Open Access Article
Open Access ArticleSolvent-free synthesis of propargylamines: an overview
Ravi Manujyothi
a,
Thaipparambil Aneeja
b and
Gopinathan Anilkumar
 *abc
*abc
aInstitute for Integrated Programmes and Research in Basic Sciences (IIRBS), Mahatma Gandhi University, Priyadarsini Hills P O, Kottayam, Kerala, 686560 India. E-mail: anilgi1@yahoo.com; anil@mgu.ac.in; Fax: +91-481-2731036
bSchool of Chemical Sciences, Mahatma Gandhi University, Priyadarsini Hills P O, Kottayam, Kerala, 686560 India
cAdvanced Molecular Materials Research Centre (AMMRC), Mahatma Gandhi University, Priyadarsini Hills P O, Kottayam, Kerala, 686560 India
First published on 30th May 2021
Abstract
Propargylamines are a class of compounds with many pharmaceutical and biological properties. A green approach to synthesize such compounds is very relevant. This review aims to describe the solvent-free synthetic approaches towards propargylamines via A3 and KA2 coupling reactions covering the literature up to 2021.
1 Introduction
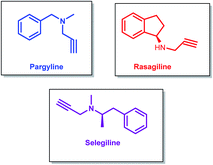
Propargylamines are one of the most versatile classes of compounds with numerous applications. Propargylamine derivatives such as pargyline, rasagiline and selegiline are used against neurodegenerative disorders like Parkinson's and Alzheimer's diseases.1–4 (−)-Deprenyl (selegiline) is found to have an antiapoptotic function,5 which makes it useful for symptomatic and neuroprotective treatment (Fig. 1).6Pargyline (N-methyl-N-propagylbenzylamine) is a monoamine oxidase inhibitor. Among the two isoforms of monoamine oxidase (MAO), MAO-B is responsible for neurodegenerative diseases like Alzheimer's disease. Pargyline acts as an irreversible selective MAO-B inhibitor drug.7 Being a MAO inhibitor pargyline is also used for treating type 1 diabetes8, and the cardiovascular complications associated with it.9 Pargyline also inhibits lysine-specific-demethylase-1 (LSD-1). When used along with the chemotherapeutic agent camptothecin, pargyline was found to enhance LSD-1 inhibition and resulted in induced senescence and growth inhibition of cancer cells.10 Pargyline was also identified to have inhibitory properties against proline-5-carboxylate reductase-1 (PYCR1), thus making it useful for cancer treatments.11
Rasagiline (N-methyl-1-(R)-aminoindan) and selegiline (N-[(2R)-1-phenylpropan-2-yl]prop-2-yn-1-amine) are also MAO-B inhibitors and are found to be effective for the treatment of Parkinson's disease.12 The neuroprotective effects of rasagiline were found to be dependent on the propargyl moiety and independent of MAO-B inhibition.13 Rasagiline prevents apoptosis by reducing oxidative stress and thus stabilizing mitochondrial membranes and has also demonstrated neurorestorative activities.14 It was found to be clinically effective as monotherapy or as an adjunct to levodopa for treating Parkinson's disease.15 Rasagiline hybrid molecules are found to have anti-Alzheimer's disease activities.16
Rasagiline has shown a higher potency in MAO-B inhibition than selegiline,17 but both are found to have similar efficacy and neuroprotective potential.18 Selegiline is found to assist in slowing down the progression of the symptoms of Parkinson's disease.19 It also has anti-depressant properties,20 and has found to be effective for treating attention deficit hyperactivity disorder (ADHD).21
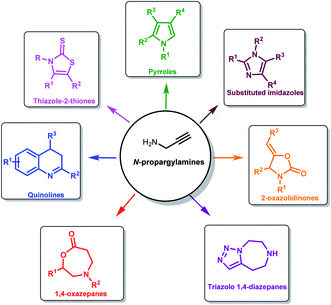
The low cost, easy availability and versatility of propargylamines makes it a perfect building block for the synthesis of diverse heterocycles like 2-oxazolidinones,22 thiazolidine-2-thione and imidazole-2-ones.23 Propargylamines are used for the synthesis of biologically active compounds like 1,4-oxazepane and 1,4-diazepane. The latter is used as an anti-depressant, anti-palatelet, anti-convulsant and more.24 Pharmaceutically and synthetically important heterocycles like imidazole and imidazolines can also be synthesized from propargylamines.25 Propargylamines are also used as an intermediate for synthesizing polyfunctional aminoderivatives.26 Synthetically important molecules such as pyrroles,27 quinolines are also generated from propargylamines (Fig. 2).28
However, with the increasing environmental concerns, a waste and hazard free synthetic approach is preferred. Most of the synthetic methodologies involves the large-scale use of volatile organic solvents. Moreover, many of the conventional solvents are toxic, corrosive or flammable.29 There is an extensive use of solvents for different purposes such as regulating temperature, cleaning equipment and clothing, isolation and purification of organic compounds via recrystallization etc.30 The role of solvents in causing a waste burden is unignorable in the production of fine chemicals and pharmaceuticals.31 Green chemistry aims to remove intrinsic hazard of particular products and processes and there by aims to decrease environmental concerns.32 Green chemistry helps to improve chemical synthesis by enhancing product selectivity, resource and energy efficiency, operational simplicity, environmental safety and health.33 A new synthetic approach is being provided by green chemistry by minimizing hazards and wastes associated with classical approach and thus making research sustainable.34
Over the past decades green chemistry has achieved great significance and conventional synthetic methods are getting replaced by green chemistry.35,36 The accomplishments in the area of green chemistry provides a great perspective that the efficiency and functionality of chemicals can be maintained while reducing the adverse effects caused by them.37 Methodologies like photocatalysis facilitated the incorporation of mild conditions and hence has widened the scope of different organic reactions.38–40
Different type of solvents like halogenated solvents were used widely regardless of the environmental and health concerns they caused. Though they have many advantages like their general lack of flammability, better solubility, low cost, their adverse effects should not be neglected and hence now they are being highly regulated.41 Solvents being used in large quantities, designing environmental friendly solvents and solventless systems have been the most active areas in green chemistry for the past decade.42,43
It is in such scenario; a solvent-free synthesis provides a green synthetic alternative.
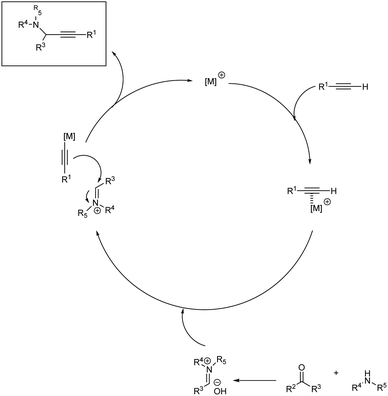
Propargylamines are mostly synthesized via A3 coupling of aldehyde, amines and terminal alkynes. When the aldehyde in this multicomponent reaction is replaced by a ketone, it is called KA2 coupling.44 Most of the synthesis of propargylamines are metal catalyzed; only a very few has been reported to be metal-free. A general mechanism for the synthesis of propargylamines is depicted in Scheme 1. There are certain challenges associated with metal-free synthesis including long reaction time and high temperature. The substrate scope provided by these reactions are also less, namely a smooth reaction with aliphatic alkynes or aliphatic ynoic acid is yet to be achieved. Recently in 2021, Biswas et al. published a review on metal-free multicomponent approaches for the synthesis of propargylamines.45 The green approach in the synthesis of propargylamines are mostly achieved through a solvent-free condition. Recently some methodologies were developed making it more environmentally benign via a solvent and metal-free approach.
For better clarity and brevity, this review is categorised based on the transition metal involved in the reaction. To the best of our knowledge, this is the first review on the solvent-free synthesis of propargylamines, and gives an overview of the solvent-free synthesis of propargylamines covering literature up to 2021.
2 Copper catalyzed solvent-free synthesis of propargylamines
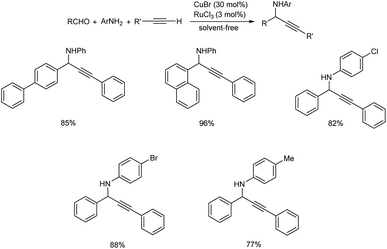
Propargylamines are mostly synthesized through multicomponent reactions catalyzed by transition metals. Copper is one of the most used metal as it is cheap and has a great potential for reactivity.A methodology for synthesizing propargylamines through Grignard-type imine addition via C–H activation was developed by Wei et al.46 The reaction was carried out under solvent-free conditions, catalyzed by Cu–Ru catalyst (Scheme 2). The reaction when performed in water also offered good results.
