Open Access Article
Open Access ArticleEthylene-bridged polysilsesquioxane/hollow silica particle hybrid film for thermal insulation material†
Satoru Tsukada ab,
Yuki Nakanishiac,
Takashi Hamada
ab,
Yuki Nakanishiac,
Takashi Hamada *a,
Kenta Okadaac,
Susumu Mineoiac and
Joji Ohshita
*a,
Kenta Okadaac,
Susumu Mineoiac and
Joji Ohshita *ade
*ade
aCollaborative Research Laboratory, Graduate School of Advanced Science and Engineering, Hiroshima University, 1-4-1 Kagamiyama, Higashi-Hiroshima, Hiroshima 739-8527, Japan. E-mail: hama@hiroshima-u.ac.jp; jo@hiroshima-u.ac.jp
bDepartment of Materials Science, Graduate School of Engineering, Chiba University, 1-33 Yayoi-cho, Inage-ku, Chiba, 263-8522, Japan
cTechnical Research Center, Mazda Motor Corporation, 3-1 Shinchi, Fuchu-cho, Aki-gun, Hiroshima 730-8670, Japan
dSmart Innovation Program, Graduate School of Advanced Science and Engineering, Hiroshima University, 1-4-1 Kagamiyama, Higashi-Hiroshima, Hiroshima 739-8527, Japan
eDivision of Materials Model-Based Research, Digital Monozukuri (Manufacturing) Education and Research Center, Hiroshima University, 3-10-32 Kagamiyama, Higashi-Hiroshima, Hiroshima 739-0046, Japan
First published on 19th July 2021
Abstract
Ethylene-bridged polysilsesquioxane (EBPSQ) was prepared by the sol–gel reaction of bis(triethoxysilyl)ethane. The whitish slurry was prepared by mixing EBPSQ and hollow silica particles (HSPs) with a median diameter of 18–65 μm at 80 °C, and it formed a hybrid film by heating at 80 and 120 °C for 1 h at each temperature, then at 200 °C for 20 min. The surface temperatures of EBPSQ films containing 10 wt% and 20 wt% of HSPs (90.2 °C–90.5 °C) were lower than those of EBPSQ films (93.6 °C), when the films on the duralumin plate were heated at 100 °C for 10 min from the bottom of the duralumin plate. The thermal conductivity/heat flux (k/q) obtained from the temperature difference between the surface temperature and bottom temperature of the films and the film thickness also decreased with adding the HSPs. EBPSQ film without HSPs exhibited T5d of 258 °C and T10d of 275 °C. However, EBPSQ film containing 20 wt% of HSPs exhibited high thermal stability, and T5d and T10d were 299 °C and 315 °C, respectively. Interestingly, T5d and T10d of the hybrid films increased with an increase in the number of HSPs. Overall, it was shown that HSPs could improve the thermal insulation properties and thermal stability.
Introduction
Currently, much energy is consumed by the heating and cooling systems in buildings. To achieve a sustainable society, it is necessary to reduce energy consumption and carbon dioxide emissions. Therefore, thermal insulator materials are crucial for reducing energy losses by preventing heat transfer through walls and roofs.1,2 Thermal insulation materials such as fiberglass and polymer foams have been developed to reduce thermal conductivity.1–4 Thermal insulation materials such as expanded polystyrene, polyurethane, and fiberglass have been used in buildings.4,5 To achieve a sustainable society, demands for advanced thermal insulator materials may be needed in the near future; for example, thermal insulator materials with even lower thermal conductivity, nonflammability, light weight, thermal stability, mechanical strength, and low cost may be required. Aerogels are porous materials that are typically made using the sol–gel and supercritical drying method.6 Since aerogels have good properties, such as high porosity, high specific surface area, low density, and low thermal conductivity, various kinds of aerogels such as silica- and polymer-based ones have been studied.7,8 Silica aerogels are good candidates for next-generation thermal insulator material because of their low thermal conductivity and high thermal stability. Therefore, silica aerogels are expected to be used as a thermal insulator in buildings. However, there are several issues with the practical application. For example, aerogels have low mechanical stability caused by the porous network structure. Furthermore, aerogels are expensive to be used as thermal insulation in the building because of their special synthetic procedure. Considering the cost, mechanical properties, thermal stability, and nonflammable properties, a new thermal insulator material alternative to conventional one will be required.The hollow silica particles (HSPs) are also expected to be applied for thermal insulation materials because it exhibits low density and high specific surface area.9 Incorporation of HSPs into the thermal insulation materials might be an effective way to decrease the thermal conductivity because of their porous structure.10,11 The HSPs may be a promising material to achieve high performance required in buildings because inorganic materials typically exhibit high thermal stability and nonflammable property. For example, the HSPs is usually prepared by templating method, and the thermal conductivity of polyethersulfone dramatically decreased by introducing the HSPs.12 To enhance the compatibility with polyurethane, the surface treatment of HSPs was conducted by 3-aminopropytriethoxysilane, and their composite film shows good thermal insulation property.13 Glass bubbles (iM16K) are HSPs consist of the shell of the silica network and the core of cavity, and iM16K are widely used to provide low density, high stiffness, and low thermal and electrical conductivity.14 Overall, the composite of polymer and HSPs is good thermal insulator material. However, there are some problems with poor processability arising from poor solubility and dispersity of HSPs to prepare composite film. Furthermore, considering the application in a difficult situation, a base material for incorporating HSPs must have high thermal stability and nonflammable properties. Therefore, a polymer as a base material is an important component in improving the thermal stability and mechanical properties composite films.
