Open Access Article
Open Access ArticleNew strategy of light quality regulation with leaf-spraying fluorescent coatings for enhancing photosynthesis efficiency†
Yankai Liua,
Shuai Zhang *b,
Fei Yanga,
Guanzhu Wanga,
Xiuli Jinga,
Xiaofei Wanga and
Chunxiang You*a
*b,
Fei Yanga,
Guanzhu Wanga,
Xiuli Jinga,
Xiaofei Wanga and
Chunxiang You*a
aNational Key Laboratory of Crop Biology, Shandong Collaborative Innovation Center of Fruit & Vegetable Quality and Efficient Production, College of Horticulture Science and Engineering, Shandong Agricultural University, Tai-An, Shandong 271018, China. E-mail: youchunxiang@126.com
bCollege of Chemistry and Material Science, Shandong Agricultural University, Tai-An, Shandong 271018, China. E-mail: hyzs@sdau.edu.cn
First published on 4th August 2021
Abstract
Fluorescent coatings are a kind of emerging light quality regulation material that can improve plant light utilization efficiency through easy manipulation at a low price. Compared with the scheme of fluorescent nanomaterials alone or those physically dispersed in polymeric materials for photosynthesis enhancement, fluorescent polymeric coatings (FPCs) originating from the covalent copolymerization of nanomaterial monomers can function stably and continuously, circumventing the high-cost manipulation of continuous leaf-spraying or hydroponics of the previous scheme in practical applications. Herein, we developed a kind of FPCs consisting of UV-to-blue light-converting nitrogen-doped carbon dots (N-CDs) as the fluorescent monomer to induce the copolymerization of N-CDs and tannic acid (TA). In the FPCs, N-CDs and TA are covalently cross-linked together. The fluorescent ability of N-CDs and the strong adhesion of TA are integrated organically to the whole to endow FPCs with excellent properties of prolonged fluorescence capacity, rain-erosion resistance and stability. After spraying FPCs on tomato leaves grown under the full spectrum, both the chlorophyll content of the leaves and effective photochemical efficiency were increased significantly, and the growth rate was promoted with 38.3% and 43.2% enhancement in the dry and fresh weight. We also analyzed the human cytotoxicity of the coating and the toxicological experiments showed that the coating did not affect the proliferation of human cells.
Introduction
Photosynthesis converts light energy into chemical energy to synthesize organic substances required for plant growth and sustaining life on earth, its activity significantly influences crop yields. During this unique process, light-harvesting complexes on the thylakoid membranes of plant chloroplasts funnel energy from sunlight into reaction centers.1,2 However, due to the limited light absorption range of photosynthetic pigments,3 only partial photons (400–500 nm, 600–700 nm) are effectively used as fuel during photosynthesis, and half of solar energy is unused. Improving the light energy utilization rate for efficient enhancement in photosynthesis and thereby grain yield can open up new opportunities to solve food security problems.4 In recent decades, light-converting plastic films and artificial supplementary light sources, have been developed and indeed stimulated crop growth and productivity, but these methods are often accompanied by environmental pollution, high-costs or other shortcomings.5–10With rapid development of nanotechnology, green nanotechnology approaches in agriculture have been developed and excellent progresses have been achieved in laboratory-scale tests.11–14 Nano-enabled approaches for augmenting photosynthesis have been a focus of recent interdisciplinary biochemical research.12 Several nanomaterial-promoted photosynthesis systems have been devised such as TiO2 nanoparticle,15 CdTe quantum dots,16 SWCNTs11 and carbon dots (CDs).12 Among these nanomaterials, CDs have being become a research hotspot and widely investigated in agricultural application because of their promising properties of photoluminescence, nontoxicity, durability and biocompatibility. CDs alone or those physically dispersed in polymers were employed by leaf-spraying and hydroponics manipulation, effectively increased photosynthesis performance in various crop species (Nicotiana tabacum, maize, mung bean and lettuce) mainly through converting useless light into blue/red light.12,16–22 On the other hand, fluorescence quenching usually took place when these CDs solution were dried on leave surface. And these CDs would be easily washed away in the practical conditions. To gain prolonged effect of CDs on photosynthesis enhancement, continuous leaf-spraying or hydroponics condition throughout plant growth process are required, resulting in high labor costs or large-scale construction of facilities, at present which is less practical to woody plants and field conditions.
Besides these strategies, in vitro spraying adhesive fluorescent coatings on the leaf surface with continuously fluorescence emission and good rain-erosion resistance provides a new tool for efficient photosynthesis enhancement, because it circumvents the requirement of continuous leaf-spraying and hydroponics manipulation. To gain the targeted fluorescent coating, covalently cross-linked polymer coatings originated from CDs and other monomers are required to endow them with prolonged fluorescent capacity, rain-erosion resistance and stability. Herein, we developed fluorescent polymer coating (FPCs) consisting of UV-excited, blue light-emitting nitrogen-doped CDs as the fluorescent body to induce the covalent copolymerization of CDs and tannic acid (TA). It was confirmed that the FPCs possessed excellent properties of fluorescence, rain-erosion resistance, stability and non-toxicity. After spraying FPCs on tomato leaves, a significant enhancement in plant photosynthesis and growth rate was obtained, indicating the promising potential of FPCs under field conditions.