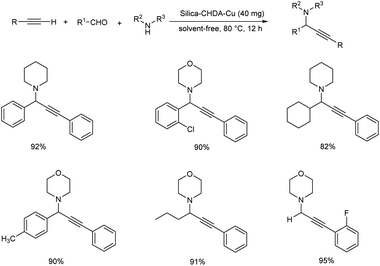
In 2007, Wang and co-workers demonstrated the synthesis of propargylamines by means of three component Mannich coupling of aldehyde, terminal alkyne and amine via C–H activation, catalyzed by silica supported copper catalyst (Scheme 3).47 The reaction afforded best results when carried out under solvent-free conditions at 80 °C using 1 mol% of silica-CHDA-Cu as catalyst. The recovered catalyst was found to be efficient up to 15 cycles.
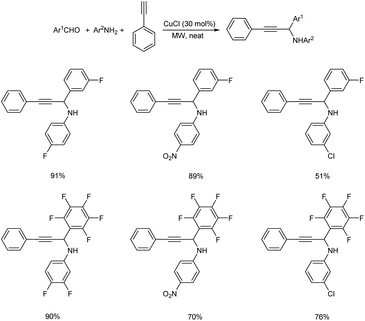
In 2008, Du et al. synthesized fluorinated propargylamines via microwave (MW) promoted one-pot three component reaction catalyzed by CuCl.48 Best results were attained under neat condition with 30 mol% of CuCl (Scheme 4). Aromatic aldehydes with electron-withdrawing groups provided good results where as those with electron-donating groups displayed poor results. From substrate scope studies it was also discovered that anilines with electron-withdrawing substituents gave higher yields than those with electron-donating groups.
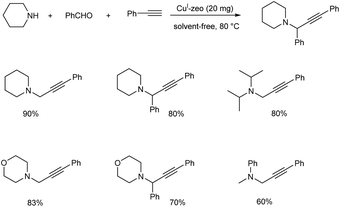
In the same year Sommer et al. achieved a green synthesis of propargylamines by using CuI-modified zeolites as catalyst.49 The best results were obtained when the reaction was catalyzed by CuI-USY at 80 °C, under solvent-free conditions (Scheme 5). Cage-type zeolites afforded better results than zeolites containing channel pores. Proposed mechanism of the reaction is depicted in (Scheme 6).
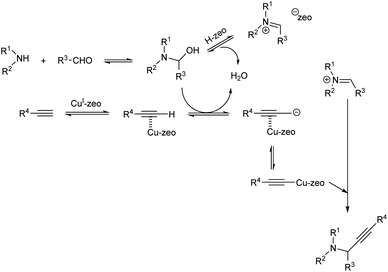 | ||
| Scheme 6 Proposed mechanism of multicomponent reaction catalyzed by CuI-USY (reproduced with permission from ref. 49). | ||
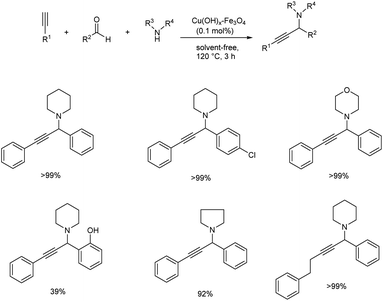
Later in 2009, Ramón and co-workers used impregnated copper on magnetite as catalyst for the synthesis of propargylamines via multicomponent acetylene-Mannich reaction of terminal alkynes, secondary amines and aldehydes.50 The optimised condition involves the use of 0.1% of copper catalyst at 120 °C under solvent-free conditions (Scheme 7). Primary amines failed to achieve the required product via this strategy. A significant reduction in the yields was observed when aldehyde was replaced by ketone which implies the selectivity of the catalyst. Water being the only by-product, the reaction displays very high atom efficiency. The catalyst was recovered and reused up to ten times without losing activity.
 | ||
| Scheme 7 Synthesis of propargylamines via multicomponent acetylene-Mannich reaction by using impregnated copper on magnetite as catalyst. | ||
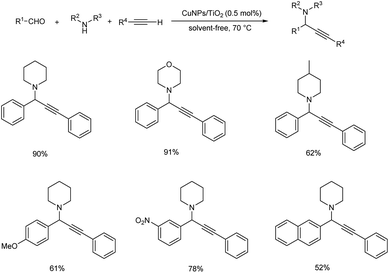
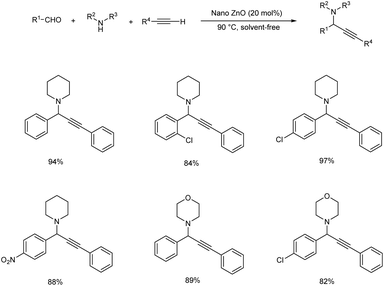
The synthesis of propargylamines through multicomponent reaction of aldehydes, alkynes and amines catalyzed by CuNPs/TiO2 was also reported (Scheme 8).51 The use of 0.5 mol% of the catalyst was found to provide best results at 70 °C under solvent-free conditions, and the desired propargylamines were obtained in moderate to excellent yields. The catalyst was recovered and reused up to four consecutive cycles.
An environmentally benign strategy for the synthesis of propargylamines was disclosed in 2014.52 This A3 coupling reaction was performed in the presence of recyclable GO-CuCl2 under microwave irradiation. The reaction was found feasible using both electron-rich and electron-deficient alkynes. The gram scale synthesis of propargylamine in 88% yield further enhances the practical application of this methodology in industrial field.
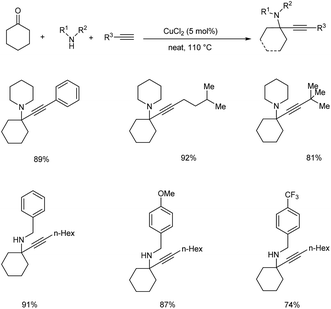
In 2015, Palchak and co-workers demonstrated the synthesis of tetrasubstituted propargylamines via copper(II) chloride catalyzed three component coupling of cyclohexanone, amines and alkynes.53 Best results were obtained when 5 mol% of CuCl2 was used at 110 °C, under solvent-free conditions (Scheme 9).
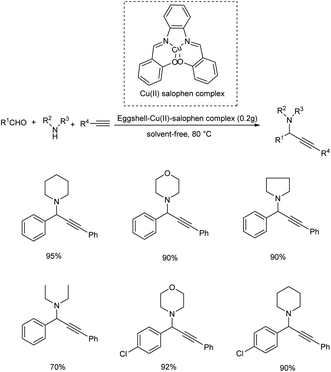
Bakherad and his co-workers reported the synthesis of propargylamines via the multi-component reactions of terminal alkynes, secondary amines and aldehydes catalyzed by egg shell-supported-Cu(II) salophen complex in 2016 (Scheme 10).54 Aromatic aldehydes bearing electron-withdrawing and electron-donating substituents showed high reactivity. Here the use of 0.2 g of catalyst at 80 °C under solvent-free conditions was identified to be the optimum reaction conditions.
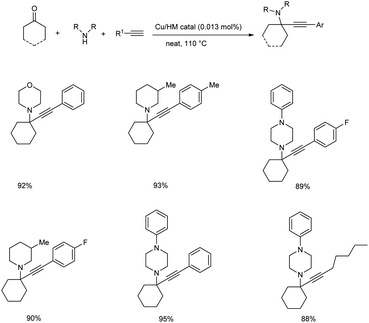
Later in the same year, Rawat and co-workers developed a methodology for the synthesis of tetrasubstituted propargylamines via KA2 coupling using hydromagnesite (Cu/HM) nanomaterial supported copper catalyst.55 The best results were obtained by the usage of 0.013 mol% of Cu/HM catalyst, under solvent-free conditions at 110 °C (Scheme 11). Here, primary amines needed longer reaction time than secondary amines.
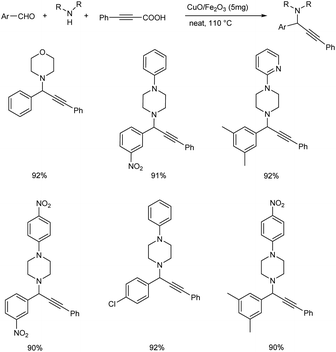
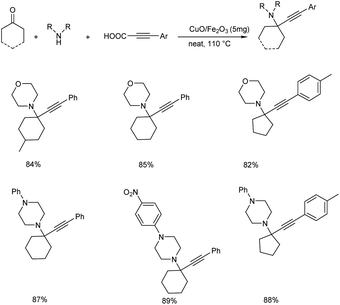
The group also synthesized propargylamines via decarboxylative A3 and KA2 coupling, catalyzed by CuO/Fe2O3 NPs later the same year.56 Decarboxylative A3 coupling was investigated by using benzaldehyde, morpholine and phenylpropiolic acid as model substrates. The optimised condition includes the use of 5 mg of CuO/Fe2O3 as the catalyst at 110 °C under solvent-free conditions (Scheme 12). Trisubstituted propargylamines were obtained in 92% yield. Decarboxylative KA2 coupling among cyclohexanone, morpholine and phenylpropiolic acid was also explored (Scheme 13). Best results were obtained upon using 5 mg of catalyst at 110 °C, under neat conditions.
 | ||
| Scheme 12 CuO/Fe2O3 catalyzed synthesis of trisubstituted propargylamines via decarboxylative A3 coupling reaction. | ||
 | ||
| Scheme 13 CuO/Fe2O3 catalyzed synthesis of trisubstituted propargylamines via decarboxylative KA2 coupling reaction. | ||
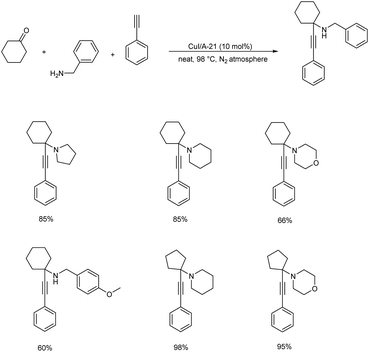
KA2 coupling catalyzed by CuI supported Amberlyst A-21 was disclosed in 2017 (Scheme 14).57 KA2 coupling is rarely employed for propargylamine synthesis since ketones are less reactive than aldehydes. The catalyst successfully catalyzed KA2 reaction involving both primary and secondary amines, aliphatic and aromatic alkynes with either cyclic or linear ketones under solvent-free conditions.
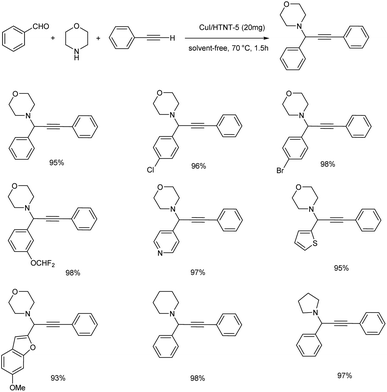
Reddy et al. synthesized propargylamines via A3 coupling, catalyzed by novel CuI catalyst supported on protonated trititanate nanotubes (CuI/HTNT).58 The optimum reaction conditions were established to be the use of 20 mg CuI/HTNT-5 (5 wt%-CuI loaded HTNT) under solvent-free conditions at 70 °C (Scheme 15). Aryl aldehydes with electron-withdrawing groups afforded excellent yields compared to electron-donating groups. Higher yields were obtained from aliphatic aldehydes. Excellent yields were given by cyclic, acyclic and heterocyclic secondary amines. Moreover, it was observed from the substrate scope studies that aniline was found inactive towards this method.
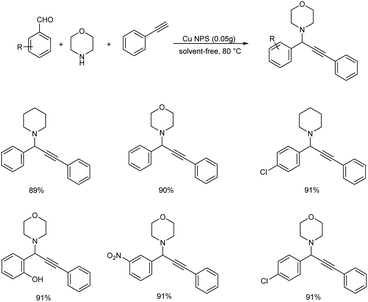
In 2017, a multicomponent reaction catalyzed by novel magnetite-supported copper nanoparticle was developed by Shouli and co-workers for synthesizing propargylamines.59 The best results were found to be obtained under solvent-free conditions at 80 °C (Scheme 16). The catalyst was magnetically recoverable and can be reused up to five times without significant loss in catalytic activity.
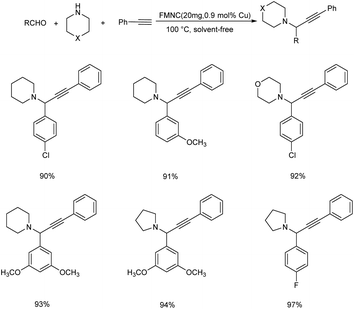
A new magnetically recoverable catalyst (FMNC) was synthesized from Cu(II) complex of dendrimer ligands on the modified MCM-41.60 This catalyst was found highly efficient in the one-pot three component reaction of aldehydes, amines and phenylacetylene to form propargylamines (Scheme 17). Pyrrolidine showed more activity than the other amines like piperidine and morpholine towards this strategy. Activity of benzaldehyde was affected by halide substituents (F > Cl > Br).
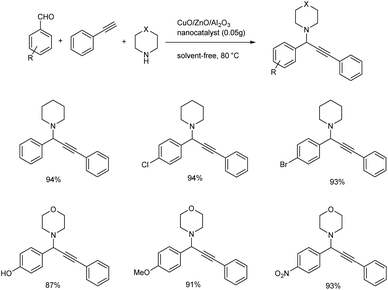
In 2018, an efficient, recyclable nanocatalyst of ZnO supported CuO/Al2O3 was prepared and it was used for the synthesis of propargylamines (Scheme 18).61 ZnO was found to be an operative promoter which helps to enhance the reducibility and distribution of copper particles. Whereas Al2O3 is added as a constitution promoter in order to increase the surface area, mechanical resistance and thermal stability of the catalyst. The reaction of 4-nitrobenzaldehyde, piperidine and phenylacetylene in the presence of 0.05 g of the nanocatalyst at 80 °C under solvent-free conditions was recognized to be the optimum. Higher yields were given by aromatic aldehydes having electron-donating or electron-withdrawing substituents, secondary amines and phenylacetylene.
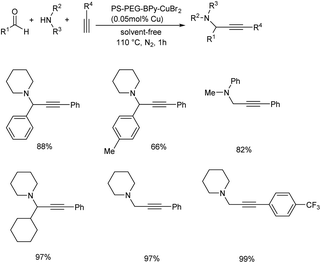
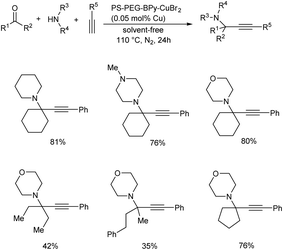
In 2019, Osako and co-workers synthesized propargylamines by the three-component coupling of aldehyde or ketone, amines and alkynes.62 They developed a copper(II)–bipyridine complex immobilized on amphiphilic polystyrene–poly (ethylene glycol) resin (PS–PEG–BPy–CuBr2) as catalyst. Benzaldehyde, piperidine and phenylacetylene were reacted in the presence of a copper loading of 500 mol ppm, under solvent-free conditions (Scheme 19). Propargylamine was obtained in 96% yield. Reaction was examined with various substrates. A three-component coupling with ketones instead of aldehydes was also investigated. KA2 coupling of cyclohexanone with secondary amines and phenylacetylene in the presence of 500 mol ppm loading of PS–PEG–BPy–CuBr2 at 110 °C gave the corresponding propargylamines (Scheme 20).
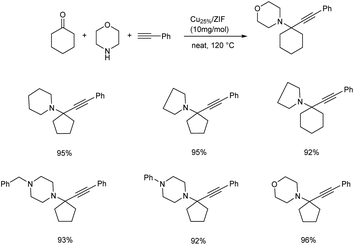
In the same year, propargylamines having quaternary carbon centre has been reported via KA2 coupling, catalyzed by Cu dopped ZIF-8.63 The optimum conditions for the reaction was identified to be under neat condition at 120 °C, with 10 mg of the catalyst (Scheme 21). It was observed that aliphatic alkynes needed a prolonged reaction time compared to phenyl acetylene. The acidic hydrogen of terminal alkynes was found to be crucial in this strategy. Good recyclability was shown by the catalyst.
