Open Access Article
Open Access ArticleTb3+–atorvastatin doped in poly(ethylene glycol) optical biosensor for selective determination of progesterone and testosterone in serum samples
Mohamed S. Attia*a,
Safwat A. Mahmoud b,
Amal M. Ahmeda,
Tarek A. Amina,
Ahmed. O. Youssefa,
Mohammed A. Amin
b,
Amal M. Ahmeda,
Tarek A. Amina,
Ahmed. O. Youssefa,
Mohammed A. Amin c,
Mona N. Abou-Omard and
Ekram H. Mohamed
c,
Mona N. Abou-Omard and
Ekram H. Mohamed *e
*e
aChemistry Department, Faculty of Science, Ain Shams University, Cairo 11566, Egypt. E-mail: Mohd_mostafa@sci.asu.edu.eg; Mohamed_sam@yahoo.com; Tel: +202 1229867311 Tel: +202 1060819022
bPhysics Department, Faculty of Science, Northern Border University, Arar, Saudi Arabia
cDepartment of Chemistry, Collage of Science, Taif University, P. O. Box 11099, Taif 21944, Saudi Arabia
dDepartment of Chemistry, Faculty of Women for Arts, Science and Education, Ain Shams University, Cairo, Egypt
ePharmaceutical Analytical, Chemistry Department, Faculty of Pharmacy, The British University in Egypt, El Sherouk City, Cairo, 11837, Egypt. E-mail: ekram.hany@bue.edu.eg
First published on 12th October 2021
Abstract
An innovative, simple and cost effective Tb3+–atorvastatin photo probe was designed and used as a core for a spectrofluorometric approach to sensitively determine two vital biological compounds in serum samples. Tb3+–atorvastatin complex displays a characteristic electrical band with λem at 545 nm with significant luminescence intensity, which is quenched in the presence of progesterone and testosterone at two variant sets of pH; 6.2 and 7.5, respectively. The conditions were optimized and the best solvent for operation was found to be acetonitrile with λex at 320 nm. Progesterone and testosterone were assessed in serum samples using the same optimal conditions within concentration ranges of 2 × 10−9 to 2.9 × 10−6 and 3.1 × 10−9 to 4.8 × 10−6 mol L−1, respectively. The proposed luminescence method was validated in accordance to ICH guidelines and found to be accurate, precise and specific and free from any interference. The cost effectiveness and applicability of the method make it a good choice for routine analysis of the two compounds and early diagnosis of chronic diseases associated with abnormalities in their physiological levels.
1. Introduction
Progesterone (PGS) is a member of the progestogen steroid hormones group, secreted mainly during the menstrual cycle by the corpus luteum preparing the body for conception in case of ova fertilization.1 PGS is used in oral contraception either in single form or combined with estrogen and as hormonal replacement therapy to alleviate menopause symptoms. Low levels of progesterone may lead to abnormal bleeding during menstruation, premature labor and miscarriage during pregnancy and is considered a sign for poly-cystic ovarian syndrome. Elevated PGS levels may increase the risk of breast cancer development and are a marker for adrenal hyperplasia. PGS concentration was recently estimated using different analytical approaches; spectroscopic,2 chromatographic,3,4 and electrochemical methods5,6 and immunological assay7,8Testosterone (TST), an anabolic steroid, is the primary male sex hormone where it regulates RBCs production, libido, fertility, spermatogenesis, fat distribution, bone and muscle mass.9,10 Imbalance in levels of TST may can cause serious body dysfunctions where diminished levels have an inverse impact on sexual drive, erection, sperm count and muscle strength. Abnormally high TST levels may trigger early puberty in males and menstrual irregularities and baldness in females.11,12 TST could also be used as a medication to replenish its insufficiency, manage breast cancer in women and enhance physique and performance, for instance in athletes.13,14 Its concentration in different matrices such as plasma, serum, and saliva was recently measured through spectroscopic15,16 chromatographic9,11,17 and electrochemical methods18 and capillary electrophoresis.19 Fig. 1 displays the structure of PGS and TST.
