DOI:
10.1039/D1RA03289E
(Review Article)
RSC Adv., 2021,
11, 24254-24281
Preparation and application of layered double hydroxide nanosheets
Received
27th April 2021
, Accepted 21st June 2021
First published on 9th July 2021
Abstract
Layered double hydroxides (LDH) with unique structure and excellent properties have been widely studied in recent years. LDH have found widespread applications in catalysts, polymer/LDH nanocomposites, anion exchange materials, supercapacitors, and fire retardants. The exfoliated LDH ultrathin nanosheets with a thickness of a few atomic layers enable a series of new opportunities in both fundamental research and applications. In this review, we mainly summarize the LDH exfoliation methods developed in recent years, the recent developments for the direct synthesis of LDH single-layer nanosheets, and the applications of LDH nanosheets in catalyzing oxygen evolution reactions, crosslinkers, supercapacitors and delivery carriers.
1 Introduction
Layered double hydroxides (LDH), also known as hydrotalcite-like anionic clay, are a class of ionic lamellar compounds consisting of positively charged brucite-like layers and charge compensating anions and solvation molecules in the interlayer region. Generally, the formula of LDH can be described as [MII1−xMIIIx(OH)2]x+(An−)x/n·mH2O, where MII represents bivalent metal cations, such as Mg2+, Zn2+ or Ni2+, and MIII represents trivalent metal, such as Al3+, Ga3+, Fe3+ or Mn3+, and x is the mole ratio of MIII/(MII + MIII), which is normally between 0.17 and 0.33,1 and subject to change for various applications. LDH can also contain M+ and M4+ cations, such as Li+ and Ti4+, but these are limited to specific examples. An− is the intercalated charge balancing anions.2–4
LDH have found widespread application in various fields, including photochemistry,5–12 adsorption,13,14 CO2 capture,15–20 drug delivery21–23 and fire retardant additives,24–29 however, the extent of their application as well as overall performance are extremely restricted by the fact that the unavoidable aggregation of the lamellar LDH flakes.
In the past decade, the synthesis of two-dimensional (2D) nanosheets of layered solids, such as metal chalcogenides,30 metal phosphates and phosphonates,31 layered metal oxides,32–35 as well as layered double hydroxide,36,37 have attracted tremendous attention. The 2D nanosheet, with a thickness of approximately one nanometer and a lateral size ranging from submicrometer to several tens of micrometers, possesses a higher specific surface area and more active sites, can be used for both fundamental studies and as a building block to fabricate a variety of functional materials.38 Exfoliation of LDH is an important route to produce positively charged thin platelets with a thickness of a few atomic layers, which can be used in series of fields. A prevailing example is as highly efficient oxygen evolution electrocatalysts.39–41 Today the synthesis of LDH nanosheets is of tremendous importance in both fundamental research and for the exponentially growing applications,42,43 such as catalysis, polymer composites, electronic materials, magnetic materials, and nanostructured materials.3 There are typically two categories of approaches to synthesize LDH nanosheets: top-down exfoliation and bottom-up direct synthesis methods. Exfoliation of layered materials is an interesting route for producing positively charged thin platelets, which typically constitutes a two-step process: the layered materials are first intercalated by macromolecule to increase the interlayer distance, and then the exfoliation step is developed to obtain exfoliated nanosheets. However, comparing with cationic clays such as lapinite® and montmorillonite which can be exfoliated into single layer sheets in aqueous suspension, LDH are more difficult to be exfoliated due to their high charge density and the high anion content. In addition, extensive interlamellar hydrogen bonding networks lead to a tight stacking of the lamellae. Therefore, new techniques need to be developed to successfully exfoliate LDH into nanosheets. While the bottom-up method is a relatively simple one-step process to directly prepare inorganic nanosheets from appropriate precursors.
LDH nanosheets synthesized by top-down exfoliation, such as in alcohols, toluene, polymers/monomers, CCl4, formamide, have been detailed discussed in previous reviews.3,44 In this review article, methods based on top-down exfoliation developed in recent years and methods to prepare LDH nanosheets based on bottom-up direct synthesis methods are discussed in detail, with a focus on the preparation mechanism. We also provide an overview of recent applications of LDH nanosheets, especially new applications in water splitting reactions, polymer nanocomposites, supercapacitors and drug delivery hosts.
2 Preparation of LDH nanosheets
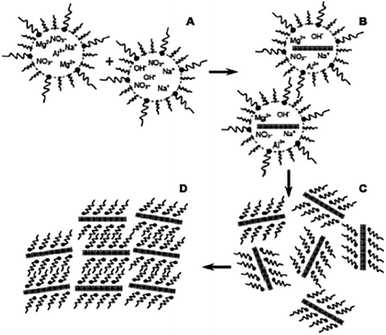
The exfoliation of LDH has always been a hot topic. Comparing with other layered inorganic compounds, LDH are difficult to be exfoliated due to their high layer charge density.45 In addition, the nanosheet dispersion obtained by exfoliation is unstable, and it is easy to self-assemble to restore the layered structure of LDH. Initially, LDH were exfoliated by inserting anionic organic guests (such as amino acids or surfactants) into the interlayer space and dispersing in a suitable solvent with the assistance of ultrasonication (Fig. 1).46 After that, exfoliation in aqueous solution by electrostatic repulsion was developed.47 Then, formamide was found as an ideal solvent, which made the direct exfoliation of LDH without being pre-intercalated by organic species in interlayer space was achieved. Presently, more and more efficient approaches to prepare LDH nanosheets were developed, such as laser ablation, the exfoliation process can be finished in several minutes. Traditional exfoliation approaches have been detailed discussed elsewhere,36,44 therefore, we will discuss the exfoliation approaches developed in recent years and the future of this area.
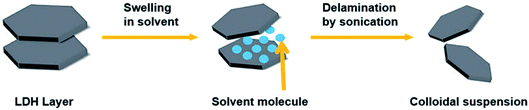 |
| | Fig. 1 A general process of exfoliating LDH.46 | |
2.1 Exfoliation or top down methods
2.1.1 Exfoliation in aqueous solution. Chen et al.48 proposed a method to prepare 2D ultrathin nanosheets of Co–Al LDH by exfoliating in aqueous solution of L-asparagine. Co–Al–CO3 LDH was pre-synthesized by a conventional coprecipitation method, after salt–acid treatment, the exfoliation of Co–Al LDH was performed: the mixture of LDH and L-asparagine saturated aqueous solution was mixed by ultrasonication, after vigorous oscillation, the unexfoliated particles was removed by centrifugation, and a translucent colloidal suspension of Co–Al LDH nanosheets was obtained. The TEM and SAED images indicated the exfoliated nanosheets were successfully obtained with intact micro-crystal structure (Fig. 2). The successful of the exfoliation was mainly due to that L-asparagine, which has a similar structure to formamide, is a water-soluble amino acid, its carbonyl groups had a strong interaction with LDH layers, and its amino groups made the interlayer interactions weakened, ultimately leaded to the exfoliation of the Co–Al LDH.
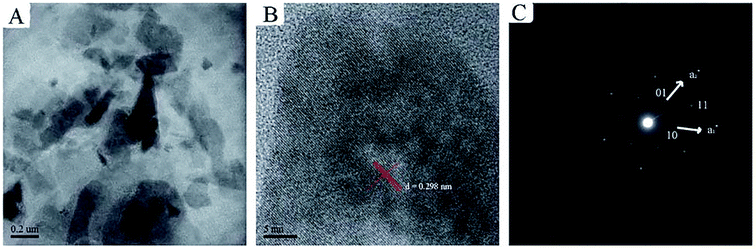 |
| | Fig. 2 (A) TEM image, (B) HRTEM image and (C) SAED pattern of the exfoliated Co–Al LDH nanosheets.48 Reproduced with permission from ref. 48. Copyright 2013. The Royal Society of Chemistry. | |
Zhang et al.49 reported a new strategy to exfoliate inorganic LDH in aqueous solution by use of zwitterionic surfactants. The zwitterionic surfactants (sodium capryloamphoacetate (SC) and sodium lauroamphoacetate (SL)) were firstly intercalated into the decarbonated LDH by ion exchange, then a certain amount of surfactant-intercalated LDH was dispersed in acid solution with initial pH values of 2.0 and the mixture was stirred at room temperature for 2 days. The yield for the exfoliated MgAl-SC-LDH and MgAl-SL-LDH were 48% and 49% respectively. The AFM images (Fig. 3) indicated that, for the MgAl-SC-LDH, the average thickness was 0.721–1.433 nm, for the MgAl-SL-LDH the average thickness was 0.871–2.194 nm, which proved the surfactant intercalated LDH were successfully exfoliated into nanosheets. The authors believed that the electrostatic repulsion was the main factor that affects the exfoliation. The zwitterionic surfactants possess positively and negatively charged head groups simultaneously, there is an isoelectric point or isoelectric area within a certain pH or pH range, at the isoelectric point or isoelectric area the anionic and cationic groups can neutralize each other.50 Above the isoelectric point, the zwitterionic exhibits an anionic character, SC or SL can be intercalated into LDH by ion exchange, when below the isoelectric point, the SC or SL converts to cationic species, resulting the exfoliation of LDH by electrostatic repulsion.
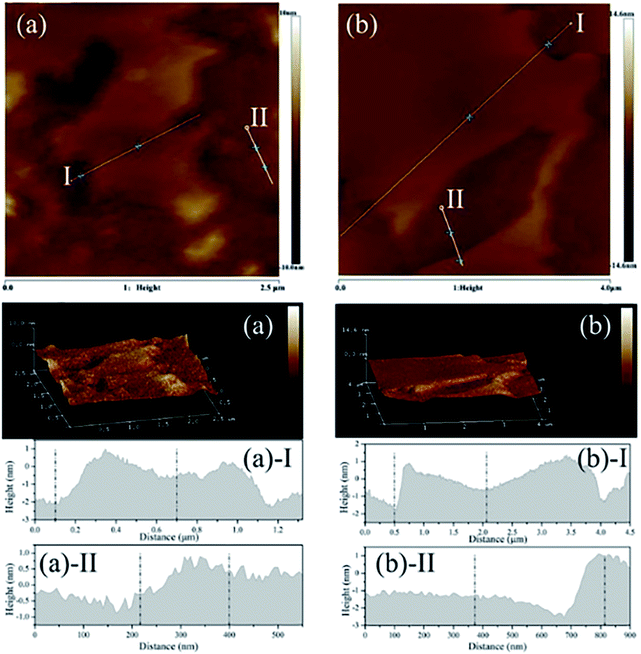 |
| | Fig. 3 The AFM image, 3D surface image and height profile of exfoliated LDH. (a) MgAl-SC-LDH; (b) MgAl-SL-LDH. Reproduced with permission from ref. 49. Copyright 2019. Taylor & Francis Group, LLC. | |
2.1.2 Exfoliation at low temperatures. Wei et al.51 developed a novel and efficient method for the exfoliation of Zn/Al–Cl−–LDH using NaOH/urea aqueous solution at low temperatures. This work was mainly taking advantage of that the alkali and urea aqueous solution can dissolve cellulose with strong inter- and intra-molecular hydrogen bonding at low temperatures,52,53 the structure of LDH and cellulose has much in common, thus the author believed that the hydrogen bond and lamellar structure of LDH could also be destroyed using NaOH/urea aqueous solution at low temperatures. In their work, a series of NaOH/urea aqueous solution with 6–9 wt% NaOH and 10–15 wt% urea concentration were prepared at room temperature and precooled to −10 °C, then a certain amount of the pre-prepared Zn–Al LDH was added to the mixed solution and stirred vigorously for 3 min. After removing the not fully exfoliated LDH by centrifugation, the exfoliated Zn–Al LDH colloidal suspension was obtained. The thickness of the obtained LDH nanosheets was ca. 0.6 nm, which corresponding to the thickness of single-layer LDH. The further study of the mechanism demonstrated that the exfoliation of LDH in NaOH/urea aqueous solution was because of the formation of hydrates in alkali and urea solution at low temperature. As shown in Fig. 4, the NaOH hydrate intercalated into the LDH interlayer and attached onto the host layers, the original hydrogen bond network between the LDH layers was broken and form a new hydrogen bond network between the hydroxyl groups on the LDH layers and NaOH hydrate. Urea hydrates can easily self-assembled at the surface of the NaOH hydrogen-bonded host layers of LDH to stabilize the LDH nanosheets suspension and preventing the aggregation. The combination of the NaOH and urea solution destroyed the original hydrogen bond network between the LDH layers, leading the rapid exfoliation of LDH.
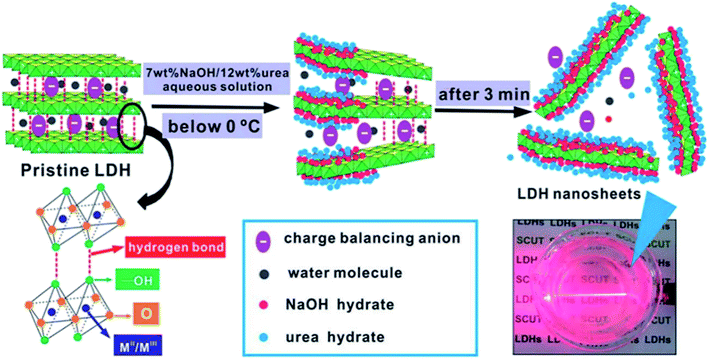 |
| | Fig. 4 The schematic diagram of delamination of LDH in precooled NaOH/urea aqueous solution.51 Reproduced with permission from ref. 51. Copyright 2014 The Royal Society of Chemistry. | |
2.1.3 Dry exfoliation. Although the liquid exfoliation can obtain single layer nanosheets, it also has certain defects, such as time consuming, expensive, and requires toxic compounds. Furthermore, the solvent molecules used in the exfoliation often adsorbed on the surface of the as-exfoliated LDH nanosheets, and once the solvent molecules have been removed to obtain the powder products, the LDH nanosheets prone to restack into bulk LDH, which limited their further application. In 2017, Wang et al.54 developed a novel method called “dry exfoliation” to obtain ultrathin CoFe LDH nanosheets. The bulk CoFe LDH with a molar ratio of Co/Fe 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were pre-synthesized by a hydrothermal method, then they were exfoliated into ultrathin LDH nanosheets by Ar plasma etching, which destroys the ionic bonds and hydrogen bonds in the interlayers of the bulk LDH, disturbing the normal charge balance and separating the positively charged brucite-like host layers from each other.
1 were pre-synthesized by a hydrothermal method, then they were exfoliated into ultrathin LDH nanosheets by Ar plasma etching, which destroys the ionic bonds and hydrogen bonds in the interlayers of the bulk LDH, disturbing the normal charge balance and separating the positively charged brucite-like host layers from each other.SEM, TEM and AFM results (Fig. 5 and 6) indicated that the pristine CoFe LDH had multilayer structure with a lateral size of 50–300 nm and a thickness of 20.6 nm. The X-ray diffraction (XRD) patterns shown that all of the diffraction peaks of the bulk CoFe LDH could be well indexed to JCPDS No. 50-0235 (Fig. 6D), which demonstrated that the bulk CoFe LDH have been synthesized successfully. The interlayer distance of the bulk CoFe LDH is 7.6 Å, this confirmed that carbonate ions were present between the layers. After treating with Ar plasma etching for 60 min, the ultrathin CoFe LDH nanosheets, marked as CoFe LDH-Ar, were produced. SEM, TEM and AFM results showed that the CoFe LDH-Ar preserved the nanosheet structure with a thickness of 0.6 nm (corresponding to the thickness of a monolayer), which demonstrated that the bulk CoFe LDH have been successfully exfoliated by the Ar plasma etching, which should destroy the ionic bonds in the interlayers of the bulk CoFe LDH. The XRD characterization (Fig. 6D) showed that the diffraction peaks corresponding to the (003) and (006) planes of CoFe LDH-Ar disappeared, which also indicated the successful exfoliation of bulk CoFe LDH into ultrathin CoFe LDH nanosheets.
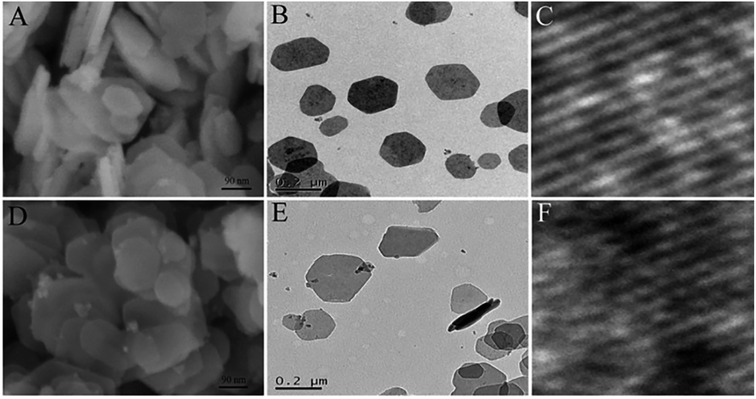 |
| | Fig. 5 SEM, TEM and HRTEM images of the bulk CoFe LDH (A–C) and the ultrathin CoFe LDH-Ar nanosheets (D–F). Reproduced with permission from ref. 54. Copyright 2017 John Wiley & Sons, Inc. | |
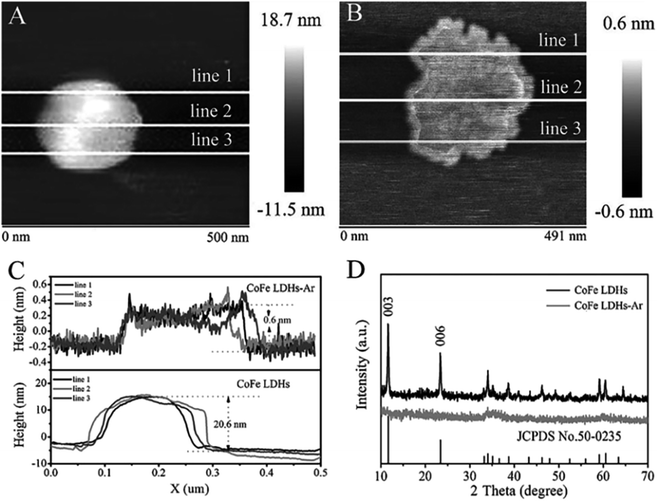 |
| | Fig. 6 AFM images of (A) bulk CoFe LDH and (B) ultrathin CoFe LDH-Ar nanosheets. (C) The corresponding height curves and (D) XRD patterns of the bulk CoFe LDH and ultrathin CoFe LDH-Ar nanosheets. Reproduced with permission from ref. 54. Copyright 2017 John Wiley & Sons, Inc. | |
Compared with liquid exfoliation, dry exfoliation has many advantages, such as clean, time-saving, non-toxic, and could avoid the adsorption of solvent molecules, it provided a new way to exfoliate 2D materials and provided more opportunities for the discovery of novel applications for LDH-based materials.
2.2 Direct synthesis methods
Bottom-up direct synthesis method is an excellent route to synthesis of LDH nanosheets, its process is relatively simple and there is no need to pre-synthesis of layered LDH. At present, the methods for directly synthesizing LDH nanosheets are mainly chemical approaches, such as creating “microreactors” utilizing the microemulsion method and mechanical methods, inhibiting the growth of layers by a layer growth inhibitor, washing the solid obtained from traditional coprecipitation method with aqueous miscible organic solvent, synthesizing directly in organic solvents, and mechanical methods, such as applying a laser beam on metals in aqueous solution, establishing a quick mixing environment using a special reactor, grinding nitrate in a suitable solvent.
2.2.1 Chemical methods. Hu et al.55 reported a facile one-step synthesis of LDH monolayers in a reverse microemulsion in 2005. In their work, the traditional aqueous co-precipitation system (magnesium salt and aluminum salt at pH ≥ 10) was adopted into an oil phase of isooctane with sodium dodecyl sulfate (SDS) as surfactant and 1-butanol as co-surfactant to prepare LDH monolayers. The synthesis process is shown in Fig. 7. The pre-prepared aqueous phase was dispersed into the oil phase to obtain droplets surrounded by dodecyl sulfate groups (DDS). And these droplets acted as nano-reactors to provide limited space and nutrients for the growth of LDH platelets, as a result, the size of the LDH platelets was controlled both in diameter and thickness.
 |
| | Fig. 7 Schematic representation of the nucleation and growth of LDH platelets. Reproduced with permission from ref. 55. Copyright 2006 The Royal Society of Chemistry. | |
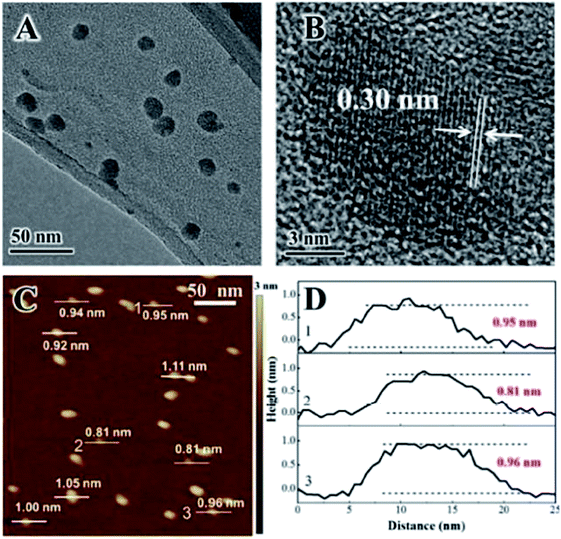
AFM was applied to characterize the obtain LDH nanosheets.55 The typical topology of isolated oval objects is presented in Fig. 8a, we can see that all the particles have a uniform diameter distribution centered around 40 nm. Fig. 8b shows that all these particles have a height of ca. 1.5 nm. The thickness of Mg2Al-LDH single layer is 0.47 nm,56–58 thus, the particles shown in Fig. 8 are corresponding to three mono-layers without considering the interlayer spacing expanded by the charge-balancing DDS anions. However, the elemental analysis result indicated the presence of the DDS anions on the LDH platelets, combining the basal spacing of DDS intercalated LDH, it can be concluded that the LDH layers formed in the reverse microemulsion have a mono-layer structure.
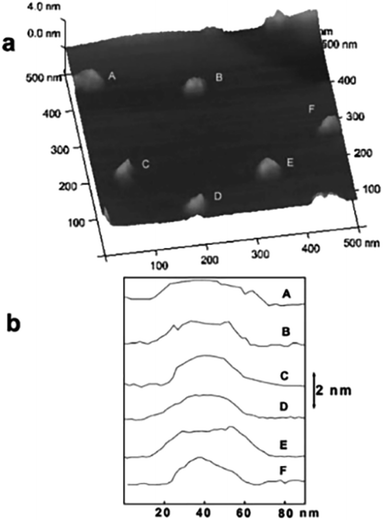 |
| | Fig. 8 (a) AFM image of the particles synthesized by a reverse micro-emulsion method deposited on a highly oriented pyrolytic graphite (HOPG) surface; (b) cross-sectional analysis of the labeled particles in (a) showing the dimensional profiles. Reproduced with permission from ref. 55. Copyright 2006 The Royal Society of Chemistry. | |
In the above synthesis method, anionic surfactants are often difficult to elute as counter anions adsorbed on the surface of LDH nanosheets, thus, in order to avoid the complex purification process, Bellezza et al.59 developed a cationic surfactant based reverse micro-emulsion method to prepare LDH nanosheets, avoiding the removal of surfactant.
In 2001, Yan et al.60 developed a novel single-step method to prepare large scale LDH nanosheets. Mg(NO3)2·6H2O, Al(NO3)3·9H2O, and urea were dissolved in 100 mL 30% H2O2, then the mixture solution was transferred into a Teflon tube Teflon-lined autoclave and heated at 150 °C for 24 h. The suggested scheme of the synthetic process was illustrated in Fig. 9A, part of H2O2 decomposed into H2O and O2 when heated, with the rising of temperature the LDH gradually formed, authors believed that during the synthetic process, part of the H2O2 and O2 could be positioned in the interlayer of LDH. The rapid decomposition of H2O2 occurred after formulation of LDH, and the violent movement of O2 in the interlayers resulted the increase of the spacing between layers and the diminished of the electrostatic interaction between the layers, leading to the separation of LDH layers. Fig. 9B is the AFM image of the prepared nanosheets, the result showed that the average thickness of the nanosheets was about 1.44 nm, corresponding to the theoretical thickness of approximately two LDH layers (0.76 × 2 = 1.52 nm).
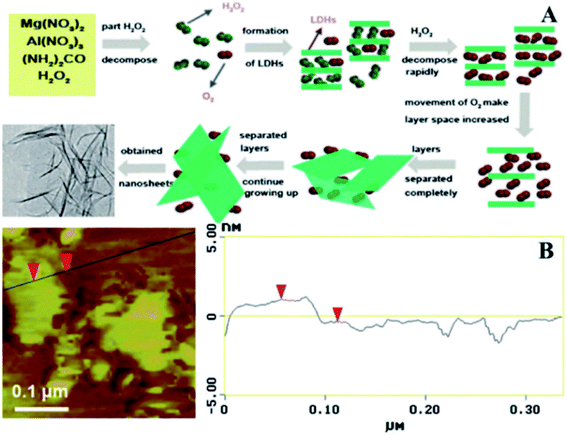 |
| | Fig. 9 (A) Proposed scheme for the preparation of exfoliated Mg2/Al–LDH nanosheets; (B) AFM image of the synthesized ultrathin sheets. Reproduced with permission from ref. 60. Copyright 2012 Elsevier Inc. | |
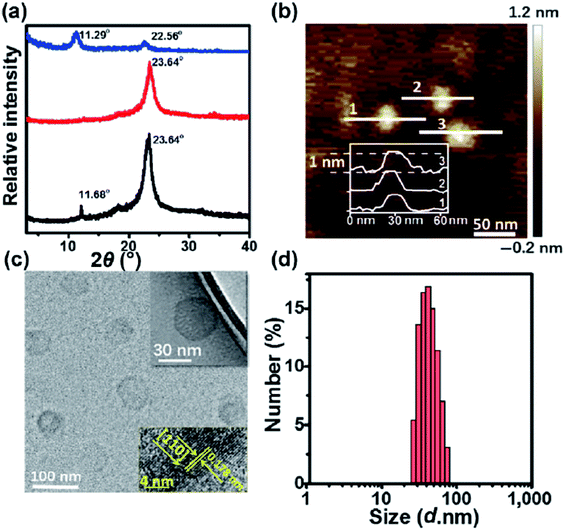
Wang et al.61 reported a facile method called aqueous miscible organic solvent treatment (AMOST) for the synthesis of LDH nanosheets. And what's interesting is that it is the first report of LDH powders that still remain exfoliated in the dry phase. Zn(NO3)2·6H2O and Al(NO3)3·9H2O solution were added dropwise to H3BO3 solution. The pH of the precipitation system was controlled at ca. 8.3 with NaOH solution. The mixture system was purged with N2 gas, followed by aging at 65 °C overnight. The obtained solid were washed with water until pH was close to 7. After that, the slurry washed by water was re-dispersed in acetone and stirred for 1 h at room temperature, then it was filtered and washed thoroughly with acetone again. At last the product was dried at 65 °C overnight. The structural changes of the samples were characterized by XRD (Fig. 10). The 00l (l = 3, 6, 9…) Bragg reflections of the conventional water washed samples could be observed obviously, while for the samples obtained by AMOST method, the 00l Bragg reflections disappeared but low intensity 012 and 110/113 Bragg reflections were observed, which indicated that the LDH were completely exfoliated.
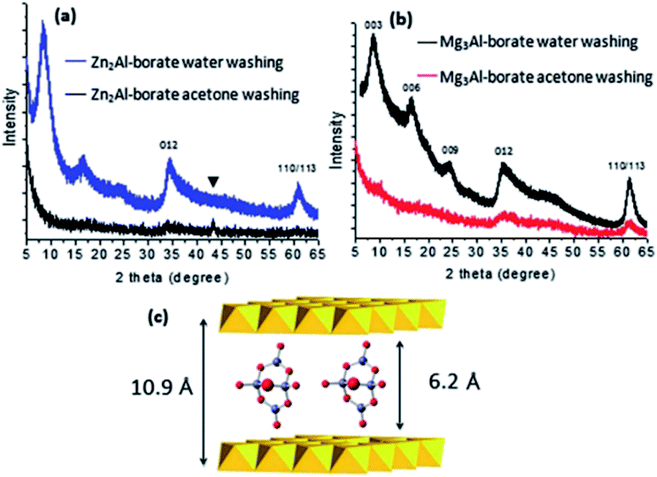 |
| | Fig. 10 (a) Comparison of the XRD patterns of Zn2Al-borate LDH synthesized by conventional co-precipitation (washing with water) and the AMOST method; (b) Mg3Al-borate LDH synthesized by conventional co-precipitation (washing with water) and the AMOST method; (c) schematic illustration of the structure of [B4O5(OH)4]2− with the LDH layers. Reflections from the sample holder. Reproduced with permission from ref. 61. The Royal Society of Chemistry. | |
The authors believed that the solvent used in the final dispersion was the key to control the hydrophobicity of the LDH platelets, and it must be 100% miscible with water. In the process of nucleation and growth of the LDH in water, the primary brucite-like metal hydroxide nanosheets were surrounded by charge compensating counter anions, and this arrangement was highly solvated by water molecules because of its high surface charge density. During the aging and washing steps, the LDH nanosheets combined with each other and stacked layer-by-layer. The water acted to both solvate the anions and also bond the individual metal hydroxide nanosheets together through extensive hydrogen bonding. When water molecules were replaced by acetone molecules, the LDH could be exfoliated and dispersed easily. Due to the low boiling point of acetone, the facile loss of interlayer acetone molecules lead to the ready collapse of an ordered stacked structure, and the formation of high surface porous materials with the specific surface areas and total pore volumes increased up to 458.6 m2 g−1 and 2.15 cm3 g−1.
