 Open Access Article
Open Access ArticleRhodamine B oxidation promoted by P450-bioinspired Jacobsen catalysts/cellulose systems†
Lucas Bomfim Bolzon *a,
Anna Karolina dos Santos Bindeiroa,
Ana Luiza Marques de Oliveira Souzaa,
Lucas Dimarô Zanattab,
Rodrigo de Paulacd,
Bruna Costa Cerqueirac and
Joicy Santamalvina dos Santosa
*a,
Anna Karolina dos Santos Bindeiroa,
Ana Luiza Marques de Oliveira Souzaa,
Lucas Dimarô Zanattab,
Rodrigo de Paulacd,
Bruna Costa Cerqueirac and
Joicy Santamalvina dos Santosa
aGrupo de Pesquisa em Bioinorgânica e Catálise (GPBioCat), Departamento de Química Geral e Inorgânica, IQ-UFBA, R. Barão de Jeremoabo 147, Campus de Ondina, 40170-115 Salvador, BA, Brazil. E-mail: lucas.bolzon@ufba.br
bLaboratório de Bioinorgânica, Departamento de Química, FFCLRP-USP, Av. Bandeirantes 3900, 14040-901, Ribeirão Preto, SP, Brazil
cCentro de Formação de Professores, UFRB, Av. Nestor de Melo Pita 535, Campus de Amargosa, 45300-000, Amargosa, BA, Brazil
dPrograma de Pós-Graduação em Química Pura e Aplicada-POSQUIPA, Universidade Federal do Oeste da Bahia, Rua Bertioga, 892, Morada Real, 47810-059, Barreiras, BA, Brazil
First published on 2nd November 2021
Abstract
In this work, we investigated the preparation of P450-bioinspired Mn(III)-Schiff base complexes supported on DEAE-cellulose ((R,R)-Jacobsen/Cell(NEt2) and (S,S)-Jacobsen/Cell(NEt2), respectively) to oxidize substrates of biological interest. Catalysts were characterized by several physical techniques. UV-Vis spectroscopy with diffuse reflectance (DR/UV-Vis) analysis featured peculiar electronic transitions for both complexes. Fourier transform infrared (FTIR) spectra evidenced the characteristic band of imine groups (HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) for bioinspired/Cell(NEt2) materials. Immobilization ratios in cellulose fibres were confirmed by atomic absorption spectroscopic (GF-AAS) analyses. Catalytic essays were conducted during rhodamine B (RhB) oxidation. Supported materials attained oxidative yields close to those of homogeneous systems, and cellulose may be stabilizing the active intermediate catalytic species. Reactions may be driven through two different intermediates: MnV(O) and MnIII(O–OH)salen. Homogeneous reactions suggest an asymmetric catalysis. Heterogeneous system reaction yields are similar, and salen complexes anchored on cellulose conformation would interfere on complex intermediate species configuration. The four possible RhB-oxidation products obtained by the reaction with the homogeneous (S,S)-Jacobsen catalyst and meta-chloroperoxybenzoic acid (m-CPBA) system were suggested by 1H NMR analysis, and a catalytic mechanism was proposed.
N) for bioinspired/Cell(NEt2) materials. Immobilization ratios in cellulose fibres were confirmed by atomic absorption spectroscopic (GF-AAS) analyses. Catalytic essays were conducted during rhodamine B (RhB) oxidation. Supported materials attained oxidative yields close to those of homogeneous systems, and cellulose may be stabilizing the active intermediate catalytic species. Reactions may be driven through two different intermediates: MnV(O) and MnIII(O–OH)salen. Homogeneous reactions suggest an asymmetric catalysis. Heterogeneous system reaction yields are similar, and salen complexes anchored on cellulose conformation would interfere on complex intermediate species configuration. The four possible RhB-oxidation products obtained by the reaction with the homogeneous (S,S)-Jacobsen catalyst and meta-chloroperoxybenzoic acid (m-CPBA) system were suggested by 1H NMR analysis, and a catalytic mechanism was proposed.
1. Introduction
By the mid-20th century, it was found that many biological processes from cellular respiration to photosynthesis are centered around redox reactions.1–3 In this context, P450 cytochromes (CYP) are a class of monooxygenase enzymes, characterized by the presence of iron(III) protoporphyrin IX, the enzyme's active site, which metabolizes several classes of organic compounds (e.g. pollutants and drugs) in biological systems.1–9 In the attempt to mimic the CYP reactions, many researchers use synthetic models called ‘bioinspired catalysts’ to reproduce reactions catalysed by these enzymes.9–32 While in nature, metalloenzyme-catalysed oxidations often exhibit exquisite substrate specificity, as well as regioselectivity and/or stereoselectivity, synthetic bioinspired or biomimetic systems may have broader substrate scope and tunable selectivity, which make them challenging protagonists of near-future environmentally friendly catalytic chemistry.9,32 Moreover, the prediction of exogenous molecule metabolism promoted by bioinspired systems can be an important task in preclinical tests, especially for drug development.9,32 Even if model systems do not provide a complete quantitative survey of in vivo situation, biomimetic catalysis allows us to obtain metabolites in a more practical synthesis, with adequate amounts for isolation. This sort of information is valuable for pharmacological and toxicological studies.9Among numerous proposals to mimic monooxygenase active sites, metal-Schiff base complexes stand out, especially Jacobsen chiral catalysts.12,33–38 These salen complexes are efficient catalysts in homogeneous and heterogeneous environment reactions such as olefin ring opening and polymerization,39,40 allyl alkylation,41 alkene epoxidation,12,42,43 alkane oxidation,38 and drug oxidation37,44–47 and act as P450 cytochromes model systems.10,12,25,38,45,48–52 An advantage of the Jacobsen complex is its simple preparation, for example, it can be synthesized on a large scale (in the order of kilograms) at a low cost (US$41.40 per gram).53
Furthermore, biomimetic chemistry has increasingly investigated the role of the protein matrix surrounding the CYP heme group,32 which controls active oxidant reactivity and prevents enzyme inactivation via possible aggregation or active site bimolecular auto-oxidation. Monooxygenase protein folding may act as a co-catalyst for electron donation/removal in redox catalysis, as well as retention of the target molecule within its cavities, allowing the creation of an ideal reaction microenvironment. In pursuit of this purpose, literature over the decades highlights the use of different supports for bioinspired catalysts.20,22–24,54–59 In this context, cellulose is the most abundant natural raw material on the planet. It is a cheap, biodegradable, and renewable polymer, which is fibrous, resistant, and insoluble in water and helps to support the structure of the cell walls of plants, oomycetes, and algae. This biomaterial primarily consists of D-glucopyranose ring units that occur in the 4C1 chair configuration, which are connected by β-1,4-glucosidic linkages, which allow for an alternate rotation of the cellulose chain axis by 180°. The hydroxyl groups in atoms C-2, C-3 and C-6 are positioned in the plane of the ring (equatorial), while the hydrogen atoms are in the vertical (axial) position, enabling the formation of various types of semi-crystalline supramolecular structures. Thus, this biopolymer has certain characteristics such as hydrophilicity, adsorbing potential, non-toxicity, easy chemical modification, and good thermo-mechanical properties. In addition to all these, cellulose and its derived products are harmless to the environment, as they safely return to the natural carbon cycle via a simple decomposition process in the presence of decomposers. These attributes are quite favorable for the development of research and application of this material in several areas of scientific and technological knowledge.60–62
Rhodamine B (RhB, Fig. 1) is an organic chloride salt and a xanthene dye, (N-[9-(2-carboxyphenyl)-6-(diethylamino)-3H-xanthen-3-ylidene]-N-ethylethanaminium), acts as the counterion. In biotechnological tests, this amphoteric dye is commonly used as a fluorochrome, a fluorescent probe, and a histological dye. Among other applications, it is possible to highlight the usage of this substance as a dyestuff of plastics, textile colorant, and dyeing paper and in printing.63 It has been reported in the literature that this compound is used in veterinary drugs,64 as well as herbicide markers.65 However, several dangers to humans and animal health such as irritation to the skin, eyes, respiratory and gastrointestinal tracts are due to this dye;66 besides, it is considered as a carcinogenic, genotoxic, and neurotoxic inducer.67–71 Rhodamine B dye is still used illegally as a food additive, which can be very harmful to consumers' health.67,72 The literature presents some strategies aimed at solutions to the pollution caused by this dye, among which methods of phase transfer removal73–75 such as photodegradation,76,77 electrocatalysis,78 and biodegradation stand out.79 However, it is important to emphasize the importance of investigating the detailed oxidation route of this dye, as well as any other pollutant, as such processes often degrade these substances in a non-selective way, in addition to the fact that many products formed can be more toxic than the initial substrate for living organisms, instead of being mineralized. In this context, the P450-biomimetic/bioinspired catalysis aims to observe the oxidative behavior of these types of compounds, to assist the study of metabolism, even though it is performed in a synthetic way.
In the light of all that has been exposed, herein we report the study of chiral metal complexes (R,R)-Jacobsen and (S,S)-Jacobsen supported in a DEAE-cellulose matrix to evaluate their oxidative P450-bioinspired profiles, as well as their enantio-reactivities toward RhB dye catalysis. In addition to the innovative design of this cellulose material, it is noteworthy that there are no reports in the literature about the oxidation of this dye via P450-bioinspired routes. Therefore, this work intends to understand high-valent metal oxo intermediate species participation, using protic and non-protic solvents and oxygen donors m-CPBA, H2O2 and PhIO. The main text of the article should appear here with headings as appropriate.
2. Experimental
2.1 Chemicals
All reagents and solvents were used without further purification. (R,R)-(−)-N,N′-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride and (S,S)-(−)-N,N′-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride ((R,R)-Jacobsen and (S,S)-Jacobsen respectively from Aldrich), cellulose-functionalized with dimethylaminoethane (DEAE-cellulose or DEAE-cell, J. T. Baker), glacial acetic acid (HAc, Synth), potassium hydroxide (KOH, Vetec), dichloromethane (DCM, CRQ), acetonitrile (ACN, CRQ), methanol (MeOH, CRQ), [9-(2-carboxyphenyl)-6-diethylamino-3-xanthenylidene]-diethylammonium chloride, 99 wt% (rhodamine B, RhB, Fig. 1, Synth), methanol, HPLC grade (MeOH, J. T. Baker), acetonitrile, HPLC grade (ACN, J. T. Baker), trifluoroacetic acid, 99% (TFA, Acros Organics), hexane (Cinética), and 3-chloroperoxybenzoic acid 70 wt% (m-CPBA, Acros Organics) were used in the experiments. Hydrogen peroxide, 50 wt% (Fluka), was checked for purity every three months by titration with permanganate. Iodosylbenzene (PhIO) was synthesized by the hydrolysis of iodobenzene diacetate (98%, Sigma-Aldrich) using a procedure adapted from the literature,80 and its purity was analyzed by iodometric titration. 5,10,15,20-Tetrakis(2,6-dichlorophenyl)porphyrin manganese(III) chloride [MnIII(TDCPP)]Cl was synthesized as reported in the literature.812.2 Synthesis of bioinspired catalysis
The synthesis of (R,R)-Jacobsen/Cell(NEt2) and (S,S)-Jacobsen/Cell(NEt2) catalysis was based on a procedure described in the literature,59 with modifications. As schematically represented in Fig. 2, in a two-neck flask, a mixture containing 25 mL of dilute acetic acid and 2.0 g DEAE-cell was maintained under magnetic stirring at room temperature for 25 min. After this period, 50 mL of deionized water was added to form a colloidal suspension. Then, 15 mg of the respective (R,R)-Jacobsen and (S,S)-Jacobsen catalysts dissolved in 10 mL of DCM were slowly added to the reaction mixture. An alkaline solution of 0.1 mol L−1 KOH was added, until colloidal suspension neutralization. After stirring for 4 h, the mixture was filtered and respective solids were washed with distinct solvents (in the following order: DCM, ACN, and MeOH). After this step, the solids were oven-dried at 65 °C. | ||
| Fig. 2 Schematic of the synthesis of (R,R)-Jacobsen/Cell(NEt2) and (S,S)-Jacobsen/Cell(NEt2) P450-bioinspired materials. | ||
2.3 Characterizations
| Step | Temperature (°C) | Ramp (°C s−1) | Time (s) | Argon flow (L min−1) |
|---|---|---|---|---|
| Drying | 90 | 5 | 15 | 2.0 |
| Drying | 120 | 5 | 15 | 2.0 |
| Pyrolysis | 1100 | 200 | 30 | 2.0 |
| Atomization | 2000 | 2000 | 5 | 0 |
| Cleaning | 2450 | 1000 | 5 | 2.0 |
2.4 Oxidation reactions
Oxidation reactions were run in 3 mL vials containing a screw cap. The standard catalyst/oxidant/substrate molar ratio for Rhodamine B oxidation was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, achieved by adding 1.25 × 10−4 mol of the substrate, 1.25 × 10−4 mol of oxidant and 2.09 × 10−6 mol of catalyst to the reaction vessel. MeOH and ACN were separately used as solvents, and the catalytic results were compared. The final volume was made up to 1.5 mL with the solvent, and the reactions were maintained under magnetic stirring at room temperature. After 24 h, reaction solutions were diluted (1
60, achieved by adding 1.25 × 10−4 mol of the substrate, 1.25 × 10−4 mol of oxidant and 2.09 × 10−6 mol of catalyst to the reaction vessel. MeOH and ACN were separately used as solvents, and the catalytic results were compared. The final volume was made up to 1.5 mL with the solvent, and the reactions were maintained under magnetic stirring at room temperature. After 24 h, reaction solutions were diluted (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v) in water before analysis using a UV-Vis spectrophotometer with a 1 cm quartz cuvette. Product reactions were analysed by UV/visible spectroscopy (Shimadzu UV-1800) using a 1 cm cuvette, and the conversions were based on intensity decrease at 554 nm band in rhodamine B spectrum after 24 h reaction. Control reactions were carried out in the absence of the catalyst for all the different studied conditions, but no products were detected in these reactions (less than 1% yield).
10 v/v) in water before analysis using a UV-Vis spectrophotometer with a 1 cm quartz cuvette. Product reactions were analysed by UV/visible spectroscopy (Shimadzu UV-1800) using a 1 cm cuvette, and the conversions were based on intensity decrease at 554 nm band in rhodamine B spectrum after 24 h reaction. Control reactions were carried out in the absence of the catalyst for all the different studied conditions, but no products were detected in these reactions (less than 1% yield).
Reactions were run using either (R,R)-Jacobsen and (S,S)-Jacobsen complexes or Mn(TDCPP)Cl porphyrin as catalysts, and PhIO, m-CPBA or H2O2 as oxidants. The reactions were performed in both homogeneous and heterogeneous media (cellulose matrix), with the exception of Mn(TDCPP)Cl, which was used only in homogeneous media. To adopt the correlate catalyst/oxidant/substrate, the materials (S,S)-Jacobsen/Cell(NEt2) and (R,R)-Jacobsen/Cell(NEt2) were weighed according to each complex amount (mol) as a function of the cellulose matrix mass (g) used as a support, using Loading data (Table 2). When m-CPBA was employed in the homogeneous catalysis with (S,S)-Jacobsen and RhB, a solid was obtained from the reaction medium (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v diluted in water as described above) and extracted with hexane. The aqueous phase was analysed by UV-Vis spectroscopy, and the hydrophobic phase containing the possible reaction products was separated, recrystallized in water, purified by extraction with acetonitrile and hexane (2
10 v/v diluted in water as described above) and extracted with hexane. The aqueous phase was analysed by UV-Vis spectroscopy, and the hydrophobic phase containing the possible reaction products was separated, recrystallized in water, purified by extraction with acetonitrile and hexane (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) v/v, dried in a desiccator for 24 h and analysed by 1H-NMR spectra using a Bruker DRX 500 MHz from Bruker Daltonics and FTIR using a device BOMEM MB102. 1H NMR (500 MHz, MeOD) δH: 7.98 (t, J = 9.7 Hz, 2H); 7.60 (m, J = 3.1 Hz, 1H); 7.59 (m, J = 2.8, 1H); 3.30 (m, J = 6.4 Hz, 3H). FTIR (ν, cm−1): 1626, 1592, 1467, 1439, 1397, 1272, 1209, 1174, 1083, 1035, 979, 937, 882.
1) v/v, dried in a desiccator for 24 h and analysed by 1H-NMR spectra using a Bruker DRX 500 MHz from Bruker Daltonics and FTIR using a device BOMEM MB102. 1H NMR (500 MHz, MeOD) δH: 7.98 (t, J = 9.7 Hz, 2H); 7.60 (m, J = 3.1 Hz, 1H); 7.59 (m, J = 2.8, 1H); 3.30 (m, J = 6.4 Hz, 3H). FTIR (ν, cm−1): 1626, 1592, 1467, 1439, 1397, 1272, 1209, 1174, 1083, 1035, 979, 937, 882.
| Catalyst | % Weight | Loading (mol g−1) |
|---|---|---|
| (S,S)-Jacobsen/Cell(NEt2) | 0.0405 | 6.38 × 10−5 |
| (R,R)-Jacobsen/Cell(NEt2) | 0.00194 | 3.05 × 10−6 |
3. Results and discussion
3.1 Characterizations
 | ||
| Fig. 3 Diffuse reflectance UV-Vis analyses of (a) DEAE-cellulose and P450-bioinspired materials: (b) (S,S)-Jacobsen/Cell(NEt2) and (c) (R,R)-Jacobsen/Cell(NEt2). | ||
In more detail, in the material analyses, three specific regions are observed, which are characteristic of the metal complexes: the first refers to π → π* salicylaldehyde benzene ring transition (237.76 and 240.61 nm for (R,R)-Jacobsen/Cell(NEt2) and (S,S)-Jacobsen/Cell(NEt2), respectively), whereas the third specifies the d → d metal central transition (420.43 and 399.96 nm for (R,R)-Jacobsen/Cell(NEt2) and (S,S)-Jacobsen/Cell(NEt2) respectively), the second is related to n → π* azomethine chromophore group transition (289.33 and 320.41 nm for (R,R)-Jacobsen/Cell(NEt2) and (S,S)-Jacobsen/Cell(NEt2), respectively). Given these results and supported by literature data, it is possible to assume that the Jacobsen catalysts were preserved in the cellulosic matrix.59
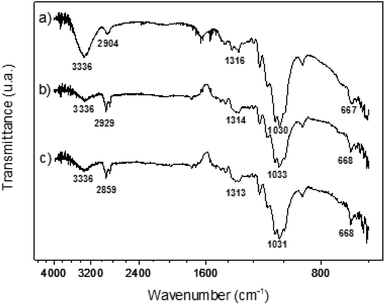 | ||
| Fig. 4 ATR-FTIR analyses of (a) DEAE-cellulose and P450-bioinspired materials: (b) (S,S)-Jacobsen/Cell(NEt2) and (c) (R,R)-Jacobsen/Cell(NEt2). | ||
According to Fig. 4a, some most characteristic regions of the DEAE-cellulose spectrum are verified: the first, peaks in the range of 1085–1025 cm−1, which could be attributed to the vibration of the C–O primary alcohol stretch and the second, at 667 cm−1 which is related to the C–O–C bond deformation, typical of fiber catenations of cellulose.60,62 Examining the spectrum of the DEAE-cellulose material (Fig. 4a), as expected, the transmittance region of 1250–1020 cm−1 relative to the C–N bond angular deformation was masked by other cellulose-attributed peaks, as described in the literature.60
Furthermore, as stated by the literature,82 the FTIR spectra of the Jacobsen catalysts have two characteristic peaks of 1600 and 1590 cm−1, which are related to imine group bond vibrations (HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), in addition to another stretch referring to the carboxy group (C–O) of these metal complexes at 1530 cm−1. In Fig. 4b and c, it was not possible to verify these vibrational modes; however, in the range of 1589–1400 cm−1, it is possible to verify a change in the spectral pattern of Jacobsen catalyst samples compared to the pure cellulose matrix (Fig. 4a), again indicating a possible structural modification of the biopolymer matrix with the metal complexes.
N), in addition to another stretch referring to the carboxy group (C–O) of these metal complexes at 1530 cm−1. In Fig. 4b and c, it was not possible to verify these vibrational modes; however, in the range of 1589–1400 cm−1, it is possible to verify a change in the spectral pattern of Jacobsen catalyst samples compared to the pure cellulose matrix (Fig. 4a), again indicating a possible structural modification of the biopolymer matrix with the metal complexes.
3.2 Oxidation reactions
Rhodamine B oxidation reactions were carried out in the presence of the catalysts (R,R) and (S,S)-Jacobsen, homogeneous and supported on the DEAE-cellulose matrix. These catalysts are well-established in the literature as efficient catalysts for hydrocarbon, drug, and herbicide oxidation,89–93 with remarkable results for asymmetric oxidations, because it is capable to conduct enantioselective epoxidation of many olefins.94–96 Mn(TDCPP)Cl, a second-generation metalloporphyrin, was chosen as a standard catalyst, because it is described in the literature as a heme model system trustworthy for biomimetic catalysis, especially for xenobiotic oxidation, and its catalytic behavior has been extensively studied.89–93RhB oxidation reactions were accomplished using either MeOH or ACN solvents, taking substrates and its metabolites solubility in this solvent into account. Catalytic performance in each milieu was compared. Homogeneous (Table 3) and heterogeneous (catalysts anchored on support, Table 4) conditions were compared. Solubility was also a determining factor for the choice of homogeneous reaction conditions, once the studied substrate and probable products were solids with limited solubility in the volume employed for the reactions (1.5 mL).
| Entry | Catalyst | Solvent | Oxidant | Yield (%) |
|---|---|---|---|---|
a Catalysis/substrate/oxidant molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 analyzed by UV-Vis (554 nm). 60 analyzed by UV-Vis (554 nm). |
||||
| 1 | (S,S)-Jacobsen | ACN | m-CPBA | 24 |
| 2 | H2O2 | 4 | ||
| 3 | PhIO | 76 | ||
| 4 | MeOH | m-CPBA | 43 | |
| 5 | H2O2 | 18 | ||
| 6 | PhIO | 28 | ||
| 7 | (R,R)-Jacobsen | ACN | m-CPBA | 5 |
| 8 | H2O2 | 11 | ||
| 9 | PhIO | 19 | ||
| 10 | MeOH | m-CPBA | 25 | |
| 11 | H2O2 | 3 | ||
| 12 | PhIO | 19 | ||
| 13 | Mn(TDCPP)Cl | ACN | m-CPBA | 25 |
| 14 | H2O2 | 23 | ||
| 15 | PhIO | 98 | ||
| 16 | MeOH | m-CPBA | 25 | |
| 17 | H2O2 | 15 | ||
| 18 | PhIO | 52 | ||
| Entry | Catalyst | Solvent | Oxidant | Yield (%) |
|---|---|---|---|---|
a Catalysis/substrate/oxidant molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 analyzed by UV-Vis (554 nm). 60 analyzed by UV-Vis (554 nm). |
||||
| 1 | (S,S)-Jacobsen/Cell(NEt2) | ACN | m-CPBA | 31 |
| 2 | H2O2 | 37 | ||
| 3 | PhIO | 98 | ||
| 4 | MeOH | m-CPBA | 7 | |
| 5 | H2O2 | 6 | ||
| 6 | PhIO | 23 | ||
| 7 | (R,R)-Jacobsen/Cell(NEt2) | ACN | m-CPBA | 36 |
| 8 | H2O2 | 65 | ||
| 9 | PhIO | 44 | ||
| 10 | MeOH | m-CPBA | <1 | |
| 11 | H2O2 | <1 | ||
| 12 | PhIO | 74 | ||
PhIO was the first oxidant to be used as an oxygen donor, and it is considered a standard and simple oxidant which contains only one oxygen atom and is well-adapted for the selective and clean formation of high-valent metal–oxo intermediates.89 m-CPBA and H2O2 were also employed. Both latter oxidants can undergo heterolytic cleavage upon coordination to the metalloporphyrin central metal ion, which gives rise to the active species oxomanganyl porphyrin π-cation radical, MIV(O)P˙+ or MV(O)P. Homolytic cleavage of the O–O bond may also occur, leading to the formation of a less reactive intermediate, MIV(OH)P, as well as RO˙ radicals, thus favoring the occurrence of radical mechanisms.91,97 Moreover, hydrogen peroxide was chosen because it is considered a “clean oxidant”, since it produces water as the only side-product, and is the oxidant employed by peroxidases, enabling comparison with these enzymes.97,98 The use of hydrogen peroxide is particularly important because it leads to the formation of catalytic intermediates that are similar to those observed in the mechanism of cytochromes P450. Knowledge of how these mechanisms function would lead to the development of more efficient and cleaner catalytic processes since H2O2 is the second most interesting oxidant for green chemistry studies, followed by O2.99
Tables 3 and 4 present the total conversion yield (R %) obtained in the rhodamine B oxidation reactions with each salen complex/oxidant system, in the heterogeneous or homogeneous milieu, under different conditions. Catalytic results for homogeneous conditions indicate that catalysis depends on the solvent employed for each reaction (protic or non-protic). The highest yields from RhB oxidation were promoted in oxidation reactions promoted in an ACN medium, especially for Mn(TDCPP)/PhIO, achieving 98% yield (Table 3, entry 15) and (S,S)-Jacobsen/PhIO, achieving 76% yield (Table 3, entry 3). Homogeneous reactions performed in MeOH presented up to 52% (MnTDCPP/PhIO, entry 18) and 43% conversion yields ((S,S)-Jacobsen/m-CPBA, entry 4). Manganese biomimetic catalysts reach their maximum activity in aprotic solvents and the presence of co-catalysts. As already mentioned, iodosylbenzene favors high-valent metal–oxo intermediate formation such as MnV(O)salen.98 Modest catalytic results were obtained by reactions performed in a MeOH medium, which can be justified using protic solvents such as methanol, which may coordinate to salen-fifth site and inactivate metal complex. It is known that in catalytic systems involving metal complexes such as metalloporphyrins and salen, in the presence of protic solvents, such as MeOH, may induce the formation of Mn(III) hydroperoxide intermediate, MnIIIOOHP, which is less active than the much more electrophilic high-valent oxo-manganese, MnV(O)salen and MnV(O)P.91,94 Hydroperoxide species may be extremely inefficient for Mn(salen) with weak electron-withdrawing groups.95 Comparing the results obtained in the use of salen complexes, reactions in which there is the participation of (S,S)-Jacobsen clearly increase catalytic conversion (compared to (R,R)-Jacobsen), which suggests possible asymmetric catalysis that could only be confirmed through analysis of oxidation products in a chiral column.
Heterogeneous reactions (Table 4) account for higher RhB conversion yields compared to homogeneous conditions (Table 3). Conversions are up to 98% yield for (S,S)-Jacobsen/Cell(NEt2)/PhIO/ACN (entry 3), (R,R)-Jacobsen/Cell(NEt2)/PhIO/MeOH (74% yield, entry 12), and (R,R)-Jacobsen/Cell(NEt2)/H2O2/ACN (65% yield, entry 8). The catalytic activity seems to be highly dependent on the nature of the oxidant, PhIO being the most efficient, followed by m-CPBA (Tables 3 and 4).97 Reactions using H2O2 afforded the lowest yields (3–46%), which can be explained by the catalysed dismutation of this oxidant in the presence of salen complexes, which was favored in these systems due to the relative inertness of the substrate rhodamine B.97,98
Similarly to P450 catalytic cycle, heterogeneous catalyst results obtained so far can be understood based on the different actions of two possible oxidation species: (i) a Mn(oxo)salen species ((S,S)-Jacobsen/Cell(NEt2)/PhIO/ACN and (R,R)-Jacobsen/Cell(NEt2)/PhIO/MeOH) and (ii) Mn(salen)-oxidation adduct ((R,R)-Jacobsen/Cell(NEt2)/H2O2/ACN). Supported catalysts would increase catalytic yields despite the use of protic or non-protic solvents because catalysis would be driven by two different mechanisms.99 One assumption is based on cellulose acting as a co-catalyst, wereas it coordinates with the Mn(salen) complex in the -trans position to the metal–oxo bond, stabilizing the active intermediate catalytic species: (i) MnV(O)salen responsible for efficient and stereoselective oxidations and preventing the fast reaction between MnV(O)salen and Mn(salen) would result in formation of MnIV(O)salen/Cell(NEt2) intermediate species, responsible for less efficient and poorly selective radical reactions, and (ii) MnIII(O-OH)salen/Cell(NEt2), in the presence of H2O2 and protic solvent. Oxidation promoted by m-CPBA seems to also form hydroperoxide species. Hydroperoxide species can transfer oxygen to RhB or can evolve to a high oxidation state forming the oxo species. The lower electrophilic character of hydroperoxy species leads to the lowest conversion yields because it is unable to transfer oxygen to the substrate.99
Even though homogeneous reactions achieve increased conversion yields to (S,S)-Jacobsen (compared to (R,R) enantiomer), suggesting probable asymmetric catalysis; there is no difference in results so far presented for heterogeneous systems. Comparable catalytic results of (S,S)-Jacobsen/Cell(NEt2) and (R,R)-Jacobsen/Cell(NEt2) may be attributed to the conformation of salen complexes anchored on cellulose, which would interfere on complex configuration, permitting a positive approximation to substrate and reflecting on higher yields for (S,S)-Jacobsen/Cell(NEt2) compared to homogeneous reactions (Fig. 7).
 | ||
| Fig. 7 Pictorial representation of how rhodamine B dye oxidation is favored by canted arrangement of the (S,S)-Jacobsen catalyst. | ||
Oxidation reaction in the presence of homogeneous (S,S)-Jacobsen/m-CPBA generated a solid that was separated, purified, and analyzed by 1H NMR and FTIR (ESI†). m-CPBA was chosen as an oxygen donor because it is very efficient to produce high-valent intermediates in hydrophobic medium and metal-hydroperoxide species in protic solvents, as discussed earlier.87,94 The possible four RhB oxidation products was suggested after assignments of 1H NMR peaks, and its possible structures (Fig. 8) were denoted as P1 ((E)-N-(9-(2-carboxyphenyl)-6-(diethylamino)-7-hydroxy-3H-xanthen-3-ylidene)-N-ethylhydroxylammonium or (E)-N-(9-(2-carboxyphenyl)-6-(diethylamino)-8-hydroxy-3H-xanthen-3-ylidene)-N-ethylhydroxylammonium, Fig. 8a), P2 ((E)-N-(9-(2-carboxy-5-hydroxyphenyl)-6-(diethylamino)-3H-xanthen-3-ylidene)-N-ethylhydroxylammonium or (E)-N-(9-(2-carboxy-4-hydroxyphenyl)-6-(diethylamino)-3H-xanthen-3-ylidene)-N-ethylhydroxylammonium) (Fig. 8b), P3 ((E)-N-(9-(2-carboxy-5-hydroxyphenyl)-6-(diethylamino)-3H-xanthen-3-ylidene)-N-(hydroxymethyl)hydroxylammonium or (E)-N-(9-(2-carboxy-4-hydroxyphenyl)-6-(diethylamino)-3H-xanthen-3-ylidene)-N-(hydroxymethyl)hydroxylammonium) and P4 ((S,E)-N-(9-(2-carboxy-5-hydroxyphenyl)-6-(diethylamino)-3H-xanthen-3-ylidene)-N-(1-hydroxyethyl)hydroxylammonium or (S,E)-N-(9-(2-carboxy-4-hydroxyphenyl)-6-(diethylamino)-3H-xanthen-3-ylidene)-N-(1-hydroxyethyl)hydroxylammonium), respectively.
 | ||
| Fig. 8 Suggestions for oxidation products of rhodamine B dye in reaction with (S,S)-Jacobsen and m-CPBA in a protic solvent medium. | ||
It is noteworthy that a single NMR spectrum is not able to provide all the characterization evidence for these products. Thereat, we state that these are suggestions and not elucidations. Confirmation of products will be further supported with mass spectrometry in the future work. In addition, it is merit remembering that in a volume of 3 mL of reaction, the amount of substrate used was exceedingly small (1.25 × 10−4 mol) and, consequently, the concentration of each product formed was much lower, and thus, the signals recorded in the 1H NMR analysis for this compound were exceptionally low. Ergo, the hinted structures are a preliminary proposal based on incomplete NMR characterization due to the low quantity obtained in the catalysis assays.
Considering our catalytic data results, we suggest a mechanism for RhB oxidation (Fig. 9), mediated by m-CPBA. At a first step, we assume the formation of a hydroperoxy species as a consequence of m-CPBA addition to the resting state of the Mn(salen). The nucleophilic species MnIII(O–OH)salen attacks the nitrogen atom from RhB imine, transferring an oxygen atom to the substrate. After terminal OH– group protonation and water loss, the salen complex returns to its resting state (Step I, Fig. 9). Then, the reactive species MnIII(O–OH)salen possibly attacked in the ortho or meta positions of the dimethylamino group attached on the phenyl ring, resulting in the transfer of the –OH group in these respective positions, thus forming the P1 product (Step II, Fig. 9). Similar to the previous step, but with the attack directed at the meta and para positions on the carboxylic group of the phenyl ring, the transfer of the –OH group by the reactive species in this region possibly generated the P2 product (Step III, Fig. 9). After this step, the MnIII(O–OH)salen species had two different attack alternatives to the ylidene-N-ethylhydroxylammonium group: one lies in the hydroxylation of β carbon, resulting in the P3 product (Step IV, Fig. 9), while the other is directed to α carbon, to form product P4 (Step V, Fig. 9).
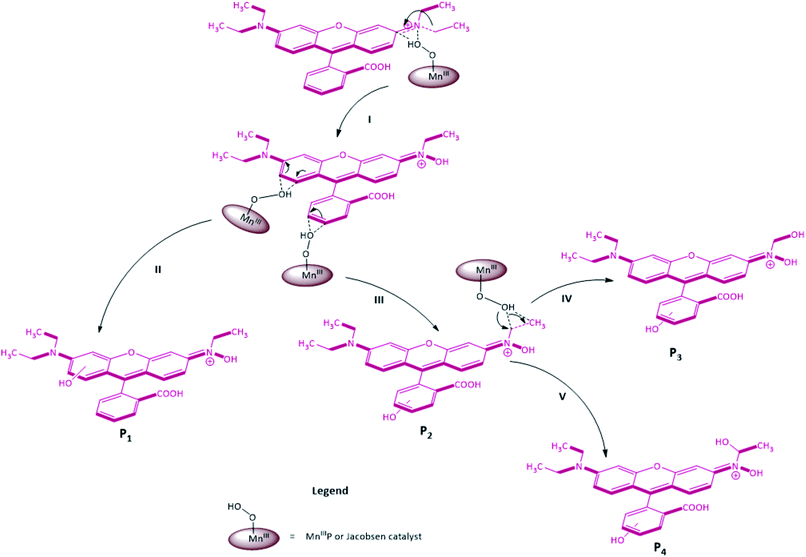 | ||
| Fig. 9 Possible rhodamine B dye oxidative pathway mediated by Mn(TDCPP)Cl and Jacobsen catalysts with m-CPBA, in protic solvent medium. | ||
4. Conclusions
(R,R)-Jacobsen/Cell(NEt2) and (S,S)-Jacobsen/Cell(NEt2) material have been roughly characterized by DR/UV-Vis, ATR-FTIR, TG/DSC and GF-AAS. DR/UV-Vis analysis confirmed that anchoring of salen complexes on DEAE-cell produced promising P450-bioinspired systems to be applied for bioactive molecules oxidation. The catalyst amount distributed in the cellulose fiber (loading) was confirmed by GF-ASS analyses, and data were used to perform catalytic essays using rhodamine B as the substrate. Cellulose as support may be acting as a co-catalyst, stabilizing the active intermediate catalytic species. Even though homogeneous reactions achieve increased conversion yields to (S,S)-Jacobsen (compared to (R,R) enantiomer), suggesting probable asymmetric catalysis; there is no difference in results so far presented for heterogeneous systems. The four oxidation products from rhodamine B dye oxidation were identified by 1H NMR analysis, and reactions seem to be driven through two different intermediates, depending on oxygen donors and solvents: (i) MnV(O)salen (non protic solvents/PhIO) and (ii) MnIII(O–OH)salen, in the presence of H2O2 or m-CPBA and protic solvents.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) - Finance Code 001. The authors are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the scholarships and Projeto Universal (427943/2018-3).References
- M. V. Doble, A. C. C. Ward, P. J. Deuss, A. G. Jarvis and P. C. J. Kamer, Bioorg. Med. Chem., 2014, 22, 5657–5677 CrossRef CAS PubMed.
- P. R. O. de Montellano, in Cytochrome P450: Structure, Mechanism, and Biochemistry, ed. P. R. O. de Montellano, Springer, London, 4th edn, 2015, part 1, ch. 4, pp. 111–176 Search PubMed.
- T. Coleman, J. E. Stok, M. N. Podgorski, J. B. Bruning, J. J. De Voss and S. G. Bell, J. Biol. Inorg Chem., 2020, 25, 583–596 CrossRef CAS PubMed.
- I. G. Denisov and S. G. Sligar, in Cytochrome P450: Structure, Mechanism, and Biochemistry, ed. P. R. O. de Montellano, Springer, London, 4th edn, 2015, part 1, ch. 3, pp. 69–109 Search PubMed.
- T. H. Yosca, J. Rittle, C. M. Krest, E. L. Onderko, A. Silakov, J. C. Calixto, R. K. Behan and M. T. Green, Science, 2013, 342, 825–829 CrossRef CAS PubMed.
- C. M. Chapman and G. B. Jones, Curr. Catal., 2015, 4, 77–110 CrossRef CAS.
- C. M. Chapman and G. B. Jones, Curr. Catal., 2015, 4, 166–213 CrossRef CAS.
- L. D. Zanatta, I. A. Barbosa, P. C. d. S. Filho, L. B. Bolzon, O. A. Serra and Y. Iamamoto, Mini-Rev. Org. Chem., 2016, 13, 281–288 CrossRef CAS.
- W. Lohmann and U. Karst, Anal. Bioanal. Chem., 2008, 391, 79–96 CrossRef CAS PubMed.
- H. Noh and J. Cho, Coord. Chem. Rev., 2019, 382, 126–144 CrossRef CAS.
- F. Fabian Schwizer, Y. Okamoto, T. Heinisch, Y. Gu, M. M. Pellizzoni, V. Lebrun, R. Reuter, V. Köhler, J. C. Lewis and T. R. Ward, Chem. Rev., 2018, 118, 142–231 CrossRef PubMed.
- J. Chen, Z. Jiang, S. Fukuzumi, W. Nam and B. Wang, Coord. Chem. Rev., 2020, 421, 213443–213471 CrossRef CAS.
- A. C. Ghosh, C. Duboc and M. Gennari, Coord. Chem. Rev., 2021, 428, 213606–213629 CrossRef CAS.
- M. C. Pigatto, M. D. C. Alves De Lima, S. L. Galdino, I. R. Pitta, R. L. Vessecchi, M. D. Assis, J. S. dos Santos, T. D. Costa and N. P. Lopes, Eur. J. Med. Chem., 2011, 46, 4245–4251 CrossRef CAS PubMed.
- D. CarvalhoDa-Silva, T. C. O. Mac Leod, A. L. Faria, J. S. dos Santos, M. E. M. D. de Carvalho, J. S. Rebouças, Y. M. Idemori and M. D. Assis, Appl. Catal., A, 2011, 408, 25–30 CrossRef CAS.
- J. S. dos Santos, V. Palaretti, A. L. Faria, E. J. Crevelin, L. A. B. Moraes and M. D. Assis, Appl. Catal., A, 2011, 408, 163–170 CrossRef.
- V. Palaretti, J. S. dos Santos, D. F. C. Guedes, L. A. B. Moraes and M. D. Assis, Appl. Catal., A, 2012, 447–448, 7–13 CrossRef CAS.
- V. P. Barros and M. D. Assis, J. Braz. Chem. Soc., 2013, 24, 830–836 CAS.
- M. Niehues, V. P. Barros, F. S. Emery, M. Dias-Baruffi, M. D. Assis and N. P. Lopes, Eur. J. Med. Chem., 2012, 54, 804–812 CrossRef CAS PubMed.
- L. B. Bolzon, H. R. Airoldi, F. B. Zanardi, J. G. Granado and Y. Iamamoto, Microporous Mesoporous Mater., 2013, 168, 37–45 CrossRef CAS.
- V. S. Silva, A. M. Meireles, D. C. S. Martins, J. S. Rebouças, G. DeFreitas-Silva and Y. M. Idemori, Appl. Catal., A, 2015, 491, 17–27 CrossRef.
- F. B. Zanardi, I. A. Barbosa, P. C. Sousa Filho, L. D. Zanatta, D. L. da Silva, O. A. Serra and Y. Iamamoto, Microporous Mesoporous Mater., 2016, 219, 161–171 CrossRef CAS.
- L. D. Zanatta, I. A. Barbosa, F. B. Zanardi, P. C. de Sousa Filho, L. B. Bolzon, A. P. Ramos, O. A. Serra and Y. Iamamoto, RSC Adv., 2016, 6, 104886–104896 RSC.
- I. A. Barbosa, P. C. de Sousa Filho, D. L. da Silva, F. B. Zanardi, L. D. Zanatta, A. J. A. de Oliveira, O. A. Serra and Y. Iamamoto, J. Colloid Interface Sci., 2016, 469, 296–309 CrossRef CAS PubMed.
- L. B. Bolzon, J. S. dos Santos, D. B. Silva, E. J. Crevelin, L. A. B. Moraes, N. P. Lopes and M. D. Assis, J. Inorg. Biochem., 2017, 170, 117–124 CrossRef CAS PubMed.
- A. M. Meireles, A. L. A. Lage, J. M. Ribeiro, M. A. N. da Silva, E. M. de Souza-Fagundes and D. C. S. Martins, Environ. Res., 2019, 177, 108615–108624 CrossRef CAS PubMed.
- V. S. Da Silva, G. M. Ucoski, S. Nakagaki, Y. M. Idemori and G. F. Silva, RSC Adv., 2015, 5, 106589–106598 RSC.
- A. R. Antonangelo, K. C. M. Westrup, L. A. Burt, C. G. Bezzu, T. Malewschik, G. S. Machado, F. S. Nunes, N. B. Mckeown and S. Nakagaki, RSC Adv., 2017, 7, 50610–50618 RSC.
- S. R. Tavares, C. Carvalho, K. M. Mantovani, F. Wypych, S. Nakagaki and A. A. Leitão, Appl. Clay Sci., 2020, 185, 105410–105417 CrossRef CAS.
- K. C. M. Westrup, R. M. Da Silva, K. M. Mantovani, L. Bach, J. F. Stival, P. G. P. Zamora, F. Wypych, G. S. Machado and S. Nakagaki, Appl. Catal., A, 2020, 602, 117708–117720 CrossRef CAS.
- A. R. Antonangelo, C. Grazia Bezzu, N. B. Mckeown and S. Nakagaki, J. Catal., 2019, 369, 133–142 CrossRef CAS.
- L. Marchetti and M. Levine, ACS Catal., 2011, 1, 1090–1118 CrossRef CAS.
- C. Baleizão and H. Hermenegildo Garcia, Chem. Rev., 2011, 106, 3987–4043 CrossRef PubMed.
- A. Martinez, C. Hemmert and B. Meunier, J. Catal., 2005, 234, 250–255 CrossRef CAS.
- N. S. Venkataramanan, G. Kuppuraj and S. Rajagopal, Coord. Chem. Rev., 2005, 249, 1249–1268 CrossRef CAS.
- L. Canali, D. C. Sherrington and H. Deleuze, React. Funct. Polym., 1999, 40, 155–168 CrossRef CAS.
- P. D. Knight, A. J. Clarke, B. S. Kimberley, R. A. Jackson and P. P. Scott, Chem. Commun., 2002, 352–402 RSC.
- R. Zhang, S. Klaine, C. Alcantar and F. Bratcher, J. Inorg. Biochem., 2020, 212, 111246–111259 CrossRef CAS PubMed.
- T. M. Ovitt and G. W. Coates, J. Am. Chem. Soc., 2002, 124, 1316–1326 CrossRef CAS PubMed.
- D. Brunel, N. Bellocq, P. Sutra, A. Cauvel, M. Laspéras, P. Moreau, F. Di Renzo, A. Galarneau and F. Fajula, Coord. Chem. Rev., 1998, 178–180, 1085–1108 CrossRef CAS.
- M. Masteri-Farahani, J. Mol. Catal. A: Chem., 2010, 316, 45–51 CrossRef CAS.
- C. Christine Bibal, J. C. Daran, S. Deroover and R. Poli, Polyhedron, 2010, 29, 639–647 CrossRef.
- T. C. O. MacLeod, M. V. Kirillova, A. J. Pombeiro, M. A. Schiavon and M. D. Assis, Appl. Catal., A, 2010, 372, 191–198 CrossRef CAS.
- T. C. O. MacLeod, V. P. Barros, A. L. Faria, M. A. Schiavon, I. V. P. Yoshida, M. E. C. Queiroz and M. D. Assis, J. Mol. Catal. A: Chem., 2007, 273, 259–264 CrossRef CAS.
- T. C. O. MacLeod, A. L. Faria, V. P. Barros, M. E. C. Queiroz and M. D. Assis, J. Mol. Catal. A: Chem., 2008, 296, 54–60 CrossRef CAS.
- T. C. O. MacLeod, V. Palaretti, V. P. Barros, A. L. Faria, T. A. Silva and M. D. Assis, Appl. Catal., A, 2009, 361, 152–159 CrossRef CAS.
- F. Moschona, I. Savvopoulou, M. Tsitopoulou, D. Tataraki and G. Rassias, Catalysts, 2020, 10, 1117–1182 CrossRef CAS.
- J. Niemeyer, J. Cloppenburg, R. Fröhlich, G. Kehr and G. Erker, J. Organomet. Chem., 2010, 695, 1801–1812 CrossRef CAS.
- T. Ben Zid, I. Khedher and A. Ghorbel, React. Kinet. Mech. Catal., 2020, 100, 131–143 Search PubMed.
- Y. Zidane, A. Ourari, T. Bataille, P. Hapiot and D. Hauchard, J. Electroanal. Chem., 2010, 641, 64–70 CrossRef CAS.
- S. Jain, K. Venkatasubbaiah, C. W. Jones and R. J. Davis, J. Mol. Catal. A: Chem., 2010, 316, 8–15 CrossRef CAS.
- D. R. Nelson, Arch. Biochem. Biophys., 1999, 369, 1–10 CrossRef CAS PubMed.
- Millipore Sigma Merck KGaA, http://www.sigmaaldrich.com, accessed February 2020.
- A. Abou-Okeil, A. Amr and F. A. Abdel-Mohdy, Carbohydr. Polym., 2012, 89, 1–6 CrossRef CAS PubMed.
- A. L. K. Roberts, J. M. Fletcher and L. Moore, Mol. Genet. Metab., 2010, 101, 208–213 CrossRef CAS PubMed.
- S. D. Starnes, D. M. Rudkevich and J. Rebek Jr, Org. Lett., 2000, 2, 1995–1998 CrossRef CAS PubMed.
- U. Lücking, J. Chen, D. M. Rudkevich and J. Rebek Jr, J. Am. Chem. Soc., 2001, 123, 9929–9934 CrossRef PubMed.
- S. Richeter and J. Rebek Jr, J. Am. Chem. Soc., 2004, 126, 16280–16281 CrossRef CAS PubMed.
- T. C. O. Mac Leod, V. Palaretti, V. P. Barros, A. L. Faria, T. A. Silva and M. D. Assis, Appl. Catal., A, 2009, 361, 152–159 CrossRef CAS.
- O. Hardick, S. Dods, B. Stevens and D. G. Bracewell, Biotechnol. Bioeng., 2013, 110, 1119–1128 CrossRef CAS PubMed.
- S. V. K. Gupta, P. J. M. Carrott, R. Singh, M. Chaudhary and S. Kushwaha, Bioresour. Technol., 2016, 216, 1066–1076 CrossRef PubMed.
- S. Gopi, P. Balakrishnan, D. Chandradhara, D. Poovathankandy and S. Thomas, Mater. Today Chem., 2019, 13, 59–78 CrossRef CAS.
- M. Alesso, G. Bondioli, M. C. Talio, M. O. Luconi and L. P. Fernandez, Food Chem., 2012, 134, 513–517 CrossRef CAS.
- J. R.-R. Fernandez and T. E. Rocke, J. Wildl. Dis., 2011, 47, 765–768 CrossRef PubMed.
- C. Tatebe, X. Zhong, T. Ohtsuki, H. Kubota, K. Sato and H. Akiyama, Food Sci. Nutr., 2014, 2, 547–556 CrossRef CAS PubMed.
- A. L. K. Roberts, J. M. Fletcher and L. Moore, Mol. Genet. Metab., 2010, 101, 208–213 CrossRef CAS PubMed.
- Y.-Y. Cheng and T.-T. Tsai, J. Agric. Food Chem., 2017, 65, 1078–1085 CrossRef CAS PubMed.
- Q. Lu, W. Ga, J. Du, L. Zhou and Y. Lian, J. Agric. Food Chem., 2012, 60, 4773–4778 CrossRef CAS PubMed.
- R. D. Combes and R. B. Haveland-Smith, Mutat. Res., 1982, 98, 101–248 CAS.
- R. Jain, M. Mathur, S. Sikawar and A. Mittal, J. Environ. Manage., 2007, 85, 956–964 CrossRef CAS PubMed.
- M. M. Islam, M. Chakraborty, P. Pandya, A. A. Masuma, N. Gupta and S. Mukhopadhyay, Dyes Pigm., 2013, 99, 412–422 CrossRef CAS.
- H. S. Lim, E. Choi, J.-H. Lee, G. Lee and M. Kim, Food Addit. Contam., Part A, 2020, 37(6), 895–904 CrossRef CAS PubMed.
- V. Kumar, M. Singh, K. Behera and S. Pandey, J. Mol. Liq., 2020, 319, 114195–114201 CrossRef CAS.
- G. J. Joshiba, P. S. Kumar, M. Govarthanan, P. T. Ngueagni, A. Abilarasu and F. Carolin C, Environ. Pollut., 2021, 269, 116173–116184 CrossRef CAS PubMed.
- P. S. Geetha Malini, P. Durgadevi, N. S. Kuma and S. Rani, Mater. Today: Proc., 2020 DOI:10.1016/j.matpr.2020.07.689.
- V. E. Podasca, T. Buruiana and E. C. Buruiana, J. Photochem. Photobiol., A, 2018, 371, 188–195 CrossRef.
- U. Ghosh and A. Pal, J. Photochem. Photobiol., A, 2020, 397, 112582–112593 CrossRef CAS.
- P. Li, Z. Bao, G. Wang, P. Xu, X. Wang, Z. Liu, Y. Guo, J. Deng and W. Zhang, J. Hazard. Mater., 2019, 362, 336–347 CrossRef CAS PubMed.
- A. L. D. da Rosa, E. Carissimi, G. L. Dotto, H. Sander and L. A. Feris, J. Cleaner Prod., 2018, 198, 1302–1310 CrossRef CAS.
- J. G. Sharefkin and H. Saltzmann, Org. Synth., 1963, 43, 60–61 CrossRef.
- R. De Paula, M. M. Q. Simões, M. G. P. M. S. Neves and J. A. S. Cavaleiro, J. Mol. Catal. A: Chem., 2011, 345, 1–11 CrossRef CAS.
- R. M. Silverstein, F. X. Webster and D. J. Kiemle, Spectrometric Identification of Organic Compounds, John Wiley & Sons, New York, 7th edn, 2005 Search PubMed.
- F. Shafizadeh and A. G. W. Bradbury, J. Appl. Polym. Sci., 1979, 23, 1431–1442 CrossRef CAS.
- S. R. Hobart, R. J. Berni and C. H. Mack, Text. Res. J., 1970, 40, 1079–1086 CrossRef CAS.
- A. D. French and G. P. Johnson, Cellulose, 2009, 16, 959–973 CrossRef CAS.
- Y. Nishiyama, G. P. Johnson and A. D. French, Cellulose, 2012, 19, 319–336 CrossRef CAS.
- U. Schnupf and F. A. Momany, Cellulose, 2011, 18, 859–887 CrossRef CAS.
- K. Conley, L. Godbout, M. A. (Tony) Whitehead and T. G. M. van de Ven, Carbohydr. Polym., 2016, 135, 285–299 CrossRef CAS PubMed.
- D. Mansuy, Catal. Today, 2008, 138, 2–8 CrossRef CAS.
- M. C. A. F. Gotardo, L. A. B. Moraes and M. D. Assis, J. Agric. Food Chem., 2006, 12, 10011–10018 CrossRef PubMed.
- B. Meunier, A. Robert, G. Pratviel and J. Bernadou, in The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, New York Academic Press, New York, 2000, vol. 4, ch. 31, pp. 119–188 Search PubMed.
- D. Mansuy, C. R. Chim., 2007, 10, 392–413 CrossRef CAS.
- S. L. H. Rebelo, M. M. Pereira, P. V. Monsanto and H. D. Burrows, J. Mol. Catal. A: Chem., 2009, 297, 35–43 CrossRef CAS.
- F. S. Vinhado, M. E. F. Gandini, Y. Iamamoto, A. M. G. Silva, M. M. Q. Simões, M. G. P. M. S. Neves, A. C. Tomé, S. L. H. Rebelo, A. M. V. M. Pereira and J. A. S. Cavaleiro, J. Mol. Catal. A: Chem., 2005, 239, 138–143 CrossRef CAS.
- L. Kurti, M. M. Blewett and E. J. Corey, Org. Lett., 2009, 11, 4592–4595 CrossRef CAS PubMed.
- M. K. Brown, M. M. Blewett, J. R. Colombe and E. J. Corey, J. Am. Chem. Soc., 2010, 132, 11165–11170 CrossRef CAS PubMed.
- M. Rocha, S. L. H. Rebelo and C. Freire, Appl. Catal., A, 2013, 460–461, 116–123 CrossRef CAS.
- B. Meunier and J. Bernardou, in Metal–Oxo and Metal–Peroxo Species in Catalytic Oxidations, ed. B. Meunier, Springer, Berlin, 2000, vol. 97, ch. 1, pp. 1–34 Search PubMed.
- S. L. H. Rebelo, M. M. Pereira, M. M. Q. Simões, M. G. P. M. S. Neves and J. A. S. Cavaleiro, J. Catal., 2005, 234, 76–87 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra04915a |
| This journal is © The Royal Society of Chemistry 2021 |



