Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
New insight into the electrochemical reduction of different aryldiazonium salts in aqueous solutions
Zahra Tavakkoli,
Hamed Goljani,
Hassan Sepehrmansourie,
Davood Nematollahi * and
Mohammad Ali Zolfigol
* and
Mohammad Ali Zolfigol
Faculty of Chemistry, Bu-Ali-Sina University, Hamedan 65174, Iran. E-mail: nemat@basu.ac.ir
First published on 27th July 2021
Abstract
Electrochemical reduction of different aryldiazonium salts in aqueous solution was studied in this work and it is shown that the aryldiazonium salts are converted to the corresponding aryl radical and aryl anion. The results of this research indicate that the reduction of aryldiazonium salts takes place in two single-electron steps. Our data show that when the substituted group on the phenyl ring is H, Cl, OH, NO2, OCH3 or SO3−, the corresponding diazonium salt shows poor adsorption characteristics, but when the substituted group is methyl, the corresponding diazonium salt shows strong adsorption characteristics. In the latter case, the voltammogram exhibits three cathodic peaks. In addition, the effect of various substitutions on the aryldiazonium reduction was studied by Hammett's method. The data are show that with increasing electron withdrawing capacity of the substituent, the reduction of corresponding diazonium salt becomes easier.
Introduction
Diazonium salts are a large group of organic compounds with the general formula R–N2+X−, where R can be alkyl or aryl and X is an organic or inorganic anion such as a halogen.1 Diazonium salts are very important chemicals that have been used as versatile building blocks for the syntheses of a broad range of organic molecules. These compounds are able to perform two types of reactions: nitrogen-removal reactions and nitrogen-retention reactions.2 In the first type, with the loss of the N2 molecule, chemical bonds such as C–C, C–X, C–S, C–P and C–P are formed.2–8 In the second type, the nitrogen atoms remain in the molecule as N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N or N–N bonds.2,5,9,10
N or N–N bonds.2,5,9,10
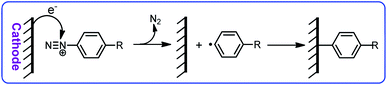
The high efficiency and high selectivity of these compounds have led to an increase in their use in the synthesis of organic compounds.11,12 Despite the large number of published papers on the synthesis of organic compounds using diazonium salts, the electrochemical behavior of these salts has not been well studied. A literature survey on electrochemical reduction of diazonium salts show that the goal of most of these studies is the reduction of the diazonium salts to produce the corresponding radical in order to modify the electrode surface (Scheme 1).13–21
These studies show that the reduction of aryldiazoniums involves a single-electron transfer from the cathode to the aryldiazonium salt, resulting in the release of a nitrogen molecule and the formation of an aryl radical followed by the bond formation between the electrode surface and the aryl group. Many researchers have worked on the grafting of aryldiazonium salts on different electrode surfaces, and obtained satisfactory results. Among them we can mention the interesting studies conducted by Pinson22–31 and Downard et al.32–41 In these published papers, the authors provided valuable information on the electrochemical reduction mechanism of aryldiazoniums in acetonitrile. Nevertheless, this study seeks to expand the frontiers of knowledge on some electrochemical properties of aryldiazonium salts in aqueous solutions. Therefore, in this paper, we want to provide some new information on the electrochemical behavior of aryldiazonium salts in water and investigate the effect of substituent groups on adsorption activity and electrochemical behavior of these compounds. This study will contribute to expand the understanding of electrochemical reduction of aryldiazonium salts in aqueous solutions.
Results and discussion
Mechanistic studies
Cyclic voltammograms of twelve aryldiazonium hydrogen sulfate salts (ADs) (10 mM) containing H, Cl, NO2, OCH3, SO3−, OH and CH3 substituent groups in aqueous solution (H2SO4, c = 1.0 M) at a temperature 4 ± 1 °C are shown in Fig. 1. The first common feature of these diazonium salts is their irreversibility. The absence of anodic peak even at high potential scan rates indicates the high reactivity of the electrode product and its participation in an irreversible fast chemical reaction. Most of these compounds (containing Cl, NO2, OCH3, SO3H, H and OH substituent groups) have two cathodic peaks (C1 and C2) and some (containing CH3 substituent group), have three cathodic peaks (C0, C1 and C2).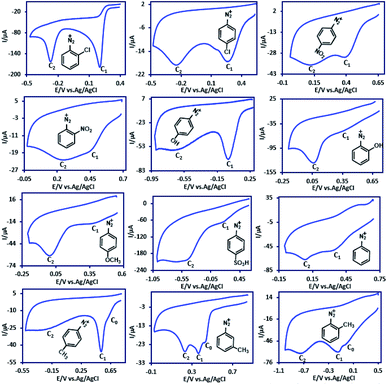 | ||
| Fig. 1 Cyclic voltammograms of 10 mM of ADs in aqueous solution (H2SO4, c = 1 M) at glassy carbon electrode. Scan rate: 100 mV s−1. Temperature: 4 ± 1 °C. | ||
To explain this difference, the linear sweep voltammograms of 2-chlorobenzenediazonium (2ClAD) and 3-methylbenzenediazonium (3MeAD) were recorded at a temperature 4 ± 1 °C, at different scan rates (Fig. 2). As can be seen, in both compounds the peak current ratio (IpC2/IpC1) changes with increasing scan rates. This shows the unequal influence of the adsorption process on two single-electron transfer steps. The examination of 2ClAD voltammograms shows that while at a scan rate of 10 mV s−1, the peak current ratio, IpC2/IpC1 is about 0.3 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IpC2/IpC1 = −0.52), with the increase of potential scan rate to 4000 mV s−1, the ratio increases sharply to about 2.4 (log
IpC2/IpC1 = −0.52), with the increase of potential scan rate to 4000 mV s−1, the ratio increases sharply to about 2.4 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IpC2/IpC1 = 0.38). These results indicate that the adsorption ability of the product of second electron transfer process is more than that of the first electron transfer process. To confirm this statement, the dependence of logarithm of the C1 and C2 peak currents (log
IpC2/IpC1 = 0.38). These results indicate that the adsorption ability of the product of second electron transfer process is more than that of the first electron transfer process. To confirm this statement, the dependence of logarithm of the C1 and C2 peak currents (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IpC1 and log
IpC1 and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IpC2) on the logarithm of potential scan rate (log
IpC2) on the logarithm of potential scan rate (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν) were examined (Fig. 2, part VI). Under these conditions, the slope of the line for a pure diffusion controlled process is 0.5,42 (p. 236) while this value is equal to 1 for a pure adsorption process.42 (p. 591) The slope of log
ν) were examined (Fig. 2, part VI). Under these conditions, the slope of the line for a pure diffusion controlled process is 0.5,42 (p. 236) while this value is equal to 1 for a pure adsorption process.42 (p. 591) The slope of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IpC1 vs. log
IpC1 vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν is 0.57. This value is higher than the theoretical value of 0.5 and is less than one, which indicate a partial adsorption for peak C1. The slope of log
ν is 0.57. This value is higher than the theoretical value of 0.5 and is less than one, which indicate a partial adsorption for peak C1. The slope of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IpC2 vs. log
IpC2 vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν is 0.92. This value is near to one which is the theoretical value for the adsorption-controlled process and confirms the greater adsorption ability of the product of second electron transfer process than that of the first electron transfer process.
ν is 0.92. This value is near to one which is the theoretical value for the adsorption-controlled process and confirms the greater adsorption ability of the product of second electron transfer process than that of the first electron transfer process.
The voltammograms of 3MeAD however, show a more complex behavior. The linear sweep voltammogram of 3MeAD at a scan rate of 10 mV s−1, shows three cathodic peaks (C0, C1 and C2). At low scan rates, IC0 and IC1 are larger than the IC2. As the potential scan rate increases, IC0 increases linearly and its shape (peak C0) becomes sharper and more symmetrical. Under these conditions, the slope of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IpC0 vs. log
IpC0 vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν is 0.96 (Fig. 2, part V, inset). This value confirms that peak C0 is an adsorption peak which is separated by a 96 mV from peak C1. This type of peak is observed when the electrode product is strongly adsorbed.42 (p. 596) The response of peaks C1 and C2 to increasing potential scan rate is generally similar to that seen for 2ClAD.
ν is 0.96 (Fig. 2, part V, inset). This value confirms that peak C0 is an adsorption peak which is separated by a 96 mV from peak C1. This type of peak is observed when the electrode product is strongly adsorbed.42 (p. 596) The response of peaks C1 and C2 to increasing potential scan rate is generally similar to that seen for 2ClAD.
The LSV of 3MeAD in aqueous solution (H2SO4, c = 1 M) is shown in Fig. 3a and has been compared with that of in acetonitrile solution containing HClO4 (1 M) (Fig. 3b). The most important feature of Fig. 3b is the presence of two peaks. Based on our findings on the adsorption nature of peak C0, this change in acetonitrile solution was predictable. The polarity of the solvent has a significant effect on the adsorption of organic compounds onto electrode surface. The higher solubility of the organic compounds in organic solvents decreases the adsorption process due to the higher affinity between organic compound and solvent.43 Therefore, in acetonitrile solution, peak C0 (a pre-peak) is removed and the voltammogram shows only peaks C1 and C2.
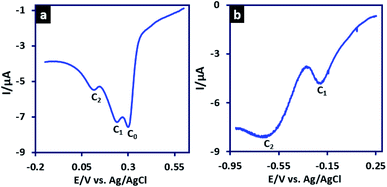 | ||
| Fig. 3 (a) LSV of 3MeAD (10 mM) in aqueous solution (H2SO4, c = 1 M) and (b) LSV of 3MeAD (10 mM) acetonitrile solution (HClO4, c = 1 M). Scan rate: 10 mV s−1. Temperature: 4 ± 1 °C. | ||
The presence of two peaks in the voltammograms of aryldiazoniums have also been reported in other studies.25,31,44–48 Downard and coworkers studied the electrochemical reduction of 4-nitrobenzenediazonium ion in [Bu4N]BF4–ACN and stated that the presence of two peaks in voltammograms is due to “a surface-catalyzed reduction step (proceeding at a clean surface only) followed by an uncatalyzed reduction at a more negative potential”.48 Since our studies were conducted in aqueous solution (H2SO4, c = 1.0 M), but Downard's research in [Bu4N]BF4–ACN, there are some important differences in our results. In the voltammograms recorded by Downard et al.,48 the ratio of the first cathodic peak to the second cathodic peak (IpC1/IpC2) increases with increasing potential scan rate, while our results are completely opposite (Fig. 2). Also in the case of some aryldiazonium salts such as 3MeAD, we clearly observed three cathodic peaks (C0, C1 and C2) while such a case was not reported by Downard et al.
In connection with the observation of two cathodic peaks in the cyclic voltammogram of aryldiazonium salts, Pinson et al. reported that (in [Bu4N]BF4–ACN) the steric effect can limit or even suppress aryl radical grafting for aryldiazonium substituted in ortho position.49 They reported that the cyclic voltammograms under these conditions show only one cathodic peak.49 However, we observed that, the position of the substituted group on the phenyl ring has no effect on the number of peaks. As shown in Fig. 1 and 2, the cyclic voltammograms of aryldiazonium salts such as 2ClAD, 2MeAD and 2NO2AD clearly show the presence of two cathodic peaks.
In this regard, Richard and co-workers investigated the electrochemical reduction of 4-nitrobenzenediazonium ion in water (0.1 M HCl) at GC electrode and announced that peak C1 corresponds to the reduction of 4NO2AD with concomitant grafting, and peak C2 corresponds the reduction of the grafted nitro group.46 This statement cannot be general, because, as shown in Fig. 1, there are two peaks in the cyclic voltammogram of aryldiazonium salts with other substituent groups, such as CH3, Cl, H or OH.
Based on our results, it is clear that the data reported for the presence of two reduction peaks in acetonitrile solution cannot be used in water. According to our results, it can be concluded that in the case of 3MeAD, we are confronted with the strong adsorption of electrochemically generated aryl radicals. This strong adsorption causes peak C0 to appear as a pre-peak in the CVs of 3MeAD.42 These data, along with the results of previously published results50 can be used to propose the following pathway for the electrochemical reduction of ADs (Scheme 2). According to the proposed scheme, in the first stage an electron transfer concerted with the cleavage of dinitrogen50 produces the aryl radical and in the second stage, the produced aryl radical is converted to related aromatic compound by taking one electron and one proton.
Hammett studies
The effect of substituents on the reduction potential of AD compound is analyzed using the Hammett equation:51
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ei = log Ei = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E0 + ρσp E0 + ρσp
| (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ei–σ graph, reflecting the sensitivity of reduction potentials to the substituent effects. The Hammett plot for AD compounds is shown in Fig. 4. As can be seen, there is a relatively good linear relationship between reduction peak potential (EpC2) and Hammett constant (σ). The slope of the line is 0.63. The positive slope indicates that the reduction potential of AD compounds is increased (easier reduction) significantly with increasing electron-withdrawing ability of the substituents. The results also show that the reduction potential of AD compounds to vary in the order 4NO2AD (σp = 0.78 (ref. 52)) > 4ClAD σp = 0.23 (ref. 52)) > 4SO3−AD (σp = 0.09 (ref. 53 and 54)) > 4MeAD (σp = −0.17 (ref. 52)) > 4OHAD (σp = −0.37 (ref. 52)). On the other hand, 4-methoxybenzenediazonium (4MeOAD) has a significant deviation from EpC2–σp plot. This can be due to the dual properties OCH3 group (resonance and inductive). The methoxide is inductively a withdrawing and resonantly a donating group. At the moment, we do not have a strong argument for diverting HAD from the EpC2–σp plot. This linear relationship shows that reduction pathway of mentioned substituents are same together.
Ei–σ graph, reflecting the sensitivity of reduction potentials to the substituent effects. The Hammett plot for AD compounds is shown in Fig. 4. As can be seen, there is a relatively good linear relationship between reduction peak potential (EpC2) and Hammett constant (σ). The slope of the line is 0.63. The positive slope indicates that the reduction potential of AD compounds is increased (easier reduction) significantly with increasing electron-withdrawing ability of the substituents. The results also show that the reduction potential of AD compounds to vary in the order 4NO2AD (σp = 0.78 (ref. 52)) > 4ClAD σp = 0.23 (ref. 52)) > 4SO3−AD (σp = 0.09 (ref. 53 and 54)) > 4MeAD (σp = −0.17 (ref. 52)) > 4OHAD (σp = −0.37 (ref. 52)). On the other hand, 4-methoxybenzenediazonium (4MeOAD) has a significant deviation from EpC2–σp plot. This can be due to the dual properties OCH3 group (resonance and inductive). The methoxide is inductively a withdrawing and resonantly a donating group. At the moment, we do not have a strong argument for diverting HAD from the EpC2–σp plot. This linear relationship shows that reduction pathway of mentioned substituents are same together.
 | ||
Fig. 4 Relationship between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EpC2 and σp for AD derivatives in aqueous solution (H2SO4, c = 1 M) at glassy carbon electrode. Temperature: 4 ± 1 °C. EpC2 and σp for AD derivatives in aqueous solution (H2SO4, c = 1 M) at glassy carbon electrode. Temperature: 4 ± 1 °C. | ||
Conclusions
In this study electrochemical reduction of AD derivatives has been studied in aqueous solution (H2SO4, c = 1 M) and in temperature 4 ± 1 °C by cyclic voltammetry technique at a glassy carbon electrode. Our results show two irreversible cathodic processes which are attributed to the reduction of ADs to the corresponding aryl radical and reduction of aryl radical to aryl anion. Our data indicate that when the substituted group on the phenyl ring is H, Cl, OH, NO2, OCH3 or SO3−, the corresponding diazonium salt shows poor adsorption characteristics. As a result, the voltammograms of these diazonium salts show only two reduction peaks (C1 and C2). But when the substituted group is methyl, the corresponding diazonium salts show strong adsorption characteristics. Therefore, the voltammograms of these diazonium salts, in addition to the peaks C1 and C2, show the adsorption peak C0. The effect of substituent groups on the reduction of ADs was investigated by Hammett studies. The data are show that with increasing electron withdrawing capacity of the substituent, the reduction of corresponding diazonium salt becomes easier. In the end, according to Downard48 “the origin of this intriguing behavior remains controversial”.Experimental section
Apparatus and reagents
Cyclic voltammetry and linear sweep voltammetry were performed using an Autolab model PGSTAT 20 potentiostat/galvanostat equipped with NOVA 1.10 software. The working electrode used in the voltammetry experiments was a glassy carbon disc (2.6 mm diameter) and a platinum wire was used as the counter electrode. The working electrode potentials were measured vs. Ag/AgCl (3.0 mol L−1 KCl) (all electrodes from AZAR electrodes). The glassy carbon electrode was polished using alumina slurry followed by washing with water and acetone. All anilines derivatives were obtained from commercial sources.Aryldiazonium salts were synthesized by adding 0.1 mmol aniline derivatives in 10 ml aqueous solution containing 1.0 M of sulfuric acid. After cooling the temperature of the solution down to 4 ± 1 °C, 0.12 mmol of sodium nitrite was added to the mixture. In order to synthesize aryldiazonium salts suitable for study in organic solvents, follows the method already described and use only perchloric acid instead of sulfuric acid. The precipitate was separated by filtration and washed several times with cold distilled water. In the following, for voltammetry in acetonitrile, 0.1 mmol of the precipitate was dissolved in 10 ml acetonitrile containing 1.0 M perchloric acid.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors wish to acknowledge the Bu-Ali Sina University Research Council for the support of this work.Notes and references
- F. Mo, G. Dong, Y. Zhang and J. Wang, Org. Biomol. Chem., 2013, 11, 1582–1593 RSC.
- M. Wang, B. C. Tang, J. C. Xiang, X. L. Chen, J. T. Ma, Y. D. Wu and A. X. Wu, Org. Lett., 2019, 21, 8934–8937 CrossRef CAS PubMed.
- Z. Ni, X. Huang and Y. Pan, Org. Lett., 2016, 18, 2612–2615 CrossRef CAS PubMed.
- F. L. Callonnec, E. Fouquet and F. X. Felpin, Org. Lett., 2011, 13, 2646–2649 CrossRef PubMed.
- H. Goljani, Z. Tavakkoli, A. Sadatnabi and D. Nematollahi, Org. Lett., 2020, 15, 5920–5924 CrossRef PubMed.
- Q. Liu, B. Sun, Z. Liu, Y. Kao, B. W. Dong, S. D. Jiang, F. Li, G. Liu, Y. Yang and F. Mo, Chem. Sci., 2018, 9, 8731–8737 RSC.
- Y. He, H. Wu and F. D. Toste, Chem. Sci., 2015, 6, 1194–1198 RSC.
- F. Mo, Y. Jiang, D. Qiu, Y. Zhang and J. Wang, Angew. Chem., Int. Ed., 2010, 49, 1846–1849 CrossRef CAS PubMed.
- M. Ramanathan, Y. H. Wang and S. T. Liu, Org. Lett., 2015, 17, 5886–5889 CrossRef CAS PubMed.
- Y. Shao, H. Zheng, J. Qian and X. Wan, Org. Lett., 2018, 20, 2412–2415 CrossRef CAS PubMed.
- J. Liu, E. Xu, J. Jiang, Z. Huang, L. Zheng and Z. Q. Liu, Chem. Commun., 2020, 56, 2202–2205 RSC.
- R. Abrams, Q. Lefebvre and J. Clayden, Angew. Chem., 2018, 57, 13587–13591 CrossRef CAS PubMed.
- H. Salehzadeh, D. Nematollahi, V. Khakyzadeh, B. Mokhtari and L. C. Henderson, Electrochim. Acta, 2014, 139, 270–280 CrossRef CAS.
- H. Salehzadeh, D. Nematollahi and S. Alizadeh, Electroanalysis, 2015, 27, 2738–2744 CrossRef CAS.
- Z. Kudas, U. Atmaca, T. Saruhan, M. Celik and D. Ekinci, Electroanalysis, 2020, 32, 1379–1390 CrossRef CAS.
- F. Lebon, R. Cornut, V. Derycke and B. Jousselme, Electrochim. Acta, 2019, 318, 754–761 CrossRef CAS.
- Y. Yang, W. Yan, Y. Guo, X. Wang, F. Zhang, L. Yu, C. Guo and G. Fang, Sens. Actuator Rep., 2020, 2, 100009 Search PubMed.
- N. Vilà and D. Bélanger, Electrochim. Acta, 2012, 85, 538–547 CrossRef.
- M. D. Raicopol, C. Andronescu, R. Atasiei, A. Hanganu, E. Vasile, A. M. Brezoiu and L. Pilan, Electrochim. Acta, 2016, 206, 226–237 CrossRef CAS.
- G. L. C. Paulus, Q. H. Wang and M. S. Strano, Acc. Chem. Res., 2013, 46, 160–170 CrossRef CAS PubMed.
- A. Kaplan, Z. Yuan, J. D. Benck, A. G. Rajan, X. S. Chu, Q. H. Wang and M. S. Strano, Chem. Soc. Rev., 2017, 46, 4530–4571 RSC.
- M. Delamar, R. Hitmi, J. Pinson and J. M. Saveant, J. Am. Chem. Soc., 1992, 114, 5883–5884 CrossRef CAS.
- P. Allongue, M. Delamar, B. Desbat, O. Fagebaume, R. Hitmi, J. Pinson and J. M. Saveant, J. Am. Chem. Soc., 1997, 119, 201–207 CrossRef CAS.
- A. Chaussé, M. M. Chehimi, N. Karsi, J. Pinson, F. Podvorica and C. Vautrin-Ul, Chem. Mater., 2002, 14, 392–400 CrossRef.
- J. Pinson and F. Podvorica, Chem. Soc. Rev., 2005, 34, 429–439 RSC.
- P. Doppelt, G. Hallais, J. Pinson, F. Podvorica, F. Podvorica and S. Verneyre, Chem. Mater., 2007, 19, 4570–4575 CrossRef CAS.
- S. Bouden, J. Pinson and C. Vautrin-Ul, Electrochem. Commun., 2017, 81, 120–123 CrossRef CAS.
- D. Hetemi, V. Noël and J. Pinson, Biosensors, 2020, 10, 4 CrossRef CAS PubMed.
- D. Belanger and J. Pinson, Chem. Soc. Rev., 2011, 40, 3995–4048 RSC.
- J. Pinson and F. I. Podvorica, Curr. Opin. Electrochem., 2020, 24, 44–48 CrossRef CAS.
- C. Combellas, F. Kanoufi, J. Pinson and F. I. Podvorica, J. Am. Chem. Soc., 2008, 130, 8576–8577 CrossRef CAS PubMed.
- P. A. Brooksby and A. J. Downard, Langmuir, 2004, 20, 5038–5045 CrossRef CAS PubMed.
- T. Menanteau, M. Dias, E. Levillain, A. J. Downard and T. Breton, J. Phys. Chem. C, 2016, 120, 4423–4429 CrossRef CAS.
- J. Lehr, B. E. Williamson, B. S. Flavel and A. J. Downard, Langmuir, 2009, 25, 13503–13509 CrossRef CAS PubMed.
- M. G. Paulik, P. A. Brooksby, A. D. Abell and A. J. Downard, J. Phys. Chem. C, 2007, 111, 7808–7815 CrossRef CAS.
- B. S. Flavel, A. J. Gross, D. J. Garrett, V. Nock and A. J. Downard, ACS Appl. Mater. Interfaces, 2010, 2, 1184–1190 CrossRef CAS PubMed.
- K. J. Bell, P. A. Brooksby, M. I. J. Polson and A. J. Downard, Chem. Commun., 2014, 50, 13687–13690 RSC.
- L. Lee, P. A. Brooksby and A. J. Downard, Electrochem. Commun., 2012, 19, 67–69 CrossRef CAS.
- A. J. Downard, Langmuir, 2000, 16, 9680–9682 CrossRef CAS.
- J. Lehr, B. E. Williamson and A. J. Downard, J. Phys. Chem. C, 2011, 115, 6629–6634 CrossRef CAS.
- D. J. Garrett, J. Lehr, G. M. Miskelly and A. J. Downard, J. Am. Chem. Soc., 2007, 129, 15456–15457 CrossRef CAS PubMed.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods, Wiley, New York, 2nd edn, 2001. pp. 236 and 595 Search PubMed.
- A. M. Dowaidar, M. S. El-Shahawi and I. Ashour, Sep. Sci. Technol., 2007, 42, 3609–3622 CrossRef CAS.
- K. K. Cline, L. Baxter, D. Lockwood, R. Saylor and A. Stalzer, J. Electroanal. Chem., 2009, 633, 283–290 CrossRef CAS.
- A. Benedetto, M. Balog, P. Viel, F. Le Derf, M. Salle and S. Palacin, Electrochim. Acta, 2008, 53, 7117–7122 CrossRef CAS.
- W. Richard, D. Evrard and P. Gros, J. Electroanal. Chem., 2012, 685, 109–115 CrossRef CAS.
- T. Menanteau, E. Levillain and T. Breton, Chem. Mater., 2013, 25, 2905–2909 CrossRef CAS.
- L. Lee, P. A. Brooksby, P. Hapiot and A. J. Downard, Langmuir, 2016, 32, 468–476 CrossRef CAS PubMed.
- C. Combellas, D. Jiang, F. Kanoufi, J. Pinson and F. I. Podvorica, Langmuir, 2009, 25, 286–293 CrossRef CAS PubMed.
- P. Andrieux and J. Pinson, J. Am. Chem. Soc., 2003, 125, 14801–14806 CrossRef PubMed.
- L. P. Hammett, J. Am. Chem. Soc., 1937, 59, 96–103 CrossRef CAS.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- E. A. Savicheva, G. Y. Mitronova, L. Thomas, M. J. Böhm, J. Seikowski, V. N. Belov and S. W. Hell, Angew. Chem., Int. Ed. Engl., 2020, 59, 5505–5509 CrossRef CAS PubMed.
- H. Zollinger, W. Büchler and C. Wittwer, Helv. Chim. Acta, 1953, 36, 1711–1722 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |