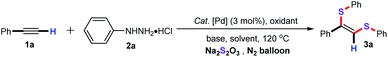Open Access Article
Open Access ArticlePalladium-catalyzed bisthiolation of terminal alkynes for the assembly of diverse (Z)-1,2-bis(arylthio)alkene derivatives†
Yin-Long Lai*a,
Shaoxi Yan‡
a,
Dan He‡b,
Li-Zhen Zhoua,
Zi-Shen Chena,
Yu-Long Dua and
Jianxiao Li *bc
*bc
aCollege of Chemistry and Civil Engineering, Shaoguan University, Shaoguan, 512005, P. R. China. E-mail: chemlaiyinlong@163.com
bKey Laboratory of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640, P. R. China. E-mail: cejxli@scut.edu.cn
cGuangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates, South China University of Technology, Guangzhou 510640, China
First published on 23rd August 2021
Abstract
An efficient and straightforward palladium-catalyzed three-component cascade bisthiolation of terminal alkynes and arylhydrazines with sodium thiosulfate (Na2S2O3) as the sulfur source for the assembly of functionalized (Z)-1,2-bis(arylthio)alkene derivatives is described. Using 0.5 mol% IPr–Pd–Im–Cl2 as the catalyst, a wide range of terminal alkynes and arylhydrazines are well tolerated, thus producing the desired products in good yields with good functional group tolerance and excellent regioselectivity. Moreover, this protocol could be readily scaled up, showing potential applications in organic synthesis and material science.
Introduction
Transition-metal-catalyzed functionalization of unsaturated hydrocarbons (such as alkenes and alkynes) is proven to be a flexible and straightforward synthetic strategy for the assembly of structurally diverse organic synthetic building blocks in organic synthesis, advanced materials, and pharmaceutical chemistry.1 Among them, the functionalization reaction of alkynes has witnessed considerable attention in recent years because of their idiographic nucleophilic and electrophilic properties.2 In this regard, transition-metal-catalyzed difunctionalization of alkynes has become one of the most powerful synthetic methodologies for the effective synthesis of diverse complex polysubstituted olefins from abundant and readily available starting materials in an atom- and step-economical manner.3 Nevertheless, this protocol required two elements of the p-block (RE–ER, E = B, S, Si, C…) as the coupling partners. In terms of green chemistry, the photo-catalyzed difunctionalization of alkynes has also attracted more attention in recent years.4 Apart from the above synthetic methods, metal-free radical addition reaction of alkynes with radical precursors has triggered a multiplication of synthetic protocols for accessing a vast array of value-added functionalized molecules.5 However, the scope of the free radical is limited to the common free radical precursors. Therefore, it is highly desirable to develop a novel and efficient synthetic approach for the straightforward difunctionalization of alkynes from readily available starting materials under eco-friendly conditions.In addition, organosulfur structural frameworks are prevalent in organic synthesis, natural products, and various bioactive molecules.6 In particular, among a whole variety of vinyl sulfide scaffolds, the 1,2-bis(arylthio)alkene derivatives exhibit remarkable biological activities and pharmaceuticals activities.7 As a result, a library of representative synthetic methodologies have been developed for the preparation of this vinyl sulfide scaffolds. Undoubtedly, transition-metal-catalyzed bisthiolation of terminal alkynes with diaryl disulfides have been identified as the extremely rapid and efficient synthetic strategy for constructing these motifs (Scheme 1a). And, several noble metal catalysts such as Pd,8 Rh,9 Ni10 have displayed a remarkable catalytic activity for this chemical transformation. For instance, Ananikov and co-workers developed a nice palladium-catalyzed addition reaction of disulfides with alkynes under solvent free conditions for the synthesis of structurally diverse (Z)-1,2-bis (arylthio)alkenes in good yields. Yamaguchi and co-workers also disclosed a rhodium-catalyzed addition reaction of dialkyl disulfides with terminal alkynes for the construction of various (Z)-bis(alkylthio)olefins with excellent stereoselectively. Additionally, in 2009, Xu and Yang and co-workers reported a cesium hydroxide catalyzed addition reaction of diaryl disulfides with terminal alkynes for constructing a series of (Z)-1,2-bis(arylthio)alkene derivatives (Scheme 1b).11 However, these reactions needed diaryl disulfides and dialkyl disulfides as the sulfenylating reagents. In 2018, our group have also successfully developed an NHC-palladium-catalyzed three-component cascade bisthiolation of terminal alkynes, K2S and diaryliodonium salts for the assembly of functionalized (Z)-1,2-bis(arylthio)alkenes derivatives with high regioselectivity (Scheme 1c).12 Despite the significance, the alternative sulfenylating reagents such as sodium thiosulfate (Na2S2O3) have rarely reported in this chemical transformation.13 To the best of our knowledge, there is no synthetic example for the synthesis of (Z)-1,2-bis(arylthio)alkenes with Na2S2O3 as the sulfenylating reagent. Inspired by our longstanding interest in Pd-catalyzed coupling reactions of alkynes,14 and organosulfur chemistry,15 we herein describe a novel palladium-catalyzed three-component cascade bisthiolation of terminal alkynes and arylhydrazines with Na2S2O3 as the sulfur source for the assembly of functionalized (Z)-1,2-bis(arylthio)alkene derivatives in good to excellent yields (Scheme 1d).
Results and discussion
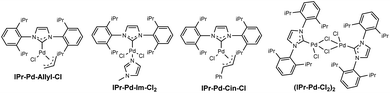
As an initial experiment, ethynylbenzene (1a), phenylhydrazine hydrochloride (2a), and sodium thiosulfate (Na2S2O3) was employed as the model substrates to screen for the optimal reaction conditions, and the representative results are summarized in Table 1. Preliminary screening revealed that IPr–Pd–Im–Cl2 as the catalyst was the most effective palladium catalysts for this protocol, while other catalysts such as PdCl2, Pd(TFA)2, Pd(PhCN)2Cl2, [Pd(allyl)Cl]2, Pd(PPh3)2Cl2, IPr–Pd–allyl–Cl, and IPr–Pd–Cin–Cl showed low efficiencies (entries 1–8). Among the oxidants tested, 35% H2O2 gave the best results (entries 10–12). Subsequently, different bases were examined, including Et3N, DBU, CsF, and Cs2CO3, the results showed that Cs2CO3 was the most effective base for this transformation (entries 7 and 13–15). Further exploration of the solvent indicated that DMSO was superior to the DMF and toluene (entries 7, 16 and 17). Inspired by our previous studies, we found that ionic liquids as the solvent showed apparent positive effects in palladium-catalyzed coupling reaction.16 Thus, we also estimated the ionic liquids as the green solvent for this transformation. It is noteworthy that ionic liquid [Bmim]PF6 as the solvent was found to be the best choice, and the desired product 3a was afforded in 90% GC yield (entry 20). In addition, the green PEG-200 and PEG-400 were also screened, however, only a trace amount of the desired product 3a was detected by GC-MS (entries 21 and 22). It is particularly noteworthy that the desired product 3a was still obtained in 90% GC yield when the reaction was performed with 0.05 mol% dosage of the IPr–Pd–Im–Cl2 catalyst (entry 25). Finally, the yield of 3a decreased dramatically when the temperature was used at 110 °C under the similar condition (entry 26).| Entry | Catalyst | Oxidant | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|---|
| a Reactions were performed with 1a (0.10 mmol), 2a (0.24 mmol), Na2S2O3 (0.20 mmol), catalyst (3 mol%), oxidant (0.20 mmol), base (0.20 mmol), solvent (1 mL) at 120 °C under N2 for 12 h. [Bmim]Cl: 1-butyl-3-methylimidazolium chloride. [Bmim]BF4: 1-butyl-3-methylimidazolium tetrafluoroborate. [Bmim]PF6: 1-butyl-3-methylimidazolium hexafluorophosphate. PEG-200: polyethylene glycol 200. PEG-400: polyethylene glycol 400.b Determined by GC using dodecane as the internal standard. The value in parentheses is the yield of isolated product.c 1 mol% IPr–Pd–Im–Cl2 was used.d 0.5 mol% IPr–Pd–Im–Cl2 was used.e At 110 °C. | |||||
| 1 | PdCl2 | H2O2 | Cs2CO3 | DMF | 0 |
| 2 | Pd(TFA)2 | H2O2 | Cs2CO3 | DMF | Trace |
| 3 | Pd(PhCN)2Cl2 | H2O2 | Cs2CO3 | DMF | 13 |
| 4 | [Pd(allyl)Cl]2 | H2O2 | Cs2CO3 | DMF | 22 |
| 5 | Pd(PPh3)2Cl2 | H2O2 | Cs2CO3 | DMF | Trace |
| 6 | IPr–Pd–allyl–Cl | H2O2 | Cs2CO3 | DMF | 37 |
| 7 | IPr–Pd–Im–Cl2 | H2O2 | Cs2CO3 | DMF | 54 |
| 8 | IPr–Pd–Cin–Cl | H2O2 | Cs2CO3 | DMF | 41 |
| 9 | (IPr–Pd–Cl2)2 | H2O2 | Cs2CO3 | DMF | 38 |
| 10 | IPr–Pd–Im–Cl2 | O2 | Cs2CO3 | DMF | Trace |
| 11 | IPr–Pd–Im–Cl2 | NFSI | Cs2CO3 | DMF | 33 |
| 12 | IPr–Pd–Im–Cl2 | PhI(OAc)2 | Cs2CO3 | DMF | 46 |
| 13 | IPr–Pd–Im–Cl2 | H2O2 | Et3N | DMF | 26 |
| 14 | IPr–Pd–Im–Cl2 | H2O2 | DBU | DMF | Trace |
| 15 | IPr–Pd–Im–Cl2 | H2O2 | CsF | DMF | 18 |
| 16 | IPr–Pd–Im–Cl2 | H2O2 | Cs2CO3 | DMSO | 56 |
| 17 | IPr–Pd–Im–Cl2 | H2O2 | Cs2CO3 | Toluene | Trace |
| 18 | IPr–Pd–Im–Cl2 | H2O2 | Cs2CO3 | [Bmim]Cl | 72 |
| 19 | IPr–Pd–Im–Cl2 | H2O2 | Cs2CO3 | [Bmim]BF4 | 65 |
| 20 | IPr–Pd–Im–Cl2 | H2O2 | Cs2CO3 | [Bmim]PF6 | 90 (84) |
| 21 | IPr–Pd–Im–Cl2 | H2O2 | Cs2CO3 | PEG-200 | Trace |
| 22 | IPr–Pd–Im–Cl2 | H2O2 | Cs2CO3 | PEG-400 | Trace |
| 23 | — | H2O2 | Cs2CO3 | [Bmim]PF6 | 0 |
| 24c | IPr–Pd–Im–Cl2 | H2O2 | Cs2CO3 | [Bmim]PF6 | 90 |
| 25d | IPr–Pd–Im–Cl2 | H2O2 | Cs2CO3 | [Bmim]PF6 | 90 |
| 26e | IPr–Pd–Im–Cl2 | H2O2 | Cs2CO3 | [Bmim]PF6 | 78 |
 |
|||||
Under the optimized conditions, the substrate scope of various alkynes was then explored, and the results are presented in Table 2. In general, both aryl alkynes and alkyl alkynes can react with phenylhydrazine hydrochloride (2a), and sodium thiosulfate (Na2S2O3) in the optimized conditions, producing the desired products 3 in good to excellent yields with good regioselectivity. As anticipated, the aryl alkynes bearing alkyl, alkoxyl, fluoro, chloro, amino, acetyl, and cyano group on the phenyl ring were perfectly accommodated in the optimized conditions to deliver the desired products 3a–3h with yields in the range of 70–92%. Specifically, this approach was compatible with the halogen atom substituent on the aryl ring such as –Cl, which might allow for further functional group derivatization by transition metal-catalyzed chemical transformation. In addition, the electronic properties of the substituents such as electron-donating group (–NMe2) and electron-withdrawing group (–Ac, CN) on the benzene ring of aryl alkynes did not have a significant influence on the reaction efficiency. Moreover, 1-ethynylnaphthalene (1i) was tolerated well, giving the corresponding product 3i in 86% yield. To our delight, 2-ethynylthiophene (1j) and 4-ethynylpyridine (1k) were also perfectly tolerated, furnishing the desired 3j and 3k in 73% and 65% yields, respectively. As for the alkyl alkynes, both the linear chain alkynes and the cyclo-alkynes were all nicely tolerated, and gave the desired products in good to excellent yields. Gratifyingly, the substrate containing vinyl group also performed well under the optimized conditions, and generated the corresponding product 3p in 91% yield. Additionally, the substrates containing free hydroxyl of linear chain alkynes also worked well, delivering the corresponding products 3q and 3s in 72% and 83% yields, respectively. Notably, ethynyltrimethylsilane (1r) was nicely compatible with the current catalytic system and afforded the corresponding 3r in 76% yield. Unfortunately, the internal alkynes such as 1,2-diphenylethyne, and pent-2-yne were not tolerated with the current system. The probable reason is that the steric hindrance of internal alkynes hindered the nucleopalladation process.17
| a Reaction conditions: 1 (0.20 mmol), 2a (0.48 mmol), Na2S2O3 (0.40 mmol), IPr–Pd–Im–Cl2 (0.5 mol%), H2O2 (0.4 mmol), Cs2CO3 (0.4 mmol), [Bmim]PF6 (2 mL) at 120 °C for 12 h. Yields referred to isolated yield. |
|---|
 |
Encouraged by the above positive results, we then evaluated the limitation and compatibility of various arylhydrazines in the optimized conditions. As illustrated in Table 3, both electron-neutral substituents (Me, tBu) and electron-withdrawing groups (F, Cl, Br, Ac) on the benzene ring were nicely tolerated and gave the expected products 4a–4f in good to high yields. It is remarkable that the substrate thiophen-3-ylhydrazine could also react smoothly to deliver the desired product 4g in 66% yield.
| a Reaction conditions: 1a (0.20 mmol), 2 (0.48 mmol), Na2S2O3 (0.40 mmol), IPr–Pd–Im–Cl2 (0.5 mol%), H2O2 (0.4 mmol), Cs2CO3 (0.4 mmol), [Bmim]PF6 (2 mL) at 120 °C for 12 h. Yields referred to isolated yield. |
|---|
 |
Subsequently, to further probe to the possible sulfur intermediate in this catalytic system, several easily available sulfur sources were investigated under the optimized conditions. As shown in Scheme 2, the S8 and Na2S were not the alternative sulfenylating reagents, and failed to furnish the desired product 3a. Amazingly, when PhSH was employed as the sulfenylating agents, the desired product 3a was detected in 57% GC yield. This observation demonstrated that PhSH might be the possible intermediate in this bisthiolation reaction. In contrast, when PhSSPh was used as the sulfenylating agent, no the desired product 3a was observed by GC-MS analysis.
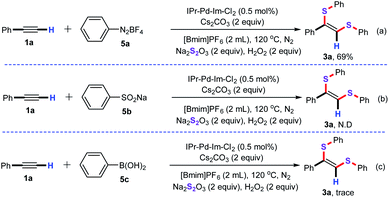
Next, various aryl reagents were explored as well to examine the substrate applicability of this synthetic strategy (Scheme 3). For instance, the cascade denitrogenative/bisthiolation of phenyldiazonium salt (5a) with ethynylbenzene (1a) allowed successfully to give the product 3a in 69% yield (Scheme 3a). Unfortunately, the desulfitative/bisthiolation of sodium benzenesulfinate (5b) with ethynylbenzene (1a) cannot carry out under the optimized conditions, and no the desired product 3a was observed by GC-MS (Scheme 3b). Similarly, the cascade Suzuki/bisthiolation of phenylboric acid (5c) with 1a also tested under the standard conditions, however, only a trace amount of the desired product 3a was detected by GC-MS (Scheme 3c).
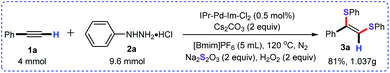
Delightfully, this approach was successfully scaled up to 4 mmol, and the desired product 3a was obtained in 81% yield (Scheme 4).
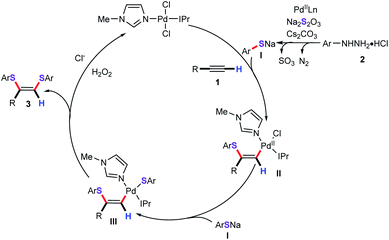
Based on these experimental results and previous literature precedents, a plausible mechanism for this cascade bisthiolation process is proposed in Scheme 5. Initially, in the presence of base, the arylhydrazine reacts with Pd(II) to give the aryl palladium species by releasing the N2. Then, ligand exchange between the aryl palladium species and Na2S2O3 to form palladium thiosulfate intermediate, which can generate the sodium thiophenolate I via the release of SO3.18 Nucleopalladation of sodium thiophenolate I with alkynes gives vinyl palladium intermediate II. Subsequently, the anion exchange process from intermediate II with ArSNa to produce the intermediate III. Finally, the reductive elimination process generates the desired products and the Pd0 active species. In light of our previously observed results, we found that ionic liquids as the solvent or cosolvent system showed apparent positive effects for the formation of the thiophenolate anion.15,19,20
Conclusions
In conclusion, we have established a novel and efficient palladium-catalyzed three-component cascade bisthiolation of terminal alkynes and arylhydrazines with Na2S2O3 as the sulfur source for the assembly of functionalized (Z)-1,2-bis(arylthio)alkene derivatives in good to excellent yields with high regioselectivity. This protocol features mild conditions, excellent regioselectivity and good functional group tolerance. Prominently, H2O2 as the green oxidant and IPr–Pd–Im–Cl2 as the catalyst play an essential role in this protocol. Notably, in the presence of 0.5 mol% of IPr–Pd–Im–Cl2 as the catalyst, a wide range of alkynes and various arylhydrazines are excellently tolerated. Preliminary mechanistic studies suggest that the sodium thiophenolate might be involved in this transformation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (21971072), the Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515010185), the Guangdong Natural Science Foundation (2018B030308007), the Science and Technology Project of Guangdong Province (Shaoguan Science and Technology Bureau [2020] No. 44), the Open Fund of Guangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates (2019B030301003), and the Innovation Projects of Department of Education of Guangdong Province (No. 2018KQNCX236) for financial support.References
- (a) X.-H. Yang, R.-J. Song, Y.-X. Xie and J.-H. Li, ChemCatChem, 2016, 8, 2429–2445 CrossRef CAS; (b) E. Kang, H. T. Kim and J. M. Joo, Org. Biomol. Chem., 2020, 18, 6192–6210 RSC; (c) R. Blieck, M. Taillefer and F. Monnier, Chem. Rev., 2020, 120, 13545–13598 CrossRef CAS PubMed; (d) S. Ghosh, D. Lai and A. Hajra, Org. Biomol. Chem., 2020, 18, 7948–7976 RSC.
- (a) S. E. Garcia-Garrido, in Modern Alkyne Chemistry, ed. B. M. Trost and C.-J. Li, Wiley, Weinheim, 2015, pp. 301–334 Search PubMed; (b) B. A. Trofimov and E. Y. Schmidt, Acc. Chem. Res., 2018, 51, 1117–1130 CrossRef CAS PubMed; (c) B. Yang, Y. Qiu and J.-E. Bäckvall, Acc. Chem. Res., 2018, 51, 1520–1531 CrossRef CAS PubMed.
- (a) H. Yoshida, ACS Catal., 2016, 6, 1799–1811 CrossRef CAS; (b) J.-J. Feng and J. Zhang, ACS Catal., 2016, 6, 6651–6661 CrossRef CAS; (c) K. Sun, X. Wang, C. Li, H. Wang and L. Li, Org. Chem. Front., 2020, 7, 3100–3119 RSC; (d) L. Peng, Z. Hu, H. Wang, L. Wu, Y. Jiao, Z. Tang and X. Xu, RSC Adv., 2020, 10, 10232–10244 RSC.
- A. B. Cuenca, R. Shishido, H. Ito and E. Fernández, Chem. Soc. Rev., 2017, 46, 415–430 RSC.
- (a) P. Sivaguru, Z. Wang, G. Zanoni and X. Bi, Chem. Soc. Rev., 2019, 48, 2615–2656 RSC; (b) Y. Li, G.-A. Pan, M.-J. Luo and J.-H. Li, Chem. Commun., 2020, 56, 6907–6924 RSC; (c) V. B. Saptal, R. Wang and S. Park, RSC Adv., 2020, 10, 43539–43565 RSC; (d) J. Liao, X. Yang, L. Ouyang, Y. Lai, J. Huang and R. Luo, Org. Chem. Front., 2021, 8, 1345–1363 RSC.
- For reviews, (a) H. Liu and X. Jiang, Chem.–Asian J., 2013, 8, 2546–2563 CrossRef CAS PubMed; (b) M. Feng, B. Tang, S. H. Liang and X. Jiang, Curr. Top. Med. Chem., 2016, 16, 1200–1216 CrossRef CAS PubMed; (c) Z. Qiao and X. Jiang, Org. Biomol. Chem., 2017, 15, 1942–1946 RSC; (d) W. Guo, K. Tao, W. Tan, M. Zhao, L. Zheng and X. Fan, Org. Chem. Front., 2019, 6, 2048–2066 RSC; (e) N. Wang, P. Saidhareddy and X. Jiang, Nat. Prod. Rep., 2020, 37, 246–275 RSC; (f) M. Pramanik, K. Choudhuri and P. Mal, Org. Biomol. Chem., 2020, 18, 8771–8792 RSC; (g) Z. Liu, A. Ebadi, M. Toughani, N. Mert and E. Vessally, RSC Adv., 2020, 10, 37299–37313 RSC. For examples, (h) Y.-C. Gao, Z.-B. Huang, L. Xu, Z.-D. Li, Z.-S. Lai and R.-Y. Tang, Org. Biomol. Chem., 2019, 17, 2279–2286 RSC; (i) J. Jiang, X. Tuo, Z. Fu, H. Huang and G.-J. Deng, Org. Biomol. Chem., 2020, 18, 3234–3238 RSC; (j) Y. Xia, H. Huang, W. Hu and G.-J. Deng, Org. Biomol. Chem., 2021, 19, 5108–5113 RSC; (k) Q.-H. Teng, Y. Yao, W.-X. Wei, H.-T. Tang, J.-R. Li and Y.-M. Pan, Green Chem., 2019, 21, 6241–6245 RSC; (l) H. Cao, X. Liu, F. Bie, Y. Shi, Y. Han, P. Yan, M. Szostak and C. Liu, J. Org. Chem., 2021, 86, 10829–10837 CrossRef CAS PubMed; (m) H. Cao, X. Liu, F. Bie, Y. Shi, Y. Han, P. Yan, M. Szostak and C. Liu, Org. Chem. Front., 2021, 8, 1587–1592 RSC.
- (a) C.-K. Ryu, R.-E. Park, M.-Y. Ma and J.-H. Nho, Bioorg. Med. Chem. Lett., 2007, 17, 2577–2580 CrossRef CAS PubMed; (b) C.-K. Ryu, Y. H. Kim, H. A. Im, J. Y. Kim, J. H. Yoon and A. Kim, Bioorg. Med. Chem. Lett., 2012, 22, 500–503 CrossRef CAS PubMed.
- J. Li, S. Yang, W. Wu and H. Jiang, Org. Chem. Front., 2020, 7, 1395–1417 RSC.
- M. Arisawa and M. Yamaguchi, Org. Lett., 2001, 3, 763–764 CrossRef CAS PubMed.
- V. P. Ananikov, K. A. Gayduk, I. P. Beletskaya, V. N. Khrustalev and M. Y. Antipin, Chem. Eur. J., 2008, 14, 2420–2434 CrossRef CAS.
- K. B. Zou, X. H. Yin, W. Q. Liu, R. H. Qiu, R. X. Li, L. L. Shao, Y. H. Li, X. Hua Xu and R. H. Yang, Synth. Commun., 2009, 39, 2464–2471 CrossRef CAS.
- J. Li, C. Li, L. Ouyang, C. Li, S. Yang, W. Wu and H. Jiang, Adv. Synth. Catal., 2018, 360, 1138–1150 CrossRef CAS.
- (a) Y. Li, J. Pu and X. Jiang, Org. Lett., 2014, 16, 2692–2695 CrossRef CAS PubMed; (b) X. Xiao, M. Feng and X. Jiang, Chem. Commun., 2015, 51, 4208–4211 RSC; (c) Y. Zhang, Y. Li, X. Zhang and X. Jiang, Chem. Commun., 2015, 51, 941–944 RSC; (d) M. Wang, Z. Qiao, J. Zhao and X. Jiang, Org. Lett., 2018, 20, 6193–6197 CrossRef CAS PubMed; (e) J. Li, Y. Wu, M. Hu, C. Li, M. Li, D. He and H. Jiang, Green Chem., 2019, 21, 4084–4089 RSC.
- For reviews, see: (a) W. Wu and H. Jiang, Acc. Chem. Res., 2014, 47, 2483–2504 CrossRef CAS PubMed; (b) J. Li, S. Yang, W. Wu and H. Jiang, Eur. J. Org. Chem., 2018, 1284–1306 CrossRef CAS; (c) J. Li, S. Yang, W. Wu and H. Jiang, Chem.–Asian J., 2019, 14, 4114–4128 CrossRef CAS PubMed; For examples, see, (d) J. Li, W. Yang, S. Yang, L. Huang, W. Wu and H. Jiang, Angew. Chem., Int. Ed., 2014, 53, 7219–7222 CrossRef CAS PubMed; (e) J. Li, W. Hu, C. Li, S. Yang, W. Wu and H. Jiang, Org. Chem. Front., 2017, 4, 373–376 RSC; (f) L. Ouyang, J. Li, J. Zheng, J. Huang, C. Qi, W. Wu and H. Jiang, Angew. Chem., Int. Ed., 2017, 56, 15926–15930 CrossRef CAS PubMed; (g) J. Li, J. Yu, W. Xiong, H. Tang, M. Hu, W. Wu and H. Jiang, Green Chem., 2020, 22, 465–470 RSC.
- (a) J. Li, C. Li, S. Yang, Y. An, W. Wu and H. Jiang, J. Org. Chem., 2016, 81, 2875–2887 CrossRef CAS PubMed; (b) J. Li, C. Li, S. Yang, Y. An, W. Wu and H. Jiang, J. Org. Chem., 2016, 81, 7771–7783 CrossRef CAS PubMed; (c) J. Li, H. Tang, Z. Lin, S. Yang, W. Wu and H. Jiang, Org. Biomol. Chem., 2020, 18, 4071–4078 RSC; (d) J. Li, Z. Lin, D. He, Z. Lin, Z. Zheng, C. Bi, W. Wu and H. Jiang, Org. Biomol. Chem., 2021, 19, 3396–3403 RSC.
- (a) J. Li, S. Yang, W. Wu and H. Jiang, Chem. Commun., 2014, 50, 1381–1383 RSC; (b) J. Li, S. Yang, W. Wu and H. Jiang, Eur. J. Org. Chem., 2018, 1284–1306 CrossRef CAS.
- (a) J. Huang, L. Zhou and H. Jiang, Angew. Chem., Int. Ed., 2006, 45, 1945–1949 CrossRef CAS PubMed; (b) J. Li, S. Yang, H. Jiang, W. Wu and J. Zhao, J. Org. Chem., 2013, 78, 12477–12486 CrossRef CAS PubMed.
- (a) Z. Qiao, H. Liu, X. Xiao, Y. Fu, J. Wei, Y. Li and X. Jiang, Org. Lett., 2013, 15, 2594–2597 CrossRef CAS PubMed; (b) Z. Qiao, J. Wei and X. Jiang, Org. Lett., 2014, 16, 1212–1215 CrossRef CAS PubMed; (c) C. Wang, Z. Zhang, Y. Tu, Y. Li, J. Wu and J. Zhao, J. Org. Chem., 2018, 83, 2389–2394 CrossRef CAS PubMed; (d) J. Li, H. Tang, Z. Lin, S. Yang, W. Wu and H. Jiang, Org. Biomol. Chem., 2020, 18, 4071–4078 RSC.
- (a) J. Li, C. Li, S. Yang, Y. An, W. Wu and H. Jiang, J. Org. Chem., 2016, 81, 2875–2887 CrossRef CAS PubMed; (b) W. Wu, Y. An, J. Li, S. Yang, Z. Zhu and H. Jiang, Org. Chem. Front., 2017, 4, 1751–1756 RSC.
- According to the previous reports, ionic liquids can behave as green solvents in comparison with conventional organic solvents, but more often they also act as ligands, co-catalysts, and stabilizing agents both for metal active species and for intermediates of catalytic systems, see reviews, (a) T. Welton, Chem. Rev., 1999, 99, 2071–2083 CrossRef CAS PubMed; (b) P. Mastrorilli, A. Monopoli, M. M. Dell'Anna, M. Latronico, P. Cotugno and A. Nacci, Top Organomet. Chem., 2015, 51, 237–286 CrossRef CAS; (c) A. S. Khan, T. H. Ibrahim, N. A. Jabbar, M. I. Khamis, P. Nancarrow and F. S. Mjalli, RSC Adv., 2021, 11, 12398–12422 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: General experimental information, and NMR spectra. See DOI: 10.1039/d1ra05773a |
| ‡ These two authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2021 |