Open Access Article
Open Access ArticleEffect of temperature fluctuation on colour change and softening of postharvest sweet cherry
Ying Xin,
Zhenzhen Liu,
Yuanwei Zhang,
Xiaofei Shi,
Fusheng Chen * and
Kunlun Liu
* and
Kunlun Liu *
*
College of Food Science and Technology, Henan University of Technology, Zhengzhou, Henan 450001, P. R. China. E-mail: knlnliu@126.com; fushengc@haut.edu.cn; Fax: +86-371-67758022; Tel: +86-371-67758022
First published on 29th June 2021
Abstract
The inevitable temperature fluctuation during cold chain transport accelerates the colour change and softening of postharvest sweet cherry, leading to further deterioration of quality and decline of the marketable value of cherries. The influences of temperature fluctuation on the contents of total anthocyanin, phenolic, malondialdehyde, and sodium carbonate-soluble pectin (SSP), as well as the activities of polyphenoloxidase (PPO) and peroxidase (POD) in sweet cherry, were assessed. In addition, the effects of temperature fluctuation on the activities of polygalacturonase (PG), pectin methyl esterase (PME), and beta-galactosidase (β-Gal) activities, and the paPG, paPME, and paPME genes expression were studied. The evolution of SSP nano-morphology was measured by atomic force microscopy. The results showed that the temperature fluctuation promoted anthocyanin synthesis, phenolic metabolism, and malondialdehyde accumulation, which immediately affected the brightness (6.2% lower than that of the cherry stored at 5 °C) of sweet cherry. Temperature fluctuation also led to a significant increase in POD and PPO activities during subsequent isothermal storage, accelerating the colour change (24.8% more than that of the cherry stored at 5 °C), which almost reached the level observed during constant 10 °C storage. In addition, temperature fluctuation not only affected the firmness (13.7% lower than that of the cherry stored at a constant temperature of 5 °C) of fruit immediately, but also, during subsequent isothermal storage, accelerated the deterioration of firmness (19.6% lower than that of the cherry stored at a constant temperature of 5 °C). This could be explained by temperature fluctuation inducing the upregulation of paPG1-3, paPME3, and paPME4 expression, which led to a 3.5 and 1.5-fold increase in PG and PME activity, respectively. This led to degradation of the aggregated SSP to its nanostructural basic units. Furthermore, temperature fluctuation resulted in upregulated expression of paβ-Gal1 and paβ-Gal3 and enhanced β-Gal activity during subsequent isothermal storage. The results provide theoretical guidance for the transportation, storage, and preservation of postharvest sweet cherry.
1. Introduction
Sweet cherry has a bright colour and distinct flavour, and is rich in physiologically active substances, such as vitamins, anthocyanins, phenolics, and carotenoids. Moreover, it has health effects of anti-gout, anti-oxidation, and prevention of cardiovascular and cerebrovascular diseases.1 Therefore, sweet cherry is appreciated worldwide and its consumption is increasing year by year. Fruit surface colour and firmness are two important appearance indexes that directly reflect the edible quality of sweet cherry after harvest. They also determine its marketable value. Dull skin colour and soft pulp negatively impact consumer purchase decisions.2 In addition, the colour changes and softening process of sweet cherries are often accompanied by flavour deterioration, nutrient content reduction, and accelerated physiological metabolism.3,4Polyphenoloxidase (PPO) and peroxidase (POD) oxidise polyphenols in fruit into anthraquinone compounds with deeper colour, which is essential in fruit browning.5 The accumulation and distribution of these polyphenols in fruit, especially anthocyanins, determines the skin colour of sweet cherry. In addition, there are five anthocyanins in cherry skin, including cyanidin 3-O-rutinoside, cyanidin 3-O-glucoside, peonidin 3-O-rutinoside, pelargonidin 3-O-rutinoside, and peonidin 3-O-glucoside, whose contents are determined mostly by the cultivar and origin.6–8 Moreover, in sweet cherry, degradation of sensory quality is also closely related to the other phenolic compounds with copigmentation effects, such as phenolic acids, including neochlorogenic acid, p-coumaroyl quinic acid, and chlorogenic acid.9,10 The softening of sweet cherries is a complex and orderly process. It was reported that the structural evolution of pectin in the intercellular layer and primary wall of fruit under the action of softening related enzymes, such as polygalacturonase (PG), pectin methyl esterase (PME), and beta-galactosidase (β-Gal), is one of the main reasons for softening.11–13 In particular, the evolution of sodium carbonate-soluble pectin (SSP) correlated positively with the softening of cherry.14 The basic structural unit of SSP from sweet cherry is rhamnogalacturonan I (RG-I), which contains arabinose, galacturonic acid, rhamnose, and galactose,15 and is susceptible to the action of PME, PG, and β-Gal. The activity of these pectin modification related enzymes was significantly correlated with the sequence expression of fruit softening related genes. Several studies have shown that ripening-related genes, such as FaPG1, FaPG2, FaPME1 and Maβ-Gal,16–18 which were up-regulated during fruits softening. Some preservation techniques maintained the intactness of cell walls through repression of FaPG1, FaPME1,19 VaPG,20 Mdβ-Gal21 transcript levels.
Low temperature cold chain transport can effectively maintain the colour and texture of sweet cherry. Great care must be taken throughout the cold chain to provide premium quality fruit for the market.22 However, there are inevitable temperature fluctuations during long-distance transportation, including improper operation, imprecise temperature control devices, and other problems.23 In addition, improper temperature control measures have implemented to reduce the transportation cost. These non-isothermal temperature transport conditions will accelerate the deterioration of the fruit. The rate of fruit and vegetable quality deterioration under non-isothermal or higher temperature conditions is much higher than that under an isothermal cold chain.24–27 Previous study has shown that non-isothermal storage, which simulates ‘broken cold chain’ and temperature fluctuations in cold chain transport, significantly accelerated the softening of cherry. Although the temperature fluctuation phase of 5 to 10 °C only accounts for 6.3% of the total duration, it could promote the SSP to be depolymerized substantially.13 Furthermore, fruit that have undergone non-isothermal transport are often stored at low temperatures before being sold. We posed the question of whether temperature fluctuation affects the colour and texture of fruit? What are the immediate effects of temperature fluctuation on fruit? In addition, how does the temperature fluctuation in the early cold chain transport affect the colour and texture characteristic of fruit during the subsequent isothermal storage?