Yu et al.62 reported a one-step direct synthesis method to synthesize LDH single-layer nanosheets by adding formamide (23 vol%) as an inhibitor. The traditional co-precipitation route was adopted with slight modified: a mixture solution (10 mL) composed of 0.040 M Mg(NO3)2 and 0.010 M Al(NO3)3 was added dropwise to the solution of NaNO3 (10.0 mL, 0.010 M) containing 23 vol% formamide, and NaOH (0.25 M) solution was added under magnetic stirring at 80 °C to adjust pH of the above-mentioned system at ca. 10. After 10 minutes, the obtained sample was centrifuged and washed three times with water, single layer LDH nanosheets were obtained by re-dispersing the slurry in water. During the synthesis process, formamide molecules were adhered to the LDH nanosheet surface, due to its preferred interaction with the LDH layer surface and high dielectric constant (which resulted the interactions between layers weakened), LDH single-layer nanosheets were prepared (Fig. 11a).
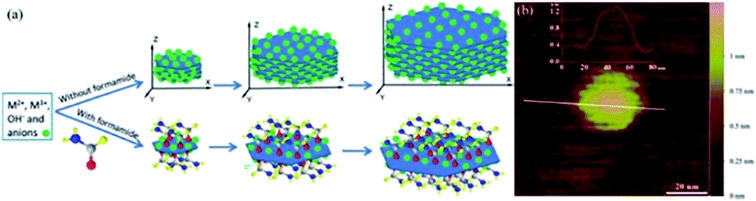 |
| | Fig. 11 (a) Direct growth of LDH single-layer nanosheets with the assistance of layer growth inhibitors (not drawn to scale); (b) AFM image of a pseudohexagonal Mg4/Al–LDH nanosheet.62 Reproduced with permission from ref. 62. Copyright 2015 The Royal Society of Chemistry. | |
The AFM image (Fig. 11b) demonstrated that the height of the prepared LDH single-layer nanosheets was ca. 0.8 nm, corresponding to the thickness of single-layer nanosheet with surface adsorbed anions and formamide molecules.
The authors further studied the effect of the concentration of formamide on the synthesis of LDH single layer nanosheets.63 The result demonstrated that LDH single layer could be better prepared with the increasing volume percentage of formamide in the reaction system. And LDH with higher layer charge was beneficial to the formation of LDH single-layer nanosheets (Fig. 12A and B). As shown in Fig. 12C and D, the single layer features of the LDH prepared in 30 vol% formamide could be observed clearly.
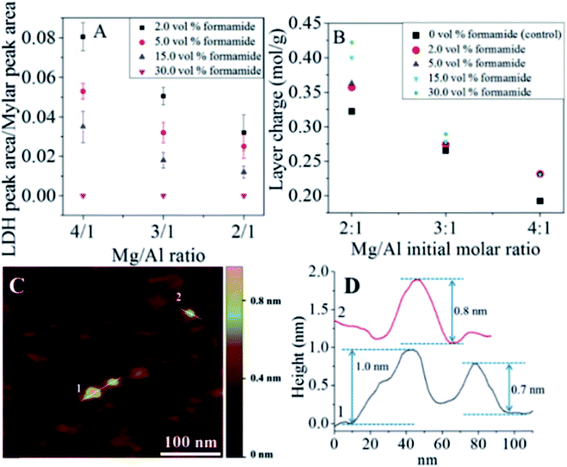 |
| | Fig. 12 (A) LDH characteristic peak to internal reference peak area ratios. (B) MgAl–LDH layer charge at different Mg/Al formulation molar ratios and formamide concentrations. (C) Representative AFM image of Mg2Al–LDH prepared in 30.0 vol% formamide and the corresponding height profile (D). Reproduced with permission from ref. 63. Copyright 2016 The American Chemistry Society. | |
The synthesis of single layer LDH with NO3− counterions has been extensively studied, however, the single layer LDH with CO32− counterions was rarely reported. In 2017, Li et al.64 first reported the simple direct synthesis of the CoAl–CO32− LDH single layer nanosheets in ethylene glycol. The synthesis process was shown in Fig. 13, a certain amount of Co(NO3)2·6H2O, Al(NO3)3·9H2O and urea were dissolved in 80 mL of ethylene glycol, and the obtained mixture solution was transferred into 100 mL Teflon-lined autoclave, following heated at 100 °C for 24 h. When preparing LDH with different Co/Al ratios, the total concentrations of metal ions and the urea concentrations were kept constant. The obtained suspension was naturally cooled to room temperature, and then centrifuged to get the sample, marked as LDH-EG. After that, the obtained LDH-EG was washed with ethanol (ET) and centrifuged for four times to get another sample, named as LDH-ET. The prepared LDH-ET sample was dried at 60 °C for 24 h and the solid sample, marked LDH-ET-D was obtained. Besides, the LDH-ET was dispersed into water by ultrasonication for 10 min and the aqueous colloids, marked as LDH-H2O, was gained, the solid content of the aqueous colloids was ∼3.87%. The solid sample, LDH-H2O-D, was obtained by drying the LDH-H2O colloid at 60 °C for 24 h. Additionally, the sample, marked as LDH-ET-D-H2O, was obtained by dispersing the LDH-ET-D in water and the sample, marked as LDH-ET-D-H2O-D, was obtained by drying the LDH-ET-D-H2O at 60 °C for 24 h.
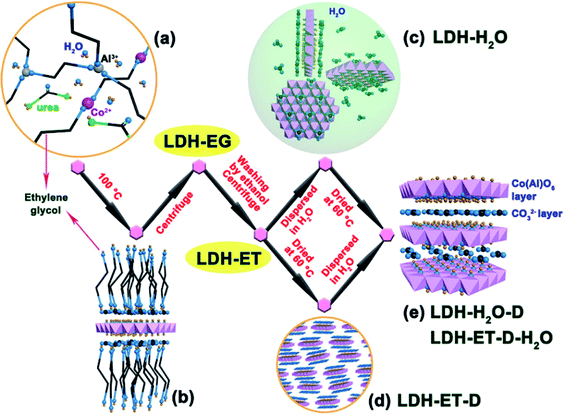 |
| | Fig. 13 Schematic illustration for formation and structure of CoAl–CO32− SL-LDH and CoAl–CO32− LDH: (a) ethylene glycol (EG) solution of reactants; (b) adsorption of EG molecules on the surface of a SL-LDH nanosheet in reaction; (c) completely dispersed SL-LDH in water; (d) disorderly stack of SL-LDH in the dried state; and (e) LDH assembly structure from generated SL-LDH. Reproduced with permission from ref. 64. Copyright 2017 The American Chemistry Society. | |
The XRD results (Fig. 14a) showed that, compared with LDH-H2O# (synthesized in water), the XRD patterns of LDH-EG, LDH-ET, LDH-H2O (gel, obtained by condensing the dilute colloid at 60 °C) and LDH-ET-D showed (110) and (012) but not (003) peaks, which indicated the presence of the single layer LDH. The AFM result (Fig. 14b) demonstrated that the single layer LDH nanosheets in LDH-H2O have a height of ∼0.85 ± 0.02 nm, corresponding to one Co(Al)O6 layer sandwiched between two CO32− layers. Authors believed that the successful preparation of CoAl–CO32− single layer LDH probably arises from three reasons: (1) EG has strong chelation to metal ions,65 which slowed the nucleation and growth of LDH crystals; (2) the growth along the [003] direction or assembly of the single layer LDH were prevented by the adsorption of EG molecules;66,67 and (3) strong electrostatic repulsions between CO32− layers and between Co(Al)O6 layers with much structurally positive charge due to the low Co/Al ratio resulted that the single layer LDHs difficult to approach to each other. In summary, the direct synthesis of CO32− LDH was reported for the first time and this work provided a simple method for the preparation of single layer LDH with CO32−.
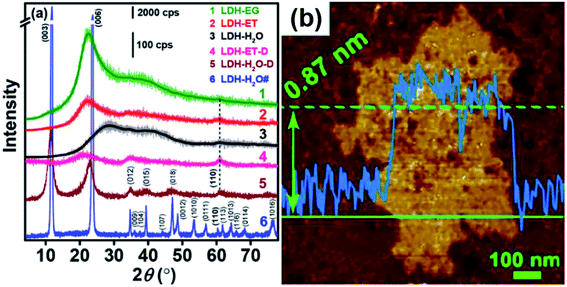 |
| | Fig. 14 (a) XRD patterns of different samples and (b) AFM image of LDH-H2O. Reproduced with permission from ref. 64. Copyright 2017 The American Chemistry Society. | |
2.3 Mechanical methods
A new method to prepare LDH nanosheets in de-ionized water by a pulsed-laser ablation method was reported by Hur et al.68 The Zn/Al, Co/Fe, Co/Al, and Mg/Fe–LDH colloidal nanosheets with a molecule thickness were obtained through this method. There are two consecutive steps in the laser ablation synthesis process: (1) laser ablation of trivalent metal target in de-ionized water at room temperature using a Q-switched Nd-yttrium aluminum garnet laser, and (2) the same laser ablation over a bivalent metal target in the pre-prepared trivalent metallic colloid. The power of the laser beam was 0.265 J cm−2 (1064 nm), the pulsed duration was 5.5 ns and the repetition rate was 10 Hz. The synthesis process took only 40 minutes. The TEM images (Fig. 15) showed that Mg/Fe and Co/Fe–LDH nanosheets displayed a rolling-and-folding morphology in the conjunction area, while the Co/Al and Zn/Al–LDH showed the edges intensively rolled. The thickness of the prepared LDH nanosheets were estimated by TEM, the Mg/Fe, Co/Fe, Co/Al, and Zn/Al–LDH nanosheets had a thickness of ca. 0.5, 0.48, 0.5, and 1.2 nm, respectively. Therefore, the laser ablation technique was deemed to have played a critical role in the formation of nanosheets.
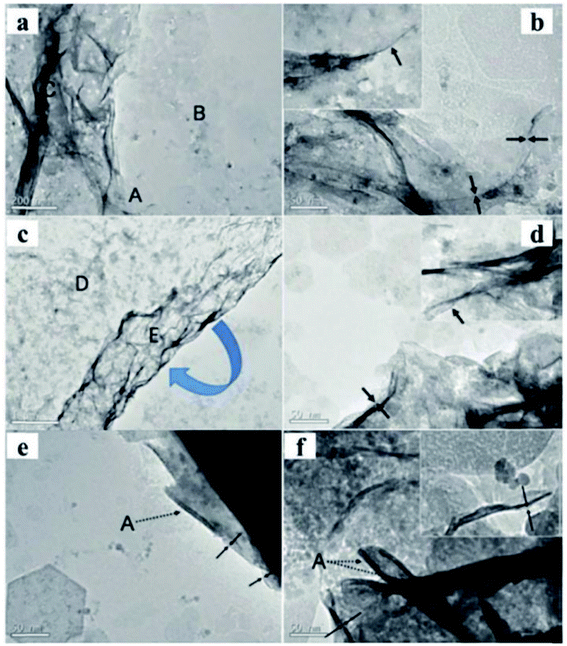 |
| | Fig. 15 TEM images of Mg1.97/Fe and Co0.96/Fe–LDH materials. Scale bars in the Mg1.97/Fe–LDH images, (a and b), represent 200 nm and 50 nm, respectively. Scale bars in the Co0.96/Fe–LDH images, (c and d), represent 1 mm and 50 nm, respectively. (e) Co1.07/Al and (f) Zn1.48/Al–LDH nanosheets. The dotted arrows marked by symbol “A” indicate the edges of the nanosheets seriously rolled because of the instability of the edges of the free-standing ultrathin LDH layers. Both scale bars represent 50 nm in (e and f). Insets in images (b, d, and f) display the corresponding side views. Reproduced with permission from ref. 68. Copyright 2010 American Institute of Physics. | |
Pang et al.69 adopted the traditional coprecipitation method in a T-type micro-channel reactor in water for the synthesis of LDH nanosheets. A mixed salt solution (contained Mg2+ and Al3+ cations) with total concentration of 0.3 mol L−1 and NH3·H2O solution (∼7 wt%) were prepared in advance. And then the prepared two solutions were pumped into the microreactor (each at a flow rate of 20 mL min−1) through two inlets. The AFM image (Fig. 16) indicated that the obtained LDH nanosheets have a diameter of 20–30 nm, and a thickness of ca. 0.68–1.13 nm, which corresponded to 1–2 brucite-like layers. This method is versatile for synthesizing various LDH nanosheets. The LDH nanosheets prepared by this method were in high quantities and could be used as building blocks for functional materials.
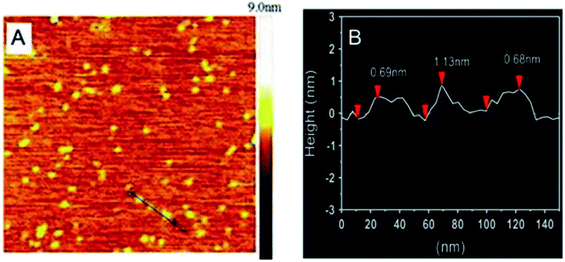 |
| | Fig. 16 (A) AFM image and height scale of the LDH nanosheets, and (B) sectional analysis along the black line marked in (A).69 Reproduced with permission from ref. 69. Copyright 2014 Elsevier Inc. | |
In 2016, our group developed a simple aqueous synthetic route to large-scale synthesis LDH single layer nanosheets.70 This route includes three steps: aqueous coprecipitation, water-washing, and redispersion in water. In a typical procedure, Mg(NO3)2/Al(NO3)3 mixed salt solution (100 mL) with a total salt concentration of 0.3 mol L−1 and Mg/Al molar ratio of 2 and NH3 H2O solution (∼7 wt%) were pre-prepared, then the two solution were added to a beaker simultaneously under stirring and N2 protection, during this period, the pH of the reaction system was kept at ∼10. After reaction for 10 min, the production was centrifuged and washed three times with water, and the LDH single layer nanosheets gel with a solid content (Cs) of ∼8.5 wt% was obtained. Then the gel was redispersed in water by ultrasonication and the LDH single layer nanosheets dispersion (Cs = 2.0 g L−1) was obtained. The TEM and AFM results showed that the LDH single layer nanosheets were successfully synthesized (Fig. 17).
