DOI:
10.1039/D1RA03022A
(Paper)
RSC Adv., 2021,
11, 19639-19646
Synthesis of tetrahydro-β-carbolines from 2-indolylmethyl azides and propargylic alcohols†
Received
18th April 2021
, Accepted 21st May 2021
First published on 1st June 2021
Abstract
A facile and efficient route to tetrahydro-β-carbolines from 2-indolylmethyl azides and propargylic alcohols via acid-catalyzed dehydrative annulation reactions is described. This reaction proceeds through a cascade sequence of Friedel–Crafts-type alkylation followed by intramolecular “Click” reaction, involving the formation of multiple chemical bonds in a single operation with excellent atom-economy and broad functional group tolerance.
Introduction
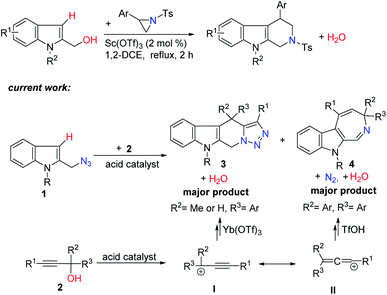
Tetrahydro-β-carboline (THBC) derivatives are very important alkaloids with various biological activities and pharmaceutical applications and with extensive occurrence in natural products.1 Consequently, intense efforts have been devoted to the development of efficient methods for their synthesis and significant progress has been achieved. However, most current methods such as the classical Pictet–Spengler reaction are limited in product scope as mostly only products with substitution at the 1-position of the THBC ring are accessible.2 Therefore, the development of methods with wide product diversity is still highly desirable.
Recently, our group have developed cascade processes using N-sulfonyl aziridines as a nitrogen source and simple electron-rich benzylic alcohols as a versatile three-carbon synthon for the synthesis of tetrahydro-β-carbolines and tetrahydroisoquinolines.3 We then reasoned that the use of 2-indolylmethyl azides as a nitrogen source and the use of readily available propargylic alcohols as a three-carbon synthon may enable access to compounds with novel structures not easily accessible by other methods. One major challenge associated with this strategy is the competitive Friedel–Crafts-type alkylations of 1 between the two resonance forms I and II of the carbocation intermediate generated from 2 in the presence of an acid catalyst (Scheme 1).4 We disclose here that the selectivity of this process hinges on both the use of different acid catalysts and the substituents on propargylic alcohols, providing a new atom-economic way to tetracyclic tetrahydro-β-carboline derivatives with a fused triazole structure and indole azepines.5
 |
| | Scheme 1 Lewis acid-catalyzed dehydrative annulation way to tetrahydro-β-carboline derivatives. | |
Results and discussion
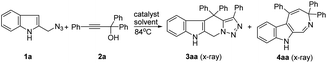
The reaction of 2-(azidomethyl)-1H-indole 1a and propargylic alcohol 2a was selected as a model reaction for optimization of reaction conditions (Table 1). Using 1,2-dichloroethane (1,2-DCE) as solvent, four different rare-earth metal triflates were screened, and two products 3aa and 4aa were generally obtained (Table 1, entries 2–5). Yb(OTf)3 was found to be the most efficient catalyst for this reaction, providing the highest overall yield of tetrahydro-β-carboline 3 and azepine 4 (Table 1, entry 5). No reaction occurred in the absence of the catalyst or when the reaction was performed at room temperature (Table 1, entries 1 and 6). Changing the solvent to toluene, DMF, 1,4-dioxane, or THF gave inferior results (Table 1, entries 7–10). Further screen of catalyst loading amount revealed that 10 mol% was optimal for the reaction, while lower (5 mol%) or higher (20 mol%) loadings all led to no improvement in both overall yield and product selectivity (Table 1, entries 11 and 12). Interestingly, when the Brønsted acid TfOH was used as the catalyst, the azepine 4aa was the major product, albeit in a relatively lower overall yield (Table 1, entry 13). It is worth mentioning that the reaction is tolerant of moisture and air and could be performed in commercial solvents under open air. The structures of the products 3aa (Fig. 1) and 4aa were additionally confirmed by X-ray crystallographic analysis (see ESI† for details).
Table 1 Screening of the reaction conditionsa
|
| Entry |
Catalyst (mol%) |
Solvent |
Time (h) |
Yieldb (%) |
| 3aa |
4aa |
| Reaction conditions: 1a (0.5 mmol), 2a (0.5 mmol), solvent (5 mL), the reaction was monitored by TLC. Yield of the isolated product. Reaction was run at 25 °C. Reaction was run at 90 °C. Reaction was run at 66 °C. Dried 1,2-DCE under argon atmosphere. |
| 1 |
No catalyst |
1,2-DCE |
24 |
0 |
0 |
| 2 |
Sc(OTf)3 (10%) |
1,2-DCE |
14 |
20 |
45 |
| 3 |
Y(OTf)3 (10%) |
1,2-DCE |
14 |
19 |
42 |
| 4 |
La(OTf)3 (10%) |
1,2-DCE |
24 |
15 |
35 |
| 5 |
Yb(OTf)3 (10%) |
1,2-DCE |
18 |
25 |
51 |
| 6c |
Yb(OTf)3 (10%) |
1,2-DCE |
24 |
0 |
0 |
| 7d |
Yb(OTf)3 (10%) |
Toluene |
18 |
15 |
46 |
| 8d |
Yb(OTf)3 (10%) |
DMF |
24 |
0 |
0 |
| 9d |
Yb(OTf)3 (10%) |
1,4-Dioxane |
18 |
16 |
41 |
| 10e |
Yb(OTf)3 (10%) |
THF |
18 |
22 |
49 |
| 11 |
Yb(OTf)3 (5%) |
1,2-DCE |
18 |
20 |
36 |
| 12 |
Yb(OTf)3 (20%) |
1,2-DCE |
18 |
23 |
50 |
| 13 |
TfOH (20%) |
1,2-DCE |
18 |
Trace |
53 |
| 14 |
TsOH (20%) |
1,2-DCE |
18 |
15 |
35 |
| 15f |
Yb(OTf)3 (10%) |
1,2-DCE |
18 |
25 |
50 |
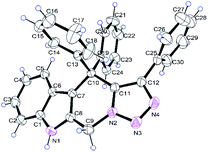 |
| | Fig. 1 Crystal structure of compound 3aa. | |
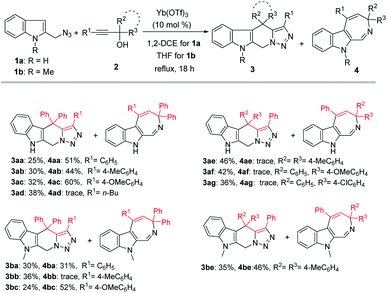
With the optimized reaction conditions in hand, a series of propargylic alcohols 2 were reacted with 2-(azidomethyl)-1H-indole 1a to examine the reaction scope with regard to the formation of tetrahydro-β-carboline 3 and azepine 4. In general, the two products were produced in poor ratios, with the yields of 3 ranging from 25% to 46%. Substrate 2d derived from an aliphatic alkyne (R1 = n-Bu) was also suitable in this reaction (Scheme 2, 3ad), while only trace of the corresponding product 4ad was detected. As for the products azepines 4, propargylic alcohols 2 bearing electron-donating substituents on the aryl group (R1) provided higher yields than others (Scheme 2, 4ac–4ad).
 |
| | Scheme 2 Scope study with different propargylic alcohols 2. aReaction conditions: 1a (0.5 mmol), 2 (0.5 mmol), Yb(OTf)3 (0.05 mmol), solvent (5 mL), reflux. bIsolated yield refers to azide. | |
Next, N-methyl-2-(azidomethyl)-1H-indole 1b was also examined in the reaction, under similar reaction conditions except that the reactions were performed at a lower reaction temperature using THF as solvent. In general, comparable results were obtained as to those obtained with 1a.
Notably, when tertiary propargylic alcohols 2 with either of the two substituents (R2, R3) being an alkyl group (Me) or secondary propargylic alcohols were used in this reaction, only tetrahydro-β-carbolines 3 were isolated in moderate yields (Scheme 3). The reduced steric hindrance in the carbocation intermediate I (Scheme 1) might be partly responsible for this product selectivity. Propargylic alcohols 2 bearing electron-donating substituents on aryl groups (R2) provided higher yields than those with electron-withdrawing ones (Scheme 3, 3ah–3ao). Moreover, when 9-fluorenyl-substituted propargylic alcohols 2p–2s were subjected to the reaction conditions, spirocyclic products 3ap–3as could be formed in the yields of 51–56% as the only products.
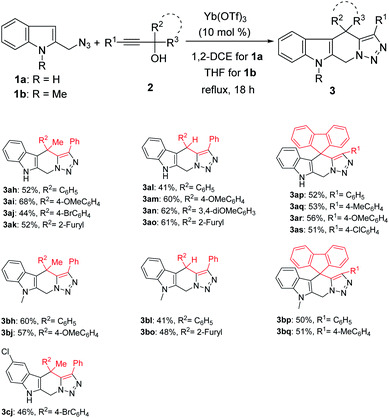 |
| | Scheme 3 Scope study with other propargylic alcohols. aReaction conditions: 1 (0.5 mmol), 2 (0.5 mmol), Yb(OTf)3 (0.05 mmol), solvent (5 mL), reflux. bIsolated yield refers to azide. | |
We also probed the reaction scope with regard to the synthesis of indole azepines via the TfOH-catalyzed formal [4 + 3]-annulation route. In general, only propargylic alcohols 2 bearing an electron-donating substituent on one of the aryl groups (R1, R2, R3) worked in this system to provide the corresponding indole azepines in moderate yields (Scheme 4). While the use of 1a or 1b also gave comparable results, the corresponding tetrahydro-β-carboline products 3 were detected in only trace amount in these cases.
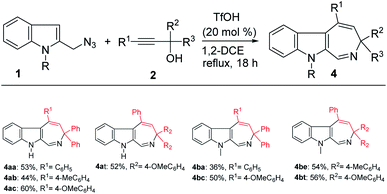 |
| | Scheme 4 Scope study on the formation of the indole azepines. aReaction conditions: 1 (0.5 mmol), 2 (0.5 mmol), TfOH (0.1 mmol), 1,2-DCE (5 mL), reflux. bIsolated yield. | |
Based on the above experimental results, a plausible mechanism for the present cascade reactions was proposed (Scheme 5). First, in the presence of an acid catalyst, propargylic alcohol 2a would be converted to the propargylic carbocation I, which is in equilibrium with the allenic form II. Subsequent Friedel–Crafts-type reaction with 1a would form the propargylic intermediate IV (from I) or allenic intermediate III (from II). Then intermediate IV would be transformed to the final product tetrahydro-β-carboline 3aa by an intramolecular click reaction, while the allenic intermediate III would be transformed to the azepine product 4aa via an acid-catalyzed allene hydroamination with the concurrent release of a molecule of N2. Both the nature of the acid catalyst and the electronic and steric effects of the substituents on the propargylic alcohols have influence on the product selectivity of the reaction.
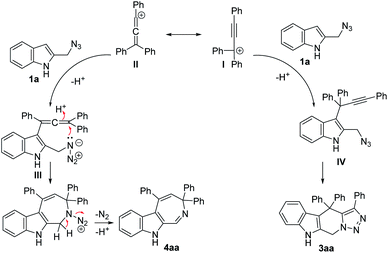 |
| | Scheme 5 A possible mechanism for the dehydrative annulations. | |
Conclusions
In summary, two cascade reactions of 2-indolylmethyl azides and propargylic alcohols were developed by using simple Lewis acid and Brønsted acid catalysts. The good atom- and step-economy, readily available starting materials, easy operation, and mild reaction conditions render this method a useful alternative way to tetrahydro-β-carbolines and indole azepines. Efforts towards the utilization of the propargylic alcohols to the synthesis of other useful cyclic compounds are underway in our laboratories.
Experimental section
General comments
Infrared spectra were obtained on a FTIR spectrometer. 1H NMR spectra were recorded on 300 MHz or 400 MHz spectrometer in DMSO-d6 or CDCl3 solution and the chemical shifts were reported relative to internal standard TMS (0 ppm). The following abbreviations are used to describe peak patterns where appropriate: br = broad, s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet. Coupling constants are reported in hertz (Hz). 13C NMR were recorded on 75 MHz or 100 MHz and referenced to the internal solvent signals (central peak is 40.00 ppm in DMSO-d6 or 77.00 ppm in CDCl3). HRMS data were obtained using ESI ionization. Melting points were measured with micro melting point apparatus.
The 2-(azidomethyl)-1H-indoles 1 were prepared from indole-2-methanols,6 and the indole-2-methanols were prepared from indole-2-carboxylates.7 The propargylic alcohols 2 were prepared from phenylacetylene and benzophenone according to the published methods.8 All commercially available reagents and solvents were used without further purification unless noted otherwise.
General procedure for the synthesis of 3
A solution of indole 1 (0.5 mmol), propargylic alcohols 2 (0.5 mmol) and Yb(OTf)3 (0.05 mmol) in 1,2-DCE (5 mL, for 1a) at 84 °C or THF (5 mL, for 1b) at 66 °C was stirred under open air for 18 h. After being cooled down to room temperature, the mixture was diluted with ethyl acetate (50 mL), washed with saturated NaCl solution (10 mL) and dried over anhydrous Na2SO4. The solvent was evaporated and the crude product was purified by silica gel column chromatography with petroleum ether/ethyl acetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
General procedure for the synthesis of 4
A solution of 2-(azidomethyl)-1H-indole 1 (0.5 mmol), the propargylic alcohols 2 (0.5 mmol) and TfOH (0.05 mmol) in 1,2-DCE (5 mL) was stirred under air at 84 °C for 18 h. After being cooled down to room temperature, the mixture was diluted with ethyl acetate (50 mL), washed with saturated NaCl solution (10 mL) and dried over anhydrous Na2SO4. The solvent was evaporated and the crude product was purified by silica gel column chromatography with petroleum ether/ethyl acetate (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
Characterization data of products
3,4,4-Triphenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3aa). White solid (55 mg, 25%); mp 349–350 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.51 (s, 1H), 7.40 (d, J = 8.1 Hz, 1H), 7.26–7.10 (m, 7H), 7.10–6.96 (m, 7H), 6.87–6.70 (m, 3H), 6.51 (d, J = 8.0 Hz, 1H), 5.89 (s, 2H); 13C NMR (75 MHz, DMSO-d6) δ 144.3, 143.2, 137.8, 137.6, 132.2, 129.6, 129.1, 129.0, 128.4, 127.9, 127.8, 127.4, 125.0, 121.8, 119.2, 119.8, 115.0, 112.3, 51.4, 45.2; IR (KBr) ν 3274, 3055, 1618, 1487, 1328, 1130, 743, 700 cm−1; HRMS: m/z calcd for ([C30H22N4 + H]+): 439.1917; found: 439.1914.
4,4-Diphenyl-3-(p-tolyl)-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3ab). White solid (68 mg, 30%); mp 275–276 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.48 (s, 1H), 7.46–7.32 (m, 1H), 7.22–7.07 (m, 6H), 7.07–6.95 (m, 5H), 6.83 (d, J = 7.9 Hz, 2H), 6.72 (dd, J = 8.0, 7.2 Hz, 1H), 6.62 (d, J = 8.0 Hz, 2H), 6.49 (d, J = 8.0 Hz, 1H), 5.86 (s, 2H), 2.18 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δ 144.3, 143.4, 137.9, 137.7, 137.2, 129.5, 129.4, 129.2, 129.0, 128.5, 128.5, 127.5, 125.1, 121.9, 120.0, 119.8, 115.0, 112.4, 51.4, 45.2, 21.3; IR (KBr) ν 3047, 2922, 1636, 1491, 1342, 1021, 743, 700 cm−1; HRMS: m/z calcd for ([C31H24N4 + H]+): 453.2074; found: 453.2071.
3-(4-Methoxyphenyl)-4,4-diphenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3ac). White solid (75 mg, 32%); mp 310–311 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.47 (s, 1H), 7.37 (d, J = 8.1 Hz, 1H), 7.21–7.08 (m, 6H), 7.07–6.95 (m, 5H), 6.73 (t, J = 7.6 Hz, 1H), 6.68–6.62 (m, 2H), 6.61–6.54 (m, 2H), 6.50 (d, J = 8.0 Hz, 1H), 5.85 (s, 2H), 3.65 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δ 144.3, 143.4, 137.9, 137.7, 137.2, 129.5, 129.4, 129.2, 129.0, 128.5, 128.5, 127.5, 125.1, 121.9, 120.0, 119.8, 115.0, 112.4, 51.4, 45.2, 21.3; IR (KBr) ν 3273, 3055, 2924, 1618, 1498, 1249, 1173, 1025, 739, 703 cm−1; HRMS: m/z calcd for ([C31H24N4O + H]+): 469.2032; found: 469.2028.
3-Butyl-4,4-diphenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3ad). White solid (80 mg, 38%); mp 257–258 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.49 (s, 1H), 7.41 (d, J = 8.1 Hz, 1H), 7.34–7.23 (m, 6H), 7.15–6.98 (m, 5H), 6.78 (t, J = 7.6 Hz, 1H), 6.51 (d, J = 8.0 Hz, 1H), 5.72 (s, 2H), 1.94 (t, J = 7.2 Hz, 2H), 1.21–1.07 (m, 2H), 1.07–0.92 (m, 2H), 0.64 (t, J = 7.2 Hz, 3H); 13C NMR (75 MHz, DMSO-d6) δ 143.9, 137.5, 136.7, 129.9, 128.9, 128.8, 127.6, 125.5, 121.8, 119.8, 114.9, 112.3, 50.9, 44.8, 30.7, 25.5, 22.4, 14.0; IR (KBr) ν 3049, 2922, 1618, 1508, 1460, 1108, 743, 624 cm−1; HRMS: m/z calcd for ([C28H26N4 + H]+): 419.2230; found: 419.2232.
3-Phenyl-4,4-di-p-tolyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3ae). White solid (107 mg, 46%); mp 358–359 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.45 (s, 1H), 7.38 (d, J = 8.1 Hz, 1H), 7.16 (t, J = 7.3 Hz, 1H), 7.11–6.97 (m, 3H), 6.97–6.83 (m, 8H), 6.81–6.69 (m, 3H), 6.55 (d, J = 8.0 Hz, 1H), 5.85 (s, 2H), 2.19 (s, 6H); 13C NMR (75 MHz, DMSO-d6) δ 144.2, 140.5, 138.2, 137.6, 136.4, 132.3, 129.6, 129.0, 128.9, 128.8, 127.7, 125.0, 121.8, 120.1, 119.7, 115.2, 112.2, 50.8, 45.1, 21.0; IR (KBr) ν 3049, 2922, 1636, 1509, 1133, 1007, 747, 620 cm−1; HRMS: m/z calcd for ([C32H26N4 + H]+): 467.2230; found: 467.2228.
4-(4-Methoxyphenyl)-3,4-diphenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3af). White solid (98 mg, 42%); mp 320–321 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.49 (s, 1H), 7.40 (d, J = 8.1 Hz, 1H), 7.28–6.98 (m, 9H), 6.95 (d, J = 8.8 Hz, 2H), 6.89–6.73 (m, 3H), 6.69 (d, J = 8.8 Hz, 2H), 6.55 (d, J = 8.0 Hz, 1H), 5.88 (s, 2H), 3.65 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δ 144.2, 140.5, 138.2, 137.6, 136.4, 132.3, 129.6, 129.0, 128.9, 128.8, 127.7, 125.0, 121.8, 120.1, 119.7, 115.2, 112.2, 50.8, 45.1, 21.0. IR (KBr) ν 2991, 2887, 1636, 1509, 1249, 1180, 750, 696 cm−1; HRMS: m/z calcd for ([C31H24N4 + H]+): 469.2023; found: 469.2024.
4-(4-Chlorophenyl)-3,4-diphenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3ag). White solid (85 mg, 36%); mp 315–316 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.54 (s, 1H), 7.40 (d, J = 8.1 Hz, 1H), 7.25–7.12 (m, 6H), 7.12–6.94 (m, 7H), 6.87–6.72 (m, 3H), 6.54 (d, J = 8.0 Hz, 1H), 5.89 (AB, J = 18.9 Hz, 2H); 13C NMR (75 MHz, DMSO-d6) δ 144.2, 140.5, 138.2, 137.6, 136.4, 132.3, 129.6, 129.0, 128.9, 128.8, 127.7, 125.0, 121.8, 120.1, 119.7, 115.2, 112.2, 50.8, 45.1, 21.0. IR (KBr) ν 3028, 2908, 1598, 1483, 1342, 1163, 661, 547 cm−1; HRMS: m/z calcd for ([C30H21ClN4 + H]+): 473.1528; found: 473.1525.
4-Methyl-3,4-diphenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3ah). White solid (98 mg, 52%); mp 310–311 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.34 (s, 1H), 7.37 (d, J = 8.1 Hz, 1H), 7.34–7.12 (m, 9H), 7.03 (t, J = 7.5 Hz, 1H), 6.90–6.74 (m, 3H), 6.03 (AB J = 16.9 Hz, 2H), 1.92 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 146.9, 142.6, 139.1, 137.9, 132.6, 129.3, 128.7, 128.4, 127.6, 127.0, 125.9, 124.1, 121.9, 119.4, 119.3, 114.0, 112.1, 45.0, 24.8. IR (KBr) ν 2987, 1625, 1132, 1242, 1007, 746, 703 cm−1; HRMS: m/z calcd for ([C25H20N4 + H]+): 377.1761; found: 377.1757.
4-(4-Methoxyphenyl)-4-methyl-3-phenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3ai). White solid (138 mg, 68%); mp 315–316 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.31 (s, 1H), 7.37 (d, J = 8.0 Hz, 1H), 7.33–7.15 (m, 6H), 7.03 (t, J = 7.5 Hz, 1H), 6.89–6.73 (m, 5H), 6.01 (AB, J = 16.9 Hz, 2H), 3.70 (s, 3H), 1.90 (s, 3H). 13C NMR (75 MHz, DMSO) δ 158.1, 142.6, 139.3, 138.9, 137.9, 132.7, 129.3, 128.7, 128.3, 128.3, 125.8, 124.2, 121.9, 119.5, 119.3, 114.3, 113.9, 112.0, 55.5, 45.0, 40.8, 25.1. IR (KBr) ν 2948, 2832, 1607, 1509, 1260, 1184,1021, 837, 739, 703 cm−1; HRMS: m/z calcd for ([C26H22N4O + H]+): 407.1866; found: 407. 1867.
4-(4-Bromophenyl)-4-methyl-3-phenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3aj). White solid (100 mg, 44%); mp 327–328 °C, 1H NMR (300 MHz, DMSO-d6) δ 11.39 (s, 1H), 7.47–7.35 (m, 3H), 7.35–7.12 (m, 6H), 7.05 (t, J = 7.6 Hz, 1H), 6.92–6.78 (m, 3H), 6.03 (AB, 16.9 Hz, 2H), 1.91 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 146.1, 142.7, 138.5, 137.9, 132.4, 131.4, 130.0, 129.4, 128.4, 126.2, 123.9, 122.0, 120.1, 119.4, 119.2, 113.5, 112.1, 45.0, 25.1. IR (KBr) ν 2942, 1621, 1487, 1328, 1075, 1010, 743, 700 cm−1; HRMS: m/z calcd for ([C25H19BrN4 + H]+): 455.0866; found: 455.0865.
4-(Furan-2-yl)-4-methyl-3-phenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3ak). White solid (95 mg, 52%); mp 290–291 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.37 (s, 1H), 7.44–7.34 (m, 3H), 7.34–7.24 (m, 3H), 7.11–7.02 (m, 1H), 7.00–6.84 (m, 3H), 6.52–6.45 (m, 1H), 6.40–6.35 (m, 1H), 5.92 (AB, J = 17.1 Hz, 2H), 1.86 (s, 3H). 13C NMR (75 MHz, DMSO) δ 157.7, 143.2, 142.5, 137.8, 135.9, 132.4, 129.2, 128.6, 128.5, 126.6, 124.2, 122.1, 119.6, 119.3, 112.2, 111.1, 110.8, 106.9, 45.0, 37.5, 25.3. IR (KBr) ν 2980, 2893, 1625, 1459, 1336, 1234, 1007, 743, 700 cm−1; HRMS: m/z calcd for ([C23H18N4O + H]+): 367.1553; found: 367.1558.
3,4-Diphenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3al). White solid (74 mg, 41%); mp 330–331 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.35 (s, 1H), 7.74 (d, J = 7.2 Hz, 2H), 7.48 (d, J = 7.8 Hz, 1H), 7.43–7.20 (m, 6H), 7.20–6.97 (m, 4H), 6.92 (t, J = 7.5 Hz, 1H), 6.24–6.02 (m, 2H), 6.00–5.81 (m, 1H), 13C NMR (75 MHz, DMSO-d6) δ 143.0, 141.9, 137.6, 133.7, 131.5, 128.8, 128.7, 128.5, 128.0, 127.3, 127.0, 125.2, 122.1, 119.4, 119.1, 111.9, 109.2, 45.3, 37.2. IR (KBr) ν 2933, 1607, 1516, 1462, 1271, 1133, 1021, 739, 700 cm−1; HRMS: m/z calcd for ([C24H18N4 + H]+): 363.1604; found: 363.1604.
4-(4-Methoxyphenyl)-3-phenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3am). White solid (118 mg, 60%); mp 282–283 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.31 (s, 1H), 7.82–7.72 (m, 2H), 7.47 (d, J = 7.8 Hz, 1H), 7.41–7.30 (m, 3H), 7.30–7.14 (m, 3H), 7.06 (t, J = 7.2 Hz, 1H), 6.92 (t, J = 7.2 Hz, 1H), 6.65 (d, J = 8.7 Hz, 2H), 6.17–6.01 (m, 2H), 5.94–5.83 (m, 1H), 3.57 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 158.2, 141.8, 137.6, 135.0, 134.0, 131.6, 129.6, 128.8, 128.0, 127.3, 126.9, 125.3, 122.1, 119.4, 119.2, 114.1, 111.9, 109.6, 55.3, 45.3, 36.4. IR (KBr) ν 2930, 1610, 1513, 1459, 1260, 1032, 743, 700 cm−1; HRMS: m/z calcd for ([C25H20N4O + H]+): 393.1710; found: 393.1710.
4-(3,4-Dimethoxyphenyl)-3-phenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3an). White solid (131 mg, 62%); mp 301–302 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.33 (s, 1H), 7.92–7.68 (m, 2H), 7.59–7.19 (m, 5H), 7.18–6.79 (m, 3H), 6.77–6.47 (m, 2H), 6.21–5.77 (m, 3H), 3.60 (s, 3H), 3.55 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 148.5, 147.7, 142.0, 137.7, 135.3, 133.9, 131.7, 128.84, 128.0, 127.5, 127.1, 125.4, 122.1, 120.4, 119.4, 119.3, 112.9, 112.1, 111.9, 109.3, 56.0, 55.7, 45.3, 36.7. IR (KBr) ν 2933, 1607, 1516, 1462, 1271, 1133, 1021, 739, 700 cm−1; HRMS: m/z calcd for ([C26H22N4O2 + H]+): 423.1816; found: 423.1815.
4-(Furan-2-yl)-3-phenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3ao). White solid (107 mg, 61%); mp 323–324 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.40 (s, 1H), 7.93–7.74 (m, 2H), 7.59 (d, J = 7.8 Hz, 1H), 7.52–7.23 (m, 5H), 7.19–6.89 (m, 2H), 6.43–6.25 (m, 2H), 6.25–6.12 (m, 1H), 6.06–5.80 (m, 2H). 13C NMR (75 MHz, DMSO-d6) δ 153.2 142.6, 142.2, 137.5, 131.4, 130.8, 128.9, 128.1, 127.7, 127.2, 125.4, 122.3, 119.6, 119.1, 112.0, 110.8, 107.5, 106.1, 45.2, 31.2. IR (KBr) ν 2929, 1636, 1459, 1328, 1148, 736, 696 cm−1; HRMS: m/z calcd for ([C22H16N4O + H]+): 353.1397; found: 353.1399.
3′-Phenyl-9′,10′-dihydrospiro[fluorene-9,4′-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole] (3ap). White solid (114 mg, 52%); mp 204–205 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.46 (s, 1H), 7.76 (d, J = 7.5 Hz, 2H), 7.39–7.19 (m, 3H), 7.12–6.96 (m, 5H), 6.94–6.75 (m, 3H), 6.55–6.40 (m, 1H), 6.33 (d, J = 7.1 Hz, 2H), 6.22 (s, 2H), 5.99 (d, J = 8.0 Hz, 1H). 13C NMR (75 MHz, DMSO-d6) δ 149.7, 142.8, 140.9, 137.8, 133.9, 131.3, 129.1, 128.8, 128.7, 128.5, 127.8, 127.6, 125.3, 123.8, 122.0, 120.7, 119.4, 118.3, 112.0, 108.1, 50.8, 45.5. IR (KBr) ν 2932, 1721, 1448, 1245, 743, 696 cm−1; HRMS: m/z calcd for ([C30H20N4 + H]+): 473.1761; found: 473.1755.
3′-(p-Tolyl)-9′,10′-dihydrospiro[fluorene-9,4′-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole] (3aq). White solid (119 mg, 53%); mp 340–341 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.45 (s, 1H), 7.80 (d, J = 7.6 Hz, 2H), 7.44–7.20 (m, 3H), 7.18–6.98 (m, 4H), 6.96–6.79 (m, 1H), 6.66 (d, J = 7.9 Hz, 2H), 6.57–6.41 (m, 1H), 6.37–6.11 (m, 4H), 5.99 (d, J = 7.9 Hz, 1H), 2.09 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 149.7, 142.6, 140.9, 137.7, 136.8, 133.6, 129.0, 128.7, 128.5, 128.4, 128.2, 125.2, 123.8, 121.9, 120.7, 119.3, 118.3, 111.9, 108.1, 50.8, 45.4, 21.2. IR (KBr) ν 2935, 1643, 1597, 1112, 1010, 743, 624 cm−1; HRMS: m/z calcd for ([C31H22N4 + H]+): 451.1917; found: 451.1919.
3′-(4-Methoxyphenyl)-9′,10′-dihydrospiro[fluorene-9,4′-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole] (3ar). White solid (131 mg, 56%); mp 313–314 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.47 (s, 1H), 7.82 (d, J = 7.5 Hz, 2H), 7.40–7.24 (m, 3H), 7.16–7.00 (m, 4H), 6.97–6.83 (m, 1H), 6.58–6.46 (m, 1H), 6.47–6.34 (m, 2H), 6.34–6.13 (m, 4H), 6.00 (d, J = 8.0 Hz, 1H), 3.61 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 158.8, 149.7, 142.4, 140.9, 137.7, 133.5, 129.8, 129.1, 128.8, 128.5, 125.2, 123.8, 123.7, 121.9, 120.7, 119.3, 118.3, 113.1, 112.0, 108.1, 55.4, 50.7, 45.4. IR (KBr) ν 2936, 1614, 1506, 1448, 1249, 1184, 830, 743 cm−1; HRMS: m/z calcd for ([C31H22N4 + H]+): 467.1866; found: 467.1865.
3′-(4-Chlorophenyl)-9′,10′-dihydrospiro[fluorene-9,4′-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole] (3as). White solid (120 mg, 51%); mp 328–329 °C; 1H NMR (300 MHz, DMSO-d6) δ 11.47 (s, 1H), 7.81 (d, J = 7.6 Hz, 2H), 7.40–7.22 (m, 3H), 7.18–6.98 (m, 4H), 6.98–6.83 (m, 3H), 6.58–6.40 (m, 1H), 1H NMR (300 MHz, DMSO) δ 11.47 (s, 1H), 7.81 (d, J = 7.6 Hz, 2H), 7.40–7.22 (m, 3H), 7.18–6.98 (m, 4H), 6.98–6.83 (m, 3H), 6.50 (t, J = 7.5 Hz, 1H), 6.37–6.26 (m, 2H), 6.22 (s, 2H), 5.99 (d, J = 8.0 Hz, 1H). 13C NMR (75 MHz, DMSO-d6) δ 149.5, 141.5, 140.8, 137.8, 134.3, 132.6, 130.4, 130.2, 129.1, 129.0, 128.7, 127.7, 125.3, 123.8, 122.0, 120.8, 119.5, 118.3, 112.0, 107.8, 50.7, 45.5. IR (KBr) ν 2933, 1632, 1448, 1328, 1090, 743 cm−1; HRMS: m/z calcd for ([C31H21ClN4 + H]+): 485.1528; found: 485.1529.
9-Methyl-3,4,4-triphenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3ba). White solid (68 mg, 30%); mp 295–296 °C; 1H NMR (300 MHz, DMSO) δ 7.46 (d, J = 8.3 Hz, 1H), 7.19–6.95 (m, 14H), 6.84–6.70 (m, 3H), 6.52 (d, J = 8.0 Hz, 1H), 5.98 (s, 2H), 3.79 (s, 3H). 13C NMR (75 MHz, DMSO) δ 144.3, 143.2, 138.4, 137.7, 132.1, 130.6, 129.6, 129.1, 128.4, 127.9, 127.8, 127.4, 124.6, 121.8, 120.1, 120.0, 114.6, 110.5, 51.4, 44.4, 30.2. IR (KBr) ν 2929, 1636, 1597, 1361, 1010, 754, 700 cm−1; HRMS: m/z calcd for ([C31H24N4 + H]+): 453.2074; found: 453.2075.
9-Methyl-4,4-diphenyl-3-(p-tolyl)-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3bb). White solid (84 mg, 36%); mp 340–341 °C; 1H NMR (300 MHz, DMSO-d6) δ 7.47 (d, J = 8.3 Hz, 1H), 7.21–6.94 (m, 11H), 6.92–6.70 (m, 3H), 6.61 (d, J = 8.0 Hz, 2H), 6.51 (d, J = 7.9 Hz, 1H), 5.97 (s, 2H), 3.79 (s, 3H), 2.18 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 144.2, 143.3, 138.4, 137.6, 137.1, 130.5, 129.4, 129.3, 129.1, 128.4, 127.4, 124.6, 121.8, 120.0, 119.9, 114.4, 110.5, 51.4, 44.4, 30.2, 21.2. IR (KBr) ν 2931, 1636, 1590, 1318, 1007, 746, 700 cm−1; HRMS: m/z calcd for ([C32H26N4 + H]+): 467.2230; found: 467.2230.
3-(4-Methoxyphenyl)-9-methyl-4,4-diphenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3bc). White solid (59 mg, 24%); mp 317–318 °C; 1H NMR (300 MHz, DMSO-d6) δ 7.49 (d, J = 8.3 Hz, 1H), 7.19–7.00 (m, 11H), 6.80 (t, J = 7.6 Hz, 1H), 6.70–6.50 (m, 5H), 5.99 (s, 2H), 3.81 (s, 3H), 3.67 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 159.0, 144.0, 143.3, 138.4, 137.4, 130.8, 130.5, 129.1, 128.4, 127.4, 124.6, 124.4, 121.8, 120.1, 119.9, 114.5, 113.3, 110.5, 55.5, 51.4, 44.4, 30.2. IR (KBr) ν 2933, 2836, 1614, 1498, 1318, 1249, 1177, 833, 703 cm−1; HRMS: m/z calcd for ([C32H26N4O + H]+): 483.2179; found: 483.2176.
9-Methyl-3-phenyl-4,4-di-p-tolyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3be). White solid (84 mg, 35%); mp 281–282 °C; 1H NMR (300 MHz, DMSO-d6) δ 7.45 (d, J = 8.0 Hz, 1H), 7.20–6.94 (m, 5H), 6.89 (s, 8H), 6.83–6.71 (m, 3H), 6.57 (d, J = 8.0 Hz, 1H), 5.95 (s, 2H), 3.78 (s, 3H), 2.16 (s, 6H). 13C NMR (75 MHz, DMSO-d6) δ 144.2, 140.5, 138.4, 138.0, 136.4, 132.2, 130.3, 129.6, 128.9, 127.8, 127.7, 124.6, 121.7, 120.2, 119.9, 114.8, 110.4, 50.8, 44.4, 30.2, 20.9. IR (KBr) ν 2934, 1636, 1509, 1361, 1010, 761, 700 cm−1; HRMS: m/z calcd for ([C33H28N4 + H]+): 481.2387; found: 481.2388.
4,9-Dimethyl-3,4-diphenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3bh). White solid (117 mg, 60%); mp 244–245 °C; 1H NMR (300 MHz, DMSO-d6) δ 7.44 (d, J = 8.2 Hz, 1H), 7.34–7.12 (m, 9H), 7.08 (t, J = 7.6 Hz, 1H), 6.85 (t, J = 7.5 Hz, 1H), 6.81–6.72 (m, 2H), 6.11 (AB, J = 17.0 Hz, 2H), 3.78 (s, 3H), 1.91 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 146.8, 142.6, 139.0, 138.6, 132.5, 129.3, 128.7, 128.3, 127.6, 127.5, 127.0, 123.7, 121.8, 119.5, 113.5, 110.2, 44.4, 40.8, 30.1, 25.0. IR (KBr) ν 2936, 1639, 1491, 1325, 1090, 739, 703 cm−1; HRMS: m/z calcd for ([C26H22N4 + H]+): 391.1917; found: 391.1919.
4-(4-Bromophenyl)-4,9-dimethyl-3-phenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3bj). White solid (134 mg, 57%); mp 295–296 °C; 1H NMR (300 MHz, DMSO-d6) δ 7.45 (d, J = 8.2 Hz, 1H), 7.42–7.33 (m, 2H), 7.33–7.04 (m, 7H), 6.93–6.74 (m, 3H), 6.10 (AB, 17.0 Hz, 2H), 3.78 (s, 3H), 1.89 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 146.0, 142.7, 138.6, 138.4, 132.4, 131.4, 129.9, 129.4, 128.4, 127.8, 123.5, 122.0, 120.1, 119.6, 119.2, 112.9, 110.3, 44.4, 40.8, 30.1, 25.2. IR (KBr) ν 2932, 1636, 1491, 1321, 1007, 739, 700 cm−1; HRMS: m/z calcd for ([C26H21N4Br + H]+): 469.1022; found: 469.1025.
9-Methyl-3,4-diphenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3bl). White solid (77 mg, 41%); mp 272–273 °C; 1H NMR (300 MHz, DMSO-d6) δ 7.89–7.67 (m, 2H), 7.62–7.43 (m, 2H), 7.43–7.20 (m, 5H), 7.22–7.07 (m, 3H), 7.07–6.90 (m, 2H), 6.26–6.02 (m, 3H), 3.81 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 143.0, 141.9, 138.4, 133.6, 131.5, 128.8, 128.8, 128.6, 128.4, 128.0, 127.3, 127.1, 124.8, 122.1, 119.6, 119.3, 110.1, 108.7, 44.7, 39.2, 37.2, 30.2. IR (KBr) ν 2934, 1632, 1470, 1321, 1007, 750, 696 cm−1; HRMS: m/z calcd for ([C25H20N4 + H]+): 377.1761; found: 377.1759.
4-(Furan-2-yl)-4,9-dimethyl-3-phenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3bo). White solid (88 mg, 48%); mp 251–252 °C; 1H NMR (300 MHz, DMSO-d6) δ 7.91–7.74 (m, 2H), 7.61 (d, J = 7.8 Hz, 1H), 7.43 (m, J = 13.1, 11.3, 7.3 Hz, 3H), 7.36–7.24 (m, 2H), 7.21–7.10 (m, 1H), 7.08–6.96 (m, 1H), 6.42–6.28 (m, 2H), 6.26–6.13 (m, 1H), 6.13–5.88 (m, 2H), 3.77 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 153.1, 142.6, 142.2, 138.2, 131.4, 130.6, 129.2, 128.9, 128.1, 127.2, 125.0, 122.2, 119.8, 119.2, 110.8, 110.2, 107.5, 105.5, 44.6, 31.2, 30.2. IR (KBr) ν 2933, 1636, 1473, 1365, 1007, 743, 689 cm−1; HRMS: m/z calcd for ([C24H20N4O + H]+): 367.1553; found: 367.1550.
9′-Methyl-3′-phenyl-9′,10′-dihydrospiro[fluorene-9,4′-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole] (3bp). White solid (113 mg, 50%); mp 271–272 °C; 1H NMR (300 MHz, CDCl3) δ 7.69–7.45 (m, 2H), 7.35–7.18 (m, 3H), 7.13–6.97 (m, 4H), 6.96–6.88 (m, 2H), 6.89–6.77 (m, 2H), 6.73–6.57 (m, 1H), 6.48–6.32 (m, 2H), 6.24 (d, J = 8.0 Hz, 1H), 6.04 (s, 2H), 3.79 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 148.8, 143.6, 140.6, 138.3, 134.0, 130.3, 128.8, 128.2, 128.0, 127.9, 127.2, 127.0, 124.6, 123.4, 122.2, 119.9, 119.8, 119.2, 108.9, 108.8, 50.5, 44.5, 29.9. IR (KBr) ν 2933, 1610, 1477, 1321, 1007, 902, 746, 696 cm−1; HRMS: m/z calcd for ([C31H22N4 + H]+): 451.1917; found: 451.1912.
9′-Methyl-3′-(p-tolyl)-9′,10′-dihydrospiro[fluorene-9,4′-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole] (3bq). White solid (118 mg, 51%); mp 352–353 °C; 1H NMR (300 MHz, DMSO-d6) δ 7.80 (d, J = 7.6 Hz, 2H), 7.40 (d, J = 8.3 Hz, 1H), 7.35–7.21 (m, 2H), 7.16–6.89 (m, 5H), 6.66 (d, J = 7.8 Hz, 2H), 6.61–6.47 (m, 1H), 6.39–6.17 (m, 4H), 6.01 (d, J = 7.9 Hz, 1H), 3.84 (s, 3H), 2.10 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 149.7, 142.6, 140.9, 138.5, 136.8, 133.5, 130.5, 128.8, 128.5, 128.4, 128.2, 125.2, 123.4, 121.9, 120.7, 119.6, 118.3, 110.2, 107.5, 50.8, 44.8, 30.3, 21.2. IR (KBr) ν 2934, 1618, 1479, 1321, 1007, 743 cm−1; HRMS: m/z calcd for ([C32H24N4 + H]+): 465.2074; found: 465.2067.
4-(4-Bromophenyl)-7-chloro-4-methyl-3-phenyl-9,10-dihydro-4H-[1,2,3]triazolo[1′,5′:1,6]pyrido[3,4-b]indole (3cj). White solid (113 mg, 46%); mp 297–298 °C; 1H NMR (400 MHz, DMSO-d6) δ 11.55 (s, 1H), 7.48 (s, 1H), 7.41 (d, J = 8.3 Hz, 2H), 7.37–7.30 (m, 1H), 7.30–7.18 (m, 4H), 7.18–7.09 (m, 1H), 6.98–6.75 (m, 3H), 6.01 (AB, 17.0 Hz, 2H), 1.90 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 146.0, 143.1, 138.7, 138.6, 132.5, 131.9, 130.2, 129.7, 128.9, 128.8, 127.7, 127.2, 123.0, 120.7, 120.6, 120.2, 114.2, 112.2, 45.3, 40.7, 25.4. IR (KBr) ν 2933, 1627, 1487, 1383, 1326, 1008, 766, 700 cm−1; HRMS: m/z calcd for ([C25H18N4BrCl + H]+): 489.0476; found: 489.0480.
3,3,5-Triphenyl-3,10-dihydroazepino[3,4-b]indole (4aa)5. White solid (109 mg, 53%); mp 276–277 °C; 1H NMR (300 MHz, CDCl3) δ 8.79 (s, 1H), 8.45 (s, 1H), 7.65–7.43 (m, 6H), 7.42–7.29 (m, 3H), 7.24–7.07 (m, 6H), 7.07–6.96 (m, 2H), 6.96–6.81 (m, 1H), 6.75 (d, J = 7.8 Hz, 1H), 5.73 (s, 1H). 13C NMR (75 MHz, CDCl3) δ 150.8, 148.0, 140.7, 138.2, 136.0, 135.8, 129.1, 128.2, 128.0, 128.0, 127.6, 127.2, 126.2, 124.9, 124.6, 122.9, 120.8, 120.0, 111.3, 71.0. IR (KBr) v 3057, 1621, 1520, 1487, 1332, 1234, 750, 703 cm−1; HRMS: m/z calcd for ([C30H22N2 + H]+): 411.1856; found: 411.1858.
3,3-Diphenyl-5-(p-tolyl)-3,10-dihydroazepino[3,4-b]indole (4ab). White solid (93 mg, 44%); mp 292–293 °C; 1H NMR (300 MHz, CDCl3) δ 8.71 (s, 1H), 8.64 (s, 1H), 7.57–7.42 (m, 4H), 7.42–7.28 (m, 2H), 7.22–7.03 (m, 8H), 7.04–6.93 (m, 2H), 6.94–6.73 (m, 2H), 5.72 (s, 1H), 2.39 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 151.0, 148.0, 138.1, 137.8, 136.0, 135.8, 129.0, 128.9, 127.6, 127.4, 127.2, 126.2, 124.8, 124.7, 122.9, 120.9, 119.9, 111.4, 71.1, 21.3. IR (KBr) ν 3055, 2836, 1614, 1506, 1448, 1253, 1184, 1018, 830, 743 cm−1; HRMS: m/z calcd for ([C31H24N2 + H]+): 425.2012; found: 425.2010.
5-(4-Methoxyphenyl)-3,3-diphenyl-3,10-dihydroazepino[3,4-b]indole (4ac)5. White solid (132 mg, 60%); mp 293–294 °C; 1H NMR (300 MHz, CDCl3) δ 8.78 (s, 1H), 8.39 (s, 1H), 7.57–7.43 (m, 4H), 7.42–7.32 (m, 2H), 7.22–7.05 (m, 6H), 7.06–6.96 (m, 2H), 6.96–6.77 (m, 4H), 5.68 (s, 1H), 3.85 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 159.6, 150.7, 148.1, 137.6, 136.1, 135.7, 133.3, 130.3, 127.6, 127.2, 127.0, 126.1, 124.9, 124.7, 123.0, 121.2, 120.0, 113.5, 111.3, 71.0, 55.3. IR (KBr) ν 3056, 2832, 1621, 1524, 1506, 1245, 1173, 1028, 754, 703 cm−1; HRMS: m/z calcd for ([C31H24N2O + H]+): 441.1961; found: 441.1965.
3,3-Bis(4-methoxyphenyl)-5-phenyl-3,10-dihydroazepino[3,4-b]indole (4at). White solid (122 mg, 52%); mp 160–161 °C; 1H NMR (300 MHz, CDCl3) δ 9.14 (s, 1H), 8.57 (s, 1H), 7.52–7.29 (m, 9H), 7.20–7.08 (m, 2H), 6.92–6.79 (m, 1H), 6.78–6.59 (m, 5H), 5.69 (s, 1H), 3.66 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 157.6, 150.9, 140.8, 140.5, 138.0, 136.1, 136.0, 129.1, 128.5, 128.4, 128.2, 128.0, 124.8, 124.6, 122.8, 120.6, 119.9, 112.9, 111.5, 70.2, 55.1. IR (KBr) ν 3056, 2835, 1610, 1506, 1234, 1028, 750, 700 cm−1; HRMS: m/z calcd for ([C32H26N2O2 + H]+): 471.2067; found: 471.2062.
10-Methyl-3,3,5-triphenyl-3,10-dihydroazepino[3,4-b]indole (4ba). White solid (76 mg, 36%); mp 280–281 °C; 1H NMR (300 MHz, CDCl3) δ 8.89 (s, 1H), 7.63–7.43 (m, 6H), 7.42–7.30 (m, 3H), 7.24–7.18 (m, 2H), 7.17–7.05 (m, 4H), 7.05–6.94 (m, 2H), 6.91–6.81 (m, 1H), 6.75 (d, J = 8.2 Hz, 1H), 5.80 (s, 1H), 3.66 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 149.7, 147.9, 140.8, 138.1, 138.0, 136.7, 129.2, 128.2, 128.0, 127.5, 127.1, 126.1, 124.5, 124.1, 122.8, 120.0, 119.7, 109.2, 71.1, 30.0. IR (KBr) ν 3055, 2825, 1639, 1618, 1130, 1235, 750, 616 cm−1; HRMS: m/z calcd for ([C31H25N2 + H]+): 425.2012; found: 425.2012.
5-(4-Methoxyphenyl)-10-methyl-3,3-diphenyl-3,10-dihydroazepino[3,4-b]indole (4bc). White solid (114 mg, 50%); mp 299–300 °C;1H NMR (300 MHz, CDCl3) δ 8.87 (s, 1H), 7.46 (d, J = 7.4 Hz, 4H), 7.42–7.35 (m, 2H), 7.27–7.13 (m, 2H), 7.13–7.03 (m, 4H), 7.02–6.93 (m, 2H), 6.93–6.79 (m, 4H), 5.74 (s, 1H), 3.83 (s, 3H), 3.62 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 159.5, 149.6, 147.9, 138.1, 137.4, 136.7, 133.4, 130.2, 127.5, 127.1, 127.0, 126.0, 124.4, 124.2, 122.9, 120.2, 119.6, 113.5, 109.2, 71.1, 55.2, 30.0. IR (KBr) ν 3056, 2833, 1603, 1484, 1253, 1097, 736, 700 cm−1; HRMS: m/z calcd for ([C32H26N2O + H]+): 455.2118; found: 455.2189.
10-Methyl-5-phenyl-3,3-di-p-tolyl-3,10-dihydroazepino[3,4-b]indole (4be). White solid (122 mg, 54%); mp 303–304 °C;1H NMR (300 MHz, CDCl3) δ 8.87 (s, 1H), 7.52–7.41 (m, 2H), 7.39–7.27 (m, 7H), 7.27–7.13 (m, 2H), 6.95–6.80 (m, 5H), 6.74 (d, J = 8.1 Hz, 1H), 5.75 (d, J = 2.1 Hz, 1H), 3.66 (s, 3H), 2.15 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 149.5, 145.2, 141.0, 137.9, 137.73, 136.8, 135.3, 129.2, 128.3, 128.2, 128.1, 127.9, 126.9, 124.3, 124.1, 122.9, 119.7, 119.6, 109.2, 70.7, 30.0, 20.9. IR (KBr) ν 3055, 2832, 1636, 1513, 1115, 1021, 754, 703 cm−1; HRMS: m/z calcd for ([C33H28N2 + H]+): 453.2325; found: 453.2323.
3,3-Bis(4-methoxyphenyl)-10-methyl-5-phenyl-3,10-dihydroazepino[3,4-b]indole (4bt). White solid (136 mg, 56%); mp 240–241 °C;1H NMR (300 MHz, CDCl3) δ 8.85 (s, 1H), 7.53–7.40 (m, 3H), 7.40–7.27 (m, 7H), 7.27–7.12 (m, 2H), 6.84 (s, 1H), 6.73 (d, J = 8.1 Hz, 1H), 6.68–6.60 (m, 3H), 5.72 (s, 1H), 3.65 (s, 3H), 3.64 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 157.5, 149.4, 140.9, 140.4, 137.8, 137.7, 136.7, 129.1, 128.5, 128.1, 128.1, 127.9, 124.4, 124.1, 122.8, 119.6, 112.80, 109.3, 70.2, 55.0, 30.0. IR (KBr) ν 3056, 2828, 1603, 1502, 1238, 1169, 1032, 833, 750 cm−1; HRMS: m/z calcd for ([C33H28N2O2 + H]+): 485.2224; found: 485.2223.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was supported by the Excellent Young Talents Fund Program of Higher Education Institutions of Anhui Province (Grant No. gxyq2019041), the National Natural Science Foundation of China (Grant No. 21602157), the College Students’ Innovation and Entrepreneurship Program of Anhui Province (S201910368095) and the Research Grant for Wannan Medical College Students (WK2020S44).
Notes and references
-
(a) P. Maity, D. Adhikari and A. K. Jana, Tetrahedron, 2019, 75, 965–1028 CrossRef CAS;
(b) R. Cao, W. Peng, Z. Wang and A. Xu, Curr. Med. Chem., 2007, 14, 479–500 CrossRef CAS PubMed;
(c) M. El-Sayed and R. Verpoorte, Phytochem. Rev., 2007, 6, 277–305 CrossRef CAS;
(d) F. E. Chen and J. Huang, Chem. Rev., 2005, 105, 4671–4706 CrossRef CAS PubMed;
(e) S. E. O'Connor and J. J. Maresh, Nat. Prod. Rep., 2006, 23, 532–547 RSC.
-
(a) M. Retini, F. Bartoccini, G. Zappia and G. Piersanti, Eur. J. Org. Chem., 2021, 2021, 825–829 CrossRef CAS;
(b) A. Nalikezhathu, V. Cherepakhin and T. J. Williams, Org. Lett., 2020, 22, 4979–4984 CrossRef CAS PubMed;
(c) H. C. Liu, F. Han, H. Li, J. P. Liu and Q. Xu, Org. Biomol. Chem., 2020, 18, 7079–7085 RSC;
(d) N. Glinsky-Olivier, S. W. Yang, P. Retailleau, V. Gandon and X. Guinchard, Org. Lett., 2019, 21, 9446–9451 CrossRef CAS PubMed;
(e) I. Khan, S. Khan, V. Tyagi, P. S. Chouhan and P. M. S. Chauhan, RSC Adv., 2015, 5, 102713–102722 RSC;
(f) N. Mittal, D. X. Sun and D. Seidel, Org. Lett., 2014, 16, 1012–1015 CrossRef CAS PubMed;
(g) D. Huang, F. Xu, X. Lin and Y. Wang, Chem.–Eur. J., 2012, 18, 3148–3152 CrossRef CAS PubMed;
(h) Q. Cai, X. W. Liang, S. G. Wang, J. W. Zhang, X. Zhang and S. L. You, Org. Lett., 2012, 14, 5022–5025 CrossRef CAS PubMed;
(i) J. Stockigt, A. P. Antonchick, F. Wu and H. Waldmann, Angew. Chem., Int. Ed., 2011, 50, 8538–8564 CrossRef CAS PubMed;
(j) E. Ascic, J. F. Jensen and T. E. Nielsen, Angew. Chem., Int. Ed., 2011, 50, 5188–5191 CrossRef CAS PubMed;
(k) M. Lorenz, M. L. Van Linn and J. M. Cook, Curr. Org. Synth., 2010, 7, 189–223 CrossRef CAS;
(l) S.-L. You, Q. Cai and M. Zeng, Chem. Soc. Rev., 2009, 38, 2190 RSC;
(m) M. E. Muratore, C. A. Holloway, A. W. Pilling, R. I. Storer, G. Trevitt and D. J. Dixon, J. Am. Chem. Soc., 2009, 131, 10796–10797 CrossRef CAS PubMed;
(n) M. J. Wanner, R. N. van der Haas, K. R. de Cuba, J. H. van Maarseveen and H. Hiemstra, Angew. Chem., Int. Ed., 2007, 46, 7485–7487 CrossRef CAS PubMed;
(o) J. Seayad, A. M. Seayad and B. List, J. Am. Chem. Soc., 2006, 128, 1086–1087 CrossRef CAS PubMed;
(p) E. D. Cox and J. M. Cook, Chem. Rev., 1995, 95, 1797–1842 CrossRef CAS.
- S. Wang, Z. Chai, S. Zhou, S. Wang, X. Zhu and Y. Wei, Org. Lett., 2013, 15, 2628–2631 CrossRef CAS PubMed.
-
(a) L. Zhang, Y. Zhu, G. Yin, P. Lu and Y. Wang, J. Org. Chem., 2012, 77, 9510–9520 CrossRef CAS PubMed;
(b) W. Yan, X. Ye, K. Weise, J. L. Petersen and X. Shi, Chem. Commun., 2012, 48, 3521–3523 RSC;
(c) T. Ishikawa, M. Okano, T. Aikawa and S. Saito, J. Org. Chem., 2001, 66, 4635–4642 CrossRef CAS PubMed.
- Liang and co-workers reported a Lewis acid-catalyzed synthesis of azepines based on the same startegy: Y. P. Han, X. R. Song, Y. F. Qiu, H. R. Zhang, L. H. Li, D. P. Jin, X. Q. Sun, X. Y. Liu and Y. M. Liang, Org. Lett., 2016, 18, 940–943 CrossRef CAS PubMed . However, these authors made no mention of the formation of the tetrahydro-β-carboline products in their work.
- D. Fischer, H. Tomeba, N. K. Pahadi, N. T. Patil and Y. Yamamoto, Angew. Chem., Int. Ed., 2007, 46, 4764–4766 CrossRef CAS PubMed.
- C. Zheng, Y. Lu, J. Zhang, X. Chen, Z. Chai, W. Ma and G. Zhao, Chem.–Eur. J., 2010, 16, 5853–5857 CrossRef CAS PubMed.
- D. A. Engel and G. B. Dudley, Org. Lett., 2006, 8, 4027–4029 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectra for newly synthesized compounds, CIF for compounds 3aa and 4aa. CCDC 2072684 and 2072685. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra03022a |
|
| This journal is © The Royal Society of Chemistry 2021 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).




