Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A novel BMSN (biologically synthesized mesoporous silica nanoparticles) material: synthesis using a bacteria-mediated biosurfactant and characterization
Raju Kumar Sharmaab,
Shau-Chun Wanga,
Jyoti Prakash Maity*bc,
Pritam Banerjeebd,
Gobinda Deybd,
Yi-Hsun Huangb,
Jochen Bundschuh be,
Ping-Gune Hsiao*f,
Tsung-Hsien Chenf and
Chien-Yen Chen
be,
Ping-Gune Hsiao*f,
Tsung-Hsien Chenf and
Chien-Yen Chen *b
*b
aDepartment of Chemistry and Biochemistry, National Chung Cheng University, 168 University Road, Min-Hsiung, Chiayi County 62102, Taiwan
bDepartment of Earth and Environmental Sciences, National Chung Cheng University, 168 University Road, Min-Hsiung, Chiayi County 62102, Taiwan. E-mail: chien-yen.chen@oriel.oxon.org
cDepartment of Chemistry, School of Applied Sciences, KIIT Deemed to be University, Bhubaneswar, Odisha 751024, India. E-mail: jyotiprakash.maityfch@kiit.ac.in; jyoti_maity@yahoo.com
dDepartment of Biomedical Science, Graduate Institute of Molecular Biology, National Chung Cheng University, 168 University Road, Min-Hsiung, Chiayi County 62102, Taiwan
eUNESCO Chair on Groundwater Arsenic within the 2030 Agenda for Sustainable Development, University of Southern Queensland (USQ), West Street, Toowoomba, QLD 4350, Australia
fDepartment of Internal Medicine, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chiayi City, Taiwan
First published on 6th October 2021
Abstract
Mesoporous materials (MMs) have recently been applied as advanced nanomaterials in different fields (separation, catalysis, adsorption etc.). Synthesis of MMs by chemical surfactants is not ecofriendly. This study focused on the biological synthesis of a MM by sol–gel method, using a Bacillus subtilis BBK006-mediated surfactant (template) and a precursor (TEOS). The biologically synthesized mesoporous silica nanoparticles (BMSN) were formed at calcination temperatures of 450–600 °C. The BMSN comprise Si and O elements with specific weights of 56.09% and 42.13% respectively, where the atomic% was detected to be 41.79% and 55.10%, respectively. The phase identity of the synthesized particles (61–300 nm uniform spherical shape; surface area: 8.2616 m2 g−1; pore diameter at 550 °C: 14.8516 nm) was confirmed with wide-angle XRD (10°–81°). A typical type IV isotherm was exhibited (BET curves) following IUPAC nomenclature and confirmed the mesoporous nature. The green-synthesized biosurfactant-mediated BMSN is an environmentally promising material to apply in biomedical science (e.g., antimicrobial activity, drug delivery, CMC, anticancer activity) and oil spill management.
1. Introduction
MMs have been receiving wide interest in the field of material science owing to their potential utilization as adsorbents, controlled drug delivery systems, sensors, catalysts in chemical reactions, cosmetics etc.1–3 Primarily, the long-chain carbon surfactants and templating agents are responsible for the pore size variation of MMs.4 Chemically and morphologically, the MMs have revealed a potential particle with highly coordinated spherical shape (2–50 nm pore diameter).5 Technologically, MMs are synthesized through numerous methods (template-assisted, sol–gel, microwave-assisted, and chemical etching),6 which are generally time-consuming and require high temperature and pressure.7In particular, the sol–gel method is popular as it is easy, less time-consuming (compared to other methods), has a low operational temperature, and is a more expedient process for synthesizing MMs.8 Numerous chemical surfactants (e.g., CTAB, P123, F127, CTAC) play an important role in the synthesis of MMs with high specific surface area and desired shape comprising well defined structural morphology.7,9,10 C19H42N+OH−/Cl− (a chemical surfactant) was used for the first time to synthesize the mesoporous spherical material MCM-41.11 With the progress of science and technology, cetyltrimethylammonium bromide (CTAB) surfactant has been applied to synthesize the mesoporous material MCM-41 due to its very low/no toxicity12 and efficiency at very low concentrations (≤1 μM);12 however, chemical surfactants (e.g. CTAB) still exhibit toxicity to living organisms at higher concentrations (>1 μM), such as cytotoxicity (human epidermal keratinocytes in HaCaT cells, lung fibroblasts in CRL-1490 cells) and chronic and subacute toxicity (male/female rats).12–14
In recent years, biosurfactants have received increasing interest in different research arenas (heavy metals removal, pharmaceuticals, food, detergents, petroleum, cosmetics, agriculture, etc.) due to their low toxicity,15–18 ecofriendly nature, low cost and easy synthesis. Microbiologically, biosurfactants are produced from microorganisms with different principal carbon sources (carbohydrates, industrial/domestic wastewater, starch-containing substrates, vegetable oils, renewable resources, etc.).19,20 Currently, a significant percentage of biosurfactant production takes place in Europe [Germany (Biotensidon, Evonik), France, Italy, Belgium (Ecover), UK, etc.], as well as India, China, Japan, Thailand, Brazil, and the USA (Jeneil Biotech) in the global market,21 where biosurfactants are mainly utilized in personal care, agriculture, the food industry, detergents, etc. The production of biosurfactants is now easy and effective due to advances with analytical instruments in industry that allow biosurfactants to be conveniently extracted from various microbes.15,16,22,23 Apart from these advantages, biosurfactants are biodegradable, environmentally friendly, economical, and renewable substrates.24 Moreover, the physical characteristics of biosurfactants are mainly dependent on the temperature and pH.24 Thus, in nanobiotechnology, natural substrates are used as precursors for synthesizing microbial-based nanoparticles to minimize the hindrance (e.g. reduce the toxicity and increase the ecofriendliness).
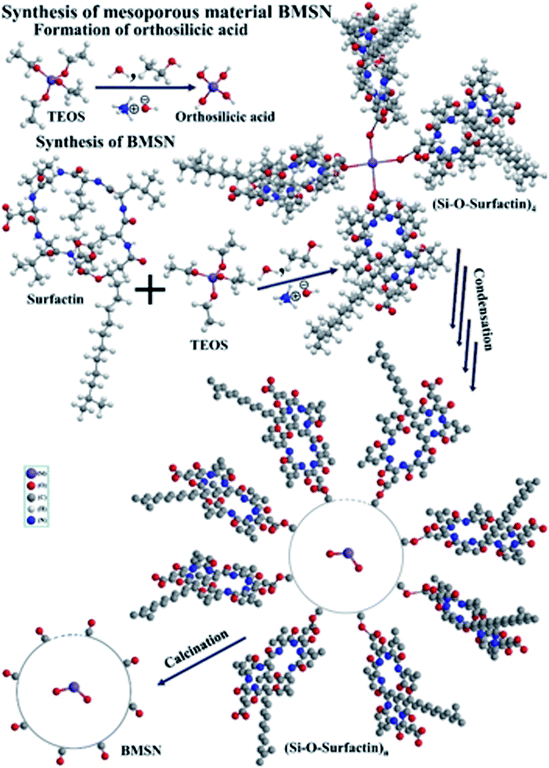
In the present study, a Bacillus subtilis BBK006-derived biosurfactant was used as the biological template to synthesize a novel material (BMSN), which is a key objective for improving the economic and environmental aspects of nanobiotechnology. A sol–gel process (bottom-up approach) with different calcining temperatures was used to synthesize the BMSN. The physicochemical characteristics of the BMSN were investigated in terms of crystal structure, chemical bonding, surface adsorption, absorptivity, morphology, pore shape and size, etc.
2. Materials and methods
2.1 Chemicals and reagents
The biosurfactant was extracted from Bacillus subtilis BBK006 (a non-pathogenic bacterium, collected from a fuel-contaminated site).15,16,22,25,26 The chemical reagents Na2HPO4 (98% purity), KH2PO4 (99.5% assay), NaCl (99.9% assay), NH4Cl (99.5% purity), MgSO4·7H2O (99.8% assay), CaCl2 (76.07% assay) and glucose (99.9% purity) were used to prepare the M9 medium for bacterial growth.15,16,22,25,26 The material was synthesized using 25 wt% of aqueous ammonia, ethyl alcohol (99.5% assay), tetraethyl orthosilicate (TEOS) (99.0% purity; Fluka) and methanol. All chemical reagents were used without further purification.2.2 Preparation of media and bacterial culture
The Bacillus subtilis BBK006 was grown in M9 broth media with the following composition: 6.78 g L−1 of Na2HPO4, 3.0 g L−1 of KH2PO4, 0.5 g L−1 of NaCl, 1.0 g L−1 of NH4Cl, 0.493 g L−1 of MgSO4·7H2O, and 0.011 g L−1 of CaCl2.15,16,22,25,26 All of these chemicals were entirely dissolved in the double deionized water separately to avoid the formation of precipitation on the surface of the transparent glass reagent bottle during the sterilization process. The dissolved components were transferred to a 1 L transparent glass reagent bottle and made up to the mark to maintain the balanced concentration. The prepared broth media was wrapped with aluminium foil for sterilization (autoclave at 121 °C for 20 min at 15 atm). The sterilized media was cooled down and placed in laminar flow hood for inoculation with Bacillus subtilis BBK006. 0.4% glucose was added to the sterilized broth media in laminar flow hood (to avoid external bacterial contamination). A small amount (2 μL at ∼1.2 OD) of bacteria was inoculated for growth.The flask containing the inoculated bacterial media was placed in an orbital shaking incubator (JSL-530) at 37 °C (200 rpm) and incubated for 24 h to increase the bacterial count per volume. The highest growth of bacteria was estimated as ∼1.2 OD (optical density in CFU mL−1 at 600 nm wavelength) by UV-vis-spectrophotometer (UV-vis, PRO-739, Prema), and was used for biosurfactant production. The scan rate of the ultraviolet-visible spectrophotometer is 2500 nm min−1 with accuracy of ±1.2 nm over the wavelength range of 320–1100 nm using a halogen bulb as the light source.
2.3 Production of biosurfactants
Bacterial population growth plays an important role in producing biosurfactants since the production rate is proportional to the amount/number of bacteria. The biosurfactant was produced following the procedure of Maity et al.16 Highly populated bacterial media (∼1.2 OD600) was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (12
000 rpm (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g) for 15 min, at 25 °C (Himac CR-22G, R20A2 Rotor, Hitachi, Japan) to collect the supernatant (without bacterial cells). The collected supernatant was adjusted to pH < 2 using 5 M hydrochloric acid (HCl) to precipitate the biosurfactant properly by incubating overnight (12 h). After precipitation, the biosurfactant was collected by centrifugation at 5000 rpm for 12 min at room temperature and the precipitated biosurfactant was then dried to a powder using a precision oven (JA-72) set to 65 °C for 24 h. Finally, the biosurfactant was obtained as a solid light brown powder, which was used to synthesize the MM.
000 × g) for 15 min, at 25 °C (Himac CR-22G, R20A2 Rotor, Hitachi, Japan) to collect the supernatant (without bacterial cells). The collected supernatant was adjusted to pH < 2 using 5 M hydrochloric acid (HCl) to precipitate the biosurfactant properly by incubating overnight (12 h). After precipitation, the biosurfactant was collected by centrifugation at 5000 rpm for 12 min at room temperature and the precipitated biosurfactant was then dried to a powder using a precision oven (JA-72) set to 65 °C for 24 h. Finally, the biosurfactant was obtained as a solid light brown powder, which was used to synthesize the MM.
2.4 Synthesis of mesoporous material
The MM was synthesized following a novel procedure. Initially, 0.57 g of biosurfactant was dissolved in 50 mL of double deionized water, and stirred continuously until a clear solution was obtained. 11 mL of 25 wt% ammonia and 76 mL of ethanol were added to the clear solution simultaneously. At the same time, 10 mL of TEOS was also added dropwise to the mixture whilst stirring the solution. The mixed solution turned into a milky gel form due to the hydrolysis of TEOS. Gradually, the mixed solution was uninterruptedly stirred for ∼1 h 45 min to complete the hydrolysis of TEOS. A white precipitate was observed in the mixture and was separated by centrifugation at 5000 rpm for 10 min, (room temperature, 25 °C). The precipitate was washed with double deionized water and methanol thrice, and then dried at 70 °C for 24 h. The dried material was finally calcined at different temperatures (450 °C, 500 °C, 550 °C, and 600 °C) in a muffle furnace to produce the MMs.2.5 Characterizations of synthesized particles
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the range of 4000–400 cm−1.
1) in the range of 4000–400 cm−1.3. Results and discussion
3.1 Properties of synthesized materials
In the non-calcinated synthesized material, the spectral bands at 2968, 2924 and 2855 cm−1 were assigned as –CH2 group stretching vibrations, which are comparable with the findings of Huo et al.33 The multiple bands at 2361 and 2343 cm−1 appeared due to N–H group stretching vibration. Strong, medium and numerous very weak intensity bands in the range of 1635–1558 cm−1 were assigned to N–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NO2 and O–H groups, which indicates the appearance of the biosurfactant in the synthesized material. Weak and medium intense peaks were also observed in the BMSN at 1402 and 1456 cm−1 for O–H and ring groups. A less intense band at 870 cm−1 represents the C–H and C–O–C functional groups with different modes of vibration (Table 1).
O, NO2 and O–H groups, which indicates the appearance of the biosurfactant in the synthesized material. Weak and medium intense peaks were also observed in the BMSN at 1402 and 1456 cm−1 for O–H and ring groups. A less intense band at 870 cm−1 represents the C–H and C–O–C functional groups with different modes of vibration (Table 1).
| Wavenumber (cm−1) before calcination | Wavenumber (cm−1) after calcination | Bond | Intensity | Mode | Notes or comments |
|---|---|---|---|---|---|
| 3700–3200 | O–H | M | STR | Broad peak | |
| 3450–3442 | O–H | S | STR | — | |
| 3500–3300 | N–H | W | STR | Secondary amine | |
| 3500–3422 | N–H | M | ASY_STR | — | |
| 3500–3300 | N–H | M | STR | — | |
| 2800–2000 | N–H | M | STR | Often multiple bands | |
| 1680–1630 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
S | STR | Amide I | |
| 1650–1550 | N–H | VW | DEF | — | |
| 1650–1590 | H–N–H | MS | DEF | — | |
| 1660–1635 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
VS | STR | — | |
| 1650–1600 | NO2 | S | ASY_STR | — | |
| 1640–1580 | NH3 | M | ASY_DEF | — | |
| 1640–1632 | OH | M | DEF | — | |
| 1545–1395 | OH | MS | DEF | Series of sharp peaks, R branch | |
| 1465–1430 | Ring | M | STR | — | |
| 1440–1395 | O–H | W | DEF | — | |
| 1435–1425 | Si–Ph | M | STR | — | |
| 1410–1310 | OH | S | DEF | — | |
| 1340–1250 | NH3 | M | SYM_DEF | — | |
| 1320–1211 | C–O (C–C–COOH) | S | STR | — | |
| 1315–1250 | C–N | MS | STR | — | |
| 1310–1250 | C–O | S | STR | — | |
| 1310–1210 | C–O–C | VS | ASY_STR | — | |
| 1305–1200 | CNH (–CO–NH–C) | MW | Combination of C–N STR and N–H BEND | Combination amide III | |
| 1300–1250 | NO2 | S | SYM_STR | — | |
| 1290–1250 | C–H | W | BEND | In-plane H bend | |
| 1285–1245 | CN | M | STR | — | |
| 1280–1240 | O–C–O | M | STR | — | |
| 1280–1250 | CH3 {Si–CH3, Si–(CH3)2, Si–(CH3)3} | S | DEF | Sharp peak | |
| 1280–1230 | C–O–C | M | SYM_STR | Mono-, di-, tri-substituted epoxy ring | |
| 1275–1250 | C–N (NH–CO–NH) | M | STR | — | |
| 1265–1200 | C–N & C–O (R–NHCOO–R) | M | STR | Amide IV | |
| 1240–1170 | C–N | WM | STR | — | |
| 1210–1100 | C–O | S | STR | Tertiary alcohol | |
| 1175–1038 | C–O–C | S | STR | Absence of strong band is good | |
| 1150–1060 | C–O–C | VS | ASY_STR | — | |
| 1140–1080 | C–N | WM | STR | — | |
| 1130–1090 | Si–Ph | S | STR | — | |
| 1125–1090 | C–O | S | STR | Secondary aliphatic alcohol | |
| 1100–1000 | Si–O–Si | VS | STR | Open chain | |
| 1110–1090 | C–O–C | S | ASY_STR | 6 ring ether | |
| 1105–1085 | C–H | W | BEND | In-plane H bend | |
| 1100–1000 | Si–O–C | S | STR | Open chain | |
| 990–945 | Si–O–C | S | STR | — | |
| 970–920 | Si–O | S | STR | — | |
| 965–950 | Ring | W | DEF | Out-of-plane ring bend | |
| 960–900 | C–H | M | DEF | 1 adjacent H out-of-plane DEF | |
| C–H | W | DEF | 4 adjacent H out-of-plane DEF | ||
| 950–815 | C–O–C | S | DEF | — | |
| C–O–C | S | ASY_STR | — | ||
| 880–830 | C–H | M | DEF | 3 adjacent H out-of-plane DEF | |
| 880–805 | C–O–C | S | DEF | — | |
| 870–860 | C–H | S | DEF | 1 adjacent H out-of-plane DEF | |
| 860–780 | C–H | S | DEF | 2 adjacent H out-of-plane DEF | |
| 850–775 | C–O–C | S | DEF | — | |
| 820–765 | C–H | M | DEF | 3 adjacent H out-of-plane DEF | |
| 840–790 | C–H (CR′R′′![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHR) CHR) |
S | DEF | CH out-of-plane DEF | |
| 845–800 | Si–H | S | WAG | — | |
| 814–800 | Si–C | V | STR | — | |
| 814–751 | N–O (R–O–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
S | STR | — | |
| 565–465 | O–H | W | DEF | Series of sharp peaks | |
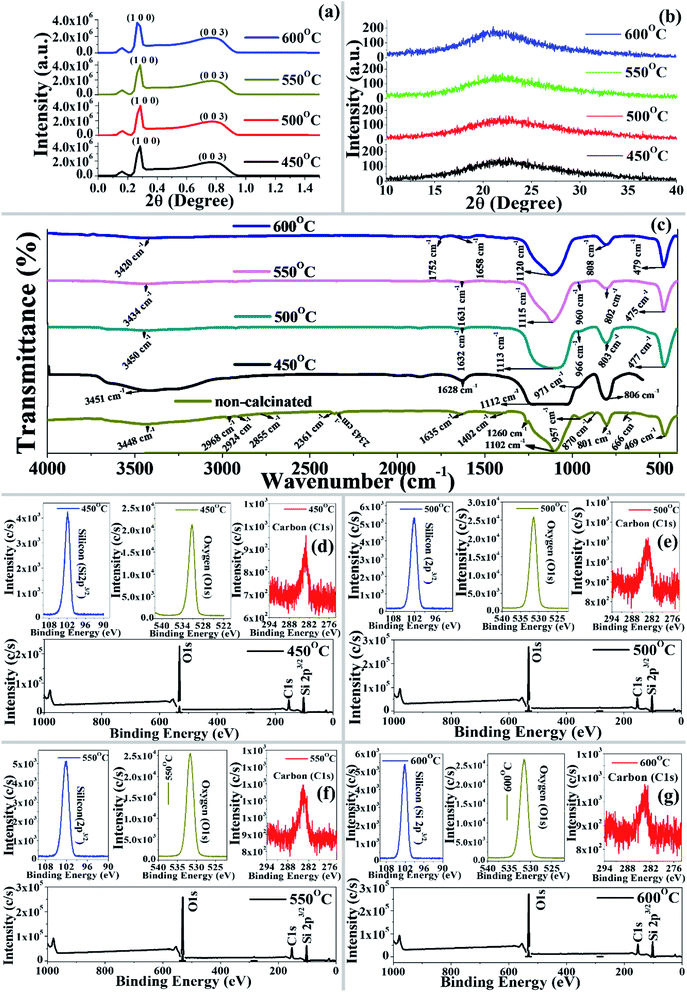
A spectral band at ∼1635 cm−1 was observed in the non-calcinated and calcinated materials due to bending vibrations of the deformational hydroxyl group (–OH), which is comparable with the findings of Salam et al.34 Spectral bands at 1102, 1105, 1113, 1115, and 1120 cm−1 were observed, representing the existence of asymmetrical vibration of a siloxane group (Si–O–Si) in the materials (non-calcinated and calcinated), which is comparable with previous literature.35 Moreover, characteristic peaks at 957, 968, 966, 960 cm−1 representing the bending vibrations of a –Si–OH functional group were observed for both the non-calcinated and calcinated materials (except 600 °C), which is similar to previous results.36 Interestingly, the spectral band of the Si–OH functional group disappeared at 600 °C calcination temperature due to the loss of hydrogen. A peak at 808 cm−1 was observed for the symmetrical stretching vibration of Si–O–Si in the non-calcinated material, whereas the peak shifted to 801, 808, 803, 802, and 808 cm−1 for the materials calcinated at 450 °C, 500 °C, 550 °C, and 600 °C, respectively. Furthermore, in the present study, the peaks at 450 cm−1 to 810 cm−1 were found to be free silica as the Si–O–Si group of synthesized particles due to bending vibrations, which is comparable with the previous results of Salam et al.34 Thus, in the calcinated synthesized material (450–600 °C), it was clearly indicated that the functional groups –H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NO2 and ring had vanished due to the eradication of the biosurfactants from synthesized material, nevertheless, Si–OH still remained and the synthesized material was designated as BMSN (biologically synthesized mesoporous silica nanoparticles).
O, NO2 and ring had vanished due to the eradication of the biosurfactants from synthesized material, nevertheless, Si–OH still remained and the synthesized material was designated as BMSN (biologically synthesized mesoporous silica nanoparticles).
| Sample | Atomic orbital | Binding energy (eV) | Atomic (%) |
|---|---|---|---|
| a Reference for database: http://www.lasurface.com/database/liaisonxps.php. | |||
| MCM-41 (450 °C) | Si 2p (2p3/2) | 101.88 (SiC) | 27.0 |
| O 1s | 531.26 (PbS–air 3′-) | 71.1 | |
| C 1s | 282.99 {C in WC (tungsten carbide)} | 1.9 | |
| MCM-41 (500 °C) | Si 2p (2p3/2) | 102.05 {natrolite (Na2Al2Si3O10)} | 27.7 |
| O 1s | 531.42 (O ds OH−) | 70.5 | |
| C 1s | 283.22 (peak attributed to carbide) | 1.8 | |
| MCM-41 (550 °C) | Si 2p (2p3/2) | 102.11 (C–O–Si) | 28.0 |
| O 1s | 531.57 (zeolite NaY, NaY) | 69.8 | |
| C 1s | 283.76 (C6H5X with X = OCH3) | 2.2 | |
| MCM-41 (600 °C) | Si 2p (2p3/2) | 102.03 {natrolite (Na2Al2Si3O10)} | 27.8 |
| O 1s | 531.47 (O in Al metal) | 71.5 | |
| C 1s | 283.56 (–(CH2–CH2)n) | 0.7 | |
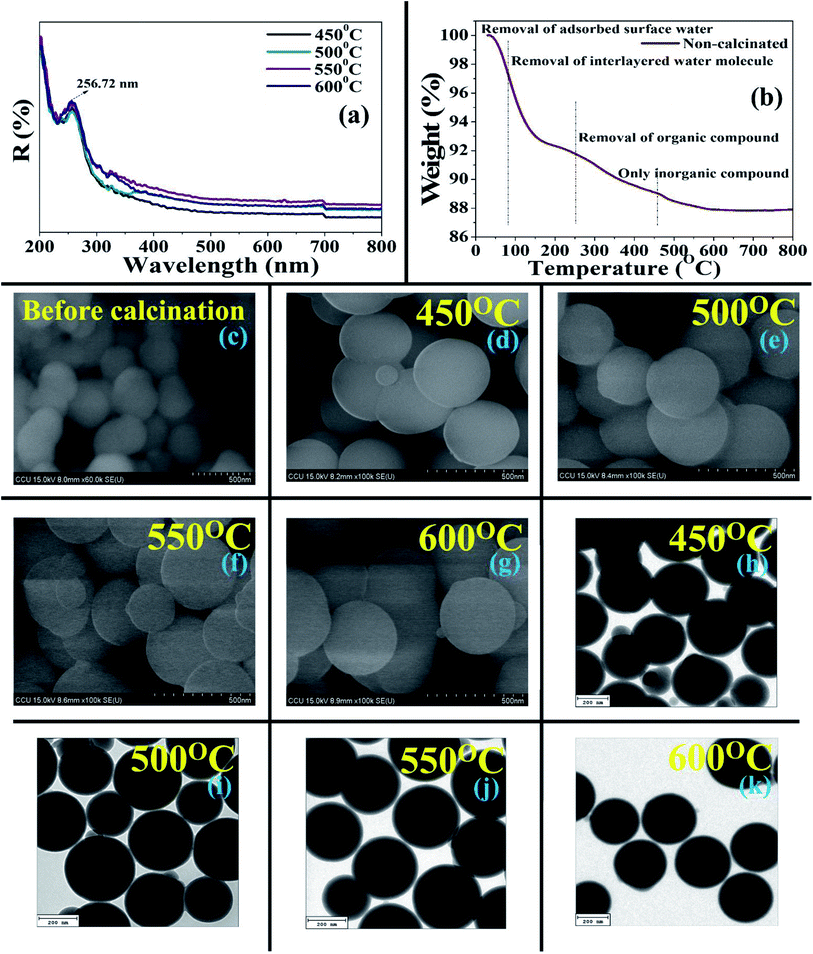
As per the HR-TEM micrographs, BMSN formed a perfectly spherical shape (Fig. 2h–k) with an average particle size of 262 nm (450–600 °C), where the particle size was almost unchanged. The importance of the BMSN particles dominated by (i) long-range order, and (ii) homogeneity and (iii) smoothness. According to previous results, the particle sizes of chemical templated materials were reported as 200–800 nm,45 whereas the size of the obtained BMSN was lower, in the range of 61–300 nm.
Furthermore, HR-TEM-EDXS confirmed the existence of silicon and oxygen in the BMSN. The weight (%) of silicon increased with increasing calcination temperature from 450 °C to 550 °C, then declined at the calcination temperature of 600 °C. This occurred due to the enhancement of the particle diameter at the calcination temperature of 600 °C and this phenomenon was illustrated by FE-SEM and HR-TEM. Hence, the elemental composition (silicon and oxygen) of the synthesized material demonstrated the formation of a MM.
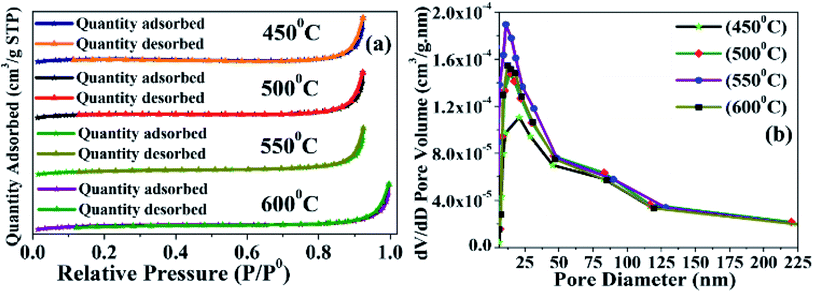 | ||
| Fig. 3 Characterization of synthesized BMSN material calcinated at different temperatures (450 °C, 500 °C, 550 °C, and 600 °C): N2 adsorption and desorption (a) and BJH curves (b). | ||
| Sample | SBET (m2 g−1) | Pore volume (cm3 g−1) | Pore diameter (nm) |
|---|---|---|---|
| MCM-41 (450 °C) | 6.5179 | 0.015763 | 29.7070 |
| MCM-41 (500 °C) | 7.7300 | 0.017399 | 20.2166 |
| MCM-41 (550 °C) | 8.2616 | 0.020407 | 14.8516 |
| MCM-41 (600 °C) | 7.9856 | 0.017394 | 16.5246 |
4. Conclusions
A novel BMSN MM was successfully synthesized by an ecofriendly and green process using Bacillus subtilis BBK006-mediated biosurfactant as a template using a sol–gel method to diminish the core toxicity in bionanotechnology and clinical studies. The calcination temperature (450–600 °C) influenced the physical and chemical characteristics of the synthesized particles. At the optimum temperature of 550 °C, the crystallographic properties of BMSN confirmed that it was an amorphous silica material with mesoporous character. In the synthesis process, the biosurfactant combines with TEOS (in the presence of NH4OH, H2O and C2H5OH) and forms Si–O–surfactin complexed molecules, which later bind with each other and produce a spherical molecule with numerous silica–O–surfactin-based complex through a condensation process. Highly complexed ordered BMSN mesoporous Si–OH material was formed to remove the biosurfactants from the silica–O–surfactin-based complex [(Si–O–surfactin)n] by calcination. BMSN mesoporous nanoparticles consist of Si–OH, where Si–O–Si linkage is observed. The absorption of BMSN was higher at the wavelength of 256.72 nm in the material calcinated at 550 °C. Effectively, 87.87% yield of BMSN was obtained (weight loss: 12.13%), using the calcination temperature range of 550–600 °C. Perfectly spherical shaped particles with regular arrangement were observed with an average particle size of 280 nm (diameter) (range: 61–300 nm), where the surface area, pore volume and pore diameter were found to be 8.2616 m3 g−1, 0.020407 cm3 g−1, and 14.8516 nm, respectively. Thus, this study suggests that materials prepared using biotemplate-based synthesis could be highly efficient and worthwhile for numerous industrial practices with very low toxicity, reduced synthesis time and cost, as well as applications in catalysis, drug delivery systems, gas separation, cosmetics, diagnostics, bio-separation, etc.Author contributions
R. K. S. prepared the manuscript. J. P. M., J. B., P. G. H., and Y. H. H. revised and gave necessary inputs. J. P. M., C. Y. C., S. C. W., H. C. C., and T. H. C. revised and gave suggestions for the manuscript. J. P. M., G. D., P. B., and S. C. W. revised the manuscript, tables, and figures.Conflicts of interest
There are no conflicts of interest.Acknowledgements
The authors would like to thank the Ministry of Science and Technology (Taiwan) and Ditmanson Medical Foundation Chiayi Christian Hospital for financial support (MOST 109-2811-M-194-502; MOST 108-2811-M-194-510; CYCH-CCU-2021-04).Notes and references
- Z. Wang, H. Ling, J. Shi, C. Stampfl, A. Yu, M. Hunger and J. Huang, J. Catal., 2018, 358, 71–79 CrossRef CAS.
- A. M. Brezoiu, M. Deaconu, I. Nicu, E. Vasile, R. A. Mitran, C. Matei and D. Berger, Microporous Mesoporous Mater., 2019, 275, 214–222 CrossRef CAS.
- D. Lu, S. Xu, W. Qiu, Y. Sun, X. Liu, J. Yang and J. Ma, J. Cleaner Prod., 2020, 264, 121644 CrossRef CAS.
- E. P. Ng, J. Y. Goh, T. C. Ling and R. R. Mukti, Nanoscale Res. Lett., 2013, 8, 1–8 CrossRef PubMed.
- S. Kamari and F. Ghorbani, Biomass Convers. Biorefin., 2020, 1–9, DOI:10.1007/s13399-020-00637-w.
- S. Kumar, M. M. Malik and R. Purohit, Mater. Today: Proc., 2017, 4, 350–357 Search PubMed.
- Y. Song, B. Li, S. Yang, G. Ding, C. Zhang and X. Xie, Sci. Rep., 2015, 5, 1–9 Search PubMed.
- H. Purwaningsih, Y. Ervianto, V. M. Pratiwi, D. Susanti and A. Purniawan, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 515, 012051 CAS.
- Y. Ma, H. Chen, Y. Shi and S. Yuan, Mater. Res. Bull., 2016, 77, 258–264 CrossRef CAS.
- M. Masteri-Farahani and N. Mosleh, Environ. Sci. Pollut. Res. Int., 2021, 28, 4615–4622 CrossRef CAS PubMed.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS.
- P. C. Ray, H. Yu and P. P. Fu, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2009, 27, 1–35 CrossRef CAS PubMed.
- B. Isomaa, J. Reuter and B. M. Djupsund, Arch. Toxicol., 1976, 35, 91–96 CrossRef CAS PubMed.
- N. A. C. Lah, R. Gray and S. Trigueros, Microb. Cell Fact., 2021, 20, 1–11 CrossRef PubMed.
- J. P. Maity, Y. M. Huang, C. M. Hsu, C. I. Wu, C. C. Chen, C. Y. Li, J. S. Jean, Y. F. Chang and C. Y. Chen, Chemosphere, 2013, 92, 1286–1293 CrossRef CAS PubMed.
- J. P. Maity, C. M. Hsu, T. J. Lin, W. C. Lee, P. Bhattacharya, J. Bundschuh and C. Y. Chen, Environ. Nanotechnol. Monit. Manag., 2018, 9, 18–28 Search PubMed.
- C. E. Drakontis and S. Amin, Curr. Opin. Colloid Interface Sci., 2020, 48, 77–90 CrossRef CAS.
- J. P. Maity, C. Y. Chen, P. Bhattacharya, R. K. Sharma, A. Ahmad, S. Patnaik and J. Bundschuh, J. Hazard. Mater., 2021, 405, 123885 CrossRef CAS PubMed.
- K. K. Gautam and V. K. Tyagi, J. Oleo Sci., 2006, 55, 155–166 CrossRef CAS.
- M. Bustamante, N. Duran and M. C. Diez, J. Plant Nutr. Soil Sci., 2012, 12, 667–687 Search PubMed.
- F. A. Bezza and E. M. Chirwa, Chem. Eng., 2020, 79, 91–96 Search PubMed.
- J. P. Maity, T. J. Lin, H. P. H. Cheng, C. Y. Chen, A. S. Reddy, S. B. Atla, Y. F. Chang, H. R. Chen and C. C. Chen, Int. J. Mol. Sci., 2011, 12, 3821–3830 CrossRef CAS PubMed.
- A. W. Zanotto, A. Valério, C. J. de Andrade and G. M. Pastore, Appl. Microbiol. Biotechnol., 2019, 103, 8647–8656 CrossRef CAS PubMed.
- I. A. Phulpoto, Z. Yu, B. Hu, Y. Wang, F. Ndayisenga, J. Li, H. Liang and M. A. Qazi, Microb. Cell Fact., 2020, 19, 1–12 CrossRef PubMed.
- C. Y. Chen, S. C. Baker and R. C. Darton, J. Chem. Technol. Biotechnol., 2006, 81, 1923–1931 CrossRef CAS.
- C. Y. Chen, S. C. Baker and R. C. Darton, J. Chem. Technol. Biotechnol., 2006, 81, 1915–1922 CrossRef CAS.
- J. P. Maity, P. R. Ho, Y. H. Huang, A. C. Sun, C. C. Chen and C. Y. Chen, Environ. Pollut., 2019, 253, 768–778 CrossRef CAS PubMed.
- H. Wu, Y. Xiao, Y. Guo, S. Miao, Q. Chen and Z. Chen, Microporous Mesoporous Mater., 2020, 292, 109754 CrossRef CAS.
- T. Popova, B. Tzankov, C. Voycheva, I. Spassova, D. Kovacheva, S. Tzankov, D. Aluani, V. Tzankova and N. Lambov, J. Drug Delivery Sci. Technol., 2021, 62, 102340 CrossRef CAS.
- P. B. Sarawade, J. K. Kim, A. Hilonga and H. T. Kim, J. Hazard. Mater., 2010, 173, 576–580 CrossRef CAS PubMed.
- P. Zhang, L. Kang, M. Zhu and B. Dai, Sustainable Energy Fuels, 2020, 4, 4293–4300 RSC.
- N. Abdullah, J. Nanosci. Nanotechnol., 2018, 18, 100–103 CrossRef CAS PubMed.
- C. Huo, J. Ouyang and H. Yang, Sci. Rep., 2014, 4, 1–9 Search PubMed.
- M. S. A. Salam, M. A. Betiha, S. A. Shaban, A. M. Elsabagh and R. M. Abd El-Aal, Egypt. J. Pet., 2015, 24, 49–57 CrossRef.
- S. H. Abbas, F. Adam and L. Muniandy, Microporous Mesoporous Mater., 2020, 305, 110192 CrossRef CAS.
- C. Huayao, L. Yueshun, Z. Hongjun, Z. Xinhua, G. Sheng and X. Hua, RSC Adv., 2016, 6, 114714–114721 RSC.
- A. Sterczyńska, A. Deryło-Marczewska, M. Zienkiewicz-Strzałka, M. Śliwińska-Bartkowiak and K. Domin, Langmuir, 2017, 33, 11203–11216 CrossRef PubMed.
- S. Wang, Y. Shi and X. Ma, Microporous Mesoporous Mater., 2012, 156, 22–28 CrossRef CAS.
- N. Mosaddegh and I. Yavari, Chem. Pap., 2018, 72, 2013–2021 CrossRef CAS.
- R. V. Sales, H. O. Moura, A. B. Câmara, E. Rodríguez-Castellón, J. A. Silva, S. B. Pergher, L. Campos, M. M. Urbina, T. C. Bicudo and L. S. de Carvalho, Catalysts, 2019, 9, 651 CrossRef CAS.
- N. Gan, P. Xiong, J. Wang, T. Li, F. Hu, Y. Cao and L. Zheng, J. Anal. Methods Chem., 2013, 2013, 482316 Search PubMed.
- L. H. Gaabour, J. Mater. Res. Technol., 2019, 8, 2157–2163 CrossRef CAS.
- R. Ouargli-Saker, N. Bouazizi, S. Lassouad, S. Ammar, J. Vieillard, F. Le Derf and A. Azzouz, J. Inorg. Organomet. Polym. Mater., 2020, 30, 5108–5117 CrossRef CAS.
- A. G. Rios, L. C. Matos, Y. A. Manrique, J. M. Loureiro, A. Mendes and A. F. Ferreira, Adsorption, 2020, 26, 75–88 CrossRef CAS.
- N. I. Vazquez, Z. Gonzalez, B. Ferrari and Y. Castro, Bol. Soc. Esp. Ceram. Vidrio, 2017, 56, 139–145 CrossRef CAS.
- S. Velmathi, U. Balakrishnan, N. Ananthi, S. S. Aldeyab, K. Ariga, T. S. Naidu and A. Vinu, Phys. Chem. Chem. Phys., 2011, 13, 4950–4956 RSC.
- Z. Taherian, M. Yousefpour, M. Tajally and B. Khoshandam, Microporous Mesoporous Mater., 2017, 251, 9–18 CrossRef CAS.
- S. S. Mohanti, N. Dash, S. P. Nanda and R. K. Dey, Der Chemica Sinica, 2015, 6, 7–11 CAS.
| This journal is © The Royal Society of Chemistry 2021 |