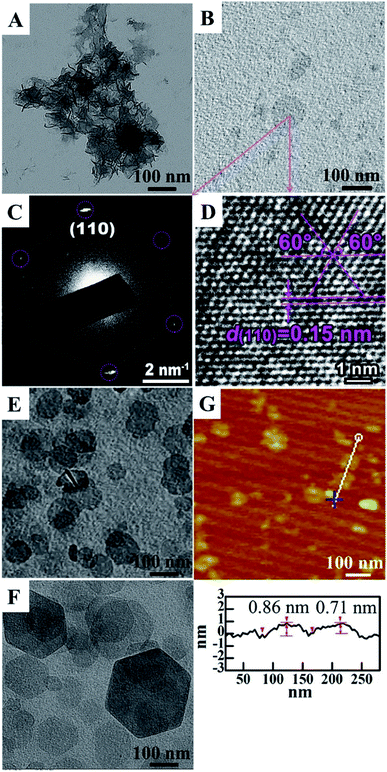 |
| | Fig. 17 TEM images of dispersions of (A) LDH single layer nanosheets gel, (B) LDH single layer nanosheets, (E) LDH single layer nanosheets after stacking at ∼25 °C, and (F) peptized LDH platelets; (C) HR-TEM image and (D) SAED pattern of LDH single layer nanosheets; (G) AFM image and sectional analysis of the LDH single layer nanosheets.70 Reproduced with permission from ref. 68. Copyright 2016 Elsevier Inc. | |
Ma et al.71 reported an ultra-large scale strategy under very moderate condition to synthesis Co–Ni LDH monolayer nanosheets. The detailed experiment process was as follows: a series of Ni(NO3)2·6H2O and Co(NO3)2·6H2O mixture with different molar ratio of Co2+ and Ni2+ were prepared by grinding in a mortar, and then morpholine was added (molar ratio of morpholine to metal ions was 4.1) and the obtained mixture was ground for 6 min, forming a sticky paste. After aging in a sealed beaker at room temperature for 10 h, the final product was washed with water and dried at 80 °C. AFM image (Fig. 18) indicated that the height of the nanosheets was inhomogeneous, the individual heights were about 1, 2 and 3 nm corresponding to one, two and three layers nanosheets respectively. For multilayered Co–Ni LDH (Fig. 19a), the multilayered structure was mainly balanced by the hydrogen bonding between the OH− in layers and water molecules existing in interlayer region. Based on this, authors proposed a “bottom-up” mechanism for the formation of monolayer nanosheets. Morpholine, an organic heterocycle compound with a chemical formula of O(CH2CH2)2NH, has both amine and ether functional groups. It could provide alkalinity through ionization reaction between its amine groups and water (Fig. 19b). The OH− released from the hydrolysis reaction of morpholine reacted with metal ions, forming Co–Ni LDH. The rapid and complete consumption of water from Ni(NO3)2·6H2O and Co(NO3)2·6H2O impeded the formation of hydrogen-bonding of interlayers (Fig. 19b), the monolayer nanosheets were obtained.
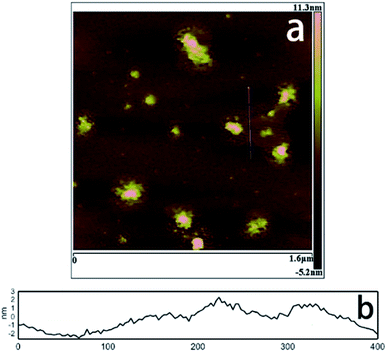 |
| | Fig. 18 AFM image (a) and height profile (b) of Co–Ni LDH monolayer nanosheets prepared at molar ratio of Co2+ to Ni2+ = 0.2. Reproduced with permission from ref. 71. Copyright 2016 Elsevier Inc. | |
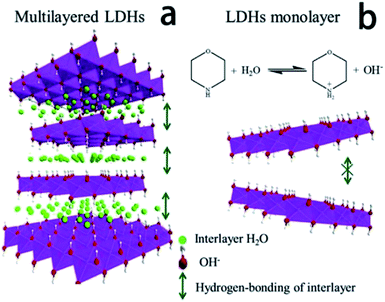 |
| | Fig. 19 Schematic illustration for the structure of multilayered Co–Ni LDH (a) and the formation mechanism of Co–Ni LDH monolayer nanosheets (b). Reproduced with permission from ref. 71. Copyright 2016 Elsevier Inc. | |
Exfoliation of LDH into nanosheets typically consists of two steps: (1) LDH were prepared by different methods; (2) intercalation of large molecules to increase the interlayer distance. Mechanical agitation to drive exfoliation and exfoliation without any solvent are adopted in the exfoliation process. Direct synthesis of LDH monolayer nanosheets mainly focused on controlling the nucleation and growth conditions. Different LDH exfoliation methods/direct growth methods are summarized in Table 1.
Table 1 A summary of LDH exfoliation/direct growth methods
| No. |
LDH |
Interlayer anion |
Dispersant |
Process |
Exfoliation |
Ref. |
| Successful: the cited methods can produce single-layer nanosheets but containing multi-layered LDH as well. |
| 1 |
CoAl-LDH |
CO32− |
L-Asparagine saturated aqueous solution |
The mixture of LDH and L-asparagine saturated aqueous solution was mixed by ultrasonication, after vigorous oscillation, the unexfoliated particles was removed by centrifugation |
Partially exfoliated |
48 |
| 2 |
MgAl-LDH |
CO32− |
Aqueous solution of zwitterionic surfactants |
Surfactant-intercalated LDH was dispersed in acid solution and stirred at room temperature for 2 days. |
MgAl-SC-LDH: 48% MgAl-SL-LDH: 49% |
49 |
| 3 |
Zn7Al3–LDH |
Cl− |
NaOH/urea aqueous solution at low temperatures |
Stirring a certain amount of Zn7/Al3–LDH in NaOH and urea solution system for 3 min |
Successfula |
51 |
| 4 |
Co2Fe-LDH |
|
Without solvent |
Bulk CoFe LDH were treated with Ar plasma with power of 100 W |
Not provided |
54 |
| Direct synthesis methods |
| No. |
LDH |
Interlayer anion |
Reaction solvent system |
Process |
Exfoliation state |
Ref. |
| 1 |
Mg2Al–LDH |
DS− |
Isooctane with SDS and 1-butanol |
LDH monolayers were synthesized in a reverse microemulsion system by a traditional aqueous co-precipitation method |
Successfula |
55 |
| 2 |
Mg2Al–LDH |
NO3− |
30 wt% H2O2 |
Mg(NO3)2·6H2O, Al(NO3)3·9H2O, and urea were dissolved in 100 mL 30% H2O2, then the mixture solution was transferred into a Teflon tube Teflon-lined autoclave and heated at 150 °C for 24 h |
Successfula |
60 |
| 3 |
Zn2Al-LDH |
BO33− |
Water |
The LDH slurry washed by water was re-dispersed in acetone and stirred for 1 h at room temperature, then it was filtered and washed thoroughly with acetone again |
Completely exfoliated |
61 |
| Mg3Al-LDH |
| 4 |
Mg2Al–LDH |
NO3− |
Water |
A mixture solution composed of Mg(NO3)2 and Al(NO3)3 was added dropwise to the solution of NaNO3 containing formamide |
Successfula |
62 and 63 |
| Mg4Al–LDH |
| 5 |
Co0.66Al0.34-LDH |
CO32− |
Ethylene glycol |
A certain amount of Co(NO3)2·6H2O, Al(NO3)3·9H2O and urea were dissolved in 80 mL of ethylene glycol, and the obtained mixture solution was transferred into 100 mL Teflon-lined autoclave, following heated at 100 °C for 24 h. |
Successfula |
64 |
| Co0.51Al0.49-LDH |
| 6 |
Zn1.48Al, Co0.96Fe, Co1.07Al, and Mg1.97Fe–LDH |
None |
Water |
Laser ablation of M(III) and M(II) metals in deionized water |
Not provided |
68 |
| 7 |
Mg2/Al–LDH |
NO3− |
Water |
A mixed salt solution (containing Mg2+ and Al3+ cations) and an alkali solution containing NH3 H2O were pumped into the reactor through two inlets each at a flow rate of 20 mL min−1 |
Successfula |
69 |
| 8 |
Mg2/Al–LDH |
NO3− |
Water |
A mixed salt solution (containing Mg2+ and Al3+ cations) and an alkali solution containing NH3 H2O were pumped into the reactor reaction for 10 min |
Successfula |
70 |
| 9 |
CoNi LDH |
NO3− |
None |
Ni(NO3)2·6H2O and Co(NO3)2·6H2O mixture with different molar ratio of Co2+ and Ni2+ were prepared by grinding in a mortar, and then morpholine was added and the obtained mixture was ground for 6 min |
Successfula |
71 |
3 Practical applications of LDH nanosheets
The applications of LDH nanosheets have been extensively studied and continues to expand. Currently, the LDH nanosheets have been used in many fields. In this section, we mainly summarize the application of LDH nanosheets in the four fields: (1) as catalysts for the oxygen evolution reaction, (2) as crosslinkers for the polymer composites, (3) as constructing materials for supercapacitors, (4) as carriers for drugs.
3.1 Catalysts
In recent years, the LDH nanosheets as catalysts for the reaction of oxygen evolution from water has been widely studied.54,72–96 Zhang et al.93 reported the preparation of porous monolayer NiFe-LDH (PM-LDH) by a facile one-step strategy, which can be used as an outstanding electrocatalyst for the oxygen evolution reaction (OER) (Fig. 20). The prepared PM-LDH showed an excellent OER electrocatalytic activity with an overpotential as low as 230 mV at a current density of 10 mA cm−2 and a Tafel slope of only 47 mV dec−1. Moreover, the OER activity could be maintained for a 100 h test period if the electrolyte was refreshed every 20 h, and the faradaic efficiency of the PM-LDH was of ≈100% over 30 min, representing one of the best OER performance yet reported for a NiFe-LDH system.
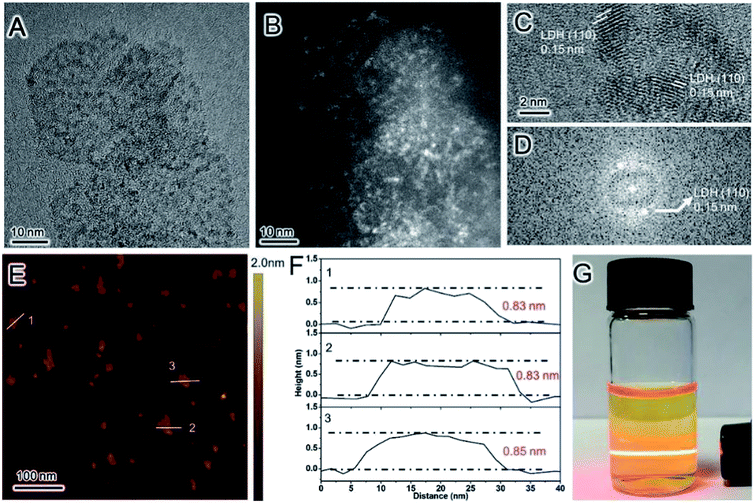 |
| | Fig. 20 (A) TEM, (B) HAADF-STEM, and (C) HRTEM image of PM-LDH; (D) the FFT pattern of the image shown in (C). (E) AFM image and (F) AFM height profiles of PM-LDH; the numbers 1–3 in (E) correspond to the profiles 1–3 in (F). (G) A dispersion of PM-LDH in ethanol displaying the Tyndall effect. Reproduced with permission from ref. 93. Copyright 2019 John Wiley & Sons, Inc. | |
Jia et al.94 studied the OER activity of ultrafine monolayer Znn/Cok–LDH (ZnCo-UF) nanosheets (with n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k molar ratios of 1
k molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 3
1 or 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), ZnCo-UF nanosheets were synthesized by exfoliating the bulk Znn/Cok–LDH (ZnCo-bulk) in formamide. The prepared ZnCo-UF with a mean size of ∼3.5 nm and thickness of ∼0.5 nm were tested as a catalyst for OER reactions (Fig. 21). ZnCo-UF (2
1), ZnCo-UF nanosheets were synthesized by exfoliating the bulk Znn/Cok–LDH (ZnCo-bulk) in formamide. The prepared ZnCo-UF with a mean size of ∼3.5 nm and thickness of ∼0.5 nm were tested as a catalyst for OER reactions (Fig. 21). ZnCo-UF (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) exhibited outstanding OER activity compared with ZnCo-bulk, with the current density of 168 mA cm−2 almost 20 times higher than that of ZnCo-bulk (8.5 mA cm−2) under a potential at 1.8 V. Moreover, comparing with ZnCo-UF prepared at Zn
1) exhibited outstanding OER activity compared with ZnCo-bulk, with the current density of 168 mA cm−2 almost 20 times higher than that of ZnCo-bulk (8.5 mA cm−2) under a potential at 1.8 V. Moreover, comparing with ZnCo-UF prepared at Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratios of 1
Co ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, ZnCo-UF (2
1, ZnCo-UF (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) displayed superior performance with a lower overpotential for OER, higher current density and the lowest OER Tafel slope of 66 mV dec−1. The prepared ZnCo-UF were active for hydrogen evolution reactions. The high current density, low OER overpotential, small charge transfer resistance and large eletroactive surface area make ZnCo-UF (2
1) displayed superior performance with a lower overpotential for OER, higher current density and the lowest OER Tafel slope of 66 mV dec−1. The prepared ZnCo-UF were active for hydrogen evolution reactions. The high current density, low OER overpotential, small charge transfer resistance and large eletroactive surface area make ZnCo-UF (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) an excellent electrocatalyst for OER.
1) an excellent electrocatalyst for OER.
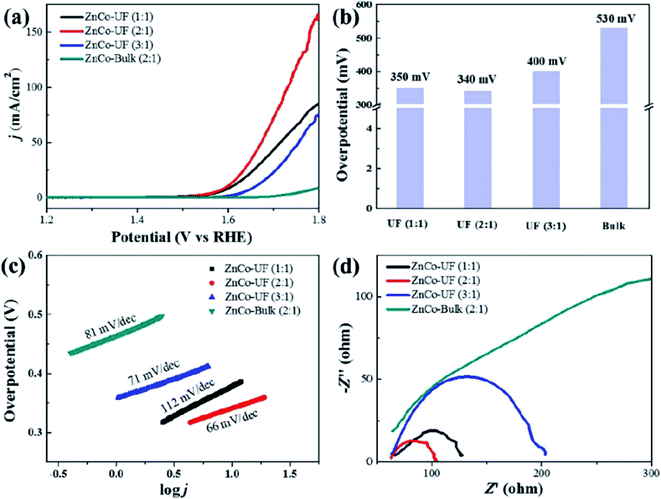 |
| | Fig. 21 (a) LSV curves for OER measured at a scan rate of 5 mV s−1; (b) the overpotential of ZnCo-UF at the current density of 5 mA cm−2; (c) corresponding Tafel plots; (d) EIS Nyquist plots at 1.8 V. Reproduced with permission from ref. 94. Copyright 2019 Elsevier Inc. | |
Li et al.95 studied the OER activity of ultrathin NiMn-LDH nanosheets synthesized by a one-step method with the assistance of ultrasonication at room temperature. The prepared NiMn-LDH nanosheets and the bulk NiMn-LDH synthesized by a typical coprecipitation method were tested as a catalyst for OER reactions (Fig. 22). Compared with the bulk NiMn-LDH, the as obtained NiMn-LDH nanosheets exhibited a twofold enhancement of the activity and a reduction of overpotential by 80 mV at 10 mA cm−2. The Tafel slope of the as-prepared NiMn-LDH nanosheets and bulk NiMn-LDH material was of ≈47 and 93 mV dec−1, respectively.
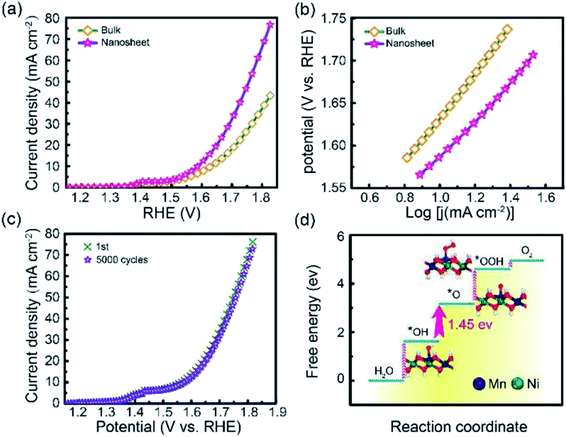 |
| | Fig. 22 (a) LSV curves for OER on bulk NiMn-LDH and ultrathin NiMn-LDH nanosheets. (b) The corresponding Tafel plots. (c) Cycles stability of the exfoliated NiMn-LDH nanosheets. (d) DFT calculated free energy diagrams for the OER process from H2O to O2 on the NiMn-LDH structures. The optimized configurations of the adsorption intermediates of *OH, *O and *OOH are shown inside. Dark blue: Mn; green: Ni; red: O; white: H. Reproduced with permission from ref. 95. Copyright 2018 John Wiley & Sons, Inc. | |
Wang et al.54 reported the preparation of ultrathin CoFe LDH nanosheets obtained by dry exfoliation in Ar plasma (CoFe LDH-Ar). The obtained CoFe LDH-Ar with multiple vacancies was an excellent electrocatalyst for the OER (Fig. 23). The CoFe LDH-Ar exhibited higher OER activity with a lower overpotential of 266 mV at 10 mA cm−2 than the bulk CoFe LDH (321 mV at 10 mA cm−2). And the CoFe LDH-Ar exhibited a smaller Tafel slope of 37.85 mV per decade than the bulk CoFe LDH (57.05 mV per decade). Furthermore, after loading the CoFe LDH-Ar onto nickel foam (denoted as CoFe LDH-Ar/NF) with a loading capacity of 0.2 mg cm−2, the obtained CoFe LDH-Ar/NF had an ultralow overpotential of 237 mV at a current density of 10 mA cm−2. And the LSV curve of CoFe LDH-Ar/NF for the OER showed negligible degradation after 2000 CV cycles, indicating a superior operational stability of the CoFe LDH-Ar.
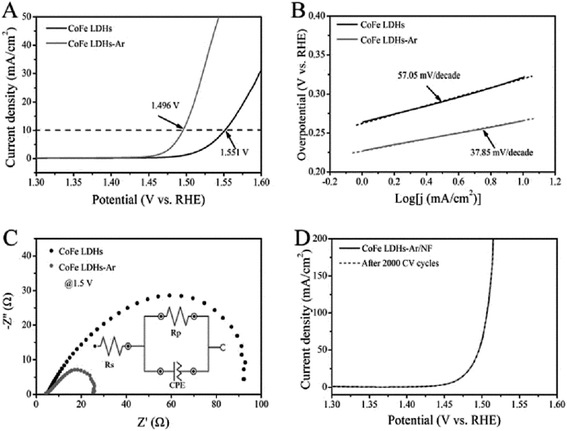 |
| | Fig. 23 The OER performance of bulk CoFe LDH and ultrathin CoFe LDH-Ar nanosheets. (A) LSV curves for the OER at a scan rate of 5 mV s−1. (B) The corresponding Tafel plots. (C) Nyquist plots at an overpotential of 270 mV. The inset gives the equivalent circuit. Rs = series resistance, Rp = charge transfer resistance, CPE = constant phase element related to the double-layer capacitance. (D) Stability test with the CoFe LDH-Ar on Ni foam. Reproduced with permission from ref. 54. Copyright 2017 John Wiley & Sons, Inc. | |
3.2 Nanocrosslinker
Due to their highly flexible and tunable chemical composition and excellent physical properties, LDH have found widespread applications in LDH/polymer nanocomposites (NC).97–103 Well dispersed LDH nanosheets in polymer matrices can greatly improve the mechanical and water-swelling properties. For example, Hu et al.97 reported a novel type of polymer NC hydrogels, LDH/polyacrylamide (PAM), prepared by means of a convenient in situ polymerization method. The obtained LDH/polymer NC hydrogels exhibited ultrahigh tensibility and excellent toughness even at low inorganic content (≤2.3 wt%), as well as unusual hierarchical porous structure, as shown in Table 2 and Fig. 24. In addition, with the increase of isethionate (HO(CH2)2SO3−)-intercalated LDH (LDH-Ise) content, the compression strengths and moduli of the LDH/PAM NC hydrogels (LmM, where m stands for the concentration 0.1 × LDH/water [g L−1]) increased significantly. This type of LDH/polymer NC hydrogels may further widen the applications of polymer hydrogels in mechanical devices such as biomedical devices, artificial muscles, and drug delivery systems.
Table 2 Tensile and compressive mechanical properties of the hydrogels, including the as-prepared ones (PAM, LmM, BnM) (the conventional chemically crosslinked hydrogels using N,N,N′,N′-methylenebis(acrylamide) as the crosslinker, where n represents 3 × BIS/water [g L−1]) and the swollen one with 90 wt% water content (L2M-S)
| Sample |
Yield strength [kPa] |
Fracture strength [kPa] |
Elongation at break [%] |
Toughnessa [MJ m−3] |
Compressive strengthb [MPa] |
Compressive modulusb [MPa] |
| Toughness was calculated from the areas under the tensile stress–strain curves. The hydrogels B1M, B2M, and PAM were too brittle to compress. The swollen hydrogel L2M-S did not break during the tensile test, accompanied by the disappearance of the yielding phenomenon. |
| L1M |
39.5 |
29.3 |
2355 |
0.69 0.5 |
4.5 |
|
| L2M |
58.4 |
43.2 |
4068 |
1.99 |
0.9 |
12.9 |
| L3M |
62.3 |
45.8 |
4361 |
2.55 |
1.2 |
16.6 |
| L2M-Sc |
No |
>34.0 |
>6236 |
>1.95 |
|
|
| B1M |
— |
41.4 |
318 |
0.10 |
— |
— |
| B2M |
— |
59.3 |
204 |
0.08 |
— |
— |
| PAM |
21.7 |
7.0 |
154 |
0.02 |
— |
— |
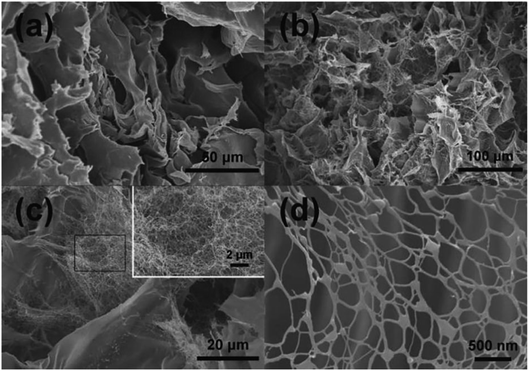 |
| | Fig. 24 Typical SEM images of the freeze-dried xerogels for the neat PAM hydrogel (a) and the LDH/PAM NC hydrogel (L1M) (b–d). The neat PAM xerogel showed the common large-pore structure at the micrometer scale (a). In contrast, the NC xerogel revealed an unusual hierarchical porous structure with interconnected pore sizes at micro- and nanometer scales (b and c). Inset in (c): enlargement of the outlined rectangular part. (d) Details of the nanometer-sized pores. Reproduced with permission from ref. 97. Copyright 2014 John Wiley & Sons, Inc. | |
Wu et al.98 developed a convenient green preparation method to achieve LDH/polyacrylamide (PAM) NC hydrogels with ultrahigh deformability. The ZnAl-LDH with amino acid (L-Ser) were first delaminated in water resulting in a transparent aqueous dispersion, then the monomer, initiator and accelerator were dissolved under stirring at 0 °C for 10 min. Subsequently, NC hydrogels were achieved via exfoliation–adsorption in situ polymerization. The prepared NC hydrogels exhibited ultrahigh deformability towards various mechanical deformations including elongation, bending, knotting, knotting and subsequent elongation, and compression. Additionally, the NC hydrogels displayed extraordinary stretchability and compressibility as shown in Table 3 and Fig. 25.
Table 3 Tensile and compression mechanical properties of the LDH/PAM NC hydrogels (LmM)
| Sample |
Yield strength (kPa) |
Fracture strength (kPa) |
Elongation at break (%) |
Tensile modulus (kPa) |
Toughnessa (MJ m−3) |
N* (mol m−3) |
104 Mc (g mol−1) |
Compressive strength (MPa) |
Compressive modulus (kPa) |
| Toughness was calculated from the areas under the tensile stress–strain curves. |
| L1M |
20.9 |
21.0 |
3800 |
36.4 |
0.64 |
10.7 |
9.38 |
0.43 |
53.7 |
| L2M |
21.7 |
>33.4 |
>3950 |
32.1 |
0.83 |
9.99 |
10.0 |
0.69 |
66.9 |
| L3M |
26.6 |
>67.0 |
>4936 |
39.5 |
1.70 |
11.2 |
8.96 |
1.02 |
78.3 |
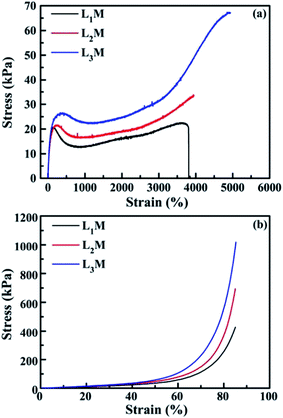 |
| | Fig. 25 Stress–strain curves of (a) tensile and (b) compression measurements for the as-prepared LDH/PAM NC hydrogels (LmM). Reproduced with permission from ref. 98. Copyright 2015 The Royal Society of Chemistry. | |
Huang et al.99 reported the facile preparation of bioactive nanocomposite PEG hydrogel crosslinked by the novel multifunctional nanocrosslinkers, polydopamine-coated LDH (PD-LDH). Fig. 26 illustrates the synthesize process, in which the LDH nanosheets were first coated by polydopamine, then the PD-LDH nanosheets were mixed with the commercially available 4 arm thiol terminated polyethylene glycol (4 arm PEG-SH) to form poly(ethylene glycol) (PEG) hydrogels. Acting as nanocrosslinkers, the catechol-rich PD-LDH nanosheets reinforced the mechanical strength of the hydrogel as well as afforded the hydrogels with bioadhesion and robust bioactivity by the cortical-mediated couplings. The prepared nanocomposite PEG hydrogels exhibited tunable mechanical properties, self-healing ability, and bioadhesion to biological tissues. Moreover, without any further bio-functionalization these hydrogels could promote the sequestration of proteins and support the osteogenic differentiation of human mesenchymal stem cells.
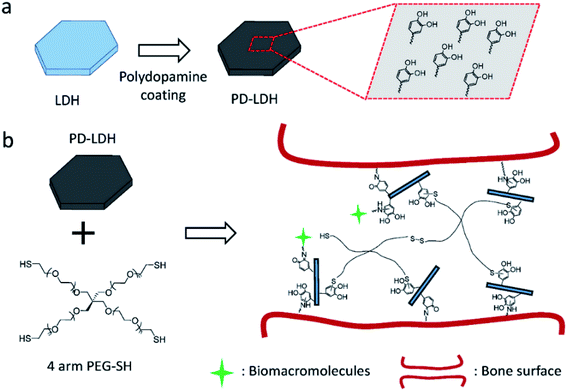 |
| | Fig. 26 (a) Schematic representation of polydopamine coating on layered double hydroxides (LDH). (b) Schematic of nanocomposite hydrogel synthesized with 4 arm thiol-terminated polyethylene glycol (4 arm PEG-SH) and polydopamine-coated LDH (PD-LDH), which is able to immobilize biomacromolecules and act as the bioadhesive for bone fracture. Reproduced with permission from ref. 99. Copyright 2016 John Wiley & Sons, Inc. | |
3.3 Supercapacitors
In recent years, due to the structural tunability, LDH as metal oxide precursors or the source of pseudo-capacitive species have attracted increasing attention in supercapacitor research.104–130 For example, a novel three-dimensional (3D) embedded hierarchical core–shell nanostructure was developed via combining ultrathin CoFe-LDH nanosheets with porous Cu3N nanowire arrays (NWAs),128 the ultrathin CoFe-LDH nanosheets in the core–shell nanostructure were grown from the interior of Cu3N nanowire cores supported on Cu foam (Cu3N@CoFe-LDH). The synthesis process of the hierarchical Cu3N@CoFe-LDH core–shell NWAs supported on Cu foam is shown in Fig. 27. The obtained Cu3N@CoFe-LDH NWA electrode exhibited a high areal capacitance of 3078 mF cm−2 at a current density of 1 mA cm−2, superior cycling stability and excellent rate capacity, which is superior to the related CuO@CoFe-LDH NWA electrodes (Fig. 28).
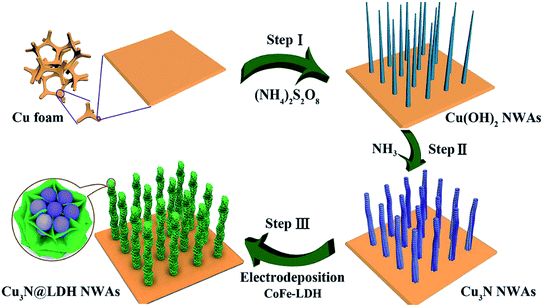 |
| | Fig. 27 Schematic illustration for the fabrication of hierarchical Cu3N@CoFe-LDH core–shell NWAs supported on copper foam. Reproduced with permission from ref. 128. Copyright 2018 The Royal Society of Chemistry. | |
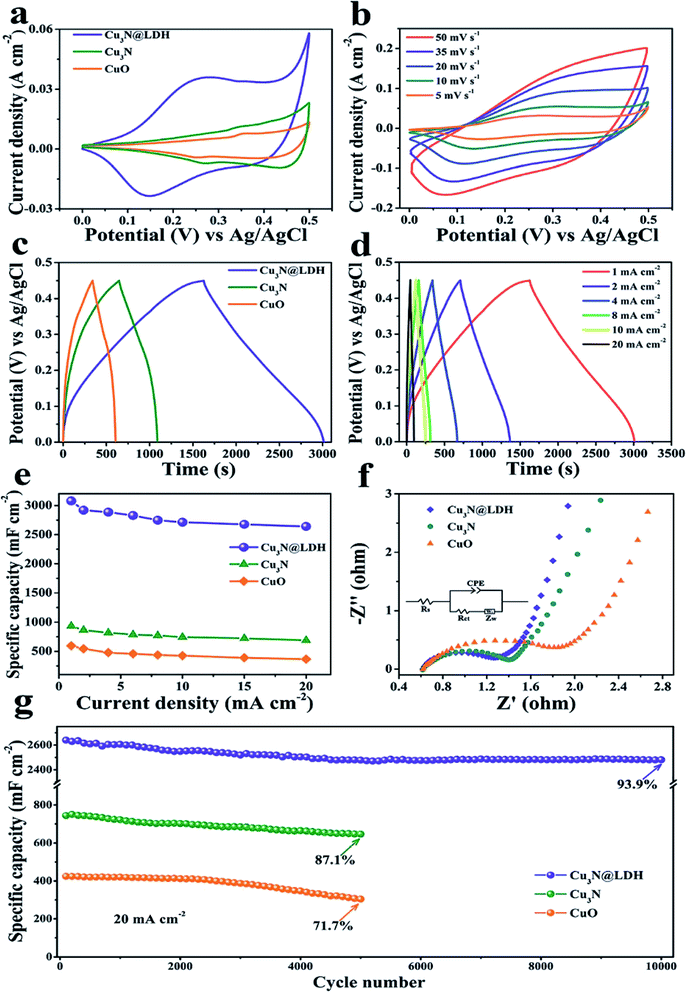 |
| | Fig. 28 (a) CV curves of CuO, Cu3N and Cu3N@CoFe-LDH at a scan rate of 5 mV s−1. (b) CV curves of Cu3N@CoFe-LDH at various scan rates. (c) GCD curves of CuO, Cu3N and Cu3N@CoFe-LDH at a current density of 1 mA cm−2. (d) GCD curves of Cu3N@CoFe-LDH at various current densities. (e) Current density dependence of the areal capacitance, (f) Nyquist plots of EIS and (g) cycling performance of the pristine CuO, Cu3N and Cu3N@CoFe-LDH. Reproduced with permission from ref. 128. Copyright 2018 The Royal Society of Chemistry. | |
Sekhar et al.129 deposited the binder-free NiCo LDH nanosheets on Ag NWs-fenced carbon cloth (NC LDH NSs@Ag@CC) by a facile electrochemical deposition method (Fig. 29). The obtained hierarchical hybrid nanocomposite led to a relatively high areal capacitance (1133.3 mF cm−2 at 1 mA cm−2) and a good cycling stability (80.47% after 2000 cycles) compared to the electrode prepared without Ag NWs. Additionally, a flexible asymmetric supercapacitor (ASC) with NC LDH NSs@Ag@CC as a positive electrode and activated carbon coated CC as a negative electrode was fabricated. The obtained ASC displayed maximum operational potential window of 1.6 V, high areal capacitance of 230.2 mF cm−2 and outstanding cycling stability of 88.1% with remarkable energy densities at all the charge–discharge conditions (78.8 μW h cm−2 at the power density of 785 μW cm−2 and 40 μW h cm−2 at the high power density of 12.1 mW cm−2, respectively).
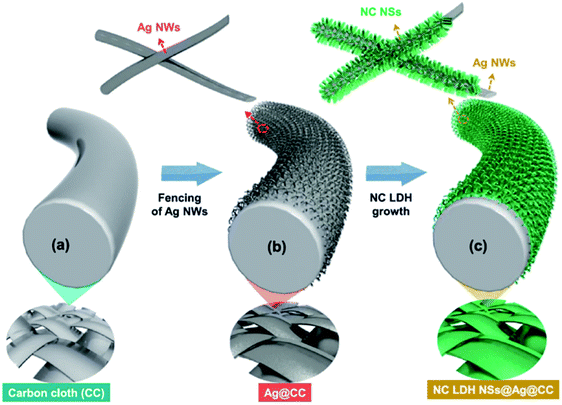 |
| | Fig. 29 (a–c) Schematic illustration showing the preparation process flow of NC LDH NSs@Ag@CC by a single-step ECD process. Reproduced with permission from ref. 129. Copyright 2017 Elsevier Inc. | |
Zhao et al.130 reported that Ni3+ doped NiTi-LDH monolayer nanosheets, which were prepared through a facile bottom-up approach, exhibited excellent supercapacitor performances. The monolayer-NiTi-LDH nanosheets had a maximum specific capacitance of 2310 F g−1, much higher than that of bulk NiTi-LDH (377 F g−1) and bulk NiAl-LDH (77 F g−1). Furthermore, the monolayer NiTi-LDH exhibited good cycling performance and excellent rate capability with 82.0% capacitance was remained at a large current density of 12 A g−1. The monolayer NiTi-LDH nanosheets were prepared by an in situ growth process in the reverse microemulsion formed by surfactant, co-surfactant and water. Fig. 30 shows the TEM and AFM images of the prepared monolayer NiTi-LDH nanosheets, which confirmed the formation of the monolayer NiTi-LDH nanosheets.
 |
| | Fig. 30 Characterizations for the monolayer-NiTi-LDH nanosheets. (A) TEM image; (B) HRTEM image; (C) AFM image and (D) the corresponding height profiles; the numbers from 1 to 3 in (D) correspond to the numbers from 1 to 3 in (C). Reproduced with permission from ref. 130. Copyright 2015 The Royal Society of Chemistry. | |
3.4 Drug delivery hosts
Because of their excellent biocompatibility, low toxicity, biodegradable and exchangeable interlayer anion, the LDH have been widely explored as inorganic composite materials for drug/gene delivery.131–137 However, compared with bulk LDH, monolayer LDH (MLDH) nanosheets exhibit much higher specific surface and more combinative sites, leading to greatly enhanced surface activity for drug loading.138–147 For example, MgAl-LDH monolayer nanosheets were employed to localize doxorubicin (DOX),148 the DOX/MLDH showed extremely high loading efficiency (3.6 mg mg−1 (w/w)) and controllable release behavior. XRD, AFM, and HRTEM confirmed the successful preparation of the MLDH nanosheets (Fig. 31). After further incorporation of folic acid (FA), the as-prepared FA-DOX/MLDH composite showed good fluorescence imaging, selective anticancer performance, and rather low cytotoxicity to normal cells. Moreover, the FA-DOX/MLDH composite exhibited high storage stability, good biocompatibility and targeting capability.
 |
| | Fig. 31 (a) XRD patterns of bulk LDH colloid (black line), MLDH colloid (red line), and the re-stacking of MLDH nanosheets (blue line). (b) AFM image, (c) HRTEM image, and (d) particle size distribution of MLDH nanosheets. The insets in (c) show a high magnification image and crystal lattice. Reproduced with permission from ref. 148. Copyright 2018 Springer. | |
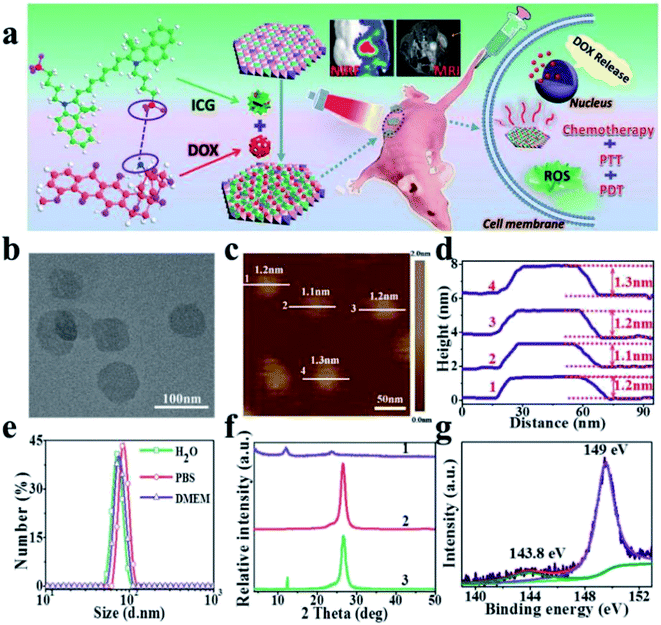
Recently, a Gd3+-doped monolayered-double-hydroxide (MLDH) nanosheets prepared through a bottom-up synthesis procedure as drug carriers was reported.149 The MLDH nanosheets exhibited ultrahigh drug loading content (LC) of 797.36% and an encapsulation efficiency (EE) of 99.67% towards doxorubicin hydrochloride (DOX) and indocyanine green (ICG) (Fig. 32a). X-ray diffraction (XRD), atomic force microscopy (AFM), high-resolution transmission electron microscopy (HRTEM) and XPS results showed that MLDH nanosheets was successfully synthesized with a uniform lateral size of about 70 nm and a thickness of about 1.2 nm (Fig. 32b–g). The DOX&ICG/MLDH composite exhibited both pH-controlled and near-infrared-irradiation-induced DOX release, resulting in the stimulated drug release performance. Furthermore, the in vitro and in vivo therapeutic evaluations demonstrated that DOX&ICG/MLDH have excellent trimode synergetic anticancer activity and superior biocompatibility.
 |
| | Fig. 32 (a) A schematic illustration for MLDH-based drug delivery system toward efficient loading and precisely controlled delivery of theranostic agents. (b) HRTEM image of MLDH nanosheets. (c) AFM image and (d) measured thickness of MLDH nanosheets. (e) Size distribution of MLDH nanosheets in water, PBS, and culture medium (high glucose Dulbecco's modified Eagle medium (DMEM)). (f) XRD patterns of: (1) bulk LDH colloid, (2) MLDH nanosheets colloid, and (3) the restacking sample of MLDH nanosheets. The peak at 26.2° is ascribed to the PET film substrate. (g) XPS spectra of MLDH nanosheets sample. Reproduced with permission from ref. 149. Copyright 2018 John Wiley & Sons, Inc. | |
Yan et al.150 developed a novel type of aqueous dispersible 2D ultrathin LDH nanosheets and modified their surface with different functional molecules, rhodamine B (RB) and poly(ethylene glycol) (PEG). After incorporating of rhodamine B, the obtained LDH-Co-RB nanosheets can disperse well in the liquid state, penetrate the cell membrane for bioimaging, and increase intracellular delivery in vitro. After tailoring with neutral PEG by covalent bonding, the obtained nanohybrids were suitable for in vivo drug delivery through systemic administration. These nanostructures were used for delivery of a negatively charged anticancer drug, methotrexate (MTX), and the in vivo anticancer therapy results (Fig. 33) showed that MTX loaded in LDH-Co-PEG nanosheets exhibits inhibition of tumor growth significantly better than that of free MTX and MTX loaded in nonmodified LDH nanoparticles MTX. In addition, higher cancer cell damage area (red stained) in LDH-MTX- and LDH-Co-PEG MTX-treated groups, indicated dramatically improved therapeutic efficacy was achieved. The further systematic in vivo safety investigation demonstrated that the new material was biocompatible.
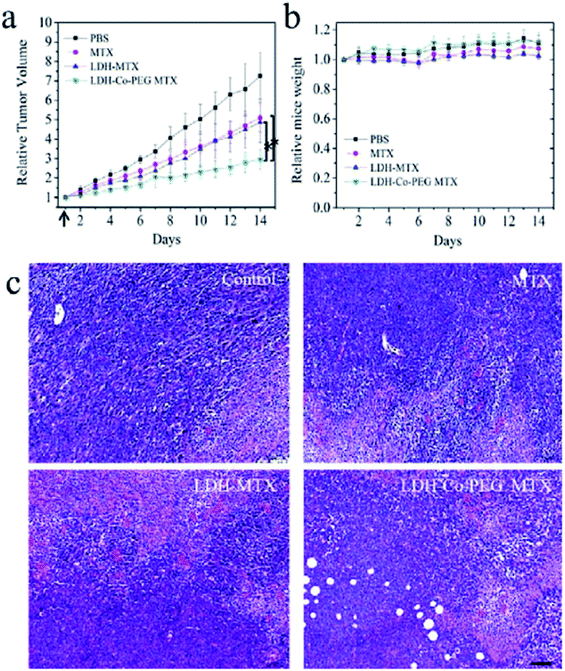 |
| | Fig. 33 In vivo anticancer activities: (a) tumor growth curves of 4T1 tumor-bearing BALB/C mice after intravenous injection with (1) PBS, (2) free MTX (MTX), (3) MTX loaded in traditional LDH nanoparticles (LDH-MTX), and (4) MTX loaded in PEG-modified ultrathin LDH nanosheets (LDH-Co-PEG MTX) (n = 5, error bar indicates standard deviation; the arrow indicates tail vein injection at day 1; * indicates p < 0.05 with Student's t test). (b) The body weight evolution of 4T1 tumor-bearing mice at different times after intravenous injection of different materials (n = 5, error bar indicates standard deviation). (c) H&E staining images of tumors slices. The tumor-bearing mice were treated with MTX, LDH-MTX, and LDH-Co-PEG MTX at day 1 and sacrificed at day 7. Higher cancer cell damage (red area indicates area of cell damage) was found in LDH MTX- and LDH-Co-PEG MTX-treated groups. Scale bar: 100 μm. Reproduced with permission from ref. 150. Copyright 2017 The American Chemistry Society. | |
4 Conclusion and outlook
In this review, we summarized the synthesis methods of LDH nanosheets, including top-down exfoliation methods developed in the recent years and the bottom-up direct synthesis methods. The top-down methods can be realized not only in aqueous solution but also in dry state. The bottom-up approaches are generally achieved by controlling the nucleation and growth conditions so that only allow the formation of dispersed nanosheets. And the bottom-up approaches favors large scale synthesis of dispersible LDH nanosheets.
In recently years, LDH nanosheets were widely applied in many fields, including polymer/LDH nanocomposites, LDH thin films, LDH hybrid magnets, LDH electrode materials, LDH catalysts and bioinorganic hybrid materials. Here, the promising results of LDH catalyzing water splitting reactions, formulating LDH/polymer nanocomposites and supercapacitors, and delivering drugs or genes as carriers were briefly summarized. At present the applications of LDH nanosheets attracted considerable attention, and many new and significant achievements have been made. However, there are still tough challenges remained to be addressed. In general, the major challenge is how to separate delaminated LDH nanosheets without aggregation from a solvent dispersion. The dispersed LDH nanosheets used in catalyzing water splitting reactions is a very active field over the last several years, LDH nanosheets can work either as support or as the active species. However, in order to obtain most suitable LDH, the exact catalytic mechanism needs to be completely revealed and more major success is anticipated. Fabricating LDH/polymer nanocomposite hydrogels need to overcome the main obstacle: the affinity of LDH with the monomer and the polymer, the surface modification of hydrophilic LDH into hydrophobic ones was needed to maximize their dispersion in polymer matrices. Research into LDH nanosheets in delivery carriers is quite extensive, therefore, its future development should focus on the functionalization of the LDH shells and explore their clinical applications. The study of the LDH nanosheets in supercapacitors is very active, and major progress in this field is anticipated in coming years. In all these applications, the selection of appropriate LDH and the complementary compounds that can deliver the desired multifunctional response is very critical. Proper exfoliation methods need to be choose to optimize the mixing and matching of the two components. Therefore, LDH should be much more attractive prospects in the coming years as they possess unique structure and performance, which remain highly attractive to researchers in chemistry, materials science, and engineering.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the Henan University of Chinese Medicine (No. 00104311-2021-1).
References
- P. J. Sideris, U. G. Nielsen, Z. H. Gan and C. P. Grey, Science, 2008, 321, 113–117 CrossRef CAS
 .
. - R. Z. Ma, Z. P. Liu, K. Takada, N. Iyi, Y. Bando and T. Sasaki, J. Am. Chem. Soc., 2007, 129, 5257–5263 CrossRef CAS PubMed
 .
. - Q. Wang and D. O'Hare, Chem. Rev., 2012, 112, 4124–4155 CrossRef CAS
 .
. - Z. P. Liu, R. Z. Ma, Y. Ebina, N. Iyi, K. Takada and T. Sasaki, Langmuir, 2007, 23, 861–867 CrossRef CAS PubMed
 .
. - C. Gomes Silva, Y. Bouizi, V. Fornes and H. Garcia, J. Am. Chem. Soc., 2009, 131, 13833 CrossRef CAS
 .
. - J. L. Gunjakar, T. W. Kim, H. N. Kim, I. Y. Kim and S. J. Hwang, J. Am. Chem. Soc., 2011, 133, 14998 CrossRef CAS PubMed
 .
. - Y. Zhao, M. Wei, J. Lu, Z. L. Wang and X. Duan, ACS Nano, 2009, 3, 4009 CrossRef CAS PubMed
 .
. - K. Parida, M. Satpathy and L. Mohapatra, J. Mater. Chem., 2012, 22, 7350 RSC
 .
. - H. Nakajima, S. Ishino, H. Masuda, T. Shimosaka, T. Nakagama, T. Hobo and K. Uchiyama, Chem. Lett., 2005, 34, 358–359 CrossRef CAS
 .
. - P. Atienzar, M. de Victoria-Rodriguez, O. Juanes, J. C. Rodriguez-Ubis, E. Brunet and H. Garcia, Energy Environ. Sci., 2011, 4, 4718–4726 RSC
 .
. - J. L. Colon, C. Y. Yang, A. Clearfield and C. R. Martin, J. Phys. Chem., 1990, 94, 874–882 CrossRef CAS
 .
. - D. S. Robins and P. K. Dutta, Langmuir, 1996, 12, 402–408 CrossRef CAS
 .
. - C. P. Chen, P. Gunawan and R. Xu, J. Mater. Chem., 2011, 21, 1218 RSC
 .
. - Y. Zhao, S. He, M. Wei, D. G. Evans and X. Duan, Chem. Commun., 2010, 46, 3031 RSC
 .
. - Q. Wang, J. Luo, Z. Zhong and A. Borgna, Energy Environ. Sci., 2011, 4, 42 RSC
 .
. - Q. Wang, H. H. Tay, Z. Zhong, J. Luo and A. Borgna, Energy Environ. Sci., 2012, 5, 7526 RSC
 .
. - Q. Wang, H. H. Tay, D. J. W. Ng, L. Chen, Y. Liu, J. Chang, Z. Zhong, J. Luo and A. Borgna, ChemSusChem, 2010, 3, 965 CrossRef CAS
 .
. - Q. Wang, Z. Wu, H. H. Tay, L. Chen, Y. Liu, J. Chang, Z. Zhong, J. Luo and A. Borgna, Catal. Today, 2011, 164, 198 CrossRef CAS
 .
. - Q. Wang, H. H. Tay, Z. Guo, L. Chen, Y. Liu, J. Chang, Z. Zhong, J. Luo and A. Borgna, Appl. Clay Sci., 2012, 55, 18 CrossRef CAS
 .
. - Q. Wang, H. H. Tay, L. Chen, Y. Liu, J. Chang, Z. Zhong, J. Luo and A. Borgna, J. Nanoeng. Nanomanuf., 2011, 1, 1 CrossRef
 .
. - A. C. S. Alcantara, P. Aranda, M. Darder and E. Ruiz-Hitzky, J. Mater. Chem., 2010, 20, 9495 RSC
 .
. - S. D. Li, J. H. Li, C. J. Wang, Q. Wang, M. Z. Cader, J. Lu, D. G. Evans, X. Duan and D. O'Hare, J. Mater. Chem. B, 2013, 1, 61 RSC
 .
. - Y. Y. Wong, K. Markham, Z. P. Xu, M. Chen, G. Q. Lu, P. F. Bartlett and H. M. Cooper, Biomaterials, 2010, 31, 8770 CrossRef CAS PubMed
 .
. - W. Wang, H. Pan, Y. Shi, Y. Pan, W. Yang, K. Liew, L. Song and Y. Hu, Composites, Part A, 2016, 80, 259–269 CrossRef CAS
 .
. - H. Pan, W. Wang, Q. Shen, Y. Pan, L. Song, Y. Hu and Y. Lu, RSC Adv., 2016, 6, 111950 RSC
 .
. - Z. Matusinovic and C. A. Wilkie, J. Mater. Chem., 2012, 22, 18701–18704 RSC
 .
. - D.-Y. Wang, A. Das, F. R. Costa, A. Leuteritz, Y.-Z. Wang, U. Wagenknecht and G. Heinrich, Langmuir, 2010, 26, 14162–14169 CrossRef CAS
 .
. - C. Nyambo, P. Songtipya, E. Manias, M. M. Jimenez-Gasco and C. A. Wilkie, J. Mater. Chem., 2008, 18, 4827 RSC
 .
. - Y. Gao, J. Wu, Q. Wang, C. A. Wilkie and D. O'Hare, J. Mater. Chem. A, 2014, 2, 10996–11016 RSC
 .
. - L. F. Nazar and A. J. Jacobson, J. Chem. Soc., Chem. Commun., 1986, 570–571 RSC
 .
. - N. Yamamoto, T. Okuhara and T. Nakato, J. Mater. Chem., 2001, 11, 1858 RSC
 .
. - L. F. Nazar, S. W. Liblong and X. T. Yin, J. Am. Chem. Soc., 1991, 113, 5889 CrossRef CAS
 .
. - T. Sasaki, M. Watanabe, H. Hashizume, H. Yamada and H. Nakazawa, J. Am. Chem. Soc., 1996, 118, 8329 CrossRef CAS
 .
. - R. E. Schaak and T. E. Mallouk, Chem. Mater., 2000, 12, 2513 CrossRef CAS
 .
. - R. E. Schaak and T. E. Mallouk, Chem. Mater., 2002, 14, 1455 CrossRef CAS
 .
. - S. O'Leary, D. O'Hare and G. Seeley, Chem. Commun., 2002, 1506–1507 RSC
 .
. - F. Song and X. Hu, Nat. Commun., 2014, 5, 4477 CrossRef CAS
 .
. - R. Ma and T. Sasaki, Adv. Mater., 2010, 22, 5082 CrossRef CAS
 .
. - K. Yan, T. Lafleur, J. J. Chai and C. Jarvis, Electrochem. Commun., 2016, 62, 24–28 CrossRef CAS
 .
. - Y. Y. Wang, Y. Q. Zhang, Z. J. Liu, C. Xie, S. Feng, D. D. Liu, M. F. Shao and S. Y. Wang, Angew. Chem., Int. Ed., 2017, 56, 1–6 CrossRef CAS
 .
. - K. Fan, H. Chen, Y. F. Ji, H. Huang, P. M. Claesson, Q. Daniel, B. Philippe, H. Rensmo, F. S. Li, Y. Luo and L. C. Sun, Nat. Commun., 2016, 7, 11981 CrossRef CAS PubMed
 .
. - M. Zubair, M. Daud, G. McKay, F. Shehzad and M. A. Al-Harthi, Appl. Clay Sci., 2017, 143, 279–292 CrossRef CAS
 .
. - H. Yin and Z. Tang, Chem. Soc. Rev., 2016, 45, 4873–4891 RSC
 .
. - J. F. Yu, Q. Wang, D. O'Hare and L. Y. Sun, Chem. Soc. Rev., 2017, 46, 5950 RSC
 .
. - M. Meyn, K. Beneke and G. Lagaly, Inorg. Chem., 1990, 29, 5201–5207 CrossRef CAS
 .
. - J. M. Hidalgo, C. Jimenez-Sanchidrian, M. Mora and J. R. Ruiz, J. Nanosci. Nanotechnol., 2010, 10, 6562–6566 CrossRef CAS PubMed
 .
. - W. Hou, L. Kang, R. Sun and Z.-H. Liu, Colloids Surf., A, 2008, 312, 92–98 CrossRef CAS
 .
. - L. J. Chen, B. Sun, X. D. Wang, F. M. Qiao and S. Y. Ai, J. Mater. Chem. B, 2013, 1, 2268–2274 RSC
 .
. - Y. Zhang, H. Y. Sun, X. L. Bai and Y. X. Zhao, J. Dispersion Sci. Technol., 2019, 40, 811–818 CrossRef CAS
 .
. - E. G. Lomax, Amphoteric Surfactants, CRC Press, New York, 1996 Search PubMed
 .
. - Y. Wei, F. Li and L. Liu, RSC Adv., 2014, 4, 18044–18051 RSC
 .
. - J. Cai and L. N. Zhang, Biomacromolecules, 2006, 7, 183–189 CrossRef CAS
 .
. - J. Cai and L. N. Zhang, Macromol. Biosci., 2005, 5, 539–548 CrossRef CAS
 .
. - Y. Y. Wang, Y. Q. Zhang, Z. J. Liu, C. Xie, S. Feng, D. D. Liu, M. F. Shao and S. Y. Wang, Angew. Chem., Int. Ed., 2017, 56, 5867–5871 CrossRef CAS
 .
. - G. Hu, N. Wang, D. O'Hare and J. Davis, Chem. Commun., 2006, 287–289 RSC
 .
. - A. I. Khan and D. O'Hare, J. Mater. Chem., 2002, 12, 3191 RSC
 .
. - Z. P. Xu and P. S. Braterman, J. Mater. Chem., 2003, 13, 268 RSC
 .
. - S. P. Newman and W. Jones, New J. Chem., 1998, 22, 105–115 RSC
 .
. - F. Bellezza, A. Cipiciani, U. Costantino, M. Nocchetti and T. Posati, Eur. J. Inorg. Chem., 2009, 2603–2611 CrossRef CAS
 .
. - Y. Yan, Q. Liu, J. Wang, J. Wei, Z. Gao, T. Mann, Z. Li, Y. He, M. Zhang and L. Liu, J. Colloid Interface Sci., 2012, 371, 15–19 CrossRef CAS PubMed
 .
. - Q. Wang and D. O'Hare, Chem. Commun., 2013, 49, 6301–6303 RSC
 .
. - J. Yu, B. R. Martin, A. Clearfield, Z. Luo and L. Sun, Nanoscale, 2015, 7, 9448–9451 RSC
 .
. - J. Yu, J. Liu, A. Clearfield, J. E. Sims, M. T. Speiegle, S. L. Suib and L. Sun, Inorg. Chem., 2016, 55, 12036–12041 CrossRef CAS PubMed
 .
. - H. P. Li, T. N. Tran, B. J. Lee, C. F. Zhang, J. D. Park, T. H. Kang and J. S. Yu, ACS Appl. Mater. Interfaces, 2017, 9, 20294–20298 CrossRef CAS PubMed
 .
. - W. Zhang, Y. Xie, D. Xiong, X. Zeng, Z. Li, M. Wang, Y.-B. Cheng, W. Chen, K. Yan and S. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 9698–9704 CrossRef CAS
 .
. - G. Xiang, Y.-G. Wang, J. Li, J. Zhuang and X. Wang, Sci. Rep., 2013, 3, 1411 CrossRef PubMed
 .
. - Q. Zhao, B. Shao, W. Lü, Y. Jia, W. Lv, M. Jiao and H. You, Cryst. Growth Des., 2014, 14, 1819–1826 CrossRef CAS
 .
. - T.-B. Hur, T. X. Phuoc and M. K. Chyu, J. Appl. Phys., 2010, 108, 114312 CrossRef
 .
. - X. Pang, M. Sun, X. Ma and W. Hou, J. Solid State Chem., 2014, 210, 111–115 CrossRef CAS
 .
. - Y. P. Zhang, H. P. Li, N. Du, R. J. Zhang and W. G. Hou, Colloids Surf., A, 2016, 501, 49–54 CrossRef CAS
 .
. - W. Ma, L. Wang, J. Y. Xue and H. T. Cui, J. Alloys Compd., 2016, 662, 315–319 CrossRef CAS
 .
. - H. Liu, Y. Wang, X. Lu, Y. Hu, G. Zhu, R. Chen, L. Ma, H. Zhu, Z. Tie, J. Liu and Z. Jin, Nano Energy, 2017, 35, 350–357 CrossRef CAS
 .
. - J. Ping, Y. Wang, Q. Lu, B. Chen, J. Chen, Y. Huang, Q. Ma, C. Tan, J. Yang, X. Cao, Z. Wang, J. Wu, Y. Ying and H. Zhang, Adv. Mater., 2016, 28, 7640–7645 CrossRef CAS PubMed
 .
. - D. Zhao, K. Jiang, Y. Pi and X. Huang, ChemCatChem, 2017, 9, 84–88 CrossRef CAS
 .
. - R. F. Chong, G. Wang, Y. Q. Du, Y. S. Jia, X. S. Wang, C. Y. Li, Z. X. Chang and L. Zhang, Chem. Eng. J., 2019, 366, 523–530 CrossRef CAS
 .
. - F. Song and X. Hu, J. Am. Chem. Soc., 2014, 136, 16481–16484 CrossRef CAS
 .
. - Y. Jia, L. Zhang, G. Gao, H. Chen, B. Wang, J. Zhou, M. T. Soo, M. Hong, X. Yan, G. Qian, J. Zou, A. Du and X. Yao, Adv. Mater., 2017, 29, 1700017 CrossRef PubMed
 .
. - T. S. Munondea, H. T. Zheng and P. N. Nomngongob, Ultrason. Sonochem., 2019, 59, 104716 CrossRef PubMed
 .
. - Z. Wang, S. Zeng, W. Liu, X. Wang, Q. Li, Z. Zhao and F. Geng, ACS Appl. Mater. Interfaces, 2017, 9, 1488–1495 CrossRef CAS
 .
. - Y. Wang, Y. Zhang, Z. Liu, C. Xie, S. Feng, D. Liu, M. Shao and S. Wang, Angew. Chem., 2017, 56, 5867–5872 CrossRef CAS
 .
. - T. R. Zhan, Y. Sun, Y. J. Wang, W. Cao, X. E. Liu, H. N. Teng and W. G. Hou, J. Colloid Interface Sci., 2019, 552, 671–677 CrossRef CAS PubMed
 .
. - B. M. Hunter, W. Hieringer, J. R. Winkler, H. B. Gray and A. M. Muller, Energy Environ. Sci., 2016, 9, 1734–1743 RSC
 .
. - W. Ma, R. Ma, C. Wang, J. Liang, X. Liu, K. Zhou and T. Sasaki, ACS Nano, 2015, 9, 1977–1984 CrossRef CAS
 .
. - X. L. Yu, J. Q. Liu, W. C. Yin, T. Wang, L. Quan, Y. Ran, J. Y. Cui, L. Wang and Y. H. Zhang, Appl. Surf. Sci., 2019, 492, 264–271 CrossRef CAS
 .
. - C. Zhang, J. Zhao, L. Zhou, Z. Li, M. Shao and M. Wei, J. Mater. Chem. A, 2016, 4, 11516–11523 RSC
 .
. - B. Chen, Z. Zhang, S. Kim, S. Lee, J. Lee, W. Kim and K. J. Yong, ACS Appl. Mater. Interfaces, 2018, 10, 44518–44526 CrossRef CAS PubMed
 .
. - K. N. Dinh, P. L. Zheng, Z. F. Dai, Y. Zhang, R. Dangol, Y. Zheng, B. Li, Y. Zong and Q. Y. Yan, Small, 2017, 1703257 Search PubMed
 .
. - Y. Y. Wang, D. F. Yan, S. E. Hankari, Y. Q. Zou and S. Y. Wang, Adv. Sci., 2018, 5, 1800064 CrossRef PubMed
 .
. - Z. L. Wang, S. M. Xu, Y. Q. Xu, L. Tan, X. Wang, Y. F. Zhao, H. H. Duan and Y. F. Song, Chem. Sci., 2019, 10, 378–384 RSC
 .
. - Y. F. Zhao, X. Zhang, X. D. Jia, G. I. N. Waterhouse, R. Shi, X. R. Zhang, F. Zhan, Y. Tao, L. Z. Wu, C. H. Tung, D. O'Hare and T. R. Zhang, Adv. Energy Mater., 2018, 1703585 CrossRef
 .
. - Y. Q. Liu, M. Zhang, D. Hu, R. Q. Li, K. Hu and K. Yan, ACS Appl. Energy Mater., 2019, 2, 1162–1168 CrossRef CAS
 .
. - Y. Hou, M. R. Lohe, J. Zhang, S. Liu, X. Zhuang and X. Feng, Energy Environ. Sci., 2016, 9, 478–483 RSC
 .
. - X. Zhang, Y. F. Zhao, Y. X. Zhao, R. Shi, G. I. N. Waterhouse and T. R. Zhang, Adv. Energy Mater., 2019, 9, 1900881 CrossRef
 .
. - X. D. Jia, X. Zhang, J. Q. Zhao, Y. F. Zhao, Y. X. Zhao, G. I. N. Waterhouse, R. Shi, L. Z. Wu, C. H. Tung and T. R. Zhang, J. Energy Chem., 2019, 34, 57–63 CrossRef
 .
. - R. Q. Li, Y. Q. Liu, H. B. Li, M. Zhang, Y. R. Lu, L. Zhang, J. P. Xiao, F. Boehm and K. Yan, Small Methods, 2019, 3, 1800344 CrossRef CAS
 .
. - X. Long, J. Li, S. Xiao, K. Yan, Z. Wang, H. Chen and S. Yang, Angew. Chem., Int. Ed., 2014, 53, 7584–7588 CrossRef CAS
 .
. - Z. Q. Hu and G. M. Chen, Adv. Mater., 2014, 26, 5950–5956 CrossRef CAS
 .
. - L. Y. Wu, Z. Q. Hu, G. M. Chen and Z. B. Li, Soft Matter, 2015, 11, 9038–9044 RSC
 .
. - H. Q. Huang, J. B. Xu, K. C. Wei, Y. J. Xu, C. K. K. Choi, M. L. Zhu and L. M. Bian, Macromol. Biosci., 2016, 16, 1019–1026 CrossRef CAS PubMed
 .
. - Z. Q. Hu and G. G. Chen, RSC Adv., 2013, 3, 12021–12025 RSC
 .
. - N. J. M. Sebri, A. F. A. Latip, R. Adnan, M. H. Hussin and T. Kobayashi, J. Appl. Polym. Sci., 2020, 137, 48637 CrossRef CAS
 .
. - X. J. Yang, P. Zhang, P. Li, Z. T. Li, W. Xia, H. Y. Zhang, Z. G. Di, M. L. Wang, H. Q. Zhang and Q. S. J. Niu, J. Mol. Liq., 2019, 280, 128–134 CrossRef CAS
 .
. - Y. P. Zhang, J. W. Ji, H. P. Li and W. G. Hou, Soft Matter, 2018, 14, 1789 RSC
 .
. - D. Zha, H. Sun, Y. Fu, X. Ouyang and X. Wang, Electrochim. Acta, 2017, 236, 18–27 CrossRef CAS
 .
. - X. Li, J. Zai, Y. Liu, X. He, S. Xiang, Z. Ma and X. Qian, J. Power Sources, 2016, 325, 675–681 CrossRef CAS
 .
. - F. Lai, Y.-E. Miao, L. Zuo, H. Lu, Y. Huang and T. Liu, Small, 2016, 12, 3235–3244 CrossRef CAS PubMed
 .
. - G. Nagaraju, G. S. R. Raju, Y. H. Ko and J. S. Yu, Nanoscale, 2016, 8, 812–825 RSC
 .
. - F. Gu, X. Cheng, S. Wang, X. Wang and P. S. Lee, Small, 2015, 11, 2044–2050 CrossRef CAS PubMed
 .
. - X. Wang, X. Li, X. Du, X. Ma, X. Hao, C. Xue, H. Zhu and S. Li, Electroanalysis, 2017, 29, 1–9 CrossRef CAS
 .
. - J. Wu, W.-W. Liu, Y.-X. Wu, T.-C. Wei, D. Geng, J. Mei, H. Liu, W.-M. Lau and L.-M. Liu, Electrochim. Acta, 2016, 203, 21–29 CrossRef CAS
 .
. - H. N. Xing, Y. Y. Lan, Y. Zong, Y. Sun, X. H. Zhu, X. H. Li and X. L. Zheng, Inorg. Chem. Commun., 2019, 101, 125–129 CrossRef CAS
 .
. - X. L. Guo, X. Y. Liu, X. D. Hao, S. J. Zhu, F. Dong, Z. Q. Wen and Y. X. Zhang, Electrochim. Acta, 2016, 194, 179–186 CrossRef CAS
 .
. - R. Zhang, H. An, Z. Li, M. Shao, J. Han and M. Wei, Chem. Eng. J., 2016, 289, 85–92 CrossRef CAS
 .
. - F. Lai, Y. Huang, Y.-E. Miao and T. Liu, Electrochim. Acta, 2015, 174, 456–463 CrossRef CAS
 .
. - M. Li, J. Cheng, J. Wang, F. Liu and X. Zhang, Electrochim. Acta, 2016, 206, 108–115 CrossRef CAS
 .
. - X. Y. Gao, Y. F. Zhao, K. Q. Dai, J. T. Wang, B. Zhang and X. J. Shen, Chem. Eng. J., 2020, 384, 123373 CrossRef CAS
 .
. - X. Wang, Y. Zheng, J. Yuan, J. Shen, J. Hu, A.-J. Wang, L. Wu and L. Niu, Electrochim. Acta, 2017, 224, 628–635 CrossRef CAS
 .
. - F. Lai, Y. E. Miao, L. Zuo, H. Lu, Y. Huang and T. Liu, Small, 2016, 12, 3199 CrossRef
 .
. - C. Wang, X. Zhang, X. Sun and Y. Ma, Electrochim. Acta, 2016, 191, 329–336 CrossRef CAS
 .
. - R. Ma, X. Liu, J. Liang, Y. Bando and T. Sasaki, Adv. Mater., 2014, 26, 4173–4178 CrossRef CAS
 .
. - Y. L. Li, L. N. Shan, Y. W. Sui, J. Q. Qi, F. X. Wei, Y. Z. He, Q. K. Meng, Y. J. Ren and J. L. Liu, J. Mater. Sci.: Mater. Electron., 2019, 30, 13360–13371 CrossRef CAS
 .
. - K. Ma, J. Cheng, J. Zhang, M. Li, F. Liu and X. Zhang, Electrochim. Acta, 2016, 198, 231–240 CrossRef CAS
 .
. - Z. Huang, S. Wang, J. Wang, Y. Yu, J. Wen and R. Li, Electrochim. Acta, 2015, 152, 117–125 CrossRef CAS
 .
. - T. Wang, S. Zhang, X. Yan, M. Lyu, L. Wang, J. Bell and H. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 15510–15524 CrossRef CAS PubMed
 .
. - Z. Yang, X. M. Wang, H. Zhang, S. H. Yan, C. Zhang and S. X. Liu, ChemElectroChem, 2019, 6, 4456–4463 CrossRef CAS
 .
. - J. Zhao, J. Chen, S. Xu, M. Shao, Q. Zhang, F. Wei, J. Ma, M. Wei, D. G. Evans and X. Duan, Adv. Funct. Mater., 2014, 24, 2938–2946 CrossRef CAS
 .
. - H. Chen, L. Hu, M. Chen, Y. Yan and L. Wu, Adv. Funct. Mater., 2014, 24, 934–942 CrossRef CAS
 .
. - X. Zhou, X. H. Li, D. J. Chen, D. Y. Zhao and X. T. Huang, J. Mater. Chem. A, 2018, 6, 24603–24613 RSC
 .
. - S. C. Sekhar, G. Nagaraju and J. S. Yu, Nano Energy, 2017, 36, 58–67 CrossRef CAS
 .
. - Y. F. Zhao, Q. Wang, T. Bian, H. J. Yu, H. Fan, C. Zhou, L. Z. Wu, C. H. Tung, D. O'Harec and T. R. Zhang, Nanoscale, 2015, 7, 7168–7173 RSC
 .
. - L. Li, W. Y. Gu, J. Liu, S. Y. Yan and Z. P. Xu, Nano Res., 2015, 8, 682–694 CrossRef CAS
 .
. - J.-H. Choy, S.-Y. Kwak, J.-S. Park, Y.-J. Jeong and J. Portier, J. Am. Chem. Soc., 1999, 121, 1399–1400 CrossRef CAS
 .
. - Z. L. Liu, D. Y. Tian, S. P. Li, X. D. Li and T. H. Lu, Int. J. Pharm., 2014, 473, 414–425 CrossRef CAS
 .
. - L. Li, W. Y. Gu, J. Z. Chen, W. Y. Chen and Z. P. Xu, Biomaterials, 2014, 35, 3331–3339 CrossRef CAS PubMed
 .
. - D. H. Park, J. E. Kim, J. M. Oh, Y. G. Shul and J. H. Choy, J. Am. Chem. Soc., 2010, 132, 16735–16736 CrossRef CAS
 .
. - S. Bégu, A. Aubert-Pouëssel, R. Polexe, E. Leitmanova, D. A. Lerner, J.-M. Devoisselle and D. Tichit, Chem. Mater., 2009, 21, 2679–2687 CrossRef
 .
. - R. Z. Liang, R. Tian, L. N. Ma, L. L. Zhang, Y. L. Hu, J. Wang, M. Wei, D. Yan, D. G. Evans and X. Duan, Adv. Funct. Mater., 2014, 24, 3144–3151 CrossRef CAS
 .
. - Y. Li, H. Y. Bi, G. W. Wang, N. Wang, C. X. Chen, Z. Z. Li and X. M. Fan, J. Dispersion Sci. Technol., 2016, 37, 366–373 CrossRef CAS
 .
. - L. Li, Z. Gu, W. Y. Gu and Z. P. Xu, RSC Adv., 2016, 6, 95518 RSC
 .
. - Y. Li, W. L. Bao, H. Y. Wu, J. Y. Wang, Y. Zhang, Y. L. Wan, D. P. Cao, D. O'Hare and Q. Wang, Sci. Bull., 2017, 62, 686–692 CrossRef CAS
 .
. - Y. P. Zhang, H. P. Li, N. Du, S. E Song and W. G. Hou, Appl. Clay Sci., 2017, 143, 336–344 CrossRef CAS
 .
. - Y. P. Zhang, X. W. Wu, H. P. Li, N. Du, S. E. Song and W. G. Hou, Colloids Surf., A, 2017, 529, 824–831 CrossRef CAS
 .
. - S. Q. Liu, S. P. Li and X. D. Li, Appl. Surf. Sci., 2015, 330, 253–261 CrossRef CAS
 .
. - Z. L. Liu, Q. Y. Jia, X. F. Zhao, S. P. Li, J. Shen and X. D. Li, Chem. Res. Chin. Univ., 2019, 35(5), 901–907 CrossRef CAS
 .
. - X. Mei, T. T. Hu, Y. J. Wang, X. S. Weng, R. Z. Liang and M. Wei, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2019, 12, e1596 Search PubMed
 .
. - J. Y. Wang, W. L. Bao, A. Umar, Q. Wang, D. O'Hare and Y. L. Wan, J. Biomed. Nanotechnol., 2016, 12, 922–933 CrossRef CAS
 .
. - X. W. Wu, H. P. Li, S. E. Song, R. J. Zhang and W. G. Hou, Int. J. Pharm., 2013, 454, 453–461 CrossRef CAS
 .
. - X. Mei, S. M. Xu, T. Y. Hu, L. Q. Peng, R. Gao, R. Z. Liang, M. Wei, D. G. Evans and X. Duan, Nano Res., 2018, 11, 195–205 CrossRef CAS
 .
. - L. Q. Peng, X. Mei, J. He, J. K. Xu, W. K. Zhang, R. Z. Liang, M. Wei, D. G. Evans and X. Duan, Adv. Mater., 2018, 1707389 CrossRef
 .
. - L. Yan, M. J. Zhou, X. J. Zhang, L. B. Huang, W. Chen, V. A. L. Roy, W. J. Zhang and X. F. Chen, ACS Appl. Mater. Interfaces, 2017, 9(39), 34185–34193 CrossRef CAS PubMed
 .
.
|
| This journal is © The Royal Society of Chemistry 2021 |
 Open Access Article
Open Access Article *,
Huifang Xu and
Song Lu
*,
Huifang Xu and
Song Lu



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were pre-synthesized by a hydrothermal method, then they were exfoliated into ultrathin LDH nanosheets by Ar plasma etching, which destroys the ionic bonds and hydrogen bonds in the interlayers of the bulk LDH, disturbing the normal charge balance and separating the positively charged brucite-like host layers from each other.
1 were pre-synthesized by a hydrothermal method, then they were exfoliated into ultrathin LDH nanosheets by Ar plasma etching, which destroys the ionic bonds and hydrogen bonds in the interlayers of the bulk LDH, disturbing the normal charge balance and separating the positively charged brucite-like host layers from each other.















![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k molar ratios of 1
k molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 3
1 or 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), ZnCo-UF nanosheets were synthesized by exfoliating the bulk Znn/Cok–LDH (ZnCo-bulk) in formamide. The prepared ZnCo-UF with a mean size of ∼3.5 nm and thickness of ∼0.5 nm were tested as a catalyst for OER reactions (Fig. 21). ZnCo-UF (2
1), ZnCo-UF nanosheets were synthesized by exfoliating the bulk Znn/Cok–LDH (ZnCo-bulk) in formamide. The prepared ZnCo-UF with a mean size of ∼3.5 nm and thickness of ∼0.5 nm were tested as a catalyst for OER reactions (Fig. 21). ZnCo-UF (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) exhibited outstanding OER activity compared with ZnCo-bulk, with the current density of 168 mA cm−2 almost 20 times higher than that of ZnCo-bulk (8.5 mA cm−2) under a potential at 1.8 V. Moreover, comparing with ZnCo-UF prepared at Zn
1) exhibited outstanding OER activity compared with ZnCo-bulk, with the current density of 168 mA cm−2 almost 20 times higher than that of ZnCo-bulk (8.5 mA cm−2) under a potential at 1.8 V. Moreover, comparing with ZnCo-UF prepared at Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratios of 1
Co ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, ZnCo-UF (2
1, ZnCo-UF (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) displayed superior performance with a lower overpotential for OER, higher current density and the lowest OER Tafel slope of 66 mV dec−1. The prepared ZnCo-UF were active for hydrogen evolution reactions. The high current density, low OER overpotential, small charge transfer resistance and large eletroactive surface area make ZnCo-UF (2
1) displayed superior performance with a lower overpotential for OER, higher current density and the lowest OER Tafel slope of 66 mV dec−1. The prepared ZnCo-UF were active for hydrogen evolution reactions. The high current density, low OER overpotential, small charge transfer resistance and large eletroactive surface area make ZnCo-UF (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) an excellent electrocatalyst for OER.
1) an excellent electrocatalyst for OER.












.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.

