material of 2 cm × 2 cm. The processed substrate materials were dipped into hybrid solutions with different polymer concentrations, and a piece of cotton cloth substrate was took out every two hours and placed in an oven at 120 °C for a certain period of time. After washing with ethanol solution, the cotton cloth substrate was dried again for 1–5 h. The water contact angle and water rolling angle of its surface was recorded. The effects of different polymer concentrations (0.3–2 mg mL−1), different immersion times (2–10 h), and different drying times (1–5 h) on the performance of superhydrophobic surfaces were explored by controlling immersion time, drying time and other conditions to determine the best super-hydrophobic surface construction conditions for the cotton fabric substrate.
In addition, in order to compare the modification effect of the hybrid solution on cotton cloth hydrophobic properties, the cotton cloth substrate was treated with polymer solution, and silica solution respectively, and the water contact angle and water rolling angle on the surface of the cotton cloth were measured.
2.5. Characterization of superhydrophobic fabric
The structure of fluoropolymer P(GMA-r-MMA)-g-PFPA and nano-SiO2 were tested by Infrared Spectroscopy (FTIR) and Nuclear Magnetic Spectra (1H NMR). For FTIR, 10 mg P(GMA-r-MMA)-g-PFPA or the modified nano-SiO2 sample were ground into fine powders in an agate mortar, mixed with dry potassium bromide powder (about 100 mg, particle size 200 mesh), put into a mold, and press into a tablet on a tablet machine. 5 mg of P(GMA-r-MMA)-g-PFPA or modified nano-SiO2 samples were packed in a nuclear magnetic tube, and 0.5 mL of deuterated THF was added to prepare samples for 1H NMR.The contact angle (CA) and rolling angle (RA) of the superhydrophobic modified material surface were tested as follow: the water contact angle test uses a water contact angle tester to test and 5 μL water solution was used. The test method of the water rolling angle was to fix the superhydrophobic material on the inclined plate, and changed the inclination angle of the inclined plate by continuously increasing or decreasing the height of the back pads. Drop a drop of solution on the sample, and when the water drop was observed rolling, the inclination angle of the inclined plate was the measured rolling angle.
Dynamic contact angle measurement method: 5 μL water droplets was dropped on the surface of modified cotton using a micro syringe. The height of the flat bottom needle of the micro syringe was adjusted and inserted into the center of the drop to increase (decrease) the liquid continuously. The advancing angle was the contact angle θA at which the gas–liquid–solid three-phase line contacting the cotton surface with the droplet will move but does not move when the volume of the droplet was increased. The receding angle θB refers to the three-phase line where the droplet contacts the solid surface when the volume of the droplet was reduced. The difference between the two Δθ = θA − θB was defined as the contact angle hysteresis.
Durability test of modified cotton cloth surface: citric acid, sodium chloride, and sodium citrate were used to prepare solutions with a concentration of 0.5 mg mL−1, the pH values were 4, 7, and 9. The modified cotton fabrics prepared under the optimal construction conditions were soaked in the three solutions. After soaking for a certain time, the cotton fabrics were taken out, rinsed with deionized water, dried at 120 °C for 2 h, and then the CA and RA on the surface of the material were measured. The soaking time was 0–5 min. The durability of the modified cotton fabric against chemical reagents were explored. 0.5 mg mL−1 detergent aqueous solution was prepared and the modified cotton fabric under the optimal construction conditions was put into the detergent aqueous solution to explore the washing durability.
Mechanical performance test: the modified and unmodified cotton cloth were placed at room temperature for 24 hours. The tensile strength and tensile modulus were tested according to the GB/T1447-2005 standard. The tensile speed of the test was 2 mm min−1. Three samples were tested, and the result was the arithmetic average. The flexural strength and flexural modulus were tested according to the GB/T1449-2005 standard, the span was set to 64 mm, and 3 samples were tested, and the result was the arithmetic average.
The wettability of different types of aqueous solutions on the surface of the substrate were tested as follow: the copper sulfate aqueous solution (1 wt%), copper nitrate aqueous solution (1 wt%), milk and beverages were select respectively as water phase. A dropper of these solution was drop on the surface of the cotton fabric before and after the modification. The hydrophobicity of the materials after the modification were compared and analyzed by taking pictures.
