Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tuning the exposure of BiVO4-{010} facets to enhance the N2 photofixation performance†
Honghao Chua,
Shisheng Zhenga,
Yang Lia,
Kuanda Xua,
Qingshui Hongb,
Tangyi Lia,
Wenju Renc,
Shunning Lia,
Zongwei Mei*a and
Feng Pan *ad
*ad
aSchool of Advanced Materials, Peking University, Shenzhen Graduate School, Shenzhen, China. E-mail: meizw@pkusz.edu.cn; panfeng@pkusz.edu.cn
bChongqing Key Laboratory of Chemical Process for Clean Energy and Resource Utilization, School of Chemistry and Engineering, Chongqing University, Chongqing, China
cSchool of Advance Manufacturing Engineering, Chongqing University of Posts and Telcommunications, Chongqing, China
dChemistry and Chemical Engineering Guangdong Laboratory, Shantou, China
First published on 27th August 2021
Abstract
Effective separation of photoexcited carriers and chemisorption of the N2 molecule are two key issues to efficient nitrogen photofixation. The spatial charge separation of BiVO4 with anisotropic exposed facets, namely the transfer of photoexcited electrons and holes to {010} and {110} facets, respectively, helps to enhance the separation ability of photogenerated carriers. Theoretical calculation results predict that a surface oxygen vacancy is easier to form on the (010) facet than on the (110) facet of BiVO4. Accordingly, in this study, enhanced N2 photofixation performance has been achieved for the first time by tuning the exposure of {010} facets of BiVO4.
Nitrogen fixation to NH3 is an important artificial synthesis in the chemical industry.1 Nowadays, NH3 is commonly produced through the Haber–Bosch process, which requires high temperature (400–500 °C) and high pressure (15–25 MPa).2 This process accounts for ∼2% of the total global energy consumption and contributes ∼1.6% of the total global emissions.3 With the growing energy needs and demand for cleaner environment, a more environmentally friendly method is needed for NH3 manufacture. Photocatalytic N2 fixation, which employs solar energy and water to produce ammonium, is a promising sustainable and green strategy for NH3 synthesis compared with the traditional Haber–Bosch process.4–9 However, the photocatalytic performance of N2 fixation is far from satisfactory due to the inefficient separation of photogenerated carriers, the high activation energy barriers and hard cleavage of the strong N
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond energy (941 kJ mol−1) of the N2 molecule.10
N triple bond energy (941 kJ mol−1) of the N2 molecule.10
Crystal facet engineering of semiconductors is a significant strategy for fine-tuning the charge separation of photocatalysts.11 Facet engineering of anatase TiO2 has been given considerable research attention for photocatalytic reaction by controlling the {001} exposure ratio.12–14 Recently, different research groups reported that the exposure of anisotropic facets of BiOX (X = Cl, Br, or I) enabled the directional transfer of photoexcited electrons and holes for spatial charge separation, accordingly improved the photocatalytic activities.15–17 Other semiconductors including SrTiO3,18 LaNbON2,19 and C3N4 (ref. 20) also demonstrated that the predominating anisotropic facet exposure could improve the separation of photogenerated carriers. Simultaneously, the spatial charge separation of BiVO4 with anisotropic exposed facets has been observed by direct imaging21 and photo-reduction or photo-oxidation reactions on {010} or {110} facets, respectively.22–24 These results indicated that the photoexcited electrons (e−) and holes (h+) would transfer to the {010} and {110} facets, respectively. Based on the special property of BiVO4, photocatalytic water splitting for O2 evolution has been greatly improved.25 However, a few researches on BiVO4 with anisotropic exposed facets have been reported for photocatalytic N2 fixation.
It is commonly understood that surface vacancies with abundant localized electrons play a critical role in N2 photofixation by capturing and activating the inert N2 molecule.26,27 Efficient transfer of the photogenerated electrons to the inert N2 molecule is also a key step for the effective photocatalytic N2 fixation.28 Considerable research results have revealed that surface oxygen vacancies could activate the N2 molecule by chemisorption, and act as the transfer bridge of photoexcited electrons from photocatalysts to the activated N2 molecule. For example, Pan et al. reported that the bond length of N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N was elongated by the interaction with the surface OVS on MoO3−x nanobelts or W18O49 nanowires, and the photocatalytic activities for the N2 fixation were directly related to the surface OVS concentration.29,30 The critical role of OVS in the photofixation of N2 was also revealed by other semiconductors including TiO2,10,31–33 BiOCl,34 BiO quantum dots,35 ultrafine Cu2O,8 and amorphous CeOx.36 However, the surface OVS on BiVO4 for N2 photofixation has been rarely studied.
N was elongated by the interaction with the surface OVS on MoO3−x nanobelts or W18O49 nanowires, and the photocatalytic activities for the N2 fixation were directly related to the surface OVS concentration.29,30 The critical role of OVS in the photofixation of N2 was also revealed by other semiconductors including TiO2,10,31–33 BiOCl,34 BiO quantum dots,35 ultrafine Cu2O,8 and amorphous CeOx.36 However, the surface OVS on BiVO4 for N2 photofixation has been rarely studied.
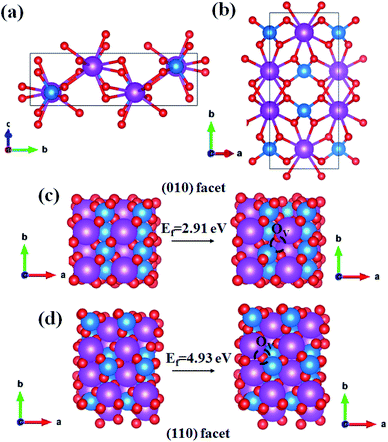
Theoretical calculation results predicted that the formation energy (Ef) of an OV on the surface of the representative (010) facet was lower than that on the surface of the typical (110) facet, accordingly there were more OVS on the surface of the (010) facet. Together with the spatial charge separation property, anisotropic exposed BiVO4 was believed to exhibit a good N2 photofixation performance. In this study, BiVO4 with anisotropic {010} and {110} facets were synthesized by a solid–liquid state reaction.37 It was first found that the as-prepared BiVO4 with higher percentage of exposed {010} facets exhibited better performance for N2 photofixation without any sacrificial reagent and cocatalyst under ambient conditions. The easier formation of a surface oxygen vacancy (OV) on the (010) facet was experimentally proved by the enhanced chemisorption of the N2 molecule based on the temperature programmed desorption (TPD) characterization. The enhanced separation of photogenerated carriers and more surface oxygen vacancies (OVS) made BiVO4 with a higher exposed ratio of {010} facets to be more efficient for photocatalytic N2 fixation.
The primitive unit cell consists of four units as shown by the side and top view of the optimized BiVO4 (Fig. 1a and b), and the optimized lattice parameters are as follows: a = 7.33 Å, b = 11.77 Å, c = 5.18 Å, and β = 134.92°. They are in good agreement with the experimental values: a = 7.25 Å, b = 11.70 Å, c = 5.09 Å, and β = 134.225°. The formation energy of the oxygen vacancy (Ef) on the surface of (010) and (110) facets were calculated by following equation:
| Ef = EOV + E½O2 − Esurface |
Based on the theoretical calculation result, BiVO4 with different exposure ratios of {010} facets was fabricated by tuning the nitric concentration in the reaction solution according to a previous report (see ESI†). The as-synthesized BiVO4-0.50, BiVO4-0.75, and BiVO4-1.0 can be indexed to the monoclinic crystal structure (PDF# 14-0688) (Fig. 2a and S1†). However, the BiVO4-0.25 sample in Fig. S1† consists of a hybrid monoclinic structure (PDF# 14-0688) and a tetragonal crystal phase (PDF# 14-0133), probably due to the low concentration of the HNO3 solution. Furthermore, the (020) peak of BiVO4-0.50 appears around 15° of 2θ (Fig. 2a). The as-synthesized BiVO4 in different concentrations of HNO3 shows different facet exposure ratios of {010} to {110}, and BiVO4-0.50 exhibits the largest exposure ratio (Fig. 2b and S2†), which is consistent with the previous report.37 The largest exposure of {010} facets must cause the appearance of the (020) peak in Fig. 2a. The typical TEM image shows the regular shape of BiVO4-0.50 (Fig. 2c). The HRTEM interplanar spacing is 0.47 nm, corresponding to the value of (110) facet (Fig. 2d). The selected area electron diffraction (SAED) rings in the inset of Fig. 2d corresponds to the (110) and (040) crystal facets of monoclinic BiVO4.
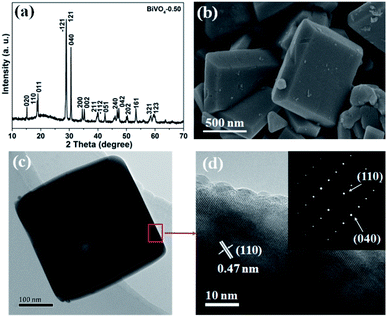 | ||
| Fig. 2 Typical XRD pattern (a), SEM image (b), TEM image (c), and HRTEM image (d) of the as-synthesized BiVO4-0.50. Inset in (d) SAED pattern. | ||
Fig. 3 exhibits the temperature programmed desorption (TPD) characterization of the N2 molecule. A single peak centering at 220 °C is observed, which is ascribed to the desorption of the chemisorbed N2, and BiVO4-0.50 exhibits the strongest chemisorption of the N2 molecule. It is consistent with the theoretical calculation result shown in Fig. 1.
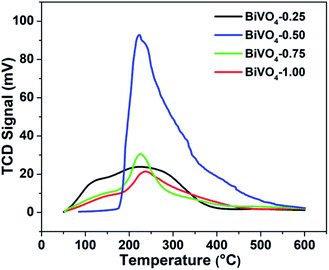 | ||
| Fig. 3 N2-TPD profiles of BiVO4 synthesized in the aqueous solution with different HNO3 concentrations. | ||
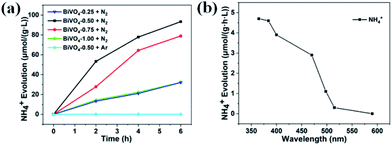
For the general photocatalytic N2 photofixation process in pure water, the photoexcited electrons are injected into the chemisorbed N2 molecule via oxygen vacancy, and then the activated N2 molecule combines with H+ from water to form NH3. Simultaneously, the photogenerated holes oxidize the OH− from water to produce O2. The standard curve from the different concentrations of NH4+ is shown in Fig. S4.† Photocatalytic performance test indicates that BiVO4-0.50 exhibits the best activity for N2 fixation among these four samples (Fig. 4a). The average NH4+ evolution rate is about 15.5 μmol g−1 L−1 h−1 for BiVO4-0.50 during a 6 h test. The average NH4+ evolution rates decrease to 13.1, 5.35, and 5.3 μmol g−1 L−1 h−1 for BiVO4-0.75, BiVO4-1.00, and BiVO4-0.25, respectively (Fig. 4a). The Brunauer–Emmett–Teller (BET) measurements indicate that the surface areas are 2.9 m2 g−1, 2.0 m2 g−1, 1.8 m2 g−1, and 1.5 m2 g−1 for BiVO4-0.25, BiVO4-0.50, BiVO4-0.75, and BiVO4-1.00, respectively. The corresponding ratios of the average NH4+ evolution rate to surface area are 4.5 μmol L−1 h−1 m−2, 7.8 μmol L−1 h−1 m−2, 3.0 μmol L−1 h−1 m−2, and 3.5 μmol L−1 h−1 m−2. It is obvious that the surface area is not the determining factor of the photocatalytic activity. In order to confirm the origination of the nitrogen element in NH4+, Ar gas was bubbled and the aqueous suspension of the BiVO4-0.50 sample was irradiated by a 300 W xenon lamp during the test time. It is found that there is no detectable NH4+ (Fig. 4a), and it can be concluded that the detected NH4+ did not originate from environmental contamination, but from the photofixation reaction of N2 molecule by the BiVO4 photocatalyst. Fig. 4b shows the light-wavelength-dependent photofixation activity of BiVO4-0.50. The photocatalytic performance decreases from 365 nm and exhibits almost no NH4+ from 515 nm, and no NH4+ is detected at 590 nm due to the light absorption range (Fig. S3†). The photocatalyst possessed photoexcited electrons with higher energy under the irradiation of the shorter wavelength light, and the photofixation of N2 was more easy to occur. However, the photofixation reaction of N2 molecule could not occur when the longer wavelength light was unable to excite the BiVO4 photocatalyst. This result further confirmed the photofixation of N2 in our study. The calculated value of quantum efficiency (QE) at 365 nm was about 0.003%.
In order to evaluate the stability of BiVO4-0.50 for N2 photofixation, three cycle tests were carried out, as exhibited in Fig. 5. After each cycle, the photocatalyst was washed by vacuum filtration with pure water for several times and dried in a vacuum oven. The photocatalytic activity gradually decreased probably due to the loss of photocatalyst during the wash process.
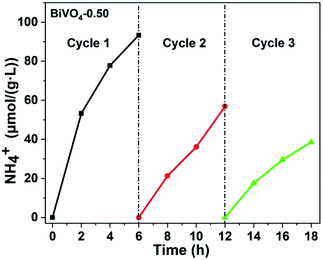 | ||
| Fig. 5 Photocatalytic stability test for BiVO4-0.50 (light source: 300 W xenon lamp; photocatalyst: 0.05 g; reaction solution: 100 ml of pure water). | ||
In summary, BiVO4 with exposed anisotropic {010} and {110} facets was synthesized, and studied for the first time for ammonia production by photocatalytic N2 fixation from pure water. It was found that the sample with the high facet exposure ratio of {010} to {110} exhibited a better photocatalytic performance for N2 fixation, which resulted from the better separation ability of the photoexcited carriers and more surface OVS on the {010} facet. The photoexcited electrons more effectively transferred to the more surface OVS on the typical (010) facet, where the N2 molecule can be activated, and accordingly benefited to the enhancement of the photocatalytic performance. Our study provides a good strategy to improve the photocatalytic activity for N2 fixation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was financially supported by the Chemistry and Chemical Engineering Guangdong Laboratory (Grant No. 1922018).References
- D. E. Canfield, A. N. Glazer and P. G. Falkowski, Science, 2010, 330, 192–196 CrossRef CAS.
- G. N. Schrauzer and T. D. Guth, J. Am. Chem. Soc., 1977, 99, 7189–7193 CrossRef CAS.
- P. Friedlingstein, M. O'Sullivan, M. W. Jones, R. M. Andrew, J. Hauck, A. Olsen, G. P. Peters, W. Peters, J. Pongratz and S. Sitch, Earth Syst. Sci. Data, 2020, 12, 3269–3340 CrossRef.
- Y. Feng, Z. Zhang, K. Zhao, S. Lin, H. Li and X. Gao, J. Colloid Interface Sci., 2021, 583, 499–509 CrossRef CAS.
- W. Gao, X. Li, S. Luo, Z. Luo, X. Zhang, R. Huang and M. Luo, J. Colloid Interface Sci., 2020, 585, 20–29 CrossRef PubMed.
- X. Hu, Y. Yong, Y. Xu, X. Hong, Y. Weng, X. Wang and X. Yao, Appl. Surf. Sci., 2020, 531, 147348 CrossRef.
- N. Ojha, A. Bajpai and S. Kumar, J. Colloid Interface Sci., 2020, 585, 764–777 CrossRef PubMed.
- S. Zhang, Y. Zhao, R. Shi, C. Zhou, G. I. Waterhouse, Z. Wang, Y. Weng and T. Zhang, Angew. Chem., Int. Ed., 2021, 60, 2554–2560 CrossRef CAS PubMed.
- Z. Zhao, C. Choi, S. Hong, H. Shen, C. Yan, J. Masa, Y. Jung, J. Qiu and Z. Sun, Nano Energy, 2020, 78, 105368 CrossRef CAS.
- H. Hirakawa, M. Hashimoto, Y. Shiraishi and T. Hirai, J. Am. Chem. Soc., 2017, 139, 10929–10936 CrossRef CAS.
- J. Bai, J. Sun, X. Zhu, J. Liu, H. Zhang, X. B. Yin and L. Liu, Small, 2020, 16, 1904783 CrossRef CAS.
- L. Ruan, X. Wang, T. Wang, Z. Ren, Y. Chen, R. Zhao, D. Zhou, G. Fu, S. Li, L. Gao, Y. Lu, Z. Wang, H. Tian, X. Kong and G. Han, ACS Appl. Mater. Interfaces, 2019, 11, 37256–37262 CrossRef CAS.
- T. Tachikawa, S. Yamashita and T. Majima, J. Am. Chem. Soc., 2011, 133, 7197–7204 CrossRef CAS PubMed.
- X. Han, Q. Kuang, M. Jin, Z. Xie and L. Zheng, J. Am. Chem. Soc., 2009, 131, 3152–3153 CrossRef CAS PubMed.
- M. Shi, G. Li, J. Li, X. Jin, X. Tao, B. Zeng, E. A. Pidko, R. Li and C. Li, Angew. Chem., Int. Ed., 2020, 59, 6590–6595 CrossRef CAS PubMed.
- T. Li, C. Wang, T. Wang and L. Zhu, Appl. Catal., B, 2020, 268, 118442 CrossRef CAS.
- M. Li, S. Yu, H. Huang, X. Li, Y. Feng, C. Wang, Y. Wang, T. Ma, L. Guo and Y. Zhang, Angew. Chem., Int. Ed., 2019, 58, 9517–9521 CrossRef CAS.
- L. Mu, Y. Zhao, A. Li, S. Wang, Z. Wang, J. Yang, Y. Wang, T. Liu, R. Chen, J. Zhu, F. Fan, R. Li and C. Li, Energy Environ. Sci., 2016, 9, 2463–2469 RSC.
- X. Wang, T. Hisatomi, J. Liang, Z. Wang, Y. Xiang, Y. Zhao, X. Dai, T. Takata, K. Domen and K. Domen, J. Mater. Chem. A, 2020, 8, 11743–11751 RSC.
- L. Lin, Z. Lin, J. Zhang, X. Cai, W. Lin, Z. Yu and X. Wang, Nat. Catal., 2020, 3, 649–655 CrossRef CAS.
- J. Zhu, F. Fan, R. Chen, H. An, Z. Feng and C. Li, Angew. Chem., Int. Ed., 2015, 54, 9111–9114 CrossRef CAS PubMed.
- H. Li, Y. Sun, B. Cai, S. Gan, D. Han, L. Niu and T. Wu, Appl. Catal., B, 2015, 170, 206–214 CrossRef.
- R. Li, H. Han, F. Zhang, D. Wang and C. Li, Energy Environ. Sci., 2014, 7, 1369–1376 RSC.
- J. Lu, Y. Lei, K. C. Lau, X. Luo, P. Du, J. Wen, R. S. Assary, U. Das, D. J. Miller and J. W. Elam, Nat. Commun., 2013, 4, 1432 CrossRef.
- D. Wang, H. Jiang, X. Zong, Q. Xu, Y. Ma, G. Li and C. Li, Chem.–Eur. J., 2011, 17, 1275–1282 CrossRef CAS PubMed.
- S. Wang, X. Hai, X. Ding, K. Chang, Y. Xiang, X. Meng, Z. Yang, H. Chen and J. Ye, Adv. Mater., 2017, 29, 1701774 CrossRef PubMed.
- T. Hou, Y. Xiao, P. Cui, Y. Huang, X. Tan, X. Zheng, Y. Zou, C. Liu, W. Zhu, S. Liang and L. Wang, Adv. Energy Mater., 2019, 9, 1902319 CrossRef CAS.
- Y. Liu, M. Cheng, Z. He, B. Gu, C. Xiao, T. Zhou, Z. Guo, J. Liu, H. He, B. Ye, B. Pan and Y. Xie, Angew. Chem., Int. Ed., 2019, 58, 731–735 CrossRef CAS.
- W. Ren, Z. Mei, S. Zheng, S. Li, Y. Zhu, J. Zheng, Y. Lin, H. Chen, M. Gu and F. Pan, Research, 2020, 3750314 CAS.
- Y. Li, X. Chen, M. Zhang, Y. Zhu, W. Ren, Z. Mei, M. Gu and F. Pan, Catal. Sci. Technol., 2019, 9, 803–810 RSC.
- X. Niu, Q. Zhu, S. Jiang and Q. Zhang, J. Phys. Chem. Lett., 2020, 11, 9579–9586 CrossRef CAS.
- J. Yang, Y. Guo, R. Jiang, F. Qin, H. Zhang, W. Lu, J. Wang and J. C. Yu, J. Am. Chem. Soc., 2018, 140, 8497–8508 CrossRef CAS PubMed.
- Y. Zhao, Y. Zhao, R. Shi, B. Wang, G. I. N. Waterhouse, L. Z. Wu, C. H. Tung and T. Zhang, Adv. Mater., 2019, 31, 1806482 CrossRef PubMed.
- Y. Shiraishi, M. Hashimoto, K. Chishiro, K. Moriyama, S. Tanaka and T. Hirai, J. Am. Chem. Soc., 2020, 142, 7574–7583 CrossRef CAS PubMed.
- S. Sun, Q. An, W. Wang, L. Zhang, J. Liu and W. A. Goddard, J. Mater. Chem. A, 2017, 5, 201–209 RSC.
- C. Zhang, Y. Xu, C. Lv, L. Bai, J. Liao, Y. Zhai, H. Zhang and G. Chen, Appl. Catal., B, 2020, 264, 118416 CrossRef CAS.
- H. L. Tan, X. Wen, R. Amal and Y. H. Ng, J. Phys. Chem. Lett., 2016, 7, 1400–1405 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra02739e |
| This journal is © The Royal Society of Chemistry 2021 |