Experimental
Reagents
Tannic acid (TA), citric acid, urea, ethanediamine (EDA), diethylenetriamine (DETA), tetrethylenepentamine (TEPA), branched polyethylenimine (average Mw ∼600, PEI-600), and branched polyethylenimine (average Mw ∼1800, PEI-1800) were purchased from Macklin Biochemical Co., Ltd (Shanghai, China) and used directly without purification.Synthesis of N-CDs
Six N-CDs, including CD-urea, CD-EDA, CD-DETA, CD-TEPA, 600-CD and 1800-CD were prepared by previously reported hydrothermal method23 and pyrolytic method.24 In a typical procedure, citric acid (0.5 g) and EDA (0.25 g) in ddH2O (10 mL) were transferred to a Teflon-equipped stainless-steel autoclave and heated at 180 °C for 4 h. The resultant solution was dried through rotary evaporation to obtain the yellow viscous oil. Then the oil was rinsed with acetone (20 mL × 3) to remove the unreacted organic molecules, followed by the freeze-drying process at reduced pressure. The obtained brown powder was denoted as CD-EDA. CD-urea, CD-DETA and CD-TEPA were also prepared by using the same mass of DETA, TETA and TEPA, instead of EDA, respectively. 600-CD and 1800-CD were prepared by pyrolytic method with citric acid (1.0 g) and PEI-600/PEI-1800 (0.5 g) as substrates.24Synthesis of FPCs
5 mL 1800-CD solution (40 mg mL−1) and 5 mL TA solution (5 mg mL−1) were mixed completely in the 10 mL sprayer, in which TA/1800-CD hybrid aggregates generated immediately. Subsequently, the mixture was evenly sprayed on the substrates (quartz glass or tomato leaves) promptly. And then, the insoluble FPC was generated and adhered to the substrate surface in 10 min. Finally, the generated FPC was cleaned with water for several times to remove the unreacted monomer.Characterization
X-ray photoelectron spectroscopy (XPS), Fourier-transform infrared spectra (FT-IR) (400–4000 cm−1), UV-vis absorption, and photoluminescence spectra were measured on a ThermoFisher EscaLab 250Xi (a monochromatic Al Kα X-ray source with an excitation of 1486.6 eV), Nicolet iS5 spectrophotometer, Shimadzu UV3600, and fluorescence spectrofluorometer (LUMINA, Thermo Scientific), respectively. Solid-state 13C nuclear magnetic resonance (13C SSNMR) spectroscopy was performed on an Agilent 600 MHz DD2 spectrometer at a resonant frequency of 150.81 MHz. Transmission electron microscopy (TEM) and atomic force microscopy (AFM) images were recorded on a JEOL2109F and Bruker Dimension Icon, respectively. TG and DSC analyses were performed on an SDT Q600 V20.9 Build 20 (room temperature to 800 °C, N2, 10 °C min−1). Dynamic light scattering measurements were performed using a Zetasizer Nano ZS90 (Malvern). Confocal images were obtained by laser scanning microscopy (LSM 880, Zeiss).Cellular toxicity
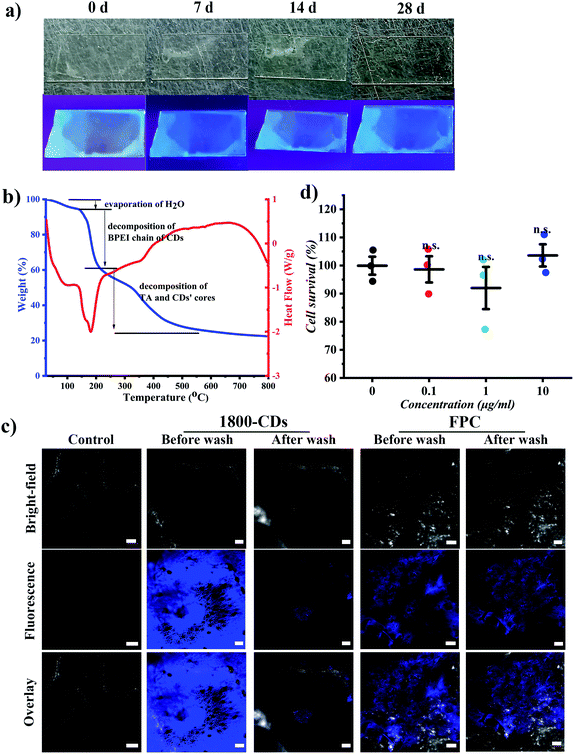
A human neuroblastoma cell line (SK cell) was used to evaluate the toxicity of FPC. SK cells were propagated in RPMI medium (GE healthcare) and maintained in a 37 °C humidified atmosphere with 5% CO2. Cell viability was measured using Cell-Counting Kit-8 (CCK8) assays. SK cells were seeded in 96-well plates and incubated for 24 h after adding different concentrations of FPC solutions (0, 0.1, 1, 10 μg mL−1). The generated FPCs have poor solubility (less than or equal to 10 μg mL−1). Therefore, the highest concentration of FPC used in this experiment was 10 μg mL−1. After 24 h, the cell solution was removed from each well and incubated with 10% CCK8 solution at 37 °C for 2 h, and then the absorbance at 450 nm was measured.Plant culture
Tomato (cv. Ailsa Craig; AC) seeds were seeded on wet filter paper and placed in 28 °C incubator to promote germination. Two days later, the germinated seeds were moved into a flowerpot filled with nutrient soil. All plants were stored at 25 °C, 60–80% relative humidity (16 h light/8 h dark). The lamp used is 32 W growth lamp (without ultraviolet) plus 20 W UV-A lamp. By adjusting the distance between lamps and seedlings, the intensity of light irradiation was set as 4 mW cm−2, which mimicked natural sunlight. 0.2 mL mixed solution (1800-CD: 40 mg mL−1, 0.1 mL; TA: 5 mg mL−1, 0.1 mL) per treatment was sprayed on tomato leaves (10 days old) every four days (due to the sprouted of new leaves) to generate the targeted FPC. 0.2 mL deionized water per treatment was used in the control treatment.Measurement of chlorophyll content and chlorophyll fluorescence
After incubation in the dark for half an hour, the chlorophyll fluorescence parameters were measured at room temperature, the method described by Zhang et al.25 was used to measure chlorophyll fluorescence on an FMS-2 pulse-modulated chlorophyll fluorometer (Hansatech, Norfolk, UK) and chlorophyll fluorescence analyzer (Dual PAM 100, WALZ).26,27The content of chlorophyll in leaves was determined by ethanol method. In short, 0.2 g fresh leaf samples were thoroughly ground with 95% ethanol as solvent and soaked in the dark for 2 hours. Absorbances were measured on a Shimadzu UV-VIS spectrophotometer UV-2600i (Shimadzu, Japan), the supernatant was collected at 645 nm and 663 nm to determine the content of chlorophyll a and chlorophyll b, respectively. The chlorophyll content of leaves was calculated according to the formula: chlorophyll a content = (12.72 × A663 − 2.59 × A645) × 10/1000/W; chlorophyll b content = (22.88 × A645 − 4.67 × A663) × 10/1000/W.28 W: fresh weight of leaves; A663 represents the absorbance value at 663 nm; A645 represents the absorbance value at 645 nm.
Statistics
For measurement of photosynthetic activity, chlorophyll content and cell survival rate, one-way analysis of variance (ANOVA) was used to evaluate the statistical significance of p < 0.05 level. In all cases, post event analysis is performed to determine multiple comparisons using the Tukey method.Results and discussion
The synthesis of blue fluorescent polymeric coating
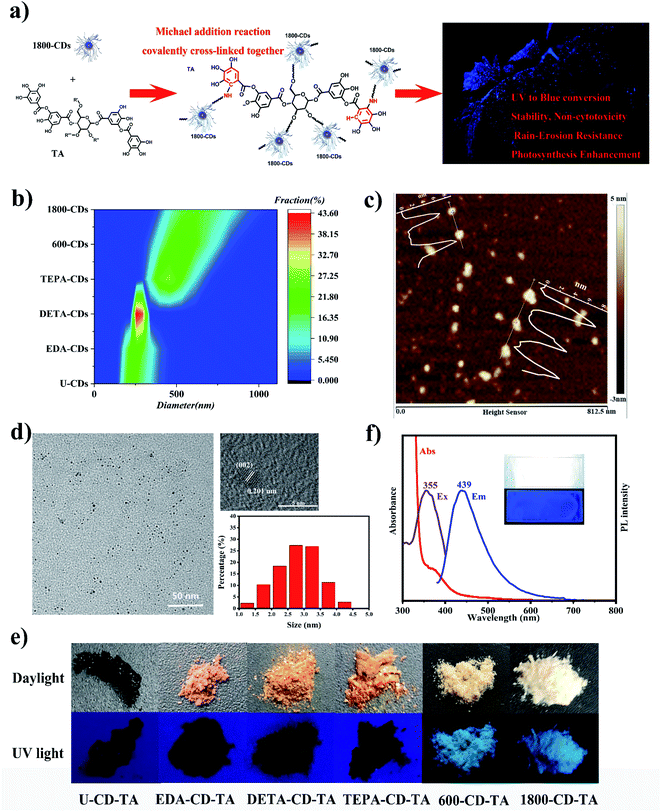
The fluorescence property of carbon dots (CDs) with high quantum yield and low cytotoxicity makes them a preferred material for improving light quality in the field. However, the fluorescence disappeared when the sprayed CDs solution was dried on leave surface (Fig. S1†). And these CDs were easily washed away by rain or dew. To obtain continuous fluorescence emission, it is necessary to keep delivering CDs by continuous leaf-spraying or hydroponics throughout plant growth in conjunction with the half-life of the materials in vivo, which is unfavorable to woody plants and field conditions. Several methods were developed to obtain in vitro effective fluorescent films or coatings,6,29,30 which continuously emitted fluorescence on the surface of substrate, such as glass and leaves. The flexibility of FPC lies in the fact that the regulation of light quality can be accomplished by spray application of fluorescent materials in vitro, without the need of hydroponics or continuous spraying manipulation. However, some serious problems usually accompanied, such as heavy metal contamination, poor adhesion and non-tolerance to water erosion. These fluorescent coatings are usually produced by physically dispersing fluorescent nanomaterials into polymers with poor adhesion, and the fluorescent nanoparticles may dissociate down from the polymers. In our work, UV-excitation, blue light-emission nitrogen-doped carbon dots (N-CDs) are employed to induce the copolymerization of tannic acid (TA) and N-CDs through Michael addition reaction31 to covalently cross-linked fluorescent polymeric coatings (FPCs) with excellent adhesion and water erosion-resistance on leave surface (Fig. 1a). The obtained FPCs provide a promising solution to the problem of rain-erosion, CD dissociation etc., to adapt to the field applicable purposes. The UV-excitation and blue-emission CDs were chosen because this combination can effectively broaden the effective light utilization range of plant photosynthesis, and the polymers generated from TA would present good adhesion.31–37 In initial study, it was found that the copolymerization between CDs and TA resulted in the disappearance of CDs fluorescence capacity (Fig. S2†), which probably because that the reaction of chromophores on the surface of CDs with TA led to the fluorescence quenching. This problem of aggregation-induced fluorescence quenching was overcome by Lei's group using long-chain polyvinyl alcohol to form a shell on the surface of CDs.32 Therefore, we synthesized a series of CDs using increased polymerization degree of polyethyleneimine (PEI) as the nitrogen source feedstock by hydrothermal method, including U-CD, EDA-CD, DETA-CD, TEPA-CD, 600-CD and 1800-CD (Experimental section, Fig. S3 and 4†), so that a protective shell composed of long-chain amines could be formed on the surface of CDs to prevent the transfer of chromophore energy. DLS shows that the kinetic radius of N-CDs increases with the polymerization degree of PEI (Fig. 1b). To further reveal the existence of PEI chains on the surface, the morphology of 1800-CD with the maximum hydration radius was characterized. Due to the degradation of PEI chains induced by the electric field and low contrast, transmission electron microscopy (TEM) was used to observe the morphology of the carbon core.37 Atomic force microscopy (AFM) was used to characterize the height of 1800-CDs by using the weak force between the probe and surface shell. The difference between the average height (7.26 ± 0.67 nm) calculated by AFM (Fig. 1c) and the average diameter (2.81 ± 0.66 nm) calculated by TEM (Fig. 1d) indicates the presence of many PEI chains on the shell around the 1800-CD carbon core. The high-resolution XPS spectra contained two N 1s peaks at 400.0 and 401.1 eV, which corresponded to amino N (C–N)32 and graphitic N,37 respectively. As expected, in the aid of the PEI shell, the fluorescence quenching was indeed overcome, the dried coating powders originated from the copolymerization of TA and 1800-CDs emitted strong blue fluorescence under UV irradiation (Fig. 1e). And the solid-state fluorescence measurement of the dried coating powders identified their excitation spectrum (320–400 nm) and emission spectrum (400–500 emission), which were in accordance with the demand in broadening the light utilization range of photosynthesis (Fig. 1f).To verify the synthetic mechanism of FPCs, that is, Michael addition reaction occurred between TA and 1800-CD (Fig. 2a), FPCs were characterized by FT-IR, 13C SSNMR and high-resolution XPS tests. The FT-IR spectra of 1800-CDs, TA, and FPC are illustrated in Fig. 2b. Notably, peaks at 600–850 cm−1 correspond to the out-of-plane C–H bending vibrations in the aromatic rings of TA. After the formation of the FPC, the peak at 760 cm−1 shifted to 616 cm−1. This result provided some evidence that Michael addition probably occurred between 1800-CD and TA, which led to the change in the number of substituents in the aromatic ring of TA. In this case, Michael addition occurred between the –NH2 groups of the PEI chain in 1800-CD and the gallol moiety of TA. In the 13C SSNMR spectra (Fig. 2c), significant changes were also observed in aromatic region from 100–160 ppm between TA and FPC. New FPC peaks appeared near 150 ppm, which corresponded to the C2 atom of the C2–N bond (Fig. 2a) formed via Michael addition between TA and 1800-CD. Slight differences in the range of 100–140 ppm (corresponding to C1, C3–C6) were also observed because of Michael addition on the aromatic ring of TA. Furthermore, the XPS spectra indicated a higher C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O content in FPC than 1800-CD and TA (Fig. S5†), which was in agreement with the reaction mechanism of poly(1800-CD-co-TA).34 These results confirmed that Michael addition reactions between 1800-CDs and TA did occur in agreement with the original design (Fig. 2a). In other words, Michael addition occurred between the amine group of PEI chains in 1800-CD shell and TA to produce poly(1800-CD-co-TA),34 which were co-deposited onto the substrate surface to generate adhesive coatings via van der Waals forces, hydrogen bonding, π–π stacking, and cation–π interactions.31,37 Compared with the strategy of physically dispersing fluorescent nanomaterials in polymers, in these fluorescent poly(1800-CD-co-TA) coatings, 1800-CDs and TA are covalently cross-linked together and integrated to the whole.
O content in FPC than 1800-CD and TA (Fig. S5†), which was in agreement with the reaction mechanism of poly(1800-CD-co-TA).34 These results confirmed that Michael addition reactions between 1800-CDs and TA did occur in agreement with the original design (Fig. 2a). In other words, Michael addition occurred between the amine group of PEI chains in 1800-CD shell and TA to produce poly(1800-CD-co-TA),34 which were co-deposited onto the substrate surface to generate adhesive coatings via van der Waals forces, hydrogen bonding, π–π stacking, and cation–π interactions.31,37 Compared with the strategy of physically dispersing fluorescent nanomaterials in polymers, in these fluorescent poly(1800-CD-co-TA) coatings, 1800-CDs and TA are covalently cross-linked together and integrated to the whole.
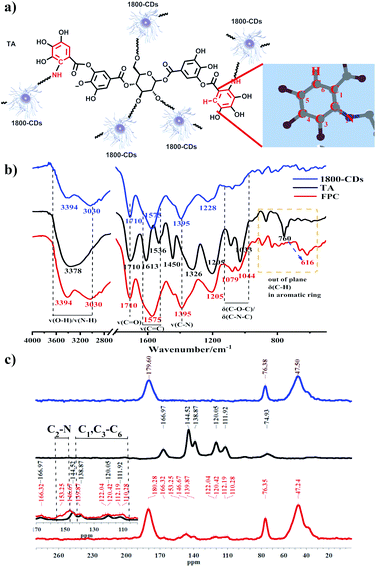 | ||
| Fig. 2 (a) Illustration of the Michael addition mechanism between 1800-CD and TA. (b) FT-IR spectra and (c) 13C solid-state NMR spectra (13C SSNMR) of 1800-CDs, TA, and FPC. | ||
Properties of FPCs
Tomato is not only a model plant, but also an edible horticultural crop, so we chose it as our experimental plant material. After spraying FPCs on tomato seedlings, numerous tiny star-shaped spots adhered on the leave surface and emitted strong blue-fluorescent under UV light (Fig. 3). FPCs continuously convert UV light into blue light (Fig. 3), expanding light absorption spectrum of photosynthetic pigments for efficient photosynthesis enhancement.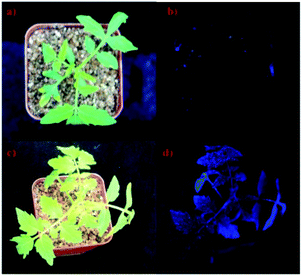 | ||
| Fig. 3 Images of tomato seedlings of the control (a and b) and the treated groups (c and d) under sunlight (a and c) and UV light (b and d). | ||
To meet the demand of practical application, such as field conditions, properties of stability, rain-erosion resistance and non-cytotoxicity are required. As shown in Fig. 4a, FPCs could maintain their fluorescence without fluorescence loss for at least 28 days under sunlight, proving their excellent light-stability. And the FPCs also showed good thermal stability in the range of room temperature to 150 °C (Fig. 4b), in which only water evaporation occurred. Subsequently, water flow as simulated rainfall was employed to test the adhesion and washing-out resistance of FPC on the smooth glass surface, and the confocal images excited at 408 nm before and after being washed for 0.5 min were displayed in Fig. S6.† Notably, after the washing of the simulated rainfall, FPC maintained a tight adhesion on the surface of glass and no loss of fluorescent FPC was observed after washing. However, for 1800-CD alone, it was completely washed away (Fig. S6†). Therefore, FPC possessed excellent water erosion tolerance, ensuring their long-term fluorescence capacity in practical applications. Most importantly, FPCs demonstrated their capacities of washing-out resistance and good adhesion on leaf surface of tomato (cv. Ailsa Craig), proving their robustness in field conditions (Fig. 4c). Due to endogenous greenish-blue fluorescence, the testing parameters of confocal laser scanning microscopy (CLSM) were tuned to reduce autofluorescence signals, thereby no fluorescence was observed in the water-treated leaves. In the treated groups were washed with ultra-pure water twice before confocal analysis. FPC showed no differences after washing and no fluorescence loss was found at the same position (Fig. 4c), suggesting FPCs had good washing-out resistance. To further simulate the situation of rain erosion more realistically, we rinsed the FPCs on the leaf surface continuously with tap water as simulated rainfall at a rate of 0.143 mL s−1. Instead of spraying directly on the surface, we used a pipette to mix FPC at certain distances apart on the leaf surface to facilitate observation. As expected, as shown in Video S1,† after being washed approximate 7 minutes, no loss of FPCs was observed, suggesting FPCs have excellent water erosion-resistance on leaf surface and can be used potentially in field conditions with good robustness. In the practical application, FPCs may be sprayed on edible plant organs, so the toxicity of FPCs was evaluated using a human neuroblastoma cell line (SK cells). After incubation with 0.1–10 μg mL−1 FPC for 24 hours, SK cells showed no significant change (survival rate was more than 90%), suggesting FPCs is not cytotoxic (Fig. 4d). On the other hand, the potential ecotoxicity of FPCs is not investigated in this work. The ecotoxicity of FPCs is also of great importance38,39 for their potential field application because FPCs enter into the local ecosystem ultimately. The potential ecotoxicity of FPCs should be closely assessed before their field application.
Efficient photosynthesis enhancement
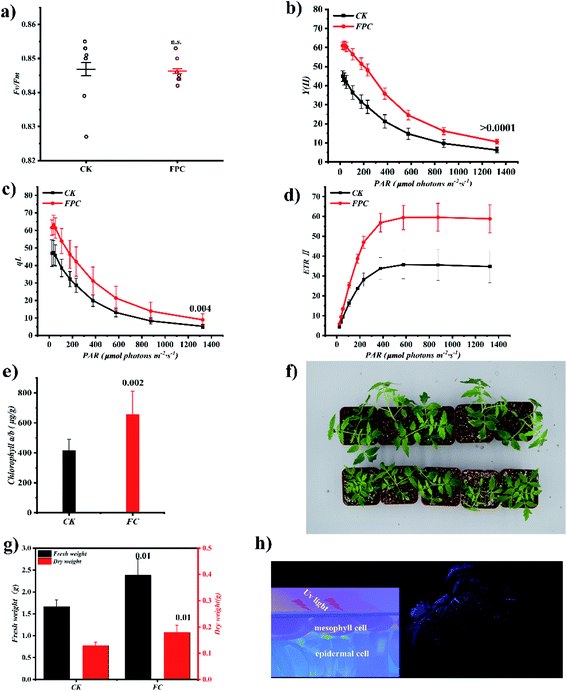
Immediately afterwards, we characterized whether FPC could promote tomato growth by increasing photosynthetic efficiency. The chlorophyll fluorescence parameters, which are important indicators that reflect the growth conditions of crops, were monitored using pot experiments to evaluate the effects of FPC on the photosynthetic performance of tomato leaves. Initially, we measured the maximum quantum yield of photosystem II (Fv/Fm) of tomato leaves treated with FPC for seven days to identify whether FPC had damage to photosynthetic apparatus, because Fv/Fm can reflect the health status of photosynthetic apparatus. As shown in Fig. 5a, FPC showed no obvious damage to the photosynthesis apparatus, and similar maximum quantum yields of photosystem II (Fv/Fm) were obtained among the control and treated groups. Previously, many studies have reported the physiological effects of different proportions of blue and red light on plants, and the mechanism of photoluminescent materials directly sprayed on plants to improve photosynthetic efficiency has been preliminarily studied.6,30,40 These studies show that photoluminescent materials can transform useless light into useful light by the way of extracellular light conversion, improve the photosynthetic electron transfer rate and increase the accumulation of assimilates. Therefore, we designed experiments to verify whether the material can effectively enhance the photosynthetic capacity of plants. Because chlorophyll is the main pigment of photosynthesis, the light absorbed by chlorophyll pigment can be used for photochemical reaction, so we use the total content of chlorophyll and chlorophyll fluorescence yield to evaluate the effect of FPC on photosynthesis of tomato. The excess energy can be converted into heat or emitted in the form of fluorescence. By measuring the yield of chlorophyll fluorescence, we can quantitatively calculate a series of indicators reflecting the state of photosynthetic apparatus, such as the effective quantum yield of PSII (YII), photochemical quenching coefficient (qL), and electron transport rate of PSII (ETRII). The chlorophyll content, effective quantum yield of PSII (YII), photochemical quenching coefficient (qL), and electron transport rate of PSII (ETRII) were significantly higher than those of the control group (Fig. 5b–e) in 20 days after application of FPC (spraying every 4 days for new leaf germination). It indicated that FPC could increase chlorophyll content and enhance the function of photosynthetic apparatus. Not surprisingly, the dry and fresh weight of tomato increased by 38.3% and 43.2%, respectively (Fig. 5f and g). In general, this fluorescent material can be attached to the surface of the leaves, as a small machine to convert ultraviolet light into blue light, and continuously provide feed for plant mesophyll cells (Fig. 5h).Conclusions
Herein, UV-excited, blue light-emitting fluorescent polymeric coatings originated from the covalently copolymerization of CDs and TA were developed, which presented excellent potential properties of continuous blue fluorescence, adhesion, rain-erosion resistance, stability and nontoxicity. After spraying FPCs on tomato leaves grown under the full spectrum, significantly improved photosynthetic activities and accelerated plant growth were observed obviously, proving the roles of FPCs in enhancing photosynthesis. These promising properties of FPCs increased their practical viability to woody plants and in field conditions. Further works on the assessment of FPCs on different plants under field conditions is smoothly ongoing.Author contributions
The manuscript was designed and written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by Natural Science Foundation of Shandong Province (ZR2020ZD43), China Postdoctoral Science Foundation (2019M652447) and Taishan Scholar Foundation of Shandong Province (2018CXGC0211, TSCY20190126).Notes and references
- T. Mirkovic, E. E. Ostroumov, J. M. Anna, R. van Grondelle, Govindjee and G. D. Scholes, Chem. Rev., 2017, 117, 249–293 CrossRef CAS PubMed.
- J. Deisenhofer, O. Epp, K. Miki, R. Huber and H. Michel, Nature, 1985, 318, 618–624 CrossRef CAS PubMed.
- X. Xia, J. H. Pan, X. Pan, L. Hu, J. Yao, Y. Ding, D. Wang, J. Ye and S. Dai, ACS Energy Lett., 2019, 4, 405–410 CrossRef CAS.
- D. Tilman, C. Balzer, J. Hill and B. L. Befort, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(50), 20260–20264 CrossRef CAS.
- Y. Qi, Y. Wang, Y. Yu, Z. Liu, Y. Zhang, Y. Qi and C. Zhou, J. Mater. Chem. C, 2016, 4, 11291–11297 RSC.
- X. Lin, Y. Li, S. Saravanakumar, Q. Tang, S. Zhang, X. Gao, Y. Hu, K. Huang and G. Han, Nano Today, 2020, 34, 100918 CrossRef CAS.
- M. Li, X. Zhang, H. Zhang, W. Chen, L. Ma, X. Wang, Y. Liu and B. Lei, J. Mater. Chem. C, 2019, 7, 3617–3622 RSC.
- W. Chen, X. Zhang, J. Zhou, H. Zhang, J. Zhuang, Z. Xia, Y. Liu, M. S. Molokeev, G. Xie and B. Lei, J. Mater. Chem. C, 2020, 8, 3996–4002 RSC.
- L. Li, Y. Luo, R. Li, Q. Zhou, W. J. G. M. Peijnenburg, N. Yin, J. Yang, C. Tu and Y. Zhang, Nat. Sustain., 2020, 3, 929–937 CrossRef.
- X.-D. Sun, X.-Z. Yuan, Y. Jia, L.-J. Feng, F.-P. Zhu, S.-S. Dong, J. Liu, X. Kong, H. Tian, J.-L. Duan, Z. Ding, S.-G. Wang and B. Xing, Nat. Nanotechnol., 2020, 15, 755–760 CrossRef CAS.
- J. P. Giraldo, M. P. Landry, S. M. Faltermeier, T. P. McNicholas, N. M. Iverson, A. A. Boghossian, N. F. Reuel, A. J. Hilmer, F. Sen, J. A. Brew and M. S. Strano, Nat. Mater., 2014, 13, 400–408 CrossRef CAS PubMed.
- Y. Li, X. Xu, Y. Wu, J. Zhuang, X. Zhang, H. Zhang, B. Lei, C. Hu and Y. Liu, Mater. Chem. Front., 2020, 4, 437–448 RSC.
- T. T. S. Lew, V. B. Koman, K. S. Silmore, J. S. Seo, P. Gordiichuk, S.-Y. Kwak, M. Park, M. C.-Y. Ang, D. T. Khong, M. A. Lee, M. B. Chan-Park, N.-H. Chua and M. S. Strano, Nat. Plants, 2020, 6, 404–415 CrossRef CAS.
- H. Wu, R. Nißler, V. Morris, N. Herrmann, P. Hu, S.-J. Jeon, S. Kruss and J. P. Giraldo, Nano Lett., 2020, 20, 2432–2442 CrossRef CAS PubMed.
- L. Zheng, M. Su, C. Liu, L. Chen, H. Huang, X. Wu, X. Liu, F. Yang, F. Gao and F. Hong, Biol. Trace Elem. Res., 2007, 119, 68–76 CrossRef CAS.
- I. Nabiev, A. Rakovich, A. Sukhanova, E. Lukashev, V. Zagidullin, V. Pachenko, Y. P. Rakovich, J. F. Donegan, A. B. Rubin and A. O. Govorov, Angew. Chem., Int. Ed., 2010, 49, 7217–7221 CrossRef.
- S. Chandra, S. Pradhan, S. Mitra, P. Patra, A. Bhattacharya, P. Pramanik and A. Goswami, Nanoscale, 2014, 6, 3647–3655 RSC.
- L. Sai, S. Liu, X. Qian, Y. Yu and X. Xu, Colloids Surf., B, 2018, 169, 422–428 CrossRef CAS PubMed.
- W. Li, Y. Zheng, H. Zhang, Z. Liu, W. Su, S. Chen, Y. Liu, J. Zhuang and B. Lei, ACS Appl. Mater. Interfaces, 2016, 8, 19939–19945 CrossRef CAS.
- Y. Zheng, G. Xie, X. Zhang, Z. Chen, Y. Cai, W. Yu, H. Liu, J. Shan, R. Li, Y. Liu and B. Lei, ACS Omega, 2017, 2, 3958–3965 CrossRef CAS.
- H. Wang, M. Zhang, Y. Song, H. Li, H. Huang, M. Shao, Y. Liu and Z. Kang, Carbon, 2018, 136, 94–102 CrossRef CAS.
- L. Hu, H. Li, C. Liu, Y. Song, M. Zhang, H. Huang, Y. Liu and Z. Kang, Nanoscale, 2018, 10, 2333–2340 RSC.
- S. Zhu, Q. Meng, L. Wang, J. Zhang, Y. Song, H. Jin, K. Zhang, H. Sun, H. Wang and B. Yang, Angew. Chem., Int. Ed., 2013, 52, 3953–3957 CrossRef CAS PubMed.
- H. Liu, R. S. Li, J. Zhou and C. Z. Huang, Analyst, 2017, 142, 4221–4227 RSC.
- C.-L. Zhang, Y.-X. Wang, X. Hu, Y.-L. Zhang, G.-L. Wang, C.-X. You, Y.-Y. Li and Y.-J. Hao, Environ. Exp. Bot., 2020, 180, 104206 CrossRef CAS.
- J.-Q. Yu, K.-D. Gu, C.-H. Sun, Q.-Y. Zhang, J.-H. Wang, F.-F. Ma, C.-X. You, D.-G. Hu and Y.-J. Hao, Plant Biotechnol. J., 2021, 19(2), 285–299 CrossRef CAS PubMed.
- S. Saroussi, D. A. J. Karns, D. C. Thomas, C. Bloszies, O. Fiehn, M. C. Posewitz and A. R. Grossman, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 11518–11527 CrossRef CAS PubMed.
- V. Shanmugam and N. Kanoujia, Biol. Contr., 2011, 57, 85–93 CrossRef.
- A. Avellan, J. Yun, Y. Zhang, E. Spielman-Sun, J. M. Unrine, J. Thieme, J. R. Li, E. Lombi, G. Bland and G. V. Lowry, ACS Nano, 2019, 13, 5291–5305 CrossRef CAS PubMed.
- X. Xu, X. Mao, J. Zhuang, B. Lei, Y. Li, W. Li, X. Zhang, C. Hu, Y. Fang and Y. Liu, ACS Sustainable Chem. Eng., 2020, 8, 3938–3949 CrossRef CAS.
- H. A. Lee, E. Park and H. Lee, Adv. Mater., 2020, 32, 1907505 CrossRef CAS.
- Y. Chen, M. Zheng, Y. Xiao, H. Dong, H. Zhang, J. Zhuang, H. Hu, B. Lei and Y. Liu, Adv. Mater., 2016, 28, 312–318 CrossRef CAS.
- M. Shin, J. H. Ryu, J. P. Park, K. Kim, J. W. Yang and H. Lee, Adv. Funct. Mater., 2015, 25, 1270–1278 CrossRef CAS.
- K. Kim, M. Shin, M.-Y. Koh, J. H. Ryu, M. S. Lee, S. Hong and H. Lee, Adv. Funct. Mater., 2015, 25, 2402–2410 CrossRef CAS.
- Y. Liu, K. Ai and L. Lu, Chem. Rev., 2014, 114, 5057–5115 CrossRef CAS.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- J. Liu, Y. Geng, D. Li, H. Yao, Z. Huo, Y. Li, K. Zhang, S. Zhu, H. Wei, W. Xu, J. Jiang and B. Yang, Adv. Mater., 2020, 32, 1906641 CrossRef CAS PubMed.
- T. Garvey, E. A. Moore, C. W. Babbitt and G. Gaustad, Clean Technol. Environ. Policy, 2019, 21, 229–242 CrossRef CAS.
- A. Bour, F. Mouchet, J. Silvestre, L. Gauthier and E. Pinelli, J. Hazard. Mater., 2015, 283, 764–777 CrossRef CAS.
- S. W. Hogewoning, G. Trouwborst, H. Maljaars, H. Poorter, W. van Ieperen and J. J. Harbinson, Exp. Bot., 2010, 61, 3107–3117 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra03695e |
| This journal is © The Royal Society of Chemistry 2021 |