Scanning electron microscopy (SEM) test of cotton fabric before and after modification: the native cotton fabric and modified cotton fabric were fixed on the SEM sample table and gold was sprayed on the surface. The scanning electron microscope pictures with scales of 5, 10, and 50 μm were taken at different magnifications.
2.6. Oil–water separation performance test of superhydrophobic fabric
The selective adsorption properties of the modified cotton fabric were tested as follow: n-hexane, n-octane, kerosene, and chloroform were selected as organic solvent for selective adsorption test. For the convenience of observation, the organic solvents was dyed with Sudan Red. 20 mL of aqueous solution was added into the sample bottle and 3 drops of organic solvent was added respectively. The modified cotton fabric was dipped into the solution, and the selective adsorption of oil and water on the modified cotton fabric was observed and explored.The oil–water separation performance of the modified cotton fabric were tested as follow: the solution copper sulfates (8%) was selected as the aqueous solution, and mixed with four different organic solvents of n-hexane, n-octane, kerosene, and chloroform. The oil–water mixture was separated by using batch separation operation and continuous separation operation respectively. The continuous separation device was also used to carry out the separation test of the emulsified oil–water mixture. Multiple separation tests were carry out and the separation efficiency for each time was recorded. When the modified cotton fabric was used continuously for many times, the oil–water separation effect of the modified cotton cloth becomes worse. The modified cotton cloth was dryed at 120° for 5 hours, and it can be used for oil–water separation again.
3 Results and analysis
3.1. Structural characterization of fluoropolymer P(GMA-r-MMA)-g-PFPA
P(GMA-r-MMA)-g-PFPA and modified nano-SiO2 samples were mixed with dry potassium bromide powder, ground into fine powder, and put into infrared press to press into the tablet. The samples were put into the inspection window of the infrared instrument for testing. The infrared spectrum analysis of fluorine-containing epoxy polymer P(GMA-r-MMA)-g-PFPA was shown in Fig. 1a. The peak at 3500 cm−1 is the stretching vibration peak of –OH, the peak of 3000 cm−1 is the stretching vibration peak of –C–H, the peak of 1230 cm−1 is –C–O– stretching vibration peak, the peak of 1750 cm−1 is –COO– stretching vibration peak, the peak of 1348 cm−1 is –C–F stretching vibration absorption peak, and the peak of 930 cm−1 is epoxy group absorption peak. The existence of these peaks proved that the fluorine-containing epoxy polymer P(GMA-r-MMA)-g-PFPA was successfully prepared.The 1H NMR spectrum of the fluorine-containing epoxy polymer P(GMA-r-MMA)-g-PFPA was shown in Fig. 1b. The absorption peak at 1.2–1.5 ppm is the H atomic absorption peak of methyl group (–(CH3)C–). The absorption peak at 1.5–2.0 ppm is the H atomic absorption peak of the methylene group (–CH2C–) on the polymer main chain. The absorption peak at 3.67 ppm is the H atom absorption peak of the chain methoxy group (CH3O–) of the MMA side of the polymer. The absorption peaks at 2.50 ppm and 3.2 ppm are the H atomic absorption peaks on the unopened GMA epoxy group methylene group (–CH2O–) and tertiary carbon (–CHO–). The absorption peaks at 5.2 ppm and 4.23 ppm are the H atomic absorption peaks of the ring-opening GMA (GMA reacted with PFPA) epoxy group tertiary carbon (–CHO–) and methylene group (–CH2O–).
3.2. Tests of contact angle and rolling angle under different superhydrophobic surface construction conditions
Cotton cloth was used as a substrate to obtain a hydrophobic or superhydrophobic surface through hydrophobic modification. Through durability research of the obtained hydrophobic or superhydrophobic surface, a superhydrophobic cotton cloth surface with excellent durability was obtained, and then used for oil–water separation research.Firstly, cotton cloth surfaces were modified by fluorine-containing epoxy polymer P(GMA-r-MMA)-g-PFPA or KH550 modified SiO2 respectively. The effects of the polymer P(GMA-r-MMA)-g-PFPA concentration and KH550 modified SiO2 concentration (from 0.3 mg mL−1 to 5 mg mL−1), soaking time (from 1 h to 10 h), drying time (from 1 h to 6 h), and drying temperature (from 60 to 140 °C) on the contact angles of the water droplets on the surface of the cotton cloth were studied, and the results were shown in Fig. 2. For Fig. 2a, when the concentration of polymer P(GMA-r-MMA)-g-PFPA or KH550 modified SiO2 increased, the contact angles increased firstly and then decreased. When the concentration of polymer was 2 mg mL−1, the contact angle was the highest and was about 90°. When the concentration of SiO2 was 3 mg mL−1, the contact angle was the highest and was 75°. For Fig. 2b, when the soaking time increased, the contact angles firstly increased and then remained stable. For Fig. 2c, when the drying time increased, the contact angles also firstly increased and then remained stable. For Fig. 2d, when the drying temperature increased, the contact angles also firstly increased and then remained stable. These date showed that the contact angles of the water droplets on the surface of the cotton cloth were only about 90° by adjusting the construction conditions, as shown in Fig. 2. These date indicated that it was difficult to achieve the super-hydrophobic effect with the contact angle above 150° by using fluorine-containing epoxy polymer P(GMA-r-MMA)-g-PFPA or KH550 modified SiO2 respectively.
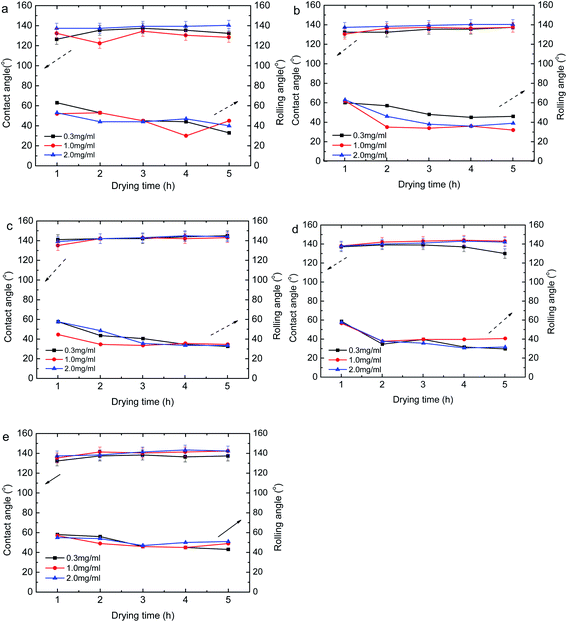
So we use fluorine-containing epoxy polymer P(GMA-r-MMA)-g-PFPA and KH550 modified SiO2 to work together to get the superhydrophobic cotton surface. The construction process of the superhydrophobic cotton cloth surface were shown in Section 2.4. When constructing the superhydrophobic surface of cotton cloth, the polymer concentration was 0.3 mg mL−1, 1 mg mL−1, and 2 mg mL−1, respectively. 5 pieces of cotton cloth substrates were soaked in the solution, and one piece was taken out every two hours, so the soaking time was 2 h, 4 h, 6 h, 8 h and 10 h. For drying each piece of sample, the CA and RA were measured every 1 h, and the drying time was 1 h, 2 h, 3 h, 4 h, and 5 h, respectively. By comparing the water contact angle and water rolling angle under different construction conditions, the best hydrophobic surface construction conditions were obtained. Fig. 3a–e showed the cotton fabric contact angles and water rolling angles under different construction conditions. For this part, the contact angles were above 150°, and the surface of modified cotton cloth was super-hydrophobic, so the water rolling angle (RA) was tested. The RA was test by following procedure: the superhydrophobic material was fixed on the inclined plate, and the inclination angle of the inclined plate was changed by continuously increasing or decreasing the height of the back pad. When testing water contact angle (CA) and RA of the surface of each sample, these angles were measured 5 times randomly and the average value was used.
It can be seen from Fig. 3a–e that the contact angle CA was larger and the rolling angle RA was smaller, when the polymer concentration was higher. However, changing the concentration has little effect on CA and RA, indicating that a polymer concentration of 0.3 mg mL−1 was sufficient to build a superhydrophobic surface. When the soaking time was changed sequentially, the CA and RA of the cotton cloth surface obtained at 2 h and 4 h changed more greatly, and the CA and RA of the cotton cloth surface obtained under the conditions of 6 h, 8 h and 10 h remain basically unchanged, so the best soaking time was 6 h. When the drying time changed, the CA on the surface of the cotton cloth gradually increased, RA gradually decreased, and then stabilized. There was little change between 4 h and 5 h, so the best drying time was 4 h. In summary, the best construction conditions were: polymer concentration 0.3 mg mL−1, soaking time 6 h, drying temperature 120°, drying time 4 h. In these experiments, the water contact angle can reach more than 120° under different conditions, the highest CA can reach 150° ± 2° as shown in Fig. 4b, and the minimum water rolling angle can reach 30° ± 2°. The water droplets were quickly absorbed by the unmodified cotton cloth, as shown in the Fig. 4, and there was no contact angle.
