 Open Access Article
Open Access ArticleMn4+-activated oxyfluoride K3TaOF6 red phosphor with intense zero phonon line for warm white light-emitting diodes†
Jiao Wu,
Bo Wang *,
Zhiyuan Liu,
Kang Zhang and
Qingguang Zeng*
*,
Zhiyuan Liu,
Kang Zhang and
Qingguang Zeng*
School of Applied Physics and Materials, Wuyi University, Jiangmen, Guangdong 529020, P. R. China. E-mail: wangbo312@mails.ucas.ac.cn; zengqg@mail.ustc.edu.cn
First published on 29th July 2021
Abstract
The intense zero phonon line (ZPL) of the Mn4+:2E→4A2 transition can further promote the color rendering and luminous efficiency for high-quality white-emitting diodes (w-LEDs). In this article, a Mn4+-activated K3TaOF6 oxyfluoride red phosphor was synthesized via a facile two-step method. Its phase and morphology were characterized by X-ray diffraction, SEM and TEM. The as-prepared K3TaOF6:Mn4+ exhibits an intense absorption of blue light and a strong emission band peaking at 628 nm with a color purity as high as 96.4%. Attributed to the distorted octahedral coordination environment of Mn4+ ions, an intense ZPL emission was detected at 620 nm. By theoretical calculation, Mn4+ ions in the K3TaOF6 host experience a strong crystal field. In addition, the temperature-dependent PL and thermoluminescence (TL) spectra suggest that thermal ionization dominates the thermal quenching phenomenon in this phosphor.
1. Introduction
With the development of lighting and display technology in the past decades, there is an increasing requirement of the color quality of white lighting-emitting diode (w-LED) illuminant sources.1–5 However, the commercially available w-LED devices, i.e., based on Y3Al5O12:Ce3+ (YAG) yellow phosphors with InGaN blue chips are unsuitable for liquid crystal display (LCD) backlights due to their limited color gamut.6–8 As an alternative, combining commercial CaAlSiN3:Eu2+ red phosphors with β-sialon:Eu2+ green phosphors has been a popular model in the LCD backlight market.9,10 Unfortunately, the excitation band of CaAlSiN3:Eu2+ is so broad that serious re-absorption between β-sialon:Eu2+ and CaAlSiN3:Eu2+ will be caused, which then brings the change of color and a reduction of luminous efficacy.11 Moreover, the LCD backlight obtained via this approach can only reach ∼82% of the required standard proposed by National Television Standards Committee (NTSC). Conceivably, it is crucial to develop phosphors with appropriate peak position, narrow emission band, high quantum efficiency (QE) and excellent thermal stability to fulfil the application.Thanks to the specific electronic structure of the Mn4+ (3d3 electron configuration) ion, obvious broadening of the color gamut of LED-lit LCD backlights has been observed by employing Mn4+-activated red phosphors.12–18 Theoretically, both Stokes and anti-Stokes photon vibrational sidebands could be easily implemented in the transition of Mn4+:2E→4A2.16,17 Whereas, only when Mn4+ ions have distorted coordination geometries can the ZPL transition of Mn4+:2E→4A2 occur.19–22 Generally, the sharpest PL emission of the Mn4+ ion is located at ∼630 nm among a group of sharp emission peaks while the observed ZPL emission in a few cases peaks at ∼620 nm.8,21,22 In fact, as part of the PL emission, the ZPL emission is of great benefit to improve the luminous efficiency because human eyes are more sensitive to shorter wavelengths.8,12,14 In spite of the absence of distorted octahedral coordination sites, the oxyfluoride compounds still can provide distorted local environments by F/O mixed-ligands, which could bring rich spectroscopic properties, thus many researchers have focused on the oxyfluoride compounds.22 For instance, new fluorotungstate red-emitting phosphors A2WO2F4:Mn4+ (A = Na, Cs) were successfully synthesized through a co-precipitation method and intense ZPL emissions were detected under blue light excitation.22,23 Similarly, in the newly obtained fluoromolybdate red phosphor Cs2MoO2F4:Mn4+, the ZPL emission is also observed despite the lower intensity compared with the phonon sidebands.24
In this work, a fluorotantalate compound, i.e., K3TaOF6 which can offer a distorted octahedral polyhedron was taken as the matrix. The host material was synthesized by the solid-state reaction and samples of K3TaOF6:Mn4+ were prepared through the hydrothermal method. The high phase purity of the as-prepared samples was demonstrated by XRD and TEM analyses. As expected, an intense ZPL at 620 nm was observed in K3TaOF6:Mn4+ upon blue light excitation. In this article, the synthetic method, luminescence property and thermal quenching of this series of phosphors will be reported.
2. Sample preparation
The raw materials used for the synthesis were Ta2O5 (A.R.), KF (A.R.), K2TaF7 (A.R.), KMnO4 (99.99%), KHF2 (A.R.), HF (49%) and H2O2 (30%).The Mn4+-activated fluorotantalate K3TaOF6:Mn4+ phosphor was prepared via the solid-state reaction and hydrothermal method in sequence (Fig. 1(a)).
 | ||
| Fig. 1 (a) Synthesis schematic diagram of K3TaOF6 and K3TaOF6:Mn4+ (b) X-ray diffraction patterns of K3TaOF6:Mn4+ samples. | ||
Step I: pure K3TaOF6 host powder was synthesized by the following stoichiometric equation:25
| 3K2TaF7 + Ta2O5 + 9KF → 5K3TaOF6 | (1) |
The mixtures were heated at 900 °C for 0.5 h under an Ar atmosphere. As presented in Fig. 1(b), the main XRD diffraction peaks of the as-obtained host are consistent with the standard XRD pattern of K3TaOF6 (PDF No. 29-1052). Then powders of the obtained K3TaOF6 and prefabricated K2MnF6 in different molar ratios were mixed and ground for 0.5 h in an agate mortar. K2MnF6 was obtained from previous work.19
Step II: loaded the mixtures into Teflon-lined autoclaves and added a drizzle of HF. The autoclaves were heated to 180 °C, kept for 1 h, and then cooled down to room temperature. Finally, the product was collected by centrifugation, subsequently washed with ethanol for three times and dried at 120 °C for 8 h.
The obtained diffraction peaks of K3TaOF6:Mn4+ also agree well with the standard data of K3TaOF6 and no impurity phase was observed with the increasing doping amount of K2MnF6, illustrating the high phase purity of the as-obtained K3TaOF6:Mn4+ samples.
3. Results and discussion
3.1 Phase, morphology and composition
Fig. 2(a) shows the XRD Rietveld refinement results of K3TaOF6:0.04Mn4+. The experimental data are consistent with calculated patterns deriving from the K3NbOF6 matrix (ICSD code: 26634). The good weight-and profile-R-parameters (Rp = 5.94% and Rwp = 8.56%) prove the high match of K3TaOF6 to K3NbOF6. Then, the cell volumes for K3TaOF6:xMn4+ as determined by Rietveld refinement of powder XRD data is presented in Fig. S1.† According to the results, the volumes gradually decrease with the change of x from 0 to 0.12, due to the Ta5+ and Mn4+ ions have the different radius. In the structure of K3TaOF6, each Ta atom may be coordinated by six F atoms and one O atom and form a highly disordered octahedron of [TaOF6]3−. Once the Mn4+ ions invade the crystal lattice of K3TaOF6, the similar ionic radii between Mn4+ and Ta5+ ions make Mn4+ ions easy to occupy the sites of Ta5+ ions. However, the substitution of Mn4+ ions for Ta5+ ions would inevitably bring some point defects. The doped phase can be represented through the formula K3Ta(1 − x)MnxO(1 − y)(Vox)yF6, with oxygen vacancies (Vox) in the lattice (alternately F vacancies). Meanwhile, Ta5+ and Mn4+ cations would coexist which would require electrical compensation with O or F vacancies. The highly-distorted octahedral coordination environment of Mn4+ ions may induce the local symmetry to lose the inversion centre, which is expected to heighten the emission intensity of ZPL, while the crystal defects would has serious effect for the thermal stability.26TEM analysis in Fig. 2(b) demonstrates that the whole particle of the K3TaOF6:0.04Mn4+ sample exhibits the crystalline nature. Moreover, with the help of HRTEM, lattice fringes could be observed clearly in Fig. 2(c) and two interplanar spacings of 5.15 Å and 4.45 Å were obtained, which are corresponding to the (1 1 1) and (2 0 0) plane of K3TaOF6, respectively. From Fig. 2(d), it can be seen that the represented particles of K3TaOF6:0.04Mn4+ have irregular morphology with the size of particle being ∼3 μm and exhibit a degree of particle agglomeration. Moreover, the energy dispersive X-ray spectroscopy (EDS) elemental mapping technique was used to confirm the composition uniformity of K3TaOF6:Mn4+, as presented in Fig. 2(e)–(i). The elemental mapping images exhibit that K, Ta, O, F and Mn are homogeneously distributed within the phosphor particle. In addition, the atom percentages of K, Ta, O and F element are 26.2%, 10.3%, 11.5% and 52.0%, respectively, which is close to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 of K3TaOF6.
5 of K3TaOF6.
3.2 Room-temperature photoluminescence
Fig. S2(a)† shows the diffuse reflection spectrum (DRS) of K3TaOF6:xMn4+ phosphors. The absorption in the blue and UV region arises from the spin-allowed transition of the Mn4+ ion in d3 configuration. Based on the DR spectrum of non-doped sample, the optical band gap (Egap) of the K3TaOF6 host calculated from the Kubelka–Munk's equations27 is approximately 4.04 eV, as denoted in Fig. S2(b).† When the Mn4+ ions are embedded into the host lattice, some well-localized energy states belonging to the ground configuration and excited configuration of impurity are introduced between the valence and the conduction bands of the host. Such a wide band gap could provide enough space to accommodate the energy levels of the activator, which can restrain the overlap between the excited state and conduction band.Fig. 3(a) displays the 3D PL emission spectra of K3TaOF6:0.04Mn4+ with different excitation wavelengths at room temperature. Under various photon irradiation, the shape and position of characteristic peaks almost unchanged, indicating the good color stability of K3TaOF6:0.04Mn4+. Then, iso-intensity contours at the longer excitation wavelengths were recorded to typical 2D photoluminescence excitation (PLE) and PL spectra (Fig. 3(b) and (c)) for further analysis. The emission spectrum excited by 470 nm blue light shows spin-forbidden 2Eg→4A2g transition peaks with phonon-coupled vibronic Stokes/anti-Stokes modes of MnOF6 octahedron. Obviously, an intense ZPL was recorded at about 620 nm, credited to the extremely distorted coordination environment of Mn4+ ions. The PLE spectrum detecting at 628 nm shows two wide excitation bands at 470 nm and 370 nm, assigned to the spin-allowed 4A2g→4T2g and 4A2g→4T1g transitions of Mn4+ ions, respectively.19,20 Notably, a substantial overlap between the broad blue absorption band and the emission region of InGaN blue chip were found, indicating that K3TaOF6:0.04Mn4+ could be a promising candidate for LCD. Moreover, the obtained CIE chromaticity coordinates of (0.691, 0.309) calculated from the emission spectrum are quite close to the red color standard values of (0.67, 0.33) and the correlated color temperature calculated to be 4451 K, as shown in Fig. 3(d).28 Besides, the color purity of this red phosphor was evaluated to be 96.4%, attributing to its narrow emission band.29 Consequently, K3TaOF6:0.04Mn4+ is a promising red phosphor which has potential application in backlit display field. To achieve the optimization of the luminescence performance of K3TaOF6:Mn4+, a group of samples with various molecular concentration of Mn4+ ions from 0.02 to 0.12 were synthesized. As displayed in Fig. S3(a),† under the 470 nm blue-light excitation, the integrated intensity exhibits an increase first and the optimal doping concentration of Mn4+ ions is 4 mol%, then concentration quenching occurs. It is accepted that non-radiative energy transfer between the adjoining luminescence centers contributed to this phenomenon. Fig. S3(b)† shows the luminescent decay curves of K3TaOF6:xMn4+ measured under excitation at 470 nm. Evidently, the luminescence of Mn4+ ions decays exponentially in K3TaOF6 host and the calculated lifetime of this group of samples declines from 4.38 to 3.31 ms with the increase of the concentration. It is believed that the gradually enhanced nonradiative transition between Mn4+ ions is responsible for the shortening of the lifetime. Specifically, a lifetime less than 5 ms could efficiently avoid the image-retention phenomenon in the application of backlight display.
The influence of crystal field strength on the energy levels of Mn4+ in K3TaOF6 could be analyzed via the Tanabe–Sugano diagram. According to Tanabe–Sugano diagram,17 the values of the crystal field strength Dq and the Racah parameters B and C of K3TaOF6:Mn4+ phosphor were calculated to be ∼2127 cm−1, 527 cm−1 and 3932 cm−1, respectively. Thus, the Dq/B is equivalent to 4.03, implying a high crystal field strength in K3TaOF6 host lattice (Fig. S4(a)†).
The nephelauxetic effect caused by the chemical bonds between Mn4+ and F−/O2−ligand often determines the emission energy of Mn4+:2E→4A2g. Therefore, a parameter β1 was adopted to quantitatively describe the nephelauxetic effect in the spectroscopy of the Mn4+:22
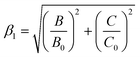 | (2) |
3.3 Temperature-dependent luminescence
To get a better study on temperature-dependent luminescence behavior of K3TaOF6:Mn4+, the PL spectra of K3TaOF6:0.04Mn4+ phosphor excited by 470 nm blue light at various temperature (290–435 K) were measured. The temperature-dependent emission intensity spectra and the corresponding intensity trend were plotted in Fig. 4(a) and (b). Clearly, the integrated PL intensity of K3TaOF6:0.04Mn4+ decreases with the increasing of temperature and the red phosphor shows a remarkable thermal quenching because of the severer non-radiative transition at higher temperature. The thermal activation energy (ΔEa) can be determined by Arrhenius equation:22
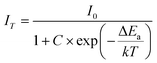 | (3) |
where I0 is the emission intensity at temperature 290 K, C and k is constant. So the calculated ΔEa can be determined to be 0.218 eV. With the increasing of temperature, most electrons at 4T2g state will absorb the heat and climb to the crossover point of the 4T2g and 4A2g states once the accumulated energy exceeds ΔEa. Then, these electrons will return to the ground state 4A2g by a non-radiative way and give rise to thermal quenching, as demonstrated in configurational coordinate diagram (Fig. 4(c))
Besides, color stability is another critical parameter for phosphors, which can be described with CIE shift (ΔE). The calculated equation is as follows:30,31
 | (4) |
Killer centers would be formed when the Mn4+ ions occupy the sites of Ta5+ ions, which may accelerate the thermal quenching process. In K3TaOF6:Mn4+, there are two kinds of killer centers, such as O or F vacancies. To further study the nature of the traps, EPR was adopted to probe the point defects and impurity ions. As shown in Fig. 5(a), the EPR spectra of the K3TaOF6:0.04Mn4+ before/after irradiation were recorded. When the specimen was illuminated with blue-light (450 nm), an almost symmetric EPR signal appeared at g = 1.998. The negative g shift is attributed to electrons trapped in K3TaOF6 host.32–34 In addition to EPR analysis, to study the thermal ionization process in K3TaOF6:0.04Mn4+, the thermoluminescence (TL) spectrum monitored by temperature (90–450 K) was collected with the sample exposure to UV light for 120 s, as shown in Fig. 5(b). Obviously, there is one broad band locating at 100–250 K, corresponding to the trapping energy level. Generally, shallow traps are easier to capture and release the electrons or holes than the deep ones. As a consequence, when the temperature reaches to a certain value, the captured electrons or holes would escape from the shallow traps, which leads to thermal quenching. In Fig. 5(b), there are two Gaussian peaks locating at around 156 K and 200 K respectively for K3TaOF6:0.04Mn4+. The phenomenon that the integrated PL intensity is sensitive to the heating temperature and decreases dramatically with the increasing temperature strongly indicates that thermal ionization is the real villain for thermal quenching of K3TaOF6:0.04Mn4+. The trap depth E was estimated to range from 0.31 to 0.40 eV according to the equation of E = Tm/500, where Tm is the peak temperature. To get a better understand of the process, the charging for TL measurement and thermal quenching induced by thermal ionization was illustrated in Fig. 5(c).
 | ||
| Fig. 5 (a) The EPR spectra of K3TaOF6:Mn4+ before/after irrational. (b) TL spectrum and theoretically fitted spectra of K3TaOF6:Mn4+ sample. (c) Schematic illustration of thermal quenching. | ||
4. Conclusions
In summary, Mn4+-activated fluorotantalate red phosphor K3TaOF6 was successfully obtained. This phosphor shows intense red emission peaking at 628 nm along with a high intensity ZPL emission under blue light excitation. The optimal doping concentration of Mn4+ ions is 4 mol%. By conducting the TL and EPR analysis, thermal ionization may play a dominant role in the thermal quenching.Conflicts of interest
The authors declared that they have no conflicts of interest to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 51902226, 61905099, 52002288), the National Natural Science Foundation of Guangdong Province (No. 2019A1515012072), the Innovation Projects of Department of Education of Guangdong Province (No. 2018KQNCX266), Innovative Leading Talents of Jiangmen [Jiangmen (2019) 7], the Science and Technology Projects of Jiangmen (No. 2020JC01012, 2020JC01018), the research and development fund of Wuyi university joint Hong Kong-Macao (No. 2019WGALH04, No. 2019WGALH09).References
- C. C. Lin and R. S. Liu, Advances in phosphors for light-emitting Diodes, Phys. Chem. Lett., 2011, 2, 1268–1277 CrossRef CAS PubMed.
- H. A. Höppe, Recent developments in the field of inorganic phosphors, Angew. Chem., Int. Ed., 2009, 48, 3572–3582 CrossRef.
- S. Ye, F. Xiao, Y. X. Pan, Y. Y. Ma and Q. Y. Zhang, Phosphors in phosphor-converted white light-emitting diodes recent advances in materials, techniques and properties, Mater. Sci. Eng., R, 2010, 71, 1–34 CrossRef.
- G. C. Adhikari, H. Y. Zhu, P. A. Vargas and P. F. Zhu, UV-green emission from organolead bromide perovskite nanocrystals, J. Phys. Chem. C, 2018, 122, 15041–15046 CrossRef CAS.
- G. C. Adhikari, S. Thapa, Y. Yue, H. Y. Zhu and P. F. Zhu, Thiocyanate based all-inorganic perovskite and their applications in white light-emitting diodes, Photonics, 2021, 8, 209 CrossRef.
- H. X. Liao, Z. Zhao, Y. Y. Zhou, M. S. Molokeev, Q. L. Liu, Q. Y. Zhang and Z. G. Xia, Polyhedron transformation toward stable narrow-band green phosphors for wide-color gamut liquid crystal display, Adv. Funct. Mater., 2019, 29, 1901988 CrossRef.
- E. H. Song, Y. Y. Zhou, Y. Wei, X. X. Han, Z. R. Tao, R. L. Qiu, Z. G. Xia and Q. Y. Zhang, A thermally stable narrow-band green-emitting phosphor MgAl2O4:Mn2+ for wide color gamut backlight display application, J. Mater. Chem. C, 2019, 7, 8192–8198 RSC.
- H. Lin, T. Hu, Q. M. Huangn, Y. Cheng, B. Wang, J. Xu, J. M. Wang and Y. S. Wang, Non-rare-earth K2XF7:Mn4+ (X = Ta, Nb): a highly-efficient narrow-band red phosphor enabling the application in wide-color-gamut LCD, Laser Photonics Rev., 2017, 11, 1700148 CrossRef.
- L. Wang, X. J. Wang, T. Kohsei, K. I. Yoshimura, M. Izumi, N. Hirosaki and R. J. Xie, Highly efficient narrow-band green and red phosphors enabling wider color-gamut LED backlight for more brilliant displays, Opt. Express, 2015, 23, 28707–28717 CrossRef CAS PubMed.
- S. X. Li, L. Wang, D. M. Tang, Y. J. Cho, X. J. Liu, X. T. Zhou, L. Lu, L. Zhang, T. Takeda, N. Hirosaki and R. J. Xie, Achieving high quantum efficiency narrow-band β-Sialon:Eu2+ phosphors for high-brightness LCD backlights by reducing the Eu3+ luminescence killer, Chem. Mater., 2018, 30, 494–505 CrossRef CAS.
- B. Wang, H. Lin, J. Xu, H. Chen and Y. S. Wang, CaMg2Al16O27:Mn4+-based red phosphor: a potential color converter for high-powered warm W-LED, ACS Appl. Mater. Interfaces, 2014, 6, 22905–22913 CrossRef CAS PubMed.
- S. G. He, L. Q. Yao, W. T. Cai, D. Wu, J. Q. Peng and X. Y. Ye, A novel Mn4+ doped oxyfluoride red phosphor for rapid-response backlights display, Dalton Trans., 2020, 49, 11290–11299 RSC.
- Q. Y. Wu, C. X. Liao, J. Q. Pan, X. Y. Ye, W. X. You and L. B. Xia, HF-free molten salt route for synthesis of highly efficient and water-resistant K2SiF6:Mn4+ for warm white LED, J. Am. Ceram. Soc., 2020, 103, 6901–6912 CrossRef CAS.
- H. Ming, L. L. Liu, S. A. He, J. Q. Peng, F. Du, J. X. Fu, F. L. Yang and X. Y. Ye, An ultra-high yield of spherical K2NaScF6:Mn4+ red phosphor and its application in ultra-wide color gamut liquid crystal displays, J. Mater. Chem. C, 2019, 7, 7237–7248 RSC.
- L. Huang, Y. W. Zhu, X. J. Zhang, R. Zou, F. J. Pan, J. Wang and M. M. Wu, HF-Free hydrothermal route for synthesis of highly efficient narrow-band red emitting phosphor K2Si1-xF6:xMn4+ for warm white Light-Emitting Diodes, Chem. Mater., 2016, 28, 1495–1502 CrossRef CAS.
- D. Q. Chen, Y. Zhou, W. Xu, J. S. Zhong, Z. G. Ji and W. D. Xiang, Enhanced luminescence of Y3Al5O12:Mn4+ red phosphor via impurity doping, J. Mater. Chem. C, 2016, 4, 1704–1712 RSC.
- B. Wang, H. Lin, F. Huang, J. Xu, H. Chen, Z. B. Lin and Y. S. Wang, Non-rare-earth BaMgAl10-2xO17:xMn4+,xMg2+: a narrow-band red phosphor for use as a high-power warm w-LED, Chem. Mater., 2016, 28, 3515–3524 CrossRef CAS.
- H. Chen, H. Lin, Q. M. Huang, F. Huang, J. Xu, B. Wang, Z. B. Lin, J. C. Zhou and Y. S. Wang, A novel double-perovskite Gd2ZnTiO6:Mn4+ red phosphor for UV-based w-LEDs: structure and luminescence properties, J. Mater. Chem. C, 2016, 4, 2374–2381 RSC.
- Y. Jin, M. H. Fang, M. Griberg, S. Mahlik, T. Lesniewski, M. G. Brik, G. Y. Luo, J. G. Lin and R. S. Liu, Narrow red emission band fluoride phosphor KNaSiF6:Mn4+ for warm white light-emitting diodes, ACS Appl. Mater. Interfaces, 2016, 8, 11194–11203 CrossRef CAS PubMed.
- A. M. Srivatava and W. W. Beers, Luminescence of Mn4+ in the distorted perovskite Gd2MgTiO6, J. Electrochem. Soc., 1996, 143, L203–L205 CrossRef.
- D. Q. Chen, Y. Zhou and J. S. Zhong, A review on Mn4+ activators in solids for warm white light-emitting diodes, RSC Adv., 2016, 6, 86285–86296 RSC.
- T. Hu, H. Lin, Y. Cheng, Q. M. Huang, J. Xu, Y. Gao, J. M. Wang and Y. S. Wang, A highly-distorted octahedron with a C2v group symmetry inducing an ultra-intense zero phonon line in Mn4+-activated oxyfluoride Na2WO2F4, J. Mater. Chem. C, 2017, 5, 10524–10532 RSC.
- S. Adachi, Photoluminescence spectra and modeling analyses of Mn4+-activated fluoride phosphors: a review, J. Lumin., 2018, 197, 119–130 CrossRef CAS.
- P. Cai, L. Qin, C. Chen, J. Wang and H. J. Seo, Luminescence, energy transfer and optical thermometry of a narrow red emitting phosphor: Cs2WO2F4:Mn4+, Dalton Trans., 2017, 46, 14331–14340 RSC.
- L. Kosa and I. Mackova, Determination of the enthalpy of fusion of K3TaF8 and K3TaOF6, Thermochim. Acta, 2006, 447, 209–211 CrossRef CAS.
- S. G. He, F. F. Xu, T. T. Han, Z. Q. Lu, W. Wang, J. Q. Peng, F. Du, F. L. Yang and X. Y. Ye, A Mn4+-doped oxyfluoride phosphor with remarkable negative thermal quenching and high color stability for warm WLEDs, Chem. Eng. J., 2020, 392, 123657 CrossRef CAS.
- B. Wang, H. W. Wang, J. H. Huang, J. C. Zhou and P. F. Liu, Trap distribution and photo-stimulated luminescence in LaSrAl3O7:Eu2+ long-lasting phosphors for optical data storage, J. Am. Ceram. Soc., 2020, 103, 315–323 CrossRef CAS.
- G. C. Adhikari, P. A. Vargas, H. Y. Zhu, A. Grigoriev and P. F. Zhu, Tetradic phosphor white light with variable CCT and superlative CRI through organolead halide perovskite nanocrystals, Nanoscale Adv., 2019, 1, 1791–1798 RSC.
- Y. Zhou, S. Zhang, X. M. Wang and H. Jiao, Structure and luminescence properties of Mn4+-activated K3TaO2F4 red phosphor for white LEDs, Inorg. Chem., 2019, 48, 4412–4419 CrossRef.
- Y. Heeyeon, K. Yoshihiro, Y. Hee Chang, J. P. Sang, H. O. Ji and R. D. Young, Sn–P–F containing glass matrix for the fabrication of phosphor-in-glass for use in high power LEDs, RSC Adv., 2016, 6, 111640–111647 RSC.
- G. C. Adhikari, S. Thape and H. Y. Zhu, Mg2+-alloyed all-inorganic halide perovskites for white light-emitting diodes by 3D-printing method, Adv. Opt. Mater., 2019, 7, 1900916 CrossRef CAS.
- V. Castaing, A. D. Sontakke, A. J. Fernandez-Carrion, N. Touati, L. Binet, M. Allix, D. Gourier and B. Viana, Persistent luminescence of ZnGa2O4:Cr3+ transparent glass ceramics: effects of excitation wavelength and excitation power, Eur. J. Inorg. Chem., 2017, 44, 5114–5120 CrossRef.
- P. F. Li, M. Y. Peng, L. Wondraczek, Y. Q. Zhao and B. Viana, Red to near infrared ultralong lasting luminescence from Mn2+-doped sodium gallium aluminum germanate glasses and (Al,Ga)-albite glass-ceramics, J. Mater. Chem. C, 2015, 3, 3406 RSC.
- T. Hu, H. Lin, J. Xu, B. Wang, J. M. Wang and Y. S. Wang, Color-tunable persistent luminescence in oxyfluoride glass and glass ceramic containing Mn2+:α-Zn2SiO4 nanocrystals, J. Mater. Chem. C, 2017, 5, 1479–1487 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra05174a |
| This journal is © The Royal Society of Chemistry 2021 |