The reported methods showed relatively high limits of detection which restricts their practical applications. Moreover, the measurement of low concentrations of, progesterone and testosterone in biological samples along with interference from some biomolecules such as uric acid (UA), ascorbic acid (AA), and different hormones requires to efficiently improve the sensitivity of chromatographic methods and the electrochemical sensors for practical applications. Therefore, developing a simple method for accurate determination of progesterone and testosterone in the presence of each other in the same sample is still of great significance. Today, the research field in which the lanthanide complexes were used as biosensors has a great interest.20–30 Luminescent optical biosensor Tb (atorvastatin)3 (Tb–Ator) complex embedded in PEG matrix have many advantages over the mentioned traditional methods. The chemical structure of atorvastatin is presented in Fig. 2. Terbium ion has sharp and precise emission bands in green light region. The terbium ion is used as photo probe for many analytes with a high selectivity depends on the excitation wavelength of terbium–analyte complex, pH and the type of solvent of the test solution. Doping of the optical sensors in the polymer matrix increases its stability and durability.24–30 The sensor can provide a constant signal response for two years, which makes it 24-fold better balance compared to the lifetime warranted for the chromatographic and electrochemical methods. The source of error of the present work eliminated as it more stables for a long time; it gives a low standard deviation value. The higher stability of the current sensor can be attributed to the doping of the optical sensor in the polymer matrix.
2. Experimental
2.1. Instrumentation
A double beam UV-Visible spectrophotometer (PerkinElmer Lambda 25), fluorescence Spectrometer (Thermo Scientific Lumina, Meslo-PN; 222-263000). pH meter (Jenway; 33300).2.2. Materials and reagents
Progesterone and testosterone, solvents including ethanol, acetonitrile, dimethylformamide (DMF), chloroform and dimethyl sulfoxide (DMSO) were purchased from Sigma Aldrich. Analytical grade ammonium hydroxide (NH4OH), hydrochloric acid (HCl), Tb (NO3)3·5H2O, atorvastatin and polyethylene glycol (PEG) were purchased from Sigma Aldrich.The human real samples were gathered from both Ain Shams Specialized and Teaching New Al-Kasr-El-Aini Hospitals, Cairo, Egypt in accordance with the approved protocol of World Health Organization (WHO) for the collection of human specimens and the use of the clinically related information and data for the purpose of research. The patients approved and were all consented before using their samples.
2.3. Preparation of standard solutions
Stock solutions of Tb (NO3)3·5H2O and atorvastatin; were prepared separately by accurately weighing and transferring 0.11 g and 0.039 g, respectively of their authentic pure forms into separate 25 mL volumetric flasks by the aid of the least amount of ethanol till dissolution and completing the volume with the same solvent to obtain final concentration of (10−2 mol L−1) for each of them.Tb3+–atorvastatin complex solution; was prepared by mixing 0.1 mL of Tb (NO3)3 stock solution with 0.3 mL of atorvastatin stock solution in 10 mL volumetric flask and completing the volume to the mark with acetonitrile.
For the two compounds under study, all stock solutions were separately prepared in 10 mL volumetric flasks in concentration of solution (10−2 mol L−1). This was achieved by dissolving for PGS and TST 0.031 g and 0.0288 g were dissolved, respectively in small amount of ethanol and then volume was diluted to the mark with acetonitrile. Further dilutions for the stock solutions using acetonitrile were performed to obtain working solutions with concentrations of 1.0 × 10−4 to 1.0 × 10−9 mol L−1 of PGS and TST.
0.1 mol L−1 of NH4OH and HCl were used to adjust the pH to 6.2, 7.5 for PGS and TST, respectively. All the prepared solutions should be kept at low temperature (2–8 °C) to remain stable.
2.4. Preparation serum samples spiked with PGS and TST
A 1.0 mL of samples of blood collected from healthy volunteers was centrifuged for 15 min at 4000 rpm to remove proteins. 0.1 mL of the serum sample was added to 1.0 mL of each drug working solution of concentration 1.0 × 10−6 mol L−1 and the volume was complete to 10 mL by acetonitrile to obtain 1.0 × 10−7 mol L−1 for each drug in four separate 10 mL measuring flasks.2.5. Preparation of Tb–Ator biosensor embedded in PEG
Tb–Ator complex was prepared in the solid state by mixing an equal volume of 1.0 × 10−4 mol L−1 Tb ion and 3.0 × 10−4 mol L−1 atorvastatin in ethanol, then evaporation near the dryness of the solution, a pale pink solid was obtained after cooling in air. The thin film was prepared by dissolving 0.1 g of the solidified and seamless complex in 3 mL ethanol and then adding 10 mL of viscose freshly prepared PEG with stirring for about one hour until a homogenous solution was obtained. A thin film was fabricated by spin-coating on a small quartz slide (width 8.5 mm, height 25 mm) to quick fit in the cuvette of the spectrofluorometer.2.6. Recommended procedure
An appropriate volume (100 μL) of various standard concentrations of progesterone and testosterone should be diluted to 3 mL with acetonitrile. The dilute solution was mixed with a thin film of biosensing Tb–Ator doped in PEG matrix in the quartz cell of a spectrofluorometer. The luminescence spectra were recorded at the excitation wavelength λex = 320 nm. After each measurement, the optical sensor was washed with acetonitrile, and the calibration curve was built by applying the Stern's Volmer equation by plotting (F/F0) the at λem = 545 nm on the y-axis versus the progesterone and testosterone concentration in mol L−1 on the x-axis.2.7. Determination of progesterone and testosterone in serum samples
The luminescence spectra of the serum samples previously prepared as described under (2.4) were measured adopting the same procedures followed under (3.2). The concentrations of each real sample were calculated using corresponding regression equation.3. Result and discussion
3.1. General features of absorption and emission spectra of Tb–Ator complex
Owing to the f–f transition forbiddance of trivalent ion (Tb3+), there is a restriction to directly absorb light which could be overcome through the antenna effect via the coupling between Tb3+ and a prominently absorbing organic ligand leading to efficient energy transfer and light absorption processes. Regarding the proposed photo probe, Tb3+ is surrounded covalently by 3 molecules of atorvastatin ligand responsible for efficient absorption of light and transfer of energy to populate 5D4 state of Tb3+.31The emission of the formed complex Tb–Ator exhibited four specific and intense bands because of the 5D4–7FJ transitions (J = 6, 5, 4 and 3).32–40
3.2. Absorption and emission spectra
The absorption spectra of atorvastatin and Tb3+ with different concentrations of atorvastatin are shown in Fig. 3. A red shift by 7 nm and the absorbance value is enhanced denoting that atorvastatin could form a stable complex with Tb3+. The absorption spectra of PGS and TST were scanned in the presence of the optical sensor are shown in Fig. 4. | ||
| Fig. 3 The absorption spectra of (1) atorvastatin (1.0 × 10−4 mol L−1), (2) 2.0 × 10−4 mol L−1 atorvastatin: Tb3+ (1.0 × 10−4), (3) 3.0 × 10−4 mol L−1 atorvastatin: Tb3+ (1.0 × 10−4 mol L−1). | ||
The emission spectra of Tb3+–atorvastatin complex after adding different concentrations of PGS and TST using acetonitrile as solvent are shown in Fig. 5 and 6, respectively. The characteristic electrical emission band of Tb3+ exhibited at λem 545 nm was quenched due to energy transfer from the optical sensor to PGS and TST.
 | ||
| Fig. 5 The emission spectra of Tb3+–Ator complex at λex = 320 nm and pH 6.2 in presence of different progesterone concentrations using acetonitrile as a solvent. | ||
 | ||
| Fig. 6 The emission spectra of Tb3+–Ator complex at λex = 320 nm and pH 7.5 in presence of different testosterone concentrations using acetonitrile as a solvent. | ||
3.3. Experimental variables
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
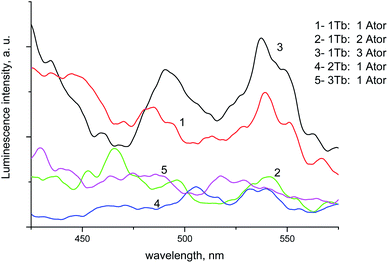
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 L indicating that the metal coordinates to the ligand at different sites of coordination not via oxygen only, Fig. 7.
3 L indicating that the metal coordinates to the ligand at different sites of coordination not via oxygen only, Fig. 7.
The pH effect on the luminescence intensity after the addition of the studied analytes to the proposed photoprobe was studied and the luminescence quenching was observed at pH 6.2 and 7.5 for PGS and TST, respectively.
3.4. Analytical performance49
The Stern–Volmer constant and critical concentration of PGS and TST values are (κsv = 3.1 and 2.2 mol L−1) and (Co = 2.49 × 10−10 and 2.4 × 10−10 mol L−1) respectively. The distance between the cited compounds and the ionophore is 2.16 Å indicating the electron transfer mechanism of quenching.
The regression equations were computed and the regression parameters in addition the LOD and LOQ were calculated, and results were presented in Table 1. [LOD = 3.3S/b and LOQ = 10S/b, (where S is the standard deviation of blank luminescence intensity values, and b is the slope of the calibration plot)].
| Parameter | Progesterone | Testosterone |
|---|---|---|
| a Where Y: intensity of luminescence, X: analyte concentration (mol L−1), a: intercept and b: slope. | ||
| λ em (nm) | 545 | |
| Linearity (mol L−1) | (2 × 10−9 to 2.9 × 10−6) | (3.1 × 10−9 to 4.8 × 10−6) |
| LOD (mol L−1) | 8.5 × 10−10 | 7.02 × 10−10 |
| LOQ (mol L−1) | 2.5 × 10−9 | 2.1 × 10−9 |
| Regression equation | (Y = a + bX)a | |
| Intercept (a) | 47.2 | 105 |
| Slope (b) | 3.1 | 2.2 |
| Standard deviation | 15.5 | 20.5 |
| Variance (S2) | 240.25 | 420.25 |
| Regression coefficient (r) | 0.99 | 0.99 |
| Sample | concentration taken (×10−7 mol L−1) | Repeatability | Intermediate precision | ||||
|---|---|---|---|---|---|---|---|
| Average found ± CLb | % REc | % RSD d | Drug average found ± CL | % RE | % RSD | ||
| a n = 3.b CL: confidence limits (ESI).c % RE: percent relative error (ESI).d RSD: relative standard deviation. | |||||||
| Progesterone in serum | 1.0 | 1.03 ± 0.15 | 3.00 | 1.39 | 1.05 ± 0.21 | 5.00 | 3.13 |
| 2.0 | 1.97 ± 0.14 | 1.50 | 1.33 | 2.04 ± 0.27 | 2.00 | 2.12 | |
| 4.0 | 4.07 ± 0.21 | 1.75 | 1.99 | 4.09 ± 0.13 | 2.25 | 2.11 | |
| Testosterone in serum | 1.0 | 1.04 ± 0.11 | 4.00 | 1.46 | 0.95 ± 0.21 | 5.00 | 3.11 |
| 2.0 | 2.04 ± 0.11 | 2.00 | 1.41 | 2.05 ± 0.26 | 2.50 | 2.06 | |
| 4.0 | 3.94 ± 0.16 | 1.50 | 1.95 | 4.08 ± 0.11 | 2.00 | 1.02 | |
The validity and selectivity were further assessed in presence of some proteins and hormones that may interfere as cortisol, thyroid stimulating hormone, norepinephrine, dopamine, and albumin within concentration range of 0.08 g L−1. The interference of 0.0.06 g L−1 urea, 0.08 g L−1 glucose, uric acid and folic acid was also studied, and the resulting data revealed that there was no significant effect on the observed luminescence activity of the proposed photo probe under optimized conditions.
In addition, the proposed optical probe was successfully applied for selective determination of PGS and TST either as single or in combination in synthetically prepared mixtures. Two synthetic mixtures were prepared by adding different concentrations of PGS and TST within their linearity range in 2 similar sets of 10 mL volumetric flasks containing 0.1 mL of the serum sample as mentioned under 2.4.
The pH of the first set was adjusted to 6.2 for selective determination of PGS in presence of TST and the pH of the second set was adjusted to 7.5 for the determination of TST in presence of PGS and the volume was completed with acetonitrile for the two sets. Thus, each mixture was prepared 2 times but at different pH (6.2 and 7.5) for selective estimation of PGS and TST, respectively. Each solution was in triplicates and yielded recovery % of 100.3 ± 1.10 and 99.6 ± 1.60 for PGS and TST, respectively.
Therefore, the luminescence of Tb3+–Ator complex with its second coordination sphere in which the dual mixture of PGS and TST is quite sensitive to two variant sets of pHs. For Tb3+–Ator–PGS was of λex = 320 and pH 6.2 and that for Tb3+–Ator–TST was of λex = 320 and pH 7.5. Thus, a dual-controlled luminescence of smoothly dynamic reversibility is achieved and a reversible on/off switchable Tb3+ emission of one system was observed by tuning its optimal values of pH to the optimal ones and the second. By this dual controlled luminescence, the dual mixture of PGS and TST was simultaneously resolved with average error < 1.8% in serum sample.
Also, the data obtained upon assaying single PGS and TST separately in serum sample, urine, and dosage form, without any interference from inactive excipients, were processed and results were tabulated as shown in Table 3 and Fig. 12. The results of the proposed method were comparable to that obtained from the reference chromatographic methods mentioned in the British pharmacopeia.50
| Sample | Added (×10−7 mol L−1) | Found (×10−7 mol L−1) | Averagea (×10−7 mol L−1) | Average recovery ± % R.S.D | B.P. (LC) |
|---|---|---|---|---|---|
| a Average of three measurements. | |||||
| Progesterone serum sample | 3.5 | 3.42, 3.58, 3.60 | 3.53 | 100.3 ± 1.1 | 98.6 ± 0.5 |
| 7.0 | 7.08, 6.95, 7.03 | 7.02 | |||
| 9.5 | 9.45, 9.56, 9.43 | 9.48 | |||
| Testosterone serum sample | 3.5 | 3.59, 3.43, 3.55 | 3.52 | 99.7 ± 1.5 | 99.2 ± 0.6 |
| 7.0 | 7.05, 6.89, 6.95 | 6.96 | |||
| 9.5 | 9.61, 9.35, 9.51 | 9.49 | |||
| Analyte | Methods | Linearity | Limit of detection | References |
|---|---|---|---|---|
| Progesterone | HPLC-MS-MS | 0.2–50 ng mL−1 | 0.2 ng mL−1 | 19 |
| Microfluidic immunosensor system | 0.5–12.5 ng mL−1 | 0.2 ng mL−1 | 22 | |
| Enzyme-linked fluorescence assay | 3–40.0 ng mL−1 | — | 23 | |
| Spectrofluorometric using Tb3+–Ator | (2.0 × 10−9 to 2.9 × 10−6) mol L−1 | 8.5 × 10−10 mol L−1 | ||
| Testosterone | HPLC in plasma | 1.6–400 ng mL−1 | 1.6 ng mL−1 | 32 |
| HPLC in serum | 1–20 ng mL−1 | 0.4 | 33 | |
| HPLC in urine | 10–500 ng mL−1 | 1 ng mL−1 | 33 | |
| HPLC in dosage form | 50–200 μg mL−1 | 5 μg mL−1 | 31 | |
| HPLC in urine | 2–300 ng mL−1 | 2 ng mL−1 | 30 | |
| Spectrofluorometric using Tb3+–Ator | (3.1 × 10−9 to 4.8 × 10−6) mol L−1 | 7.02 × 10−10 mol L−1 |
4. Conclusion
The proposed analytical method based on the use of Tb3+–atorvastatin complex is simple and economic and can be successfully applied for sensitive and accurate determination of progesterone and testosterone in different matrices including dosage forms, urine, and serum. The analysis of the PGS and TST in biological samples can contribute to early diagnosis of some chronic diseases associated with their abnormal levels.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Authors thank Taif University Researchers Supporting Project (TURSP-2020/03), Taif University, Taif, Saudi Arabia, for supporting this work.References
- A. L. Attar, M. P. Redekar and N. R. Jadhav, Curr. Pharm. Res., 2016, 6, 1842–1847 CrossRef.
- D. Maliwal, P. Jain, A. Jain and V. Patidar, J. Young Pharma., 2009, 1, 371–374 CrossRef CAS.
- W. Zhuping, Z. Chao, Y. Chengdui, Z. Xinrong and W. Erruo, Analyst, 2000, 125, 2201–2205 RSC.
- P. Naderi and F. Jalali, J. Electrochem. Soc., 2020, 167, 027524, DOI:10.1149/1945-7111/ab6a7f.
- C. Esmaeili, M. S. Karimi, P. Norouzi, F. Wu, M. R. Ganjali and E. Safitri, J. Electrochem. Soc., 2020, 167, 067513, DOI:10.1149/1945-7111/ab828e.
- A. F. Javier, M. G. Alejandro, M. P. Gabriela, Z. M. Alicia, R. Julio and F. Héctor, Talanta, 2010, 80, 1986–1992 CrossRef PubMed.
- N. Brugger, C. Otzdorff, B. Walter, B. Hoffmann and J. Braun, Reprod. Domestic Animals, 2011, 46, 870–873 CrossRef CAS PubMed.
- C. Yu, Y. Mehrdad, R. H. Barry, P. D. Eleftherios and W. Pui-Yuen, Clin. Biochem., 2009, 42, 484–490 CrossRef PubMed.
- J. Bain, Clin. Interv. Aging, 2007, 2, 567–576 CrossRef CAS PubMed.
- D. French, Clin. Chim. Acta, 2013, 415, 109–117 CrossRef CAS PubMed.
- R. S. Tan and S. J. Pu, The Aging Male, 2003, 6, 13–17 CrossRef CAS PubMed.
- B. A. Andre, M. D. Julia, A. S. Elizabeth, M. M. Hassan, T. G. Lin and A. W. Gary, J. Clin. Endocrin. Metab., 2011, 96, 3007–3019 CrossRef PubMed.
- S. P. Tuck and R. M. Francis, Front. Hormone Res., 2009, 37, 123–132 CAS.
- N. Bassil, S. Alkaade and J. E. Morley, Therap. Clin. Risk Manag., 2009, 5, 427–448 CAS.
- A. J. Kadhem, S. Xiang, S. Nagel, C.-H. Lin and M. Fidalgo de Cortalezzi, Polymers, 2018, 10, 349–352 CrossRef PubMed.
- M. M. Abd-Elzaher, M. A. Ahmed, A. B. Farag, M. S. Attia, A. O. Youssef and S. M. Sheta, Sensor Lett, 2017, 15, 977–981 CrossRef.
- L. Konieczna, A. Plenis, I. Olędzka, P. Kowalski and T. Bączek, Talanta, 2011, 83, 804–814 CrossRef CAS PubMed.
- C. He, S. Li, H. Liu, K. Li and F. Liu, J. Chromatogr., A, 2005, 1082, 143–149 CrossRef CAS PubMed.
- R. K. Gupta, R. Roy, A. Chaudhary and A. N. Jha, Biosci. Biotech. Res. Asia., 2010, 7, 505–511 CAS.
- K. H. Yuen and B. H. Ng, J. Chromatogr.B., 2003, 793, 421–426 CrossRef.
- V. Loi, M. Vertzoni, A. Vryonidou and C. Phenekos, J.Pharma. Biomed. Anal., 2006, 41, 527–532 CrossRef CAS PubMed.
- J. Albrethsen, M. L. Ljubicic and A. Juul, J. Clin. Endocrin. Metabol., 2020, 105(10), dgaa496 Search PubMed.
- S. Alvi and M. Hammami, J. Advanced Pharma. Techn. Res., 2020, 11, 64, DOI:10.4103/japtr.japtr_162_19.
- W. C. Chang, D. A. Cowan, C. J. Walker, N. Wojek and A. D. Brailsford, J. Chroma. A, 2020, 1628, 461445, DOI:10.1016/j.chroma.2020.461445.
- X. Li, T. Yuan, T. Zhao, X. Wu and Y. Yang, J. Chroma. Sci., 2020, 58(9), 880–886, DOI:10.1093/chromsci/bmaa051.
- W. Xu, H. Li, Q. Guan, Y. Shen and L. Cheng, JCLA J. Clinic. Lab. Anal., 2017, 31, e22102, DOI:10.1002/jcla.22102.
- R. Heidarimoghadam, O. Akhavan, E. Ghaderibi, E. Hashemi, S. S. Mortazavi and A. Farmany, Materials Sci. Eng: C., 2016, 61, 246–250 CrossRef CAS PubMed.
- K. H. Liu, D. O'Hare, J. L. Thomas, H. Z. Guo, C. H. Yang and M. H. Lee, Biosensors, 2020, 10, 16, DOI:10.3390/bios10030016.
- B. Du, J. Zhang, Y. Dong, J. Wang, L. Lei and R. Shi, Steroids, 2020, 161, 108691, DOI:10.1016/j.steroids.2020.108691.
- U. Turpeinen, S. Linko, O. Itkonen, E. Hämäläinen and E. Scandinavian, J. Clin. Lab. Invest., 2008, 68, 50–57 CrossRef CAS PubMed.
- M. S. Attia, W. H. Mahmoud, A. O. Youssef and M. S. Mostafa, Cilostazol Determination by the Enhancement of the Green Emission of Tb3+ Optical Sensor, J. Fluoresc., 2011, 21, 2229–2235, DOI:10.1007/s10895-011-0927-y.
- M. S. Attia, M. H. Khalil, M. S. A. Abdel-Mottaleb, M. B. Lukyanova, Yu. A. Alekseenko and B. Lukyanov, Effect of Complexation with Lanthanide Metal Ions on the Photochromism of (1, 3, 3-Trimethyl-5_-Hydroxy-6_-Formyl- Indoline-Spiro2, 2-[2H] chromene) in Different Media, Int. J. Photoenergy, 2006, 1–9, DOI:10.1155/ijp/2006/42846.
- M. S. Attia, A. A. Essawy, A. O. Youssef and M. S. Mostafa, Determination of Ofloxacin using a Highly Selective Photo Probe Based on the Enhancement of the Luminescence Intensity of Eu3+ - Ofloxacin Complex in Pharmaceutical and Serum Samples, J. Fluoresc., 2012, 2, 557–564, DOI:10.1007/s10895-011-0989-x.
- M. S. Attia, A. M. Othman, A. O. Youssef and E. El-Raghi, Excited state interaction between Hydrochlorothiazide and Europium ion in PMMA polyme, and its application as optical sensor for Hydrochlorothiazide in tablet and serum samples, J. Lumines., 2012, 132, 2049–2053, DOI:10.1016/j.jlumin.2012.03.012.
- M. S. Attia, A. O. Youssef and A. A. Essawy, A novel method for tyrosine assessment in vitro by using fluorescence enhancement of the ion-pair tyrosine- neutral red dye photo probe, Anal. Methods, 2012, 4, 2323–2328, 10.1039/c2ay25089f.
- M. S. Attia, A. O. Youssef and R. H. El-Sherif, Durable diagnosis of seminal vesicle and sexual gland diseases using the nano optical sensor thin film sm-doxycycline complex, Anal. Chim. Acta, 2014, 835, 56–64, DOI:10.1016/j.aca.2014.05.016.
- M. S. Attia, M. Diab and M. F. El-Shahat, Diagnosis of some diseases related to the histidine level in human serum by using the nano optical sensor Eu–Norfloxacine complex, Sensors and Actuators B, 2015, 207, 756–763, DOI:10.1016/j.snb.2014.10.132.
- M. S. Attia, A. O. Youssef, Z. A. Khan and M. N. Abou-Omar, Alpha fetoprotein assessment by using a nano optical sensor thin film binuclear Pt-2-aminobenzimidazole-Bipyridine for early diagnosis of liver cancer, Talanta, 2018, 186, 36–43, DOI:10.1016/j.talanta.2018.04.043.
- M. S. Attia and N. S. Al-Radadi, Progress of pancreatitis disease biomarker alpha amylase enzyme by new nano optical sensor, Biosens. Bioelect., 2016, 86, 413–419, DOI:10.1016/j.bios.2016.06.079.
- M. S. Attia and N. S. Al-Radadi, Nano optical sensor binuclear Pt-2-pyrazinecarboxylic acid-bipyridine for enhancement of the efficiency of 3-nitrotyrosine biomarker for early diagnosis of liver cirrhosis with minimal hepatic encephalopathy, Biosens. Bioelect., 2016, 86, 406–412, DOI:10.1016/j.bios.2016.06.074.
- M. S. Attia, Nano optical probe samarium tetracycline complex for early diagnosis of histidinemia in newborn children, Biosens. Bioelect., 2017, 94, 81–86, DOI:10.1016/j.bios.2017.02.018.
- M. S. Attia, K. Ali, M. El-Kemary and W. M. Darwish, Phthalocyanine-doped polystyrene fluorescent nanocomposite as a highly selective biosensor for quantitative determination of cancer antigen 125, Talanta, 2019, 201, 185–193, DOI:10.1016/j.talanta.2019.03.119.
- M. S. Attia, M. N. Ramsis, L. H. Khalil and S. G. Hashem, Spectrofluorimetric assessment of chlorzoxazone and Ibuprofen in pharmaceutical formulations by using Eu-tetracycline HCl optical sensor doped in sol–gel matrix, J. Fluoresc., 2012, 22, 779–788, DOI:10.1007/s10895-011-1013-1.
- A. A. Elabd and M. S. Attia, A new thin film optical sensor for assessment of UO22+ based on the fluorescence quenching of Trimetazidine doped in sol gel matrix, J. Lumines., 2015, 165, 179–184, DOI:10.1016/j.jlumin.2015.04.024.
- A. A. Elabd and M. S. Attia, Spectroflourimetric assessment of UO22+ by the quenching of the fluorescence intensity of Clopidogrel embedded in PMMA matrix, J. Lumines., 2016, 169, 313–318, DOI:10.1016/j.jlumin.2015.04.024.
- A. A. Essawy and M. S. Attia, Novel application of pyronin Y fluorophore as high sensitive optical sensor of glucose in human serum, Talanta, 2013, 107, 18–24, DOI:10.1016/j.talanta.2012.12.033.
- E. Hamed, M. S. Attia and K. Bassiony, Synthesis, spectroscopic and thermal characterization of Copper (II) complexes of folic acid and their absorption efficiency in the blood, J. Bioinorg. chem. appl., 2009, 1–7, DOI:10.1155/2009/979680.
- M. S. Attia, K. Ali, M. El- Kemary and W. M. Darwish, Phthalocyanine-doped polystyrene fluorescent nanocomposite as a highly selective biosensor for quantitative determination of cancer antigen 125, Talanta, 2019, 201, 185–193 CrossRef CAS PubMed.
- I. H. T. Guideline, Validation of analytical procedures: text and methodology. Q2 (R1), 1, 1–15, 2005.
- Stationery Office (Great Britain), British pharmacopoeia 2009, Stationery Office, London, 2008 Search PubMed.
| This journal is © The Royal Society of Chemistry 2021 |