 | ||
| Fig. 4 Surface contact angle pictures of unmodified cotton cloth (a) and superhydrophobic cotton cloth (b). | ||
3.3. Surface properties of superhydrophobic cotton cloth
The contact angles with time increasing were studied by dropping the water droplets on the surface of native cotton cloth and super-hydrophobic cotton cloth and let them stand at room temperature to observe the continuous changes of the water droplets on the surface of the cotton material. The results were shown in Fig. 5. It can be seen from Fig. 5a that the water droplets on the super-hydrophobic cotton cloth surface continue to volatilize over time, but the extremely high contact angle can still be maintained until the water was completely volatilized, and the water droplets can stay on the super-hydrophobic cotton cloth surface for 90 min. Fig. 5b showed that the dynamic contact angle of unmodified cotton fabric. The water was completely absorbed by the cotton fabric after 5 seconds. | ||
| Fig. 5 The contact angles of unmodified cotton fabric (a), superhydrophobic cotton cloth (b), the advancing angle θA (c) and the receding angle θB (d). | ||
The dynamic contact angle were measurement by dropping 5 μL water droplets on the surface of modified cotton using a micro syringe. The height of the flat bottom needle of the micro syringe was adjusted and inserted into the center of the drop to increase (decrease) the liquid continuously. The advancing angle θA was 148°. The receding angle θB was 153°. The contact angle hysteresis Δθ = θA – θB was 5°.
The modified cotton cloth prepared in this study has good hydrophobic properties. The durability and chemical stability of the modified cotton cloth were shown in Fig. 6. The cotton fabric was soaked and formed a protective film on the surface of the cotton fabric in an acid–base solution of sodium citrate, sodium chloride, and citric acid. So that the acid–base solution cannot be impregnated and corroded the polymer coating on the surface. Therefore, it has little effect on the CA and RA on the modified cotton cloth surface. The GMA block in the polymer prepared in this study can chemically cross-link the low surface energy substance TFEMA and aminated modified nano-SiO2 on the surface of the cotton fabric, so that the surface of the cotton fabric has a good hydrophobic effect and good acid-alkali resistance. However, the washing action of detergent has a significant influence on the hydrophobic effect of the modified cotton cloth. CA decreased from 152° ± 2° to 140° ± 2°, and RA increased from 8° ± 2° to 14° ± 2°. This is because the external force of the detergent and magnetic stirring destroys the hydrophobic coating on the surface of the HC. The aqueous detergent solution is immersed into the interior of the modified cotton fabric, part of the fluoropolymer is corroded, and the hydrophobic ability of the HC is reduced.
 | ||
| Fig. 6 Chemical stability of hydrophobically modified cotton fabric (CA means sodium citrate, NS means sodium chloride, SSC means citric acid, washing means detergent and magnetic stirring). | ||
In order to characterize the influence of superhydrophobic materials on the mechanical properties of cotton cloth, the mechanical properties of cotton cloth were tested according to the national standards of cotton cloth composites. The mechanical properties of cotton cloth before and after super-hydrophobic treatment were shown in Fig. 7. The tensile strength and tensile modulus were shown in Fig. 7a. The flexural strength and flexural modulus are shown in Fig. 7b. From the analysis of the figure, it can be seen that the tensile strength and tensile modulus of modified cotton cloth corner untreated cotton cloth materials were increased by 1.4 MPa and 12 MPa respectively, and the flexural strength and flexural modulus were also increased by 2.47 MPa and 471 MPa respectively. Through mechanical data analysis, the super-hydrophobic modified material has basically no effect on the mechanical properties of cotton cloth.
3.4. Scanning electron microscope (SEM) of the modified cotton fabric
The cotton fabric before and after the superhydrophobic modification was fixed on the SEM sample stage, sprayed with gold, and SEM pictures with scales of 5 μm, 10 μm, and 50 μm were taken at different magnifications. The SEM results were shown in Fig. 8, where (a)–(c) were the surface fiber morphology of the unmodified cotton fabric. It can be seen from Fig. 8c that the cotton fabric fibers are arranged in whole clusters. It can be seen from Fig. 8a and b that the fiber surface of the unmodified cotton fabrics are smooth. Fig. 8d–f showed the morphology of the fibers on the surface of the superhydrophobic modified cotton fabric. It can be seen from Fig. 8f that the fibers on the surface of the superhydrophobic modified cotton fabric are also arranged in whole clusters, and there is no adhesion between the fibers. Comparing Fig. 8f of the superhydrophobic modified cotton fabric with Fig. 8c of the unmodified cotton fabric, there is no difference in the fiber morphology, the fibers are clearly visible, and there is no adhesion between the fibers. This phenomenon indicates that the superhydrophobic modification has no effect on the performance of cotton fabrics such as air permeability and softness, and also indicates that the super-hydrophobic modification has no effect on the application of superhydrophobic modified cotton fabrics.Comparing Fig. 8d, e and a, b, it can be found that the fiber surface of the superhydrophobic cotton cloth surface becomes rough. For the magnified observation of the fibers of the superhydrophobic cotton fabric, as shown in Fig. 8d and e, there are layers of uniformly arranged white particles with a particle size of 50–100 nm on the surface of the fiber, which may be silica. After further careful observation, we found that the silica did not fall off, and the silica was nested on the surface of the fiber. This is because the role of fluorine-containing epoxy polymer. On the one hand, the fluorine-containing epoxy polymer reacted with the surface amino groups of the modified silica, and on the other hand, it reacts with the active groups on the surface of the cotton fabric to combine and firmly fixed the fluorine-containing substance and silica on the fiber surface to achieve super hydrophobic properties.
3.5. The wettability test of aqueous solutions
Milk, beverages, copper sulfate aqueous solution (1 wt%), and copper nitrate aqueous solution (1 wt%) were selected for the wettability test. The drops of four different kinds of liquid were drop on the surfaces of the cotton fabric before and after the modification, take photos to compare and analyze the hydrophobicity of the modified material, and the wetting situation were observed as shown in Fig. 9. Fig. 9a showed the droplets of milk, beverages, copper sulfate aqueous solution, and copper nitrate aqueous solution on the surface of unmodified cotton cloth. Various droplets quickly wet the surfaces of the cotton cloth and spread out. This phenomenon indicates that the surface of the unmodified cotton cloth has a lot of hydrophilic properties. | ||
| Fig. 9 Surface wettability test (a) of cotton fabrics with different types of aqueous solutions before and after modification (the pictures in Fig. 5a are respectively the surface test diagrams of cotton fabrics before modification with milk, Fanta, copper sulfate aqueous solution (1 wt%) and copper nitrate aqueous solution (1 wt%), and the surface test diagrams of cotton fabrics after modification) and persistence of water droplets on the surface of cotton cloth (b). | ||
Fig. 9a also showed the droplets of milk, beverage, copper sulfate aqueous solution, and copper nitrate aqueous solution on the surface of the superhydrophobic modified cotton cloth. Various droplets stand on the surface of the superhydrophobic cotton cloth in a spherical shape and cannot wet the surface of the superhydrophobic cotton cloth. This phenomenon indicates that the surface of super-hydrophobic cotton cloth has good hydrophobic properties.
Water droplets can stay on the surface of the superhydrophobic cotton cloth for a long time without penetrating the surface of the cotton cloth, as shown in Fig. 5b. It can be seen from the figure that 0.5 mL of water droplets can stay for more than 120 minutes until the water droplets are completely volatilized.
3.6. Superhydrophobic cotton fabric used for selective adsorption of oil–water mixture
n-Hexane, n-octane, kerosene, and chloroform were selected as organic solvents for selective adsorption test. For the observation convenience, the organic solvents were dyed with Sudan Red. 20 mL of aqueous solution and 3 drops of organic solvents were added to the sample bottles respectively, and the modified cotton fabrics were immersed in the solution. The selective adsorption of the modified cotton fabric to oil and water were observed, and found that the modified cotton fabrics have good hydrophobicity–lipophilicity. The test results were shown in Fig. 10. In the Fig. 10a, e, i, and m, four organic solvents that are dyed with Sudan Red, were added drop-wise to the aqueous solution. Among them, the density of n-hexane, n-octane, and kerosene is less than that of water, and floated on the upper layer. And the density of chloroform is greater than that of water, so the organic solvent was at the bottom of the bottle. The Fig. 10b, f, j and n, are the adsorption process diagrams of the modified cotton fabric. It can be seen that the organic matter is well “captured” on the surface of the modified cotton fabric, and the cotton fabric shows good lipophilicity. Fig. 10c, g, k, and o are cotton fabrics after adsorption. Organic matters were well absorbed on the surface of the cotton fabric, and the surfaces of the cotton fabric have no water droplets attached, showing good hydrophobicity. Fig. 10d, h, l and p are the water left in the sample bottle after the adsorption experiment. The organic matters were completely “captured” by the modified cotton fabric. The water in the bottle are clear and there is no residual organic solvent after dyeing.3.7. Intermittent oil–water separation performance test of modified cotton fabric
The blue copper sulfate aqueous solution used as the water phase. Chloroform, kerosene, n-octane and n-hexane dyed with Sudan Red used as the oil phase. 10 mL of copper sulfate aqueous solution was mixed with 10 mL of four organic solvents to prepare four kinds of oil–water mixtures. The super-hydrophobic modified cotton fabric was placed on the mouth of the collection bottle and fixed to form a groove. The oil and water mixture was drop into the groove of the superhydrophobic cotton cloth. The oil phase would penetrate the cotton fabric and reached the collection bottle. The water phase wound stay in the groove of the superhydrophobic cotton fabric, and the water phase was collected and weighed to calculate the efficiency of oil–water separation. The oil–water separation effect of the four organic solvents and water mixtures were shown in Fig. 11. After multiple separations, the separation efficiency was still as high as 98% or more. It can be seen that the prepared super-hydrophobic cotton fabric has a good oil–water separation effect. | ||
| Fig. 11 The separation efficiency diagram of chloroform, kerosene, n-octane, n-hexane and water mixture. | ||
When the modified cotton fabric was used continuously for many times, the oil–water separation effect of the modified cotton cloth becomes worse. And the oil–water seperation efficiency was lower than 90%. The modified cotton cloth was dryed at 120° for 5 hours, and it can be used for oil–water separation again, and the oil–water seperation efficiency were higher than 98%, and can be operated for another 6 times. It can be inferred from this phenomenon that nano-SiO2 and fluoropolymers still remain on the surface of the modified cotton cloth after the modified cotton cloth has been recycled for many times. This is because the fluoropolymer and silica are chemically bonded to the surface of the cotton fabric. The possible process is shown in the figure.
3.8. Continuous oil–water separation performance test of modified cotton fabric
The intermittent operation of oil–water mixture separation requires more operating time, and the separated amount of oil–water mixture is limited. The continuous operation of oil–water mixture separation is further studied. The experimental process is shown in Fig. 12. Fig. 12a, e, i and m are the mixtures of n-octane, n-hexane, kerosene, chloroform and copper sulfate aqueous solutions, respectively. The red solution are organic solvent and the blue solution is water. The superhydrophobic cotton fabric is placed at the entrance of the latex tube, and the latex tube is connected to the peristaltic pump, and the collection bottle is connected to the outlet of the latex tube to form a continuous oil–water separation device. The inlet of the latex tube is placed at the phase interface of the oil–water mixture. Under the power of the peristaltic pump, the blue aqueous solution will not pass through the super-hydrophobic cotton fabric and did not enter the collection bottle via the peristaltic pump. While the red organic solvent will pass through the superhydrophobic cotton fabric and enter the collection bottle via the peristaltic pump, as shown in Fig. 12b, f, j and n. As the continuous separation progresses, the water phase still cannot penetrate the superhydrophobic cotton fabric, and almost all the oil phase reaches the collection bottle, regardless of whether the oil phase is located in the upper or lower layer, as shown in Fig. 12c, g, k and o. Collect the remaining copper sulfate aqueous solution in a graduated cylinder, as shown in Fig. 12d, h, l and p. It can be observed that the collected copper sulfate solution are greater than 9.8 mL, and the separation efficiency exceeds 98%.3.9. Separation performance of emulsified oil–water mixture of modified cotton fabric
Under the action of the emulsifier, 10 mL of chloroform dyed with Sudan Red and 1 mL of copper sulfate aqueous solution were added together and formed an opaque oil–water emulsified mixture using 0.05 g sodium dodecyl sulfonate as emulsifier, as shown in Fig. 13a. The super-hydrophobic modified cotton fabric was packed at the entrance of the latex tube, and then connected the latex tube to the peristaltic pump. Under the power of the peristaltic pump, the separation process of the emulsion is shown in Fig. 13b. The chloroform dyed with Sudan Red in the right bottle was separated from the emulsion, passed through the superhydrophobic modified cotton cloth, and reached the left side through the peristaltic pump. The collection bottle turned into a transparent red solution, and the blue copper sulfate aqueous solution in the right bottle gradually separated from the emulsion and floated on the red chloroform solution, as shown in Fig. 13b. At the end of the separation, the volume of the red chloroform solution in the left collection bottle reached the maximum, and the peristaltic pump could not completely suck all the emulsion in the right bottle to the left collection bottle, but there was some blue copper sulfate solution in the upper part of the right bottle. It can be seen that the super-hydrophobic modified cotton fabric can be used for the separation of oil–water mixture emulsions and has a wide range of application prospects.4. Conclusion
The fluorine-containing epoxy polymer P(GMA-r-MMA)-g-PFPA was prepared by traditional free radical polymerization, and the hybrid assembly was assembled with KH-550 modified nano-SiO2 to prepare hybrid assembly solutions with different concentrations. The superhydrophobic surface was prepared by using solution immersion method. By controlling the construction conditions such as polymer concentration, immersion time, drying time, etc., the best hydrophobic surface construction conditions are obtained, and the results were 0.3 mg mL−1 polymer concentration, immersion time 6 h, drying temperature 120°, drying time 4 h. The maximum water contact angle can reach 150° ± 2°, and the minimum water rolling angle is 30° ± 2°. It has good hydrophobic effect on different types of aqueous solutions. For the oil–water separation experiment, in batch operation, the separation efficiency can reach 99%, and can maintain 5 consecutive high-efficiency separations; in continuous separation, the separation efficiency can reach 98; for oil–water mixed emulsions, it also has a good oil–water separation effect. Although the preparation method is simple and easy to implement, its durability is still not high, and the efficiency begins to decrease after five separations, which cannot meet industrial needs, so further research is needed.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Nature Science Foundation of China (no. 51803167).References
- L. Han, Y. Z. Tan, T. Netke, A. G. Fane and J. W. Chew, Understanding oily wastewater treatment via membrane distillation, J. Membr. Sci., 2017, 539(1), 284–294 CrossRef CAS.
- X. Xu, W. Liu, S. Tian, W. Wang, Q. Qi, P. Jiang, X. Gao, F. Li, H. Li and H. Yu, Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: a perspective analysis, Front. Microbiol., 2018, 9(1), 2885 CrossRef PubMed.
- O. Carmody, R. Frost, Y. Xi and S. Kokot, Adsorption of hydrocarbons on organo-clays—Implications for oil spill remediation, J. Colloid Interface Sci., 2007, 305(1), 17–24 CrossRef CAS PubMed.
- X. Yue, Z. Li, T. Zhang, D. Yang and F. Qiu, Design and fabrication of super wetting fiber-based membranes for oil/water separation applications, Chem. Eng. J., 2019, 364(5), 292–309 CrossRef CAS.
- S. Rasouli, N. Rezaei, H. Hamedi, S. Zendehboudi and X. Duan, Superhydrophobic and superoleophilic membranes for oil–water separation application: A comprehensive review, Mater. Des., 2021, 204(6), 109599 CrossRef CAS.
- K. Zhu, Application of liquid membrane separation technology in pharmaceutical and chemical industry, Chem. Eng. Des. Commun., 2018, 44(08), 200–201 RSC.
- J. Usman, M. H. D. Othman, A. F. Ismail, M. A. Rahman, J. Jaafar, Y. O. Raji, A. O. Gbadamosi, T. H. El Badawy and K. A. M. Said, An overview of superhydrophobic ceramic membrane surface modification for oil–water separation, J. Mater. Res. Technol., 2021, 12(3), 643–667 CrossRef CAS.
- X. Li, M. Wang, C. Wang, C. Cheng and X. Wang, Facile Immobilization of Ag Nanocluster on Nanofibrous Membrane for Oil/Water Separation, ACS Appl. Mater. Interfaces, 2014, 6(17), 15272–15282 CrossRef CAS.
- D. Liang, F. Lefeng and Z. Baicun, Application of nano-superhydrophilic membrane, Shanghai Building Materials, 2006,(01), 10–11 Search PubMed.
- J. Ju, T. Wang and Q. Wang, A facile approach in fabricating superhydrophobic and superoleophilic poly (vinylidene fluoride) membranes for efficient water–oil separation, J. Appl. Polym. Sci., 2015, 132(24), 42077 Search PubMed.
- M. Cao, X. Luo, H. Ren and J. Feng, Hot water-repellent and mechanically durable super-hydrophobic mesh for oil/water separation, J. Colloid Interface Sci., 2018, 512(3), 567–574 CrossRef CAS.
- H. Tseng, J. Wu, Y. Lin and G. Zhuang, Superoleophilic and superhydrophobic carbon membranes for high quantity and quality separation of trace water-in-oil emulsions, J. Membr. Sci., 2018, 559(8), 148–158 CrossRef CAS.
- S. Wang, C. Liu, G. Liu, M. Zhang, J. Li and C. Wang, Fabrication of superhydrophobic wood surface by a sol–gel process, Appl. Surf. Sci., 2011, 258(2), 806–810 CrossRef CAS.
- D. Lopez-Torres, C. Elosua, M. Hernaez, J. Goicoechea and F. J. Arregui, From superhydrophilic to superhydrophobic surfaces by means of polymeric Layer-byLayer films, Appl. Surf. Sci., 2015, 351, 1081–1086 CrossRef CAS.
- Ni Wen, X. Miao, X. Yang, M. Long, W. Deng, Q. Zhou and W. Deng, An alternative fabrication of under oil superhydrophobic or underwater superoleophobic stainless steel meshes for oil-water separation: Originating from one-step vapor deposition of polydimethylsiloxane, Sep. Purif. Technol., 2018, 204, 116–126 CrossRef CAS.
- J. Wang, L. Wang and L. Feng, One-step fabrication of fluoropolymer transparent films with superhydrophobicity by dry method, J. Appl. Polym. Sci., 2011, 120(1), 524–529 CrossRef CAS.
- Z. Xu, D. Jiang, Z. Wei, J. Chen and J. Jing, Fabrication of superhydrophobic nano aluminum films on stainless steel meshes by electrophoretic deposition for oilwater separation, Appl. Surf. Sci., 2018, 427, 253–261 CrossRef CAS.
- W. Qing, X. Shi, Y. Deng, W. Zhang, J. Wang and C. Y. Tang, Robust superhydrophobicsuperoleophilic polytetrafluoroethylene nanofibrous membrane for oil/water separation, J. Membr. Sci., 2017, 540, 354–361 CrossRef CAS.
- Ci Cheng, F. Wang, B. Zhao, Y. Ning, Y. Lai and L. Wang, Acid/base treatment of monolithic activated carbon for coating silver with tunable morphology, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2017, 32(4), 760–765 CrossRef CAS.
- X. J. Lee, P. L. Show, T. Katsuda, W.-H. Chen and Jo-S. Chang, Surface grafting techniques on the improvement of membrane bioreactor: state-of-the-art advances, Bioresour. Technol., 2018, 269, 489–502 CrossRef CAS PubMed.
- K. Tang, J. Yu, Y. Zhao, Y. Liu, X. Wang and R. Xu, Fabrication of super-hydrophobic and super-oleophilic boehmite membranes from anodic alumina oxide film via a twophase thermal approach, J. Mater. Chem., 2006, 16(18), 1741–1745 RSC.
- F. Chen, J. Song, Z. Liu, J. Liu, H. Zheng, S. Huang, J. Sun, W. Xu and X. Liu, Atmospheric pressure plasma functionalized polymer mesh: an environmentally friendly and efficient tool for oil/water separation, ACS Sustainable Chem. Eng., 2016, 4(12), 6828–6837 CrossRef CAS.
- D.-H. Kim and D.-H. Lee, Effect of irradiation on the surface morphology of nanostructured superhydrophobic surfaces fabricated by ion beam irradiation, Appl. Surf. Sci., 2019, 477(31), 154–158 CrossRef CAS.
- M. Xi, J. Yong, F. Chen, Q. Yang and X. Hou, A femtosecond laser-induced superhygrophobic surface: beyond superhydrophobicity and repelling various complex liquids, RSC Adv., 2019, 9(12), 6650–6657 RSC.
| This journal is © The Royal Society of Chemistry 2021 |