Open Access Article
Open Access ArticleAssembly of iron oxide nanosheets at the air–water interface by leucine–histidine peptides†
Nina Hoinkisa,
Helmut Lutz*a,
Hao Lua,
Thaddeus W. Golbekb,
Mikkel Bregnhøj b,
Gerhard Jakob
b,
Gerhard Jakob c,
Mischa Bonn
c,
Mischa Bonn a and
Tobias Weidner
a and
Tobias Weidner *b
*b
aMax Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany. E-mail: helmut.lutz@googlemail.com
bDepartment of Chemistry, Aarhus University, Langelandsgade 140, 8000 Aarhus C, Denmark. E-mail: weidner@chem.au.dk
cUniversity of Mainz, Institute of Physics, Staudinger Weg 7, 55128 Mainz, Germany
First published on 18th August 2021
Abstract
The fabrication of inorganic nanomaterials is important for a wide range of disciplines. While many purely inorganic synthetic routes have enabled a manifold of nanostructures under well-controlled conditions, organisms have the ability to synthesize structures under ambient conditions. For example, magnetotactic bacteria, can synthesize tiny ‘compass needles’ of magnetite (Fe3O4). Here, we demonstrate the bio-inspired synthesis of extended, self-supporting, nanometer-thin sheets of iron oxide at the water–air interface through self-assembly using small histidine-rich peptides.
Since the discovery of magnetotactic bacteria, researchers have been fascinated by the ability of these specialized organisms to orient within the Earth's magnetic field.1 To be able to react to magnetic fields, magnetotactic bacteria use specialized compartments, called magnetosomes, to produce the iron oxide magnetite (Fe3O4). Magnetosomes have a typical size of 30–120 nm and establish a permanent magnetic moment along a fixed axis within the bacteria.2,3 The magnetization is used to navigate and orient within the terrestrial magnetic field to find favorable living conditions.4 It has been shown that the size and morphology of magnetic particles used are tightly genetically controlled and vary for different species.5,6 The biomimetic synthesis of magnetic nanomaterials has been of substantial interest in the past years. Potential applications include biotechnology,7 medicine8 and data storage.9 While the biomimetic preparation of magnetic particles and surface patterns using proteins derived from magnetotactic bacteria has been reported before,10–12 the biomimetic production of extended 2D magnetic materials using proteins has not been shown. At the same time, 2D nanomaterials have received much attention as ultrathin, high fidelity materials with new properties.13 Such 2D structures hold great promise as a material that can be self-assembled using effective, low-cost, bottom-up fabrication methods and then tuned chemically to the application.14 2D materials can often be synthesized from simple building blocks and are very flexible in terms of surface morphology, porosity, chemical functionality, electronic and magnetic properties. It has been shown that functional 2D inorganic materials can be templated by organic precursor structures at interfaces.15 In nature, proteins act as the surface “engineers” and steer the growth of minerals at interfaces.16 2D materials based on silica, calcium carbonate, and calcium oxalate have been assembled previously by biomimetic peptides at interfaces.17–19 In this study, we describe the preparation of magnetic 2D materials using peptides mimicking the precipitation of magnetite within magnetosomes.
The processes leading to the production of magnetite is complex and not entirely understood. Faivre et al. have suggested the involvement of bi- and trivalent iron ions taken up from sediments and that magnetite is then synthesized via templated ferrihydrite precursors.3 Proton pumps maintain the basic pH required for magnetite formation within the magnetosomes. In analogy to biomineralization of bone, teeth, and shells, the crystal phase and structure of the iron oxide generated have been hypothesized to be controlled by specialized proteins within the magnetosome.10,12 Sone et al. have shown that, within these proteins, histidine sites play an important role because of their ability to chelate Fe2+-ions.6
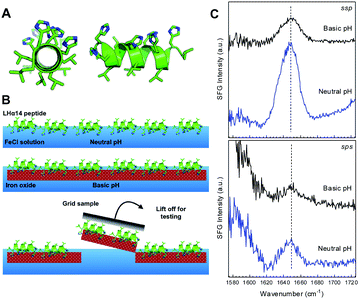
The rationale of this study has been to mimic the precipitation mechanism used by bacteria with a peptide that can precipitate iron oxide 2D sheets and also can effectively bind the air–water interface. We chose a synthetic peptide with the amino acid sequence Ac-LHHLLHLLHHLLHL (short: LHα14, see Fig. 1A), which contains only histidine (H) and leucine (L). In analogy with leucine–lysine (LK) peptides designed by deGrado and Lear,20 the leucine side chains are intended to bind to the air–water interface, while histidines are expected to be exposed to the water phase and available for interactions with iron ions. The hydrophobic periodicity of 3.5 should favor an α-helical secondary structure at an interface and provide a stable platform for interfacial assembly.21 The peptide design allows a maximum number of histidines to be exposed to the solution phase, while the leucine are intended to stabilize the peptide at the water surface.
We used SFG spectroscopy and surface tension measurements to follow the surface assembly (schematic in Fig. 1B and S1†): first, LHα14 is injected into a Teflon trough with the iron chloride solution at neutral pH. The surface tension increased to 19.1 ± 0.8 mN m−1 (measured of 3000 s after stabilization of the film assembly) upon peptide injection, indicating full monolayer coverage of the water surface. The structure of the LHα14 monolayer was determined using sum-frequency generation (SFG) spectroscopy in the amide I region.22 SFG can probe the secondary structure and orientation of peptides and proteins at liquid surfaces. As second-order nonlinear spectroscopy, SFG is intrinsically surface-specific due to selection rules: SFG is only allowed where inversion symmetry is broken, which is typically the case within an interfacial layer between two bulk isotropic media, such as water and air.23 Amide I SFG spectra of LHα14 at the FeCl solution surface collected with ssp (s-polarized SFG, s-polarized visible, p-polarized infrared) and sps polarization, are shown in Fig. 1C. Both ssp and sps SFG spectra show a pronounced amide I band centered near 1644 cm−1, which can be assigned to α-helical structure in D2O.24
Following surface adsorption, the subphase was diluted by a factor of eight to reduce the bulk peptide concentration and avoid precipitation in the volume. Subsequently, the pH is increased using ammonia vapor to trigger the precipitation of iron oxide by protonating the histidine sites. SFG spectra recorded in this state (Fig. 1C) show a reduction of the signal level but the resonance position remains unchanged at basic pH. This implies that the secondary structure of LHα14 remains unchanged.
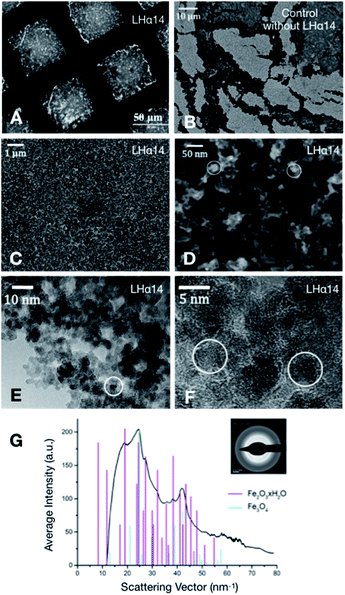
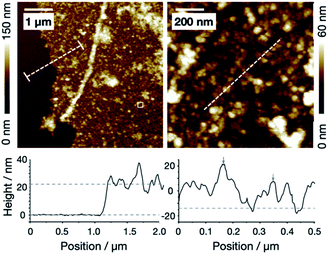
The interaction of protonated LHα14 with iron ions leads to the precipitation of sheets of iron oxide which can be lifted off the solution surface with transmission electron microscopy (TEM) grids (Fig. 2A). A TEM image of a film transferred from the air–water is shown in Fig. 2A. The sheet covers the openings within the TEM grid and is stable on a length scale of tens of micrometers. Control samples, where iron oxide was precipitated without LHα14, resulted in fragmented and unstable films, as can be seen in the SEM image of a film deposited onto a silicon wafer surface in Fig. 2B. Clearly, the peptides are key to stabilize the iron oxide layer. Higher-resolution SEM images in Fig. 2C and D show the LHα14-precipitated films have a sponge-like porous structure consisting of ∼15 nm particles interconnected by wire-like protrusions. Atomic force microscopy (AFM) showed the particles are approximately 20 nm high, leading to the conclusion the particles are roughly spherical (see Fig. 3). The films have a thickness of 22.2 ± 4.3 nm according to AFM line scans across edges of the film.
High-resolution TEM images of an edge in the film are displayed in Fig. 2E. It can be clearly seen that the films are freestanding nanosheets and stable without substrate support. The sheets consist of amorphous areas without crystalline structure and crystalline domains of an approximate size of the particles (Fig. 2F). The respective electron diffraction pattern (Fig. 2G) shows the diffraction rings related to the polycrystalline structures. The average intensities as a function of the diffraction angle are plotted along with reference peak positions for ferrihydrite (red) and magnetite (cyan).25 The ferrihydrite diffraction peaks are generally in good agreement with the experimental peak positions. Some diffraction peaks are not fully resolved within the resolution of the experiment and appear as shoulders (e.g. the peak near 27 nm−1). This is likely also the reason the first two ferrihydrite peaks are not visible in the experimental data. Several peak positions and their relative intensities match the typical magnetite diffraction peaks near 24, 37, 39 and 42 nm−1. The data show that the nanosheets contain both magnetite and its precursor ferrihydrite.
X-ray photoelectron spectroscopy (XPS) shows that the sheets contain significant amounts of iron and oxygen as well as the expected nitrogen and carbon from the peptides in the film (Table 1). No contaminations of the sheets have been detected.
| Fe | O | N | C | Si | |
|---|---|---|---|---|---|
| LHα14 | 7.6 (0.5) | 38.6 (1.0) | 2.0 (0.2) | 30.4 (0.8) | 21.4 (1.2) |
| Control | 10.8 (0.5) | 46.4 (2.4) | 0.4 (0.1) | 27.2 (0.1) | 15.1 (1.9) |
The carbon and nitrogen contents of the nanosheets is comparable to that of peptide-based silica and calcium carbonate biomimetic 2D materials.18,19 Control samples prepared without peptides showed increased amounts of iron and oxygen and negligible nitrogen signals. The carbon signal observed for the control samples is typical for surface preparation under ambient conditions and most likely related to impurity hydrocarbons adsorbed at the air–water interface and the silicon wafer surface (Table 2).
| Fe/Si | Thickness/nm | |
|---|---|---|
| LHα14 | 0.4 | 22.2 (4.3) |
| Control | 0.7 | 38.8 (6.0) |
High-resolution Fe 2p XPS spectra (see Fig. S3 in the ESI†) show peaks near the energy positions expected for the iron oxides Fe2O3 and Fe3O4 at 724.7 eV and 711.0 eV, related to the Fe 2p1/2 and Fe 2p3/2 emission, respectively.26 In addition, a satellite peak observed near 719 eV is commonly assigned to Fe2O3 and the magnetite precursor ferrihydrite. Together, the XPS data show that histidines stabilize iron oxides at the interface.
Conclusions
Histidine-rich LHα14 peptides can precipitate composite iron oxide nanosheets at the air–water interface. The sheets are self-supported, approximately 20 nm thin and at least 50 μm across. The results show that peptide-directed assembly of inorganic materials is a promising approach for the potential fabrication of thin magnetic structures: typical film thicknesses achieved using standard synthesis yield materials with film thicknesses closer to several hundred nanometers.In future applications, the versatility of peptide amino acid sequences will open up possibilities for tuning the morphology and polymorphism of the nanosheets. The synthesis is, in principle, also compatible with strategies to fabricate 3D structures at the interfaces within emulsions. Generally, the new approach to synthesize nanometer-thin iron oxides may pave the way towards the fabrication of biomimetic magnetic 2D sheets and a low-cost approach to growing magnetic materials with new properties for data storage, sensing and medical applications.
Author contributions
N. H., H. L., H. L., T. W. G., M. B and G. J. performed experiments. All authors analysed data, discussed conclusions and wrote the article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Villum Foundation (Experiment Grant 22956) and the Novo Nordisk Foundation (Facility Grant NanoScat, No. NNF18OC0032628) for financial support. TWG thanks the Lundbeck Foundation for a postdoc fellowship (R322-2019-2461).References
- R. Blakemore, Science, 1975, 190, 377 CrossRef CAS PubMed.
- D. A. Bazylinski and R. B. Frankel, Nat. Rev. Microbiol., 2004, 2, 217 CrossRef CAS PubMed.
- D. Faivre and D. Schüler, Chem. Rev., 2008, 108, 4875 CrossRef CAS PubMed.
- (a) D. Schüler and R. B. Frankel, Appl. Microbiol. Biotechnol., 1999, 52, 464 CrossRef PubMed; (b) K. O. Konhauser, Earth-Sci. Rev., 1998, 43, 91 CrossRef CAS.
- (a) D. Bertrand, P. Mihály, H. Xin, D. A. Bazylinski, R. B. Frankel and P. R. Buseck, Am. Mineral., 1998, 83, 1387 CrossRef; (b) A. Scheffel, A. Gärdes, K. Grünberg, G. Wanner and D. Schüler, J. Bacteriol., 2008, 190, 377 CrossRef CAS PubMed.
- E. D. Sone and S. I. Stupp, Chem. Mater., 2011, 23, 2005 CrossRef CAS.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 3995 CrossRef CAS PubMed.
- S. Mornet, S. Vasseur, F. Grasset, P. Veverka, G. Goglio, A. Demourgues, J. Portier, E. Pollert and E. Duguet, Prog. Solid State Chem., 2006, 34, 237 CrossRef CAS.
- R. F. Service, Science, 2000, 287, 1902 CrossRef CAS.
- L. Wang, T. Prozorov, P. E. Palo, X. Liu, D. Vaknin, R. Prozorov, S. Mallapragada and M. Nilsen-Hamilton, Biomacromolecules, 2012, 13, 98 CrossRef PubMed.
- S. M. Bird, A. E. Rawlings, J. M. Galloway and S. S. Staniland, RSC Adv., 2016, 6, 7356 RSC.
- T. Prozorov, S. K. Mallapragada, B. Narasimhan, L. Wang, P. Palo, M. Nilsen-Hamilton, T. J. Williams, D. A. Bazylinski, R. Prozorov and P. C. Canfield, Adv. Funct. Mater., 2007, 17, 951 CrossRef CAS.
- A. Turchanin and A. Gölzhäuser, Adv. Mater., 2016, 28, 6075 CrossRef CAS PubMed.
- H. Zhao, Y. Zhu, F. Li, R. Hao, S. Wang and L. Guo, Angew. Chem., Int. Ed., 2017, 56, 8766 CrossRef CAS PubMed.
- (a) D. Anselmetti and A. Gölzhäuser, Angew. Chem., Int. Ed. Engl., 2014, 53, 12300 CAS; (b) H. Qin, F. Li, D. Wang, H. Lin and J. Jin, ACS Nano, 2016, 10, 948 CrossRef CAS PubMed; (c) E. J. Robertson, A. Battigelli, C. Proulx, R. V. Mannige, T. K. Haxton, L. Yun, S. Whitelam and R. N. Zuckermann, Acc. Chem. Res., 2016, 49, 379 CrossRef CAS PubMed; (d) E. J. Robertson, G. K. Oliver, M. Qian, C. Proulx, R. N. Zuckermann and G. L. Richmond, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 13284 CrossRef CAS PubMed; (e) S. Schrettl, C. Stefaniu, C. Schwieger, G. Pasche, E. Oveisi, Y. Fontana, A. F. i. Morral, J. Reguera, R. Petraglia and C. Corminboeuf, et al., Nat. Chem., 2014, 6, 468 CrossRef CAS PubMed.
- (a) M. Hildebrand, Chem. Rev., 2008, 108, 4855 CrossRef CAS PubMed; (b) H. Pyles, S. Zhang, J. J. de Yoreo and D. Baker, Nature, 2019, 571, 251 CrossRef CAS PubMed.
- (a) H. Lu, A. Schäfer, H. Lutz, S. J. Roeters, I. Lieberwirth, R. Muñoz-Espí, M. A. Hood, M. Bonn and T. Weidner, J. Phys. Chem. Lett., 2019, 10, 2170 CrossRef CAS PubMed; (b) H. Lutz, V. Jaeger, M. Bonn, J. Pfaendtner and T. Weidner, J. Pept. Sci., 2017, 23, 141 CrossRef CAS PubMed.
- H. Lu, H. Lutz, S. J. Roeters, M. A. Hood, A. Schäfer, R. Muñoz-Espí, R. Berger, M. Bonn and T. Weidner, J. Am. Chem. Soc., 2018, 140, 2793 CrossRef CAS PubMed.
- H. Lutz, V. Jaeger, L. Schmüser, M. Bonn, J. Pfaendtner and T. Weidner, Angew. Chem., Int. Ed., 2017, 56, 8277 CrossRef CAS PubMed.
- (a) W. F. DeGrado and J. D. Lear, J. Am. Chem. Soc., 1985, 107, 7684 CrossRef CAS; (b) W. F. DeGrado, Z. R. Wasserman and J. D. Lear, Science, 1989, 243, 622 CrossRef CAS PubMed.
- T. Weidner, J. S. Apte, L. J. Gamble and D. G. Castner, Langmuir, 2010, 26, 3433 CrossRef CAS PubMed.
- S. Hosseinpour, S. J. Roeters, M. Bonn, W. Peukert, S. Woutersen and T. Weidner, Chem. Rev., 2020, 120, 3420–3465 CrossRef CAS PubMed.
- (a) Y. R. Shen, The principles of nonlinear optics, Wiley-Interscience, New York, 1984 Search PubMed; (b) A. G. Lambert, P. B. Davies and D. J. Neivandt, Appl. Spectrosc. Rev., 2005, 40, 103 CrossRef CAS; (c) S. Roy, P. A. Covert, W. R. FitzGerald and D. K. Hore, Chem. Rev., 2014, 114, 8388 CrossRef CAS PubMed; (d) B. Ding, J. Jasensky, Y. Li and Z. Chen, Acc. Chem. Res., 2016, 49, 1149 CrossRef CAS PubMed.
- B. R. Singh, in Infrared analysis of peptides and proteins, ACS Symposium Series, Washington D. C., 1999 Search PubMed.
- JCPDS International Centre for Diffraction Data, PDF-2 Database, Newton Square, USA, 2004 Search PubMed.
- T. Yamashita and P. Hayes, Appl. Surf. Sci., 2008, 254, 2441 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra04733g |
| This journal is © The Royal Society of Chemistry 2021 |