The aim of this paper was to evaluate the influence of temperature fluctuation on the colour and texture characteristic of harvested sweet cherry. These effects on the quality of cherries were observed not only during temperature fluctuation, but also during subsequent isothermal storage. At present, the temperature fluctuation range of land cold chain transportation is mostly 5–10 °C, and the transportation time is up to 3 days. Therefore, this study simulated and amplified this temperature fluctuation pattern. The colour changes and softening of fruit after temperature fluctuation (5–10 °C) and during subsequent isothermal storage (5 °C) were determined. Meanwhile, the total anthocyanin, phenolic, malondialdehyde (MDA) and SSP contents were examined. The activities of enzymes related to browning (POD and PPO) and softening (PME, PG, and β-Gal) were also measured. Furthermore, the nanostructural characterisation of SSP and the expression of pectin-related genes were determined. The results explained how temperature fluctuations accelerate browning and softening of sweet cherry, and revealed their regulatory mechanisms. The results provided theoretical guidance for the transportation, storage, and preservation of sweet cherries after harvest.
2. Materials and methods
2.1 Materials
Fresh commercial maturity sweet cherry fruit (Prunus avium L. cv. ‘Hongdeng’) were hand-picked from a local orchard and delivered to the laboratory in May 2020. We selected sweet cherries with a uniform transverse diameter, shape, colour, and no disease, and then cleaned them for subsequent experiments.2.2 Storage temperature conditions
All sweet cherry samples were precooled at 5 °C for 24 h, then the samples were randomly stored under three different storage conditions. The first group was kept under fluctuating temperature conditions (TF). Sweet cherry samples were first stored at 5 ± 1 °C for 12 h, and then stored at 10 ± 1 °C for 12 h, which was repeated twice (simulation and amplification of temperature fluctuation in cold chain transport); thereafter, they were finally stored at 5 ± 1 °C (simulation of the subsequent isothermal storage). The other two groups were stored at constant temperatures of 5 ± 1 °C (CT-5) and 10 ± 1 °C (CT-10), respectively. All samples were stored for up to 28 d.2.3 Colour and firmness
Colour of fruit samples was determined using a handheld digital colourimeter (Illuminant D65, CR-400 Minolta Chromameter, Minolta Co., Tokyo, Japan) with the CIELab colourimetric system. The overall colour changes of fruit samples during different storage conditions were calculated as follow:| ΔE = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2 | (1) |
A TA-XT2i texture analyser (Stable Micro Systems Ltd, Godalming, UK) was used to determine the firmness of each group of fruit (n = 20 per group). The specific test parameters were determined according to the report of Xin et al.14
2.4 Total anthocyanin, phenolics, and MDA compounds
For each sample, 2.0 g of sweet cherry flesh from fifteen fruit was added to 20 mL of acidified methanol (HCl, 1%, v/v). The mixture then kept in the dark at 4 °C for 30 min and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 30 min at 4 °C. The supernatant was used to test anthocyanin and phenolic compounds. The total anthocyanin content was extracted according to Albishi's method.29 The total anthocyanin concentration was calculated from a calibration curve of cyanidin-3-glucoside and was expressed as mg per kg fresh weight. Total phenolics were estimated using the Folin–Ciocalteu method and expressed as milligram of gallic acid equivalent per kg of fresh weight.30 The MDA concentration was determined using the thiobarbituric acid (TBA) method described by Wang et al.31 by measuring the absorbance changes of the extract at 450, 532, and 600 nm after adding 10% (w/v) trichloroacetic acid (TCA) and 0.6% (w/v) TBA. The MDA contents were expressed as mol per kg fresh weight.
000 × g for 30 min at 4 °C. The supernatant was used to test anthocyanin and phenolic compounds. The total anthocyanin content was extracted according to Albishi's method.29 The total anthocyanin concentration was calculated from a calibration curve of cyanidin-3-glucoside and was expressed as mg per kg fresh weight. Total phenolics were estimated using the Folin–Ciocalteu method and expressed as milligram of gallic acid equivalent per kg of fresh weight.30 The MDA concentration was determined using the thiobarbituric acid (TBA) method described by Wang et al.31 by measuring the absorbance changes of the extract at 450, 532, and 600 nm after adding 10% (w/v) trichloroacetic acid (TCA) and 0.6% (w/v) TBA. The MDA contents were expressed as mol per kg fresh weight.
2.5 Pectin extraction, determination, and nanostructural characterisation
The SSP fraction of sweet cherry flesh was separated and determined using a previously published method.15 Carbazole colourimetry was used to determine the extracted SSP content. The nanostructure and quantitative parameters of SSP were measured using a Multimode NanoScope IIIa AFM (ZhuoLun MicroNano Equipment Co., Ltd Shanghai, China) in tapping mode at a scan rate of 0.5–2 Hz. The samples were treated according to the method of Xin et al.15 Approximately 10 μL of diluted sample was distributed on a mica sheet and dried using an aurilave. Atomic force microscopy (AFM) images for samples from each treatment were analysed offline using the AFM software (Bruker Corp., Santa Barbara, CA). The reliable results were obtained from the analysis of at least 80 single chains for each sample.2.6 Analysis of enzyme activities
POD (EC 1.11.1.7) and PPO (EC 1.14.18.1) were extracted and determined according to previously published protocols.4,32 The crude extract was added with 10 mmol L−1 H2O2 and 10 mmol L−1 guaiacol to determine the POD activity by spectrophotometry at 470 nm. PPO activity was measured spectrophotometrically at 420 nm in a reaction mixture containing 0.1 mol L−1 sodium phosphate buffer (pH 6.0) and 65 mmol L−1 pyrocatechol. One unit of POD or PPO activity was defined as the change of A470 min−1 or A420 min−1, respectively.PG (exo-PG; EC 3.2.1.67 and endo-PG; EC 3.2.1.15) and PME (EC 3.1.1.11) were extracted and their activities measured using the method described by Lohani et al.33 A solution including the enzyme extract, 1% polygalacturonic acid, and 200 mmol L−1 sodium acetate (pH 4.5) was prepared to determine the PG activity at 540 nm. The PG activity unit was expressed as the amount of enzyme necessary to release 1 μmol galacturonic acid per min per g fresh weight. A mixture of 3 mmol L−1 potassium phosphate buffer (pH 7.5), 2.5 mmol L−1 galacturonic acid, 0.5% pectin solution, and bromothymol blue were added to the enzyme extract to measure the PME activity at 620 nm. One unit of PME activity was defined as the amount of enzyme that liberated 1 μmol of methylester per min under standard assay conditions.
β-Gal (EC 3.2.1.23) was extracted and its activity measured according to the method reported by Ranjbar et al.34 The β-Gal activity was determined by the chemical reaction between the crude enzyme solution and p-nitrophenyl-β-D-galactopyranoside. Then the absorption value of the P-nitrophenol produced by the chemical reaction was measured at 405 nm to calculate the β-Gal activity. One unit of enzyme was defined as the amount of enzyme that catalyses the liberation of 1 μmol of p-nitrophenol per min at 37 °C.
2.7 Expression levels of pectin-related genes
Total RNA extraction, reverse transcription, and cDNA synthesise were performed according to the method of Xin et al.14 To evaluate the expression of pectin-related genes, 18S rRNA (XM_021960154) was chosen as the reference gene. Target genes in sweet cherry were chosen in comparison with the whole genome of Arabidopsis thaliana in GenBank. Primer 5.0 software (Premier Biosoft, Palo Alto, CA, USA) and NCBI Primer BLAST were used to design and confirm the primer pairs for the target genes, as shown in Table 1. Real-time reverse transcription PCR (RT-qPCR) was carried out using the DNA binding dye SYBR green I to detect the PCR products. The RT-qPCR cycling conditions were set according to those detailed by Xin et al.14| Primer name | Forward primer sequence (5′–3′) | Reverse primer sequence (5′–3') |
|---|---|---|
| 18S | ACATGGGTCAGGGCTTAGGA | CACTTGAAATGCTGCTCTTGC |
| PG1 | TAGGGTGCGTGGAGTGA | CCAAGGCTACCGATACTG |
| PG2 | CAACTGCAAACCGTCAG | ATCGCTTCCTCCTCATT |
| PG3 | GCATTTGTGCCAGAACC | TGAAGGAAGCAAGGGAG |
| PME1 | GCCATTACCAACCAGGAGACT | TGCAGAGTTGTTCTACGTGTACCT |
| PME2 | GGTAATGGCTCAAATGTTCCG | CCTGTTGCTCCCACTCCTCT |
| PME3 | CTATGGCAAAGTGGACGAGC | TTGAGTGAAGCGGATGGAGT |
| PME4 | CAAAACCGTAAATCCCGTCAC | TCTGCAAACCCTTCTCGACAT |
| PME5 | TTTCATCTTCGGCAACGC | CAAGATGGAAATGCCAGTGTT |
| β-Gal1 | TGGCTGGTTTTCGGAGTTT | AGCGTCATAGTCATAGCTGGTAGT |
| β-Gal2 | GTTGAGTGGATTCAGGGGTCT | GTTACAGGCACCACAACTACCA |
| β-Gal3 | AGAATTTGCTAGCTATGGTACTCCT | AGAACCGCATCTTGCCTCA |
2.8 Statistical analysis
The experiments were conducted in triplicate independently. Differences between groups were assessed using analysis of variance (ANOVA) and Duncan's test in the SAS software (SAS Institute Inc., Cary, NC, USA) with P < 0.05 being considered as significant.3. Results and discussion
3.1 The colour changes of sweet cherries due to temperature fluctuations
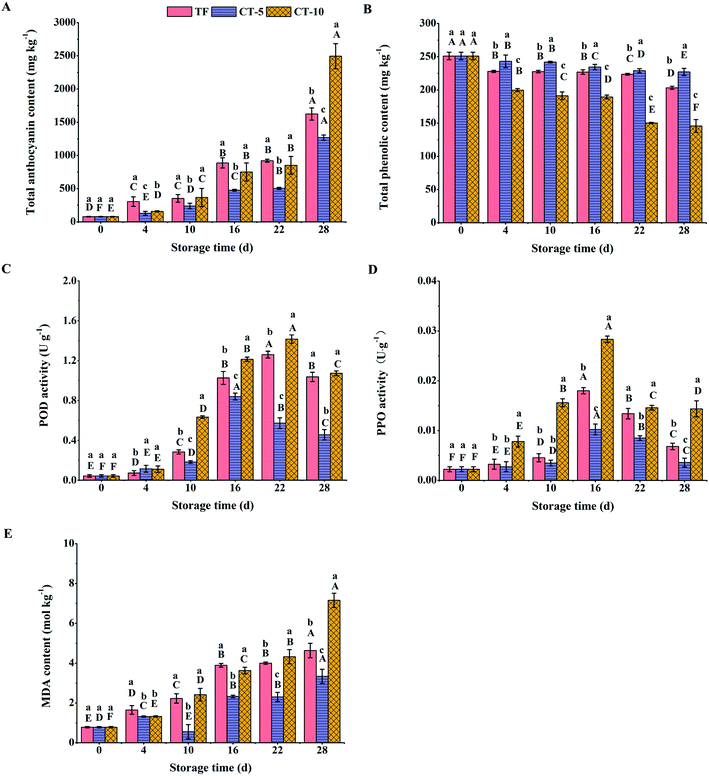
Fig. 1A shows that temperature fluctuations accelerated the transition of sweet cherries from bright red to dark red. After temperature fluctuation (4 d), except for the L* value, there was no significant difference in the colour parameters between the TF group and the CT-5 group (Fig. 1B). The L* value of the TF samples was lower after 3 days of fluctuating temperature storage, indicating that temperature fluctuations yielded lower L* values than the constant temperature treatments. During the subsequent storage at a constant temperature of 5 °C, the L* and b* values of the TF samples were lower than those of CT-5 at 10 d (Fig. 1B and D), and the ΔE value of TF was larger than that of CT-5 (Fig. 1E). In addition, the a* value was significantly lower at the end of storage (Fig. 1C). The results showed that temperature fluctuation not only affected the brightness of sweet cherries immediately, but also promoted the colour change of cherries during subsequent storage at constant temperature.3.2 The anthocyanin and phenolic compounds contents of sweet cherries under temperature fluctuations
The skin colour of sweet cherry is mainly determined by the type and contents of anthocyanin. The higher the anthocyanin content, the darker the skin colour of the fruit.35 As can be seen from Fig. 2A, during the colour transformation of sweet cherry, the anthocyanin contents of the TF, CT-5, and CT-10 groups increased by 19.8, 15.3, and 31.0 times, respectively. This accumulation of anthocyanins in sweet cherries during postharvest storage is in agreement with the results reported by Gonçalves et al.,36 who studied the anthocyanin content during the colour evolution in sweet cherries under different storage temperatures. After the end of the temperature fluctuation (4 d), the anthocyanin content in the TF group was almost twice that in the CT-5 group. In addition, during subsequent constant temperature storage, the anthocyanin content in the TF group reached the level of the CT-10 group at 10 to 22 d. This indicated that temperature fluctuation could promote secondary metabolism in sweet cherry and accelerated the synthesis of anthocyanins.The colour transformation of postharvest sweet cherry was positively and significantly associated with phenolic compounds.5,32 Phenolic substances can not only generate anthraquinones under the action of browning-related enzymes to change the colour of the fruit, but also act as antioxidants to remove free radicals generated by an adverse storage environment and delay the maturation of the fruit.37 As can be seen from Fig. 2B, in our study, the total phenolic content of sweet cherries in all treatment groups decreased continuously. At the end of the temperature fluctuation (4 d), the total phenolic content of sweet cherries was the highest in CT-5 group, followed by TF group, and then the CT-10 group, which remained unchanged during subsequent storage. This indicated that temperature fluctuations accelerated the metabolism of phenolic substances in sweet cherries.
3.3 The peroxidase (POD) and polyphenoloxidase (PPO) activities, and malondialdehyde (MDA) contents of sweet cherries under temperature fluctuations
The change in colour of sweet cherry was significantly related to enzyme activity, especially POD and PPO.5,32 Fig. 2C and D show the variance in browning enzyme activities (POD and PPO) among different samples. It was reported that browning caused by oxidation of phenolic compounds by PPO is one of the symptoms of senescence in sweet cherry.38 Meanwhile, POD, as a candidate browning enzyme for oxidation of phenolics, cannot be neglected, because single electron oxidation of diverse antioxidant compounds could be catalysed by POD in the presence of H2O2.39 As shown in Fig. 2C, the POD activities in both the TF and CT group samples tended to peak and then decline. Similar trends were reported by Yang et al.40 Compared with that in the CT-5 group, the POD activity of sweet cherries in the CT-10 group was higher and increased faster. Interestingly, during the constant temperature storage stage, the POD activity in the TF group was always higher than that in the CT-5 group. In particular, the POD activity in the TF group reached the level of the CT-10 group at the end of storage.PPO in sweet cherry in all groups increased quickly during the first 16 d of storage, and then decreased progressively. Although there was no significant difference in PPO activity between the TF and CT-5 groups at the end of the temperature fluctuation (4 d), the enzyme activity of the TF group was higher than that of the CT-5 group from storage day 16. The results for the POD and PPO activities indicated that temperature fluctuation occurring in the early stage could accelerate the activities of browning-related enzymes in the subsequent constant temperature storage of sweet cherries, accompanied by a decrease in the contents of anthocyanins and phenolics (Fig. 2A and B), which were the main reasons for the colour transformation of sweet cherries.
It was reported that the degree of browning of sweet cherries was closely related to the continuous accumulation of MDA,41 which was explained by the oxidative damage of the cytoplasmic membrane under senescence stress during storage of fruit caused by excessive reactive oxygen species (ROS), including H2O2 and O2˙−.42 The rapid accumulation in MDA is generally linked to increased ROS production, which leads to dysfunction of cell membrane, ultimately causing browning symptoms.40 As demonstrated in Fig. 2E, a similar progressive increase in the MDA content was observed in all samples. Fluctuating temperature conditions resulted in increased accumulation of MDA. For example, after the temperature fluctuations, the MDA content of sweet cherries in the TF group was markedly higher than that in the CT-5 group and even the CT-10 group. Although the increase in MDA accumulation slowed down during later constant temperature storage, it was still significantly higher than that of the CT-5 group.
Polyphenols mainly exist in the vacuoles of plant cells, while browning-related enzymes are distributed in the cytoplasm (such as plasmids or mitochondria) and cytoplasmic membrane.43,44 During fruit maturation, the generation of ROS will cause oxidative damage to the cytoplasmic membrane, which can lead to an accumulation of MDA and a number of secondary reactions, including increased respiration, ethylene release, and blocked energy production.32,40,45 Ultimately, enhanced membrane permeability and the destruction of membrane integrity lead to contact between phenolics and enzymes, resulting in colour change. Collectively, the results of the present study showed that temperature fluctuation not only promoted the immediate accumulation of MDA, but also accelerated the increase of PPO and POD activity and the MDA content during subsequent isothermal storage. These physiological and metabolic reactions promoted the transformation of polyphenols, which ultimately accelerated the change in the skin colour of sweet cherry.
3.4 The softening of sweet cherries under temperature fluctuations
Softening is one of the symptoms of senescence in sweet cherry. The results shown in Table 2 demonstrate that the firmness of sweet cherries in the CT-10 group decreased continuously and significantly, while there was a gradual decline in the TF and CT-5 groups at 16 days before flattening during later storage. The firmness of sweet cherry after three days of temperature fluctuation treatment was significantly lower than that of the CT-5 group. For example, on the 4th day, the firmness of the TF group was only 86.3% of that of the CT-5 group. During subsequent constant temperature storage, the firmness of the TF group was consistently lower than that of CT-5 group and was almost as same as that of the CT-10 group.| Storage time (d) | Firmness (N) | SSP content (mg kg−1) | ||||
|---|---|---|---|---|---|---|
| TFd | CT-5 | CT-10 | TF | CT-5 | CT-10 | |
| a Values of firmness are means ± standard error (n = 20) and values of SSP content are means ± standard error (n = 3).b The different lowercase superscript letters indicate that there are significant differences among three storage temperature groups at the same storage time (P < 0.05, Duncan's test). The different capital superscript letters indicate that the same storage temperature group has a significant difference at different storage times (P < 0.05, Duncan's test).c Standard error.d TF means sweet cherry treated with temperature fluctuation and then stored at 5 ± 1 °C; CT-5 denotes sweet cherry stored constantly at 5 ± 1 °C; CT-10 denotes sweet cherry stored constantly at 10 ± 1 °C. | ||||||
| 0 | 9.95aAb ± 0.36c | 9.95aA ± 0.36 | 9.95aA ± 0.36 | 1035.8aA ± 70.7 | 1035.8aA ± 70.7 | 1035.8aA ± 70.7 |
| 4 | 7.36cB ± 0.07 | 8.53aB ± 0.59 | 7.61bB ± 0.11 | 831.4bB ± 62.8 | 907.2aB ± 66.6 | 705.7bB ± 66.6 |
| 10 | 6.58bC ± 0.23 | 7.99aB ± 0.64 | 6.34bC ± 0.12 | 605.1bD ± 70.8 | 835.9aC ± 66.5 | 552.4bC ± 69.9 |
| 16 | 5.76bD ± 0.14 | 7.19aC ± 0.70 | 5.39bD ± 0.24 | 566.5bE ± 71.3 | 743.5aD ± 62.4 | 481.6bD ± 70.2 |
| 22 | 4.78bE ± 0.29 | 6.22aD ± 0.64 | 4.29bE ± 0.27 | 614.7bC ± 70.7 | 763.3aD ± 61.3 | 441.7cE ± 64.1 |
| 28 | 4.65bE ± 0.39 | 5.78aD ± 0.43 | 3.83cF ± 0.30 | 576.2bE ± 62.4 | 756.0aD ± 62.0 | 381.6cF ± 70.0 |
Inevitably, under the temperature change, the respiration and transpiration of sweet cherry are affected significantly, causing the loss of water, the reduction of cell turgor and the degradation of the cell wall, which eventually leads to the fruit softening.46–49 In addition, the firmness of postharvest sweet cherry correlated more positively and significantly with the content of pectin, as the skeleton material of cell wall, especially SSP.14,15 The SSP constant in the TF and CT-5 groups decreased as time progressed and levelled out at storage day 16, tending to remain stable during subsequent storage. Meanwhile, the SSP content of the CT-10 group continued to decrease. Consistent with the changing trend of firmness, temperature fluctuation treatment accelerated the reduction of SSP content significantly in sweet cherries. For example, the SSP contents in the TF group were only 91.6% and 76.2% of that in the CT-5 group at 4 d and 16 d, respectively, and then decreased almost to the level of the CT-10 group. At the end of storage, the CT-5, TF, and CT-10 groups retained 73.0%, 55.6%, and 36.8% of their SSP content, respectively. These results indicated that softening of sweet cherries would be accelerated under temperature fluctuation, which also had a negative effect on the storage stability of sweet cherries in the subsequent constant temperature storage period.
3.5 Nanostructure changes of SSP in sweet cherries under temperature fluctuations
In addition to the SSP content, the evolution of heterogeneous and intricate nanostructures of SSP also significantly affects the texture characteristics of sweet cherries.14,15 In fresh and filled sweet cherries, the morphological information of SSP mainly includes the large polymer (Lp), visible as an amorphous phase, concentrated around linear molecules and formed by the interconnected SSPs to maintain fruit firmness (Fig. 3A(a)). In addition, the quantitative characteristics of SSP showed that in fresh sweet cherries, the SSP chain widths were mainly distributed between 60 and 90 nm, with some chains being larger than 90 nm (Fig. 3B). The width of the SSP chains in fruit includes several basic units, such as 15 nm, 23 nm, and 37 nm. These basic units are cross-linked, entangled, and aggregated with each other according to the nanostructural rules of SSP formation.50 When stored at 5 °C, the Lp structure of sweet cherries gradually depolymerized, resulting in a loose structure (Ls), and then separated into a long chain (Lc) structure which interrupted by local bend points or branches (Fig. 3A(c)), thereafter further dispersing into multiple blocks (Fig. 3A(f)). The Lc are identified as a section of unbranched homogalacturonan. And arabinose plays an important role in the aggregation of multiple pectin chains. The bend points and branches on the Lc were most likely to be formed by a single rhamnose unit, which connected the three homogalacturonan chains or short sections of rhamnogalacturonan-I with a homogalacturonan branch chain.51 At the same time, the Lps almost disappeared when sweet cherry was stored at 10 °C, and a large number of Lcs and short chains (Scs) appeared (Fig. 3A(d and g)). In the meantime, the proportion of SSP chains with a width less than 60 nm gradually increased as time progressed and the storage temperature increased (Fig. 3B).Temperature fluctuation treatment significantly accelerated the evolution of the SSP nanostructure and the dissociation of the aggregated basic units. Compared with that in the CT-5 group, few Lps were observed in the TF group, and more Scs and Lcs were distributed in the different blocks (Fig. 3A(b)). At the end of storage, the morphology of SSP in the TF group was basically consistent with that in the CT-10 group (Fig. 3A(e)). The same changes in the SSP chain width were observed (Fig. 3B), for example, there were significantly fewer pectin chains with a width of 80 to 90 nm in TF group than in the CT-5 group. Moreover, the SSP chain width values of the TF and CT-10 group were concentrated in the 40 to 50 nm range, while those of the CT-5 group were mainly distributed at 50 to 60 nm at the end of storage.
3.6 The pectin-related enzyme activities and gene expression of sweet cherries under temperature fluctuations
It is extremely challenging to clearly elucidate the trends of enzymatic modification of softening-related pectin, because the enzymatic action involves the synergistic cooperation among a large number of proteins.52 In earlier studies, the co-existing PME, PG, and β-Gal in postharvest sweet cherry were observed to contribute to the cell wall modification.52–54 Moreover, after being de-esterified by PME, pectin is usually susceptible to the action of PG and β-Gal on both the pectin backbone and side chain.55In the present study, the activity of PME in sweet cherry increased significantly (Fig. 4A). The activity of PME in the TF group was enhanced significantly, increasing to 1.8 times that of the CT-5 group. Thereafter, the PME activity continued to increase in the TF group during the subsequent constant temperature storage, reaching the level of the CT-10 group at 22 d.
As shown in Fig. 4B, the PG activities of the CT-5 and CT-10 groups increased in the early stage of storage and peaked at 16 d and 10 d, respectively, before stabilising. The activity of PG in the TF group was significantly higher than that in the CT-5 group after the end of temperature fluctuation (4 d), and continued to rise in the initial stage of constant temperature storage. Finally, the PG activity of the TF reached the level of the CT-10 group at the end of storage.
Fig. 4C shows that the β-Gal in postharvest sweet cherry remained active, which is consistent with an earlier study by Gerardi et al.54 who reported that the activity of β-Gal, as a key ripening-related enzyme, increased noticeably during sweet cherry ripening. β-Gal increases cell wall porosity by depolymerizing the galactose side chains, which then allows the binding of PG and PME. Among the samples, the activity of β-Gal in the CT-10 group barely changed and remained active during the whole storage period. Under the action of low temperature, the β-Gal activity of cherries stored at 5 °C was markedly lower than that of cherries stored at 10 °C. Interestingly, temperature fluctuation did not immediately induce an increase in β-Gal activity. However, in the later stage of constant temperature storage, the β-Gal activity of TF samples was always greater than that of the CT-5 group.
To identify whether any of the candidate pectin-related enzyme genes were affected by temperature fluctuations during sweet cherry softening, we determined the gene expression levels of candidate genes in the different groups. The expression levels of most candidate genes were differently up or downregulated during the storage of sweet cherries, indicating that they are involved in the evolution of pectin (Fig. 5A). After three days of temperature fluctuation treatment, the expression levels of paPME3 and paPME4 (Fig. 5B(c and d)) in the TF group were enhanced significantly. In addition, during constant temperature storage (16 d), the expression levels of paPME1, paPME3, and paPME4 (Fig. 5B(a, c and d)) in the TF group were always higher than those in the CT-5 group. Furthermore, at the end of storage, the expression of paPME2 was increased by several fold in the TF group (Fig. 5B(b)).
The expression levels of paPG genes in the CT-5 and CT-10 groups decreased after peaking at 16 d, which was consistent with the stabilisation of PG activity after this time point shown in Fig. 4B. This trend was in agreement with the previous study on blueberry during softening.56 This may be due to the synergistic effect of biochemical metabolism of the organism, which produces negative feedback on the expression of paPG genes. The expression levels of paPG genes in the TF group also increased on the 4th day. Moreover, during constant temperature storage, the expression levels of paPG genes in the TF group remained high. Especially at the end of storage, the expression levels of paPG1 and paPG3 were significantly higher than those in the CT-5 group (Fig. 5B(f and h)). Therefore, the PG enzymes in the TF group were always more active than those in the CT-5 group (Fig. 3B).
Compared with that in fresh cherries, paβ-Gal expression was downregulated in the CT-5 and CT-10 groups during storage (Fig. 5A). This was consistent with the trend of β-Gal activity observed in Fig. 3C. Although the paβ-Gal expression level in the TF group did not increase significantly after temperature fluctuation treatment, the expression levels of paβ-Gal1 and paβ-Gal3 (Fig. 5B(i and k)) were upregulated compared with those in the CT-5 group at the beginning of the subsequent constant temperature storage. In addition, their expression levels had doubled by the end of storage in the TF group.
Changes in the expression patterns of the selected pectin related genes suggested that the expression levels of paPG1-3, paPME3, and paPME4 in sweet cherries increased immediately under temperature fluctuation, and the expression levels of paPGs, paPME1-4, paβ-Gal1, and paβ-Gal3 genes were also upregulated during subsequent constant temperature storage. The results of the analysis of the gene expression data were consistent with those of the PME, PG, and β-Gal enzyme activities (Fig. 3A–C).
3.7 Temperature fluctuation accelerated browning and softening of postharvest sweet cherry
The cellular structure of fresh cherries was intact, and browning and softening-related enzymes were distributed in the cytoplasm and cytoplasmic membrane. Polyphenols were distributed in vacuoles, and SSP, as the skeletal structure of cell wall, was intertwined in the form of aggregates (Fig. 6A and J).When sweet cherry experienced temperature fluctuations, the MDA content was increased continuously, cell membrane permeability was enhanced, and the anthocyanin and phenolic contents changed rapidly, which was ultimately reflected in the change in the apparent colour of sweet cherry (Fig. 6D). In particular, the temperature fluctuation significantly accelerated the synthesis of anthocyanins, and its content increased significantly compared with that of CT-5 and CT-10 groups (Fig. 6B and F). At the same time, temperature fluctuation also induced the upregulation of paPG1-3, paPME3 and paPME4 expression, which regulated the PG and PME activities. These changes resulted in the demethylation and depolymerisation of the SSP aggregates that maintain the hardness of sweet cherry, resulting in a significant decrease in the firmness of cherry (Fig. 6M). At the same time, the monosaccharides produced by pectin degradation could combine with anthocyanins to form chromogenic substances that changes the colour of cherries (Fig. 6I). Under the action of PME and PG, the depolymerization and degradation degree of SSP in TF group was significantly higher than that in CT-5 (Fig. 6K) group and almost reached the level of CT-10 group (Fig. 6O).
Temperature fluctuation not only accelerated the colour and texture deterioration of cherry immediately, but also further aggravated the deterioration of sweet cherry sensory quality during subsequent constant temperature storage (Fig. 6E). Although temperature fluctuation had no immediate effect on POD, PPO, and β-Gal activities, those enzymes activities increased significantly and the expression levels of paGal1 and paGal3 were upregulated during subsequent isothermal storage. These changes accelerated the development of enzymatic browning (Fig. 6H) and the further degradation of SSP (Fig. 6N). The anthocyanin synthesis, phenolic metabolism, malondialdehyde accumulation and pectin evolution in TF group were significantly faster than that in CT-5 group (Fig. 6C and L), and were close to that in CT-10 group (Fig. 6G and P). Finally, at the end of storage, the ΔE value of the TF group was 24.8% higher than that in CT-5 group, and the firmness was 19.6% lower than that in the CT-5 group. Although they only experienced a temperature fluctuation of 5 °C for 3 days, the colour and firmness change of the cherries in TF group almost reached the levels observed in cherries stored at a constant 10 °C.
In summary, timely regulation of temperature fluctuations in the cold chain is the key to avoid the accelerated deterioration of the colour and texture of postharvest sweet cherry.
4. Conclusions
To evaluate the influence of temperature fluctuation on the storage stability of sweet cherry, the colour changes and softening of sweet cherries after temperature fluctuation (5–10 °C) and during subsequent isothermal storage (5 °C) were studied. Results showed that the temperature fluctuation could not only immediately affect the brightness (6.2% lower than the control) and firmness (13.7% lower than the control) of the fruit, but also, during the subsequent isothermal storage, accelerated the colour change (24.8% more than the control) and firmness deterioration (19.6% lower than the control), almost reaching the level of samples stored at a constant 10 °C.Temperature fluctuation promoted anthocyanin synthesis, phenolic metabolism, and MDA accumulation, which immediately affected the brightness of sweet cherry. Meanwhile, temperature fluctuation also induced upregulation of paPG1-3, paPME3, and paPME4 expression, which led to increased PG and PME activity and the degradation of aggregated SSP into its nanostructural basic units. These changes accelerated the softening of sweet cherry. In addition, during the subsequent isothermal storage, temperature fluctuation also led to significant increases in POD and PPO activity, further accelerating the colour change. Furthermore, temperature fluctuation resulted in upregulated expression of paβ-Gal1 and paβ-Gal3 genes, which enhanced β-Gal activity. The results provide theoretical guidance for the transportation, storage, and preservation of postharvest sweet cherries.
Author contributions
Kunlun Liu designed the study and interpreted the results. Ying Xin collected test data and drafted the manuscript. Zhenzhen Liu and Yuanwei Zhang carried out the experiment in detail. Xiaofei Shi assisted in completing the experiment. Fusheng Chen revised the manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by National Natural Science Foundation of China (31601519), Cultivation Programme for Young Backbone Teachers in Henan University of Technology (21420089), and National Key Research and Development Program of China (2018YFD0401102).References
- F. Blandoa and B. D. Oomahb, Sweet and sour cherries: origin, distribution, nutritional composition and health benefits, Trends Food Sci. Technol., 2019, 86, 517–529 CrossRef.
- M. Li, H. Zhi and Y. Dong, Influence of preharvest and postharvest applications of glycine betaine on fruit quality attributes and storage disorders of ‘lapins’ and ‘regina’ cherries, Hortscience, 2019, 54, 1540–1545 CAS.
- E. Aglar, B. Ozturk, S. K. Guler, O. Karakaya, S. Uzun and O. Saracoglu, Effect of modified atmosphere packaging and ‘Parka’ treatments on fruit quality characteristics of sweet cherry fruits (Prunus avium L. ‘0900 Ziraat’) during cold storage and shelf life, Sci. Hortic., 2017, 222, 162–168 CrossRef CAS.
- J. Giné-Bordonaba, G. Echeverria, D. Ubach, I. Aguiló-Aguayo, M. L. López and C. Larrigaudière, Biochemical and physiological changes during fruit development and ripening of two sweet cherry varieties with different levels of cracking tolerance, Plant Physiol. Biochem., 2017, 111, 216–225 CrossRef PubMed.
- Z. S. Wu, M. Zhang and S. Wang, Effects of high pressure argon treatments on the quality of fresh-cut apples at cold storage, Food Control, 2012, 23, 120–127 CrossRef CAS.
- G. Ballistreri, A. Continella, A. Gentile, M. Amenta, S. Fabroni and P. Rapisarda, Fruit quality and bioactive compounds relevant to human health of sweet cherry (Prunus avium L.) cultivars grown in Italy, Food Chem., 2013, 140, 630–638 CrossRef CAS PubMed.
- J. Cao, Q. Jiang, J. Lin, X. Li, C. Sun and K. Chen, Physicochemical characterisation of four cherry species (Prunus spp.) grown in China, Food Chem., 2015, 173, 855–863 CrossRef CAS PubMed.
- A. A. Hayaloglu and N. Demir, Physicochemical characteristics, antioxidant activity, organic acid and sugar contents of 12 sweet cherry (Prunus avium L.) cultivars grown in Turkey, J. Food Sci., 2015, 80, C564–C570 CrossRef CAS PubMed.
- H. Kelebek and S. Selli, Evaluation of chemical constituents and antioxidant activity of sweet cherry (Prunus avium L.) cultivars, Int. J. Food Sci. Technol., 2011, 46, 2530–2537 CrossRef CAS.
- A. T. Serra, R. O. Duarte, M. R. Bronze and C. M. M. Duarte, Identification of bioactive response in traditional cherries from Portugal, Food Chem., 2011, 125, 318–325 CrossRef CAS.
- L. Chen, Y. Zhou, Z. He, Q. Liu, S. Lai and H. Yang, Effect of exogenous ATP on the postharvest properties and pectin degradation of mung bean sprouts (Vigna radiata), Food Chem., 2018, 251, 9–17 CrossRef CAS PubMed.
- S. G. Gwanpua, B. E. Verlinden, M. L. A. T. M. Hertog, B. M. Nicolai, M. Hendrickx and A. Geeraerd, A transcriptomics-based kinetic model for enzyme-induced pectin degradation in apple (Malus×domestica) fruit, Postharvest Biol. Technol., 2017, 130, 64–74 CrossRef CAS.
- Y. Lin, H. Lin, H. Wang, M. Lin, Y. Chen, Z. Fan, Y. Hung and Y. Lin, Effects of hydrogen peroxide treatment on pulp breakdown, softening, and cell wall polysaccharide metabolism in fresh longan fruit, Carbohydr. Polym., 2020, 242, 116427 CrossRef CAS PubMed.
- Y. Xin, Z. Jin, F. Chen, S. Lai and H. Yang, Effect of chitosan coatings on the evolution of sodium carbonate-soluble pectin during sweet cherry softening under non-isothermal conditions, Int. J. Biol. Macromol., 2020, 154, 267–275 CrossRef CAS PubMed.
- Y. Xin, F. Chen, S. Lai and H. Yang, Influence of chitosan-based coatings on the physicochemical properties and pectin nanostructure of Chinese cherry, Postharvest Biol. Technol., 2017, 133, 64–71 CrossRef CAS.
- M. A. Quesada, R. Blanco-Portales, S. Posé, J. A. Garcıá-Gago, S. Jiménez-Bermúdez, A. Muñoz-Serrano, J. L. Caballero, F. Pliego-Alfaro, J. A. Mercado and J. Muñoz-Blanco, Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening, Plant Physiol., 2009, 150, 1022–1032 CrossRef CAS PubMed.
- N. M. Villarreal, H. G. Rosli, G. A. Martínez and P. M. Civello, Polygalacturonase activity and expression of related genes during ripening of strawberry cultivars with contrasting fruit firmness, Postharvest Biol. Technol., 2008, 47, 141–150 CrossRef CAS.
- J. Zhuang, J. Su, X. Li and W. Chen, Cloning and expression analysis of beta-galactosidase gene related to softening of banana (Musa sp.) fruit, J. Plant Physiol. Mol. Biol., 2006, 4, 9–411 Search PubMed.
- K. Wang, T. Li, S. Chen, Y. Li and A. Rashid, The biochemical and molecular mechanisms of softening inhibition by chitosan coating in strawberry fruit (Fragaria x ananassa) during cold storage, Sci. Hortic., 2020, 271, 109483 CrossRef CAS.
- H. Wang, X. Cheng, C. Wu, G. Fan, T. Li and C. Dong, Retardation of postharvest softening of blueberry fruit by methyl jasmonate is correlated with altered cell wall modification and energy metabolism, Sci. Hortic., 2021, 276, 109752 CrossRef CAS.
- C. Liu, C. Chen, Y. Zhang, A. Jiang and W. Hu, Aqueous ozone treatment inhibited degradation of cellwall polysaccharides in fresh-cut apple during cold storage, Innovative Food Sci. Emerging Technol., 2021, 67, 102550 CrossRef CAS.
- S. Chockchaisawasdee, J. B. Golding, Q. V. Vuong, K. Papoutsis and C. E. Stathopoulos, Sweet cherry: composition, postharvest preservation, processing and trends for its future use, Trends Food Sci. Technol., 2016, 55, 72–83 CrossRef CAS.
- E. M. Gonçalves, J. Pinheiro, M. Abreu, T. R. S. Brandão and C. L. M. Silva, Kinetics of quality changes of pumpkin (Curcurbita maxima L.) stored under isothermal and non-isothermal frozen conditions, J. Food Eng., 2011, 106, 40–47 CrossRef.
- M. Göransson, F. Nilsson and Å. Jevinger, Temperature performance and food shelf-life accuracy in cold food supply chains-insights from multiple field studies, Food Control, 2018, 86, 332–341 CrossRef.
- Z. Huang, L. Guo, H. Wang, H. Qu, S. Ma, Y. Liu, H. Huang and Y. Jiang, Energy status of kiwifruit stored under different temperatures or exposed to long-term anaerobic conditions or pure oxygen, Postharvest Biol. Technol., 2014, 98, 56–64 CrossRef CAS.
- T. Theofania, D. Efimia, G. Marianna, G. Eleni, K. George and T. Petros, Shelf-life prediction models for ready-to-eat fresh cut salads: testing in real cold chain, Int. J. Food Microbiol., 2016, 240, 131–140 Search PubMed.
- V. Vicent, F. T. Ndoye, P. Verboven, B. M. Nicolaï and G. Alvarez, Quality changes kinetics of apple tissue during frozen storage with temperature fluctuations, Int. J. Refrig., 2018, 92, 165–175 CrossRef CAS.
- R. N. Barbagallo, M. Chisari and G. Caputa, Effects of calcium citrate and ascorbate as inhibitors of browning and softening in minimally processed ‘Birgah’ eggplants, Postharvest Biol. Technol., 2012, 73, 107–114 CrossRef CAS.
- T. Albishi, J. A. John, A. S. Al-Khalifa and F. Shahidi, Antioxidative phenolic constituents of skins of onion varieties and their activities, J. Funct. Foods, 2013, 5, 1191–1203 CrossRef CAS.
- V. L. Singleton, R. Orthofer and R. M. Lamuela-Raventós, Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent, Methods Enzymol., 1999, 299C, 152–178 Search PubMed.
- Y. Wang, X. Xie and L. E. Long, The effect of postharvest calcium application in hydro-cooling water on tissue calcium content, biochemical changes, and quality attributes of sweet cherry fruit, Food Chem., 2014, 160, 22–30 CrossRef CAS PubMed.
- H. Zhao, B. Liu, W. Zhang, J. Cao and W. Jiang, Enhancement of quality and antioxidant metabolism of sweet cherry fruit by near-freezing temperature storage, Postharvest Biol. Technol., 2019, 147, 113–122 CrossRef CAS.
- S. Lohani, P. K. Trivedi and P. Nath, Changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: effect of 1-MCP, ABA and IAA, Postharvest Biol. Technol., 2004, 31, 119–126 CrossRef CAS.
- S. Ranjbar, M. Rahemi and A. Ramezanian, Comparison of nano-calcium and calcium chloride spray on postharvest quality and cell wall enzymes activity in apple cv, Red Delicious, Sci. Hortic., 2018, 240, 57–64 CrossRef CAS.
- D. González-Gómez, M. Lozano, M. F. Fernández-León, M. J. Bernalte and A. B. R. Ayuso, Sweet cherry phytochemicals: identification and characterization by HPLC-DAD/ESI-MS in six sweet-cherry cultivars grown in Valle del Jerte (Spain), J. Food Compos. Anal., 2010, 23, 533–539 CrossRef.
- B. Gonçalves, A. P. Silva, J. Moutinho-Pereira, E. Bacelar, E. Rosa and A. S. Meyer, Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.), Food Chem., 2007, 103, 976–984 CrossRef.
- Y. Liu, X. Liu, F. Zhong, R. Tian, K. Zhang, X. Zhang and T. Li, Comparative study of phenolic compounds and antioxidant activity in different species of cherries, J. Food Sci., 2011, 76, C633–C638 CrossRef CAS PubMed.
- M. S. Pasquariello, D. D. Patre, F. Mastrobuoni, L. Zampella, M. Scortichini and M. Petriccione, Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit, Postharvest Biol. Technol., 2015, 109, 45–56 CrossRef CAS.
- M. Petriccione, F. De Sanctis, M. S. Pasquariello, F. Mastrobuoni, P. Rega, P. Scortichini and F. Mencarelli, The effect of chitosan coating on the quality and nutraceutical traits of sweet cherry during postharvest life, Food Bioprocess Technol., 2015, 8, 394–408 CrossRef CAS.
- Y. Q. Yang, X. Zhang, F. Wang and Q. Zhao, Effect of pressurized argon combined with controlled atmosphere on the postharvest quality and browning of sweet cherries, Postharvest Biol. Technol., 2019, 147, 59–67 CrossRef.
- Y. Zhao, X. Zhu, Y. Hou, X. Wang and X. Li, Postharvest nitric oxide treatment delays the senescence of winter jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit during cold storage by regulating reactive oxygen species metabolism, Sci. Hortic., 2020, 261, 109009 CrossRef CAS.
- A. A. Lo'ay and M. A. Taher, Effectiveness salicylic acid blending in chitosan/PVP biopolymer coating on antioxidant enzyme activities under low storage temperature stress of ‘Banati’ guava fruit, Sci. Hortic., 2018, 238, 343–349 CrossRef.
- K. Amaki, E. Saito, K. Taniguchi, K. Joshita and M. Murata, Role of chlorogenic acid quinone and interaction of chlorogenic acid quinone and catechins in the enzymatic browning of apple, Biosci., Biotechnol., Biochem., 2011, 75, 829–832 CrossRef CAS PubMed.
- J. Li, X. Ding, S. Han, T. He, H. Zhang, L. Yang, S. Yang and J. Gai, Differential proteomics analysis to identify proteins and pathways associated with male sterility of soybean using iTRAQ-based strategy, J. Proteomics, 2016, 138, 72–82 CrossRef CAS PubMed.
- H. J. Rui, S. F. Cao, H. T. Shang, P. Jin, K. T. Wang and Y. H. Zheng, Effects of heat treatment on internal browning and membrane fatty acid in loquat fruit in response to chilling stress, J. Sci. Food Agric., 2010, 90, 1557–1561 CrossRef CAS PubMed.
- P. M. Allan-Wojtas, C. F. Forney, S. E. Carbyn and K. Nicholas, Microstructural indicators of quality-related characteristics of blueberries-an integrated approach, LWT--Food Sci. Technol., 2001, 34, 23–32 CrossRef CAS.
- J. A. Heyes and D. F. Sealey, Textural changes during nectarine (Prunus persica) development and ripening, Sci. Hortic., 1996, 65, 49–58 CrossRef.
- A. John, J. Yang, J. Liu, Y. Jiang and B. Yang, The structure changes of water-soluble polysaccharides in papaya during ripening, Int. J. Biol. Macromol., 2018, 115, 152–156 CrossRef CAS PubMed.
- A. C. Paniagua, A. R. East, J. P. Hindmarsh and J. A. Heyes, Moisture loss is the major cause of firmness change during postharvest storage of blueberry, Postharvest Biol. Technol., 2013, 79, 13–19 CrossRef.
- F. Chen, H. Liu, H. Yang, S. Lai, X. Cheng, Y. Xin, B. Yang, H. Hou, Y. Yao, S. Zhang, G. Bu and Y. Deng, Quality attributes and cell wall properties of strawberries (Fragaria annanassa Duch.) under calcium chloride treatment, Food Chem., 2011, 126, 450–459 CrossRef CAS.
- P. M. Pieczywek, A. Kozioł, W. Płazinski, J. Cybulska and A. Zdunek, Resolving the nanostructure of sodium carbonate extracted pectins (DASP) from apple cell walls with atomic force microscopy and molecular dynamics, Food Hydrocolloids, 2020, 104, 105726 CrossRef CAS.
- B. Belge, L. F. Goulao, E. Comabella, J. Graell and I. Lara, Refrigerated storage and calcium dips of ripe ‘Celeste’ sweet cherry fruit: combined effects on cell wall metabolism, Sci. Hortic., 2017, 219, 182–190 CrossRef CAS.
- B. Belge, E. Comabella, J. Graell and I. Lara, Post-storage cell wall metabolism in two sweet cherry (Prunus avium L.) cultivars displaying different postharvest performance, Food Sci. Technol. Int., 2014, 21, 416–427 CrossRef PubMed.
- C. Gerardi, F. Blando and A. Santino, Purification and chemical characterisation of a cell wall-associated-galactosidase from mature sweet cherry (Prunusavium L.) fruit, Plant Physiol. Biochem., 2012, 61, 123–130 CrossRef CAS PubMed.
- L. F. Goulao and M. C. Oliveira, Cell wall modifications during fruit ripening: when a fruit is not the fruit, Trends Food Sci. Technol., 2008, 19, 4–25 CrossRef CAS.
- H. Wang, X. Cheng, C. Wu, G. Fan, T. Li and C. Dong, Retardation of postharvest softening of blueberry fruit by methyl jasmonate is correlated with altered cell wall modification and energy metabolism, Sci. Hortic., 2021, 276, 109752 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |