Open Access Article
Open Access ArticleFabrication and application of a MIL-68(In)–NH2 incorporated high internal phase emulsion polymeric monolith as a solid phase extraction adsorbent in triazine herbicide residue analysis
Jinhua Luo†
a,
Liping Jiang†ab,
Guihua Ruan b,
Chengyong Li*ac and
Fuyou Du
b,
Chengyong Li*ac and
Fuyou Du *ab
*ab
aCollege of Biological and Environmental Engineering, Changsha University, Changsha 410022, China. E-mail: lyong92@163.com; dufu2005@126.com; Fax: +86-731-84250583; Tel: +86-731-84261506
bCollege of Chemistry and Bioengineering, Guilin University of Technology, Guangxi 541004, China
cHunan Provincial Key Laboratory of Nutrition and Quality Control of Aquatic Animals, Changsha University, Changsha 410022, China
First published on 8th June 2021
Abstract
In this work, a metal–organic framework MIL-68(In)–NH2 incorporated high internal phase emulsion polymeric monolith (MIL-68(In)–NH2/polyHIPE) was prepared and applied as a solid phase extraction adsorbent for the extraction and detection of trace triazine herbicides in environmental water samples by coupling with HPLC-UV detection. The fabricated material showed good adsorption for simazine, prometryn, and prometon in water samples because of π–π interactions and hydrogen bonding interactions. Under optimal conditions, the maximum adsorption capacity of simazine, prometon and prometryn was 800 μg g−1, 800 μg g−1 and 6.01 mg g−1, respectively. The linearities were 10–800 ng mL−1 for simazine, prometon and prometryn. The limits of detection were 31–97 ng L−1, and the recoveries were 85.6–118.2% at four spiked levels with relative standard deviations lower than 5.0%. The method has a high sensitivity for the determination of three triazine herbicides in environmental water samples.
1. Introduction
In order to prevent weed growth and increase crop yields, triazine herbicides have been widely used in agricultural production all over the world because of their high efficiency and broad-spectrum weed control in a variety of crops.1 However, their continued and indiscriminate use has resulted in many potential hazards to the countryside and the surrounding environment, and therefore many countries and organizations have regulated the maximum residue limits (MRL) for triazine herbicides in a variety of samples. For example, the European Union (EU) has set the MRL of a single triazine herbicide in drinking water to 0.1 ng mL−1 and the total amount of multiple triazine herbicides to 0.5 ng mL−1.2,3 Therefore, it is highly important to develop novel separation and analysis methods for monitoring the concentrations of triazine herbicide residues in environmental water samples.Generally, herbicide residues are likely to exist in different water samples at low concentration, therefore, effective sample preparation is necessary before instrumental detection in order to improve method sensitivity and accuracy and/or prevent the used apparatus from damaging by other interferents.4–10 Among various sample preparation, solid phase extraction (SPE) is the most widely used extraction technique for extracting triazine herbicides from water samples because of its advantages of high recovery, high enrichment factor, low solvent consumption, and short extraction time.2–6 As a result, various SPE adsorbents, including oxidized single-walled carbon nanohorns,2 triazine rings-containing porous aromatic frameworks,3 poly(high internal phase emulsions) (polyHIPEs),5,6 molecular imprinted polymers,4,11,12 carbazole-based porous organic polymers,13 hydrazone-based covalent triazine polymers,14 and metal–organic frameworks (MOFs)15–18 have been fabricated and applied in the SPE of triazine herbicides in environmental samples. Among these SPE adsorbents, MOFs were possessed of high adsorption and separation performance, owing to their remarkable merits like large surface area, adjustable pore size, diverse structures, as well as great chemical and thermal stability.19–21 On the other hand, polyHIPEs were also highly interconnected porous materials with distinct characteristics such as highly interconnected pore architecture, tunable porosity, controllable cavity, large surface areas, and adjustable functions,22–24 however, depending on the high porosity polyHIPEs for separation applications usually suffer from insufficient mechanical properties (strength, modulus and ductility).25–27 Thus, enhancement of mechanical properties by using a variety of approaches, such as selecting new functional monomer systems, using medium internal phase emulsions instead of HIPEs to obtain higher density polyHIPEs, and especially combining HIPE templates with organic or inorganic particles to form composite structures, was very important to achieve improved polyHIPEs for separation applications.25–27 Based on their advantages of MOFs and polyHIPEs, MOFs as particle stabilizers were introduced to fabricate MOFs functionalized polyHIPEs (MOFs–polyHIPEs), which exhibited synergistic advantages in separation applications.28–30 In addition, HPLC method is a rapid, sensitive and reproducible way to determine trace analytes in complex samples,31–34 and thus has been used to analyse triazine herbicides.3,5,6,12,18 To the best of our knowledge, however, MOFs–polyHIPEs as SPE adsorbents have not been reported in the separation and analysis of triazine herbicides by combining with HPLC method.
In this work, an amine functionalized indium-based MOF MIL-68(In)–NH2 was prepared and then used as particle stabilizers to fabricate MIL-68(In)–NH2 functionalized polyHIPEs (MIL-68(In)–NH2/polyHIPEs) by polymerization of Pickering high internal phase emulsions (HIPEs) of MIL-68(In)–NH2, surfactant sorbitan monooleate (Span 80), 2-ethylhexyl acrylate (EHA) and divinylbenzene (DVB). The resulting MIL-68(In)–NH2/polyHIPEs as SPE adsorbents were used to separate some triazine herbicides from environmental water samples. The three most commonly used triazine herbicides (simazine, prometon and prometryn) in local pesticide store were chosen as the analytes. After monolithic SPE, the analytes were detected by HPLC-UV, and a satisfactory result was achieved.
2. Materials and methods
2.1 Chemicals and materials
Simazine, prometryn, prometon, EHA, DVB, Span 80, indium nitrate hydrate (In(NO3)3·xH2O), 2-amino terephthalic acid (H2ATA), N,N-dimethylfomamide (DMF), and azo-bisisobutyronitrile (AIBN) were purchased from Aladdin Chemistry Co. Ltd. (Shanghai, China). HPLC grade acetonitrile and methanol were obtained from Thermo Fisher Scientific. Deionized water was used for all experiments unless otherwise mentioned.2.2 Preparation of MIL-68(In)–NH2
MIL-68(In)–NH2 was prepared by solvothermal synthesis method.35,36 Briefly, 3.4641 g In(NO3)3·xH2O and 0.5348 g H2ATA were mixed with 30 mL DMF to prepare homogeneous dispersion solution with ultrasonication. The mixture solution was transferred into a 100 mL Teflon liner with a stainless steel autoclave and then kept at 125 °C for 5 h. After that, the obtained yellow powders were purified with DMF and fresh methanol for three times. Finally, the prepared MIL-68(In)–NH2 were filtered and dried under vacuum at 80 °C overnight.2.3 Preparation of MIL-68(In)–NH2/polyHIPEs
MIL-68(In)–NH2/polyHIPEs monoliths were fabricated by polymerization of Pickering HIPEs as previously reported.5,6,30 Briefly, 300.0 μL EHA, 150.0 μL DVB, and 150.0 μL Span 80 were mixed with ultrasonication as organic phase in a 10 mL polypropylene centrifuge tube. 40 mg MIL-68(In)–NH2 and 19.0 mg AIBN were dispersed in 3.5 mL water under ultrasonication as water phase in a 10 mL polypropylene centrifuge tube. Then, 400.0 μL of water phase mixture was added to the organic phase at one time and added again after homogeneous mixing with the help of an IKA MS-3B homogenizer (IKA, Germany) until all the aqueous phase mixture were mixed with the organic phase. Finally, 1.0 mL emulsion was transferred into a 3.0 mL blank SPE column and sealed for the following heating at 65 °C for 18 h. After reaction, the fabricated MIL-68(In)–NH2/polyHIPEs monoliths were washed with methanol and water in turn for three times at least.2.4 Enrichment of triazine herbicides
Before the extraction, the MIL-68(In)–NH2/polyHIPEs monoliths were washed with 10 mL methanol and 6 mL water, separately, and then loaded with 30 mL water samples at a flow rate of 1.0 mL min−1. After the enrichment, 2.0 mL 70% (v/v) acetonitrile was used to elute the adsorbed triazine herbicides on the monoliths. The elution solution was collected and then filtered by 0.22 μm nylon syringe filters for the following HPLC-UV analysis. In this work, the recovery rates were calculated using the equation:where C0 and Ce (ng mL−1) are the concentrations of the analyte in original sample solution and elution solution, respectively. V0 and Ve (mL) indicates the volume of sample solution and the eluate, respectively.
2.5 HPLC analysis
The detection of triazine herbicides was carried out by LC-20A system (Shimadzu, Japan) equipped with a SPD-20 UV-vis detector at 220 nm of wavelength. Separations of triazine herbicides were performed on a ZORBAX Eclipse Plus C18 column (4.6 × 250 mm, 5 μm, Agilent) at 30 °C with a flow rate of 0.8 mL min−1. The elution was performed for 0–13 min with 60% acetonitrile and 40% water. The injection volume was 10 μL.3. Results and discussion
3.1 Characterization of MIL-68(In)–NH2/polyHIPEs monoliths
The fabricated MIL-68(In)–NH2/polyHIPEs were characterized by Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM). As can be seen from Fig. 1A, the two bands at 3446 and 3370 cm−1 were assigned to the symmetric and asymmetric stretching vibrations of primary amines, respectively, and the adsorption band at 1255 cm−1 was corresponded to N–C stretching vibrations.35,36 The peaks at 1563 and 1381 cm−1 were ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of aromatic rings and aromatic C
C stretching vibration of aromatic rings and aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration. Compared with FTIR of the In-based composites,37,38 the characteristic peaks at 434, 542 and 765 cm−1 can be attributed to the stretching vibrations of O–In–O bending modes, In–O-bridging and In–O-nonbridging oxygen atoms in the composites, respectively. The intensity of the adsorption peaks at 1563, 1381, and 1255 cm−1 was decreased, and the two peaks at 3446 and 3370 cm−1 were overlapped by the broad peaks (around 3450 cm−1) of the O–H stretching vibration in MIL-68(In)–NH2/polyHIPEs in comparison with the FTIR spectra of MIL-68(In)–NH2. In addition, the stretching vibrations of –CH2– group (2963, 2927, and 2860 cm−1) and –C
C stretching vibration. Compared with FTIR of the In-based composites,37,38 the characteristic peaks at 434, 542 and 765 cm−1 can be attributed to the stretching vibrations of O–In–O bending modes, In–O-bridging and In–O-nonbridging oxygen atoms in the composites, respectively. The intensity of the adsorption peaks at 1563, 1381, and 1255 cm−1 was decreased, and the two peaks at 3446 and 3370 cm−1 were overlapped by the broad peaks (around 3450 cm−1) of the O–H stretching vibration in MIL-68(In)–NH2/polyHIPEs in comparison with the FTIR spectra of MIL-68(In)–NH2. In addition, the stretching vibrations of –CH2– group (2963, 2927, and 2860 cm−1) and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the –COOH group (1721 cm−1) were attributed to the poly(EHA/DVB)HIPEs in MIL-68(In)–NH2/polyHIPEs composites. The above FTIR results demonstrated that the MIL-68(In)–NH2/polyHIPEs were successfully fabricated.
O of the –COOH group (1721 cm−1) were attributed to the poly(EHA/DVB)HIPEs in MIL-68(In)–NH2/polyHIPEs composites. The above FTIR results demonstrated that the MIL-68(In)–NH2/polyHIPEs were successfully fabricated.
 | ||
| Fig. 1 Fourier transform infrared (FTIR) spectra (A) and scanning electron microscopy (SEM) image (B) of MIL-68(In)–NH2/polyHIPEs (a, MIL-68(In)–NH2; b, MIL-68(In)–NH2/polyHIPEs). | ||
According to the SEM images shown in Fig. 1B, MIL-68(In)–NH2/polyHIPEs were cross-linked porous materials with an open-cell structure, which make them very promising materials for separation application because of their superiority in high permeability and fast mass transfer.
3.2 Optimization of SPE conditions for extraction of triazine herbicides
A series of factors affecting the SPE process including sample pH, sample flow rate, the type and volume of desorption solvent, and sample volume were investigated, respectively, and the obtained results were shown in Fig. 2 and 3.The sample pH value can affect extraction efficiency by influencing the existing forms of simazine (pKa 1.62), prometon (pKa 4.36) and prometryn (pKa 4.05) because of the protonation and de-protonation reactions,3,5,6 thus the effect of the sample pH on their recoveries was investigated. As a result in Fig. 2A, the extraction recovery of simazine decreased slowly with pH increasing from 4 to 8 and then increased with further increasing pH to 10, while the recoveries of prometon and prometryn were not obviously changed with increase of pH from 4 to 11, which were different from the reported results.3,5 The main reason was attributed to the strong π–π interactions and hydrogen bonding interactions between the investigated triazine herbicides and MIL-68(In)–NH2/polyHIPEs.36 Subsequently, the sample pH was kept at 4.0 for subsequent experiments.
Fig. 2B showed that the recoveries of simazine, prometon and prometryn remained unchanged when the sample flow rate increased from 0.2 to 0.8 mL min−1, and then decreased with further increase of sample flow rate to 1.2 mL min−1. When the sample flow rate was 1.0 mL min−1, the recoveries of the three triazine herbicides were higher than 85%, therefore, 1.0 mL min−1 was chosen in order to reasonably reduce the extraction time.
A satisfactory desorption solvent should efficiently desorb the adsorbed analytes, and thus different desorption solvent including methanol, acetone, acetonitrile, 30% (v/v) and 70% (v/v) acetonitrile were investigated to elute the three triazine herbicides. The results shown in Fig. 2C revealed that all five solvents could desorb triazine herbicides from MIL-68(In)–NH2/polyHIPEs, however, methanol and 30% (v/v) acetonitrile were not good for elution of prometryn. On the other hand, acetonitrile was a component of the mobile phase in this work, therefore, acetonitrile was finally chosen as desorption solvent. Furthermore, the volume of acetonitrile varying in 1.0–4.0 mL was investigated in order to effectively elute the adsorbed triazine herbicides. The obtained results showed that simazine and prometon were completely eluted when 2.0 mL acetonitrile was used, while the extraction recovery of prometryn increased from 72.8% to 107.0% with increase of acetonitrile volume from 1.0 to 4.0 mL (Fig. 2D). When the volume of acetonitrile was 3.0 mL, the extraction recoveries of three triazine herbicides were higher than 95.6%, so 3.0 mL acetonitrile was chosen for the elution in this work.
To obtain high concentration factor, different sample volumes ranging from 5 to 35 mL were investigated. As can be observed from Fig. 2E, high recoveries (>85.5%) were obtained for all three triazine herbicides when the sample volumes were not higher than 30 mL, and a slight decline of recoveries was observed for the three analytes with further increase of sample volumes to 35 mL. Thus, 30 mL of sample solution was chosen in this work.
Under the optimized conditions, the mean recoveries of simazine, prometon and prometryn in water samples by using the MIL-68(In)–NH2/polyHIPEs monoliths were 85.6%, 90.2%, and 92.5%, respectively, which indicated that the selected extraction conditions were appropriate for the SPE of trace triazine herbicides from environmental water samples. In addition, the maximum adsorption capacities of MIL-68(In)–NH2/polyHIPEs and polyHIPEs monoliths for triazine herbicides were evaluated, and the obtained results showed that the maximum adsorption capacity of MIL-68(In)–NH2/polyHIPEs towards simazine, prometon and prometryn was 800 μg g−1, 800 μg g−1 and 6.01 mg g−1, respectively, higher than that of polyHIPEs towards simazine (400 μg g−1), prometon (662.6 μg g−1) and prometryn (5.20 mg g−1), which demonstrated that the incorporated MIL-68(In)–NH2 could obviously improve the extraction ability of polyHIPEs.
3.3 Method validation
To investigate the analytical performance of the MIL-68(In)–NH2/polyHIPEs based SPE-HPLC method, several analytical parameters including linearity, precisions, limits of detection (LODs), and recovery were evaluated by using standard aqueous solution under the optimized conditions, and the results were summarized in Tables 1 and 2.| Analyze | Spiked (ng mL−1) | River water | Lake water | Pond water | Farmland water | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Found (±SD, ng mL−1) | Recovery (%) | RSD (%) | Found (±SD, ng mL−1) | Recovery (%) | RSD (%) | Found (±SD, ng mL−1) | Recovery (%) | RSD (%) | Found (±SD, ng mL−1) | Recovery (%) | RSD (%) | ||
| a All water samples were collected from Guilin, China. | |||||||||||||
| Simazine | 0 | 4.90 (±0.10) | 2.1 | 6.65 (±0.13) | 1.9 | 11.96 (±0.47) | 3.9 | 17.58 (±0.47) | 2.7 | ||||
| 5 | 9.78 (±0.26) | 97.6 | 2.7 | 12.01 (±0.25) | 107.2 | 2.1 | 17.09 (±0.60) | 102.6 | 3.5 | 22.35 (±0.45) | 95.4 | 2.0 | |
| 20 | 27.33 (±0.30) | 112.1 | 1.1 | 27.92 (±0.31) | 106.3 | 1.1 | 31.42 (±1.16) | 97.3 | 3.7 | 37.11 (±0.26) | 97.6 | 0.7 | |
| 50 | 49.99 (±0.70) | 90.2 | 1.4 | 57.60 (±0.81) | 101.9 | 1.4 | 59.78 (±2.39) | 95.6 | 4.0 | 63.43 (±1.14) | 91.7 | 1.8 | |
| 100 | 117.14 (±0.82) | 112.2 | 0.7 | 122.29 (±1.83) | 115.6 | 1.5 | 111.19 (±3.22) | 99.2 | 2.9 | 105.92 (±0.85) | 88.3 | 0.8 | |
| Prometon | 0 | 4.37 (±0.08) | 1.9 | 3.91 (±0.12) | 3.1 | 4.01 (±0.10) | 2.6 | 2.99 (±0.14) | 4.6 | ||||
| 5 | 9.99 (±0.18) | 112.4 | 1.8 | 9.08 (±0.31) | 103.4 | 3.4 | 8.72 (±0.23) | 94.2 | 2.6 | 7.68 (±0.31) | 93.8 | 4.1 | |
| 20 | 28.02 (±0.20) | 118.2 | 0.7 | 24.19 (±0.56) | 101.4 | 2.3 | 22.40 (±0.38) | 92.0 | 1.7 | 21.43 (±0.92) | 92.2 | 4.3 | |
| 50 | 53.02 (±0.53) | 95.3 | 1.0 | 53.96 (±0.43) | 100.1 | 0.8 | 49.66 (±1.19) | 91.3 | 2.4 | 45.77 (±0.23) | 85.6 | 0.5 | |
| 100 | 96.41 (±1.06) | 92.0 | 1.1 | 104.04 (±0.94) | 100.2 | 0.9 | 96.45 (±1.25) | 92.4 | 1.3 | 95.73 (±1.53) | 92.7 | 1.6 | |
| Prometryn | 0 | 1.77 (±0.05) | 2.6 | 1.64 (±0.04) | 2.7 | 1.60 (±0.06) | 3.5 | 0.96 (±0.05) | 4.8 | ||||
| 5 | 6.73 (±0.21) | 99.2 | 3.1 | 6.28 (±0.18) | 92.8 | 2.8 | 6.72 (±0.21) | 102.4 | 3.1 | 5.82 (±0.23) | 97.2 | 3.9 | |
| 20 | 23.27 (±0.51) | 107.5 | 2.2 | 19.46 (±0.39) | 89.1 | 2.0 | 21.31 (±0.15) | 98.6 | 0.7 | 19.39 (±0.78) | 92.2 | 4.0 | |
| 50 | 55.71 (±1.39) | 107.9 | 2.5 | 46.77 (±0.70) | 90.3 | 1.5 | 51.29 (±1.80) | 99.4 | 3.5 | 51.09 (±0.77) | 100.3 | 1.5 | |
| 100 | 101.75 (±0.31) | 100.0 | 0.3 | 103.52 (±2.59) | 101.9 | 2.5 | 112.27 (±0.79) | 110.7 | 0.7 | 105.30 (±0.63) | 104.3 | 0.6 | |
The calibration curves were attained by analyzing a series of standard solution (10–800 ng mL−1), and good linearity was achieved with correlation coefficient (R2) higher than 0.9990. The LODs, calculated based on signal-to-noise ratios (S/N) of 3, were in the range of 0.031–0.097 ng mL−1, which were below the MRLs for the target triazine herbicides in environmental water samples legislated by European Union (0.10 ng mL−1).2,3 The intra-day and inter-day precisions were evaluated by analyzing five replicated spiked samples (20, 50 and 100 ng mL−1) for a day and once a day for five consecutive days. The obtained results showed that the recoveries were 90.2–118.5%, the intra-day and inter-day precisions (expressed as relative standard deviations, RSDs) were 1.7–2.5% and 2.1–5.3%, respectively, which indicated that the method precision was good.
To verify the accuracy of the proposed method, four real water samples including river water, lake water, pond water and farmland water spiked at four concentrations (5, 20, 50, and 100 ng mL−1) were analyzed. The recoveries were in the range of 85.6–118.2% with RSDs of 0.30–4.8% for all water samples (Table 2). In addition, the same MIL-68(In)–NH2/polyHIPEs monoliths were repeatedly used for extraction of triazine herbicides from water samples, and the results was shown in Fig. 2F. Based on the recovery results, the extraction ability of MIL-68(In)–NH2/polyHIPEs monoliths towards simazine, prometon and prometryn was not obviously decreased after replicate extraction 20 times. By comparing with the SEM images of MIL-68(In)–NH2/polyHIPEs before and after 20 circle times, similar microstructure was observed, which suggested that the MIL-68(In)–NH2/polyHIPEs monoliths have stability and reusability along with a potential in practical applications.
Compared with the other reported methods presented in Table 3, the proposed method in this work has a desirable LOD, recovery, and repeatability with UV detection for the simultaneous determination of multiple triazine herbicides, therefore, the MIL-68(In)–NH2/polyHIPEs based SPE-HPLC method was sensitive, reliable and practically feasible for simultaneously separating and analyzing the trace levels of multiple triazine herbicides in water samples.
| Sample matrix | Sample volume (mL) | Extraction time (min) | SPE adsorbent | Detection method | LOD (μg L−1) | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|
| Water | 10 | 2 | Single-walled carbon nanohorns | GC-MS | 0.015–0.100 | 87–94 | 2 |
| Maize leaf | 10 | 55 | Porous aromatic frameworks | HPLC-DAD | 0.037–0.089 | 85.1–115 | 3 |
| Water | 2 | 10 | Carbonized polyGO/HIPEs | HPLC-DAD | 2.5–5.6 | >90 | 5 |
| Soil | 20 | 10 | polyHIPEs-carboxylated carbon nanotube | HPLC-UV | — | 87.56–97.67 | 6 |
| Tobacco | 2 | — | Simetryn imprinted nanoparticles | HPLC-MS/MS | 6–30 | 84.03–119.05 | 12 |
| Water | 5 | About 9 | MIL-101 (Cr)/chitosan sponge column | HPLC-MS/MS | 0.014–0.045 | 78.9–118.6 | 18 |
| Water | 30 | 30 | MIL-68(In)–NH2/polyHIPEs | HPLC-UV | 0.031–0.097 | 85.6–118.2 | This work |
3.4 Sample analysis
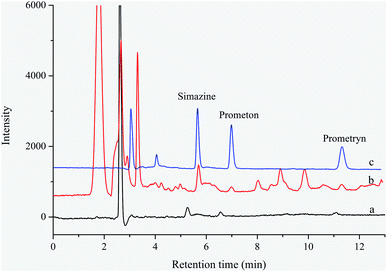
The developed MIL-68(In)–NH2/polyHIPEs based SPE-HPLC method was applied to determine triazine herbicides in environmental water samples, including river water, lake water, pond water and farmland water samples, and the typical HPLC chromatograms were shown in Fig. 3. As shown as in Table 2, the concentration of simazine, prometon and prometryn was 4.90–17.58 ng mL−1, 2.99–4.37 ng mL−1, and 0.96–1.77 ng mL−1 in collected water samples, respectively, which exceeded the MRL for single triazine herbicide in drinking water established by EU (0.1 ng mL−1), which suggested that the collected water samples might be contaminated with triazine herbicides.4. Conclusions
The MIL-68(In)–NH2/polyHIPEs were successfully prepared and applied to extract triazine herbicides from environmental water samples, and the performance including rapid extraction, high stability, and excellent reusability was observed. The good precisions and satisfactory recoveries demonstrated that the proposed MIL-68(In)–NH2/polyHIPEs based SPE-HPLC-UV method was valid for the separation and analysis of trace triazine herbicides in water samples, and has shown great potential in hazardous residue analysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by National Natural Science Foundation of China (21964006), the Hunan Province Natural Science Foundation of China (2020JJ4640), the Scientific Research Fund of Hunan Provincial Education Department (20A050), and the Scientific Research Fund of Changsha University (SF1934), respectively.References
- H. Piao, Y. Jiang, X. Li, P. Ma, X. Wang, D. Song and Y. Sun, J. Sep. Sci., 2019, 42, 2123–2130 CrossRef CAS PubMed.
- J. M. Jimenez-Soto, S. Cardenas and M. Valcarcel, J. Chromatogr., 2012, 1245, 17–23 CrossRef CAS PubMed.
- S. Zheng, M. He, B. Chen and B. Hu, J. Chromatogr., 2020, 1614, 460728 CrossRef CAS PubMed.
- M. Yan, Y. She, X. Cao, J. Ma, G. Chen, S. Hong, Y. Shao, A. M. Abd EI-Aty, M. Wang and J. Wang, Microchim. Acta, 2019, 186, 143 CrossRef PubMed.
- W. Zhang, G. Ruan, X. Li, X. Jiang, Y. Huang, F. Du and J. Li, Anal. Chim. Acta, 2019, 1071, 17–24 CrossRef CAS PubMed.
- X. Jiang, G. Ruan, H. Deng, Z. Gan, W. Zhang, F. Du and Z. Chen, Chem. Eng. J., 2021, 415, 129005 CrossRef CAS.
- Z. A. ALOthman, Materials, 2012, 5, 2874–2902 CrossRef CAS.
- M. A. Habila, Z. A. ALOthman, E. Yilmaz and M. Soylak, Int. J. Environ. Anal. Chem., 2018, 98, 171–181 CrossRef CAS.
- Z. A. ALOthman and S. M. Wabaidur, Arabian J. Chem., 2019, 12, 633–651 CrossRef CAS.
- Z. A. ALOthman, M. A. Habila, E. Yilmaz, E. A. Alabdullkarem and M. Soylak, Measurement, 2020, 153, 107394 CrossRef.
- S. Wang, Y. She, S. Hong, X. Du, M. Yan, Y. Wang, Y. Qi, M. Wang, W. Jiang and J. Wang, J. Hazard. Mater., 2019, 367, 686–693 CrossRef CAS PubMed.
- Z. Tong, Y. Han, L. Gu, Z. Li, K. Du, G. Kong, D. Liu, J. Peng and J. Shi, J. Sep. Sci., 2020, 43, 1107–1118 CrossRef CAS PubMed.
- G. Li, X. Meng, J. Wang, Q. Wang, J. Zhou, C. Wang, Q. Wu and Z. Wang, Food Chem., 2020, 309, 125618 CrossRef CAS PubMed.
- N. Mokhtari, M. M. Khataei, M. Dinari, B. H. Monjezi, Y. Yamini and M. Hatami, Microchem. J., 2021, 160, 105634 CrossRef CAS.
- X. Li, J. Xing, C. Chang, X. Wang, Y. Bai, X. Yan and H. Liu, J. Sep. Sci., 2014, 37, 1489–1495 CrossRef CAS PubMed.
- Y. Jiang, H. Piao, Z. Qin, X. Li, P. Ma, Y. Sun, X. Wang and D. Song, J. Sep. Sci., 2019, 42, 2900–2908 CrossRef CAS PubMed.
- J. Wang, Q. Du, X. You, Y. Lv, W. Bi, H. Li and D. Y. Chen, Anal. Chim. Acta, 2019, 1071, 8–16 CrossRef CAS PubMed.
- Y. Jiang, Z. Qin, F. Liang, J. Li, Y. Sun, X. Wang, P. Ma and D. Song, J. Chromatogr., 2021, 1638, 461887 CrossRef CAS PubMed.
- R. Krishna, RSC Adv., 2015, 5, 52269–52295 RSC.
- S. Ramanayaka, M. Vithanage, A. Sarmah, T. An, K. H. Kim and Y. S. Ok, RSC Adv., 2019, 9, 34359–34376 RSC.
- S. Rojas and P. Horcajada, Chem. Rev., 2020, 120, 8378–8415 CrossRef CAS PubMed.
- M. S. Silverstein, Prog. Polym. Sci., 2014, 39, 199–234 CrossRef CAS.
- T. Zhang, R. A. Sanguramath, S. Israel and M. S. Silverstein, Macromolecules, 2019, 52, 5445–5479 CrossRef CAS.
- C. M. Yu, X. H. Zhuang, S. W. Zeng, Q. X. Dong, Z. X. Jing, P. Z. Hong and Y. Li, RSC Adv., 2019, 9, 17543–17550 RSC.
- K. M. L. Taylor-Pashow and J. G. Pribyl, Solvent Extr. Ion Exch., 2019, 37, 1–26 CrossRef CAS.
- J. Luo, Z. Huang, L. Liu, H. Wang, G. Ruan, C. Zhao and F. Du, J. Sep. Sci., 2020, 4 4, 169–187 Search PubMed.
- Y. Zhu, W. Wang, H. Yu and A. Wang, J. Environ. Sci., 2020, 88, 217–236 CrossRef PubMed.
- B. Zhang, J. Zhang, C. Liu, L. Peng, X. Sang, B. Han, X. Ma, T. Luo, X. Tan and G. Yang, Sci. Rep., 2016, 6, 21401 CrossRef CAS PubMed.
- H. Zhu, Q. Zhang and S. Zhu, Chem.–Eur. J., 2016, 22, 8751–8755 CrossRef CAS PubMed.
- F. Du, L. Sun, W. Tan, Z. Wei, H. Nie, Z. Huang, G. Ruan and J. Li, Anal. Bioanal. Chem., 2019, 411, 2239–2248 CrossRef CAS PubMed.
- S. M. Wabaidur, A. AlAmmari, A. Aqel, S. A. AL-Tamrah, Z. A. Alothman and A. Y. B. H. Ahmed, J. Chromatogr. B, 2016, 1031, 109–115 CrossRef CAS PubMed.
- M. R. Khan, S. M. Wabaidur, Z. A. Alothman, R. Busquets and M. Naushad, Talanta, 2016, 152, 513–520 CrossRef CAS PubMed.
- N. A. AlFaris, S. M. Wabaidur, Z. A. Alothman, J. Z. Altamimi and T. S. Aldayel, J. Sep. Sci., 2020, 43, 2079–2087 CrossRef CAS PubMed.
- N. A. AlFaris, J. Z. ALTamimi, Z. A. ALOthman, S. M. Wabaidur, A. A. Ghafar and T. S. Aldayel, J. King Saud Univ., Sci., 2020, 32, 2414–2418 CrossRef.
- L. Wu, M. Xue, S. L. Qiu, G. Chaplais, A. Simon-Masseron and J. Patarin, Microporous Mesoporous Mater., 2012, 157, 75–81 CrossRef CAS.
- Y. Lv, R. Zhang, S. Zeng, K. Liu, S. Huang, Y. Liu, P. Xu, C. Lin, Y. Cheng and M. Liu, Chem. Eng. J., 2018, 339, 359–368 CrossRef CAS.
- J. Bielecki, S. F. Parker, D. Ekanayake, S. M. H. Rahman, L. Börjessona and M. Karlsson, J. Mater. Chem. A, 2014, 2, 16915–16924 RSC.
- N. Tarasova, I. Animitsa, T. Denisova and R. Nevmyvako, Solid State Ionics, 2015, 275, 47–52 CrossRef CAS.
Footnote |
| † Jinhua Luo and Liping Jiang contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |