Open Access Article
Open Access ArticleGreen synthesis and characterization of gold nanoparticles and their application for the rapid detection of glycyrrhizin with immunochromatographic strips
Dan-Ni Feng,
A-She Fang,
Tie-Ying Zhang,
Ming-Ze Ma,
Zhi-Hua Xu,
Yi-Xiao Sun,
Meng-Ting Zhang and
Feng Shi *
*
College of Life Science, Shihezi University, Shihezi, Xinjiang 832003, P. R. China. E-mail: shifeng2314@yeah.net
First published on 6th July 2021
Abstract
In this study, a facile and environmentally friendly synthesis process was proposed without regular chemical additives. We successfully synthesized spherical gold nanoparticles (AuNPs) coated with glycyrrhizin (GL) by using GL as both a reductant and a stabilizer to reduce chloroauric acid. The obtained NPs were approximately 35 nm in size. The formation of these GL-AuNPs was verified by the presence of a surface plasmon resonance band at 526 nm. We also experimentally determined that in terms of chemical structure, GL can be used as a reducing agent to obtain colloidal gold. The D-glucuronic acid structure, rather than glycyrrhetinic acid (GA), plays an important reducing role in colloidal gold production. From this, we hypothesized that other compounds with sugar structures in Glycyrrhiza may also have the ability to reduce chloroauric acid. To mitigate the high cost and low efficiency of current GL detection methods, we applied AuNPs to the immunochromatographic system. Then, a colloidal gold immunochromatographic test strip based on the indirect competition method was developed for the rapid detection of GL, and the detection limit of this strip was 25 ng mL−1. The cross-test showed that the strip has high specificity. The test results are consistent with the data obtained by high-performance liquid chromatography (HPLC) with a coincidence rate of up to 100%. The rapid test strip is simple, fast, highly efficient and inexpensive, making it suitable for large-scale, rapid glycyrrhizin content determination.
Introduction
Nanomaterials have been intensively studied because of their broad application prospects in many different disciplines. It has been reported that the specific particle size of nanomaterials imparts surface effects and small size effects. Therefore, compared with conventional materials, nanomaterials have unique properties, such as photoelectric, and thermomagnetic properties.1 We have seen the potential of these novel materials with nanoscale properties for use in diverse applications, such as pharmaceutical activities,2 bioimaging3 and biosensing.4 Metallic nanoparticles, especially gold nanoparticles (AuNPs), are at the forefront of development in this field. AuNPs are considered to be a useful platform to create a valid and efficient carrier system due to their large surface area-to-volume ratio, diverse morphology, facile synthesis and remarkable biocompatibility.5For the synthesis of AuNPs, conventional methods, such as the sodium citrate reduction method,6 are extensively used to prepare AuNPs, particularly in the biological and medical fields. In recent years, research on new reductants for hydrochloroauric acid, such as chitosan,7 polyols,8 ascorbic acid,9 tartaric acid,10 and gallic acid,11 has continuously attracted much attention. These reductants usually have hydroxyl and carboxyl groups because the hydroxyl group can reduce AuCl4− ions, while the carboxyl group can adsorb onto the surface of AuNPs, giving them electrostatic stability.12 Thus, these reductants are actually both reductants and stabilizers.
Glycyrrhiza is a perennial herbaceous plant of the genus Licorice in Papilionatae Taub of the Leguminosa family. The traditional Chinese medicine Glycyrrhiza has antiviral, antioxidant, anticancer and other pharmacological activities.13 The triterpenoid saponins of Glycyrrhiza are mainly found in the roots and the rhizome, with high activity and high abundance. Glycyrrhizin (GL) and glycyrrhetinic acid (GA) are the most widely studied compounds in Glycyrrhiza. GL is the main active factor in Glycyrrhiza and has high medicinal value. GL, a ternary carboxylic acid that also contains free hydroxyl and carboxylic groups, is composed of 1 molecule of GA and 2 molecules of D-glucuronic acid (Fig. 1).14 GA, another widely studied saponin with multiple biological activities, is a secondary metabolite of GL,15 and it exists as 18α-glycyrrhetinic acid and 18β-glycyrrhetinic acid. GA has extensive biological activities, but its poor water-solubility leads to weakened pharmacological properties. GL, as a natural carboxylic acid from plants, has a molecular structure similar to that of citric acid. We propose that GL can be a promising alternative reagent to synthesize stable and uniform AuNPs without the use of any additional reagents. Therefore, this study attempted to use GL as an important substitute for sodium citric acid as a reducing agent and stabilizer for the rapid synthesis of AuNPs.
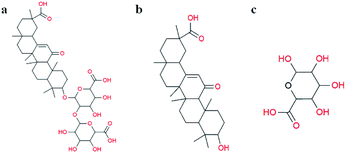 | ||
| Fig. 1 Molecular structures of (a) glycyrrhizin, (b) β-glycyrrhetinic acid, and (c) D-glucuronic acid. | ||
Immunoassay strip detection technology is a new real-time detection technology based on monoclonal antibody technology, immunolabelling technology and immunochromatography technology. This technique can be used for the qualitative, quantitative and semi-quantitative detection of antigens, haptens and antibodies. It is simple, specific, rapid and sensitive, moreover, immunoassay strip detection does not require professional technicians. With increasing practical needs and developments in the field of biology, the discovery of new biomarkers based on immunochromatography detection has attracted much attention from several fields of study. Currently, nanoparticles have been widely used in immunochromatography. At present, the immunochromatographic markers that are most widely used are colloidal gold, lanthanide elements,16 quantum dots,17 carbon nanotubes,18 organic nanocrystals, etc. These immunochromatographic markers, with the help of colorimetric, optical, electrical, magnetic and other signals, play a role in indicating and tracking biological responses and signal amplification for biological detection. The use of nanomaterial markers in biological detection can further improve the sensitivity of existing detection technologies and improve existing detection types and, to a certain extent, can solve difficult problems such as quantitative analysis or multiple detections that were previously challenging. Thus, AuNPs are excepted to be used more widely in immunochromatography.
The GL content is a prerequisite for the quality control and research of cultivated Glycyrrhiza. The Chinese Pharmacopoeia (2010) regards the GL content as an important index to measure the quality of Glycyrrhiza. Therefore, it is very important to determine the GL content for optimal utilization of GL resources. Currently, the most common methods for the detection of GL are liquid chromatography (LC), high-performance liquid chromatography (HPLC), ultraviolet (UV)-visible spectrophotometry, thin-layer chromatography (TLC), high-performance capillary electrophoresis (HPCE) and enzyme-linked immunosorbent assay (ELISA). At present, GL content analysis is mainly achieved by means of HPLC. Although this method has high sensitivity, accuracy and repeatability, it is expensive, complicated, time-consuming and laborious, and it is difficult to meet the requirements of spot checks for commercial products.19 ELISA is a simple and sensitive assay that can be used to analyse a large number of samples. However, this method is not reproducible. Moreover, ELISA is easily affected by interference as a result of various factors, such as autoantibodies, instruments and manual operation, resulting in false positive results. Overall, these methods are mostly laborious, time-consuming, and easily disrupted by various factors, and they require complicated detection procedures and sophisticated technical equipment. Compared with the abovementioned instrumental methods, colloidal gold immunochromatography test strips have the advantages of rapid detection, simple result analysis, low cost, high efficiency, and strong resistance to matrix interference. Therefore, this technique is a good rapid detection method for on-site screening in non-laboratory settings. This technology yields results by visual inspection of the presence or depth of colour on the strip, which can achieve highly sensitive qualitative and semi-quantitative analysis of GL. Based on this technique, we attempted to establish a simple and accurate new technique for immunochromatographic GL detection.
In this paper, GL was selected as the reducing agent and stabilizer to synthesize AuNPs with adsorbed GL molecules in one step. We explored the influence of some synthesis reaction conditions on the characteristic of the resultant AuNPs. The as-synthesized NPs were characterized by enzyme-labelling analysis, particle size analysis, transmission electron microscopy (TEM) and FT-IR spectroscopy. Then, GL-coated AuNPs were used as markers to establish a colloidal gold immunochromatographic test strip based on the indirect competition law. The colloidal gold rapid test strip was optimized, perfected and used for the rapid semi-quantitative detection of GL.
Materials and methods
Materials
Hydrochloroauric acid (HAuCl4) was purchased from Aldrich Chemical Co. and used without further purification. Silver nitrate (AgNO3) was purchased from Sigma. Glycyrrhizin (molecular weight: 822.93), quercetin and liquiritigenin were obtained from Beijing Balinway Technology Co., Ltd (purity ≥ 98%) Beijing, China). 18β-Glycyrrhetinic acid was purchased from Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China). D-Glucuronic acid was purchased from Shanghai Macklin Biochemical Technology Co., Ltd (Shanghai, China). All other chemical reagents were of analytical grade and used as received. We used ultrapure water from a Milli-Q A10 water purification system for all experiments.The nitrocellulose film (NC film) was purchased from Sartorius Corporation. Glass fibre film, a PVC bottom plate and absorbent paper were purchased from Shanghai Jieyi Biotechnology Co., Ltd. Monoclonal antibodies against GL and antibodies against BSA were provided by our laboratory.
Methods
GL is composed of 1 molecule of GA and 2 molecules of D-glucuronic acid. To further explore the mechanism by which GL can be used as a reducing agent to obtain colloidal gold, we used these two components of GL's chemical composition (GA and D-glucuronic acid) as reducing agents to verify the site of reduction in glycyrrhizin. Therefore, we also prepared glycyrrhetinic acid and D-glucuronic acid solutions at different concentrations to determine whether they could reduce chloroauric acid to obtain AuNPs.
A typical experimental procedure involved the following steps: solid GL and D-glucuronic acid of a certain quantity were mixed with ultrapure water respectively to prepare GL solutions and D-glucuronic acid solutions of different concentrations. A GA solution at a certain concentration was prepared by mixing a certain quantity of solid GA with absolute ethyl alcohol. Aqueous solutions of chloroauric acid and the three reductants described above were added to boiling ultrapure water under continuous and rapid agitation (the total reaction volume was 50 mL). Then, the reaction was carried out at a predetermined temperature, and the colour change in the solution was observed. When the reactants were completely discoloured and clear, the reaction was stopped and cooled to room temperature. Finally, the mixture was stored at 4 °C.
The as-prepared colloidal gold (3 mL) was analysed by an enzyme-labelling instrument with quartz cuvettes. The absorbance spectra were collected from 300 nm to 700 nm with 1 nm steps. Thus, the maximum absorption peak wavelength and peak width were determined.
The particle size distribution, molecular weight and zeta potential of colloidal gold particles under different conditions were measured by a Malvern particle size analyser. Therefore, the mean particle size, the width of the particle size distribution and the stability of the colloidal dispersion system were characterized.
A drop of the colloidal gold solution was placed on carbon-coated copper grids. After drying at room temperature, the mean particle size, surface morphology and dispersion of the as-prepared colloidal gold were determined by TEM.
The prepared colloidal gold was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min, and the precipitate was ground into fine powder, which was evenly mixed with dried KBr and pressed into thin pieces. Then, FT-IR analysis was conducted over a scanning range of 400–4000 cm−1.
000 rpm for 30 min, and the precipitate was ground into fine powder, which was evenly mixed with dried KBr and pressed into thin pieces. Then, FT-IR analysis was conducted over a scanning range of 400–4000 cm−1.
The colloidal gold immunolabelling rapid detection strips were then assembled. A NC film was sprayed with monoclonal antibody against GL as the detection line and the anti-BSA antibody as the quality control line and then dried at room temperature for 2 h. The NC film sprayed with the quality control line and detection line, two layers of absorbent paper, the bonding pad sprayed with colloidal gold, and the sample pad were successively pasted onto the PVC bottom plate. Then, the assembled PVC bottom plate was cut into a width of 4 mm by a programmable slicer to form the colloidal gold immunochromatography strip for rapid detection (Fig. 3).
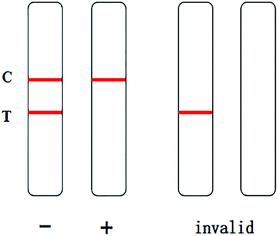
The assembled strips were evaluated with samples. 80 μL of extracted GL (10 μL of sample solution and 70 μL of 0.02 M Tris–HCl buffer at pH 8.5) was added to the sample pad of the test strip, and the results were observed within 10 min. In the chromatography process, the NC membrane with the detection line and control line was fixed as the fixed phase, and the mobile phase was the liquid to be detected once combined with the marker. Through capillary action, the liquid to be detected moved up the chromatographic strip. The specific immune reaction with the liquid to be detected occurred at the detection line, and the present substance reacted with the immune response target at the control line. Qualitative or quantitative detection was carried out according to the chromogenic strip of the marker, which is visible to the naked eye. Only when a stripe is present at the control line can the test strip be considered as effective. If there is a red stripe on the test line, the sample is negative. If there is no red stripe on the test line, the sample is positive.
Sensitivity of the test strip. GL reference solutions of 0, 5, 10, 15, 20, and 25 ng mL−1 were detected with a quick test strip, and each concentration was analysed in triplicate. The sensitivity of the test strip was determined according to the colour developed during the reactions at the control line and the test line.
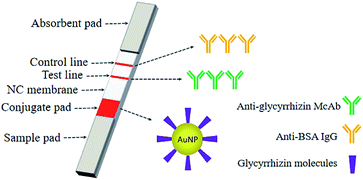 | ||
| Fig. 3 An immunochromatographic system for the determination of GL using the AuNPs with glycyrrhizin molecules adsorbed on their surfaces as markers. | ||
Specificity of the test strip. Appropriate amounts of reference substances, such as GA, quercetin and liquiritigenin, were precisely weighed. These compounds are the chemical components that with high abundance in Glycyrrhiza. A 1 μg mL−1 solution was prepared with methanol and filtered through a 0.45 μm microporous membrane to obtain the reference solution. The reference solution of each chemical component was diluted with methanol to form solutions of 15, 25, 50, and 100 ng mL−1. Colloidal gold immunochromatography for rapid detection was used to detect the above chemical components, and each concentration was repeated 3 times. The specificity of the test strip was determined according to the colour developed during the reactions at the control line and the test line.
Stability of the test strip. The strips were sealed and stored at 4 °C, 25 °C and 37 °C. In addition, the strips were kept in the dark under drying conditions without vacuum treatment. Five test strips were randomly selected every 2 months to evaluate the limit of detection for a total of 18 months. The effect of different temperatures and storage times on the test strip was observed to determine the stability of the strip.
Sample analysis with the test strip. The GL content of wild Glycyrrhiza roots in 6 areas (Xingjiang, Gansu, Ningxia, Inner Mongolia, Shaanxi and Shanxi) was determined. A Glycyrrhiza root powder sample was weighed precisely to 0.100 g and stabilized in 50 mL of deionized water. The samples were centrifuged at 3500 rpm for 10 min.20 The supernatant was diluted with deionized water to form samples with ratios of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, 1
100, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, 1
1000, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 1
000, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 according to the concentration gradient to obtain the test solutions, which were used for the rapid test strip to determine the GL content. This result was compared with the HPLC results.
000 according to the concentration gradient to obtain the test solutions, which were used for the rapid test strip to determine the GL content. This result was compared with the HPLC results.
Results
Synthesis and characterization of AuNPs
Colloidal gold presents a ruby red colour in aqueous solution due to the surface plasmon resonance (SPR) of metal nanoparticles.21 Herein, colour variations and SPR were used as tools to confirm the formation of gold particles.12 In this experiment, a GL solution was mixed with a chloroauric acid solution. The synthesis reaction started within a few minutes, and the colour of the solution changed from light yellow to bright wine red, preliminarily indicating the formation of AuNPs. The synthesis of AuNPs was further confirmed by UV-vis spectroscopic studies. The synthesized AuNPs had the largest absorption band in the range of 300–700 nm. This was due to the SPR of AuNPs with a maximum absorption peak at 526 nm (Fig. 4a).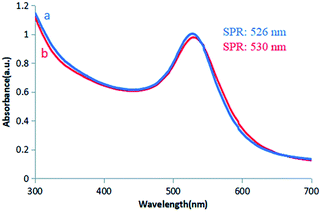 | ||
| Fig. 4 UV/vis spectra of (a) GL-reduced AuNPs. (b) GL-reduced AuNPs after being placed at room temperature for 2 months. | ||
Colloidal gold prepared under several different reaction conditions (such as different concentrations of the reducing agent and different reaction temperatures) exhibited different shades of wine red under natural light. However, they were all clear and transparent and remained stable at room temperature for two months without precipitation. There was no significant change in the position of the absorption peak or the maximum peak in the UV-vis spectrum (Fig. 4b), indicating stability and non-agglomeration of the formed nanoparticles. This may be because GL not only acts as a reducing agent in the synthesis of AuNPs, but also acts as a stabilizer to cap the surface of AuNPs.
Colloidal gold prepared under different concentrations of D-glucuronic acid solution exhibited different shades of bluish violet under natural light, but they were all clear and transparent, and the SRP observed at 535 nm confirmed AuNPs synthesis. The gold nanoparticles remained stable at room temperature for two months without precipitation, and no significant variation in the UV-vis spectrum was observed (Fig. 5b). However, GA solution did not react with the chloroauric acid solution, and thus could not reduce an aqueous solution of gold chloride trihydrate to obtain colloidal gold. GL is composed of 1 molecule GA and 2 molecules of D-glucuronic acid. The above results indicate that in terms of chemical structure, GL can be used as a reducing agent to reduce the aqueous solution of chloroauric acid to obtain colloidal gold. D-Glucuronic acid plays an important reducing role in colloidal gold production, while GA does not.
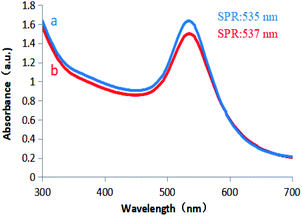 | ||
| Fig. 5 UV/vis spectra of (a) D-glucuronic acid-reduced AuNPs. (b) D-Glucuronic acid-reduced AuNPs after being placed at room temperature for 2 months. | ||
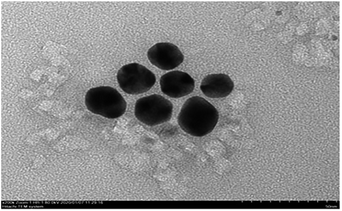
TEM images of the AuNPs synthesized in this study show the existence of spherical particles with an average size of 35 nm (Fig. 6). The TEM images show that the nanoparticles were approximately spherical with uniform size, good dispersion, and no agglomeration, indicating stabilization of the prepared AuNPs due to the capping stabilization effect of gold nanoparticles by GL molecules. Active groups such as carboxyl and hydroxyl groups in GL interacted strongly with the surface of AuNPs, which effectively inhibited the aggregation tendency of AuNPs and enhanced the stability of AuNPs in liquid.
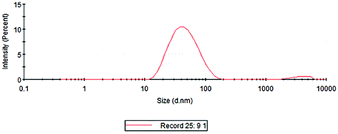
The particle size analysis results of the prepared colloidal gold are shown in Fig. 7. It can be seen from the figure that the particle size of the AuNPs was between 30 and 50 nm, and the average particle size was 35.08 nm. The results were basically consistent with those of TEM characterization. The colloidal gold particles had a narrow particle size distribution and good dispersion. The polydispersity index was 0.380, indicating that the colloidal gold particle size distribution was concentrated. Then, the zeta potential was measured to obtain information about the surface charge and stability of the as-prepared AuNPs.
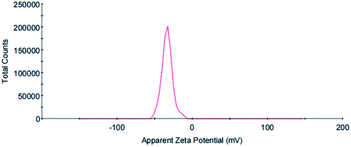
The zeta potential is an important parameter to characterize the stability of colloidal dispersions and predict the long-term stability of nanoparticles. The value of the zeta potential in a sample determines whether particles in a liquid are stable or tend to flocculate and stick together. If the absolute value of the zeta potential is large, then a particle has many negative or positive charges. This promotes particle separation to increase the stability of the whole system. Generally, the boundary of particle dispersion stability is considered to be ±30 mV. If the zeta potential of these particles is higher than +30 mV or lower than −30 mV, then the dispersion system is relatively stable. In this study, the zeta potential of the synthesized AuNPs with GL molecules on the surface was −33.7 mV, indicating that the nanoparticle suspension showed moderate stability. The electronegativity of the suspension was mainly attributed to the GL molecules on the AuNP surface (Fig. 8).
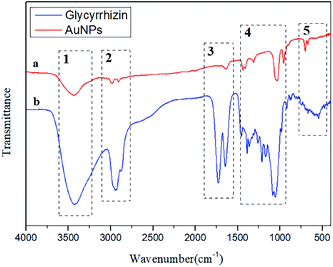
FT-IR spectra were recorded to characterize the possible role of functional groups in the synthesis of AuNPs. To determine the possible interaction sites between GL and AuNPs, the FT-IR spectra of GL and AuNPs synthesized with GL were measured and are shown in Fig. 9. The FT-IR spectra of GL (Fig. 9a) include typical bands at 3427, 2933, 1730, and 1647 cm−1. The peak at 3427 cm−1 was assigned to the stretching vibration of the hydroxy group. The signal at 2933 cm−1 was indicative of a carboxyl group. The band at 1730 cm−1 was indicative of a carbonyl group. The peak at 1647 cm−1 could be assigned to the vibrational modes of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds of GL. Fig. 9b shows that after the synthesis of AuNPs, the peaks at 3433 and 3427 cm−1, the stretching vibration signals of hydroxyl and carboxyl groups were reduced in intensity. The signal of the stretching vibration of the carbonyl group disappeared. This indicated that these functional groups were involved in the reduction process. This result also suggested the electronic interaction of the AuNPs with other functional groups. All the above results confirmed that the GL molecules adsorbed on the AuNPs surfaces.
C double bonds of GL. Fig. 9b shows that after the synthesis of AuNPs, the peaks at 3433 and 3427 cm−1, the stretching vibration signals of hydroxyl and carboxyl groups were reduced in intensity. The signal of the stretching vibration of the carbonyl group disappeared. This indicated that these functional groups were involved in the reduction process. This result also suggested the electronic interaction of the AuNPs with other functional groups. All the above results confirmed that the GL molecules adsorbed on the AuNPs surfaces.
Immunochromatographic systems for the determination of GL
GL-coated AuNPs were used as markers to establish a colloidal gold immunochromatographic test strip based on the indirect competition law. A red stripe on the NC film was the indicator of the assembled rapid test strip. When a red stripe appeared on the strip (only the control line shows the red stripe), indicating that the sample contained GL and the content exceeded the detection limit of the strip. When there were two red stripes on the test strip, the sample did not contain GL, or the content of GL did not reach the detection limit of the strip (Fig. 10).A specially treated sample pad (the sample pad was pretreated with 0.02 M Tris–HCl buffer at pH 8.5) was subjected to a sample of Glycyrrhiza root extract. The sample flowed through the conjugate pad to the NC membrane and developed in the superlayer of the film. The sample continued to move on the NC membrane (the detection line of the NC membrane was coated with a monoclonal antibody against glycyrrhizin as the coating agent. The control line of the NC membrane was coated with anti-BSA antibodies). When the content of GL in the sample exceeded the detection limit of the strip, it reacted with monoclonal antibodies against GL on the NC membrane to saturate the antibody binding site. Therefore, the colloidal gold with glycyrrhizin molecules on the surface did not react with monoclonal antibodies against GL on the NC film, and it did not show red bands. In contrast, when the content of GL in the sample did not exceed the detection limit of the strip, the colloidal gold with GL molecules on the surface reacted with the monoclonal antibodies against GL on the NC membrane and resulted in red bands. When the sample passed through the binding pad to the quality control line by capillary action, the BSA on the surface of colloidal gold bound to the anti-BSA antibody coated on the quality control line, forming a visible band.
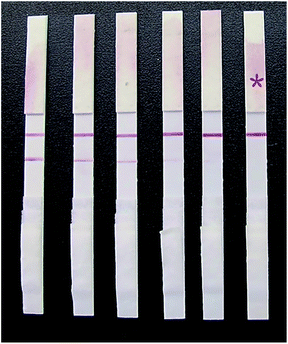 | ||
| Fig. 11 Detection results of different concentrations of glycyrrhizin on the strip. The contents of glycyrrhizin from left to right were 0, 5, 10, 15, 20, and 25 ng mL−1. | ||
When a 0 ng mL−1 GL sample was tested, a dark red stripe appeared on the test line. However, with increasing concentration of the GL reference solution, the red stripe on the test line gradually disappeared. When the mass concentration of the GL reference solution increased to 20 ng mL−1, the red stripe on the test line was still visible, but it was significantly reduced. This was still considered a negative result. When the mass concentration of GL reference solution increased to 25 ng mL−1, no red stripe appeared on the test line (Fig. 11). This was considered a positive result. Thus, we determined that the detection limit of the strip was 25 ng mL−1. The above results are valid only if the quality control line of the rapid test strip has a dark red stripe. Otherwise, the rapid test strip is invalid.
Specificity of the test strip
Fig. 12 shows that only when a GL solution was used as the sample could the test strips yield positive results (when the concentration of GL exceeded 25 ng mL−1). When a different control solution was used as the sample, the test strips yielded only negative results (Table 1). This indicated that the rapid test strip for GL has no cross reaction with other analogues and exhibits only specific binding with GL. Thus, the rapid test strip has good specificity.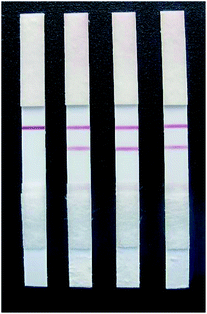 | ||
| Fig. 12 The specificity of the colloidal gold strips. From left to right, the test results of 25 ng mL−1 glycyrrhizin, glycyrrhetinic acid, quercetin and liquiritigenin. | ||
| Compound abundant in Glycyrrhiza (ng mL−1) | 15 | 25 | 50 | 100 |
|---|---|---|---|---|
| a Key: “+”, positive result, an obvious red band was observed; “−”, negative result, no band was observed. | ||||
| Glycyrrhizin | − | + | + | + |
| Glycyrrhetinic acid | − | − | − | − |
| Quercetin | − | − | − | − |
| Liquiritigenin | − | − | − | − |
Stability of the test strip
The rapid test strips were stored at 4 °C, 25 °C and 37 °C, and their detection limits were regularly tested. The results showed that the detection limit of the strip placed at 4 °C for 18 months did not change significantly. The detection limit of the strips stored at 25 °C for 14 months gradually increased (in the fourteenth month, the detection limit increased to 50 ng mL−1). The detection limit of the strips stored at 37 °C for 4 months increased significantly (in the fourth month, the detection limit increased to 1000 ng mL−1). This indicates that the rapid strips can be stably stored at 37 °C for 3 months and can be stably stored at room temperature for more than 1 year. The strips can be stored stably for approximately a year and a half at 4 °C, which proves that the strips have good stability.Sample test results
Wild Glycyrrhiza roots from the six regions of Xinjiang, Gansu, Ningxia, Inner Mongolia, Shaanxi and Shanxi were prepared in test solutions. The solutions were analysed by the HPLC method according to the Chinese Pharmacopoeia, and then, GL test strips were used for the determination. The test results were compared to verify the accuracy of the strip. The detection results of the rapid test strips for GL are shown in Fig. 13. It can be seen from the figure that the GL samples from four of the regions gave positive detection results on the test strips, indicating that the GL content in the GL roots from these four regions exceeded 25 ng mL−1. The samples from Ningxia and Shanxi gave negative results with the strips, indicating that the GA content in the Glycyrrhiza roots from these two regions did not exceed 25 ng mL−1. This was consistent with the HPLC detection results (Table 2). This indicates that the rapid test strip for GL is a fast, accurate and reliable test method that can meet the rapid screening requirements for GL production on a large scale.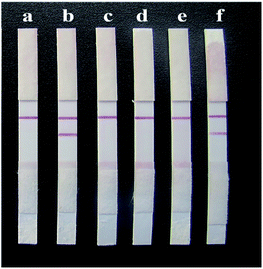 | ||
| Fig. 13 Detection results of samples from different provinces on the strips. (a) Xinjiang, (b) Gansu, (c) Ningxia, (d) Inner Mongolia, (e) Shaanxi, and (f) Shanxi. | ||
| Sample source | Result/(ng mL−1) | |
|---|---|---|
| HPLC | Glycyrrhizin test strips | |
| a Key: “+”, positive result, an obvious red band was observed; “−”, negative result, no band was observed. | ||
| Xinjiang | 39.32 | + |
| Shanxi | 21.60 | − |
| Inner Mongolia | 44.79 | + |
| Shaanxi | 33.30 | + |
| Gansu | 40.94 | + |
| Ningxia | 17.45 | − |
Discussion
Conventional methods for the synthesis of AuNPs require hazardous chemicals and large amounts of heat. In addition, this process may produce hazardous byproducts that have both ecological and environmental risks. Therefore, it is of great significance to study the synthesis of colloidal gold with clean, non-toxic, environmentally friendly and mild reaction conditions. Plant-based synthesis of nanoparticles has generated worldwide interest because of its cost-effectiveness, eco-friendly nature and plethora of applications. Various plant materials and their extracts provide an attractive platform for the synthesis of nanoparticles, as they are easily available, safe and free from toxic chemicals. Moreover, these compounds provide natural capping agents for the stabilization of nanoparticles.This study found that the plant extract GL can be used as a reducing agent to rapidly synthesize AuNPs with uniform particle size and good dispersibility without the addition of other reducing agents and stabilizers. Several phytoconstituents have been explored previously for the preparation of AuNPs. Phenolic and carboxylic groups have been reported to act as reducing and capping agents during the synthesis of AuNPs. GL is composed of 1 molecule of GA and 2 molecules of D-glucuronic acid. The carboxylic groups (–COOH) and hydroxyl groups (–OH) that are abundant on D-glucuronic acid possibly reduce gold ions to gold particles. The GL molecules adsorbed on the surface of the AuNPs provide colloidal stability owing to their negative charge. Thus, GL acts as both the reducing agent and stabilizing agent in our reaction.
To further elucidate the mechanism of GL preparation of AuNPs. GA and D-glucuronic acid, the individual components of GA, were used independently as reducing agents to reduce gold chloride acid in this study. The results show that D-glucuronic acid could reduce chloroauric acid to obtain AuNPs that could be recreated, while GA could not reduce chloroauric acid. This indicates that the D-glucuronic acid structure in GL molecules plays an important role in reduction. Thus, we hypothesized that other compounds with sugary structures in Glycyrrhiza may also have the ability to reduce chloroauric acid. In addition, other compounds with similar sugar structures in plant extracts may have the potential to be used as reductants to prepare AuNPs, which will widen the availability of reducing agents for the green preparation of AuNPs.
At present, the quality control of cultivated GL is based on the content of GL. Because the analysis and determination of a large number of samples is often difficult, it is particularly important to establish a new detection technology with simple steps, high sensitivity, high throughput, low instrumental requirements and low cost. The surfaces of the AuNPs prepared in this study contained GL molecules as observed via characterization analysis. Thus, these particles can be directly used as marker materials in an immunochromatography system to establish a rapid detection technology based on the indirect competition law. An immunochromatographic strip with high efficiency and low cost was developed for the rapid determination of the GL content on a large scale. This technical system provides a theoretical basis and technical support for the quality research and scientific use of GL.
The previously reported rapid detection of GL by colloidal gold immunochromatography utilized GL molecules in a solution to be tested and GL conjugates competitively binding to monoclonal antibodies.20 In this process, GL is a hapten due to its relatively small molecular weight and weak immunogenicity and must be coupled with the corresponding carrier protein to stimulate the body to produce an immune response as an immunogen. Therefore, colloidal gold is first labelled with a monoclonal antibody against GL to form a colour marker and then sprayed on the binding pad. The detection line on the NC film was coated with GL protein conjugate, while the quality control line was coated with anti-gold target secondary antibody. Compared with the detection method established in this study, monoclonal antibody labelling of colloidal gold with GL is an additional process with cumbersome procedures and increased costs. However, the detection method established in this study requires only the bonding pad to be sprayed by the as-prepared colloidal gold. A monoclonal antibody against GL was used as the detection line, and the antibody against BSA was used as the control line. During this detection, the GL molecules in the substance and the GL molecules attached to the surface of AuNPs competitively bound to monoclonal antibodies against GL.
Conclusions
In this study, we describe a green and facile method for the synthesis of well-dispersed spherical AuNPs using GL as a reducing and stabilizing agent. The synthesized monodispersed nanoparticles showed a narrow size distribution with an average of 35 nm and GL molecules on the surface. The nanoparticles also had high stability in aqueous colloidal solutions.In addition, this study explored the reaction conditions of the rapid test strip for GL, and established a sensitive, specific, and simple detection method that is suitable for the separation and screening of GL in large quantities. This study laid a foundation for the rapid and efficient separation, extraction and purification of GL from Glycyrrhiza samples, and provided a new biotechnological means for the application research of high-yield and high-quality cultivation and biochemical regulation technology of Glycyrrhiza and variety breeding.
The rapid immunochromatographic technique for GL established in this study has the following advantages: (a) the sample treatment method is simple and safe, requiring only water immersion without toxic or harmful chemical reagents. (b) This method is suitable for large sample determination, which can greatly improve work efficiency and reduce work intensity. (c) This detection method is inexpensive and can quickly identify the quality of GL, which has obvious advantages for basic units and occasions where large samples need to be analysed.
Author contributions
Dan-Ni Feng: responsible for the main data curation and writing the original draft. A-She Fang: responsible for the conceptualization and methodology. Tie-Ying Zhang: responsible for part of the data curation. Ming-Ze Ma: responsible for part of the data curation. Zhi-Hua Xu: responsible for reviewing and editing the paper. Yi-Xiao Sun: responsible for part of the data curation. Meng-Ting Zhang: responsible for reviewing and editing the paper. Feng Shi: responsible for writing, reviewing and editing the paper, funding acquisition, designing the experimental, instructing the students to complete their experiments and revising the full paper. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (32060224).References
- A. B. Jindal, Int. J. Pharm., 2017, 532, 450–465 CrossRef CAS PubMed.
- P. Ghosh, G. Han, M. De, C. K. Kim and V. M. Rotello, Adv. Drug Delivery Rev., 2008, 60, 1307–1315 CrossRef CAS PubMed.
- E. Boisselier and D. Astruc, Chem. Soc. Rev., 2009, 38, 1759–1782 RSC.
- T. Špringer, X. Chadtová Song, M. L. Ermini, J. Lamačová and J. Homola, Anal. Bioanal. Chem., 2017, 409, 4087–4097 CrossRef PubMed.
- F. Danhier, O. Feron and V. Préat, J. Controlled Release, 2010, 148, 135–146 CrossRef CAS PubMed.
- J. Ftouni, M. Penhoat, A. Addad, E. Payen, C. Rolando and J. S. Girardon, Nanoscale, 2012, 4, 4450–4454 RSC.
- J. Esther and V. Sridevi, Gold Bull., 2017, 50, 1–5 CrossRef CAS.
- C. Li, K. L. Shuford, M. Chen, E. J. Lee and S. O. Cho, ACS Nano, 2008, 2, 1760–1769 CrossRef CAS PubMed.
- B. J. Morrow, E. Matijević and D. V. Goia, J. Colloid Interface Sci., 2009, 335, 62–69 CrossRef CAS PubMed.
- N. Liu, K. Wang, Y. Gao, D. Li, W. Lin and C. Li, Colloids Surf., A, 2017, 535, 251–256 CrossRef CAS.
- B. Moyo, S. Oyedemi, P. J. Masika and V. Muchenje, Meat Sci., 2012, 91, 441–447 CrossRef CAS.
- K. P. Kumar, W. Paul and C. P. Sharma, Process Biochem., 2011, 46, 2007–2013 CrossRef CAS.
- N. A. Mamedov and D. Egamberdieva, Plant Hum. Health, 2019, 3, 1–21 Search PubMed.
- R. Fan, N. Li, H. Xu, J. Xiang, L. Wang and Y. Gao, Food Chem., 2016, 190, 912–921 CrossRef CAS.
- J. Y. Yang, J. H. Koo, Y. G. Song, K. B. Kwon, J. H. Lee, H. S. Sohn, B. H. Park, E. C. Jhee and J. W. Park, Acta Pharmacol. Sin., 2006, 27, 1467–1473 CrossRef CAS PubMed.
- Q. You, M. Liu, Y. Liu, H. Zheng, Z. Hu, Y. Zhou and B. Wang, ACS Sens., 2017, 2, 569–575 CrossRef CAS PubMed.
- C. Wang, F. Hou and Y. Ma, Biosens. Bioelectron., 2015, 68, 156–162 CrossRef CAS.
- L. L. Meng, T. T. Song and X. Mao, Talanta, 2017, 167, 379–384 CrossRef CAS.
- J. Zhao, G. Li, B. M. Wang, W. Liu, T. G. Nan, Z. X. Zhai, Z. H. Li and Q. X. Li, Anal. Bioanal. Chem., 2006, 386, 1735–1740 CrossRef CAS PubMed.
- P. Sun, J. Zhang, J. Zheng, B. Jia and F. Shi, Chin. J. Pharm. Anal., 2017, 37(10), 1769–1775 Search PubMed.
- S. Rajeshkumar, C. Malarkodi, G. Gnanajobitha, K. Paulkumar, M. Vanaja, C. Kannan and G. Annadurai, J. Nanostruct. Chem., 2013, 3(1), 1–7 Search PubMed.
| This journal is © The Royal Society of Chemistry 2021 |