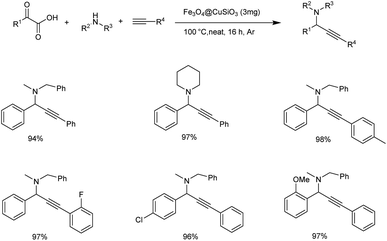
Later the same year Zhang and co-workers achieved the synthesis of propargylamines through decarboxylative A3 coupling of α-ketoacid, amine and alkyne catalyzed by magnetic copper catalyst (Scheme 22).64 From the substrate scope studies it was found that alkynes with electron-donating and electron-withdrawing groups gave the product in good yields. Aliphatic alkynes also gave the desired product in excellent yields. Acyclic, cyclic and aliphatic amines gave products with good to excellent yields. The reaction carried out under solvent-free conditions with 3 mg catalyst at 100 °C under Argon atmosphere was found to be optimum, the catalyst could be reused up to six times.
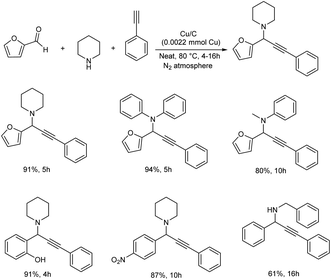
Kim and co-workers synthesized a biomass-derived copper catalyst (Cu/C) from glucose. This catalyst was found highly efficient towards the synthesis of propargylamines.65 An A3 coupling reaction was carried out using furfural, piperidine and phenylacetate as model substrates and was found that solvent-free conditions gave higher yields (Scheme 23). Cyclic, acyclic and secondary amines provided good yields. Aromatic aldehydes with –NO2, –Cl and –OH functional groups showed good to excellent yields.
Recently, Li et al. achieved the synthesis of propargylamines through A3 coupling catalyzed by CuSO4 nanoparticles anchored on the surface of polymeric composites.66 This novel catalyst produced the desired propargylamines in good to excellent yields under solvent-free conditions. The catalyst was recovered and reused upto five times without considerable loss in catalytic efficiency.
3 Zinc catalyzed solvent-free synthesis of propargylamines
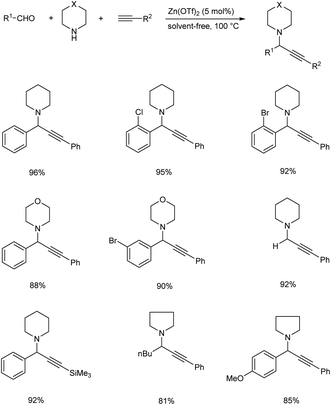
Satyanarayana and co-workers synthesized recyclable ZnO nanoparticles as a catalyst for synthesizing propargylamines via A3 coupling (Scheme 24).67 Benzaldehyde, piperidine and phenylacetylene were used as model substrates. The best results were obtained in the presence of 20 mol% of ZnO NPs. The catalyst was separated and reused up to ten runs without significant loss of catalytic activity.In 2016, a three component coupling of aldehydes, terminal alkynes and amines catalyzed by Zn(OTf)2 for the synthesis of propargylamines was established by Chandak and his co-workers.68 Excellent results were obtained at 100 °C under neat conditions (Scheme 25). Aldehydes with electron-donating groups required longer reaction time, while those with electron-withdrawing groups reacted rapidly. The desired product was not obtained from heteroaromatic aldehydes. Five and six membered cyclic secondary amines yielded desired products in good yields.
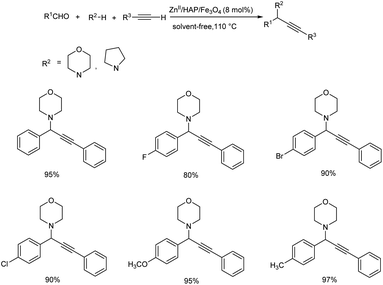
Later, a magnetically recoverable nanocatalyst, Zn(II) anchored onto the magnetic natural hydroxyapatite (ZnII/HAP/Fe3O4) was reported.69 This nanocatalyst was used for the synthesis of propargylamines, through one-pot, three components A3 coupling. Here, the use of 8 mol% of ZnII/HAP/Fe3O4 at 110 °C, under solvent-free conditions was identified as optimum reaction condition (Scheme 26). Benzaldehydes substituted with electron-withdrawing groups needed more reaction time to achieve the desired product. Whereas, benzaldehydes bearing electron-donating groups afforded desired products in good to excellent yields, in shorter reaction time. Phenylacetylenes gave good or moderate yields irrespective of electron-withdrawing or electron-donating substituents.
 | ||
| Scheme 26 Synthesis of propargylamines in the presence of ZnII/HAP/Fe3O4 under solvent-free conditions. | ||
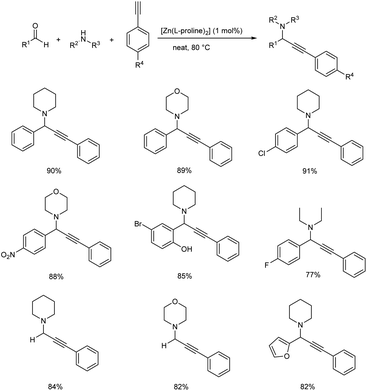
A one pot, three component A3 coupling for propargylamine synthesis, via C–H activation of alkynes catalyzed by [Zn(L-proline)2] was reported in 2018 (Scheme 27).70 Aryl aldehydes substituted with electron-withdrawing groups showed better results than those with electron-donating groups. More reactivity was observed with 4-chlorobenzaldehyde than 4-methoxybenzaldehyde or 4-methylbenzaldehyde. Heterocyclic and acyclic aldehydes afforded good yields. Though secondary amines provided good yields, aromatic amines such as aniline gave no product. Both substituted and unsubstituted aromatic alkynes generated good to excellent yields of product.
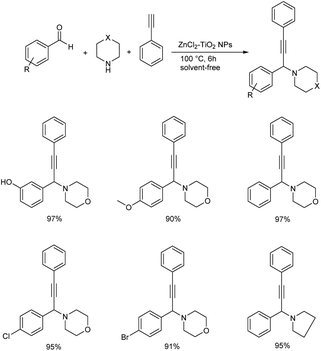
Later in 2019, Kanade and co-workers used nano-crystalline ZnCl2–TiO2 as heterogenous catalyst for the synthesis of propargylamines from aromatic aldehydes, secondary amines and phenylacetylene (Scheme 28).71 Excellent results were obtained when the reaction was performed using 15% ZnCl2 loaded TiO2 nanoparticles as the catalyst at 100 °C for 6 h. Better yields and high reactivity were shown by aromatic aldehydes with halogen and electron-donating substituents. Aryl aldehydes with methoxy group at para position did not show excellent results compared to meta or ortho substituted hydroxy aldehydes. 2-Chloro and 4-chlorobenzaldehydes were slightly more reactive than 4-flurobenzaldehyde and 4-bromobenzaldehyde. The catalyst was recycled and used for five successive runs without any significant loss in the catalytic activity. The tentative mechanism is shown in (Scheme 29).
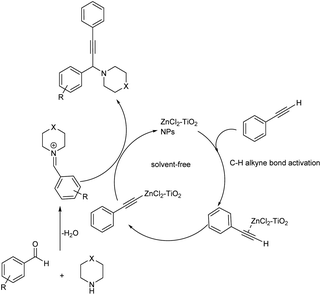 | ||
| Scheme 29 A tentative mechanism for the synthesis of propargylamine catalyzed by ZnCl2–TiO2 nanoparticles (NPs) (reproduced with permission from ref. 71). | ||
4 Gold catalyzed solvent-free synthesis of propargylamines
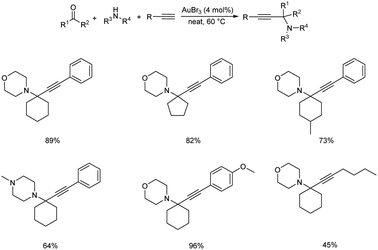
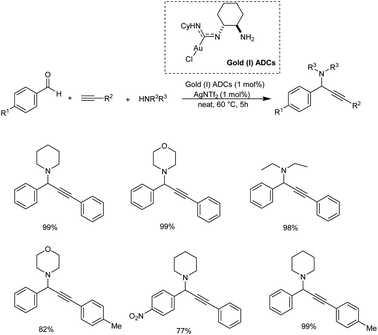
Gold being a biocompatible metal provides an eco-friendly metal catalysis. Cheng and co-workers developed a novel methodology for synthesizing propargylamines with quaternary carbon center.72 Propargylamines were synthesized via gold catalyzed direct intermolecular coupling of ketones, secondary amines and alkynes. Cyclohexanone, morpholine and phenylacetylene were taken as model substrates and the optimum condition was obtained when 4 mol% of AuBr3 was employed under neat conditions at 60 °C (Scheme 30). The substrate scope studies displayed the inability of aromatic ketones to give the desired products. This might be due to the special stability of conjugated aromatic ketones, which may result in difficulty in the formation of the intermediate.Gimeno et al. prepared mononuclear gold(I) acyclic diaminocarbenes (ADCs) and gold(I) ADCs were used as catalyst in the three-component coupling of aldehydes, amines and alkynes for the synthesis of propargylamines and indolizines.73 Catalytic synthesis of propargylamines were explored (Scheme 31). Secondary amines like piperidine, pyrrolidines, morpholines showed better results. Au(I) was used instead of Au(III), in the absence of solvent and inert atmosphere, adding to operational simplicity. This green protocol afforded the propargylamines in high yields. The authors also proposed a plausible mechanism for the reaction (Scheme 32).
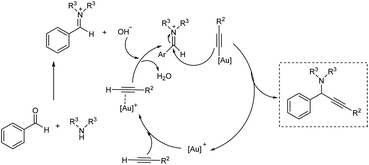 | ||
| Scheme 32 Plausible mechanism of synthesis of propargylamines (reproduced with permission from ref. 73). | ||
5 Solvent-free synthesis of propargylamines: using other metals
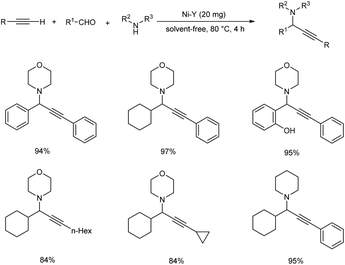
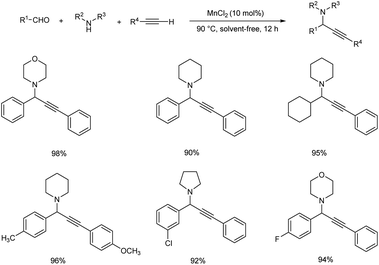
A nickel-catalyzed three component coupling of aldehyde, alkyne and amine via C–H activation was demonstrated by Namitharan et al. for the synthesis of propargylamines (Scheme 33).74 This heterogenous catalyst was supported on zeolite. Optimization studies showed that the use of nickel-exchanged zeolite at 80 °C under solvent-free conditions gave the best results. The catalyst was recovered and reused up to eight cycles.Chen and co-workers accomplished the synthesis of propargylamines via one-pot three component coupling catalyzed by manganese(II) chloride.75 Reactions catalyzed by 10 mol% MnCl2 at 90 °C under solvent-free conditions achieved good yields of the desired products (Scheme 34).
In 2015, Schneider and co-workers developed an astonishing methodology towards the synthesis of propargylamines using silica-xerogel-supported indium(III) composite (In/SiO2) as catalyst.76 They carried out one-pot A3 coupling under conventional and microwave-assisted method (Schemes 35 and 36). This reaction was completed within 10 min. Using microwave assisted method and at the same time conventional method yielded the desired product in 10 h. The catalyst was found efficient, and good yields of product were obtained even after four cycles.
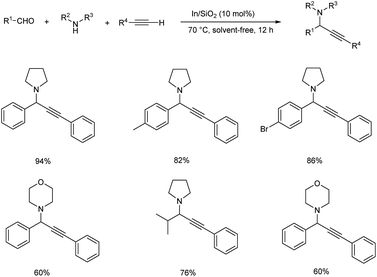 | ||
| Scheme 35 Synthesis of propargylamines using silica-xerogel-supported indium(III) composite (In/SiO2) under conventional method. | ||
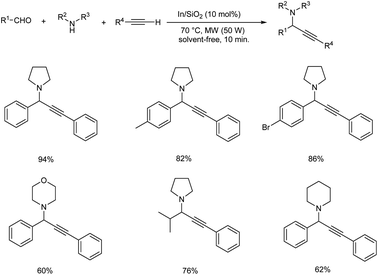 | ||
| Scheme 36 Synthesis of propargylamine using silica-xerogel-supported indium(III) composite (In/SiO2) under microwave assisted method. | ||
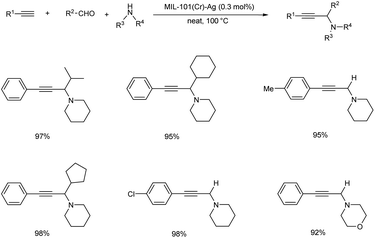
Gao et al. prepared a catalyst, MIL-101(Cr)–SO3Ag, for synthesizing propargylamines via A3 coupling in 2016 (Scheme 37).77 The reaction was efficiently catalyzed with a turn over frequency up to 6600 h−1. The para substituted aromatic alkynes reacted slower than phenylacetylene, irrespective of the nature of the substituents. Piperidine was found to be more reactive than amines like morpholine and diethylamine, however the yields can be improved by prolonged reaction time.
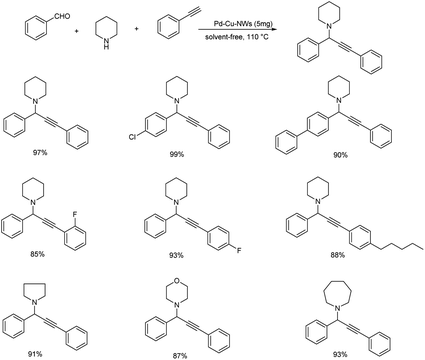
Yi et al. proposed A3 coupling of aldehydes, alkynes and amines, catalyzed by Pd–Cu NWs for the synthesis of propargylamines.78 The reaction, when conducted at 110 °C showed excellent result and the required product was obtained in 10 minutes (Scheme 38). Benzaldehyde with electron-withdrawing group reacted better compared to electron-donating group. Due to steric hindrance, ortho substituted aryl aldehydes showed a reduction in reactivity. The catalytic system was tolerant to various functional groups. High reactivity was shown by aromatic alkynes with both electron-donating and electron-withdrawing substituents. Mono-substituted phenylacetylenes showed high reactivity in the order para > meta > ortho. Ethyl, bulky n-pentyl and tert-butyl substituted phenylacetylenes provided good yields and high reactivity. The plausible mechanism indicates the initiation of reaction by CH bond activation by the coordination of terminal alkynes to Pd–Cu NWs (Scheme 39). Pd–Cu acetylide intermediate formed undergo nucleophilic addition with the iminium ion generated in situ from aldehyde and secondary amine and gave the corresponding propargylamines. The catalyst was easily recoverable and can be used up to at least five cycles without significant loss in catalytic activity.
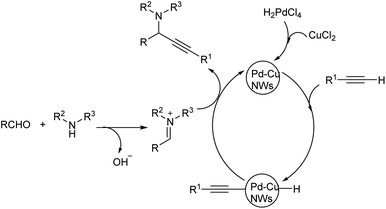 | ||
| Scheme 39 Plausible mechanism of A3 coupling reaction catalyzed by Pd–Cu NWs (reproduced with permission from ref. 78). | ||
6 Synthesis of chiral propargylamines
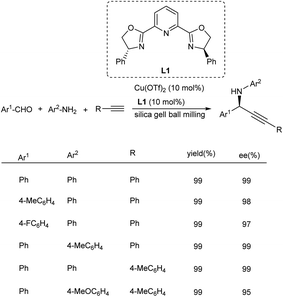
Propargylamines being a unique class of compounds have achieved great significance in organic synthesis. Efforts have been made to synthesize chiral propargylamines in its enantiomerically pure form. There is a considerable amount of development in asymmetric synthesis of propargylamines.79 Some of the solvent-free methodologies for the synthesis of chiral propargylamines are discussed here.Cu(OTf)2/Ph-Pybox catalyzed synthesis of chiral propargylamines was established by Su and co-workers in 2015.80 This protocol involves the reaction between aldehydes, alkynes and amines under solvent-free ball milling condition for 60 min (Scheme 40). Aldehydes with electron-donating and electron-withdrawing substituents participated well in his reaction and afforded the desired products in good yields.
A novel methodology for the synthesis of tetrasubstituted propargylamine using copper(I) chloride was successfully established by Periasamy and co-workers.81 Highly diastereomerically pure chiral propargylamines were achieved in moderate to good yields using optically active N-methyl camphanyl piperazine and 2-benzyl morpholine.
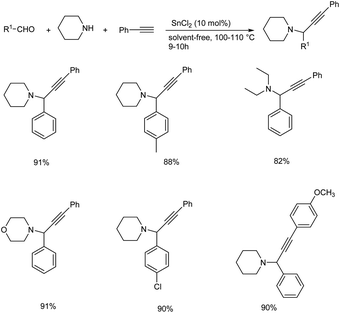
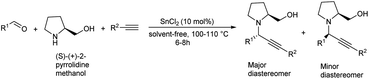
Tin(II) chloride catalyzed synthesis of propargylamines via three components A3 coupling was reported in 2016.82 A3 coupling reaction catalyzed by 10 mol% of SnCl2 under solvent-free condition at 110 °C gave the best result (Scheme 41). Chiral propargylamines were synthesized by incorporating chiral amine, (S)-(+)-2-pyrrolidinemethanol in A3 coupling reaction (Scheme 42). This method exhibits excellent diastereoselectivity.
Ortiz and co-workers synthesized a C–S-cycloaurated complex through tin(IV)–Au(III) transmetallation from the corresponding chlorodimethylstannyl derivative.83 Au(I) nanoparticles were used as a catalyst for the synthesis of propargylamines via A3 coupling. The use of 0.1 mol% of catalyst and heating the reaction mixture at 60 °C for 8 h was found to be the optimum condition. Here chiral propargylamines were synthesized by using (S)-2-(methoxymethyl)pyrrolidine as chiral reagent, with excellent diastereoselectivity.
7 Miscellaneous
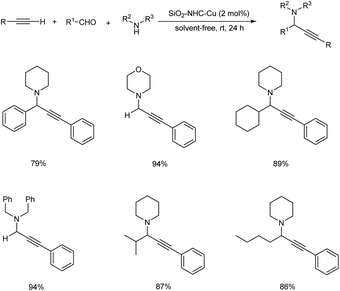
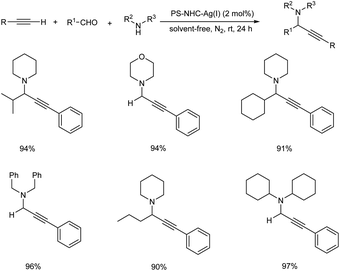
In 2008, Wang et al. established an efficient silica-immobilized NHC–CuI complex catalyzed three-component coupling reaction under solvent-free condition.84 They carried out the reaction using aliphatic aldehydes, alkynes and amines in the presence of 2 mol% of SiO2–NHC–CuI catalyst at room temperature under solvent-free condition (Scheme 43). The environmentally benign condition and reusability of this catalyst further enhanced the significance of this method.Later the same year, Wang et al. also developed a polystyrene–supported N-heterocyclic carbene–Ag(I) catalyst (PS–NHC–Ag(I)) for synthesizing propargylamines via A3 coupling.85 The optimised conditions were found to be the use of 2 mol% PS–NHC–Ag(I) catalyst under neat condition at room temperature (Scheme 44). Aromatic aldehydes containing both electron-donating and electron-withdrawing substituents gave good to excellent results. Aliphatic alkynes were less reactive than aromatic alkynes. The catalyst was recovered and reused up to 12 cycles without loss in catalytic activity.
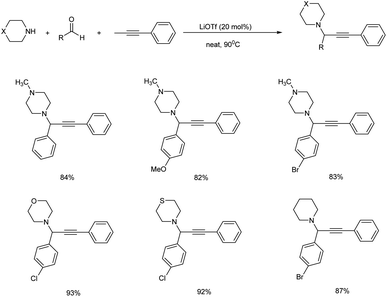
Jeong and co-workers used lithium triflate (LiOTf) for catalysing A3 coupling reaction of aldehyde, secondary alicyclic amine and alkyne for the synthesis of propargylamines under solvent-free conditions.86 The optimization studies showed that 20 mol% of the catalyst at 90 °C under neat conditions provided the best result (Scheme 45). The reaction proceeds through C–H bond activation of the terminal alkynes.
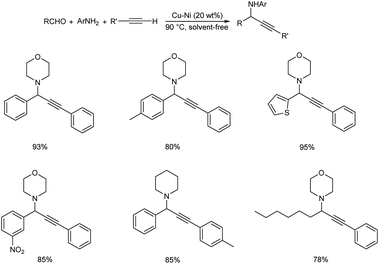
A reusable Cu–Ni bimetallic catalyst was synthesized by Jayaram et al.87 The bimetallic catalyst afforded propargylamines through multicomponent reaction of aldehydes, secondary amines and phenylacetylene by C–H activation (Scheme 46). Best results were obtained under solvent-free conditions at 90 °C with 20 wt% of Cu–Ni. The catalyst was magnetically recovered and reused up to five times without considerable loss in catalytic performance.
In 2018, Cirujano et al. developed a methodology in which MOF-derived metal oxide cluster in porous aluminosilicates were used for the synthesis of bioactive aza-heterocycles. Propargylamines were synthesized through multi component reaction between alkynes, aldehydes and amines.88
In 2019, Koukabi et al. synthesized fibroin-functionalized magnetic carbon nanotube supported silver nanoparticles as biocatalyst. Propargylamines were synthesized by A3 coupling of aldehydes and amines with phenylacetylene.89 Reactions carried out at 80 °C for 2 h under solvent-free conditions with 0.02 g CNT–Fe3O4–fibroin–Ag as catalyst was found to be optimum (Scheme 47). The catalyst was retrieved eight times without losing the catalytic activity.
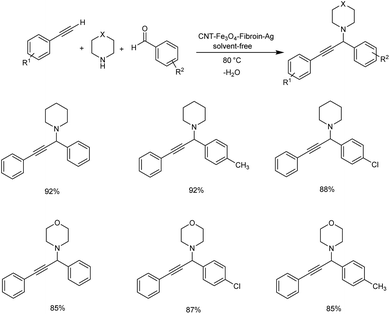 | ||
| Scheme 47 Synthesis of propargylamines catalyzed by CNT–Fe3O4–fibroin–Ag under solvent-free conditions. | ||
Hydroxylated propargylamines were synthesized by decarboxylative A3 coupling of ortho-hydroxy benzaldehydes, secondary amines and alkynoic acids under metal and solvent-free conditions (Scheme 48).90 Utility of recyclable chitosan-supported copper catalyst was explored. This catalyst eliminated the necessity for a N2 atmosphere and reaction proceeded well without forming any homocoupling product. The reaction was performed using equimolar amounts ortho-hydroxy benzaldehyde, morpholine and phenylpropiolic acid at 140 °C. Good yields were obtained with ortho-hydroxy benzaldehydes bearing electron-releasing and electron-with drawing substituents. Reaction did not work when ortho-hydroxy benzaldehyde was replaced by 2-hydroxy acetophenone. Primary amines failed to give the desired product. The reaction conditions were found to be selective with secondary amines. A gram scale synthesis of ortho-hydroxy propargylamines was performed for demonstrating reproducibility and commercial viability. It was divulged through mechanistic investigations that the reaction proceeds via Eschweiler–Clarke type of decarboxylation of the in situ generated ortho quinonoid intermediate. This methodology provides a green alternative to the contemporary metal catalyzed synthesis of ortho-hydroxy propargylamines.
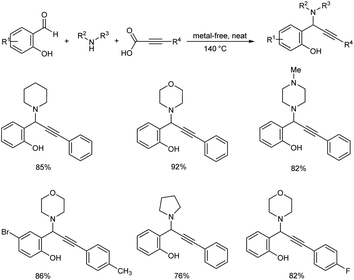 | ||
| Scheme 48 Synthesis of propargylamines by decarboxylative A3 coupling under metal and solvent-free conditions. | ||
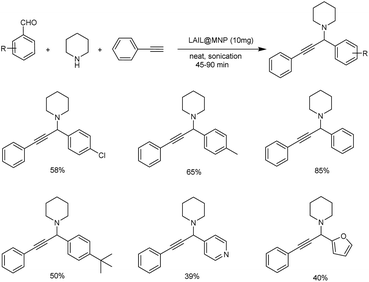
In 2020, protocols for one-pot synthesis polyhydroquinolines and propargylamines using nano-sized Fe3O4-supported Lewis acid ionic liquid catalyst (LAIL@MNP) was established. Here, one-pot multicomponent reaction under solvent-free sonication was used for the synthesis of propargylamines.91 Under solvent-free ultra sound irradiation phenylacetate, piperidine and aldehyde along with 10 mg LAIL@MNP as catalyst afforded the corresponding propargylamines in excellent yields (Scheme 49). The catalyst was recovered and reused for up to five cycles without considerable degradation in catalytic activity.
8 Conclusions
This review summarises the recent developments in solvent-free synthesis of propargylamines. Since propargylamines have wide range of applications in pharmaceutical field, a greener approach for its synthesis is of great importance. Nowadays, scientific community are more interested in developing environmentally benign synthetic strategies. Solvent-free method has become an emerging greener approach in organic synthesis and much work has been ongoing in this field.Most of the reported works for the synthesis of propargylamines involve A3 coupling of aldehydes, alkynes and amines. Majority of the synthesis of propargylamines under solvent-free conditions are catalyzed by transition metals and most of the catalysts were copper based. A greener approach could be provided if the reactions were metal free. Metal-free synthesis of propargylamines and metal-free along with a solvent-free pathway and KA2 coupling reaction for propargylamines are yet to be explored.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
TA thanks the Council of Scientific and Industrial Research (CSIR), New Delhi for the award of junior research fellowship. GA thanks the Kerala State Council for Science, Technology & Environment (KSCSTE), Trivandrum for a research grant (No. 341/2013/KSCSTE dated 15.03.2013).References
- K. Lauder, A. Toscani, N. Scalacci and D. Castagnolo, Chem. Rev., 2017, 117, 14091–14200 CrossRef CAS PubMed
.
- A. A. Boulton, B. A. Davis, D. A. Durden, L. E. Dyck, A. V. Juorio, X. M. Li, I. A. Paterson and P. H. Yu, Drug Dev. Res., 1997, 42, 150–156 CrossRef CAS
.
- J. Marco-Contelles, M. Unzeta, I. Bolea, G. Esteban, R. R. Ramsay, A. Romero, R. Martínez-Murillo, M. C. Carreiras and L. Ismaili, Front. Neurosci., 2016, 10, 1–7 Search PubMed
.
- J. J. Chen, D. M. Swope and K. Dashtipour, Clin. Ther., 2007, 29, 1825–1849 CrossRef CAS PubMed
.
- W. Maruyama, M. B. H. Youdim and M. Naoi, Ann. N. Y. Acad. Sci., 2001, 939, 320–329 CrossRef CAS PubMed
.
- A. A. Boulton, Mech. Ageing Dev., 1999, 111, 201–209 CrossRef CAS PubMed
.
- H. L. Yang, P. Cai, Q. H. Liu, X. L. Yang, F. Li, J. Wang, J. J. Wu, X. B. Wang and L. Y. Kong, Eur. J. Med. Chem., 2017, 138, 715–728 CrossRef CAS PubMed
.
- E. P. Cai, Y. Ishikawa, W. Zhang, N. C. Leite, J. Li, S. Hou, B. Kiaf, J. Hollister-Lock, N. K. Yilmaz, C. A. Schiffer, D. A. Melton, S. Kissler and P. Yi, Nat. Metab., 2020, 2, 934–945 CrossRef CAS PubMed
.
- S. Deshwal, M. Forkink, C. H. Hu, G. Buonincontri, S. Antonucci, M. Di Sante, M. P. Murphy, N. Paolocci, D. Mochly-Rosen, T. Krieg, F. Di Lisa and N. Kaludercic, Cell Death Differ., 2018, 25, 1671–1685 CrossRef CAS PubMed
.
- Y. He, Y. Zhao, L. Wang, L. R. Bohrer, Y. Pan, L. Wang and H. Huang, Oncogene, 2018, 37, 534–543 CrossRef CAS PubMed
.
- K. Milne, J. Sun, E. A. Zaal, J. Mowat, P. H. N. Celie, A. Fish, C. R. Berkers, G. Forlani, F. Loayza-Puch, C. Jamieson and R. Agami, Bioorg. Med. Chem. Lett., 2019, 29, 2626–2631 CrossRef CAS PubMed
.
- E. Cereda, R. Cilia, M. Canesi, S. Tesei, C. B. Mariani, A. L. Zecchinelli and G. Pezzoli, J. Neurol., 2017, 264, 1254–1263 CrossRef CAS PubMed
.
- O. Weinreb, T. Amit, O. Bar-Am, O. Chillag-Talmor and M. B. H. Youdim, Ann. N. Y. Acad. Sci., 2005, 1053, 348–355 CrossRef CAS PubMed
.
- P. L. McCormack, CNS Drugs, 2014, 28, 1083–1097 CrossRef CAS PubMed
.
- N. R. Bali and P. S. Salve, Int. J. Biol. Macromol., 2020, 164, 1006–1024 CrossRef CAS PubMed
.
- O. Weinreb, S. Mandel, O. Bar-Am, M. Yogev-Falach, Y. Avramovich-Tirosh, T. Amit and M. B. H. Youdim, Neurotherapeutics, 2009, 6, 163–174 CrossRef CAS PubMed
.
- K. Magyar, Int. Rev. Neurobiol., 2011, 100, 65–84 CrossRef CAS PubMed
.
- É. Szökő, T. Tábi, P. Riederer, L. Vécsei and K. Magyar, J. Neural Transm., 2018, 125, 1735–1749 CrossRef PubMed
.
- S. Pålhagen, E. Heinonen, J. Hägglund, T. Kaugesaar, O. Mäki-Ikola and R. Palm, Neurology, 2006, 66, 1200–1206 CrossRef PubMed
.
- C. U. Pae, H. K. Lim, C. Han, A. Neena, C. Lee and A. A. Patkar, Prog. Neuro-Psychopharmacol. Biol. Psychiatry, 2007, 31, 1153–1163 CrossRef CAS PubMed
.
- S. Akhondzadeh, R. Tavakolian, R. Davari-Ashtiani, F. Arabgol and H. Amini, Prog. Neuro-Psychopharmacol. Biol. Psychiatry, 2003, 27, 841–845 CrossRef CAS PubMed
.
- S. Arshadi, E. Vessally, M. Sobati, A. Hosseinian and A. Bekhradnia, J. CO2 Util., 2017, 19, 120–129 CrossRef CAS
.
- V. A. Peshkov, O. P. Pereshivko, A. A. Nechaev, A. A. Peshkov and E. V. Van Der Eycken, Chem. Soc. Rev., 2018, 47, 3861–3898 RSC
.
- E. Vessally, A. Hosseinian, L. Edjlali, A. Bekhradnia and M. D. Esrafili, RSC Adv., 2016, 6, 99781–99793 RSC
.
- S. Tong, Q. Wang, M.-X. Wang and J. Zhu, Angew. Chem., 2015, 127, 1309–1313 CrossRef
.
- Y. Wang, M. Mo, K. Zhu, C. Zheng, H. Zhang, W. Wang and Z. Shao, Nat. Commun., 2015, 6, 2–10 Search PubMed
.
- E. Vessally, RSC Adv., 2016, 6, 18619–18631 RSC
.
- E. Vessally, L. Edjlali, A. Hosseinian, A. Bekhradnia and M. D. Esrafili, RSC Adv., 2016, 6, 49730–49746 RSC
.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC
.
- I. T. Horváth, Green Chem., 2008, 10, 1024–1028 RSC
.
- P. J. Dunn, Chem. Soc. Rev., 2012, 41, 1452–1461 RSC
.
- R. A. Gross, J. Gu, D. Eberiel, S. P. McCarthy, M. Poliakoff, J. Michael Fitzpatrick, T. R. Farren and P. T. Anastas, J. Polym. Sci., Polym. Lett. Ed., 1995, 32, 193 Search PubMed
.
- C. J. Li and B. M. Trost, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 13197–13202 CrossRef CAS PubMed
.
- C. J. Li, Green Chem., 2008, 10, 151–152 RSC
.
- M. H. Haider, N. F. Dummer, D. W. Knight, R. L. Jenkins, M. Howard, J. Moulijn, S. H. Taylor and G. J. Hutchings, Nat. Chem., 2015, 7, 1028–1032 CrossRef CAS PubMed
.
- R. V. Jagadeesh, H. Junge and M. Beller, Nat. Commun., 2014, 5, 1–8 Search PubMed
.
- J. B. Zimmerman, P. T. Anastas, H. C. Erythropel and W. Leitner, Science, 2020, 367, 397–400 CrossRef CAS PubMed
.
- X. A. Liang, L. Niu, S. Wang, J. Liu and A. Lei, Org. Lett., 2019, 21, 2441–2444 CrossRef CAS PubMed
.
- H. Tian, H. Yang, C. Tian, G. An and G. Li, Org. Lett., 2020, 22, 7709–7715 CrossRef CAS PubMed
.
- M. Zhang, L. Yang, H. Yang, G. An and G. Li, ChemCatChem, 2019, 11, 1606–1609 CrossRef CAS
.
- A. Jordan, P. Stoy and H. F. Sneddon, Chem. Rev., 2021, 121, 1582–1622 CrossRef CAS PubMed
.
- P. T. Anastas and M. M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686–694 CrossRef CAS PubMed
.
- S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275–3279 CrossRef CAS PubMed
.
- L. P. Zorba and G. C. Vougioukalakis, Coord. Chem. Rev., 2021, 429, 213603 CrossRef CAS
.
- S. Ghosh and K. Biswas, RSC Adv., 2021, 11, 2047–2065 RSC
.
- C. J. Li and C. Wei, Chem. Commun., 2002, 2, 268–269 RSC
.
- P. Li and L. Wang, Tetrahedron, 2007, 63, 5455–5459 CrossRef CAS
.
- W. Q. Du, J. M. Zhang, R. Wu, Q. Liang and S. Z. Zhu, J. Fluorine Chem., 2008, 129, 695–700 CrossRef CAS
.
- M. K. Patil, M. Keller, B. M. Reddy, P. Pale and J. Sommer, Eur. J. Org. Chem., 2008, 26, 4440–4445 CrossRef
.
- M. J. Aliaga, D. J. Ramón and M. Yus, Org. Biomol. Chem., 2010, 8, 43–46 RSC
.
- M. J. Albaladejo, F. Alonso, Y. Moglie and M. Yus, Eur. J. Org. Chem., 2012, 2012, 3093–3104 CrossRef CAS
.
- X. Xiong, H. Chen and R. Zhu, Catal. Commun., 2014, 54, 94–99 CrossRef CAS
.
- Z. L. Palchak, D. J. Lussier, C. J. Pierce and C. H. Larsen, Green Chem., 2015, 17, 1802–1810 RSC
.
- M. Bakherad, A. Keivanloo, A. H. Amin, R. Doosti and O. Hoseini, Iran. J. Catal., 2016, 6, 325–332 CAS
.
- U. Chinna Rajesh, U. Gulati and D. S. Rawat, ACS Sustainable Chem. Eng., 2016, 4, 3409–3419 CrossRef CAS
.
- U. Gulati, U. C. Rajesh and D. S. Rawat, Tetrahedron Lett., 2016, 57, 4468–4472 CrossRef CAS
.
- G. Bosica and R. Abdilla, J. Mol. Catal. A: Chem., 2017, 426, 542–549 CrossRef CAS
.
- B. R. P. Reddy, P. V. G. Reddy, M. V. Shankar and B. N. Reddy, Asian J. Org. Chem., 2017, 6, 712–719 CrossRef CAS
.
- A. Shouli, S. Menati and S. Sayyahi, C. R. Chim., 2017, 20, 765–772 CrossRef CAS
.
- N. Gharibpour, M. Abdollahi-Alibeik and A. Moaddeli, ChemistrySelect, 2017, 2, 3137–3146 CrossRef CAS
.
- Z. Sotoudehnia, J. Albadi and A. R. Momeni, Appl. Organomet. Chem., 2019, 33, 1–8 CrossRef
.
- S. Yan, S. Pan, T. Osako and Y. Uozumi, ACS Sustainable Chem. Eng., 2019, 7, 9097–9102 CrossRef CAS
.
- P. Ramesh, M. S. Reddy, V. R. Madduluri, L. R. Chowan and N. S. Kumar, ChemistrySelect, 2019, 4, 9045–9049 CrossRef CAS
.
- F. Wang, H. Feng, H. Li, T. Miao, T. Cao and M. Zhang, Chin. Chem. Lett., 2020, 31, 1558–1563 CrossRef CAS
.
- P. V. Rathod, J. M. C. Puguan and H. Kim, Appl. Organomet. Chem., 2020, 34, 1–15 CrossRef
.
- W. Jiang, J. Xu, W. Sun and Y. Li, Appl. Organomet. Chem., 2021, 35, e6167 CAS
.
- K. V. V. Satyanarayana, P. A. Ramaiah, Y. L. N. Murty, M. R. Chandra and S. V. N. Pammi, Catal. Commun., 2012, 25, 50–53 CrossRef CAS
.
- P. B. Sarode, S. P. Bahekar and H. S. Chandak, Synlett, 2016, 27, 2209–2212 CrossRef CAS
.
- Z. Zarei and B. Akhlaghinia, RSC Adv., 2016, 6, 106473–106484 RSC
.
- S. Layek, B. Agrahari, S. Kumari, Anuradha and D. D. Pathak, Catal. Lett., 2018, 148, 2675–2682 CrossRef CAS
.
- D. B. Bankar, R. R. Hawaldar, S. S. Arbuj, M. H. Moulavi, S. T. Shinde, S. P. Takle, M. D. Shinde, D. P. Amalnerkar and K. G. Kanade, RSC Adv., 2019, 9, 32735–32743 RSC
.
- M. Cheng, Q. Zhang, X. Y. Hu, B. G. Li, J. X. Ji and A. S. C. Chan, Adv. Synth. Catal., 2011, 353, 1274–1278 CrossRef CAS
.
- M. Aliaga-Lavrijsen, R. P. Herrera, M. D. Villacampa and M. Concepción Gimeno, ACS Omega, 2018, 3, 9805–9813, DOI:10.1021/acsomega.8b01352
.
- K. Namitharan and K. Pitchumani, Eur. J. Org. Chem., 2010, 2010, 411–415 CrossRef
.
- S. N. Afraj, C. Chen and G. H. Lee, RSC Adv., 2014, 4, 26301–26308 RSC
.
- T. L. Da Silva, R. S. Rambo, D. D. S. Rampon, C. S. Radatz, E. V. Benvenutti, D. Russowsky and P. H. Schneider, Mol. Catal., 2015, 399, 71–78 CrossRef
.
- W. J. Sun, F. G. Xi, W. L. Pan and E. Q. Gao, Mol. Catal., 2017, 430, 36–42 CrossRef CAS
.
- R. Yi, Z. J. Wang, Z. Liang, M. Xiao, X. Xu and N. Li, Appl. Organomet. Chem., 2019, 33, 1–6 Search PubMed
.
- F. Zhao, S. Kim and D. Castagnolo, Propargylamines: Recent Advances in Asymmetric Synthesis and Use as Chemical Tools in Organic Chemistry, Wiely, 2021, pp. 103–154 Search PubMed
.
- Z. Li, Z. Jiang and W. Su, Green Chem., 2015, 17, 2330–2334 RSC
.
- M. Periasamy, P. O. Reddy, I. Satyanarayana, L. Mohan and A. Edukondalu, J. Org. Chem., 2016, 81, 987–999 CrossRef CAS PubMed
.
- S. N. Afraj and C. Chen, Asian J. Org. Chem., 2016, 5, 257–263 CrossRef CAS
.
- E. Belmonte Sánchez, M. J. Iglesias, H. El Hajjouji, L. Roces, S. García-Granda, P. Villuendas, E. P. Urriolabeitia and F. López Ortiz, Organometallics, 2017, 36, 1962–1973 CrossRef
.
- M. Wang, P. Li and L. Wang, Eur. J. Org. Chem., 2008, 49, 2255–2261 CrossRef
.
- P. Li, L. Wang, Y. Zhang and M. Wang, Tetrahedron Lett., 2008, 49, 6650–6654 CrossRef CAS
.
- S. D. Dindulkar, B. Kwan, K. T. Lim and Y. T. A. E. Jeong, J. Chem. Sci., 2013, 125, 101–107 CrossRef CAS
.
- S. V. Katkar and R. V. Jayaram, RSC Adv., 2014, 4, 47958–47964 RSC
.
- N. Martín, M. Dusselier, D. E. De Vos and F. G. Cirujano, ACS Catal., 2019, 9, 44–48 CrossRef
.
- P. Akbarzadeh and N. Koukabi, Appl. Organomet. Chem., 2020, 34, 1–16 Search PubMed
.
- P. Kaur, B. Kumar, K. K. Gurjar, R. Kumar, V. Kumar and R. Kumar, J. Org. Chem., 2020, 85, 2231–2241 CrossRef CAS PubMed
.
- H. T. Nguyen, V. A. Truong and P. H. Tran, RSC Adv., 2020, 10, 25358–25363 RSC
.
| This journal is © The Royal Society of Chemistry 2021 |