Open Access Article
Open Access ArticleSynthesis and biological evaluation of a new class of multi-target heterocycle piperazine derivatives as potential antipsychotics†
Lanchang Gao‡
ab,
Chao Hao‡b,
Ru Maa,
Jiali Chenb,
Guisen Zhangab and
Yin Chen *a
*a
aJiangsu Key Laboratory of Marine Biological Resources and Environment, Jiangsu Key Laboratory of Marine Pharmaceutical Compound Screening, School of Pharmacy, Jiangsu Ocean University, Lianyungang 222005, China. E-mail: 2019000015@jou.edu.cn; Fax: +86-518-85586628; Tel: +86-518-85586628
bDepartment of Biomedical Engineering, College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan 430074, China
First published on 7th May 2021
Abstract
In this study, we designed and synthesized a novel series of multi-receptor ligands as polypharmacological antipsychotic agents by using a multi-receptor affinity strategy. Among them, 3w combines a multi-receptor mechanism with high mixed affinities for D2, 5-HT1A, 5-HT2A and H3 receptors, and low efficacy at the off-target receptors (5-HT2C, H1 and α1 receptor) and human ether-à-go-go-related gene (hERG) channel. In addition, compound 3w exhibits favorable antipsychotic drug-like activities in in vivo assessment. An animal behavioral study revealed that compound 3w significantly reverses apomorphine-induced climbing and MK-801-induced hyperactivity, and avoidance behavior in the CAR test, with a high threshold for catalepsy. Moreover, compound 3w demonstrates memory enhancement in a novel object recognition task and low liabilities for weight gain and hyperprolactinemia in a long-term metabolic adverse effects model. Thus, 3w was selected as an antipsychotic candidate for further development.
1. Introduction
Schizophrenia is a chronic and complex psychotic mental disorder that affects around 1% of people. It is characterized by a combination of positive, negative and cognitive impairment.1 Although current antipsychotics on the market have brought about great progress in the treatment of schizophrenia, such as typical antipsychotics (chlorpromazine and haloperidol, Fig. 1) that have been proven to be effective treatments in controlling positive symptoms, their strong and nonselective blockade of dopaminergic transmission causes numerous side effects, such as tardive dyskinesia (TD), extrapyramidal symptoms (EPS) and hyperprolactinemia, and even exacerbates negative and cognitive symptoms.2,3 Atypical antipsychotics, such as clozapine and risperidone, are less tightly bound to the dopamine D2 receptor. Besides this, their affinities for various 5-hydroxytryptamine (5-HT) receptors means that they display more clinical advantages over typical antipsychotics in the treatment of positive symptoms, as well as having small effect size advantages in the improvement of negative cognitive symptoms and in promoting relapse prevention and cognitive impairment. However, patients who take antipsychotic drugs still suffer from long-term side effects, such as QT (Q wave and the end of the T wave on electrocardiograms) interval prolongation, hyperprolactinemia and weight gain.4 Therefore, there is still a great clinical need for the development of safer, more effective novel antipsychotics.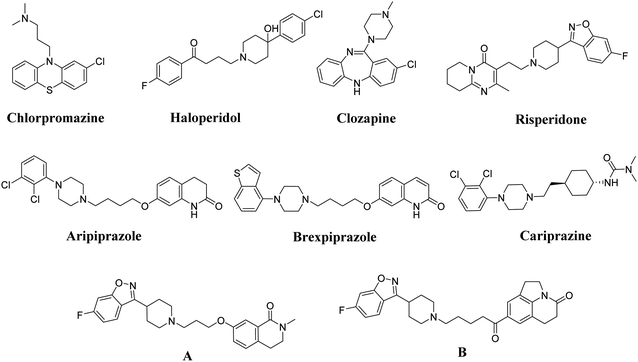 | ||
| Fig. 1 The structure of representative antipsychotic drugs and representative compounds from our previous study. | ||
From the perspective of targets, activation of the 5-HT1A receptor in the frontal cortex will enhance the functions of the mesocortical dopamine pathway, which may improve the negative symptoms and cognitive deficits in patients who have schizophrenia.5,6 An inverse agonist of 5-HT2A could counteract excessive D2 receptor blockade, which is not only conducive to alleviating extrapyramidal effects but also enhances the efficacy against negative symptoms.7–9 Also, there is plenty of evidence that strongly supports that combined effects on D2 and 5-HT2A receptors are beneficial to the improvement of both negative symptoms and symptoms positive of schizophrenia.10,11 Currently, a large number of literature studies have detailed the design of multi-target ligands that can simultaneously modulate and balance their activities at several specific targets to overcome the shortcomings of conventional antischizophrenic drugs, such as the novel antipsychotics aripiprazole,12 brexpiprazole and cariprazine,13,14 which differ from previous antipsychotics in that they are more inclined to achieve balance and coordinate multiple biological targets (Fig. 1). For example, both brexpiprazole and cariprazine modulate and stabilize DA (dopamine) neurotransmission via synergistic effects rather than by complete antagonism of D2 alone.15,16 In addition, these drugs are partial agonists of D2, 5-HT1A, 5-HT2A, and 5-HT2C (particularly of 5-HT1A and 5-HT2A) and exhibit higher levels of intrinsic activity at 5-HT1A.17,18 These combined actions effectively relieve the behavioral and psychological symptoms of schizophrenia, significantly reduce severe side effects, are associated with a favorable safety profile, and improve cognitive symptoms to some extent.19
As schizophrenia has varied and numerous different symptoms, developing multi-target ligands with a polypharmacological profile has become a widely used therapeutic approach.20 Also, exploring novel multi-target ligands that can accurately modulate and balance activities at dopaminergic and serotoninergic receptors, especially the D2, 5-HT1A and 5-HT2A receptors, and simultaneously decrease the affinity for other off-target receptors associated with side effects has been our long-standing research interest.21,22 In our previous study, we explored a series of multi-target ligands, as shown in Fig. 1, where compounds A and B exhibited a high affinity for dopamine D2 and serotonin 5-HT1A, 5-HT2A, 5-HT6 receptors, and low affinity for 5-HT2C, histamine H1, and adrenergic α1, which can be regarded as related to side effects. Both compound A and B showed negligible effects on the human ether-à-go-go-related gene channel (hERG; associated with QT interval prolongation). In the study of animal models, compounds A and B show favorable anti-schizophrenic activity, such as markedly inhibited APO (apomorphine)-induced hyperlocomotion, and MK-801-induced hyperactivity. In addition, compounds A and B display a high threshold for acute toxicity, a low tendency to induce catalepsy, as well as negligible side effects (hyperprolactinemia and weight gain) when compared to risperidone. To our satisfaction, compounds A and B display precognition properties in the novel object recognition task (NOR) in rats, giving them an advantage over most of the conventional antischizophrenic drugs.23,24
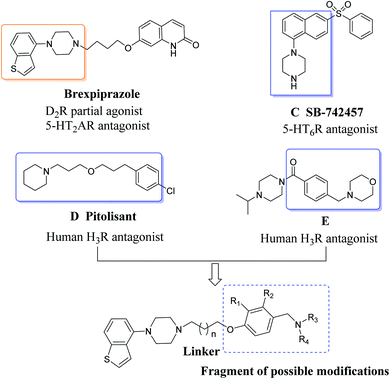
Inspired by these findings, here we describe a series of new compounds prepared using a molecular hybridization method, the design concept of which is shown in Fig. 2. Their general structure consists of benzothiophenylpiperazine, which is a crucial DA and 5-HT pharmacophore of the novel antipsychotic brexpiprazole, and a special pharmacophore (pyrrolidine, piperidine or morpholine connected to a benzene ring via a methylene group), derived from reported H3 and 5-HT6 receptor antagonists (pitolisant, compounds C and E SB-7424) that showed potential to improve cognitive deficits in clinical trials,25–27 expect that a mixed DA/5-HT/H3 receptor affinity profile is beneficial for the cognitive function of schizophrenia. Finally, the two central pharmacophores are connected via an appropriate linker, according to the literature, as well as our own experience. Besides this, the effects that different substituents on the benzene ring of the new compounds have on the receptor affinity were investigated. Subsequently, these new derivatives were subject to in vitro evaluation and in vivo behavioral studies. Among these new derivatives, compound 3w demonstrates a high level of multi-target activities at D2, 5-HT1A, 5-HT2A, H3 and lacks the receptors associated with side effects (5-HT2C, α1, and H1).28–30 In behavioral studies, compound 3w was found to significantly attenuate MK-801-induced hyperlocomotion as well as apomorphine-induced climbing, exhibiting negligible liability in terms of inducing weight gain, and resulted in no significant hyperprolactinemia compared to risperidone. In addition, NOR testing demonstrated that compound 3w shows pro-cognitive properties in rats, which conform to the original expectations. Thus, the present study identifies compound 3w as a potential antipsychotic candidate for the treatment of schizophrenia.
2. Results and discussion
2.1. Chemistry
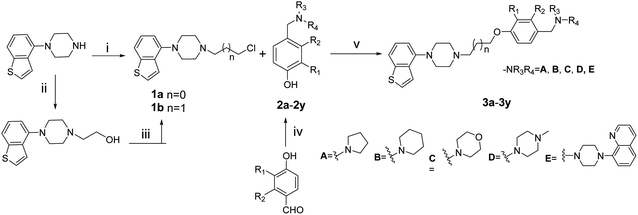
The general procedures for the synthesis of the intermediates (1a, 1b, 2a–2y) and novel compounds 3a–3y are illustrated in Scheme 1. The substitution of alkyl groups into the aryl-piperazines was achieved through the reaction of benzothiophenylpiperazine with 1-bromo-3-chloropropane in the presence of anhydrous potassium carbonate to afford 1a. The benzothiophenylpiperazine reacts with 2-bromoethanol following reflux in a mixture of thionyl chloride and dichloromethane to given intermediate 1b. The intermediates 2a–2y were obtained using p-hydroxybenzaldehyde and its derivatives to react with pyrrolidine, piperidine, morpholine and their derivatives in the presence of sodium triacetoxyborohydride. The desired compounds 3a–3y were synthesized via the reaction of 1a or 1b and 2a–2y using anhydrous potassium carbonate as a base and a trace of potassium iodide as a catalyst.2.2. Biological studies
2.3. In vitro evaluation of new compounds
The effect of modifications on the functional activities of the five target receptors was preliminarily evaluated at the beginning of this study. As the results in Table 1 show, all of the compounds in the present study (3a–3y) exhibit affinities for D2, 5-HT1A and 5-HT2A to some extent, especially compounds 3t and 3w, which exhibit high affinity for the D2, 5-HT1A and 5-HT2A receptors, attributed to the privileged structure of benzothiophenylpiperazine.
| Cmpd | R1 | n | R2 | NR3R4 | Receptor affinity Ki ± SEMa (nM) | ||||
|---|---|---|---|---|---|---|---|---|---|
| D2 | 5-HT1A | 5-HT2A | 5-HT6 | H3 | |||||
| a Ki values obtained from three experiments, recorded as means ± SEM. | |||||||||
| 3a | CH3 | 1 | H | 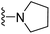 |
94.1 ± 5.6 | 154.7 ± 10.5 | 205.6 ± 23.0 | 463.5 ± 43.0 | 61.3 ± 3.9 |
| 3b | H | 1 | CH3 | 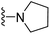 |
95.6 ± 11.2 | 163.8 ± 13.0 | 276.4 ± 24.2 | 481.9 ± 52.2 | 66.9 ± 3.0 |
| 3c | F | 1 | H | 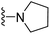 |
100.1 ± 7.0 | 154.6 ± 15.2 | 258.6 ± 14.0 | 382.8 ± 34.6 | 92.9 ± 3.6 |
| 3d | H | 1 | F | 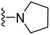 |
98.7 ± 6.8 | 144.1 ± 10.4 | 262.4 ± 17.5 | 354.2 ± 65.8 | 54.4 ± 2.0 |
| 3e | Cl | 1 | H | 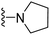 |
101.2 ± 10.1 | 157.3 ± 9.8 | 248.6 ± 22.9 | 418.5 ± 85.4 | 58.9 ± 5.5 |
| 3f | H | 1 | Cl | 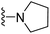 |
120.1 ± 15.0 | 184.6 ± 16.3 | 268.5 ± 20.2 | 375.6 ± 58.3 | 75.30 ± 8.5 |
| 3g | OCH3 | 1 | H | 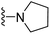 |
99.5 ± 8.4 | 149.0 ± 11.7 | 128.7 ± 15.1 | 462.4 ± 55.7 | 62.5 ± 5.0 |
| 3h | OCH3 | 0 | H | 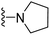 |
82.5 ± 5.8 | 354.6 ± 19.3 | 156.9 ± 12.6 | 764.3 ± 94.6 | 164.3 ± 14.5 |
| 3i | H | 1 | H | 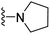 |
91.1 ± 4.5 | 134.7 ± 10.5 | 185.6 ± 20.1 | 384.0 ± 51.3 | 53.0 ± 6.3 |
| 3j | CH3 | 1 | H | 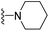 |
138.2 ± 30.1 | 305.4 ± 28.2 | 324.4 ± 27.5 | 385.4 ± 51.0 | 85.0 ± 9.8 |
| 3k | H | 1 | CH3 | 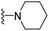 |
249.6 ± 37.5 | 394.5 ± 38.2 | 286.1 ± 19.3 | 403.7 ± 87.8 | 103.2 ± 24.6 |
| 3l | F | 1 | H | 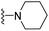 |
197.4 ± 15.5 | 285.4 ± 15.7 | 175.4 ± 25.3 | 389.5 ± 63.2 | 88.4 ± 10.1 |
| 3m | H | 1 | F | 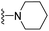 |
110.2 ± 8.9 | 213.2 ± 24.3 | 145.9 ± 11.5 | 364.5 ± 55.6 | 81.5 ± 6.5 |
| 3n | Cl | 1 | H | 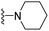 |
125.2 ± 10.2 | 253.1 ± 23.1 | 152.71 ± 15.6 | 375.4 ± 42.2 | 89.4 ± 10.6 |
| 3o | H | 1 | Cl | 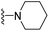 |
155.3 ± 13.0 | 200.4 ± 22.1 | 176.5 ± 14.5 | 395.7 ± 48.8 | 95.7 ± 8.2 |
| 3p | OCH3 | 1 | H | 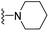 |
144.2 ± 15.0 | 228.5 ± 25.8 | 197.6 ± 16.9 | 415.5 ± 81.3 | 49.3 ± 36.5 |
| 3q | H | 1 | H | 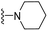 |
138.2 ± 20.7 | 210.3 ± 31.6 | 205.5 ± 24.7 | 438.3 ± 58.1 | 47.2 ± 6.4 |
| 3r | CH3 | 1 | H | 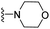 |
90.2 ± 12.0 | 14.6 ± 2.0 | 12.4 ± 1.3 | 295.9 ± 42.6 | 56.2 ± 5.1 |
| 3s | F | 1 | H | 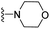 |
78.5 ± 16.5 | 14.8 ± 28.6 | 11.5 ± 1.5 | 304.0 ± 38.6 | 61.0 ± 5.8 |
| 3t | H | 1 | F | 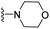 |
24.6 ± 8.7 | 11.7 ± 1.9 | 12.0 ± 2.4 | 322.2 ± 53.9 | 54.2 ± 7.3 |
| 3u | H | 1 | Cl | 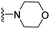 |
85.6 ± 9.6 | 145.9 ± 15.3 | 156.7 ± 19.1 | 356.3 ± 67.2 | 43.1 ± 3.5 |
| 3v | OCH3 | 1 | H | 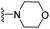 |
73.0 ± 6.4 | 128.4 ± 12.6 | 118.4 ± 10.4 | 341.7 ± 64.5 | 48.2 ± 4.2 |
| 3w | H | 1 | H | 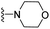 |
17.5 ± 1.9 | 16.7 ± 1.8 | 5.6 ± 6.5 | 336.4 ± 65.3 | 12.1 ± 1.5 |
| 3x | H | 1 | H | 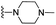 |
86.2 ± 15.0 | 109.2 ± 12.4 | 119.5 ± 15.4 | 313.6 ± 61.0 | 39.4 ± 26.0 |
| 3y | H | 1 | H | 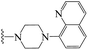 |
30.8 ± 5.6 | 58.5 ± 4.2 | 83.4 ± 9.7 | 33.1 ± 5.8 | 753.1 ± 98.5 |
The effects of N-heterocycles (aralkyl pyrrolidine, piperidine, morpholine and piperazine fragments) and 8-(piperazin-1-yl)quinoline connected to a benzene ring were assessed, and the results suggest that the N-heterocycles play a significant role in the SARs. According to the results, compounds (3a–3h) with pyrrolidineyl disubstitution show higher affinity for the D2 receptor than compounds with piperidyl substitution (3j–3q), but show lower affinity for the D2 receptor than compounds with morpholinyl, piperazinyl and 8-(piperazin-1-yl)quinolineyl substitution. Similar results were also observed for the 5-HT1A receptor. Except for the compounds with piperazinyl (3x) and 8-(piperazin-1-yl)quinolineyl substitution (3y), most of the compounds show moderate to low affinity for the 5-HT2A receptor. Compounds 3a–3x show moderate to high affinity for the H3 receptor, but only 3y exhibits high affinity for the 5-HT6 receptor, which may be due to the introduction of the privileged structures in the H3 and 5-HT6 receptor antagonists.
In the present study, the effects that different electron-withdrawing group of the substituents of the benzene ring, such as –F, –Cl, –CH3, and –OCH3 groups, have on the various receptors were also evaluated. When electron-donating (–CH3) and electron-withdrawing (–F, –Cl, –OCH3) groups were introduced at different positions of the benzene ring, of the compounds 3a–3i that bear N-methyl pyrrolidinyl substituents, the substituted compounds 3a–3f display decreased affinities for three receptors (D2, 5-HT1A and 5-HT2A) compared with 3i, with only the –OCH3-substituted derivative 3g showing increased affinity for the 5-HT1A receptor. However, the presence of different substituents on the benzene ring does not have any obvious impact on the affinity for the 5-HT6 and H3 receptors. In terms of the compounds bearing N-methyl piperidyl substituents (3j–3q), meta and ortho positioned –CH3 substituted 3j and 3k exhibit diminished affinities for the 5-HT1A and 5-HT2A receptors. However, when –F, –Cl, –CH3, and –OCH3 groups are introduced to the N-methyl morpholinyl substituted derivatives, the formed compounds 3r–3v show a decline in their affinities for the D2 receptor. Compared to compound 3w, –F and –CH3-substituted 3r–3t show improved affinities for 5-HT1A, but a negative effect for 5-HT2A receptors. Disappointingly, –Cl and –OCH3 group substituted 3u and 3v exhibit a dramatic decline in affinities for the D2, 5-HT1A and 5-HT2A receptors, and compounds with –F, –Cl, –CH3, and –OCH3 substituents (3s–3v) show slight differences in affinity for the 5-HT6 receptor and less H3 affinity.
Based on the literature and our previous experience, the space between benzothiophenylpiperazine and the phenoxy structure features three carbons. On replacement of the chain lengths of 3g from three carbons to two carbons, the generated compound 3h displays diminished affinities for the 5-HT1A, 5-HT2A, 5-HT6 and H3 receptors, but slightly enhanced affinity for D2 receptor.
In order to obtain compounds that show balance and high affinity, as well as high selectivity for further biological evaluation, herein, we set up three primary selection filter conditions: (a) high potency for the D2, 5-HT1A and 5-HT2A receptors (D2, Ki ≤ 30 nM; 5-HT1A, Ki ≤ 20 nM; and 5-HT2A, Ki ≤ 20 nM); (b) an affinity for H3 ≤ 15 nM and 5-HT6 ≤ 350 nM (c) with balanced activities at the D2, 5-HT1A and 5-HT2A receptors, with a potency ratio for any two receptors of ≤2. As a result, compounds 3t and 3w were selected for further biological evaluation, including safety and behavioral studies.
2.4. hERG channel blockade
Cardiotoxicity is a serious side effect that usually occurs due to the interactions between drugs and various voltage-gated ion channels in the heart, particularly the hERG channel. The hERG potassium channel mediates the delayed rectified potassium current and drugs that inhibit the hERG channel may cause prolonged QT intervals34 and increase the occurrence of potentially lethal Torsades de Pointes arrhythmia.35 Thus, the inhibition of hERG is an effective and widely used indicator for the prediction of the cardiotoxicity of candidate drugs. To predict the cardiotoxicity of compounds 3t and 3w, their inhibitory actions on the hERG channel were evaluated in a patch-clamp assay in vitro. Compound 3w (IC50 = 1765 nM) displays a lower hERG channel inhibition than compound 3t (IC50 = 1158 nM) (Table 2), which suggests that compound 3w has a low propensity to elicit treatment-induced QT interval prolongation than 3t and risperidone (IC50 = 1480 nM).To sum up, compound 3w displays excellent in vitro profiles with favorable affinity for the desired target receptors (D2, 5-HT1A, 5-HT2A, H3 and 5-HT6) and lower affinity for the off-target receptors (5-HT2C, H1 and α1) and hERG channel. Therefore, compound 3w was subjected to intrinsic activity, safety assessment and animal behavioral studies to verify its effect on schizophrenia.
2.5. Intrinsic activity
Compound 3w was chosen for further functional characterization because of its excellent in vitro activity and favorable safety profile. The results (ESI† Table 1) show that compound 3w displays feeble agonist activity against the D2, 5-HT1A, 5-HT2A, 5-HT6 and H3 receptors. In the antagonist assays, compound 3w shows potent antagonism of the five receptors, higher than 85% efficiency. Compound 3w shows potent D2 (IC50 = 11.6 nM) antagonism, and moderate 5-HT1A (IC50 = 218.5 nM), 5-HT2A (IC50 = 141.8 nM), 5-HT6 (IC50 = 413.5 nM) and H3 (IC50 = 232.3 nM) antagonism.2.6. Acute toxicity
The acute toxicities of 3w were assessed based on lethal dose, 50% (LD50) analyses (Table 3). The LD50 value of 3w is over 1000 mg kg−1, whereas those of haloperidol and risperidone are 21.0 and 82.0 mg kg−1, respectively. Thus, 3w has a better safety profile than haloperidol and risperidone.| Cmpd | LD50 | APOa | MK-801b | CATc | CARd | CAT/APO | CAT/MK-801 |
|---|---|---|---|---|---|---|---|
| a APO: apomorphine-induced climbing (ED50, mg kg−1, po (per os)).b MK-801: MK-801-induced hyperactivity (ED50, mg kg−1, po).c CAT: catalepsy (ED50, mg kg−1, po).d CAR: conditioned avoidance response (ED50, mg kg−1, po). | |||||||
| 3w | >1000 | 0.28 | 0.17 | 27.5 | 2.1 | 91.07 | 161.76 |
| Haloperidol | 21.0 | 0.09 | 0.11 | 0.12 | — | 5.67 | 4.63 |
| Risperidone | 82.0 | 0.05 | 0.02 | 0.51 | 0.65 | 10.4 | 32.5 |
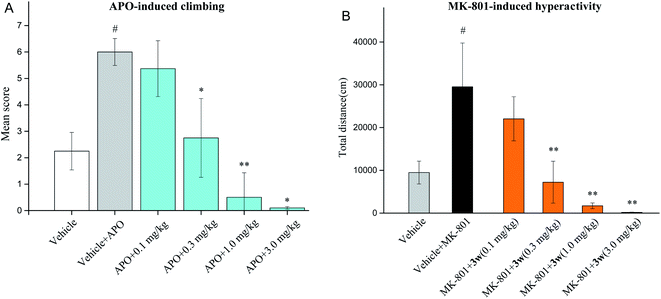
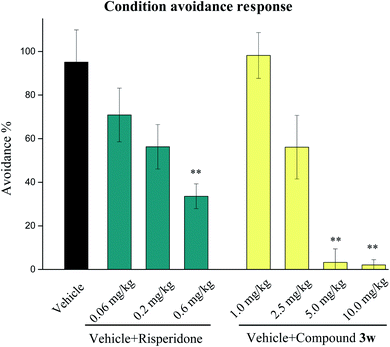
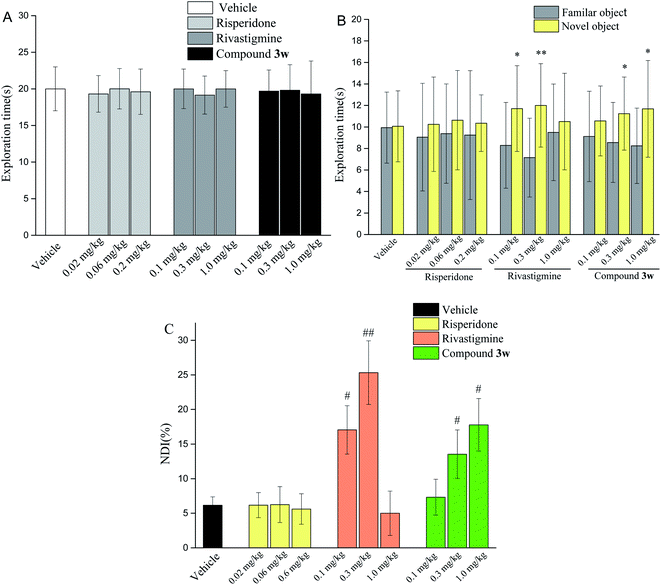
2.7. Evaluations of antipsychotic drug-like activities in animal models
Based on in vivo and acute oral toxicity evaluation, compound 3w was chosen as a candidate for in vivo behavioral study and long-term side effects evaluation to verify its antipsychotic-like activities.Taken together, compound 3w exhibits good anti-schizophrenic activity in terms of the above-mentioned behavioral testing, especially in terms of memory enhancement in the NOR testing, which is probably due to the addition of H3 receptor antagonism to its mixed dopamine and 5-hydroxytryptamine receptor antagonist profile.
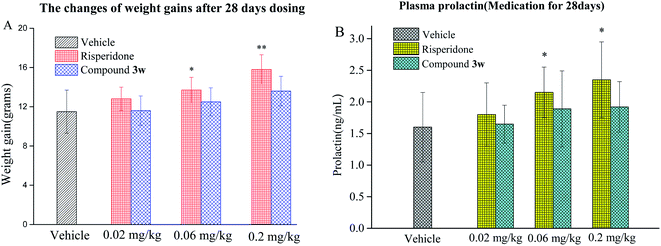
2.8. Weight gain and serum prolactin levels
To determine whether compound 3w has adverse long-term metabolic adverse, it was assessed in terms of its ability to induce weight gain and hyperprolactinemia in an animal model of chronic administration. After 28 days, the mice given compound 3w exhibit negligible weight gain, while the mice given risperidone show significant weight gain (Fig. 6A and B). Besides this, risperidone induces a significant increase in serum prolactin levels, but compound 3w does not, which means that 3w shows a lower incidence of treatment-related side effects than the representative atypical antischizophrenic drug risperidone, which might result from its desirable selectivity profile against the 5-HT2C and H1 receptors that are related to the adverse effects of marketed antipsychotics.3. Conclusions
We are engaged in the synthesis and biological evaluation of new arylpiperazine derivatives that have facilitated the discovery of compound 3w with a favorable antipsychotic profile, combining affinities for the D2, 5-HT1A, 5-HT2A 5-HT6 and H3 receptors. Besides this, compound 3w also exhibits low affinities for the 5-HT2C, H1 and α1 receptors and hERG inhibitory activity. The multi-receptor antagonist profile of 3w means that it has a significant effect in the inhibition of schizophrenia-like symptoms, including reversing APO-induced hyperlocomotion and MK-801-induced hyperactivity in mice and restraining the CAR in rats, and has a high threshold in terms of acute toxicity and a lower tendency to induce catalepsy. Moreover, compared to the representative conventional antipsychotic risperidone and the memory-enhancing rivastigmine, compound 3w demonstrates superior effectiveness in cognitive enhancement in NOR tests. Taken together, compound 3w is similar to the compounds A and B that were reported previously by our research group, and is in alignment with our original expectations. Thus, compound 3w was selected for the treatment of schizophrenia and deserves further development.Funding
We are grateful for support from the National Natural Science Foundation of China (No. 81602971).Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We are grateful for support from Jiangsu Nhwa Pharmaceutical Co., Ltd. and the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.References
- R. S. Kahn, I. E. Sommer, R. M. Murray, A. Meyer-Lindenberg, D. R. Weinberger, T. D. Cannon, M. O'Donovan, C. U. Correll, J. M. Kane, J. van Os and T. R. Insel, Schizophrenia, Nat. Rev. Dis. Primers, 2015, 1, 15067 CrossRef PubMed.
- J. R. Dequardo and R. Tandon, Review Do atypical antipsychotic medications favorably alter the long-term course of schizophrenia?, J. Psychiatr. Res., 1998, 32, 229–242 CrossRef CAS PubMed.
- G. W. Arana, An overview of side effects caused by typical antipsychotics, J. Clin. Psychiatry, 2000, 61(suppl. 8), 5–11 CAS.
- A. Serretti, D. De Ronchi, C. Lorenzi and D. Berardi, New antipsychotics and schizophrenia: a review on efficacy and side effects, Curr. Med. Chem., 2004, 11, 343–358 CrossRef CAS PubMed.
- S. O. Ogren, The role of 5-HT1A receptors in learning and memory, Behav. Brain Res., 2008, 195, 54–77 CrossRef PubMed.
- M. J. Millan, Improving the treatment of schizophrenia: focus on serotonin (5HT)(1A) receptors, J. Pharmacol. Exp. Ther., 2001, 295, 853–861 Search PubMed.
- C. J. Schmidt, S. M. Sorensen, J. H. Kehne, A. A. Carr and M. G. Palfreyman, The role of 5-HT2A receptors in antipsychotic activity, Life Sci., 1995, 56, 2209–2222 CrossRef CAS PubMed.
- G. Zhang and R. W. Stackman, The role of serotonin 5-HT2A receptors in memory and cognition, Front. Pharmacol., 2015, 6, 225 Search PubMed.
- H. Y. Meltzer and B. W. Massey, The role of serotonin receptors in the action of atypical antipsychotic drugs, Curr. Opin. Pharmacol., 2011, 11, 59–67 CrossRef CAS PubMed.
- S. Miyamoto, G. E. Duncan, C. E. Marx and J. A. Lieberman, Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs, Mol. Psychiatry, 2005, 10, 79–104 CrossRef CAS PubMed.
- M. C. Mauri, S. Paletta, M. Maffini, A. Colasanti, F. Dragogna, C. D. Pace and A. Altamura, Clinical pharmacology of atypical antipsychotics: an update, Clin. Pharmacokinet., 2014, 13, 1163–1191 CAS.
- A. B. Casey and C. E. Canal, Classics in chemical neuroscience: aripiprazole, ACS Chem. Neurosci., 2017, 8, 1135–1146 CrossRef CAS PubMed.
- J. E. Frampton, Brexpiprazole: a review in schizophrenia, Drugs, 2019, 79, 189–200 CrossRef CAS PubMed.
- S. Caccia, R. W. Invernizzi, A. Nobili and L. Pasina, A new generation of antipsychotics: pharmacology and clinical utility of cariprazine in schizophrenia, Ther. Clin. Risk Manage., 2013, 9, 319–328 CrossRef CAS PubMed.
- A. Yee, Brexpiprazole for the treatment of schizophrenia, Expert Rev. Neurother., 2016, 16, 109–122 CrossRef CAS PubMed.
- D. Mamo, A. Graff, R. Mizrahi, C. M. Shammi and S. Kapur, Differential effects of aripiprazole on D2, 5-HT2, and 5-HT1Areceptor occupancy in patients with schizophrenia: a triple tracer PET study, Am. J. Psychiatry, 2007, 164, 1411–1417 CrossRef PubMed.
- S. Natesan, G. E. Reckless, J. N. Nobrega, P. J. Fletcher and S. Kapur, Dissociation between in vivo occupancy and functional antagonism of dopamine D2 receptors: comparing aripiprazole to other antipsychotics in animal models, Neuropsychopharmacol, 2006, 31(9), 1854–1863 CrossRef CAS PubMed.
- R. Mailman and V. Murthy, Third generation antipsychotic drugs: partial agonism or receptor functional selectivity?, Curr. Pharm. Des., 2010, 16, 488–501 CrossRef CAS PubMed.
- R. H. Campbell, M. Diduch, K. N. Gardner and C. Thomas, Review of cariprazine in management of psychiatric illness, Ment. Health Clin., 2018, 7, 221–229 CrossRef PubMed.
- M. Kondej, P. Stępnicki and A. A. Kaczor, Multi-target approach for drug discovery against schizophrenia, Int. J. Mol. Sci., 2018, 19, 3105 CrossRef PubMed.
- Y. Chen, S. Wang, X. Xu, X. Liu, M. Yu, S. Zhao, S. Liu, Y. Qiu, T. Zhang, B. F. Liu and G. Zhang, Synthesis and biological investigation of coumarin piperazine (piperidine) derivatives as potential multireceptor atypical antipsychotics, J. Med. Chem., 2013, 56, 4671–4690 CrossRef CAS PubMed.
- Y. Chen, Y. Lan, S. Wang, H. Zhang, X. Xu, X. Liu, M. Yu, B. F. Liu and G. Zhang, Synthesis and evaluation of new coumarin derivatives as potential atypical antipsychotics, Eur. J. Med. Chem., 2014, 74, 427–439 CrossRef CAS PubMed.
- J. Jin, K. Zhang, F. Dou, C. Hao, Y. Zhang, X. Cao, L. Gao, J. Xiong, X. Liu, B. F. Liu, G. Zhang and Y. Chen, Isoquinolinone derivatives as potent CNS multi-receptor D2/5-HT1A/5-HT2A/5-HT6/5-HT7 agents: synthesis and pharmacological evaluation, Eur. J. Med. Chem., 2020, 112709 CrossRef CAS PubMed.
- X. Cao, Y. Zhang, Y. Chen, Y. Qiu, M. Yu, X. Xu, X. Liu, B. F. Liu and G. Zhang, Synthesis and biological evaluation of fused tricyclic heterocycle piperazine (piperidine) derivatives as potential multireceptor atypical antipsychotics, J. Med. Chem., 2018, 61, 10017–10039 CrossRef CAS PubMed.
- J. C. Schwartz, The histamine H3 receptor: from discovery to clinical trials with pitolisant, Br. J. Pharmacol., 2011, 163, 713–721 CrossRef CAS PubMed.
- N. Upton, T. T. Chuang, A. J. Hunter and D. J. Virley, 5-HT6 receptor antagonists as novel cognitive enhancing agents for Alzheimer's disease, Neurotherapeutics, 2008, 5, 458–469 CrossRef CAS PubMed.
- M. A. Letavic, L. Aluisio, R. Apodaca, M. Bajpai, A. J. Barbier, A. Bonneville, P. Bonaventure, N. I. Carruthers, C. Dugovic, I. C. Fraser, M. L. Kramer, B. Lord, T. W. Lovenberg, L. Y. Li, K. S. Ly, H. Mcallister, N. S. Mani, K. L. Morton, A. Ndifor, S. D. Nepomuceno, C. R. Pandit, S. B. Sands, C. R. Shah, J. E. Shelton, S. S. Snook, D. M. Swanson and W. Xiao, Novel benzamide-based histamine H3 receptor antagonists: the identification of two candidates for clinical development, ACS Med. Chem. Lett., 2015, 6, 450–454 CrossRef CAS PubMed.
- M. D. Wood, C. Heidbreder, C. Reavill, C. R. Ashby Jr and D. N. Middlemiss, 5-HT2C receptor antagonists: potential in schizophrenia, Drug Dev. Res., 2001, 54, 88–94 CrossRef CAS.
- M. He, C. Deng and X. F. Huang, The role of hypothalamic H1 receptor antagonism in antipsychotic-induced weight gain, CNS Drugs, 2013, 27(6), 423–434 CrossRef CAS PubMed.
- P. A. Gwirtz and H. L. Stone, Coronary blood flow and myocardial oxygen consumption after alpha adrenergic blockade during submaximal exercise, J. Pharmacol. Exp. Ther., 1981, 217, 92–98 CAS.
- S. C. Chang and M. L. Lu, Metabolic and Cardiovascular Adverse Effects Associated with Treatment with Antipsychotic Drugs, J. Exp. Clin. Med., 2012, 4, 103–107 CrossRef CAS.
- H. A. Nasrallah, Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles, Mol. Psychiatry, 2008, 13, 27–35 CrossRef CAS PubMed.
- J. Leung, A. M. Barr, R. M. Procyshyn, W. G. Honer and C. Pang, Cardiovascular side-effects of antipsychotic drugs: the role of the autonomic nervous system, Pharmacol. Ther., 2012, 135, 113–122 CrossRef CAS PubMed.
- M. Recanatini, E. Poluzzi, M. Masetti, A. Cavalli and F. De Ponti, QT prolongation through hERG K+ channel blockade: current knowledge and strategies for the early prediction during drug development, Med. Res. Rev., 2005, 25, 133–166 CrossRef CAS PubMed.
- E. Raschi, L. Ceccarini, F. D. Ponti and M. Recanatini, hERG-related drug toxicity and models for predicting hERG liability and QT prolongation, Expert Opin. Drug Metab. Toxicol., 2009, 5, 1005–1021 CrossRef CAS PubMed.
- C. A. Jones, D. Watson and K. Fone, Animal models of schizophrenia, Br. J. Pharmacol., 2011, 164, 1162–1194 CrossRef CAS PubMed.
- E. W. Tuplin, M. R. Stocco and M. R. Holahan, Attenuation of MK-801-induced behavioral perseveration by typical and atypical antipsychotic pretreatment in rats, Behav. Neurosci., 2015, 129, 399–411 CAS.
- D. A. Sykes, H. Moore, L. Stott, N. Holliday, J. A. Javitch, J. R. Lane and S. J. Charlton, Extrapyramidal side effects of antipsychotics are linked to their association kinetics at dopamine D2 receptors, Nat. Commun., 2017, 8, 763 CrossRef PubMed.
- M.-L. Wadenberg, Conditioned avoidance response in the development of new antipsychotics, Curr. Pharm. Des., 2010, 16, 358–370 CrossRef CAS PubMed.
- M. Feng, J. Gao, N. Sui and M. Li, Effects of central activation of serotonin 5-HT2A/2C or dopamine D2/3 receptors on the acute and repeated effects of clozapine in the conditioned avoidance response test, Psychopharmacology, 2015, 232, 1219–1230 CrossRef CAS PubMed.
- L. Rajagopal, B. W. Massey, M. Huang, Y. Oyamada and H. Y. Meltzer, The Novel Object Recognition Test in Rodents in Relation to Cognitive Impairment in Schizophrenia, Curr. Pharm. Des., 2014, 20(31), 5104–5114 CrossRef CAS PubMed.
- M. Horiguchi, K. E. Hannaway, A. E. Adelekun, M. Huang, K. Jayathilake and H. Y. Meltzer, D-1 receptor agonists reverse the subchronic phencyclidine (PCP)-induced novel object recognition (NOR) deficit in female rats, Behav. Brain Res., 2013, 238, 36–43 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Receptor binding studies; hERG affinity; intrinsic activity; acute toxicity; behavioral studies; 1H NMR, 13C NMR and HR-MS of 3w. See DOI: 10.1039/d1ra02426d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |