Open Access Article
Open Access ArticleRecent advances in bismuth oxyhalide photocatalysts for degradation of organic pollutants in wastewater†
Yang Lia,
Haiyan Jiangb,
Xu Wanga,
Xiaodong Hong*c and
Bing Liangd
aCollege of Materials Science and Engineering, Liaoning Technical University, Fuxin 123000, China
bBasic Department, Liaoning Institute of Science and Technology, Benxi, 117004, China
cSchool of Materials Science and Hydrogen Energy, Foshan University, Foshan 528000, China. E-mail: hongxiaodong@lntu.edu.cn
dCollege of Materials Science and Engineering, Shenyang University of Chemical Technology, Shenyang 110142, China
First published on 6th August 2021
Abstract
Photocatalysis has been considered as an environmental-friendly strategy for degradation of organic pollutants to the nontoxic products of H2O and CO2. Compared to metal oxide semiconductors, BiOX (X = Cl, Br and I) photocatalysts exhibit some advantages, such as, unique layered structure, good chemical stability and superior photocatalytic activity. This review provides an overview on the controllable synthesis of BiOX-based photocatalysts and their application in photodegradation of organic pollutants. Firstly, the controllable synthesis of BiOX is introduced, including hydrothermal, solvothermal, hydrolysis, precipitation, two-phase methods, ultrasonic/microwave-assisted methods, and physical methods. Then, the doping and surface modification of BiOX are summarized, including non-metal doping, metal doping, dual doping, and the modification by introducing surface terminations or carriers. In addition, the heterojunctions of BiOX/BiOY and BiOX/BimOnXz are introduced. At last, the promising research trends of BiOX-based photocatalysts are put forward. The main purpose is providing practical guidelines for developing high-performance BiOX photocatalysts.
1. Introduction
The rapid development of modern industries causes environmental pollution, including air pollution, soil pollution and water pollution, which have been receiving much more attention in recent years. Water is a precious resource for the survival of animals and humans. Therefore, the treatment of water pollutants is an urgent task for the technicians and researchers. Among the strategies for solving wastewater pollution, photocatalysis is considered as an environmental-friendly method to obtain nontoxic degradation products. Under the irradiation of visible light or UV light, oxygen in the air is directly used as oxidant under normal reaction conditions, and organic pollutants in wastewater can be degraded into H2O and CO2, with no secondary pollutants. Moreover, the semiconductor photocatalysts have stable chemical properties, low cost, strong redox, and long service life.1,2 Therefore, photocatalysis is particularly suitable for the purification of wastewater containing organic matter. Photocatalysis technique has been adopted as an effective strategy for degrading various organic contaminants, including antibiotics, organic dyes, organic acids, amines or phenols, and so on. Nowadays, developing high-performance photocatalysts has become a research hotspot in the photocatalysis field. Various metal oxide semiconductors, including TiO2, SnO2, ZnO, bismuth oxychloride (BiOX) have been synthesized and widely applied for the wastewater treatment.3–5 Compared to traditional metal oxides, BiOX (X = Cl, Br, I) has some advantages,6–8 such as, chemical stability, unique layered structure, easy preparation, and superior photocatalytic activity. In the unique lamellar structure of BiOX, the [Bi2O2] layer is interleaved by X22− slabs, and Bi–O and Bi–X bonds are connected by strong covalent bonding and weak Van der Waals interactions.9 The band gap of BiOX decides the light absorption range. As shown in Fig. 1a, BiOCl has a broad band gap of ∼3.3 eV, and exhibits a strong UV light absorption capability. Compared to BiOCl, BiOBr and BiOI have a low band gap of ∼2.64 eV and ∼1.77 eV, which enable them a strong visible light activity. The light absorption capability decides the photocatalytic activity of BiOX. Under the irradiation of light, when the absorbed light energy is greater than or equal to the energy gap of semiconductors, the electrons in the valence band (VB) can be excited and transferred to the conduction band (CB), leaving relatively stable holes in the VB, then electron–hole pairs are generated. During a photocatalytic reaction process, the electrons and holes participate in the redox reaction for degradation of organic pollutants. Due to the recombination of electrons and holes, the photocatalytic efficiency of semiconductors will be decreased. Therefore, the reduction of band gap energy and inhibiting the recombination of electrons and holes are the major strategies for improving the photocatalytic efficiency of BiOX.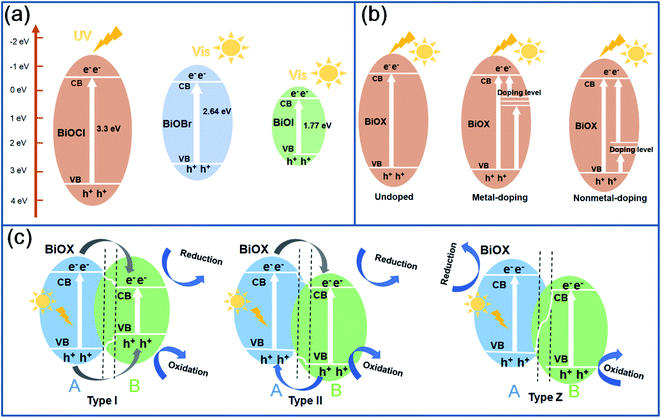 | ||
| Fig. 1 The band gap structure of BiOCl, BiOBr and BiOI (a), the effect of heteroatom doping of BiOX (b) and construction of different types of BiOX heterojunctions (c). | ||
In order to reduce the band gap of BiOX, the microstructure design, heteroatom doping and the construction of heterojunction are the effective measures.10 In the field of microstructure design, the control of morphology and surface defects including oxygen vacancies and exposed (001) facets have been widely reported. From the relationship between heteroatom doping and band gap structure in Fig. 1b, metal-doping can be adopted for adjusting the edge of the CB, while non-metal doping changes the edge of the VB. In addition, the formation of a heterojunction not only adjusts the band gap energy, enhancing the light absorption capability, but also accelerates the transfer of electrons and/or holes, preventing the recombination of electron–hole pairs. According to different transfer pathways, as given in Fig. 1c, BiOX heterojunctions can be classified into Type I, Type II and Type Z.11,12 From a Type I heterojunction, the electrons and holes transfer to the CB and VB of B from substance A, and the electrons in the CB and holes in the VB participate in the redox reaction. From a Type II heterojunction, the electrons transfer to the CB of B from substance A, and the holes transfer to the VB of A from B, and the electrons in the CB of B and holes in the VB of A participate in the redox reaction. From a Type Z heterojunction, the electrons transfer to the VB (hole) of A from substance B. The holes remained in B and the electrons in A participate in the redox reaction. Among the three heterojunctions, Z-scheme heterojunctions are regarded as one of the best ways to improve photocatalytic activity.9 Besides the construction of heterojunctions, the decoration of BiOX by inorganic/organic frameworks, carbon materials, metal particles and carriers has been reported for enhancing the electronic conductivity, and promoting the transfer and separation rate of carriers, which restrains the recombination of electron–hole pairs.
Besides the design of photocatalysts, the selection of target organic pollutants also affects the photocatalytic activity. Up to now, various organic dyes are widely used, including Rhodamine B (Rh B), Methylene blue (MB), Methyl orange (MO), brilliant blue K-NR, and Alizarin Red S (ARS), etc. Besides organic dyes, all kinds of antibiotics have been selected as target pollutants, such as, ciprofloxacin (CIP), tetracycline (TC), tetracycline hydrochloride (TCH or TC-HCl), carbamazepine (CBZ), norfloxacin (NOR), atenolol (ATL), sulfamethoxazole (SMZ), levofloxacin (LVX), metronidazole (MTZ), ofloxacin (OFX), doxycycline (DOX), chlortetracycline hydrochloride, and so on. In addition, abundant organics can be degraded by BiOX-based photocatalysts, including phenol, bisphenol A (BPA), estriol, lindane, perfluorooctanoic acid (PFOA), 4-chlorophenol (4-CP), methanol, 4-dihydroxybenzoate (MDB), benzoic acid (BA), p-chloroaniline (PCA), diclofenac (DCF), benzyl alcohol, nitrobenzene, 17-ethinyl estradiol (EE2), 2,4-dihydroxybenzoate (MDB), and so on.
Herein, we introduce the recent progress on the different BiOX photocatalysts in degradation of organic pollutants. As shown in Fig. 2, synthetic methods decide the microstructure and the specific surface area of BiOX, which affect the photocatalytic activity. So the controllable synthesis of BiOX was introduced firstly, including hydrothermal or solvothermal method, hydrolysis, precipitation, two-phase method, ultrasonic/microwave assisted method, and physical method. Then, in the second part, the doping and surface modification of BiOX was summarized. The heteroatom doping is devided into non-metal doping, metal doping and dual-doping. While in the respect of surface modification, different decoration terminations and carriers involved in modified BiOX were introduced. In the third part, the heterojunctions including BiOX/BiOY type and BiOX/BimOnXz system are summarized. At last, some promising research trends for developing high-performance BiOX photocatalyst are put forward. We expect that this review would provide practical guideline for the beginners in photocatalysis field.
2. Controllable synthesis of BiOX
The photocatalytic activity of BiOX photocatalysts mainly depends on their size, morphology, specific surface area and crystalline structure. Up to now, various BiOX species, including spheres, nanoflakes, sea urchins, flower spheres, hollow balls, and so on, have been synthesized for photocatalysts. In this section, we introduce the synthesis methods of different BiOX species, including hydrothermal, solvothermal, hydrolysis, precipitation method, two-phase method, ultrasonic/microwave-assisted method and physical method.2.1. Hydrothermal method
In a typical hydrothermal process, the reactants including Bi source of Bi(NO3)3·5H2O or NaBiO3·2H2O, and the halogen source of NaX, KX, HX or ionic liquids are dissolved in water, and transferred into polytetrafluoroethylene (PTFE)-lined autoclave. Under a condition of high temperature and high pressure, various BiOX nanostructures were synthesized. During a reaction process, besides the precursors and their ratios, reaction temperature/time, the pH value of solution greatly affects the size and morphology of BiOX. In this field, Zhang et al.13 confirmed that the BiOCl sheets prepared in alkaline solvent had the best photocatalytic oxidation performance on gaseous Hg0 under UV light, due to the large specific surface area and a good growth orientation of crystal BiOCl. Similarly, BiOBr flakes were prepared via a hydrothermal method.14 The sample prepared at the pH value of 9 displayed the highest degradation efficiency for removal of ciprofloxacin (CIP) under the irradiation of visible light. In addition, Intaphong et al.15 found that pH value greatly affected the morphology, crystal structure of BiOBr and their photocatalytic performance. When the pH value was 8, the BiOBr micro-flowers were conducive to the uniform adsorption of Rh B molecules, which presented the best photodegradation performance for degradation of Rh B. Feng et al.16 synthesized BiOBr by hydrothermal method, and changed the internal stress of BiOBr nanosheets through adjusting pH value. The low strain energy changed the symmetry and band structure of BiOBr, which enhanced the charge separation rate and the degradation rate of Rh B and MO dye.During a hydrothermal process, various surfactants have been added to control the microstructure of BiOX. In this respect, Dou et al.17 synthesized BiOI photocatalysts in glacial acetic acid by using EO106PO70EO106 (F127) as a surfactant. A large specific surface area and much more hydroxyls on F127-BiOI accelerated the rapid separation of photogenerated carriers and promoted the formation of O2−. Zhou et al.18 synthesized BiOCl nanosheets by using dulcitol (C6H14O6) as surfactants. The thickness of BiOCl nanosheets were adjusted by changing the pH of solution. The BiOCl prepared at the pH value of 4 had a smaller thickness, which exhibited the best photocatalytic activity in the degradation of Rh B and TC-HCl, due to the best oxygen vacancy concentration and exposed (001) crystal plane. By using cationic polyacrylamide (C-PAM) as a surfactant and chlorine source, Li et al.19 synthesized tetragonal BiOCl nanosheets with exposed (001) facets. The BiOCl sample prepared at 150° for 12 h presented the highest degradation efficiency for the removal of MO. Through adjusting the amount of xylitol surfactants, Cai et al.20 synthesized BiOCl nanosheets with different sizes. The sample prepared with 0.1 g xylitol showed a narrow band gap, a special polycrystalline structure, and a concentration of grain boundaries and oxygen vacancies, which degraded 98% Rh B in 20 min under visible light. Therefore, the selection of surfactants is crucial for enhancing the photocatalytic activity of BiOX.
2.2. Solvothermal method
Different from the aqueous solution in hydrothermal reaction, organic solvents are used in solvothermal reaction. Except for reaction conditions, the effect of solvent is indispensable. Various alcohols are adopted to synthesize BiOX. Among these alcohols, ethylene glycol is the commonly-used solvent.By using EG-mediated solvothermal reaction, Gao et al.21 prepared BiOCl microspheres for removal of carbamazepine (CBZ) under visible light. Tian et al.22 synthesized core–shell hierarchical BiOCl microspheres. Rich oxygen vacancies formed on the BiOCl surface reduced the band gap and extended the light absorption range. The sample displayed the highest photocatalytic performance for degradation of norfloxacin (NOR) under visible light. By using EG as solvent, Mera et al.23 synthesized BiOBr microspheres by using KBr precursor at 145° for 18 h, and the optimized sample degraded 97% MO in 60 min at the pH value of 2.
Mera et al.24 synthesized two kinds of BiOI by using KI and 1-butyl-3-methylimidazolium iodide ([bmim]I) as iodine sources respectively. Through a comparison, the BiOI prepared with [bmim]I presented the best photocatalytic activity for removal of gallic acid, due to the highly exposed (001) facets. By using EG solvent, Jiang et al.25 prepared hollow flower-shaped BiOI (h-BiOI). Compared to bulk BiOI, h-BiOI nanosheets with 2 nm thickness exhibited a larger specific surface area and stronger oxidation ability, which degraded 99% of Rh B within 60 min, much faster than that of bulk BiOI. Besides the single-phased BiOX, Zhang et al.26 synthesized BiOBrxI1−x nanoplates with exposed (001) crystal plane. Through decreasing the ratio of Br/I, the band gap reduced from 2.87 to 1.89 eV, which enhanced the light absorption. The optimized BiOBr0.8I0.2 showed the highest photocatalytic activity for degradation of Rh B, which was 5.4 and 1.7 times higher than that of BiOI and BiOBr, respectively.
During hydrothermal process, the type of solvent affects on the morphology, surface structure and performance of BiOCl. Zhao et al.27 synthesized BiOCl with oxygen vacancies (OV-BOC) by using EG as solvent. Compared to pure BiOCl prepared with ethanol, the OV-BOC had a higher photocatalytic performance for degradation of Rh B, MO and phenol for the stronger oxidation capacity. In another work, ethanol, EG and glycerol was selected as solvents to synthesize BiOCl photocatalysts with different morphologies.28 Through a comparison, BiOCl hierarchical microspheres prepared in EG solvent showed the highest photocatalytic activity for degradation of carbamazepine (CBZ), due to the enhanced adsorption capability and high separation efficiency. Xing et al.29 synthesized different BiOBr photocatalysts by using water, ethanol, isobutanol, ethylene glycol, and glycerin. When used for photodegradation of reactive brilliant blue (KN-R), flower-like BiOBr prepared in glycerin presented the highest activity for the small size, exposed active face and abundant oxygen vacancies. Wei et al.30 synthesized several BiOBr photocatalysts by using ethanol, ethylene glycol, tert-butanol, benzyl alcohol, and 2-methoxyethanol solvent. As a result, thin BiOBr nanosheets synthesized in benzyl alcohol presented the outstanding photocatalytic activity for degradation of Rh B, due to the exposed (001) facets. In addition, various BiOI photocatalyts were also synthesized by using water, ethanol, ethylene glycol and glycerin, respectively.31 Among these samples, 3D mesoporous hierarchitectured BiOI prepared in glycerol displayed excellent visible-light photocatalytic activity for the removal of As(III), due to the largest specific surface area, mesoporous structure and highly exposed (001) facets. Dehghan et al.32 prepared two kinds of BiOI photocatalysts by hydrolysis method and solvothermal method, and the sample prepared by solvothermal method showed an obvious superiority for degradation of tetracycline hydrochloride (TCH). Gao et al.33 synthesized 3D mesoporous nanostructured BiOCl by adding PEG1000 into PEG400 solvent. Abundant mesopores and vacancies generated on thin BiOCl nanosheets (3–6 nm) effectively improved the photocatalytic activity for degradation of bisphenol A (BPA).
Besides the type of alcohols, the ratio of water/methanol also affects the morphology and performance of BiOCl. For example, Xu et al.34 synthesized BiOCl nanosheets with different sizes and shapes through adjusting the water/methanol ratios. When the water content was 10%, the prepared BiOCl exhibited a small size and thickness, with largely exposed {001} facets, which presented superior photocatalytic activity for degradation of MO and Rh B. In addition, Lee et al.35 synthesized BiOX (X: Cl, Br, I) microspheres by using ethanol/EG mixture solvent. When used for photocatalytic hydrogen evolution, flower-like BiOI microspheres exhibited the highest photocatalytic activity for the lower fluorescence intensity.
2.3. Hydrolysis method
In a typical hydrolysis process, Bi(NO3)3·5H2O, BiBr3 or NaBiO3·2H2O is often used as Bi sources. After the solution of halide sources was dropped to the solution of Bi source, the BiOX can be produced under the mechanical stirring. The precursor types and their concentrations have a great influence on the morphology and performance of BiOX. Moreover, solvent affects the growth of BiOX crystals, which also decides the photocatalytic performance. By using a hydrolysis method, Hu et al.36 synthesized BiOCl photocatalyst for degradation of atenolol (ATL), and the removal efficiency reached nearly 100% within 60 min. Song et al.37 synthesized BiOCl nanosheets for degradation of perfluorooctanoic acid (PFOA). Under the irradiation of UV light, the BiOCl decomposed nearly 100% PFOA within 12 h, and the degradation rate constant was 14.6 and 1.7 times higher than that of commercial TiO2 (P25) and In2O3. Zhang et al.38 synthesized thin BiOCl nanosheets with a thickness of 20 nm. When used for degradation of Rh B, the BiOCl exhibited a higher photocatalytic activity than degradation of MO and MB. Yuan et al.39 synthesized ultrathin BiOBr nanoflakes, and the ultrathin nanocrystals exhibited a higher photocatalytic activity for degradation of Rh B under visible light, much better than that of 3D BiOBr microspheres and P25-TiO2. By using BiBr3 as precursor, Li et al.40 synthesized nanosheet, honeycomb, and flower-like BiOBr photocatalysts by using water, ethanol (EtOH) and isopropyl alcohol (IPA), respectively. Through a comparison, the BiOBr nanosheets prepared in water presented the best crystallinity and superior photocatalytic activity for degradation of MO. By controlling the amount of water, Lu et al.41 prepared BiOI with surface heterojunction between (001) facets and (110) facets. The interface junction in BiOI promoted the separation of electron–hole pairs, and enhanced the photocatalytic activity for degradation of MO. In addition, Ahern et al.42 synthesized BiOCl and BiOI via a hydrolysis method for degradation of 17-ethinyl estradiol (EE2) and estriol in water. Through a comparison, BiOI displayed the highest activity under 350 nm light source, while the BiOCl had the highest activity under 254 nm light source.2.4. Precipitation method
In a typical precipitation method, alkali solution containing halide sources was slowly dropped into the Bi-containing precursors. Under the stirring, BiOX were precipitated. Followed by this method, Zhang et al.43 synthesized BiOCl, BiOBr and BiOI photocatalysts respectively. When used for the removal of Hg0 under the irradiation of fluorescent light, the activity sequence was BiOI > BiOBr > BiOCl, and BiOI exhibited the best photocatalytic performance. Huang et al.44 synthesized Br− substituted BiOI (Br-BiOI) photocatalyst by introduction of Br− into BiOI lattice. The replacement by Br− reduced the valence band potential and enlarged band gap, which suppressed the recombination of electron–hole pairs and enhanced the photocatalytic activity of BiOI. In addition, the photocatalytic activity of BiOX can be improved by adjusting the band engineering. In this respect, Bi24O31Cl10 powders were synthesized via a chemical precipitation method.45 The Bi24O31Cl10 had a narrow band gap, which presented a superior photocatalytic activity for degradation of Rh B under visible light.2.5. Two-phase method
Besides traditional synthetic methods, two-phase reaction was used to synthesize BiOCl nanosheet at the water–air interface.46 The resulting BiOCl nanosheets with exposed (010) facets presented a high photocatalytic activity for degradation of methyl orange (MO). In another two-phase method,47 octadecene (ODE), oleic acid (OA) and oleylamine (OLA) was acted as oil phase to dissolve Bi(NO3)3·5H2O, and the aqueous solution of KX (X = Cl, Br, I) was acted as water phase. After the mixed solution was refluxed at 170 °C, ultrathin 2D BiOX nanosheets were synthesized. During the preparation process, acidic condition enabled the formation of ultrathin BiOX structure with highly exposed {001} facets.2.6. Ultrasonic/microwave-assisted method
Based on traditional chemical bath method, Intaphong et al.48 synthesized BiOI nanoplates via a sonochemical method in ultrasonic bath at 80 °C, and the BiOI nanoplates prepared in the pH of 12 exhibited the highest efficiency for degradation of Rh B under visible light. Furthermore, microwave-assisted method was used to synthesize BiOI and BiOBr in a short time. Montoya-Zamora et al.49 synthesized BiOI photocatalyst via microwave-assisted method with EDTA retarder. The sample prepared with 40% of EDTA at 110 °C had the highest specific surface area, which presented the highest photocatalytic activity in degradation of Rh B. In addition, Miao et al.50 synthesized BiOBr hierarchical microcubes via microwave-assisted ionothermal self-assembly method. Multi-layered BiOBr nanosheets were formed and exhibited a strong light harvesting ability. Chen et al.51 synthesized porous and hollow BiOBr microspheres via a microwave-assisted solvothermal route within 20 min. Compared to the hollow microspheres, porous BiOBr spheres showed a higher photocatalytic activity for degradation of Rh B, due to the large surface area, small particle size and narrow band gap.2.7. Physical methods
Some physical methods, including combustion or calcinations, ultrasonic exfoliation, and mechanical grinding have been reported for synthesizing BiOX photocatalysts. A simple one-step combustion method was developed to synthesize BiOBr nanosheets with exposed {001} facets.52 The proportion of exposed {001} facets could be adjusted by changing the amount of ammonium bromide, which further narrowed the band gap energy of BiOBr. The BiOBr-5 sample with a thickness of 193 nm presented the highest photocatalytic activity for degradation of Rh B under visible light. In addition, electrospinning method53 was adopted to generate nanofibers containing BiBr3 precursors firstly, and the post calcination was conducted at 500 °C in air to prepare BiOBr with lamellae nanostructures. Through adjusting BiBr3 content in PAN solution, the optimized BiOBr with 3% BiBr3 displayed the best performance for degradation of Alizarin Red S (ARS).Ultrasonic exfoliation method54 was developed to fabricate monolayered BiOBr nanosheets with a thickness of ∼0.85 nm in formamide solvent. The monolayered BiOBr exhibited a higher adsorption and photodegradation performance. A simple solvent-free mechanical grinding method was developed for preparing BiOCl, BiOBr, and BiOI hierarchical flower-like nanostructures with a thickness of 5 min.55 Three BiOX samples possessed excellent photocatalytic activities for photodecomposition of MB and Rh B. Due to the short preparation time and excellent photocatalytic activity, the solvent-free grinding strategy can be adopted for achieving the mass production of BiOX.
2.8. Summary
Synthetic method decides the microstructure and performance of BiOX photocatalysts. In this section, we introduce the controllable synthesis of BiOX photocatalysts. In order to make a comparsion, synthesis method, morphology and photocatalytic performance of BiOCl, BiOBr and BiOI are listed in Table S1.† Among these methods, hydrothermal and solvothermal method are the most popular route for synthesizing BiOX. Compared to hydrothermal method, the selection of solvents is really important in solvothermal reaction. Various alcohols, including methanol, ethanol, ethylene glycol, tert-butanol, benzyl alcohol, and PEG-400 have been used as solvents for synthesizing BiOX. Through a comparison, the BiOX prepared with EG usually exhibits a superior photocatalytic activity than the sample prepared by monohydric alcohols. Besides the influence of the reaction temperature/time, surfactants and halide precursors are also essential for the morphology and performance of BiOX. Halide-containing ionic liquids are often used for fabricating hierarchical spherical BiOX with a high photocatalytic activity. However, these ionic liquids are much more expensive than traditional halide source KX or NaX, further limiting their wide application. Two-phase method and precipitation method can be adopted to prepare BiOX nanosheets with highly exposed (010) facets, while these methods are seldom accepted by researchers. Based on solution-based reaction, microwave-assisted strategy greatly shortens the reaction time, and improves the production efficiency. Different from solution-based synthetic methods, some physical methods including calcinations and mechanical grinding method, exhibit a promising application potential for the mass production of BiOX photocatalysts.3. The doping and surface modification of BiOX
Except for the microstructure control of BiOX, the heteroatom doping and surface modification can be adopted for improving the photocatalytic activity of BiOX. The heteroatom doping is introducing nonmetal or metal heteroatoms to the crystal plane, further reducing the band gap, extending the light absorbance range and promoting the separation of photogenerated carriers.56 According to the species of doped element, we classify the doping into non-metal doping, metal doping and dual doping, and recent advance of doped BiOX photocatalysts was summarized. Different from the heteroatom doping, surface modification of BiOX has a wide research range. On the one hand, surface defects or surface terminations can be introduced on the surface of BiOX. On the other hand, various decorators including inorganic/organic frameworks, carbon materials, metal particles, and carriers can be used to hybridize with BiOX. After the surface modification, the electronic conductivity and the light absorption capability of BiOX will be improved, which further accelerate the transfer and separation of charge carriers, and effectively inhibit the recombination of electron–hole pairs.3.1. The doping of BiOX
In addition, C doping also accelerates the transfer and separation of photo-generated charges separation. In this respect, Zeng et al.60 synthesized C-doped BiOI (C-BiOI) photocatalysts by using glucose as carbon sources, as shown in Fig. 3a. Doped carbon clusters embedded into the interlayers of BiOI structure increased the specific surface area, adjusted the lattice periodicity, and produced vacancies, which reduced the band gap and enhanced charge separation efficiency. When used for degradation of methyl orange (MO), the degradation rate of optimized C-BiOI was 4.44 times higher than that of pure BiOI. Qu et al.61 synthesized graphene oxide (GO) and carbon nanodots (CDots) co-doped BiOBr (GO/CDots/BiOBr) composites. In this ternary composite, GO was acting as the electron-transfer and electron-reservoir center and suppressed the recombination of electron–hole pairs. The CDots enhanced the visible light harvesting and utilizing ability of BiOBr. So the GO/CDots/BiOBr exhibited the highest photocatalytic activity for degradation of 4-chlorophenol (4-CP) under visible light. In addition, N-doped BiOBr nanosheets were decorated on the surface of carbon fibers (CFs) through a solvothermal method.62 Due to the strong visible-light absorption from all directions, 3D N-doped BiOBr/CFs exhibited an outstanding photocatalytic activity for degradation of Rh B and methanol.
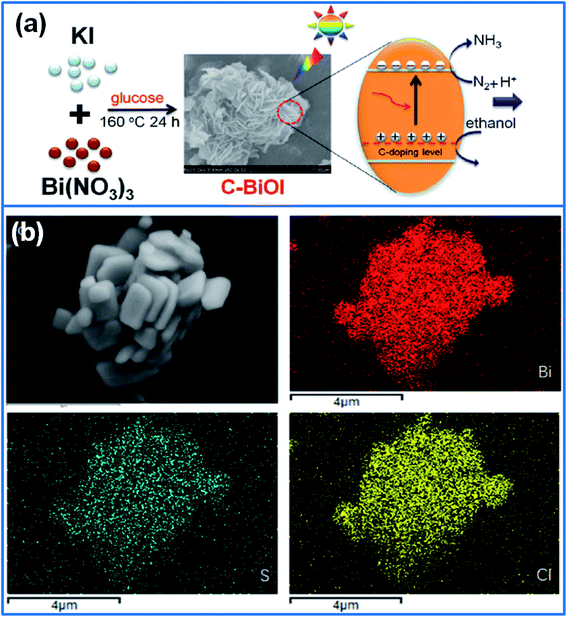 | ||
| Fig. 3 (a) Preparation of C-BiOI photocatalysts by using glucose as carbon sources for degradation of organic pollutants.60 Copyright 2019 Elsevier. (b) The microstructure of BiOCl-S and its element mapping.63 Copyright 2018 Elsevier. | ||
S-doped BiOCl and BiOBr have been reported in recent years. For instance, Shang et al.63 synthesized S-doped BiOCl (BiOCl-S, Fig. 3b) by using thioacetamide as sulfur source. The crystal structure of S-doped BiOCl had no change. While S doping broadened the light absorption edge, which enhanced the visible light absorption ability. Moreover, S doping suppressed the recombination of electron–hole pairs of BiOCl. So the BiOCl-S exhibited a superior photocatalytic activity for degradation of Rh B and phenol. Jiang et al.64 prepared BiOCl-S photocatalysts via a solvothermal method. The uniform dispersion of S elements on BiOCl extended the light absorption range and promoted the separation of electron–hole pairs. In addition, S-doping can be adopted to tailor the band structure of BiOBr. In this field, Wang et al.65 synthesized S-doped BiOBr with a thickness of ∼10 nm via hydrothermal method. S doping narrowed the band gap of BiOBr, and enabled the BiOBr with a strong visible-light response. Furthermore, S doping improved the charge separation efficiency. As a result, optimized S0.2-BiOBr nanosheets presented the highest activity for degradation of bisphenol A (BPA) under visible light irradiation. Zhang et al.66 prepared I-doped BiOCl microspheres by a microwave-assisted method. I doping increased the specific surface area of BiOCl, narrowed the band gap, and promoted the separation of photogenerated carriers. Therefore, I-doped BiOCl achieved a high removal ratio of 91.2% in 60 min for degradation of 2,4-dihydroxybenzoate (MDB).
Sn-doped BiOCl was synthesized with a tetragonal crystal structure by using SnCl2 as reactant via an oxidation–reduction method.70 After doping with Sn, the surface of BiOCl was rougher, and the band gap energy was narrowed. Tetra-valence Sn (10%) doped BiOCl enhanced the photocatalytic activity for degradation of Rh B and benzoic acid (BA). Li et al.71 synthesized In-doped BiOI (In-BiOI) photocatalysts with exposed {001} facets via co-precipitation and hydrothermal method. In-doping did not change the specific surface area, microstructure or light absorption range, only improved the charge separation efficiency. The optimized In-BiOI exhibited an enhanced photocatalytic activity for degradation of p-chloroaniline (PCA) and MO under visible light. In addition, noble metal ion Pt4+ was filled into Bi defects on the BiOCl surface to adjust the surface atoms.72 Pt4+ doping enhanced the light absorption and separation efficiency of electron–hole pairs, so PtO/Pt4+-BiOCl photocatalyst exhibited an excellent degradation performance for removal of sulfamethoxazole (SMZ).
Among the transition metals, Co, Fe, Ti, Mn, W, and Nb have been used for preparing doped BiOX. For example, Wang et al.73 synthesized Co-doped BiOCl nanosheets via hydrothermal method. The Co-doping accelerated the charge transfer by producing a doping energy level in the band gap of BiOCl. Moreover, Co-doped BiOCl exhibited a strong visible light response through extending the light absorption range. When used for degradation of BPA under visible light, Co-doped BiOCl nanosheets presented 3.5 times higher degradation rate than that of BiOCl.
In addition, Fe-doped BiOCl nanosheets were synthesized for degradation of levofloxacin (LVX) under LED light irradiation.74 As shown in Fig. 4a, Fe-doping adjusted the energy band structure, expanded visible light absorption range, and promoted the charge separation. As a result, Fe-doped BiOCl achieved the fast degradation of 95% LVX in 60 min, with a superior reusability for five cycles. Liu et al.75 synthesized Ti-doped BiOI microspheres (Fig. 4b) for degradation of diclofenac (DCF) under visible light. The Ti doping broadened the energy gap through producing more negative conduction band edge, which induced the generation of more ˙O2− radicals during photocatalysis process. The optimized sample removed 99.2% of DCF within 90 min, and the kinetics constant was ∼12 times higher than that of pure BiOI.
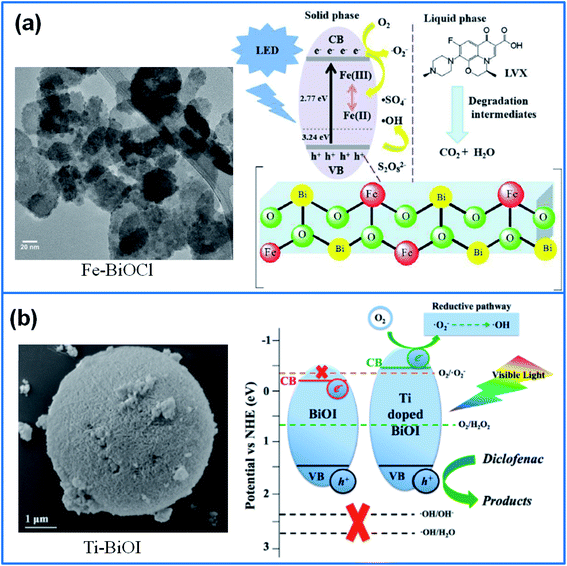 | ||
| Fig. 4 (a) TEM image of Fe-BiOCl nanosheets and their photocatalytic mechanism.74 Copyright 2020 Elsevier. (b) SEM image of Ti doped BiOI and the photocatalytic mechanism.75 Copyright 2019 Elsevier. | ||
Manganese (Mn) can be used as a suitable dopant for BiOCl, due to multiple valence states and low cost. The experiment results confirmed that Mn-doping promoted the separation of photoinduced carriers, and enhanced the light absorption region through forming an intermediate energy level. Moreover, Mn cations in doped BiOCl generated abundant active oxygen species.76 Therefore, Mn-doped BiOCl photocatalysts have been reported for degrading 94.3% metronidazole (MTZ) in 15 min under simulated sunlight, degrading 98% malachite green in 120 min under visible light,77 and removing 58.8% Rh B in 120 min under UV irradiation.78 Therefore, Mn-BiOCl can be used to treat various wastewaters containing antibiotics or organic dyes. Mokhtari et al.79 synthesized W-doped BiOCl nanosheets through a hydrothermal method. W-doped BiOCl exhibited a high photocatalytic activity for degradation of Rh B under both visible light and UV irradiation, due to the reduced band gap and expanded light absorption region. In addition, niobium-doped BiOBr (Nb-BiOBr, Fig. 4b) were prepared for degradation of Rh B and ofloxacin (OFX) under visible light.80 Nb-doping increased the specific surface area, extended the light absorption range, and promoted the separation of photogenerated carriers. The optimized 1.25Nb-BiOBr sample showed the highest photocatalytic activity in degradation of Rh B and OFX. Yu et al.81 synthesized Bi-modified Nb-doped oxygen defective BiOCl microflowers via solvothermal method. The deposited Bi on BiOCl surface acted as the medium for transferring electrons, while the doping of Nb5+ was served as electron traps by adjusting the energy level of the CB of BiOCl. The doping of Nb5+ and modification by Bi0 expanded the visible light absorption, promoted the transfer of electrons and the separation of electron–hole pairs. Therefore, the modified BiOCl with the Nb/Bi of 1/7 delivered a superior photocatalytic activity for degradation of Rh B and Tetracycline (TC) under visible light, and the kinetics constant increased by 134% (Rh B) and 82% (TC), respectively.
In view of the up-conversion luminescence induced by lanthanides ions (Ln-ions),82 lanthanide metals including La, Ce, Y, Yb, and Er, have been used to dope BiOX by combining the photocatalysis with luminescent emission. In this field, Xu et al.83 prepared La-doped BiOCl nano-/micro-structures with ginkgo-leaf-like “petals” based on Ostwald ripening mechanism. The La-doped BiOCl film showed narrow band gap energy, which delivered a superior photocatalytic activity for degradation of Rh B under simulated sunlight. In another work, La3+-doped BiOBr microspheres were fabricated by using 1-hexadecyl-3-methylimidazolium bromide ([C16mim]Br)-assisted solvothermal method.84 La3+ doping reduced the band gap and promoted the fast separation of electron–hole pairs, which presented an enhanced photocatalytic activity for degradation of Rh B and ciprofloxacin (CIP).
In addition, Ce-doped BiOBr nanoplates were prepared via hydrothermal method for degrading Rh B under visible light.85 Ce3+ ions were embedded into BiOBr crystal lattice, which increased the specific surface area, induced the blue shift of the adsorption edge and broadened the band gap of BiOBr. Ce-doped BiOBr achieved the fast degradation of 99.22% Rh B in 40 min, and the kinetic constant was 3 times higher than that of pure BiOBr. Zhong et al.86 synthesized yttrium-doped BiOCl (Y-BiOCl) for removal of tetracycline hydrochloride (TC). The doped Y3+ displaced Bi3+ ions in BiOCl lattices and caused the lattice distortion, which increased the concentration of oxygen defects and holes. Furthermore, Y3+-doping reduced the bandgap energy of BiOCl, and suppressed the recombination of photocarriers. As a result, 15 wt% Y-doped BiOCl degraded 90.3% TC within 60 min under visible light irradiation. Ytterbium ions (Yb3+) doped BiOI 3D nanoflowers were also synthesized via solvothermal method.87 Yb3+ ions doping enabled the BiOI with a capacity to up-convert near-IR light into visible light, and enhanced the separation efficiency of electron–hole pairs. The degradation efficiency of 2%-Yb: BiOI was two times higher than that of pure BiOI for degrading Rh B. Moreover, Yb3+-doped BiOI also showed a high activity for photodegradation of herbicide isoproturon. Peng et al.88 prepared Er3+ doped BiOI nanosheets by using water and ethylene glycol as mixed solvent via microwave-assisted solvothermal route. The doping of Er3+ ions decreased the energy level of BiOI conduction band, which enhanced the electron capture capability of BiOI, and prevented the recombination of the electron–hole pairs. Therefore, Er3+ doped BiOI nanosheets presented an excellent photocatalytic activity for degradation of Rh B and MO under visible light.
In the field of two metals doping, Nussbaum et al.92 synthesized Fe, Nb co-doped BiOCl by a co-precipitation method. The Fe, Nb co-doped BiOCl generated a strong interaction between the adsorbent and adsorbate (dye molecules), which contributed to the enhanced photocatalytic activity for degradation of Rh B. Yu et al.93 synthesized Er3+ and/or Yb3+ ions doped BiOCl via hydrothermal route. With an increase of the Ln doping amount, the thickness of BiOCl sheets decreased to ∼80 nm from ∼140 nm. The BiOCl sheets doped with 2.0% Yb3+ and 0.5% Er3+ showed the highest photocatalytic activity under visible light, which degraded 99.5% Rh B in 20 min. The degradation efficiency was 2.8 times higher than that of pure BiOCl. Niu et al.94 synthesized 3D flower-like Ho3+/Yb3+ and Er3+/Yb3+ co-doped BiOCl photocatalysts via a hydrothermal route respectively. The doping by 0.5% Ho3+/10% Yb3+ and 0.5% Er3+/10% Yb3+ ion pairs reduced the band gap to 2.65 and 2.58 eV from original 3.1 eV of pure BiOCl, which effectively suppressed the recombination of electron–hole pairs. Furthermore, co-doping extended the UV absorption to near infrared light and blue light. Therefore, Ho3+/Yb3+ and Er3+/Yb3+ co-doped BiOCl exhibited a superior photocatalytic performance for removal of Rh B under simulated sunlight and 450 nm blue light.
3.2. Surface modification
Surface modification of BiOX involves a wide research range. For example, we can introduce surface defects or surface terminations on the surface of BiOX. Furthermore, inorganic/organic frameworks, carbon materials, metal particles, and different carriers can be adopted to modify BiOX.In view of the stability and conductivity of noble metals, Ag, Pd, Au, Rh and Pt nanoparticles have been used to decorate BiOX. In this field, Yu et al.113 selectively deposited Ag nanoparticles on different surface regions of BiOCl single-crystal nanosheets, and compared the photocatalytic activity of different Ag/BiOCl samples. The Ag/BiOCl-(UV) sample with Ag nanoparticles deposited on exposed {001} facets presented the highest photocatalytic activity for degradation of MO and phenol, much better that the sample with Ag deposited on the lateral surfaces of BiOCl and the sample with Ag nanoparticles randomly deposited on BiOCl. In addition, Ag and Bi loaded BiOCl (Ag/Bi/BiOCl) microflowers were synthesized by in situ solvothermal method for degradation of Rh B.114 Coexistence of Ag, Bi nanoparticles and oxygen vacancies enhanced the light absorption range and promoted the charge-carrier separation. Therefore, the Ag/Bi/BiOCl showed a higher photocatalytic activity than that of P25 and pure BiOCl under visible light and UV irradiation. Meng et al.115 synthesized palladium (Pd) nanoparticles on BiOBr surface via electrostatic assembly method. A Mott–Schottky junction formed between Pd and BiOBr promoted the separation of photogenerated carriers. Furthermore, the synergetic effect between SPR and Mott–Schottky junction contributed to the enhanced photocatalytic activity for removal of phenol under visible light. Li et al.116 synthesized Au–BiOCl and Pd–BiOCl photocatalysts by loading Au or Pd nanoparticles on ultrathin BiOCl (001) nanosheets for oxidation of benzyl alcohol. After decorating with noble metal nanoparticles, much more oxygen vacancies and active sites were introduced into BiOCl nanosheets, which facilitated the adsorption and activation of alcohols. Moreover, strong interface cooperation was formed between Pd and BiOCl (001) surface by the electronic coupling. The strong surface activity of Pd nanoparticles and abundant oxygen vacancies on BiOCl (001) synergistically improved the adsorption-activation of alcohol molecules and O2, which enhanced the photocatalytic activity of BiOCl. In addition, noble metals (Rh, Pd, Pt)-deposited BiOX (Cl, Br, I) photocatalysts were synthesized via solution-photodeposition method.117 Decorated Pd and Pt existed in metallic state, while Rh existed in both metallic and oxidized states. The decoration of three noble metals reduced the band gaps and inhibited the recombination of the electron–hole pairs. Under the irradiation of UV light, the descending activity order was Pd(0.5%)/BiOBr > Pt(1%)/BiOCl > Pd(2%)/BiOCl. Under the visible light, the activity order changed to Pd(4%)/BiOBr > Pd(0.5%)/BiOI > Rh(1%)/BiOCl.
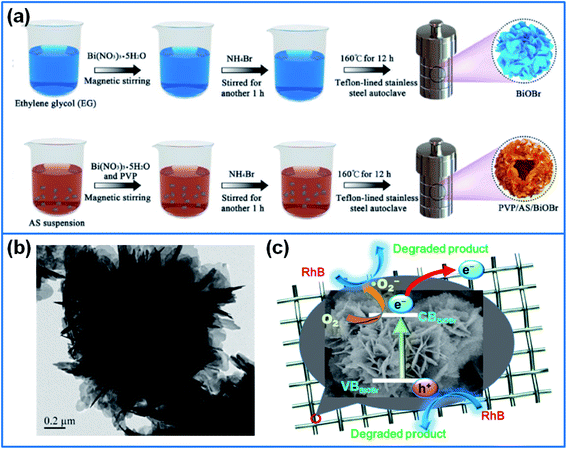 | ||
| Fig. 6 (a) Schematic illustration for preparing BiOBr and PVP/AS/BiOBr composite.119 Copyright 2019 Elsevier. (b) TEM image of flower-like BiOBr, and (c) photodegradation mechanism of Rh B dye by loading BiOBr on metal wire mesh.125 Copyright 2019 Elsevier. | ||
Cui et al.123 synthesized I-deficient BiOI thin film on glass substrate via a solvothermal method under the assistance of ([C6Mim]I) ionic liquid. The calcinations of BiOI-coated substrate produced I-deficient BiOI. The iodine vacancies effectively trapped the photogenerated holes, which inhibited the recombination of photogenerated electron–hole pairs. Therefore, the I-deficient BiOI thin film displayed a superior photocatalytic performance for degradation of BPA, and a good reusability for practical wastewater treatment. Ge et al.124 synthesized BiOBr on superhydrophobic Cu mesh, and BiOBr-decorated Cu mesh exhibited an excellent cycling stability for degradation of Rh B, MB and ciprofloxacin drug. In addition, Chang et al.125 synthesized flower-like BiOBr on stainless steel wire mesh via solvothermal method and calcinations (Fig. 6b and c). After heated at 250 °C, the (001) facets of BiOBr were generated, which reduced the energy gap and enhanced the photocurrent density. The metal mesh/BiOBr showed an enhanced photocatalytic activity for degradation of Rh B.
3.3. Summary
To sum up, the doping and surface modification of BiOX was summarized in this section. The heteroatom doping effectively adjusts the band gap of BiOX and promotes the transfer and separation of photogenerated electron–hole pairs. Among non-metal elements, boron (B), carbon (C), nitrogen (N), sulfur (S) and iodine(I) have been reported as dopants for improving the photocatalytic activity of BiOX. However, up to now, there are no related reports about Si, P, and F doped BiOX, which would become a promising research direction. Besides non-metal dopants, metal dopants including normal metals (Al, Zn, Sn, In, and Pt), transition metals (Co, Fe, Ti, Mn, W, and Nb), and lanthanides metals (La, Ce, Y, Yb, and Er), have been reported for preparing doped BiOX. In view of the up-conversion luminescence of lanthanides ions, Ln-doped BiOX displayed an outstanding photocatalytic activity. However, considering the high cost of rare earth elements, some transition metals show obvious advantage for the high performance and low cost. In addition, dual doping by non-metal and metal or two metals also exhibits an excellent performance for playing the synergistic effect. In addition, surface modification of BiOX involves much more research contents, such as, generation of surface defects or oxygen vacancies, introduction of active sites or hydroxyl groups, decoration with inorganic/organic frameworks, carbon materials, metal particles, and other carriers, and so on. Some metal decorators or conductive carriers have been widely reported to improve the photocatalytic performance of BiOX. In consideration of the reusability in wastewater treatment, free-standing Cu mesh decorated with BiOX shows a promising application prospect for the simple post-treatment.4. Heterojunction of different compounds
Besides heteroatom doping and surface modification, the construction of heterojunction is widely reported for enhancing the photocatalytic activity of single-component BiOX. Compared to single BiOX, the combination of different BiOX species effectively adjusts the band gap energy and increases the visible light absorption capability. Furthermore, the heterojunction achieves the fast transfer of electrons and holes, suppresses the recombination of electron–hole pairs, and enhances the photocatalytic activity. In this section, we classify the BiOX heterojunction into two parts. The first is BiOX/BiOY type heterojunction, including BiOCl/BiOBr, BiOCl/BiOI, BiOBr/BiOI, BiOF/BiOBr, and some ternary composites containing BiOX/BiOY. The second is BiOX/BimOnXz type heterojunction.4.1. BiOX/BiOY heterojunction
Due to the structure similarity of BiOCl and BiOBr, one-step hydrothermal or solvothermal method was used to prepare BiOCl/BiOBr heterojunction, and the morphology and the ratio of BiOCl/BiOBr are the main factors for deciding the photocatalytic activity. In this field, Cui et al.126 synthesized BiOCl nanoparticles on BiOBr nanosheets via hydrothermal method, and the BiOBr/BiOCl hybrid nanosheets with the weight ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exhibited the best photocatalytic activity for degradation of Rh B. Under the assistance of ethylene glycol (EG), Zhang et al.127 prepared nanosheet-assembled BiOCl/BiOBr microspheres (Fig. 7a). Through adjusting the ratio of BiOBr/BiOCl, the composite of 40% BiOCl/BiOBr displayed the best photocatalytic efficiency for degradation of MB under LED light. In addition, 3D hierarchical microspheres of BiOBr/BiOCl were prepared by solvothermal method.128 The BiOBr/BiOCl composite with the mole ratio of 2
1 exhibited the best photocatalytic activity for degradation of Rh B. Under the assistance of ethylene glycol (EG), Zhang et al.127 prepared nanosheet-assembled BiOCl/BiOBr microspheres (Fig. 7a). Through adjusting the ratio of BiOBr/BiOCl, the composite of 40% BiOCl/BiOBr displayed the best photocatalytic efficiency for degradation of MB under LED light. In addition, 3D hierarchical microspheres of BiOBr/BiOCl were prepared by solvothermal method.128 The BiOBr/BiOCl composite with the mole ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 presented the best photodegradation performance for degradation of Rh B, and the degradation rate constant was 4.5 times and 8.8 times higher than pure BiOBr and pure BiOCl, respectively. Li et al.129 synthesized flower-like BiOBr/BiOI composite via solvothermal method and discussed the effect of BiOBr/BiOI molar ratios on the degradation efficiency. The composite with BiOBr/BiOI ratio of 3
3 presented the best photodegradation performance for degradation of Rh B, and the degradation rate constant was 4.5 times and 8.8 times higher than pure BiOBr and pure BiOCl, respectively. Li et al.129 synthesized flower-like BiOBr/BiOI composite via solvothermal method and discussed the effect of BiOBr/BiOI molar ratios on the degradation efficiency. The composite with BiOBr/BiOI ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 delivered the highest degradation activity, which degraded 99.8% Rh B in 80 min under visible light. Jiang et al.130 prepared BiOBr/BiOF composites with different Br/F ratios. As shown in Fig. 7b, the introduction of BiOF reduced the crystalline size and suppressed the recombination of electron–hole pairs, and the synergistic effect between BiOF and BiOBr enhanced the photocatalytic activity for degradation of Rh B and nitrobenzene.
1 delivered the highest degradation activity, which degraded 99.8% Rh B in 80 min under visible light. Jiang et al.130 prepared BiOBr/BiOF composites with different Br/F ratios. As shown in Fig. 7b, the introduction of BiOF reduced the crystalline size and suppressed the recombination of electron–hole pairs, and the synergistic effect between BiOF and BiOBr enhanced the photocatalytic activity for degradation of Rh B and nitrobenzene.
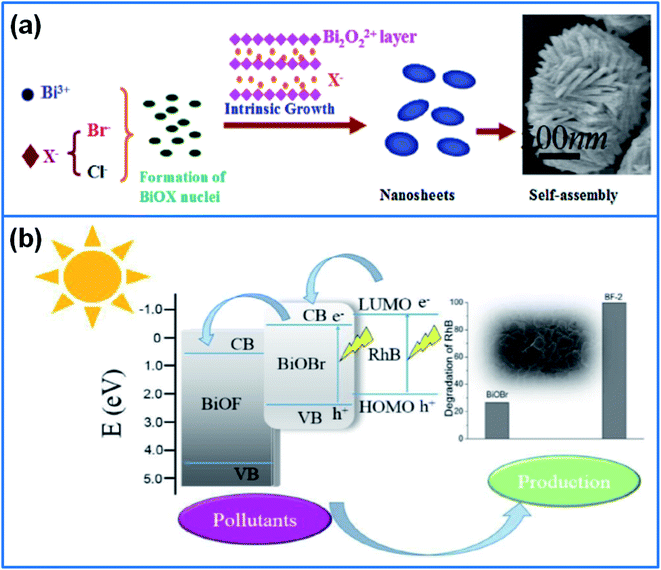 | ||
| Fig. 7 (a) Schematic illustration for fabricating BiOCl/BiOBr microsphere.127 Copyright 2018 Elsevier. (b) Schematic illustration of the charge transfer and separation of BiOBr/BiOF heterojunction.130 Copyright 2017 Elsevier. | ||
Compared to inorganic KCl, KBr or KI precursors, Cl-, Br- or I-containing ionic liquids can be served as precursor, solvent and template for synthesizing BiOX. Under the presence of 1-hexadecyl-3-methylimidazolium chloride ([C16mim]Cl) and 1-hexadecyl-3-methylimidazolium bromide ([C16mim]Br) ionic liquids, Zhang et al.131 synthesized BiOCl/BiOBr flower-like microspheres via solvothermal method. This composite achieved the complete decomposition of Rh B, due to the excellent visible light absorption capability. In addition, Yang et al.132 synthesized flower-like BiOBr/BiOCl heterojunction by using 1-allyl-3-methylimidazolium bromide ([AMIm]Br) ionic liquid and KCl as reactants. When used for degradation of Rh B, the photocatalytic performance of BiOBr/BiOCl composite was six times higher than that of pure BiOCl. By using 1-propyl-3-methylimidazolium iodide ([PrMIm]I) ionic liquid (IL) as solvent and I source, Liu et al.133 prepared BiOI/BiOCl heterojunction photocatalysts via a hydrothermal method. Compared to pure BiOCl and BiOI, BiOI/BiOCl heterojunction presented a higher separation efficiency of photo-generated charge pairs. When the mass ratio of [PrMIm]I/BiOCl is 8%, the composite showed the highest photocatalytic activity for degradation of Rh B under simulated sunlight, and the degradation rate was 7.7 and 5.8 times higher than that of BiOCl and BiOI.
In addition, self-assembled BiOI/BiOCl microflowers134 and hierarchical porous flower-like BiOI/BiOCl composite were synthesized by a template-free method.135 In this hierarchical porous BiOI/BiOCl composite, tri-model mesopores were formed by the aggregation of self-assembled nanoplates. Due to the large surface area and pore volume, and modified band structure, this composite displayed an enhanced visible light activity for removal of NO in air.
Besides hydrothermal or solvothermal method, microwave-assisted precipitation method was used to synthesize BiOCl/BiOBr composite.136 Through adjusting the molar ratios of Cl/Br, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 BiOCl/BiOBr composite presented the best visible-light photocatalytic activity for degradation of Rh B, due to the strong visible light absorption capability and the low recombination rate of electron–hole pairs. Through doping with I− ions, flower-like I-BiOCl/I-BiOBr composite was synthesized by a deposition–precipitation method.137 The doping of I− ions not only enhanced the visible light absorption capability of BiOCl and BiOBr, but also formed type-II heterojunction through adjusting their energy level of valence bands. Due to the strong visible light absorption and efficient separation of electrons and holes, the 20% I-BiOCl/I-BiOBr composite displayed the highest photocatalytic activity for degradation of MO and phenol under visible light.
5 BiOCl/BiOBr composite presented the best visible-light photocatalytic activity for degradation of Rh B, due to the strong visible light absorption capability and the low recombination rate of electron–hole pairs. Through doping with I− ions, flower-like I-BiOCl/I-BiOBr composite was synthesized by a deposition–precipitation method.137 The doping of I− ions not only enhanced the visible light absorption capability of BiOCl and BiOBr, but also formed type-II heterojunction through adjusting their energy level of valence bands. Due to the strong visible light absorption and efficient separation of electrons and holes, the 20% I-BiOCl/I-BiOBr composite displayed the highest photocatalytic activity for degradation of MO and phenol under visible light.
In addition, Lin et al.138 synthesized BiOI/BiOBr composite photocatalyst via a facile anion-exchange method through replacing partial of Br− by I−. The BiOI/BiOBr heterojunction presented much higher photocurrent intensity than pristine BiOI and BiOBr, which contributed to the higher photocatalytic activity for degradation of MO dye under visible light. Jamil et al.139 synthesized BiOI0.5Br0.5 heterojunction by a facile chemical etching method. When used for degradation of Lindane pesticide, the heterojunction presented much higher photocatalytic activity than pure BiOBr and BiOI. Bai et al.140 prepared BiOBrxI1−x/BiOBr heterojunction photocatalyst by coupling BiOBrxI1−x with BiOBr monomer. The BiOBrxI1−x/BiOBr exhibited a high molecular oxygen activation capacity, which enhanced the photocatalytic performance for degradation of Rh B, phenol and BPA under visible light.
Oxygen vacancies (OVs) could increase the surface dangling bonds and active sites on the BiOX. Moreover, the defects induced by OVs effectively suppress the recombination of electron–hole pairs.141 In view of the function of OVs, Liu et al.142 synthesized BiOCl/BiOBr heterojunction with rich OVs via ultrasound and photoinduced method. Abundant OVs in BiOCl/BiOBr provided active sites for photocatalytic reaction, and enhanced the light absorption range. Furthermore, the interface electric field derived from BiOCl/BiOBr heterostructure accelerated the electron transfer and inhibited the recombination of photogenerated electron–hole pairs. Therefore, the composite presented a high photocatalytic activity for degradation of carbamazepine under visible light. In addition, under the assistance of PVP K30, ultrathin BiOBr/BiOI (BiOBr/BiOI-U) photocatalyst with OVs was synthesized via a solvothermal method,143 and the BiOBr/BiOI-U composite exhibited high photocatalytic efficiency for removal of NO.
Besides abundant experimental studies, density functional calculations were adopted to investigate the structural, electronic, and optical properties of BiOX/BiOY heterojunction systems, including BiOF, BiOCl, BiOBr, and BiOI.144 The results confirmed that all the BiOX/BiOY superlattice systems were indirect bandgap semiconductors, and the bandgap of BiOX/BiOY system was between two bandgap values of BiOX and BiOY. The maximum absorption wavelength had a sequence of BiOF < BiOF/BiOCl < BiOCl < BiOF/BiOBr < BiOCl/BiOBr < BiOBr < BiOF/BiOI < BiOCl/BiOI < BiOBr/BiOI < BiOI. The bandgap of BiOF/BiOI, BiOCl/BiOBr, BiOCl/BiOI, and BiOBr/BiOI was 2.74, 2.99, 2.30, and 2.23 eV, respectively. The performance comparison of different heterojunctions provided guidelines for the design of high-active heterojunctions.
In order to further improve the activity of BiOX heterojunction, some carriers or decorators, such as, conductive carbon materials, cellulose, noble metal and stainless steel wire mesh have been used for preparing ternary composites. Among carbon materials, carbon quantum dots (CQDs) has superior electron transfer/reservoir properties, and photoluminescence (PL) up-conversion effect, which can be used to expand the light utilization range and promote photo-induced electron transfer.145 Zhao et al.146 synthesized BiOBr/BiOCl/CQDs heterostructure microspheres via a solvothermal method. When used for degradation of Rh B, the BiOBr/BiOCl/CQDs-4% exhibited a high photocatalytic activity under visible light, and the photodegradation rate was 2.8 times higher than that of BiOBr/BiOCl, due to the strong light harvesting capacity and outstanding electron transferability between BiOCl and CQDs. Besides CQDs, Su et al.147 synthesized BiOCl/BiOI flower-like hollow microspheres on rGO sheets for photodegradation of Rh B. The introduction of GO changed the morphology of 50% BiOCl/BiOI from solid microspheres to hollow microspheres, which enhanced the light absorption. Moreover, the high conductivity of rGO and the strong interfacial interaction between rGO and BiOCl/BiOI effectively promoted the separation of photogenerated carriers. Therefore, the incorporation of rGO showed over twice degradation rate than that of pristine 50% BiOCl/BiOI under visible light.
Due to the remarkable compatibility between cellulose and BiOBr/BiOI, Du et al.148 prepared BiOBr/BiOI/cellulose composite by using pulp board as the cellulose source. Meanwhile, the combination of three components broadened the visible light absorption, and improved the migration efficiency of the electron–holes. So BiOBr/BiOI/cellulose composite displayed a high degradation activity for removal of Rh B, fluorescein dye (FL) and TCH under visible light.
Considering the function of noble metal in improving the photocatalytic activity, Ren et al.149 developed flower-like Pd/BiOCl/BiOI photocatalyst via a solvothermal method. The deposition of Pd on BiOCl/BiOI suppressed the recombination of photoinduced electrons and holes, and enhanced the photodegradation performance. The composite of 3% Pd/BiOCl/BiOI displayed the best photocatalytic activity for degradation of Rh B under the irradiation of 350 W Xe lamp. In order to improve the reusability of photocatalysts, Wang et al.150 prepared BiOI/BiOBr heterostructure film on 304 stainless steel wire mesh. The free-standing composite film prepared with 50 mM TBAB exhibited high photodegradation efficiency for removal of Rh B. Meanwhile, this heterostructure film showed high mechanical strength and cycling stability.
4.2. BiOX/BimOnXz heterojunction
In the family of bismuth oxyhalide, besides the BiOX (X = F, Cl, Br, I), newly discovered non-stoichiometric bismuth oxyhalides (BimOnXz) have been reported, such as, Bi24O31Br10,151 Bi12O17Cl2,152 Bi3O4Br,153 Bi5O7I,154 and Bi12O15Cl6.155 Recently, BiOX/BimOnXz composites have been developed for enhancing the photocatalytic activity. In this field, a novel 3D flower-like Bi3O4Cl/BiOCl heterostructure was prepared by a co-precipitation method for degradation of antibiotic drug.156 The composite exhibited a high photocatalytic activity in visible light range for the efficient charge-separation properties, which degraded 87% levofloxacin in 180 min. In addition, mesoporous-mixed-phase bismuth oxychlorides was fabricated by using one-pot sorbitol-nitrate solution autocombustion method,157 and the intra-heterojunction containing 59% BiOCl and 41% Bi24O31Cl10 displayed a high activity for degradation of antibiotic ofloxacin, due to the enhanced light absorption and surface reactions by a high porosity.Liu et al.158 synthesized Bi3O4Br/BiOCl heterojunction photocatalyst. The composite presented a high photocatalytic activity in degradation of Rh B, and the degradation rate was 6.3 and 3.1 times higher than that of pure Bi3O4Br and BiOCl. Wang et al.159 prepared heterostructured 2D/2D BiOBr/Bi12O17Cl2 photocatalysts via a chemical deposition–precipitation method (Fig. 8a). The BiOBr/Bi12O17Cl2 heterostructure presented a high photocatalytic activity for degradation of MO, 4-chlorophenol (4-CP) and TC under visible light. Heidari et al.160 synthesized 3D-flower-like BiOCl/BiOBr/Bi24O31Br10 composite via sono-assisted solvothermal method (Fig. 8b). The generated type-II heterojunction enhanced the photocatalytic activity for removal of levofloxacin, ofloxacin, norfloxacin and ciprofloxacin.
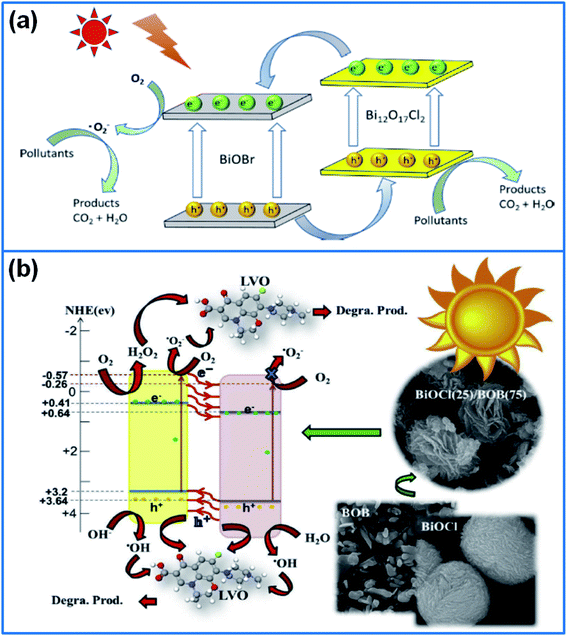 | ||
| Fig. 8 (a) Schematic illustration for the transport and separation of the photo-induced electron–hole pairs and the degradation mechanism of BiOBr/Bi12O17Cl2 composite.159 Copyright 2020 Elsevier. (b) Degradation mechanism for removal of levofloxacin by using 3D flower-like BiOCl(25)/BOB(75) heterojunction photocatalysts.160 Copyright 2020 Elsevier. | ||
Sun et al.161 synthesized Bi4O5BrxI2−x photocatalysts by a facile precipitation method, and discussed the influence of I/Br molar ratio on the photocatalytic performance. When used for decomposing resorcinol, o-phenylphenol, and 4-tert-butylphenol, the Bi4O5Br0.6I1.4 sample presented the best activity, due to the enhanced charge separation efficiency and enlarged visible light absorption. In another work, Du et al.162 synthesized BiOClxBr1−x sheets with different Cl/Br ratios via a precipitation method, and the composite with Cl/Br ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 presented the highest activity for degradation of Rh B. The degradation ratio reached 99% in 30 min under visible light irradiation. Moreover, Xu et al.163 confirmed that the BiOCl0.5Br0.5 nanoplates had the highest photocatalytic activity for degradation of Rh B under simulated sunlight. Considering the influence of microstructure on the photocatalytic activity, Kim et al.164 synthesized 3D flower-like and 2D plate-like BiOClxI1−x with different Cl/I ratios respectively. With an increase of I content, the specific surface area and adsorption ability of BiOClxI1−x greatly increased. When used for degradation of MO and Rh B under visible light, the sample with flower-like structure showed a better adsorption and photodegradation performance than 2D plate-like structure.
1 presented the highest activity for degradation of Rh B. The degradation ratio reached 99% in 30 min under visible light irradiation. Moreover, Xu et al.163 confirmed that the BiOCl0.5Br0.5 nanoplates had the highest photocatalytic activity for degradation of Rh B under simulated sunlight. Considering the influence of microstructure on the photocatalytic activity, Kim et al.164 synthesized 3D flower-like and 2D plate-like BiOClxI1−x with different Cl/I ratios respectively. With an increase of I content, the specific surface area and adsorption ability of BiOClxI1−x greatly increased. When used for degradation of MO and Rh B under visible light, the sample with flower-like structure showed a better adsorption and photodegradation performance than 2D plate-like structure.
4.3. Summary
In order to further enhance the photocatalytic activity of single-component BiOX, the heterojunction of BiOX have been widely developed in recent years. According to the component of heterojunction, we classify the BiOX heterojunction into BiOX/BiOY and BiOX/BimOnXz type heterojunction. In the section of BiOX/BiOY heterojunction, BiOCl/BiOBr, BiOCl/BiOI, BiOBr/BiOI, and BiOBr/BiOF, have been summarized and applied for degradation of organic pollutants. The selection of halide and their ratio are the research topics for deciding the photocatalytic activity. Moreover, some carriers or decorators including carbon quantum dots, rGO, cellulose, noble metal Pd and stainless steel wire mesh, have been hybridized with BiOX/BiOY heterojunction. These decorated heterojunctions usually exhibit superior electron transfer/separation properties and visible light absorption ability. The results confirm that the decoration of BiOX/BiOY heterojunction is another effective way for enhancing the photocatalytic activity. Different from BiOX, BimOnXz has different ratios of Bi/O/X, abundant active sites and oxygen vacancies, which facilitate the enhancement of photocatalytic activity. There are more types of BimOnXz species, such as, Bi24O31Br10, Bi12O17Cl2, Bi3O4Br, Bi3O4Br, BiOClxBr1−x, BiOClxI1−x, Bi4O5BrxI2−x, and so on. When hybridized with these special BimOnXz species, the BiOX/BimOnXz heterojunctions present outstanding photocatalytic activity for removal of organic dyes and pharmaceuticals, even better than traditional BiOX/BiOY heterojunction. Therefore, developing novel BimOnXz species and BiOX/BimOnXz heterojunctions is a promising prospect for designing high-performance BiOX photocatalysts.5. Conclusions and prospects
In view of the important role of BiOX in semiconductor photocatalysts, herein, we summarize the recent advances on BiOX photocatalysts, including the controllable synthesis, doping and surface modification, and heterojunction of different BiOX species. Crystal structure, size and specific surface area are the main factors for deciding the photocatalytic activity of BiOX. While synthetic methods decide the physical properties of BiOX, so the synthetic methods of BiOX are introduced firstly, in which, seven methods are classified, including hydrothermal, solvothermal, hydrolysis method, precipitation method, two-phase method, ultrasonic/microwave-assisted method and physical method. Among these synthetic methods, hydrothermal and solvothermal method are most popular for the uniform morphology and easy operation. In the hydrothermal/solvothermal reaction, reaction conditions (temperature and time), halide precursors, surfactants, and solvent systems greatly affect the microstructure and performance of BiOX. The preferred morphology is thin BiOX nanosheets with highly exposed (010) facets, or hierarchical BiOX spheres/flowers with a large specific surface area. In addition, microwave-assisted method can be adopted to shorten the reaction time and improve production efficiency. Compared to solution-based synthetic methods, calcinations method and mechanical grinding method show some advantages in the industrial-scale production. In the second part, we introduce the doping and surface modification of BiOX. The doping is introducing heteroatoms into the crystal planes of BiOX, which effectively adjusts the band gap and enhances the visible light absorption capability. The doping elements and doping dosage greatly affect the photocatalytic activity of BiOX. Various non-metal elements, including B, C, N, S and I, have been used for doping different BiOX species. Compared to non-metals, metal elements including Al, Zn, Sn, In, Pt, Co, Fe, Ti, Mn, W, Nb, and lanthanides metal (La, Ce, Y, Yb, and Er) have been reported for preparing metal-doped BiOX. In addition, dual-doping has been reported by introducing non-metal and metal or two metals, such as, Bi quantum dots implanted C-doped BiOCl, Ag and carbon dots co-doped BiOI microspheres, Fe and N co-doped BiOBr, Fe, Nb co-doped BiOCl, Er3+ an Yb3+ ions doped BiOCl, and Ho3+/Yb3+ and Er3+/Yb3+ co-doped BiOCl. Compared to non-metal doping or metal doping, dual doping of BiOX achieves the synergistic effect of two dopants. Moreover, dual-doping provides much more choices for developing novel doped BiOX. Different from heteroatom doping, surface modification introduces different surface terminations on the surface of BiOX, or decorate BiOX with different carriers. For example, alcohols or amines modified BiOX exhibits a good hydrophilic feature, which facilitates the adsorption of organic molecules. Furthermore, oxygen vacancies or defects generated on BiOX would greatly enhance the photocatalytic activity. Various carriers, including inorganic/organic frameworks, carbon materials, metal nanoparticles, cellulose, sepiolite, conductive polymers, glass substrate, metal mesh have been reported for loading BiOX photocatalysts. The decoration not only adjusts the microstructure, increases the specific surface area of BiOX, but also promotes the transfer and separation of photogenerated carriers and charges, further enhancing the photocatalytic performance. Among the carriers, free-standing Cu mesh can be used for preparing self-supporting photocatalysts, which exhibits an excellent reusability in wastewater treatment. In the third section, different BiOX heterojunctions are summarized, including BiOX/BiOY heterojunction and BiOX/BimOnXz heterojunction. In the field of BiOX/BiOY heterojunctions, BiOCl/BiOBr, BiOCl/BiOI, BiOBr/BiOI, and BiOBr/BiOF have been synthesized and applied for degradation of organic dyes and antibiotics. The halide types and their ratio of BiOX/BiOY greatly affect the photocatalytic performance. Furthermore, some carriers or decorators including carbon quantum dots, rGO, cellulose, noble metal Pd and stainless steel wire mesh, have been hybridized with BiOX/BiOY heterojunction to fabricate ternary composite photocatalysts. These ternary composites exhibit superior electron transfer/separation properties and visible light absorption ability, much better than that of BiOX/BiOY. Different from BiOX, BimOnXz has different ratios of Bi/O/X. Abundant active sites and oxygen vacancies enable the BimOnXz with much higher photocatalytic activity. Up to now, Bi24O31Br10, Bi12O17Cl2, Bi3O4Br, Bi3O4Br, BiOClxBr1−x, BiOClxI1−x and Bi4O5BrxI2−x, have been reported for hybridizing with BiOX, and exhibit a superior photocatalytic activity.Up to now, a great progress has been achieved in the field of BiOX photocatalysts. However, there are some tough problems to be solved, such as, the controllable synthesis of BiOX, most efficient modification method, and the relationship between the microstructure and photocatalytic activity. These existing problems just provide potential research directions, which can be summarized as following:
(1) Developing novel synthetic methods for preparing BiOX photocatalysts with particular microstructures. The preferred morphologies include hierarchical flowers/spheres with high specific surface area, ultrathin nanosheets with highly exposed {001} facets, and hollow nanostructure. In addition, the cost of raw materials, preparation periods and mass-production are the essential factors for consideration.
(2) Effective doping or modification on the facet or surface of BiOX. Heteroatom doping and surface modification are effective strategies for enhancing the photocatalytic activity of BiOX. Based on existing reports, the main tasks are developing dual-doped BiOX and selecting suitable carriers for hybridizing with BiOX.
(3) Developing novel BimOnXz species and BiOX/BimOnXz heterojunctions. BimOnXz displays much higher photocatalytic activity than traditional BiOX for rich active sites and oxygen vacancies. Therefore, developing novel BimOnXz species and BiOX/BimOnXz heterojunctions is a promising research topic.
(4) Disclosing the relationship between microstructure and photocatalytic performance. Theory computation about the band gap and energy level of BiOX would provide theoretical instruction for designing the microstructure and selecting suitable matching component. Therefore, the combination of experiment and computation is essential for developing BiOX-based photocatalyts.
At last, photocatalysis is an environmental-friendly strategy for solving the wastewater problem. In view of the high photocatalytic activity of BiOX, the development of BiOX-based photocatalysts is becoming a major task in photocatalytic field, which would greatly promote the development of environmental protection industries.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by National Natural Science Foundation of China (No. 51403094).References
- X. Wei, M. U. Akbar, A. Raza and G. Li, Nanoscale Adv., 2021, 3, 3353 RSC.
- J. Di, J. Xia, H. Li, S. Guo and S. Dai, Nano Energy, 2017, 41, 172 CrossRef CAS.
- P. Raizada, A. Sudhaik, P. Singh, P. Shandilya, V. K. Gupta, A. Hosseini-Bandegharaei and S. Agrawal, J. Photochem. Photobiol., A, 2019, 374, 22 CrossRef CAS.
- P. Singh, P. Shandilya, P. Raizada, A. Sudhaik, A. Rahmani-Sani and A. Hosseini-Bandegharaei, Arabian J. Chem., 2020, 13, 3498 CrossRef CAS.
- A. Kumar, P. Raizada, P. Singh, R. V. Saini, A. K. Saini and A. Hosseini-Bandegharaei, Chem. Eng. J., 2020, 391, 123496 CrossRef CAS.
- P. Raizada, A. Sudhaik, P. Singh, A. Hosseini-Bandegharaei and P. Thakur, Sep. Purif. Technol., 2019, 227, 115692 CrossRef CAS.
- V. Dutta, P. Singh, P. Shandilya, S. Sharma, P. Raizada, A. K. Saini, V. K. Gupta, A. Hosseini-Bandegharaei, S. Agarwal and A. Rahmani-Sani, J. Environ. Chem. Eng., 2019, 7, 103132 CrossRef CAS.
- L. Ye, Y. Su, X. Jin, H. Xie and C. Zhang, Environ. Sci.: Nano, 2014, 1, 90 RSC.
- A. Kumar, P. Raizada, V. Kumar Thakur, V. Saini, A. Aslam Parwaz Khan, N. Singh and P. Singh, Chem. Eng. Sci., 2021, 230, 116219 CrossRef CAS.
- G. Boczkaj and A. Fernandes, Chem. Eng. J., 2017, 320, 608–633 CrossRef CAS.
- A. Kumar, P. Raizada, A. Hosseini-Bandegharaei, V. K. Thakur, V. H. Nguyen and P. Singh, J. Mater. Chem. A, 2021, 9, 111 RSC.
- Sonu, V. Dutta, S. Sharma, P. Raizada, A. Hosseini-Bandegharaei, V. Kumar Gupta and P. Singh, J. Saudi Chem. Soc., 2019, 23, 1119 CrossRef CAS.
- J. Zhan, J. Wu, P. Lu, Q. Liu, T. Huang, H. Tian, R. Zhou, J. Ren, B. Yuan and X. Sun, Mater. Lett., 2017, 186, 353–356 CrossRef.
- X. Zhang, R. Li, M. Jia, S. Wang, Y. Huang and C. Chen, Chem. Eng. J., 2015, 274, 290–297 CrossRef CAS.
- P. Intaphong, A. Phuruangrat, K. Karthik, P. Dumrongrojthanath, T. Thongtem and S. Thongtem, J. Inorg. Organomet. Polym., 2020, 30, 714–721 CrossRef CAS.
- H. Feng, Z. Xu, L. Wang, Y. Yu, D. Mitchell, D. Cui, X. Xu, J. Shi, T. Sannomiya and Y. Du, ACS Appl. Mater. Interfaces, 2015, 7, 27592–27596 CrossRef CAS.
- L. Dou, D. Ma, J. Chen, J. Li and J. Zhong, J. Solid State Sci., 2019, 90, 1–8 CrossRef CAS.
- Z. Zou, H. Xu, D. Li, J. Sun and D. Xia, Appl. Surf. Sci., 2019, 463, 1011–1018 CrossRef CAS.
- K. Li, Y. Liang, J. Yang, Q. Gao, Y. Zhu, S. Liu, R. Xu and X. Wu, J. Alloys Compd., 2017, 695, 238–249 CrossRef CAS.
- Y. Cai, D. Li, J. Sun, M. Chen, Y. Li, Z. Zou, H. Zhang, H. Xu and D. Xia, Appl. Surf. Sci., 2018, 439, 697–704 CrossRef CAS.
- X. Gao, W. Peng, G. Tang, Q. Guo and Y. Luo, J. Alloys Compd., 2018, 757, 455–465 CrossRef CAS.
- J. Tian, Z. Chen, X. Deng, Q. Sun, Z. Sun and W. Li, Appl. Surf. Sci., 2018, 453, 373–382 CrossRef CAS.
- A. C. Mera, H. Váldes, F. J. Jamett and M. Meléndrez, Solid State Sci., 2017, 65, 15–21 CrossRef CAS.
- A. C. Mera, Y. Moreno, D. Contreras, N. Escalona, M. F. Meléndrez, R. V. Mangalaraja and H. D. Mansilla, Solid State Sci., 2017, 63, 84–92 CrossRef CAS.
- Z. Jiang, X. Liang, Y. Liu, T. Jing, Z. Wang, X. Zhang, X. Qin, Y. Dai and B. Huang, Appl. Catal., B, 2017, 211, 252–257 CrossRef CAS.
- X. Zhang, C. Y. Wang, L. W. Wang, G. X. Huang, W. K. Wang and H. Q. Yu, Sci. Rep., 2016, 6, 1–10 CrossRef CAS.
- H. Zhao, X. Liu, Y. Dong, Y. Xia and H. Wang, Appl. Catal., B, 2019, 256, 117872 CrossRef CAS.
- X. Gao, Q. Guo, G. Tang, W. Zhu and Y. Luo, J. Solid State Chem., 2019, 277, 133–138 CrossRef CAS.
- H. Xing, H. Ma, Y. Fu, X. Zhang, X. Dong and X. Zhang, J. Renewable Sustainable Energy, 2015, 7, 063120 CrossRef.
- X. X. Wei, B. Cui, X. Wang, Y. Cao, L. Gao, S. Guo and C. M. Chen, CrystEngComm, 2019, 21, 1750–1757 RSC.
- J. Hu, S. Weng, Z. Zheng, Z. Pei, M. Huang and P. Liu, J. Hazard. Mater., 2014, 264, 293–302 CrossRef CAS.
- A. Dehghan, M. H. Dehghani, R. Nabizadeh, N. Ramezanian, M. Alimohammadi and A. A. Najafpoor, Chem. Eng. Res. Des., 2018, 129, 217–230 CrossRef CAS.
- S. Q. Guo, X. H. Zhu, H. J. Zhang, B. C. Gu, W. Chen, L. Liu and P. J. Alvarez, Environ. Sci. Technol., 2018, 52, 6872–6880 CrossRef CAS.
- Y. Xu, X. Hu, H. Zhu and J. Zhang, J. Mater. Sci., 2016, 51, 4342–4348 CrossRef CAS.
- G. J. Lee, Y. C. Zheng and J. J. Wu, Catal. Today, 2018, 307, 197–204 CrossRef CAS.
- J. Hu, X. Jing, L. Zhai, J. Guo, K. Lu and L. Mao, Chemosphere, 2019, 220, 77–85 CrossRef CAS.
- Z. Song, X. Dong, N. Wang, L. Zhu, Z. Luo, J. Fang and C. Xiong, Chem. Eng. J., 2017, 317, 925–934 CrossRef CAS.
- D. Zhang, L. Chen, C. Xiao, J. Feng, L. Liao, Z. Wang and T. Wei, J. Nanomater., 2016, 2016, 5697672 Search PubMed.
- Q. L. Yuan, Y. Zhang, H. Y. Yin, Q. L. Nie and W. W. Wu, J. Exp. Nanosci., 2016, 11, 359–369 CrossRef CAS.
- R. Li, X. Gao, C. Fan, X. Zhang, Y. Wang and Y. Wang, Appl. Surf. Sci., 2015, 355, 1075–1082 CrossRef CAS.
- J. Lu, J. Wu, W. Xu, H. Cheng, X. Qi, Q. Li, Y. Zhang, Y. Guan, Y. Ling and Z. Zhang, Mater. Lett., 2018, 219, 260–264 CrossRef CAS.
- J. C. Ahern, R. Fairchild, J. S. Thomas, J. Carr and H. H. Patterson, Appl. Catal., B, 2015, 179, 229–238 CrossRef CAS.
- A. Zhang, W. Xing, D. Zhang, H. Wang, G. Chen and J. Xiang, Catal. Commun., 2016, 87, 57–61 CrossRef CAS.
- H. Huang, X. Li, X. Han, N. Tian, Y. Zhang and T. Zhang, Phys. Chem. Chem. Phys., 2015, 17, 3673–3679 RSC.
- L. Wang, J. Shang, W. Hao, S. Jiang, S. Huang, T. Wang, Z. Sun, Y. Du, S. Dou, T. Xie, D. Wang and J. Wang, Sci. Rep., 2014, 4, 7384 CrossRef PubMed.
- Z. Xu, Ferroelectrics, 2018, 527, 37–43 CrossRef CAS.
- Z. Wang, Z. Chu, C. Dong, Z. Wang, S. Yao, H. Gao, Z. Liu, Y. Liu, B. Yang and H. Zhang, ACS Appl. Nano Mater., 2020, 3, 1981–1991 CrossRef CAS.
- P. Intaphong, A. Phuruangrat, S. Thongtem and T. Thongtem, Mater. Lett., 2018, 213, 88–91 CrossRef CAS.
- J. M. Montoya-Zamora, A. Martínez-de la Cruz and E. L. Cuéllar, Res. Chem. Intermed., 2017, 43, 2545–2563 CrossRef CAS.
- Y. Miao, Z. Lian, Y. Huo and H. Li, Chin. J. Catal., 2018, 39, 1411–1417 CrossRef CAS.
- Z. Chen, J. Zeng, J. Di, D. Zhao, M. Ji, J. Xia and H. Li, Green Energy Environ., 2017, 2, 124–133 CrossRef.
- M. Gao, D. Zhang, X. Pu, H. Li, J. Li, X. Shao and K. Ding, Mater. Lett., 2015, 140, 31–34 CrossRef CAS.
- V. J. Babu, M. Sireesha, R. S. R. Bhavatharini and S. Ramakrishna, Mater. Lett., 2016, 169, 50–53 CrossRef CAS.
- H. Yu, H. Huang, K. Xu, W. Hao, Y. Guo, S. Wang, X. Shen, S. Pan and Y. Zhang, ACS Sustainable Chem. Eng., 2017, 5, 10499–10508 CrossRef CAS.
- Y. Long, Q. Han, Z. Yang, Y. Ai, S. Sun, Y. Wang, Q. Liang and M. Ding, J. Mater. Chem. A, 2018, 6, 13005–13011 RSC.
- X. C. Song, Y. F. Zheng, H. Y. Yin, J. N. Liu and X. D. Ruan, New J. Chem., 2016, 40, 130–135 RSC.
- C. Yu, H. He, Q. Fan, W. Xie, Z. Liu and H. Ji, Sci. Total Environ., 2019, 694, 133727 CrossRef CAS PubMed.
- Z. Liu, J. Liu, H. Wang, G. Cao and J. Niu, J. Colloid Interface Sci., 2016, 463, 324–331 CrossRef CAS PubMed.
- M. M. Obeid, C. Stampfl, A. Bafekry, Z. Guan, H. R. Jappor, C. V. Nguyen, M. Naseri, D. M. Hoat, N. N. Hieu, A. E. Krauklis, T. V. Vu and D. Gogova, Phys. Chem. Chem. Phys., 2020, 22, 15354–15364 RSC.
- L. Zeng, F. Zhe, Y. Wang, Q. Zhang, X. Zhao, X. Hu, Y. Wu and Y. He, J. Colloid Interface Sci., 2019, 539, 563–574 CrossRef CAS.
- S. Qu, Y. Xiong and J. Zhang, J. Colloid Interface Sci., 2018, 527, 78–86 CrossRef CAS.
- G. Jiang, X. Li, Z. Wei, T. Jiang, X. Du and W. Chen, Powder Technol., 2014, 260, 84–89 CrossRef CAS.
- J. Shang, T. Chen, X. Wang, L. Sun and Q. Su, Chem. Phys. Lett., 2018, 706, 483–487 CrossRef CAS.
- Z. Jiang, Y. Liu, T. Jing, B. Huang, Z. Wang, X. Zhang, X. Qin and Y. Dai, RSC Adv., 2015, 5, 47261–47264 RSC.
- C. Y. Wang, Q. Zeng and G. Zhu, Chemosphere, 2021, 268, 128854 CrossRef CAS PubMed.
- L. Zhang, F. Liu, X. Xiao, X. Zuo and J. Nan, Environ. Sci. Pollut. Res., 2019, 26, 28871–28883 CrossRef CAS.
- J. Zhang, K. Zhu, Y. Zhu, C. Qin, L. Liu, D. Liu, Y. Wang, W. Gan, X. Fu and H. Hao, Chem. Phys. Lett., 2020, 750, 137483 CrossRef CAS.
- W. T. Li, W. Z. Huang, H. Zhou, H. Y. Yin, Y. F. Zheng and X. C. Song, J. Alloys Compd., 2015, 638, 148–154 CrossRef CAS.
- J. Guo, X. Liao, M. H. Lee, G. Hyett, C. C. Huang, D. W. Hewak, S. Mailis, W. Zhou and Z. Jiang, Appl. Catal., B, 2019, 243, 502–512 CrossRef CAS.
- F. Xie, X. Mao, C. Fan and Y. Wang, Mater. Sci. Semicond. Process., 2014, 27, 380–389 CrossRef CAS.
- H. Li, Z. Yang, J. Zhang, Y. Huang, H. Ji and Y. Tong, Appl. Surf. Sci., 2017, 423, 1188–1197 CrossRef CAS.
- J. Liu, D. Li, R. Li, Y. Wang, Y. Wang and C. Fan, Chem. Eng. J., 2020, 395, 123954 CrossRef CAS.
- C. Y. Wang, Y. J. Zhang, W. K. Wang, D. N. Pei, G. X. Huang, J. J. Chen, X. Zhang and H. Q. Yu, Appl. Catal., B, 2018, 221, 320–328 CrossRef CAS.
- X. Zhong, K. X. Zhang, D. Wu, X. Y. Ye, W. Huang and B. X. Zhou, Chem. Eng. J., 2020, 383, 123148 CrossRef CAS.
- F. Liu, J. Liang, L. Chen, M. Tong and W. Liu, J. Mol. Liq., 2019, 275, 807–814 CrossRef CAS.
- J. Cao, J. Li, W. Chu and W. Cen, Chem. Eng. J., 2020, 400, 125813 CrossRef CAS.
- B. Pare, B. Sarwan and S. B. Jonnalagadda, Appl. Surf. Sci., 2011, 258, 247–253 CrossRef CAS.
- R. Zhao, X. Li, K. Lin and X. Gao, Res. Chem. Intermed., 2016, 42, 7031–7043 CrossRef CAS.
- F. Mokhtari and N. Tahmasebi, J. Phys. Chem. Solids, 2021, 149, 109804 CrossRef CAS.
- Z. Wei, X. Dong, N. Zheng, Y. Wang, X. Zhang and H. Ma, J. Mater. Sci., 2020, 55, 16522–16532 CrossRef CAS.
- H. Yu, D. Ge, Y. Wang, S. Zhu, X. Wang, M. Huo and Y. Lu, J. Alloys Compd., 2019, 786, 155–162 CrossRef CAS.
- R. Adhikari, G. Gyawali, S. H. Cho, R. Narro-García, T. Sekino and S. W. Lee, J. Solid State Chem., 2014, 209, 74–81 CrossRef CAS.
- K. Xu, X. Fu and Z. Peng, Mater. Res. Bull., 2018, 98, 103–110 CrossRef CAS.
- S. Yin, W. Fan, J. Di, T. Wu, J. Yan, M. He, J. Xia and H. Li, Colloids Surf., A, 2017, 513, 160–167 CrossRef CAS.
- M. Hu, A. Yan, X. Wang, F. Huang, Q. Cui, F. Li and J. Huang, Mater. Res. Bull., 2019, 116, 89–97 CrossRef CAS.
- S. Zhong, X. Wang, Y. Wang, F. Zhou, J. Li, S. Liang and C. Li, J. Alloys Compd., 2020, 843, 155598 CrossRef CAS.
- L. Zhang, Z. Ma, H. Xu, R. Xie, Y. Zhong, X. Sui, B. Wang and Z. Mao, Solid State Sci., 2018, 75, 45–52 CrossRef CAS.
- J. H. Peng, Y. J. Zhao, Q. Ul Hassan, H. Y. Li, Y. B. Liu, S. H. Ma, D. L. Mao, H. Q. Li, L. C. Meng and M. Hojamberdiev, Adv. Powder Technol., 2018, 29, 1158–1166 CrossRef CAS.
- Y. He, J. Li, K. Li, M. Sun, C. Yuan, R. Chen, J. Sheng, G. Leng and F. Dong, Chin. J. Catal., 2020, 41, 1430–1438 CrossRef CAS.
- Y. Guo, C. H. Lay, D. Zhou, S. Dong, J. Zhang and N. Ren, Environ. Sci. Pollut. Res., 2020, 27, 17516–17529 CrossRef CAS.
- K. LópezVelázquez, J. L. Guzmán-Mar, A. Hernández-Ramírez, E. González-Juárez and M. Villanueva-Rodríguez, Mater. Sci. Semicond. Process., 2021, 123, 105499 CrossRef.
- M. Nussbaum, N. Shaham-Waldmann and Y. Paz, J. Photochem. Photobiol., A, 2014, 290, 11–21 CrossRef CAS.
- N. Yu, Y. Chen, W. Zhang, M. Wen, L. Zhang and Z. Chen, Mater. Lett., 2016, 179, 154–157 CrossRef CAS.
- S. Niu, R. Zhang, X. Zhou, X. Zhao, H. Suo, Y. Jiao, H. Yao and C. Guo, Dyes Pigm., 2018, 149, 462–469 CrossRef CAS.
- S. Wu, W. Sun, J. Sun, Z. D. Hood, S. Z. Yang, L. Sun, P. R. C. Kent and M. F. Chisholm, Chem. Mater., 2018, 30, 5128–5136 CrossRef CAS.
- Z. Wei, R. Li and R. Wang, RSC Adv., 2018, 8, 7956–7962 RSC.
- S. R. Zhu, Q. Qi, Y. Fang, W. N. Zhao, M. K. Wu and L. Han, Cryst. Growth Des., 2018, 18, 883–891 CrossRef CAS.
- J. D. Xiao and H. L. Jiang, Acc. Chem. Res., 2019, 52, 356–366 CrossRef CAS PubMed.
- H. Yang, M. Zhao, J. Zhang, J. Ma, P. Wu, W. Liu and L. Wen, J. Mater. Chem. A, 2019, 7, 20742–20749 RSC.
- W. Jiang, Z. Li, C. Liu, D. Wang, G. Yan, B. Liu and G. Che, J. Alloys Compd., 2021, 854, 157166 CrossRef CAS.
- J. Di, S. Li, Z. Zhao, Y. Huang, Y. Jia and H. Zheng, Chem. Eng. J., 2015, 281, 60–68 CrossRef CAS.
- S. Li, S. Hu, K. Xu, W. Jiang, J. Liu and Z. Wang, Nanomaterials, 2017, 7, 22 CrossRef.
- D. Ma, J. Zhong, J. Li, L. Wang and R. Peng, Appl. Surf. Sci., 2018, 443, 497–505 CrossRef CAS.
- D. Ma, X. Tang, X. Liu, M. Zhao, R. Peng, J. Li, J. Zhong and R. Duan, Mater. Res. Bull., 2019, 118, 110521 CrossRef CAS.
- Y. Shirota and H. Kageyama, Chem. Rev., 2007, 107, 953–1010 CrossRef CAS PubMed.
- F. Dong, T. Xiong, Y. Sun, Z. Zhao, Y. Zhou, X. Feng and Z. Wu, Chem. Commun., 2014, 50, 10386–10389 RSC.
- Y. Yu, C. Cao, H. Liu, P. Li, F. Wei, Y. Jiang and W. Song, J. Mater. Chem. A, 2014, 2, 1677–1681 RSC.
- F. Dong, T. Xiong, S. Yan, H. Wang, Y. Sun, Y. Zhang, H. Huang and Z. Wu, J. Catal., 2016, 344, 401–410 CrossRef CAS.
- H. Wang, W. Zhang, X. Li, J. Li, W. Cen, Q. Li and F. Dong, Appl. Catal., B, 2018, 225, 218–227 CrossRef CAS.
- H. Ma, M. Zhao, H. Xing, Y. Fu, X. Zhang and X. Dong, J. Mater. Sci.: Mater. Electron., 2015, 26, 10002–10011 CrossRef CAS.
- X. Zhang, G. Ji, Y. Liu, X. Zhou, Y. Zhu, D. Shi, P. Zhang, X. Cao and B. Wang, Phys. Chem. Chem. Phys., 2015, 17, 8078–8086 RSC.
- F. Cao, J. Wang, Y. Wang, J. Zhou, S. Li, G. Qin and W. Fan, Nanoscale Adv., 2019, 1, 1124–1129 RSC.
- H. Yu, C. Cao, X. Wang and J. Yu, J. Phys. Chem. C, 2017, 121, 13191–13201 CrossRef CAS.
- Y. Huang, H. Xu, D. Luo, Y. Zhao, Y. Fang, Y. Wei, L. Fan and J. Wu, Solid State Sci., 2019, 89, 74–84 CrossRef CAS.
- X. Meng, Z. Li and Z. Zhang, Mater. Res. Bull., 2018, 99, 471–478 CrossRef CAS.
- B. Li, L. Shao, R. Wang, X. Dong, F. Zhao, P. Gao and Z. Li, J. Mater. Chem. A, 2018, 6, 6344–6355 RSC.
- C. Yu, F. Cao, G. Li, R. Wei, J. C. Yu, R. Jin and Q. Fan, Sep. Purif. Technol., 2013, 120, 110–122 CrossRef CAS.
- C. Tian, S. Luo, J. She, Y. Qing, N. Yan, Y. Wu and Z. Liu, Appl. Surf. Sci., 2019, 464, 606–615 CrossRef CAS.
- Y. Wang, Q. Yang, X. Wang, J. Yang, Y. Dai, Y. He, W. Chen and W. Zhang, Mater. Sci. Eng., B, 2019, 244, 12–22 CrossRef CAS.
- D. S. Dhawale, R. R. Salunkhe, V. S. Jamadade, D. P. Dubal, S. M. Pawar and C. D. Lokhande, Curr. Appl. Phys., 2010, 10, 904–909 CrossRef.
- C. Yan, Z. Zhang, W. Wang, T. Ju, H. She and Q. Wang, J. Mater. Sci.: Mater. Electron., 2018, 29, 18343–18351 CrossRef CAS.
- J. Xu, Y. Hu, C. Zeng, Y. Zhang and H. Huang, J. Colloid Interface Sci., 2017, 505, 719–727 CrossRef CAS PubMed.
- S. Cui, G. Shan and L. Zhu, Appl. Catal., B, 2017, 219, 249–258 CrossRef CAS.
- B. Ge, L. Han, X. Liang, F. Li, X. Pu, X. Zhu, Z. Zhang, X. Shao, C. Jin and W. Li, Appl. Surf. Sci., 2018, 462, 583–589 CrossRef CAS.
- C. J. Chang, P. Y. Chao and K. S. Lin, Appl. Surf. Sci., 2019, 494, 492–500 CrossRef CAS.
- Z. Cui, H. Song, S. Ge, W. He and Y. Liu, Appl. Surf. Sci., 2019, 467–468, 505–513 CrossRef CAS.
- J. Zhang, J. Lv, K. Dai, C. Liang and Q. Liu, Appl. Surf. Sci., 2018, 430, 639–646 CrossRef CAS.
- C. Zhao, Y. Liang, W. Li, X. Chen, Y. Tian, D. Yin and Q. Zhang, J. Mater. Sci.: Mater. Electron., 2020, 31, 1868–1878 CrossRef CAS.
- J. Li, Q. Zhou, F. Yang, L. Wu, W. Li, R. Ren and Y. Lv, New J. Chem., 2019, 43, 14829–14840 RSC.
- T. Jiang, J. Li, Y. Gao, L. Li, T. Lu and L. Pan, J. Colloid Interface Sci., 2017, 490, 812–818 CrossRef CAS PubMed.
- J. Zhang, J. Xia, S. Yin, H. Li, H. Xu, M. He, L. Huang and Q. Zhang, Colloids Surf., A, 2013, 420, 89–95 CrossRef CAS.
- C. Yang, J. Zhong, J. Li, S. Huang and R. Duan, Mater. Lett., 2020, 259, 126766 CrossRef CAS.
- H. Liu, C. Yang, J. Huang, J. Chen, J. Zhong and J. Li, Chem. Commun., 2020, 113, 107806 CAS.
- X. Sun, J. Lu, J. Wu, D. Guan, Q. Liu and N. Yan, J. Colloid Interface Sci., 2019, 546, 32–42 CrossRef CAS PubMed.
- F. Dong, Y. Sun, M. Fu, Z. Wu and S. C. Lee, J. Hazard. Mater., 2012, 219–220, 26–34 CrossRef CAS.
- S. Zhang and J. Yang, Ind. Eng. Chem. Res., 2015, 54, 9913–9919 CrossRef CAS.
- X. Jia, J. Cao, H. Lin, M. Zhang, X. Guo and S. Chen, Appl. Catal., B, 2017, 204, 505–514 CrossRef CAS.
- H. Lin, H. Ye, X. Li, J. Cao and S. Chen, Ceram. Int., 2014, 40, 9743–9750 CrossRef CAS.
- T. S. Jamil, E. S. Mansor and R. A. Nasr, Desalin. Water Treat., 2016, 57, 14750–14761 CrossRef CAS.
- Y. Bai, X. Shi, P. Wang, L. Wnag, K. Zhang, Y. Zhou, H. Xie, J. Wang and L. Ye, Chem. Eng. J., 2019, 356, 34–42 CrossRef CAS.
- S. H. Li, N. Zhang, X. Xie, R. Luque and Y. J. Xu, Angew. Chem., Int. Ed., 2018, 57, 13082–13085 CrossRef CAS PubMed.
- G. Liu, H. Xu, D. Li, Z. Zou, Q. Li and D. Xia, Eur. J. Inorg. Chem., 2019, 2019, 4887–4893 CrossRef CAS.
- X. Shi, P. Wang, W. Li, Y. Bai, H. Xie, Y. Zhou and L. Ye, Appl. Catal., B, 2019, 243, 322–329 CrossRef CAS.
- G. Wang, X. Luo, Y. Huang, A. Kuang, H. Yuan and H. Chen, RSC Adv., 2016, 6, 91508–91516 RSC.
- J. Di, J. Xia, Y. Ge, H. Li, H. Ji, H. Xu, Q. Zhang, H. Li and M. Li, Appl. Catal., B, 2015, 168–169, 51–61 CrossRef CAS.
- C. Zhao, Y. Liang, W. Li, Y. Tian, X. Chen, D. Yin and Q. Zhang, RSC Adv., 2017, 7, 52614–52620 RSC.
- X. Su, J. Yang, X. Yu, Y. Zhu and Y. Zhang, Appl. Surf. Sci., 2018, 433, 502–512 CrossRef CAS.
- M. Du, Y. Du, Y. Feng, Z. Li, J. Wang and N. Jiang, Cellulose, 2019, 26, 5543–5557 CrossRef CAS.
- L. Ren, D. Zhang, X. Hao, X. Xiao, Y. Jiang, J. Gong, F. Zhang, X. Zhang and Z. Tong, Mater. Res. Bull., 2017, 94, 183–189 CrossRef CAS.
- Y. Wang, Y. Long, Z. Yang and D. Zhang, J. Hazard. Mater., 2018, 351, 11–19 CrossRef CAS PubMed.
- X. Xiao, C. Zheng, M. Lu, L. Zhang, F. Liu, X. Zuo and J. Nan, Appl. Catal., B, 2018, 228, 142–151 CrossRef CAS.
- C. Y. Wang, X. Zhang, H. B. Qiu, W. K. Wang, G. X. Huang, J. Jiang and H. Q. Yu, Appl. Catal., B, 2017, 200, 659–665 CrossRef CAS.
- R. Li, J. Liu, X. Zhang, Y. Wang, Y. Wang, C. Zhang, X. Zhang and C. Fan, Chem. Eng. J., 2018, 339, 42–50 CrossRef CAS.
- Y. Bai, L. Ye, T. Chen, L. Wang, X. Shi, X. Zhang and D. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 27661–27668 CrossRef CAS PubMed.
- C. Y. Wang, X. Zhang, X. N. Song, W. K. Wang and H. Q. Yu, ACS Appl. Mater. Interfaces, 2016, 8, 5320–5326 CrossRef CAS.
- G. Gupta and S. K. Kansal, Process Saf. Environ., 2019, 128, 342–352 CrossRef CAS.
- M. Shabani, M. Haghighi and D. Kahforoushan, J. Cleaner Prod., 2019, 207, 444–457 CrossRef CAS.
- X. Liu, X. Jiang, Z. Chen, J. Yu and Y. He, Mater. Lett., 2018, 210, 194–198 CrossRef CAS.
- L. Wang, X. Min, X. Sui, J. Chen and Y. Wang, J. Colloid Interface Sci., 2020, 560, 21–33 CrossRef CAS.
- S. Heidari, M. Haghighi and M. Shabani, J. Cleaner Prod., 2020, 259, 120679 CrossRef CAS.
- S. Meng, Y. Bi, T. Yan, Y. Zhang, T. Wu, Y. Shao and D. Wei, J. Hazard. Mater., 2018, 358, 20–32 CrossRef CAS PubMed.
- D. Du, W. Li, S. Chen, T. Yan, J. You and D. Kong, New J. Chem., 2015, 39, 3129–3136 RSC.
- H. Y. Xu, X. Han, Q. Tan, K. J. Wu and S. Y. Qi, Front. Mater. Sci., 2017, 11, 120–129 CrossRef.
- W. J. Kim, D. Pradhan, B. K. Min and Y. Sohn, Appl. Catal., B, 2014, 147, 711–725 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra05796k |
| This journal is © The Royal Society of Chemistry 2021 |