Polysilsesquioxanes (PSQs) are known as organic–inorganic hybrids since it has an organic group on the silicon atom and an inorganic siloxane network. The organic group on the silicone atom improves solubility and miscibility, and the inorganic siloxane network improves thermal and mechanical properties,15 PSQs have attracted interest in materials chemistry.16 PSQs can be easily made from alkoxysilane as a starting material using the sol–gel reaction, which involves hydrolysis and polycondensation.15 Furthermore, PSQs have good film formability, and coating films can be made using a solution technique. Bridged PSQs, which included an organic bridged structure between two silanes, attracted particular attention due to their unique structure; for example, the organic bridged structure was used to apply to separation membranes and low dielectric constant materials.17–19
To date, we have applied bridged PSQ to reverse osmosis (RO) membrane for water purification.20 Interestingly, water permeability was improved depending on the rigidity of the bridged spacer; namely, the membrane pore size increased by the rigidity of the bridged spacer. Based on these findings, we have expected that bridged structure provides porosity, resulting in an excellent thermal insulator property. Indeed, recently, we reported the preparation of ethylene-bridged polysilsesquioxane (EBPSQ) film by hydrosilylation reaction, and their ethylene-bridged structure improved the thermal insulating property.21 To further enhance the thermal insulating properties, we conceived to introduce HSPs containing a hollow cavity into EBPSQ film. Furthermore, because of its excellent thermal properties, EBPSQ is a suitable base polymer for preparing a composite film containing HSPs. Unfortunately, the hydrosilylation reaction used to prepare the EBPSQ film is a multistep and complicated due to the need to synthesize oligosilsesquioxanes with a silyl and vinyl groups. Bis(triethoxysilyl)ethane is a suitable monomer for the preparation of EBPSQ film in terms of the preparation process. As a result, we attempted to make an EBPSQ film from bis(triethoxysilyl)ethane and combine it with HSPs to make a hybrid film.
This paper reports the facile preparation of EBPSQ/HSPs hybrid film for thermal insulator material. We investigated their thermal insulation property and thermal stability to demonstrate the effect of HSPs in the EBPSQ film. Additionally, to demonstrate the effect of ethylene-bridged structure on the thermal insulation property, the thermal insulation property of EBPSQ film was compared with polymethylsilsesquioxane (PMSQ) film.
Experimental
Materials
Bis(triethoxysilyl)ethane was purchased from Oakwood Products, Inc. (Estill, SC, USA) and used as received. Triethoxymethylsilane was purchased from Tokyo Chemical Industry Co., Ltd (Tokyo, Japan) and used as received. Tetrahydrofuran (THF) and ethanol (super dehydrated) was purchased from FUJIFILM Wako Pure Chemical Co., Ltd (Osaka, Japan) and used as received. 6 mol L−1 hydrochloric acid was purchased from FUJIFILM Wako Pure Chemical Co., Ltd (Osaka, Japan) and used as received without purification. Hollow silica particles with a median diameter of 18–65 μm (3M™ Glass Bubbles iM16K) were purchased from 3 M Japan Ltd (hereafter, hollow silica particles of iM16K were named as HSPs). The water was purified by a Millipore Mill-Q UV system and had a resistance of 18.2 MΩ cm and total organic carbon content of <10 ppb.Measurements
The 1H nuclear magnetic resonance (NMR) measurements in deuterium chloroform (CDCl3) were performed on a Varian 400 MHz spectrometer and residual chloroform was used as an internal standard (7.26 ppm) for a chemical shift. A Shimadzu LC-20AD system equipped with a RID-10A detector and three directly connected TSKgel G6000H/G4000H/G2000H columns was used to conduct gel permeation chromatography (GPC). THF was used as the eluent at 40 °C with a flow rate of 1 mL min−1 and polystyrene standards were used for calibration. A Shimazu IR Affinity-1 spectrometer equipped with an attenuated total reflectance (ATR) unit was used to calculate Fourier transform infrared (FTIR) spectra. Thermogravimetric analysis (TGA) data were obtained using an SII EXSTAR TG-DTA6200 thermal analyzer at a heating rate of 10 °C min−1 under airflow of 100 mL min−1. Field emission scanning electron microscopy (FE-SEM) was performed on a Hitachi S-5200 microscope. The sample was coated with platinum by sputtering to prevent charging.Synthesis of EBPSQ and PSQ by nitrogen flow method
EBPSQ was synthesized according to the procedure in a previous report.22,23 In a 100 mL four-necked flask equipped with a mechanical stirrer, nitrogen inlet tube, and an outlet tube, the solution of bis(triethoxysilyl)ethane (3.55 g, 10.0 mmol) and ethanol (4.61 g, 100 mmol) was stirred at a rotation rate of 150 rpm in an ice/water bath for 10 min under a nitrogen flow rate of 360 mL min−1. For hydrolysis, a solution of 0.192 g of 6 mol L−1 HCl and 0.243 g of water (molar ratio of HCl/monomer = 0.105 and water/monomer = 2.2) was slowly added dropwise and stirred at 0 °C for 10 min and at room temperature for 10 min, successively. For the polycondensation reaction, the reaction mixture was then heated for 3 h at 80 °C to afford EBPSQ as a colorless viscous liquid. PMSQ was also synthesized similarly (water/monomer = 2.0).Preparation of hybrid film
A typical preparation of hybrid film is as follows: 0.8865 g of HSPs was added to a colorless viscous liquid of EBPSQ prepared from 3.55 g (10.0 mmol) of bis(triethoxysilyl)ethane, and the mixture was mixed at 80 °C. The mixture turned to whitish slurry after 20 min, and the hot slurry was immediately poured into a poly(tetrafluoroethylene-co-perfluoroalkyl vinyl ether) (PFA) vial with an inner diameter of 18.4 mm after the formation of whitish slurry and heated at 80 °C and 120 °C for 1 h at each temperature, then at 200 °C for 20 min to form gel film. PMSQ film containing HSPs was also prepared in the same procedure.Evaluation of thermal insulation properties of the hybrid film
The thermal insulation property was tested using a handmade technique (Fig. S1†): a hot white slurry of EBPSQ and 20 wt% HSPs was poured onto a duralumin plate and heated at 80 °C and 120 °C for 1 h at each temperature, then at 200 °C for 20 min. The films on the duralumin plate were heated at 100 °C from the bottom of the duralumin plate for the thermal insulation property assessment, and the surface temperature of the film was measured using a surface thermometer. After 10 min of heating the duralumin plate at 100 °C from one side only, the surface temperature of the duralumin plate on the opposite side (t1) was 98 °C. As a result, t1 was set to 98 °C, and after 10 min, the surface temperature (t2) of the film on the duralumin plate was measured.According to Fourier's law of heat conduction,24 the thermal insulation property of films was evaluated by the following eqn (1):
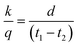 | (1) |
Results and discussion
Preparation of EBPSQ and hybrid films
First, EBPSQ was prepared from bis(triethoxysilyl)ethane (Scheme 1). As reported in a previous paper, the molecular weight of EBPSQ was easily controlled by changing the molar ratio of water and monomer in the sol–gel reaction by the nitrogen flow method.22,23 Unlike the sol–gel reaction under reflux, this method is useful for preparing PSQ with high molecular weight because simultaneous removal of water, catalyst, and solvent provides moderate polycondensation for preventing the gelation and increases the concentration during the polycondensation.25 The weight average molecular weight (Mw) and polydispersity index (Mw/Mn) of EBPSQ with high molecular weight (EBPSQ-HM) were estimated to be 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 and 8.80 by the GPC in case of molar ratio of water/monomer of 2.2. The residual ethoxy group in the polymer was determined to be 25% by 1H NMR.
200 and 8.80 by the GPC in case of molar ratio of water/monomer of 2.2. The residual ethoxy group in the polymer was determined to be 25% by 1H NMR.
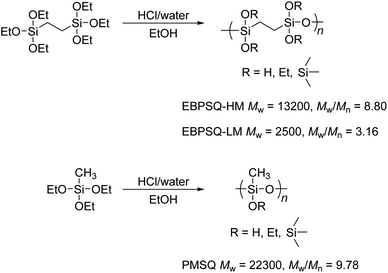 | ||
| Scheme 1 Preparation of EBPSQ and PMSQ by the sol–gel reaction of bis(triethoxysilyl)ethane and triethoxymethylsilane. | ||
However, when the molar ratio of water/monomer was 1.8, the molecular weight of EBPSQ with low molecular weight (EBPSQ-LM) considerably decreased to Mw = 2500 and Mw/Mn = 3.16, and the residual ethoxy group increased to 31%. This suggested that the amount of water in the sol–gel reaction by the nitrogen flow method influenced the molecular weight and residual ethoxy group. To investigate the effect of ethylene-bridged structure in EBPSQ on the thermal properties, PMSQ was also prepared with the molar ratio of water/monomer of 2.0, and it showed Mw of 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 and Mw/Mn of 9.78, as shown in Scheme 1.
300 and Mw/Mn of 9.78, as shown in Scheme 1.
As reported in a previous paper,22 EBPSQ-HM formed the bulk gel by heating at 80 °C and 120 °C for 1 h at each temperature, then at 200 °C for 20 min. The obtained EBPSQ film was transparent and colorless. Next, the viscous liquid of EBPSQ-HM was mixed with 20 wt% of HSPs (against the total mixture of bis(triethoxysilyl)ethane and HSPs) at 80 °C. The mixture of EBPSQ-HM and 20 wt% of HSPs turned to whitish slurry after 20 min. The whitish slurry showed no fluidity solid at room temperature, but it interestingly returned to the highly viscous slurry at 80 °C. To prepare the film, hot whitish slurry was immediately poured into a PFA vial after the formation of whitish slurry and heated at 80 °C and 120 °C for 1 h at each temperature, then at 200 °C for 20 min to obtain the gel film. The whitish slurry changed to solid-like film after heat treatment; namely, EBPSQ-HM and 20 wt% of HSPs formed stiff film (hybrid 1–20). The film was hereafter denoted hybrid n–X, where n and X denote the hybrid film species and the number of HSPs in hybrid film, respectively. It was noted that hybrid film was readily prepared by mixing EBPSQ-HM and HSPs at 80 °C, and good processability was presented. This might be due to the good miscibility of EBPSQ-HM and HSPs; namely, EBPSQ-HM would react with silanol units on the surface of HSPs, forming siloxane bonds, improving miscibility. According to the literature, it was reported to modify the surface treatment of HSPs with (3-aminopropyl)triethoxysilane for enhancing the compatibility between the silica particles and poly(ε-caprolactone).26 As confirmed by the ATR-FTIR spectrum of hybrid 1–20 (vide infra), condensation reactions occurred between the remaining ethoxy or silanol in EBPSQ-HM and the surface silanol on HSPs similar to the surface treatment by (3-aminopropyl)triethoxysilane.26 Additionally, the hybrid film could be easily prepared by the applicator because the whitish slurry was soluble in 1-methoxy-2-propyl acetate. To investigate the concentration dependence of HSPs, EBPSQ film and hybrid 1–10 were prepared from a 20 wt% THF solution of EBPSQ-HM and a whitish slurry of EBPSQ-HM and 10 wt% of HSPs, respectively, under the same conditions.
Fig. 1 shows the ATR-FTIR spectra of HSPs, EBPSQ film, and hybrid 1–20. For HSPs, the absorption peaks attributed to the Si–O–Si bond were observed at 1030 cm−1. In the ATR-IR of EBPSQ film, the distinct peaks attributed to the silanol group and the Si–O–Si bond were observed at 3300 cm−1 and 1100–900 cm−1. This indicates that the curing reaction proceeded by heating EBPSQ-HM at 80 and 120 °C for 1 h at each temperature, then at 200 °C for 20 min. Indeed, the viscous liquid of EBPSQ-HM changed to gel film with sufficient hardness. The peaks at 2980 and 2880 cm−1 in the spectrum of EBPSQ-HM were due to CH stretching of the ethane and unreacted ethoxy groups. The shoulder peak assigned to the SiCH2CH2Si bond was observed at 1160 cm−1, overlapping with the peak of the Si–O–Si bond. However, hybrid 1–20 prepared from EBPSQ-HM and 20 wt% of HSPs also showed similar absorption peaks. As shown in Fig. 1, the absorption peaks attributed to HSPs were simple, and its peaks were almost overlapped with the Si–O–Si bond in EBPSQ film. However, the silanol peak of EBPSQ film was interestingly disappeared in the hybrid 1–20. This seems to indicate that EBPSQ-HM reacted with the silanol on the surface of HSPs, as mentioned above.
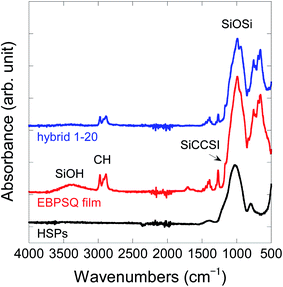 | ||
| Fig. 1 Attenuated total reflectance-Fourier transform infrared spectra of HSPs, EBPSQ film, and hybrid 1–20. | ||
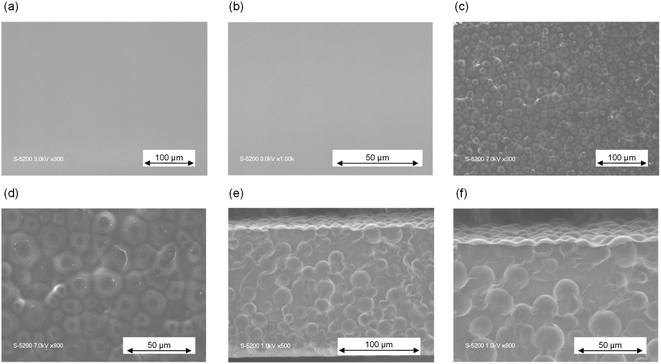
To confirm the morphology of HSPs in films, hybrid 1–20 was prepared on Kapton film by the applicator from the slurry of EBPSQ-HM and 20 wt% of HSPs in 1-methoxy-2-propyl acetate. Fig. 2 shows FE-SEM images with different magnifications of the EBPSQ film and hybrid 1–20. As shown in Fig. 2(a) and (b), no cracks and dimples were observed in the FE-SEM image of EBPSQ film surface. The obtained EBPSQ film was entirely uniform, and EBPSQ-HM exhibited good film formability. In the FE-SEM image of the surface of hybrid 1–20, cracks and dimples were not observed, and it revealed that HSPs with an average size of 18–65 μm were well dispersed in the EBPSQ film (Fig. 2(c) and (d)). Similarly, HSPs were well dispersed in the perpendicular direction of the hybrid 1–20, as shown in Fig. 2(e) and (f). Interestingly, pinholes were not observed even in the cross-section of hybrid 1–20. This indicated that EBPSQ worked as a binder to react with silanol on the surface of HSPs. Indeed, the obtained hybrid 1–20 with a thickness of about 170 μm was self-standing, and it has a certain level of stiffness.
The thermal insulation property of hybrid films
The white slurry of EBPSQ-HM and 20 wt% HSPs was poured onto a duralumin plate and heated at 80 °C and 120 °C for 1 h, then at 200 °C for 20 min to shape its solid film to investigate the effect of HSPs in EBPSQ film on the thermal insulation property. The film's thermal insulation was tested as stated in the Experimental section (see above), and the thermal conductivity/heat flux (k/q) was calculated using eqn (1). Other films were tested in the same way, and the measured k/q, as well as film thickness and surface temperature, are summarized in Table 1.| Entry | Polymer | HSPs (wt%) | Film | Thickness (μm) | Surface temperature (°C) | k/q (m K−1) |
|---|---|---|---|---|---|---|
| 1 | EBPSQ-HM | 0 | EBPSQ | 479 | 93.6 | 109 × 10−6 |
| 2 | EBPSQ-HM | 10 | Hybrid 1–10 | 465 | 90.5 | 62 × 10−6 |
| 3 | EBPSQ-HM | 20 | Hybrid 1–20 | 594 | 90.2 | 76 × 10−6 |
| 4 | EBPSQ-LM | 20 | Hybrid 2–20 | 468 | 93.5 | 107 × 10−6 |
| 5 | PMSQ | 0 | PMSQ | 576 | 94.4 | 160 × 10−6 |
| 6 | PMSQ | 20 | Hybrid 3–20 | 487 | 92.2 | 84 × 10−6 |
The surface temperatures (t2) of the EBPSQ film, hybrid 1–10, and hybrid 1–20 on the duralumin plate reached 90.2 °C–93.6 °C after 10 min when heated at 100 °C from the bottom of the duralumin plate (Table 1). Interestingly, the surface temperatures decreased with an increase in the number of HSPs in EBPSQ film (entry 1–3). This indicates that the heat is unlikely transferred among the EBPSQ films containing HSPs, compared with EBPSQ film. Even if the film thickness was considered, the k/q also decreased from 109 × 10−6 to 76 × 10−6 m K−1; namely, the introduction of HSPs increased thermal insulation properties. Other groups observed similar behavior. For example, Liang et al. investigated the effect of hollow glass bead in polypropylene composites on thermal conductivity, and the thermal conductivity decreased linearly with an increase in the volume fraction of hollow glass bead.27 In urethane acrylate resin, the thermal conductivity decreased with the addition of hollow glass microspheres, as reported by Villoria's group.28 On the other hand, Wu's group also investigated the effect of hollow glass bead in the silicone rubber foam.29 Interestingly, the thermal conductivity increased with the content and the size of a hollow silica glass bead. In their difference in the effect of hollow glass beads, they have suggested the influence of morphology of the composite.
The heat transfer mechanism is complicated in porous materials, and heat transport is mainly governed by solid conduction, convection, and gaseous conduction.10 When EBPSQ film was heated at 100 °C, the heat was transferred among the siloxane network, namely, the transportation of heat occurred by solid conduction. In the case of hybrid films, heat transfer occurred through solid conduction and convection, and gaseous conduction. The hollow cavity provided by HSPs was effective in increasing the thermal insulation property, as observed by another group.27,28 It was concluded that HSPs apparently improved the thermal insulation property by a similar conductivity mechanism.
However, EBPSQ film containing 20 wt% of HSPs (hybrid 2–20) prepared from EBPSQ-LM exhibited a high surface temperature of 93.5 °C (entry 4) higher than the surface temperature of hybrid 1–20. Although the increase in surface temperature was not clear, there are two possibilities: (1) this might be due to the influence of the molecular weight of EBPSQ-LM, namely, EBPSQ-LM with the low molecular weight filled the pore, and the free volume formed by polymer was small in the film. (2) The remaining ethoxy group in EBPSQ-LM (31%) was higher than that of EBPSQ-HM (25%). The ethoxy group in the film might enhance the thermal conductivity. The mechanism for thermal conduction in PSQ films needs to be further studied, and it is underway. Additionally, to investigate the influence of ethylene-bridged structure on the thermal conductivity, PMSQ film and PMSQ film containing 20 wt% of HSPs (hybrid 3–20) was prepared under the same conditions. Interestingly, the surface temperature of the PMSQ film was 94.4 °C (entry 5), and it was higher than that of the EBPSQ film. This indicated that EBPSQ film possesses high thermal insulating properties compared with PMSQ film. This might be due to the ethylene-bridged structure, as mentioned in the introduction; namely, the bridged spacer between silicon atoms might make the void spaces. Furthermore, the effect of HSPs on the thermal insulating property was also observed in PMSQ film. The surface temperature and k/q of hybrid 3–20 decreased by the effect of HSPs, as shown in Table 1 (entries 5 and 6).
Thermal stability of hybrid films
To investigate the effect of HSPs, the thermal stabilities of EBPSQ film, hybrid 1–10, and hybrid 1–20 with different number of HSPs were measured by thermogravimetric- and derivative thermogravimetric analysis in air (Fig. S2†) and the data are shown in Table 2, along with that of hybrid 2–20 and hybrid 3–20. EBPSQ film prepared from EBPSQ-HM exhibited 5% weight loss temperature (T5d) of 258 °C and 10% weight loss temperature (T10d) of 275 °C (entry 1). On the other hand, hybrid 1–20 exhibited high T5d of 299 °C and T10d of 315 °C (entry 3). Interestingly, T5d and T10d of hybrid films increased with increasing the amounts of HSPs. Indeed, the peak apparently shifted to a higher temperature region in the derivative thermogravimetric analysis of the EBPSQ film, hybrid 1–10, and hybrid 1–20. Similarly, hybrid 2–20 prepared from EBPSQ-LM/20 wt% of HSPs also showed high thermal stability, but their T5d and T10d were slightly lower than hybrid 1–20 prepared from EBPSQ-HM/20 wt% of HSPs (entry 4). This might be due to the remaining ethoxy group, namely, the elimination of ethoxy group decreased polymer weight. To understand further the effect of HSPs, exothermic peaks for EBPSQ film, hybrid 1–10, and hybrid 1–20 is shown in Fig. 3, and the exothermic peak temperatures are shown in Table 2. Exothermic peaks were shifted to higher temperatures as the number of HSPs increased, as shown in Fig. 3. Surprisingly, the exothermic peak temperature of hybrid 1–20 prepared from EBPSQ-HM/20 wt% of HSPs increased by 27 °C, compared with EBPSQ film prepared from EBPSQ-HM. However, the thermal stability of hybrid 3–20 prepared from PMSQ and 20 wt% of HSPs was lower than that of hybrid 1–20, as shown in Table 2 (entry 5).| Entry | Polymer | HSPs (wt%) | Film | T5d (°C) | T10d (°C) | Exothermic peak (°C) |
|---|---|---|---|---|---|---|
| a Measured at a heating rate of 10 °C min−1 under air flow of 100 mL min−1. | ||||||
| 1 | EBPSQ-HM | 0 | EBPSQ | 258 | 275 | 290 |
| 2 | EBPSQ-HM | 10 | Hybrid 1–10 | 275 | 290 | 298 |
| 3 | EBPSQ-HM | 20 | Hybrid 1–20 | 299 | 315 | 317 |
| 4 | EBPSQ-LM | 20 | Hybrid 2–20 | 287 | 303 | 305 |
| 5 | PMSQ | 20 | Hybrid 3–20 | 275 | 527 | 302 |
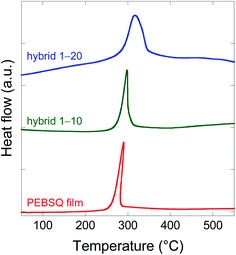 | ||
| Fig. 3 Hollow silica particles content dependence of exothermic peak for EBPSQ film, hybrid 1–10, and hybrid 1–20. | ||
To improve the mechanical and thermal stability, additives such as silica nanoparticles and polyhedral oligomeric silsesquioxane were added to a polymer such as poly(vinyl alcohol),30 methyl acrylic polymer,31 phenolic resin,32 and poly(butylene adipate-co-terephthalate).33 Interestingly, thermal properties such as decomposition temperature and glass transition temperature increased by adding the silica component. In the EBPSQ film, T5d and T10d increased with an increase in the number of HSPs, and exothermic peaks were shifted to a higher temperature region. HSPs could enhance the thermal properties. Similar behavior was observed in the polypropylene/intumescent flame retardants composite, and iM16K improved the thermal stability.34
For example, the interaction such as hydrogen bonding between polymer and silanol, the formation of confined structure, and the heating barrier by SiO2 component might enhance the thermal stability.30,31,33 In the case of hybrid films, the thermal stability might be improved by forming covalent bonds as strong interaction between EBPSQ-HM and HSPs. Furthermore, as discussed in the section on thermal insulation property, HSPs exhibited the effect of increasing the thermal insulation property, namely, the thermal stability of hybrid films might be enhanced by delaying the heat transfer. In the PSQ film, the improvement of thermal stability by the addition of HSPs was of interest.
Conclusions
EBPSQ and PMSQ were prepared by the sol–gel reaction of bis(triethoxysilyl)ethane and triethoxymethylsilane. The whitish slurry was easily prepared by mixing EBPSQ and HSPs at 80 °C, and it formed a hybrid film by heating at 80 and 120 °C for 1 h at each temperature, then at 200 °C for 20 min.The surface temperatures of hybrid 1–10 and hybrid 1–20 (90.2–90.5 °C) were lower than that of EBPSQ film (93.6 °C) when the films on the duralumin plates were heated at 100 °C from the duralumin plate. The k/q obtained from the temperature difference between the surface temperature and bottom temperature of films and the film thickness also decreased from 109 × 10−6 to 62 × 10−6 m K−1 by adding the HSPs. The surface temperatures of PMSQ film and PMSQ film containing 20 wt% of HSPs were 94.4 °C and 92.2 °C, respectively. The effect of HSPs on the thermal insulation property was observed in the PMSQ film. Additionally, the thermal insulation property of EBPSQ film was higher than that of PMSQ film due to the ethylene-bridged structure.
EBPSQ film exhibited T5d and T10d of 258 °C and 275 °C, respectively. However, hybrid 1–20 exhibited high T5d and T10d of 299 °C and 315 °C. Interestingly, T5d and T10d of hybrid films increased with increasing number of HSPs. And the exothermic temperatures were shifted to a higher temperature region with increasing the number of HSPs.
Overall, HSPs could enhance the thermal insulation property and thermal stability.
Author contributions
Satoru Tsukada designed the research and drafted the paper. Yuki Nakanishi contributed to all of the experimental work. Takashi Hamada analyzed the experimental results, wrote the paper, and edited the paper with contributions from all the authors. Kenta Okada and Susumu Mineoi contributed to the analysis of the hybrid film. Joji Ohshita directed this study and edited the paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by Japan Science and Technology Agency (JST) Adaptable and Seamless Technology transfer Program through Target-driven R&D (A-STEP) (grant number JPMJTM20G3) and JSPS KAKENHI (Grant Number JP16K17951 and JP19K15637). We thank Dr M. Maeda, the Natural Science Center for Basic Research and Development (N-BARD), Hiroshima University, for measuring FE-SEM. The authors would like to thank Enago for the English language review.References
- B. Abu-Jdayil, A.-H. Mourad, W. Hittini, M. Hassan and S. Hameedi, Traditional, state-of-the-art and renewable thermal building insulation materials: An overview, Constr. Build. Mater., 2019, 214, 709–735 CrossRef.
- S. Wi, J. H. Park, Y. U. Kim, S. Yang and S. Kim, Thermal, hygric, and environmental performance evaluation of thermal insulation materials for their sustainable utilization in buildings, Environ. Pollut., 2021, 272, 116033 CrossRef CAS PubMed.
- K. W. Suh, C. P. Park, M. J. Maurer, M. H. Tusim, R. D. Genova, R. Broos and D. P. Sophiea, Lightweight Cellular Plastics, Adv. Mater., 2000, 12, 1779–1789 CrossRef CAS.
- B. P. Jelle, Traditional, state-of-the-art and future thermal building insulation materials and solutions – Properties, requirements and possibilities, Energy Build, 2011, 43, 2549–2563 CrossRef.
- D. Illera, J. Mesa, H. Gomez and H. Maury, Cellulose Aerogels for Thermal Insulation in Buildings: Trends and Challenges, Coatings, 2018, 8, 345 CrossRef.
- A. Du, B. Zhou, Z. Zhang and J. Shen, A Special Material or a New State of Matter: A Review and Reconsideration of the Aerogel, Materials, 2013, 6, 941–968 CrossRef CAS PubMed.
- J. Wang, D. Petit and S. Ren, Transparent thermal insulation silica aerogels, Nanoscale Adv., 2020, 2, 5504–5515 RSC.
- Z. Liu, Y. Ran, J. Xi and J. Wang, Polymeric hybrid aerogels and their biomedical applications, Soft Matter, 2020, 16, 9160–9175 RSC.
- J. Sharma and G. Polizos, Hollow Silica Particles: Recent Progress and Future Perspectives, Nanomaterials, 2020, 10, 1599 CrossRef CAS PubMed.
- P. Ruckdeschel, A. Philipp and M. Retsch, Understanding Thermal Insulation in Porous, Particulate Materials, Adv. Funct. Mater., 2017, 27, 1702256 CrossRef.
- Z. Jia, Z. Wang, D. Hwang and L. Wang, Prediction of the effective thermal conductivity of hollow sphere foams, ACS Appl. Energy Mater., 2018, 1, 1146–1157 CrossRef CAS.
- L. Ernawati, T. Ogi, R. Balgis, K. Okuyama, M. Stucki, S. C. Hess and W. J. Stark, Hollow Silica as an Optically Transparent and Thermally Insulating Polymer Additive, Langmuir, 2016, 32, 338–345 CrossRef CAS PubMed.
- Y. Liao, X. Wu, Z. Wang, R. Yue, G. Liu and Y. Chen, Composite thin film of silica hollow spheres and waterborne polyurethane: Excellent thermal insulation and light transmission performances, Mater. Chem. Phys., 2012, 133, 642–648 CrossRef CAS.
- S. E. Amos and B. Yalcin, Hollow glass microspheres for plastics, elastomers, and adhesives compounds, Elsevier, Oxford, UK, 2015 Search PubMed.
- N. Ahmed, H. Fan, P. Dubois, X. Zhang, S. Fahad, T. Aziz and J. Wan, Nano-engineering and micromolecular science of polysilsesquioxane materials and their emerging applications, J. Mater. Chem. A, 2019, 7, 21577–21604 RSC.
- J. G. Croissant, X. Cattoën, J.-O. Durand, M. W. C. Man and N. M. Khashab, Organosilica hybrid nanomaterials with a high organic content: syntheses and applications of silsesquioxanes, Nanoscale, 2016, 8, 19945–19972 RSC.
- K. J. Shea and D. A. Loy, Bridged Polysilsesquioxanes. Molecular-Engineered Hybrid Organic–Inorganic Materials, Chem. Mater., 2001, 13, 3306–3319 CrossRef CAS.
- H.-C. Liu, W.-C. Su and Y.-L. Liu, Self-assembled benzoxazine-bridged polysilsesquioxanes exhibiting ultralow-dielectric constants and yellow-light photoluminescent emission, J. Mater. Chem., 2011, 21, 7182–7187 RSC.
- K. Yamamoto and J. Ohshita, Bridged polysilsesquioxane membranes for water desalination, Polym. J., 2019, 51, 1103–1116 CrossRef CAS.
- K. Yamamoto, S. Koge, K. Sasahara, T. Mizumo, Y. Kaneko, M. Kanezashi, T. Tsuru and J. Ohshita, Preparation of Bridged Polysilsesquioxane Membranes from Bis[3-(triethoxysilyl)propyl]amine for Water Desalination, Bull. Chem. Soc. Jpn., 2017, 90, 1035–1040 CrossRef CAS.
- T. Hamada, Y. Nakanishi, K. Okada and J. Ohshita, ACS Omega, 2021, 6, 8430–8437 CrossRef CAS PubMed.
- K. Yamamoto, J. Ohshita, T. Mizumo and T. Tsuru, Polymerization behavior and gel properties of ethane, ethylene and acetylene-bridged polysilsesquioxanes, J. Sol-Gel Sci. Technol., 2014, 71, 24–30 CrossRef CAS.
- T. Ishimoto, S. Tsukada, S. Wakitani, K. Sato, D. Saito, Y. Nakanishi, S. Takase, T. Hamada, J. Ohshita and H. Kai, Model-based research toward design of innovative materials: molecular weight prediction of bridged polysilsesquioxanes, RSC Adv., 2020, 10, 28595–28602 RSC.
- E. Espinel-Blanco, J. H. Arévalo-Ruedas and E. Florez-Solano, Development of an automated system for the determination of thermal conductivity in granular materials, J. Phys.: Conf. Ser., 2020, 1587, 012028 CrossRef.
- N. Takamura, T. Gunji, H. Hatano and Y. Abe, Preparation and Properties of Polysilsesquioxanes: Polysilsesquioxanes and Flexible Thin Films by Acid-Catalyzed Controlled Hydrolytic Polycondensation of Methyl- and Vinyltrimethoxysilane, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 1017–1026 CrossRef CAS.
- A. Vignali, S. Iannace, G. Falcone, R. Utzeri, P. Stagnaro and F. Bertini, Lightweight Poly(ε-caprolactone) Composites with Surface Modified Hollow Glass Microspheres for Use in Rotational Molding: Thermal, Rheological and Mechanical Properties, Polymers, 2019, 11, 624 CrossRef PubMed.
- J. Z. Liang and F. H. Li, Measurement of thermal conductivity of hollow glass-bead-filled polypropylene composites, Polym. Test., 2006, 25, 527–531 CrossRef CAS.
- L. C. Herrera-Ramírez, M. Cano and R. G. de Villoria, Low thermal and high electrical conductivity in hollow glass microspheres covered with carbon nanofiber-polymer composites, Compos. Sci. Technol., 2017, 151, 211–218 CrossRef.
- J. Gao, J. Wang, H. Xu and C. Wu, Preparation and properties of hollow glass bead filled silicone rubber foams with low thermal conductivity, Mater. Des., 2013, 46, 491–496 CrossRef CAS.
- Z. Peng, L. X. Kong and S.-D. Li, Thermal Properties and Morphology of a Poly(vinyl alcohol)/Silica Nanocomposite Prepared with a Self-Assembled Monolayer Technique, J. Appl. Polym. Sci., 2005, 96, 1436–1442 CrossRef CAS.
- Z. H. Huang and K. Y. Qiu, The effects of interactions on the properties of acrylic polymers/silica hybrid materials prepared by the in situ sol-gel process, Polymer, 1997, 38, 521–526 CrossRef CAS.
- Y. Zhang, S. Lee, M. Yoonessi, K. Liang and C. U. Pittman, Phenolic resin-trisilanolphenyl polyhedral oligomeric silsesquioxane (POSS) hybrid nanocomposites: Structure and properties, Polymer, 2006, 47, 2984–2996 CrossRef CAS.
- R. Venkatesan and N. Rajeswari, Nanosilica-reinforced poly(butylene adipate-co-terephthalate) nanocomposites: preparation, characterization and properties, Polym. Bull., 2019, 76, 4785–4801 CrossRef CAS.
- B.-H. Kang, X.-Y. Yang and X. Lu, Effect of hollow glass microsphere on the flame retardancy and combustion behavior of intumescent flame retardant polypropylene composites, Polym. Bull., 2020, 77, 4307–4324 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1: photograph of handmade thermal insulation property tester: surface thermometer (left) and duralumin plate (right); Fig. S2: thermogravimetric analysis curves for EBPSQ film, hybrid 1–10, and hybrid 1–20 measured at a heating rate of 10 °C min−1 under air. See DOI: 10.1039/d1ra04301c |
| This journal is © The Royal Society of Chemistry 2021 |