Open Access Article
Open Access ArticleUltradispersed IrxNi clusters as bifunctional electrocatalysts for high-efficiency water splitting in acid electrolytes†
Xiaojie Zhao,
Ying Chang*,
Jiang Ji,
Jingchun Jia * and
Meilin Jia
* and
Meilin Jia
College of Chemistry and Environmental Science, Inner Mongolia Key Laboratory of Green Catalysis and Inner Mongolia Collaborative Innovation Center for Water Environment Safety, Inner Mongolia Normal University, Hohhot, 010022, China. E-mail: changying@imnu.edu.cn; jjc1983@126.com
First published on 8th October 2021
Abstract
Design and synthesis of electrocatalysts with high activity and low cost is an important challenge for water splitting. We report a rapid and facile synthetic route to obtain IrxNi clusters via polyol reduction. The IrxNi clusters show excellent activity for the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) in acidic electrolytes. The optimized Ir2Ni/C clusters exhibit an electrochemical active area of 18.27 mF cm−2, with the overpotential of OER being 292 mV and HER being 30 mV at 10 mA cm−2, respectively. In addition, the Ir2Ni/C used as the cathode and anode for the H-type hydrolysis tank only needs 1.597 V cell voltages. The excellent electrocatalytic performance is mainly attributed to the synergistic effect between the metals and the ultra-fine particle size. This study provides a novel strategy that has a broad application for water splitting.
1. Introduction
Hydrogen energy as a clean energy has become a research hotspot in recent years. Electrolytic water1,2 and fuel cells3,4 are important devices for hydrogen energy generation and conversion.5,6 These new technologies have been deemed to be clean, environmentally friendly and renewable, and they play extremely important roles in hydrogen energy technology. The use of these technologies is a promising strategy for the production of clean energy by electrocatalytic water splitting,7 which involves the cathode HER8–10 and the anode OER.11–14 The product of electrolyzed water can be used as raw material for fuel cells. However, the OER is usually a complex electron transfer process with multistep coupling, which often shows hysteresis reaction kinetics,15 thereby substantially affecting the reaction efficiency of water electrolysis.16Pt,17 Ru,18 Pd19 and other precious metal catalysts are used in both electrolytic water and fuel cells. However, owing to their expense and poor durability, large-scale commercial development is difficult. We attempt to introduce other elements and reduce particle size to enhance catalytic activity while reducing the amount of precious metals. Bimetallic electrocatalysts usually combine precious metals with transition metals, such as IrCu.20,21 Ir-based22–24 catalysts have higher activity and stability in alkaline electrolytes, but it is difficult to realize under acidic conditions because they easily dissolve and agglomerate. To overcome these barriers, it is necessary to design efficient and stable electrocatalysts in acidic media. The synergistic effect that is introduced by alloying reduces the adsorption energy of intermediates and further improves the catalytic performance.
Recently, the unique electronic structures and high surface atomic ratios have made metal clusters a new type of catalysts.25,26 Researchers have successfully prepared Pt and Ru clusters of smaller particle size for OER. Moreover, these structures27–30 can stabilize the metal clusters31,32 during the removal of ligands. However, in conventional synthesis methods, clusters tend to agglomerate, which reduces their catalytic performance. Recent studies have shown that carbon supports33,34 can overcome this obstacle by regulating charge transfer pathways and pores. Since the particle sizes of metal clusters are very small, more active sites can be exposed;35 thus, our research group conducted relevant studies on them.
This study report that the synthesis of an IrxNi cluster catalyst in which a simple oil bath was used to control the element composition ratio by adjusting the proportion of the metal precursor, thereby avoiding complex processes, such as high-temperature annealing, and time-consuming steps.36–38 The synthesized IrxNi clusters were distributed on a thin layer of carbon (IrxNi/C). Due to differences in atom sizes, the metals forms clusters by bonding; thus, the ultra-fine particle size (2 nm) can increase active sites. Based on the rapid synthetic applications of this work; it can be extended to other Ir-based clusters materials.
2. Experimental section
2.1 Experimental materials
Trisodium citrate dehydrate (C6H5Na3O7·2H2O, ≥99.0%), iridium(III) chloride hydrate (IrCl3·xH2O, 99.9%, Ir: 60%), nickel(II) chloride hexahydrate (NiCl2·6H2O, ≥98.0%), Vulcan XC-72R carbon black, ethylene glycol (C2H6O2, 99%), and potassium hydroxide (KOH, ≥85.0%) and Nafion solution (∼5 wt%) were all purchased from Aladdin Industrial Corp. All the chemicals were used without further purification. The water (18.25 MΩ cm−1) that was used in all experiments was prepared via passage through an ultrapure purification system (aqua solutions).2.2 Materials synthesis
For the Ir2Ni/C, 40 mg C6H5Na3O7·2H2O was added into a round-bottomed flask of 30 mL ethylene glycol and ultrasonic dispersion to transparency. Then, 268 μL NiCl2·6H2O (0.2 g mL−1) and 1445 μL IrCl3·xH2O (0.1 g mL−1) were added in to a flask, meanwhile add the magnetons and stir well. Then, 1 M KOH was added drop by drop to adjust pH to 9. Next, 40 mg XC-72R was added and dispersed evenly using ultrasound, and the oil bath is conducted under set conditions (160 °C, 300 rpm). The resulting mixture was washed via ethanol/ultrapure water and dried under vacuum. The preparations of IrNi/C, Ir3Ni/C and Ir/C were similar to the preparation of Ir2Ni/C except that 723 μL, 2168 μL, and 1667 μL IrCl3·xH2O (0.1 g mL−1) were used for IrNi/C, Ir3Ni/C and Ir/C, respectively.2.3 Materials characterization
Transmission electron microscopy (TEM) and High-resolution TEM (HRTEM, FEI Tecnai G2 F30) were conducted to morphology and structure. X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi) and X-ray diffraction (XRD, Rigaku Ultima IV) were conducted to chemical state and structure, the scan speed was set as 4° min−1 with 2θ range of 5–90°.2.4 Electrochemical measurements
The electrochemical workstation was CHI 660E (Chenhua, Shanghai, China), and all tests were performed in three-electrode electrolytic tank. The working electrode was carbon paper (area: 0.25 cm2), reference electrode (Ag/AgCl) and counter electrode (graphite rod) were used. The mixing Nafion (5%), ultrapure water and ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) into slurry to prepared working electrode. Then, 5 mg catalyst was weighed and dissolved in 1 mL prepared slurry to obtain a uniform dispersion. A total of 16 μL catalyst ink was measured and spread evenly on square carbon paper (loading amount: 0.32 mg cm−2). The linear sweep voltammetry (LSV) were measured at a sweep rate of 5 mV s−1. The Cdl was in the test range between potentials of 1–1.1 V with various sweep rates (10–100 mV s−1). Electrochemical impedance spectroscopy (EIS) of the OER was performed a test potential of 1.4 V and a frequency range (100–0.01 Hz). The chronopotentiometry parameter (CP) test was conducted at 10 mA cm−2.
10) into slurry to prepared working electrode. Then, 5 mg catalyst was weighed and dissolved in 1 mL prepared slurry to obtain a uniform dispersion. A total of 16 μL catalyst ink was measured and spread evenly on square carbon paper (loading amount: 0.32 mg cm−2). The linear sweep voltammetry (LSV) were measured at a sweep rate of 5 mV s−1. The Cdl was in the test range between potentials of 1–1.1 V with various sweep rates (10–100 mV s−1). Electrochemical impedance spectroscopy (EIS) of the OER was performed a test potential of 1.4 V and a frequency range (100–0.01 Hz). The chronopotentiometry parameter (CP) test was conducted at 10 mA cm−2.
3. Results and discussion
The IrxNi/C (x = 1, 2, 3) clusters were prepared by polyol reduction (Fig. S1†). The composition ratio of IrxNi/C was determined by adjusting the mole ratio of the precursor (see Experimental section for details). X-ray diffraction (XRD) (Fig. 1a) pattern resemble that of the amorphous carbon support, only two broad diffraction peaks at 24.5° and 44.5° were readily observed, and there was no evidence of metal or metal oxide formation. In addition, with increasing Ni content, the peak strength of the clusters gradually decreased, and the half-peak width gradually increased (Fig. S2†), which indicate that the clusters were of low crystallinity and ultrafine sizes.8,20,21 Moreover, compared with pure XC-72R, the peak strength decreased significantly; hence, the clusters were successfully loaded onto the carbon support. The change in peak strength also further demonstrates the occurrence of a synergistic effect in the cluster phase, which may have been caused by the replacement of Ni by Ir.X-ray photoelectron spectroscopy (XPS) (Fig. S3†) was used to further investigate the changes in the electronic structure of the Ir2Ni/C clusters. The Ir 4f spectrum shows that iridium was present mainly in the valence states of Ir0 and Ir4+, and the peaks at 61.1, 62.5, 64.0 and 66.5 eV correspond to Ir0 4f7/2, Ir4+ 4f7/2, Ir0 4f5/2, and Ir4+ 4f5/2, respectively; which indicating the surface of Ir2Ni/C is mainly composed of Ir0 (Fig. S3b†). As shown in Fig. S3c,† due to the low Ni content and insufficient signal-to-noise ratio, there are disorderly peaks in Ni 2p. In addition, the small peak of O 1s in the spectrum may have been partially oxidized exposure to air, and C was involved in a single bond and a double bond according to Fig. S3d.† Moreover, the Ir 4f peaks of Ir/C show a positive shift as presented in Fig. S4.† Fig. 1b shows that the Ir 4f peaks of Ir2Ni/C clusters were significantly negatively shifted compared with Ir/C. The results demonstrate that Ir and Ni underwent electron transfer due to strong electron interactions.
The preparation of Ir2Ni/C was monitored via transmission electron microscopy (TEM) (Fig. 1c). The results demonstrated that the clusters were uniformly distributed on the carbon support. Fig. 1d shows high-resolution TEM (HRTEM) image of Ir2Ni/C, in which clusters are circled in yellow, and irregular clusters and defective surfaces can expose more active sites. The HRTEM image shows a continuous lattice fringe, which indicates the crystalline properties of the clusters, and the lattice fringe spacings were 0.2151 nm and 0.1945 nm,21 which correspond to the (111) crystal plane and (200) crystal plane, respectively, of the face-centered cubic (fcc) Ir–Ni clusters (the inset of Fig. 1d). Therefore, the previous hypothesis of an amorphous phase was rejected, which may be because a low metal content leads to poor crystallinity. In combination with the previous XRD and XPS diagrams, the formation of Ir2Ni/C clusters was further demonstrated, and the previous hypothesis of an amorphous phase was also rejected; due to the low metal content, there was no readily observable diffraction peak. Fig. 1e clearly shows the size of the clusters, which have a particle size of nearly 2 nm; therefore, clusters structure has been successfully formed. The electron diffraction spectrum (EDS) (Fig. 1f) shows that the distinct Ir, Ni and C peaks (from the yellow area in Fig. 1g) support the presence of Ir, Ni, and C. The Ir2Ni/C clusters were characterized by high-angle circular dark field scanning (HAADF-STEM) (Fig. 1g), and the white particles in the figure clearly indicate the presence of metals. In addition, clusters are uniformly distributed without growth. The element mappings of Ir2Ni/C showed that Ir, Ni and C are evenly distributed among the clusters, which further demonstrates the presence of iridium and nickel in the clusters. Compared to IrNi/C (Fig. S5†) and Ir3Ni/C (Fig. S6†), Ir2Ni/C is more uniformly distributed and has a smaller particle size than IrNi/C (4.3 nm) and Ir3Ni/C (2.3 nm) (Fig. S7†).
Ir-based catalysts are considered excellent materials for OER in alkaline media.24,39 However, their development in acidic media has been hindered by slow reaction kinetics, strong corrosion, and easy dissolution. Therefore, it is necessary to identify a catalyst that can overcome the acid barrier. Apart from that the Ir/C and Pt/C were used as the comparison samples of OER and HER, respectively. Fig. 2a exhibits the polarization curves of the OER. The Ir2Ni/C clusters only require overpotential of 292 mV at 10 mA cm−2, which is less than Ir/C (30 mV) and RuO2 (48 mV) (Fig. 2b and Fig. S8†); hence, it has higher activity. Table S2† summarizes the performance of Ir-based catalysts of OERs in recent years. Moreover, by adding a suitable amount of nickel, the polarization curve is negatively shifted to a low potential, thereby reducing the reaction overpotential, which is mainly caused by the synergistic interaction between Ir and Ni species. Table S1† presents the metal content in IrxNi/C determined via inductively coupled plasma emission spectrometry (ICP-OES), with commercial contrast catalyst as references. The results show that low noble metal loading can still show excellent catalytic activity. In order to gain further insight into the catalytic ability and structural changes of OER, we performed XPS (Fig. S9†) characterization of Ir2Ni/C after OER. It can be clearly seen in Fig. S9† that the binding energy of the Ir 4f peak becomes higher during the OER process, indicating the surface oxidation of Ir. These results suggest that Ir2Ni/C forms IrOx species on the catalyst surface during the OER process.40,41
In addition, compared to IrNi/C and Ir3Ni/C (Fig. S5 and S6†), the enhancement of Ir2Ni/C activity is also due to its uniform dispersion and ultrafine particle size (Fig. S7†). The Tafel slopes (Fig. 2c) of Ir2Ni/C, Ir3Ni/C, Ir/C, Pt/C and IrNi/C are 47.83, 48.54, 49.05, 80.70 and 187.84 mV dec−1, respectively. The smaller Ir2Ni/C indicated the low overpotential that is required in the reaction, which accelerated the kinetics. To further investigate the OER kinetic performance, the electrochemical impedance was tested. Charge transfer resistance (Rct) occurred due to the strong interactions between atoms in the electrocatalytic process, and a smaller semicircle diameter indicated faster charge transfer and better catalytic performance (Fig. 2d). Furthermore, in the hydrogen adsorption/desorption zone;42 the electrochemical double-layer capacitances (Cdl), which are linearly related to the electrochemical surface areas (ECSAs) were measured via cyclic voltammetry to evaluate the catalytic activity. The capacitance can be indirectly characterized by measuring the CV at various sweeping speeds (Fig. S10†). The Cdl (Fig. 2e) of Ir2Ni/C is as high as 18.27 mF cm−2, which represents a 2-fold improvement in Cdl over that of Ir/C. Ir2Ni/C shows significantly enhanced ECSA compared with Ir/C, which further demonstrated that the appropriate amount of Ni exposed more active sites. In the chronopotentiometry test (Fig. 2f), the Ir2Ni/C demonstrated excellent stability for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s (10 mA cm−2) in acidic media. Then, we compared the polarization curves of Ir2Ni/C before and after durability to reflect its activity reproducibility (Fig. S11†). After 10
000 s (10 mA cm−2) in acidic media. Then, we compared the polarization curves of Ir2Ni/C before and after durability to reflect its activity reproducibility (Fig. S11†). After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s durability test, its activity decreased by only 25 mV at the 10 mA cm−2. In addition, the current density represents a 2.4-fold improvement in the polarization curve (Fig. S12a†), a 3.4-fold improvement in Cdl (Fig. S12d†), and lower Tafel slopes (Fig. S12b†) and EIS (Fig. S12c†) in alkaline medium. The results show that the IrxNi clusters are more suitable for acidic medium.
000 s durability test, its activity decreased by only 25 mV at the 10 mA cm−2. In addition, the current density represents a 2.4-fold improvement in the polarization curve (Fig. S12a†), a 3.4-fold improvement in Cdl (Fig. S12d†), and lower Tafel slopes (Fig. S12b†) and EIS (Fig. S12c†) in alkaline medium. The results show that the IrxNi clusters are more suitable for acidic medium.
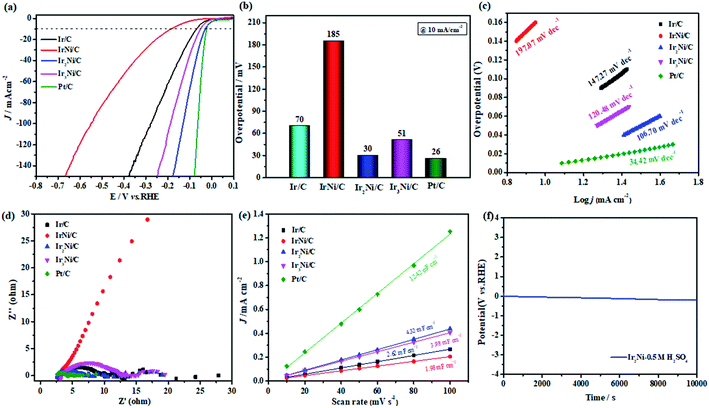
The prepared catalyst was evaluated for HER in the same medium. Pt/C is considered the best catalyst for the HER,43 especially because its overpotential tends to zero and its Tafel slope is much lower in acidic solutions. Iridium, which is an independent metal under typical conditions, is often insensitive to HER, but the formation of clusters of IrxNi/C can significantly improve its catalytic performance.44 Fig. 3a shows the LSV curves of HER in acidic medium. Ir2Ni/C provided higher activity (only a 30 mV overpotential) (Fig. 3b) and low Tafel slope (106.70 mV dec−1) (Fig. 3c). Table S3† describes in detail the Ir-based catalysts that have been used in HER in recent years. Moreover, in the electrochemical impedance spectroscopy (EIS) test (Fig. 3d), the small Rct indicates that it has superior charge transfer capability and kinetics.45 Although the double electric layer capacitance (Fig. 3e) of Ir2Ni/C, which is less than Pt/C, it represents 1.6-fold improvement over the Cdl of Ir/C. Soon afterwards, the same test was conducted in an alkaline medium. Due to differences in the HER paths and the influence of high pH, the HER activity in alkaline media is slightly inferior. Although a high current density (Fig. S13a†) could be realized, the initial potential and Tafel slope value (Fig. S13b†) were large due to the large background current. Compared with the acidic conditions, the double electric layer capacitance (Cdl) showed a downward trend (Fig. S13d†). However, the electrochemical impedance (Fig. S13c†) of Ir2Ni/C is better than commercial Pt/C, which may be acceleration of the reaction kinetics by the electron synergy between Ir and Ni atoms. Ir2Ni/C also demonstrates excellent stability. As shown in Fig. 3f, we further assess the long-term stability of Ir2Ni/C. Ir2Ni/C is stable up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s (10 mA cm−2) with no significant potential change to be negligible, thus, Ir2Ni/C is stable for HER in highly corrosive acidic solution. This is due to the rough surfaces of the clusters and the reduction of the metal Ni–Ni bond length and the chemical reaction activation energy after alloying, which further improves the long-term stability of catalysts.
000 s (10 mA cm−2) with no significant potential change to be negligible, thus, Ir2Ni/C is stable for HER in highly corrosive acidic solution. This is due to the rough surfaces of the clusters and the reduction of the metal Ni–Ni bond length and the chemical reaction activation energy after alloying, which further improves the long-term stability of catalysts.
Considering that the self-supported46 Ir2Ni/C material has excellent bifunctional properties, we used Ir2Ni/C as cathode and anode, respectively, to fabricate an electrolyzer (Ir2Ni/C‖Ir2Ni/C) toward overall water splitting (the inset of Fig. 4a), which used as cathode and anode for H-type hydrolysis tank only needs 1.597 V cell voltages well below Ir/C‖Pt/C (1.690 V) and other reported articles (Fig. 4a). In addition, the electrolyzer is durable up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s (Fig. 4b).47 The Faradaic efficiency (FE) of Ir2Ni/C‖ Ir2Ni/C was experimentally measured to be 99%, and the gas production (H2
000 s (Fig. 4b).47 The Faradaic efficiency (FE) of Ir2Ni/C‖ Ir2Ni/C was experimentally measured to be 99%, and the gas production (H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2) being close to 2
O2) being close to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 tested by drainage method (Fig. S14†). The Ir2Ni/C is an outstanding bifunctional catalyst for overall water splitting.
1 tested by drainage method (Fig. S14†). The Ir2Ni/C is an outstanding bifunctional catalyst for overall water splitting.
Compared to the previously reported Ir-based materials (Tables S2 and S3†), Ir2Ni/C has excellent catalytic properties for OER and HER, which main reasons can be summarized as follows: (1) the strong electron interaction between the precious metal Ir and the 3d transition metal Ni promotes electron transfer and accelerates the reaction kinetics. (2) Cluster structures are formed with nanometer particle sizes of nearly 2 nm, which can increase the ECSAs and further improve the catalytic performance. (3) Carbon support not only increases the electrical conductivity, but also inhibits the agglomeration of metal clusters into larger particles.
4. Conclusions
In summary, IrxNi/C cluster catalysts were synthesized via simple method. IrxNi/C clusters with average particle sizes of less than 2 nm were successfully prepared, and the abundant active sites and rough surface enhance the catalytic activity. The synthesized Ir2Ni/C performs excellent bifunctional catalyst, the Ir2Ni/C used as cathode and anode for H-type hydrolysis tank only needs 1.597 V cell voltages with long-term stability. This study provides valuable information and a novel strategy for relevant industrial production by preparing Ir2Ni/C bifunctional catalysts, so this strategy has broad application prospects in water splitting.Conflicts of interest
No competing economic interests are declared by the authors.Acknowledgements
We would like to thank the National Natural Science Foundation of China (Project No. 21703249), the Natural Science Foundation of Inner Mongolia of China (2019MS2017), the Research Innovation Foundation for Graduate of Inner Mongolia Normal University (No. CXJJS20105), the Research Innovation Foundation for Graduate of Inner Mongolia Autonomous Region (No. SZ2020116) and the Research Foundation for Advanced Talents of Inner Mongolia Normal University (No. 2018YJRC001 and 2018YJRC012) for financial support.References
- Q. Wang, C. Q. Xu, W. Liu, S. F. Hung, H. Bin Yang, J. Gao, W. Cai, H. M. Chen, J. Li and B. Liu, Nat. Commun., 2020, 11, 4246–4258 CrossRef CAS PubMed
.
- H. N. Nong, T. Reier, H.-S. Oh, M. Gliech, P. Paciok, T. H. T. Vu, D. Teschner, M. Heggen, V. Petkov, R. Schlögl, T. Jones and P. Strasser, Nat. Catal., 2018, 1, 841–851 CrossRef CAS
.
- Y. Li, Z. Kang, J. Mo, G. Yang, S. Yu, D. A. Talley, B. Han and F.-Y. Zhang, Int. J. Hydrogen Energy, 2018, 43, 11223–11233 CrossRef CAS
.
- O. Panchenko, E. Borgardt, W. Zwaygardt, F. J. Hackemüller, M. Bram, N. Kardjilov, T. Arlt, I. Manke, M. Müller, D. Stolten and W. Lehnert, J. Power Sources, 2018, 390, 108–115 CrossRef CAS
.
- X. Shi, H. Wang, P. Kannan, J. Ding, S. Ji, F. Liu, H. Gai and R. Wang, J. Mater. Chem. A, 2019, 7, 3344–3352 RSC
.
- L. Rößner and M. Armbrüster, ACS Catal., 2019, 9, 2018–2062 CrossRef
.
- J. Feng, F. Lv, W. Zhang, P. Li, K. Wang, C. Yang, B. Wang, Y. Yang, J. Zhou, F. Lin, G.-C. Wang and S. Guo, Adv. Mater., 2017, 29, 1703798 CrossRef PubMed
.
- Q. Wang, M. Ming, S. Niu, Y. Zhang, G. Fan and J.-S. Hu, Adv. Energy Mater., 2018, 8, 1801698 CrossRef
.
- S. Li, P. Ren, C. Yang, X. Liu, Z. Yin, W. Li, H. Yang, J. Li, X. Wang, Y. Wang, R. Cao, L. Lin, S. Yao, X. Wen and D. Ma, Sci. Bull., 2018, 63, 1358–1363 CrossRef CAS
.
- S. Li, R. Cao, M. Xu, Y. Deng, L. Lin, S. Yao, X. Liang, M. Peng, Z. Gao and Y. Ge, Natl. Sci. Rev., 2021 DOI:10.1093/nsr/nwab026
.
- Y. Liu, Y. Ying, L. Fei, Y. Liu, Q. Hu, G. Zhang, S. Y. Pang, W. Lu, C. L. Mak, X. Luo, L. Zhou, M. Wei and H. Huang, J. Am. Chem. Soc., 2019, 141, 8136–8145 CrossRef CAS PubMed
.
- J. Hu, S. Li, J. Chu, S. Niu, J. Wang, Y. Du, Z. Li, X. Han and P. Xu, ACS Catal., 2019, 9, 10705–10711 CrossRef CAS
.
- Y. Li, Z. Wang, J. Hu, S. Li, Y. Du, X. Han and P. Xu, Adv. Funct. Mater., 2020, 30, 1910498 CrossRef CAS
.
- S. Niu, X.-P. Kong, S. Li, Y. Zhang, J. Wu, W. Zhao and P. Xu, Appl. Catal., B, 2021, 297, 120440 CrossRef
.
- O. Matselko, R. R. Zimmermann, A. Ormeci, U. Burkhardt, R. Gladyshevskii, Y. Grin and M. Armbrüster, J. Phys. Chem. C, 2018, 122, 21891–21896 CrossRef CAS
.
- Q. Jiang, J. Xu, Z. Li, C. Zhou, X. Chen, H. Meng, Y. Han, X. Shi, C. Zhan, Y. Zhang, Q. Zhang, X. Jia and R. Zhang, Adv. Mater. Interfaces, 2021, 20, 2002034 CrossRef
.
- J. Liu, M. Jiao, B. Mei, Y. Tong, Y. Li, M. Ruan, P. Song, G. Sun, L. Jiang, Y. Wang, Z. Jiang, L. Gu, Z. Zhou and W. Xu, Angew. Chem., Int. Ed., 2019, 58, 1163–1167 CrossRef CAS PubMed
.
- J. Xu, J. Li, Z. Lian, A. Araujo, Y. Li, B. Wei, Z. Yu, O. Bondarchuk, I. Amorim, V. Tileli, B. Li and L. Liu, ACS Catal., 2021, 11, 3402–3413 CrossRef CAS
.
- J. Zhu, M. Xie, Z. Chen, Z. Lyu, M. Chi, W. Jin and Y. Xia, Adv. Energy Mater., 2020, 10, 1904114 CrossRef CAS
.
- F. Wang, K. Kusada, D. Wu, T. Yamamoto, T. Toriyama, S. Matsumura, Y. Nanba, M. Koyama and H. Kitagawa, Angew. Chem., Int. Ed., 2018, 57, 4505–4509 CrossRef CAS
.
- Y. Pi, J. Guo, Q. Shao and X. Huang, Chem. Mater., 2018, 30, 8571–8578 CrossRef CAS
.
- X. Shi, H. Zhu, J. Du, L. Cao, X. Wang and H.-P. Liang, Electrochim. Acta, 2021, 370, 137710 CrossRef CAS
.
- J. Shan, C. Ye, S. Chen, T. Sun, Y. Jiao, L. Liu, C. Zhu, L. Song, Y. Han, M. Jaroniec, Y. Zhu, Y. Zheng and S. Z. Qiao, J. Am. Chem. Soc., 2021, 143, 5201–5211 CrossRef CAS PubMed
.
- S. M. Alia, S. Shulda, C. Ngo, S. Pylypenko and B. S. Pivovar, ACS Catal., 2018, 8, 2111–2120 CrossRef CAS
.
- P. Li, Z. Jin, Y. Qian, Z. Fang, D. Xiao and G. Yu, ACS Energy Lett., 2019, 4, 1793–1802 CrossRef CAS
.
- L. Liu and A. Corma, Chem. Rev., 2018, 118, 4981–5079 CrossRef CAS
.
- P. Li, Z. Jin, Z. Fang and G. Yu, Angew. Chem., Int. Ed., 2020, 59, 22610–22616 CrossRef CAS PubMed
.
- J. Li, G. Zhan, J. Yang, F. Quan, C. Mao, Y. Liu, B. Wang, F. Lei, L. Li, A. W. M. Chan, L. Xu, Y. Shi, Y. Du, W. Hao, P. K. Wong, J. Wang, S. X. Dou, L. Zhang and J. C. Yu, J. Am. Chem. Soc., 2020, 142, 7036–7046 CrossRef CAS PubMed
.
- J. Zhang, H. Zhang and Y. Huang, Appl. Catal., B, 2021, 297, 120453 CrossRef CAS
.
- S. L. Zhang, X. F. Lu, Z. P. Wu, D. Luan and X. W. D. Lou, Angew. Chem., Int. Ed., 2021, 60, 19068–19073 CrossRef CAS PubMed
.
- Z. Jin and A. J. Bard, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 12651–12656 CrossRef CAS PubMed
.
- J. Zhang, F. Xing, H. Zhang and Y. Huang, Dalton Trans., 2020, 49, 13962–13969 RSC
.
- M. Liu, F. Hof, M. Moro, G. Valenti, F. Paolucci and A. Penicaud, Nanoscale, 2020, 12, 20165–20170 RSC
.
- Y. Huang, S. L. Zhang, X. F. Lu, Z. P. Wu, D. Luan and X. W. D. Lou, Angew. Chem., Int. Ed., 2021, 60, 11841–11846 CrossRef CAS PubMed
.
- P. Li, Z. Jin, Z. Fang and G. Yu, Energy Environ. Sci., 2021, 14, 3522–3531 RSC
.
- Z. Liu, J. Mao, T.-H. Liu, G. Chen and Z. Ren, MRS Bull., 2018, 43, 181–186 CrossRef
.
- L. Fu, G. Cheng and W. Luo, J. Mater. Chem. A, 2017, 5, 24836–24841 RSC
.
- W. H. Lee, J. Yi, H. N. Nong, P. Strasser, K. H. Chae, B. K. Min, Y. J. Hwang and H. S. Oh, Nanoscale, 2020, 12, 14903–14910 RSC
.
- P. Jiang, J. Chen, C. Wang, K. Yang, S. Gong, S. Liu, Z. Lin, M. Li, G. Xia, Y. Yang, J. Su and Q. Chen, Adv. Mater., 2018, 30, 1705324 CrossRef PubMed
.
- L. Fu, F. Yang, G. Cheng and W. Luo, Nanoscale, 2018, 10, 1892–1897 RSC
.
- D. Weber, L. M. Schoop, D. Wurmbrand, S. Laha, F. Podjaski, V. Duppel, K. Müller, U. Starke and B. V. Lotsch, J. Mater. Chem. A, 2018, 6, 21558–21566 RSC
.
- F. Li, G. F. Han, H. J. Noh, J. P. Jeon, I. Ahmad, S. Chen, C. Yang, Y. Bu, Z. Fu, Y. Lu and J. B. Baek, Nat. Commun., 2019, 10, 4060–4067 CrossRef PubMed
.
- J. N. Tiwari, S. Sultan, C. W. Myung, T. Yoon, N. Li, M. Ha, A. M. Harzandi, H. J. Park, D. Y. Kim, S. S. Chandrasekaran, W. G. Lee, V. Vij, H. Kang, T. J. Shin, H. S. Shin, G. Lee, Z. Lee and K. S. Kim, Nat. Energy, 2018, 3, 773–782 CrossRef CAS
.
- S. Liu, Z. Hu, Y. Wu, J. Zhang, Y. Zhang, B. Cui, C. Liu, S. Hu, N. Zhao, X. Han, A. Cao, Y. Chen, Y. Deng and W. Hu, Adv. Mater., 2020, 3, 2006034 CrossRef
.
- D. Wang, Q. Li, C. Han, Q. Lu, Z. Xing and X. Yang, Nat. Commun., 2019, 10, 3899–3911 CrossRef PubMed
.
- F. Godínez-Salomón, L. Albiter, S. M. Alia, B. S. Pivovar, L. E. Camacho-Forero, P. B. Balbuena, R. Mendoza-Cruz, M. J. Arellano-Jimenez and C. P. Rhodes, ACS Catal., 2018, 8, 10498–10520 CrossRef
.
- Y. Peng, Q. Liu, B. Lu, T. He, F. Nichols, X. Hu, T. Huang, G. Huang, L. Guzman, Y. Ping and S. Chen, ACS Catal., 2021, 11, 1179–1188 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra06136d |
| This journal is © The Royal Society of Chemistry 2021 |