Open Access Article
Open Access ArticleSynthesis of functionalized benzo[1,3]dioxin-4-ones from salicylic acid and acetylenic esters and their direct amidation†
Rasmi P. Bhaskaran ,
Kalinga H. Nayak
,
Kalinga H. Nayak and
Beneesh P. Babu
and
Beneesh P. Babu *
*
Department of Chemistry, National Institute of Technology Karnataka – NITK, Surathkal 575025, Mangalore, India. E-mail: pbbeneesh@nitk.edu.in; Tel: +91-824-2473219
First published on 13th July 2021
Abstract
Direct synthesis of 4H-benzo[d][1,3]dioxin-4-one derivatives from salicylic acids and acetylenic esters (both mono- and disubstituted) has been described. The reaction is mediated by CuI and NaHCO3 in acetonitrile. Room temperature amidation of the synthesized 1,3-benzodioxinones with primary amines readily afforded the corresponding salicylamides in moderate to good yields.
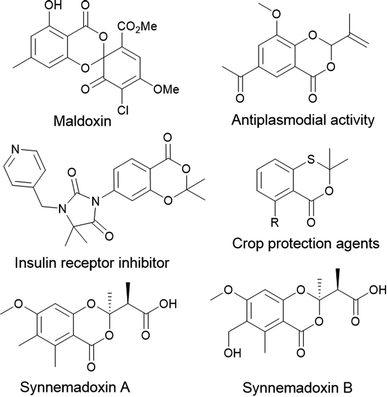
Heterocycles and their derivatives play a pivotal role in natural products and synthetic organic chemistry. Isolation, bio and chemical synthesis, and investigation of pharmacological and biological properties of diverse heterocycles have attracted both organic and medicinal chemists.1 It is a highly vibrant and ever-expanding field of research. Besides, heterocycle scaffolds contribute significantly towards the development of novel organic materials for luminescent applications due to their unique photophysical properties, which can be tuned to suit diverse applications.2 Benzodioxans are oxygen-based isomeric heterocycles with various applications in medicinal, agricultural, and synthetic chemistry. Among the isomeric benzodioxans, 1,3-benzodioxane and its derivatives are used in medicinal and agrochemicals chemistry research.3 In addition, they are potential synthetic intermediates in multistep organic synthesis.4 Among the various 1,3-benzodioxane derivatives, 4H-benzo[d][1,3]dioxin-4-one has been identified as an active core in many biologically active molecules such as nucleoside base transport inhibitor, topoisomerase I inhibitor, antiplasmodial and cytotoxic drugs, etc., and its thio derivatives find applications as an insecticide, crop protection agents, and fungicides.5 Few examples have been shown in Fig. 1.
Salicylic acid and its derivatives are widely used to access the 4H-benzo[d][1,3]dioxin-4-one scaffolds, and several approaches have been reported in the literature.6 In a similar line, reports in which thiosalicylic acid yielding the corresponding benzo-1,3-oxathiine derivatives are also reported.7 For example, conversion of salicylic acid to benzo[d][1,3]dioxin-4-ones using dichloromethane as the methylene donor was reported by Xiuling Cui under catalyst-free condition using potassium phosphate.8 Later, Liu et al. developed a Cu(OAc)2 catalyzed approach based on ortho-halobenzoic acid, KOH, and NaHCO3.9 Morpholine catalyzed conversion of salicylic acid to the benzodioxinone scaffold was reported by Qiu and co-workers treating with ynones.10 In 2017, Kawatsura et al. reported the synthesis of 1,3-oxathiine derivatives via an iron-catalyzed intermolecular reaction between thiosalicylic acid and internal alkynes.11 Very recently, Muthusamy et al. reported a catalyst-free synthesis of 1,3-oxathiine derivatives by treating thiosalicylic acid and substituted propargylic alcohols.12 Due to our continued interest in the development of novel and modified synthetic routes to heterocycles,13 we herein report a modified route to synthesize benzo[d][1,3]dioxin-4-ones from salicylic acid and acetylenic esters. To the best of our knowledge, this is the first report on the synthesis of benzo[d][1,3]dioxin-4-ones from salicylic acid and acetylenic esters though the corresponding benzo-1,3-oxathiine route has already been documented.11 Interestingly, both mono- and disubstituted acetylenic esters were equally effective in the reaction. The benzo[d][1,3]dioxin-4-ones derivatives synthesized further readily converted to amides when treated with primary amines at room temperature.
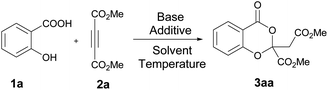
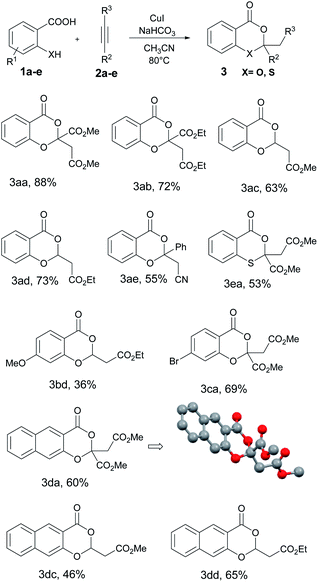
From the literature, we understand that the reaction between salicylic acid and acetylenic esters is not yet explored in detail though the analogous thiosalicylic acid-based methods have been well documented. We started our investigations using the salicylic acid 1a and dimethyl acetylenedicarboxylate (DMAD) 2a as the model substrates and the reaction optimization observations of the pilot experiment are summarized in Table 1. Stirring 1a (0.6 mmol) and 2a (0.5 mmol) together in a Schlenk tube using acetonitrile (2 ml) as the solvent resulted in no characteristic reaction at room temperature as well as at 80 °C. We further introduced a base into the reaction medium. Both organic and inorganic bases were screened to identify the best condition. Organic bases such as pyridine and DABCO failed to afford the expected product under hot conditions. Interestingly, inorganic bases succeeded in yielding the expected benzo[d][1,3]dioxin-4-one with moderate conversion. NaHCO3 (1.2 equiv.) was proved to be the best base for the reaction, yielding 55% of the product 3aa (Table 1, entry 5). We further studied the effect of different metal salts such as CuI, Pd(OAc)2, CuCl, FeCl3, NiCl2, and MnCl2 in the reaction. Among the various metal salts screened, CuI showed a remarkable influence on the outcome of the reaction as summarized in Table 1. Stoichiometric CuI (1 equiv.) as an additive in presence of NaHCO3 (1.2 equiv.) base enhanced the yield of the final benzo[d][1,3]dioxin-4-one derivative to 88% in 24 h (entry 8). We noticed that the presence of both NaHCO3 and CuI was crucial for the reaction as withdrawal of any one of the reagents affected the overall conversion (entries 5 and 9). Acetonitrile was finalized as the best solvent for the reaction after screening various solvents. THF and 1,2-DCE yielded the product in trace amount while methanol and DMSO failed to promote the reaction. In short, after a series of optimization experiments, we identified the best reaction condition for the synthesis of 3aa as treating 1a and 2a in acetonitrile in presence of CuI (1 equiv.) and NaHCO3 (1.2 equiv.) at 80 °C for 24 h.
| S. no. | Base | Additive | Solvent | T (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a All reactions were carried out in a Schlenk tube in 0.5 mmol scale. Reaction conditions: 1a (1.2 equiv.), 2a (1 equiv.), base (1.2 equiv.), additive (1 equiv.), solvent (2 ml), 24 h, 80 °C.b Isolated yield after column chromatography is reported.c nr: no reaction. | |||||
| 1 | Pyridine 10 mol% | — | CH3CN | 80 | nrc |
| 2 | DABCO 10 mol% | — | CH3CN | 80 | nr |
| 3 | K2CO3 20 mol% | — | CH3CN | 80 | Trace |
| 4 | NaHCO3 20 mol% | — | CH3CN | 80 | 40 |
| 5 | NaHCO3 1.2 equiv. | — | CH3CN | 80 | 55 |
| 6 | NaHCO3 1.2 equiv. | CuI 20 mol% | CH3CN | 80 | <60 |
| 7 | NaHCO3 1.2 equiv. | Pd(OAc)2 10 mol% | CH3CN | 80 | 45 |
| 8 | NaHCO3 1.2 equiv. | CuI 1 equiv. | CH3CN | 80 | 88 |
| 9 | — | CuI 1 equiv. | CH3CN | 80 | 30 |
| 10 | NaHCO3 1.2 equiv. | CuI 1 equiv. | 1,2-DCE | 80 | Trace |
| 11 | NaHCO3 1.2 equiv. | CuI 1 equiv. | CH3OH | 60 | nr |
| 12 | NaHCO3 1.2 equiv. | CuI 1 equiv. | THF | 60 | Trace |
| 13 | NaHCO3 1.2 equiv. | CuI 1 equiv. | DMSO | 100 | Sluggish reaction |
| 14 | NaHCO3 1.2 equiv. | FeCl3 1 equiv. | CH3CN | 80 | nr |
| 15 | NaHCO3 1.2 equiv. | CuCl 1 equiv. | CH3CN | 80 | Trace |
| 16 | NaHCO3 1.2 equiv. | NiCl2 1 equiv. | CH3CN | 80 | nr |
| 17 | NaHCO3 1.2 equiv. | MnCl2 1 equiv. | CH3CN | 80 | nr |
With the optimized reaction conditions in hand, we subsequently explored the substrate scope of the reaction using various 2-hydroxy aromatic acids and acetylenic esters. Interestingly, both disubstituted acetylenic esters – such as DMAD 2a and diethyl acetylenedicarboxylate 2b – and monosubstituted acetylenic esters – such as methyl propiolate 2c and ethyl propiolate 2d – were equally effective in the optimized conditions affording the benzo[d][1,3]dioxin-4-one derivatives in moderate to good yields (Table 2). Surprisingly, the hindered 3-phenylpropiolonitrile 2e in place of acetylenic esters also afforded the expected product in 55% yield. However, ethyl phenylpropiolate, phenylacetylene and acetylenic ketone such as 4-phenylbut-3-yn-2-one failed to yield the product. Apart from salicylic acid, substituted salicylic acids – 4-methoxy derivative 1b and 4-bromo derivative 1c – were also used in the reaction. The former afforded 36% of the final product while the latter yielded 69%. Electron deficient 2-hydroxy-3-nitrobenzoic acid and 3-hydroxypicolinic acid were not successful in the reaction as the former yielded a sluggish reaction mixture while the latter didn't react at all. Interestingly, the hindered 3-hydroxy-2-naphthoic acid 1d reacted efficiently with both DMAD and ethyl propiolate with good conversion. However, with thiosalicylic acid 1e, conversion was moderate with DMAD and very low with methyl propiolate.
| a All reactions were carried out in a Schlenk tube. Reaction conditions: 1a (1.2 equiv.), alkyne (1 equiv.), NaHCO3 (1.2 equiv.), CuI (1 equiv.), acetonitrile (2 ml), 24 h, 80 °C. Isolated yield after column chromatography is reported. |
|---|
 |
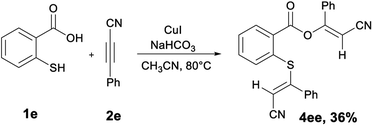
Surprisingly, an unexpected reactivity was observed when thiosalicylic acid is treated with 3-phenylpropiolonitrile 2e under the optimized reaction conditions. Contrary to the expected 1,3-benzothiinone, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adduct 2-cyano-1-phenylvinyl(2-(2-cyano-1,2-diphenylvinyl)-thio)benzoate (4ee) was obtained as the final product (Scheme 1).
2 adduct 2-cyano-1-phenylvinyl(2-(2-cyano-1,2-diphenylvinyl)-thio)benzoate (4ee) was obtained as the final product (Scheme 1).
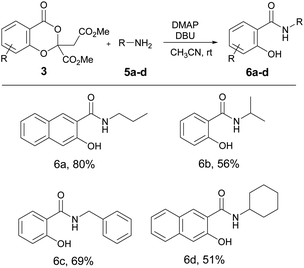
The 4H-benzo[d][1,3]dioxin-4-one derivatives are widely used in synthetic organic chemistry as a versatile synthetic intermediate. A spectrum of compounds such as amide, ester, alcohol, aldehyde etc. can be easily accessed from the 1,3-benzodioxinone derivatives.14 We were particularly interested in their conversion to amides, because salicylamides are widely used in medicinal chemistry as various receptor inhibitors.15 Keeping this in mind, we treated the final 1,3-benzodioxinones with various amines expecting easy access to salicylamides. Following the literature procedure of amide synthesis,16 we treated 3aa and n-propylamine 5a in presence of DMAP (10 mol%) and DBU (1 equiv.) in acetonitrile at room temperature for 8 h. The reaction was successful and the expected salicylamide was formed in good yield (Table 3). The strategy was quite versatile and other primary amines such as isopropylamine 5b, benzylamine 5c and cyclohexylamine 5d readily afforded the salicylamide in moderate to good yield. At the same time, secondary amines such as diethylamine and diphenylamine were not very effective. 1,3-Benzodioxinones derived from both salicylic acid and 3-hydroxy-2-naphthoic acid yielded the amide at room temperature. However, attempt to perform the amide synthesis in a one-pot, two step process, without isolating the 1,3-benzodioxinone was less effective as the amide 6a was formed in 35% yield. It is noteworthy that the previous reports on the conversion of 1,3-benzodioxinones to amide involved treating the reagents in refluxing toluene while the present methodology made it feasible at room temperature itself.15 However, our initial results show that the method is best suited for aliphatic primary amines as the poor conversion was observed with anilines and secondary amines.
| a Reaction conditions: 3 (1 equiv.), amine (1.1 equiv.), DMAP (10 mol%), DBU (1 equiv.), acetonitrile (4 ml) 8 h, rt. Isolated yield after column chromatography is reported. |
|---|
 |
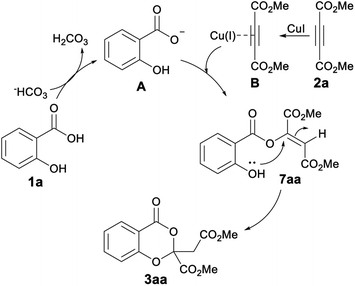
A plausible reaction mechanism is shown in Scheme 2 based on the control experiment conducted. The initial event is the acid deprotonation of 1a by the base.17 The carboxylate then adds to the Cu(I) coordinated alkyne to afford the linear adduct 7aa which was isolated, characterized and compared with the literature reports.18 The linear adduct subsequently undergoes intramolecular cyclization with ortho-hydroxyl group generating the final product.
Conclusions
In summary, an efficient and modified strategy for 4H-benzo[d][1,3]dioxin-4-one derivatives has been developed. The method involves a CuI mediated addition between salicylic acid and acetylenic esters in a basic medium followed by intramolecular cyclization. Disubstituted acetylenic esters and monosubstituted propionic esters are equally effective in the reaction. The 4H-benzo[d][1,3]dioxin-4-one derivatives were further converted into salicylamides by treating with primary amines at room temperature. Investigation to expand the scope of this reaction further is underway in our lab.Conflicts of interest
There are no conflicts to declare.Acknowledgements
B. P. B. acknowledges the Department of Science and Technology (DST), Government of India for an INSPIRE Faculty Award (IFA-13 CH-98). B. P. B., R. P. B., and K. H. N. thank NITK, Surathkal for providing laboratory facility and research fellowships for R. P. B. and K. H. N. The authors also thank NIIST-CSIR Trivandrum, STIC-CUSAT Kochi, CIF-Manipal and NMR Centre, Mangalore University for spectra.Notes and references
- (a) Special Issue: Heterocyclic Chemistry, Eur. J. Org. Chem., 2019, 31–32, 4973–4975 Search PubMed; (b) T. Kunied and H. Mutsanga, in The Chemistry of Heterocyclic Compounds, Academic Press, Palmer, 2002 Search PubMed; (c) N. Panda, P. Mishra and I. Mattan, J. Org. Chem., 2016, 81, 1047–1056 CrossRef CAS PubMed.
- (a) X. Ke, L. Meng, X. Wan, Y. Sun, Z. Guo, S. Wu, H. Zhang, C. Li and Y. Chen, Mater. Chem. Front., 2020, 4, 3594–3601 RSC; (b) A. Pron, P. Gawrys, M. Zagorska, D. Djurado and R. Demadrille, Chem. Soc. Rev., 2010, 39, 2577–2632 RSC; (c) A. Mishra and P. Bäuerle, Angew. Chem., Int. Ed., 2012, 51, 2020–2067 CrossRef CAS PubMed.
- (a) R. G. Dushin and S. J. Danishefsky, J. Am. Chem. Soc., 1992, 114, 655–659 CrossRef CAS; (b) S. W. Kang, C. M. Gothard, S. Maitra, A.-t. Wahab and J. S. Nowick, J. Am. Chem. Soc., 2007, 129, 1486–1487 CrossRef CAS PubMed; (c) G. C. Clososki, C. J. Rohbogner and P. Knochel, Angew. Chem., Int. Ed., 2007, 46, 7681–7684 CrossRef CAS PubMed.
- (a) T. Yoshino, I. Sato and M. Hirama, Org. Lett., 2012, 14, 4290–4292 CrossRef CAS PubMed; (b) D. C. Elliott, T. K. Ma, A. Selmani, R. Cookson, P. J. Parsons and A. G. M. Barrett, Org. Lett., 2016, 18, 1800–1803 CrossRef CAS PubMed; (c) P. A. Evans, M. H. Huang, M. J. Lawler and S. Maroto, Nat. Chem., 2012, 4, 680–684 CrossRef CAS PubMed.
- (a) R. Cookson, T. N. Barrett and A. G. M. Barrett, Acc. Chem. Res., 2015, 48, 628–642 CrossRef CAS PubMed; (b) G. Attardo, B. Zacharie, R. Rej, J. F. Lavallee, L. Vaillancourt, R. Denis and S. Levesque, US Pat., US2003013660(A1), 2003 Search PubMed; (c) M. A. Tasdelen and Y. Yagci, ACS Macro Lett., 2017, 6, 1392–1397 CrossRef CAS; (d) T. S. Cooper, B. Atrash, P. Sheldrake, P. Workman and E. McDonald, Tetrahedron Lett., 2006, 47, 2241–2243 CrossRef CAS.
- (a) J. S. S. Rountree and P. V. Murphy, Org. Lett., 2009, 11, 871–874 CrossRef CAS PubMed; (b) J. K. Augustine, Y. A. Naik, A. B. Mandal, N. Chowdappa and V. B. Praveen, J. Org. Chem., 2007, 72, 9854–9856 CrossRef CAS PubMed; (c) M. J. Fan, G. Q. Li and Y. M. Liang, Tetrahedron, 2006, 62, 6782–6791 CrossRef CAS; (d) H. H. Wang, T. Shi, W. W. Gao, H. H. Zhang, Y. Q. Wang, J. F. Li, Y. S. Hou, J. H. Chen, X. Peng and Z. Wang, Org. Biomol. Chem., 2017, 15, 8013–8017 RSC; (e) L. Qui, X. He and M. Wang, Faming Zhuanli Shenqing, Chines Patent, CN 108383827, 2018 Search PubMed.
- Y. Nishina and J. Miyata, Synthesis, 2012, 44, 2607–2613 CrossRef CAS.
- F. Lin, Q. Song, Y. Gao and X. Cui, RSC Adv., 2014, 4, 19856–19860 RSC.
- Y. Liu, M. Huang and L. Wei, New J. Chem., 2017, 41, 4776–4778 RSC.
- (a) X. He, Y. Li, M. Wang, H. X. Chen, B. Chen, H. Liang, Y. Zhang, J. Pang and L. Qiu, Org. Biomol. Chem., 2018, 16, 5533–5538 RSC; (b) V. K. Tripathi, P. S. Venkataramani and G. Mehta, J. Chem. Soc., Perkin Trans. 1, 1979, 36–41 RSC.
- T. Sonehara, S. Murakami, S. Yamazaki and M. Kawatsura, Org. Lett., 2017, 19, 4299–4302 CrossRef CAS PubMed.
- S. Muthusamy, M. Malarvizhi and E. Suresh, Org. Biomol. Chem., 2021, 19, 1508–1513 RSC.
- (a) J. C. Janardhanan, R. K. Mishra, G. Das, S. Sini, P. Jayamurthy, C. H. Suresh, V. K. Praveen, N. Manoj and B. P. Babu, Asian J. Org. Chem., 2018, 7, 2094–2104 CrossRef CAS; (b) J. C. Janardhanan, K. James, A. Puthuvakkal, R. P. Bhaskaran, C. H. Suresh, V. K. Praveen, N. Manoj and B. P. Babu, New J. Chem., 2019, 43, 10166–10175 RSC; (c) U. Amrutha, B. P. Babu and S. Prathapan, J. Heterocycl. Chem., 2019, 56, 3236–3243 CrossRef CAS; (d) R. P. Bhaskaran, J. C. Janardhanan and B. P. Babu, ChemistrySelect, 2020, 5, 4822–4825 CrossRef CAS.
- (a) O. Soltani and J. K. De Brabander, Angew. Chem., Int. Ed., 2005, 44, 1696–1699 CrossRef CAS PubMed; (b) N. Bajwa and M. P. Jennings, J. Org. Chem., 2006, 71, 3646–3649 CrossRef CAS PubMed; (c) J. Spengler, J. Ruíz-Rodríguez, F. Yraola, M. Royo, M. Winter, K. Burger and F. Albericio, J. Org. Chem., 2008, 73, 2311–2314 CrossRef CAS PubMed.
- (a) K. D. Combrink, H. B. Gulgeze, K. L. Yu, B. C. Pearce, A. K. Trehan, J. Wei, M. Deshpande, M. Krystal, A. Torri, G. Luo, C. Cianci, S. Danetz, L. Tiley and N. A. Meanwell, Bioorg. Med. Chem. Lett., 2000, 10, 1649–1652 CrossRef CAS; (b) M. S. Deshpande, J. Wei, G. Luo, C. Cianci, S. Danetz, A. Torri, L. Tiley, M. Krystal, K. L. Yu, S. Huang, Q. Gao and N. A. Meanwell, Bioorg. Med. Chem. Lett., 2001, 11, 2393–2396 CrossRef CAS; (c) D. Balan, C. J. Burns, N. G. Fisk, H. Hügel, D. C. S. Huang, D. Segal, C. White, J. Wagler and M. A. Rizzacasa, Org. Biomol. Chem., 2012, 10, 8147–8153 RSC.
- X. Yang and V. B. Birman, Org. Lett., 2009, 11, 1499–1502 CrossRef CAS PubMed.
- (a) H. Liang, X. He, Y. Zhang, B. Chen, J. Ouyang, Y. Li, B. Pan, C. V. S. Reddy, W. T. K. Chan and L. Qiu, Chem. Commun., 2020, 56, 11429–11432 RSC; (b) X. Xu, R. Sun, S. Zhang, X. Zhang and W. Yi, Org. Lett., 2018, 20, 1893–1897 CrossRef CAS PubMed.
- See ESI† for more details about control experiment and spectral data of the intermediate.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental information and procedure, full characterization data and spectra (1H and 13C NMR) of all the products are available. CCDC 2084126. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra05032j |
| This journal is © The Royal Society of Chemistry 2021 |