Open Access Article
Open Access ArticleTCCA-mediated oxidative rearrangement of tetrahydro-β-carbolines: facile access to spirooxindoles and the total synthesis of (±)-coerulescine and (±)-horsfiline†
Manda Sathish‡
ad,
Akash P. Sakla‡b,
Fabiane M. Nachtigallc,
Leonardo S. Santos *d and
Nagula Shankaraiah
*d and
Nagula Shankaraiah *b
*b
aInstituto de Investigación Interdisciplinaria, Vicerrectoría Académica, Universidad de Talca, Campus Lircay, Talca 3460000, Chile
bDepartment of Medicinal Chemistry, National Institute of Pharmaceutical Education and Research (NIPER), Hyderabad 500 037, India. E-mail: shankar@niperhyd.ac.in
cInstituto de Ciencias Químicas Aplicadas, Universidad Autónoma de Chile, Sede Talca 3467987, Chile
dLaboratory of Asymmetric Synthesis, Instituto de Química de Recursos Naturales, Universidad de Talca, Campus Lircay, Talca 3460000, Chile. E-mail: lssantos@utalca.cl
First published on 5th May 2021
Abstract
Multi-reactive centered reagents are beneficial in chemical synthesis due to their advantage of minimal material utilization and formation of less by-products. Trichloroisocyanuric acid (TCCA), a reagent with three reactive centers, was employed in the synthesis of spirooxindoles through the oxidative rearrangement of various N-protected tetrahydro-β-carbolines. In this protocol, low equivalents of TCCA were required to access spirooxindoles (up to 99% yield) with a wide substrate scope. Furthermore, the applicability and robustness of this protocol were proven for the gram-scale total synthesis of natural alkaloids such as (±)-coerulescine (1) and (±)-horsfiline (2) in excellent yields.
Introduction
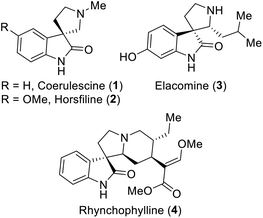
Spirooxindoles are exceptional and versatile scaffolds, and thus have been extensively studied in the fields of synthetic and pharmaceutical chemistry.1 The unique three-dimensional spiro system may be the main cause for the bioactivities of spirooxindoles.1,2 Especially, spirooxindoles have been considered to exhibit antitumor,3 antimicrobial,4 antioxidant,5 anti-inflammatory,6 antiviral1a and other bioactivities. Moreover, many of their derivatives have been extensively used in clinical trials,7 and some representative examples of bioactive spirooxindoles are depicted in Fig. 1. For example, horsfiline (2) is an intoxicating snuff,8 rhynchophylline (4) is potent towards various cancer cell lines,9 spirotryprostatin A prevents G2M progression in mammalian tsFT210 cells,10 and corynoxine and corynoxine B show potential for the treatment of Parkinson's disease.11 Due to all these pharmaceutical potencies of spirooxindole derivatives, chemists have been inspired to establish various synthetic routes for this class of compounds.Several approaches have been proposed in the literature to build spirooxindoles, which mainly involve two ways: (i) multistep synthesis1d,8,12 and (ii) oxidative rearrangement.13 However, oxidative rearrangement reaction is more beneficial than multistep synthesis, because it only involves a single step, avoids the use of various toxic reagents, and ultimately time saving. A few examples of oxidants have been reported previously to afford spirooxindoles, which involve the use of mild organic oxidants such as oxone (Fig. 2),13f N-bromosuccinimide (NBS),13c t-BuOCl13a,13d and transition metal oxidants such as Pb(OAc)4,13a CrO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13f and OsO4.13b However, the latter oxidants are more likely to produce highly toxic by-products. In continuation of our efforts in the development of new synthetic strategies,14 we established a simple method employing the safer multi-reactive centered reagent (MRCR) trichloroisocyanuric acid (TCCA), a highly desirable catalyst that requires less equivalents, thereby reducing the by-products.
13f and OsO4.13b However, the latter oxidants are more likely to produce highly toxic by-products. In continuation of our efforts in the development of new synthetic strategies,14 we established a simple method employing the safer multi-reactive centered reagent (MRCR) trichloroisocyanuric acid (TCCA), a highly desirable catalyst that requires less equivalents, thereby reducing the by-products.
Particularly, TCCA is inexpensive and produces essentially the nontoxic cyanuric acid as a by-product, which can be easily separated from the reaction mixture.15 TCCA is a versatile reagent with three reactive N–Cl centers, which helps to utilize only 0.33–0.5 equivalents in the reaction. In scaled-up reactions, it is also possible to re-generate TCCA by passing chlorine gas in the aqueous mixture of cyanuric acid.16 Moreover, it is widely used in several applications such as chlorination and mild oxidation.17 Studer and co-workers18 utilized TCCA to obtain α-chloro aldehydes and α-chloro ketones from their corresponding 1° and 2° alcohols. Bathini and co-workers also reported the TCCA-mediated decarboxylative/dehydrogenative aromatization of tetrahydro-β-carbolines (THBCs), which was applied for the total synthesis of β-carboline alkaloids.19 Veisi and co-workers developed a method to produce nitriles through the direct oxidation of the corresponding amines, alcohols, aldehydes, and benzyl halides using TCCA.20 Thus, based on these significant findings such as bioactivity and research interest on the synthesis of spirooxindoles, we developed an efficient protocol to construct spirooxindole via oxidative rearrangement using TCCA, which is a cost effective and versatile reagent.
Results and discussion
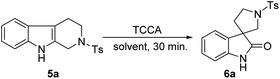
All the key substrates 5a–z were synthesized according to known reports.21–23 Initially, we performed a model reaction with tetrahydro-β-carboline (5a) and TCCA (1 equiv.) in an acidic solvent mixture of THF/water/AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 4 h at 0 °C. The formation of spirooxindole 6a was observed with 45% yield (entry 1, Table 1). Next, we considered to avoid the use of acidic solvents and executed another reaction without AcOH, which afforded spirooxindole 6a with slightly improved yield (58%, entry 2, Table 1). We realized that the acidic nature of AcOH and HCl liberated from excess equivalents of TCCA may affect the yield of 6a. Then, after reducing the TCCA equivalents to 0.33 at 0 °C for 2 h (entry 3, Table 1), we achieved 88% of 6a. However, a slight improvement in the yield (90%) was seen after minimizing the reaction time to 30 min at room temperature (entry 4, Table 1). Considering our enthusiasm, another trial with similar reaction conditions was performed by taking 0.35 equiv. of TCCA, and surprisingly 6a was isolated with excellent yield (99%, entry 5, Table 1). Hence, we established that 0.35 equiv. of TCCA is optimal for this oxidative rearrangement reaction. Further investigation using other immiscible solvent mixtures such as DCE/water, DCM/water, and EtOAc/water achieved poor isolated yields of 6a (entries 6, 7 and 8, respectively, Table 1), thus showing the incompatibility of immiscible solvent mixtures for this reaction. However, considerable yields of 6a were identified in the case of miscible solvent mixtures such as MeOH/water (82%) and MeCN/water (90%) (entries 9 and 10, respectively, Table 1).
1) for 4 h at 0 °C. The formation of spirooxindole 6a was observed with 45% yield (entry 1, Table 1). Next, we considered to avoid the use of acidic solvents and executed another reaction without AcOH, which afforded spirooxindole 6a with slightly improved yield (58%, entry 2, Table 1). We realized that the acidic nature of AcOH and HCl liberated from excess equivalents of TCCA may affect the yield of 6a. Then, after reducing the TCCA equivalents to 0.33 at 0 °C for 2 h (entry 3, Table 1), we achieved 88% of 6a. However, a slight improvement in the yield (90%) was seen after minimizing the reaction time to 30 min at room temperature (entry 4, Table 1). Considering our enthusiasm, another trial with similar reaction conditions was performed by taking 0.35 equiv. of TCCA, and surprisingly 6a was isolated with excellent yield (99%, entry 5, Table 1). Hence, we established that 0.35 equiv. of TCCA is optimal for this oxidative rearrangement reaction. Further investigation using other immiscible solvent mixtures such as DCE/water, DCM/water, and EtOAc/water achieved poor isolated yields of 6a (entries 6, 7 and 8, respectively, Table 1), thus showing the incompatibility of immiscible solvent mixtures for this reaction. However, considerable yields of 6a were identified in the case of miscible solvent mixtures such as MeOH/water (82%) and MeCN/water (90%) (entries 9 and 10, respectively, Table 1).
| Entry | TCCA (equiv.) | Solvent | Temp. (°C) | Yield (%) |
|---|---|---|---|---|
| a All reactions were performed using tetrahydro-β-carboline 5a (1 equiv.) and TCCA for 30 min.b The reaction was stirred for 2 h.c The remaining starting material was recovered. | ||||
| 1 | 1 | THF/water/AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0 | 45b |
| 2 | 1 | THF/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0 | 58b |
| 3 | 0.33 | THF/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0 | 88b |
| 4 | 0.33 | THF/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
rt | 90 |
| 5 | 0.35 | THF/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
rt | 99 |
| 6 | 0.35 | DCE/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
rt | 50 |
| 7 | 0.35 | DCM/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
rt | 52 |
| 8 | 0.35 | EtOAc/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
rt | 55 |
| 9 | 0.35 | MeOH/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
rt | 82 |
| 10 | 0.35 | MeCN/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
rt | 90 |
| 11 | 0.35 | Water | rt | 40c |
| 12 | 0.35 | THF | rt | — |
Moreover, two reactions were tested in pure THF and distilled water as solvents, but only 40% of 6a was isolated in the case of water (entry 11, Table 1) and no product (6a) was observed in THF (entry 12, Table 1). Based on all these observations, the optimized protocol for the oxidative rearrangement of tetrahydro-β-carbolines to spirooxindoles is 0.35 equiv. of TCCA and THF/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent at room temperature for 30 min. During the reaction optimization, we identified that a homogenous reaction mixture is required before adding TCCA to achieve the complete conversion of the substrate. Accordingly, it is recommended to use 2
1) as the solvent at room temperature for 30 min. During the reaction optimization, we identified that a homogenous reaction mixture is required before adding TCCA to achieve the complete conversion of the substrate. Accordingly, it is recommended to use 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of THF/water if precipitation of tetrahydro-β-carbolines occurs in a 1
1 of THF/water if precipitation of tetrahydro-β-carbolines occurs in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of THF/water to ensure a homogeneous reaction mixture is obtained.
1 mixture of THF/water to ensure a homogeneous reaction mixture is obtained.
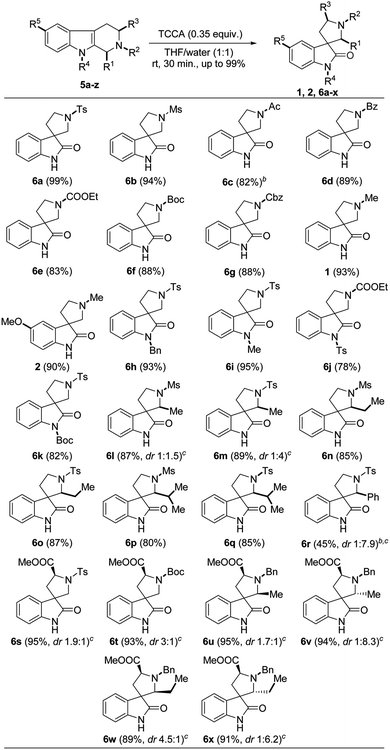
Having the optimized protocol in hand, we explored a sequential study on different substituted/protected THBCs 5a–z, which delivered the corresponding spirooxindoles 6a–x, 1 and 2 in good to excellent yields (78–99%). Totally, the protocol was executed on five types of THBC substrates based on their substitution/protection pattern as follows: (i) simple THBCs with N2-protection (5a–i); (ii) THBCs with N2-protection and N9-protection (5j–m); (iii) THBCs with C1-substitution and N2-protection (5n–t); (iv) THBCs with N2-protection and C3-substitutions (5u and 5v); and (v) THBCs with C1-substitution, N2-protection and C3-substitutions (5w–z).
The sulfonyl groups (R2 = tosyl and mesyl) on N2 of THBCs (5a and 5b) smoothly underwent oxidative rearrangement to produce the corresponding spirooxindoles 6a and 6b with excellent yields (99% and 94%, Table 2), respectively. However, other electron-withdrawing groups (EWG) such as acetyl, benzoyl, CO2Et, Boc and Cbz at N2-position of simple THBCs (5c–g) were only slightly affected to give lower yields (82%, 81%, 83%, 88% and 88%) of the corresponding spirooxindoles (6c–g, Table 2), respectively, which may be due to the acid sensitivity of the amide and carbamate functionalities. Furthermore, the reaction of 5c with TCCA was performed at 0 °C, and a low yield (50%) was observed at room temperature, affording de-protected 5c. However, an electron-donating group (EDG) such as methyl on N2 of THBC (5h and 5i) gave good yields of natural spirooxindoles (±)-coerulescine (1, 93%) and (±)-horsfiline (2, 90%), respectively, as depicted in Table 2.
Further, our attempts on the oxidative rearrangement of THBCs 5k–m with indole nitrogen protection succeeded and achieved the corresponding spirooxindoles 6h–k in high yields, respectively. The EDGs (R4 = benzyl and methyl) at the indole nitrogen of THBCs delivered 6h and 6i with satisfactory yields (93% and 95%), respectively. Interestingly, our protocol also worked well on EWG (R4 = tosyl and Boc)-protected THBCs (5l and 5m) to provide spirooxindoles 6j (78%) and 6k (82%) without affecting the protecting groups (Table 2), respectively. A recent report13f stated that the presence of EDGs such as hydrogen, alkyl and benzyl at the indolyl nitrogen (N–R4) is essential to achieve oxidative rearrangement. It is noticeable that our protocol is very advantageous given that it works well on both EDGs and EWGs on the indole nitrogen. Next, we explored our protocol on C1 alkyl (R1 = methyl, ethyl and isopropyl)- and phenyl-substituted THBCs 5n–t, but only alkyl-substituted spirooxindoles 6l–q were obtained in appreciable yields (80–89%, Table 2). A low yield was observed for 6r (27%) at room temperature from 1-phenyl substituted THBC (5t), and interestingly another ring-opened side product (6r′, 64%) was isolated and confirmed by comparing its NMR data (for NMR and plausible mechanism see ESI†) with the available literature.21 However, a slight improvement in yield (45%) was observed with good diastereoselectivity (6r, dr 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.9, Table 2) when the reaction was carried out at 0 °C and the yield of the ring-opened side product (6r′) decreased to 50%. The diastereomers of spirooxindoles 6l–q could not be separated by column chromatography and the ratio was determined by 1H NMR. Nevertheless, the THBCs 5u and 5v generated from L-tryptophan smoothly underwent oxidative rearrangement to produce spirooxindoles 6s and 6t in excellent yields and good diastereoselectivities (95%, dr 1.9
7.9, Table 2) when the reaction was carried out at 0 °C and the yield of the ring-opened side product (6r′) decreased to 50%. The diastereomers of spirooxindoles 6l–q could not be separated by column chromatography and the ratio was determined by 1H NMR. Nevertheless, the THBCs 5u and 5v generated from L-tryptophan smoothly underwent oxidative rearrangement to produce spirooxindoles 6s and 6t in excellent yields and good diastereoselectivities (95%, dr 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 93%, dr 3
1 and 93%, dr 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 respectively, Table 2). Finally, the diastereomers of 5w–z with C1 (R1 = methyl or ethyl) and C3 (R3 = CO2Me) substitutions were easily separated by column chromatography and employed for oxidative rearrangement to afford spirooxindole products 6u–x in good yields with prominent diastereomeric ratios (95%, dr 1.7
1 respectively, Table 2). Finally, the diastereomers of 5w–z with C1 (R1 = methyl or ethyl) and C3 (R3 = CO2Me) substitutions were easily separated by column chromatography and employed for oxidative rearrangement to afford spirooxindole products 6u–x in good yields with prominent diastereomeric ratios (95%, dr 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 94%, dr 1
1, 94%, dr 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.3, 89%, dr 4.5
8.3, 89%, dr 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 91%, dr 1
1 and 91%, dr 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.2, respectively) as shown in Table 2.
6.2, respectively) as shown in Table 2.
Further, we tested this protocol on C1-substituted (R1 = methyl and phenyl) and N2-unprotected (R2 = hydrogen) THBCs 7a and 7b and isolated dihydro-β-carbolines 8a and 8b, albeit with very poor yields (35% and 30%, respectively) and no oxidative rearrangement product was observed. This can be attributed to dehydrohalogenation (I and II, Scheme 1A) instead of chlorohydrin formation, oxidative rearrangement, and thereby stabilization of the dihydro-β-carbolines by conjugation from imine, as shown in Scheme 1A.
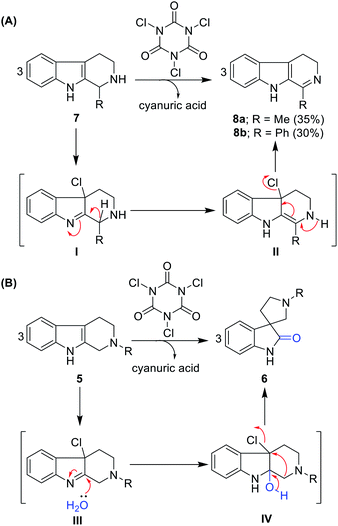 | ||
| Scheme 1 (A) Imine formation of N2 unprotected THBCs. (B) Plausible reaction mechanism for the oxidative rearrangement of N2-protected THBCs. | ||
Notably, in the reaction path of oxidative rearrangement, one mole of TCCA is sufficient to produce three moles of spirooxindoles. The reaction mechanism can be explained as initial TCCA chlorination of THBC to form Cl-THBC (III, Scheme 1B), formation of chlorohydrin (IV, Scheme 1B) and dehydrohalogenation followed by semi-pinacol-type rearrangement. The by-product cyanuric acid can be easily separated in the work-up given that it is soluble in water.
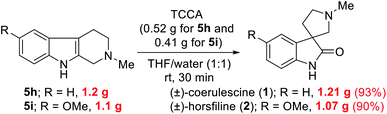
To highlight the utility of this protocol, we accomplished the gram-scale total synthesis of two bioactive spirooxindole natural products, (±)-coerulescine (1) and (±)-horsfiline (2) from 5h and 5i (Scheme 2), respectively. The gram-scale reaction of bioactive natural products is highly desirable from a commercial perspective. Specifically, 5h (1.2 g) and 5i (1.1 g) were reacted with TCCA to produce (±)-coerulescine (1) and (±)-horsfiline (2) in high yields (1.21 g, 93% and 1.07 g, 90%, respectively). It is important to highlight that the quantity of TCCA used for this gram-scale synthesis is very low (0.52 g for 5h and 0.41 g for 5i), whereas these reactions need large quantities of other earlier reported oxidants13 when performing gram-scale synthesis (>1 g).
Conclusions
In conclusion, we developed an operationally simple and high yield protocol for the synthesis of spirooxindoles from the corresponding tetrahydro-β-carbolines (THBCs) using the inexpensive TCCA. The reaction proceeds via oxidative rearrangement and produced spirooxindoles in good to excellent yields (up to 99%). Diversely substituted spirooxindoles and naturally occurring (±)-coerulescine and (±)-horsfiline were furnished in excellent yields using the optimized reaction conditions. To demonstrate the commercial importance of this protocol, we also carried out gram-scale reactions to produce (±)-coerulescine (1) and (±)-horsfiline (2) with yields of 93% and 90%, respectively. In addition, this synthetic strategy is amenable for the generation of a library spiro-compounds and their derivatives, which can be further utilized in the drug discovery process.Experimental
General
All solvents used for the reactions and purifications were of commercial grade or were purified prior to use when necessary. NMR spectral analyses were done on a Bruker 400 MHz or 500 MHz spectrometer for 1H and 100 MHz or 125 MHz spectrometer for 13C spectra, and calibrated to either TMS (δ = 0 for 1H) or residual DMSO (δ = 2.50 for 1H and δ = 39.51 for 13C) and residual CHCl3 (δ = 7.26 for 1H and δ = 77.23 for 13C). Spin multiplicities are described as s (singlet), br s (broad singlet), d (doublet), t (triplet), dd (double doublet), td (triple doublet), q (quartet), and m (multiplet) and the coupling constants are reported in hertz (Hz). TLC analyses for the reactions and chromatography purifications were performed using silica gel plates (0.25 mm, E. Merck, 60 F254) with iodine and a UV lamp for visualization. Mass spectral measurements were recorded via electrospray ionization mass spectrometry (ESI-MS). HRMS was performed on a Varian QFT-ESI instrument. Melting points were measured on an electrothermal melting point apparatus and are uncorrected.All the THBCs 5a,21a,22a 5b,21b 5c,21b 5d, 5e,21b 5f,21b 5g,21c 5h,13e 5i,13e 5j, 5k,21a 5l, 5m, 5n, 5o,22a 5p, 5q,21b 5r, 5s,22b 5t,23 5u,23a 5v,23b 5w,23c 5x,23c 5y (ref. 23d and e) and 5z (ref. 23d and e) were synthesized using known procedures in the literature from their corresponding starting materials such as tryptamine (1a), tryptophan methyl ester (1b) and 5-methoxy tryptamine (1c). For the preparation of 5a–i and 5n–z, initially we reacted 1 with the respective aldehydes to achieve Pictet-Spengler cyclized products (NH-THBCs). Later, N-protection of THBCs was performed with the corresponding protecting groups in the presence of bases such as triethylamine and N,N-diisopropylethylamine, as mentioned in the respective literature reports.13e,21-23
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane or MeOH
hexane or MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 solvent systems and the ninhydrin charring technique. After completion, the reaction was quenched with saturated NH4Cl solution and extracted with EtOAc and the combined organic phases were washed with water. The organic phase was evaporated in vacuo after drying over Na2SO4 to give the crude product, which was purified by silica gel column chromatography using 20–50% EtOAc/hexane to afford the pure products 5j, 5k and 5m. The same procedure was used for the synthesis of 5l from 5e using Boc2O as the protecting group.
CH2Cl2 solvent systems and the ninhydrin charring technique. After completion, the reaction was quenched with saturated NH4Cl solution and extracted with EtOAc and the combined organic phases were washed with water. The organic phase was evaporated in vacuo after drying over Na2SO4 to give the crude product, which was purified by silica gel column chromatography using 20–50% EtOAc/hexane to afford the pure products 5j, 5k and 5m. The same procedure was used for the synthesis of 5l from 5e using Boc2O as the protecting group.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), TCCA (0.35 equiv.) was added at room temperature in one batch. The resulting reaction mixture was stirred at room temperature for 30 min. After the reaction was completed, as monitored by TLC analysis (EtOAc
1), TCCA (0.35 equiv.) was added at room temperature in one batch. The resulting reaction mixture was stirred at room temperature for 30 min. After the reaction was completed, as monitored by TLC analysis (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane or MeOH
hexane or MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 solvent systems), it was quenched by addition of saturated NaHCO3 and extracted with EtOAc three times. The combined organic phases were washed with water, dried over Na2SO4, filtered, and evaporated under reduced pressure. The resulting crude product was purified by flash column chromatography (EtOAc/hexane = 1
CH2Cl2 solvent systems), it was quenched by addition of saturated NaHCO3 and extracted with EtOAc three times. The combined organic phases were washed with water, dried over Na2SO4, filtered, and evaporated under reduced pressure. The resulting crude product was purified by flash column chromatography (EtOAc/hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 1
10 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide the pure spirooxindole. Note: This reaction was performed at 0 °C in the case of THBCs 5c and 5t.
1) to provide the pure spirooxindole. Note: This reaction was performed at 0 °C in the case of THBCs 5c and 5t.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 rotamers CDCl3), mp: 75–78 °C; 1H NMR (500 MHz, CDCl3): δ 9.38 and 9.22 (s, 1H), 7.29–7.20 (m, 1H), 7.19–7.10 (s, 1H), 7.09–7.00 (s, 1H), 6.95 (ddd, J = 21.6, 14.1, 8.0 Hz, 1H), 4.05–3.95 (m, 1H), 3.93–3.80 (m, 2H), 3.68 (dd, J = 65.0, 15.0 Hz, 1H), 2.54–2.40 (m, 1H), 2.29–2.21 (m, 1H), 2.19 and 2.10 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 180.5, 179.4, 169.7, 169.5, 140.7, 140.4, 132.5, 131.7, 128.8, 128.7, 123.1, 122.9, 122.7, 110.4, 110.3, 55.5, 53.9, 53.6, 51.9, 46.7, 45.2, 36.5, 35.1, 22.6, 22.4; HRMS (ESI) calcd for C15H15N2O2 m/z 231.1128 [M + H]+, found 231.1108.
50 rotamers CDCl3), mp: 75–78 °C; 1H NMR (500 MHz, CDCl3): δ 9.38 and 9.22 (s, 1H), 7.29–7.20 (m, 1H), 7.19–7.10 (s, 1H), 7.09–7.00 (s, 1H), 6.95 (ddd, J = 21.6, 14.1, 8.0 Hz, 1H), 4.05–3.95 (m, 1H), 3.93–3.80 (m, 2H), 3.68 (dd, J = 65.0, 15.0 Hz, 1H), 2.54–2.40 (m, 1H), 2.29–2.21 (m, 1H), 2.19 and 2.10 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 180.5, 179.4, 169.7, 169.5, 140.7, 140.4, 132.5, 131.7, 128.8, 128.7, 123.1, 122.9, 122.7, 110.4, 110.3, 55.5, 53.9, 53.6, 51.9, 46.7, 45.2, 36.5, 35.1, 22.6, 22.4; HRMS (ESI) calcd for C15H15N2O2 m/z 231.1128 [M + H]+, found 231.1108.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 rotamers in CDCl3), mp: 83–85 °C; 1H NMR (500 MHz, CDCl3): δ 8.07 and 8.03 (s, 1H), 7.63 (d, J = 7.0 Hz, 1H), 7.52 (d, J = 7.5 Hz, 1H), 7.47–7.43 (m, 1H), 7.41–7.32 (m, 2H), 7.27–7.20 (s, 2H), 7.17–7.01 (m, 1H), 6.91 (dd, J = 22.2, 7.7 Hz, 1H), 4.25–4.02 (m, 2H), 3.99–3.56 (m, 2H), 2.66–2.31 (m, 1H), 2.27–2.10 (m, 1H); 13C NMR (125 MHz, CDCl3): δ 180.6, 178.8, 170.2, 170.1, 140.6, 140.1, 136.4, 132.4, 130.3, 128.8, 128.7, 128.5, 127.3, 123.2, 122.7, 110.3, 57.6, 54.2, 53.6, 51.9, 48.7, 45.6, 36.9, 35.0; HRMS (ESI) calcd for C18H17N2O2S2 m/z 293.1285 [M + H]+, found 293.1253.
38 rotamers in CDCl3), mp: 83–85 °C; 1H NMR (500 MHz, CDCl3): δ 8.07 and 8.03 (s, 1H), 7.63 (d, J = 7.0 Hz, 1H), 7.52 (d, J = 7.5 Hz, 1H), 7.47–7.43 (m, 1H), 7.41–7.32 (m, 2H), 7.27–7.20 (s, 2H), 7.17–7.01 (m, 1H), 6.91 (dd, J = 22.2, 7.7 Hz, 1H), 4.25–4.02 (m, 2H), 3.99–3.56 (m, 2H), 2.66–2.31 (m, 1H), 2.27–2.10 (m, 1H); 13C NMR (125 MHz, CDCl3): δ 180.6, 178.8, 170.2, 170.1, 140.6, 140.1, 136.4, 132.4, 130.3, 128.8, 128.7, 128.5, 127.3, 123.2, 122.7, 110.3, 57.6, 54.2, 53.6, 51.9, 48.7, 45.6, 36.9, 35.0; HRMS (ESI) calcd for C18H17N2O2S2 m/z 293.1285 [M + H]+, found 293.1253.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 rotamers in CDCl3); 1H NMR (500 MHz, CDCl3): δ 9.11 and 9.04 (s, 1H), 7.27–7.22 (m, 1H), 7.17 (t, J = 5.0 Hz, 1H), 7.05 (t, J = 7.4 Hz, 1H), 6.97–6.90 (m, 1H), 4.23–4.12 (m, 2H), 3.95–3.87 (m, 1H), 3.85–3.74 (m, 2H), 3.64 (dd, J = 18.0, 9.1 Hz, 1H), 2.48–2.40 (m, 1H), 2.16–2.07 (m, 1H), 1.32 (t, J = 7.0 Hz, 1.3H), 1.24 (t, J = 7.0 Hz, 1.7H); 13C NMR (125 MHz, CDCl3): δ 180.2, 179.9, 155.2, 155.1, 140.3, 140.2, 132.9, 132.5, 128.6, 123.4, 123.1, 123.1, 122.8, 111.2, 110.2, 61.6, 61.5, 54.3, 54.1, 53.4, 52.4, 45.7, 45.3, 36.5, 35.6, 14.9, 14.8; HRMS (ESI) calcd for C14H17N2O3 m/z 261.1234 [M + H]+, found 269.1246.
44 rotamers in CDCl3); 1H NMR (500 MHz, CDCl3): δ 9.11 and 9.04 (s, 1H), 7.27–7.22 (m, 1H), 7.17 (t, J = 5.0 Hz, 1H), 7.05 (t, J = 7.4 Hz, 1H), 6.97–6.90 (m, 1H), 4.23–4.12 (m, 2H), 3.95–3.87 (m, 1H), 3.85–3.74 (m, 2H), 3.64 (dd, J = 18.0, 9.1 Hz, 1H), 2.48–2.40 (m, 1H), 2.16–2.07 (m, 1H), 1.32 (t, J = 7.0 Hz, 1.3H), 1.24 (t, J = 7.0 Hz, 1.7H); 13C NMR (125 MHz, CDCl3): δ 180.2, 179.9, 155.2, 155.1, 140.3, 140.2, 132.9, 132.5, 128.6, 123.4, 123.1, 123.1, 122.8, 111.2, 110.2, 61.6, 61.5, 54.3, 54.1, 53.4, 52.4, 45.7, 45.3, 36.5, 35.6, 14.9, 14.8; HRMS (ESI) calcd for C14H17N2O3 m/z 261.1234 [M + H]+, found 269.1246.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 rotamers in DMSO-d6), mp: 123–125 °C; 1H NMR (500 MHz, DMSO-d6): δ 10.50 (s, 1H), 7.25–7.19 (m, 2H), 6.97 (t, J = 6.5 Hz, 1H), 6.86 (d, J = 7.6 Hz, 1H), 3.67–3.57 (m, 2H), 3.51–3.43 (m, 2H), 2.18–2.09 (m, 2H), 1.44 and 1.39 (s, 9H); 13C NMR (125 MHz, DMSO-d6): δ 179.3, 153.5, 141.7, 131.8, 128.2, 122.8, 121.5, 109.4, 78.6, 53.8, 53.5, 52.2, 51.3, 45.0, 44.9, 35.5, 34.6, 28.1, 28.0; HRMS (ESI) calcd for C16H20N2O3Na m/z 311.1366 [M + Na]+, found 311.1416.
49 rotamers in DMSO-d6), mp: 123–125 °C; 1H NMR (500 MHz, DMSO-d6): δ 10.50 (s, 1H), 7.25–7.19 (m, 2H), 6.97 (t, J = 6.5 Hz, 1H), 6.86 (d, J = 7.6 Hz, 1H), 3.67–3.57 (m, 2H), 3.51–3.43 (m, 2H), 2.18–2.09 (m, 2H), 1.44 and 1.39 (s, 9H); 13C NMR (125 MHz, DMSO-d6): δ 179.3, 153.5, 141.7, 131.8, 128.2, 122.8, 121.5, 109.4, 78.6, 53.8, 53.5, 52.2, 51.3, 45.0, 44.9, 35.5, 34.6, 28.1, 28.0; HRMS (ESI) calcd for C16H20N2O3Na m/z 311.1366 [M + Na]+, found 311.1416.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 rotamers in CDCl3) 1H NMR (500 MHz, CDCl3): δ 8.65 and 8.59 (s, 1H), 7.43–7.37 (m, 2H), 7.33–7.32 (m, 3H), 7.24 (t, J = 12.8 Hz, 1H), 7.15 (dd, J = 15.2, 7.4 Hz, 1H), 7.05–7.03 (m, 1H), 6.93 (d, J = 7.8 Hz, 1H), 5.21 (d, J = 2.8 Hz, 1H), 5.16 (s, 1H), 3.97–3.90 (m, 1H), 3.87–3.78 (m, 2H), 3.69 (dd, J = 23.0, 11.1 Hz, 1H), 2.47–2.41 (dt, J = 12.7, 8.1 Hz, 1H), 2.16–2.08 (m, 1H); 13C NMR (125 MHz, CDCl3): δ 179.7, 154.8, 140.1, 136.9, 136.7, 132.7, 132.3, 128.6, 128.6, 128.1, 128.1, 128.0, 127.9, 123.1, 122.9, 110.1, 67.2, 54.5, 54.2, 53.3, 52.4, 45.8, 45.4, 36.5, 35.6; HRMS (ESI) calcd for C19H19N2O3 m/z 323.1390 [M + H]+, found 323.1349.
48 rotamers in CDCl3) 1H NMR (500 MHz, CDCl3): δ 8.65 and 8.59 (s, 1H), 7.43–7.37 (m, 2H), 7.33–7.32 (m, 3H), 7.24 (t, J = 12.8 Hz, 1H), 7.15 (dd, J = 15.2, 7.4 Hz, 1H), 7.05–7.03 (m, 1H), 6.93 (d, J = 7.8 Hz, 1H), 5.21 (d, J = 2.8 Hz, 1H), 5.16 (s, 1H), 3.97–3.90 (m, 1H), 3.87–3.78 (m, 2H), 3.69 (dd, J = 23.0, 11.1 Hz, 1H), 2.47–2.41 (dt, J = 12.7, 8.1 Hz, 1H), 2.16–2.08 (m, 1H); 13C NMR (125 MHz, CDCl3): δ 179.7, 154.8, 140.1, 136.9, 136.7, 132.7, 132.3, 128.6, 128.6, 128.1, 128.1, 128.0, 127.9, 123.1, 122.9, 110.1, 67.2, 54.5, 54.2, 53.3, 52.4, 45.8, 45.4, 36.5, 35.6; HRMS (ESI) calcd for C19H19N2O3 m/z 323.1390 [M + H]+, found 323.1349.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6), mp: 140–142 °C; 1H NMR (500 MHz, DMSO-d6): major isomer: δ 10.52 (s, 1H), 7.43 (d, J = 7.4 Hz, 1H), 7.22 (t, J = 8.3 Hz, 1H), 7.01 (t, J = 7.5 Hz, 1H), 6.84 (d, J = 7.7 Hz, 1H), 3.89–3.75 (m, 2H), 3.69–3.62 (m, 1H), 3.08 (s, 3H), 2.24–2.16 (m, 1H), 2.10 (dd, J = 6.4, 2.1 Hz, 1H), 1.07 (d, J = 6.4 Hz, 3H); minor isomer: δ 10.58 (s, 1H), 7.33 (d, J = 7.4 Hz, 1H), 7.22 (t, J = 8.3 Hz, 1H), 7.01 (t, J = 7.5 Hz, 1H), 6.88 (d, J = 7.7 Hz, 1H), 3.77–3.70 (m, 2H), 3.60 (dd, J = 7.0, 3.1 Hz, 1H), 3.01 (s, 3H), 2.14 (dd, J = 12.9, 6.1 Hz, 1H), 2.07 (dd, J = 6.3, 1.9 Hz, 1H), 1.02 (d, J = 6.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 180.3, 178.9, 140.7, 130.0, 129.0, 128.7, 125.5, 123.2, 123.0, 122.8, 110.3, 110.0, 63.1, 61.4, 57.8, 57.4, 47.3, 47.2, 40.3, 35.1, 34.8, 34.3, 20.0, 15.8; HRMS (ESI) calcd for C13H17N2O3S m/z 281.0954 [M + H]+, found 281.0968.
1.6), mp: 140–142 °C; 1H NMR (500 MHz, DMSO-d6): major isomer: δ 10.52 (s, 1H), 7.43 (d, J = 7.4 Hz, 1H), 7.22 (t, J = 8.3 Hz, 1H), 7.01 (t, J = 7.5 Hz, 1H), 6.84 (d, J = 7.7 Hz, 1H), 3.89–3.75 (m, 2H), 3.69–3.62 (m, 1H), 3.08 (s, 3H), 2.24–2.16 (m, 1H), 2.10 (dd, J = 6.4, 2.1 Hz, 1H), 1.07 (d, J = 6.4 Hz, 3H); minor isomer: δ 10.58 (s, 1H), 7.33 (d, J = 7.4 Hz, 1H), 7.22 (t, J = 8.3 Hz, 1H), 7.01 (t, J = 7.5 Hz, 1H), 6.88 (d, J = 7.7 Hz, 1H), 3.77–3.70 (m, 2H), 3.60 (dd, J = 7.0, 3.1 Hz, 1H), 3.01 (s, 3H), 2.14 (dd, J = 12.9, 6.1 Hz, 1H), 2.07 (dd, J = 6.3, 1.9 Hz, 1H), 1.02 (d, J = 6.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 180.3, 178.9, 140.7, 130.0, 129.0, 128.7, 125.5, 123.2, 123.0, 122.8, 110.3, 110.0, 63.1, 61.4, 57.8, 57.4, 47.3, 47.2, 40.3, 35.1, 34.8, 34.3, 20.0, 15.8; HRMS (ESI) calcd for C13H17N2O3S m/z 281.0954 [M + H]+, found 281.0968.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), mp: 195–199 °C; 1H NMR (400 MHz, CDCl3): major isomer: δ 8.58 (s, 1H), 7.84 (d, J = 8.2 Hz, 2H), 7.40 (d, J = 8.0 Hz, 2H), 7.19 (t, J = 7.7 Hz, 1H), 6.95–6.85 (m, 2H), 6.57 (d, J = 7.4 Hz, 1H), 4.08–4.00 (m, 1H), 3.93–3.85 (m, 1H), 3.80–3.68 (m, 1H), 2.49 (s, 3H), 2.21–2.13 (m, 1H), 1.80–1.74 (m, 1H), 1.33 (d, J = 6.5 Hz, 3H); minor isomer: δ 8.44 (s, 1H), 7.79 (d, J = 8.2 Hz, 2H), 7.40 (d, J = 8.0 Hz, 2H), 7.09 (t, J = 7.6 Hz, 1H), 6.95–6.85 (m, 2H), 6.57 (d, J = 7.4 Hz, 1H), 4.08–4.00 (m, 1H), 3.93–3.85 (m, 1H), 3.80–3.68 (m, 1H), 2.46 (s, 3H), 2.21–2.13 (m, 1H), 1.80–1.74 (m, 1H), 1.14 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 178.9, 178.5, 143.9, 143.8, 140.6, 140.2, 135.7, 133.5, 130.6, 129.9, 128.8, 127.9, 127.6, 125.2, 123.0, 122.9, 122.6, 110.2, 110.0, 63.7, 62.3, 58.4, 57.8, 48.4, 48.0, 34.8, 33.8, 23.9, 21.7, 17.5, 17.3; HRMS (ESI) calcd for C19H21N2O3S m/z 357.1267 [M + H]+, found 357.1269.
4), mp: 195–199 °C; 1H NMR (400 MHz, CDCl3): major isomer: δ 8.58 (s, 1H), 7.84 (d, J = 8.2 Hz, 2H), 7.40 (d, J = 8.0 Hz, 2H), 7.19 (t, J = 7.7 Hz, 1H), 6.95–6.85 (m, 2H), 6.57 (d, J = 7.4 Hz, 1H), 4.08–4.00 (m, 1H), 3.93–3.85 (m, 1H), 3.80–3.68 (m, 1H), 2.49 (s, 3H), 2.21–2.13 (m, 1H), 1.80–1.74 (m, 1H), 1.33 (d, J = 6.5 Hz, 3H); minor isomer: δ 8.44 (s, 1H), 7.79 (d, J = 8.2 Hz, 2H), 7.40 (d, J = 8.0 Hz, 2H), 7.09 (t, J = 7.6 Hz, 1H), 6.95–6.85 (m, 2H), 6.57 (d, J = 7.4 Hz, 1H), 4.08–4.00 (m, 1H), 3.93–3.85 (m, 1H), 3.80–3.68 (m, 1H), 2.46 (s, 3H), 2.21–2.13 (m, 1H), 1.80–1.74 (m, 1H), 1.14 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 178.9, 178.5, 143.9, 143.8, 140.6, 140.2, 135.7, 133.5, 130.6, 129.9, 128.8, 127.9, 127.6, 125.2, 123.0, 122.9, 122.6, 110.2, 110.0, 63.7, 62.3, 58.4, 57.8, 48.4, 48.0, 34.8, 33.8, 23.9, 21.7, 17.5, 17.3; HRMS (ESI) calcd for C19H21N2O3S m/z 357.1267 [M + H]+, found 357.1269.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.9), mp: 169–173 °C; 1H NMR (400 MHz, DMSO-d6): major isomer: δ 10.43 (s, 1H), 7.72 (d, J = 8.0 Hz, 2H), 7.47 (d, J = 7.9 Hz, 2H), 7.23–6.92 (m, 6H), 6.66 (dd, J = 14.1, 7.4 Hz, 2H), 6.55 (d, J = 7.4 Hz, 1H), 4.81 (s, 1H), 4.00–3.92 (m, 1H), 3.90–3.82 (m, 1H), 2.45 (s, 3H), 2.07–1.96 (m, 1H), 1.92–1.80 (m, 1H); minor isomer: δ 10.55 (s, 1H), 7.84 (d, J = 8.0 Hz, 2H), 7.57 (d, J = 7.9 Hz, 2H), 7.23–6.92 (m, 6H), 6.66 (dd, J = 14.1, 7.4 Hz, 2H), 6.55 (d, J = 7.4 Hz, 1H), 4.83 (s, 1H), 4.28–4.00 (m, 2H), 2.45 (s, 3H), 2.19–2.08 (m, 2H); 13C NMR (100 MHz, DMSO-d6): δ 177.8, 143.5, 141.3, 138.7, 133.7, 129.7, 128.4, 128.1, 127.7, 127.4, 127.2, 126.8, 125.0, 121.0, 109.2, 69.1, 58.5, 47.9, 34.5, 21.1; HRMS (ESI) calcd for C24H23N2O3S m/z 419.1424 [M + H]+, found 419.1428.
7.9), mp: 169–173 °C; 1H NMR (400 MHz, DMSO-d6): major isomer: δ 10.43 (s, 1H), 7.72 (d, J = 8.0 Hz, 2H), 7.47 (d, J = 7.9 Hz, 2H), 7.23–6.92 (m, 6H), 6.66 (dd, J = 14.1, 7.4 Hz, 2H), 6.55 (d, J = 7.4 Hz, 1H), 4.81 (s, 1H), 4.00–3.92 (m, 1H), 3.90–3.82 (m, 1H), 2.45 (s, 3H), 2.07–1.96 (m, 1H), 1.92–1.80 (m, 1H); minor isomer: δ 10.55 (s, 1H), 7.84 (d, J = 8.0 Hz, 2H), 7.57 (d, J = 7.9 Hz, 2H), 7.23–6.92 (m, 6H), 6.66 (dd, J = 14.1, 7.4 Hz, 2H), 6.55 (d, J = 7.4 Hz, 1H), 4.83 (s, 1H), 4.28–4.00 (m, 2H), 2.45 (s, 3H), 2.19–2.08 (m, 2H); 13C NMR (100 MHz, DMSO-d6): δ 177.8, 143.5, 141.3, 138.7, 133.7, 129.7, 128.4, 128.1, 127.7, 127.4, 127.2, 126.8, 125.0, 121.0, 109.2, 69.1, 58.5, 47.9, 34.5, 21.1; HRMS (ESI) calcd for C24H23N2O3S m/z 419.1424 [M + H]+, found 419.1428.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9); 1H NMR (400 MHz, CDCl3): δ 8.62 and 8.40 (s, 1H), 7.81 (d, J = 8.0 Hz, 2H), 7.53 (d, J = 7.4 Hz, 0.5H), 7.34 (d, J = 8.0 Hz, 2H), 7.26–7.18 (m, 1H), 7.06 (t, J = 7.6 Hz, 0.5H), 6.92–6.85 (m, 1.5H), 6.77 (d, J = 7.5 Hz, 0.5H), 4.79 (t, J = 8.1 Hz, 0.66H), 4.57 (t, J = 7.9 Hz, 0.34H), 3.83 and 3.72 (s, 3H), 3.82–3.57 (m, 2H), 2.68–2.60 (m, 1H), 2.46 and 2.43 (s, 3H), 2.42–2.34 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 179.4, 177.6, 172.0, 171.5, 144.2, 144.0, 140.2, 139.8, 136.1, 133.9, 132.3, 130.5, 129.8, 129.8, 129.0, 128.8, 128.2, 127.6, 124.1, 123.4, 123.2, 123.0, 110.3, 110.1, 60.9, 60.5, 56.8, 56.5, 53.0, 52.8, 52.7, 52.1, 41.5, 40.6, 21.7, 21.7; HRMS (ESI) calcd for C20H21N2O5S m/z 401.1166 [M + H]+, found 401.1172.
1.9); 1H NMR (400 MHz, CDCl3): δ 8.62 and 8.40 (s, 1H), 7.81 (d, J = 8.0 Hz, 2H), 7.53 (d, J = 7.4 Hz, 0.5H), 7.34 (d, J = 8.0 Hz, 2H), 7.26–7.18 (m, 1H), 7.06 (t, J = 7.6 Hz, 0.5H), 6.92–6.85 (m, 1.5H), 6.77 (d, J = 7.5 Hz, 0.5H), 4.79 (t, J = 8.1 Hz, 0.66H), 4.57 (t, J = 7.9 Hz, 0.34H), 3.83 and 3.72 (s, 3H), 3.82–3.57 (m, 2H), 2.68–2.60 (m, 1H), 2.46 and 2.43 (s, 3H), 2.42–2.34 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 179.4, 177.6, 172.0, 171.5, 144.2, 144.0, 140.2, 139.8, 136.1, 133.9, 132.3, 130.5, 129.8, 129.8, 129.0, 128.8, 128.2, 127.6, 124.1, 123.4, 123.2, 123.0, 110.3, 110.1, 60.9, 60.5, 56.8, 56.5, 53.0, 52.8, 52.7, 52.1, 41.5, 40.6, 21.7, 21.7; HRMS (ESI) calcd for C20H21N2O5S m/z 401.1166 [M + H]+, found 401.1172.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); 1H NMR (400 MHz, CDCl3) Major isomer: δ 8.71 (m, 1H), 7.35–7.21 (m, 1H), 7.16–7.02 (m, 2H), 6.99–6.88 (m, 1H), 4.88–4.71 (m, 1H), 3.94–3.68 (m, 2H), 3.79 (s, 3H), 2.74–2.48 (m, 2H), 1.46 (s, 9H); minor isomer: δ 7.63 (m, 1H), 7.35–7.21 (m, 1H), 7.16–7.02 (m, 2H), 6.99–6.88 (m, 1H), 4.66–4.59 (m, 1H), 3.94–3.68 (m, 2H), 3.80 (s, 3H), 2.45–2.26 (m, 2H), 1.48 (s, 9H); 13C NMR (100 MHz, CDCl3): δ 178.0, 173.4, 172.5, 172.2, 154.4, 153.5, 140.8, 139.8, 133.3, 133.1, 129.4, 129.0, 128.8, 123.4, 123.2, 123.1, 122.5, 110.4, 110.1, 81.0, 80.7, 59.1, 58.7, 55.4, 54.9, 54.5, 52.4, 52.3, 41.0, 40.6, 40.3, 39.7, 28.4, 28.3; HRMS (ESI) calcd for C18H23N2O5 m/z 347.1601 [M + H]+, found 347.1613.
3); 1H NMR (400 MHz, CDCl3) Major isomer: δ 8.71 (m, 1H), 7.35–7.21 (m, 1H), 7.16–7.02 (m, 2H), 6.99–6.88 (m, 1H), 4.88–4.71 (m, 1H), 3.94–3.68 (m, 2H), 3.79 (s, 3H), 2.74–2.48 (m, 2H), 1.46 (s, 9H); minor isomer: δ 7.63 (m, 1H), 7.35–7.21 (m, 1H), 7.16–7.02 (m, 2H), 6.99–6.88 (m, 1H), 4.66–4.59 (m, 1H), 3.94–3.68 (m, 2H), 3.80 (s, 3H), 2.45–2.26 (m, 2H), 1.48 (s, 9H); 13C NMR (100 MHz, CDCl3): δ 178.0, 173.4, 172.5, 172.2, 154.4, 153.5, 140.8, 139.8, 133.3, 133.1, 129.4, 129.0, 128.8, 123.4, 123.2, 123.1, 122.5, 110.4, 110.1, 81.0, 80.7, 59.1, 58.7, 55.4, 54.9, 54.5, 52.4, 52.3, 41.0, 40.6, 40.3, 39.7, 28.4, 28.3; HRMS (ESI) calcd for C18H23N2O5 m/z 347.1601 [M + H]+, found 347.1613.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δ 8.96–8.73 (m, 1H), 7.80 (d, J = 7.4 Hz, 1H), 7.41–7.29 (m, 4H), 7.28–7.18 (m, 2H), 7.14–7.03 (m, 1H), 6.95–6.88 (m, 1H), 4.03–3.80 (m, 1H), 3.79–3.60 (m, 3H), 3.50 (s, 2H), 3.17 (dd, J = 12.1, 6.0 Hz, 1H), 2.76–2.66 (m, 0.6H), 2.51–2.45 (m, 0.4H), 2.30 (dd, J = 13.3, 9.2 Hz, 0.4H), 2.11 (dd, J = 13.4, 6.0 Hz, 0.6H), 0.75 and 0.73 (d, J = 10.3 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 180.9, 180.3, 174.3, 174.0, 140.6, 140.3, 139.2, 137.4, 132.8, 132.5, 129.5, 128.7, 128.4, 128.2, 128.0, 127.9, 127.2, 126.3, 125.0, 122.8, 122.5, 109.8, 109.6, 67.0, 64.5, 64.2, 60.4, 57.9, 57.3, 55.8, 51.8, 51.5, 50.9, 38.6, 38.2, 14.5, 14.3; HRMS (ESI) calcd for C21H23N2O3 m/z 351.1703 [M + H]+, found 351.1711.
1); 1H NMR (400 MHz, CDCl3): δ 8.96–8.73 (m, 1H), 7.80 (d, J = 7.4 Hz, 1H), 7.41–7.29 (m, 4H), 7.28–7.18 (m, 2H), 7.14–7.03 (m, 1H), 6.95–6.88 (m, 1H), 4.03–3.80 (m, 1H), 3.79–3.60 (m, 3H), 3.50 (s, 2H), 3.17 (dd, J = 12.1, 6.0 Hz, 1H), 2.76–2.66 (m, 0.6H), 2.51–2.45 (m, 0.4H), 2.30 (dd, J = 13.3, 9.2 Hz, 0.4H), 2.11 (dd, J = 13.4, 6.0 Hz, 0.6H), 0.75 and 0.73 (d, J = 10.3 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 180.9, 180.3, 174.3, 174.0, 140.6, 140.3, 139.2, 137.4, 132.8, 132.5, 129.5, 128.7, 128.4, 128.2, 128.0, 127.9, 127.2, 126.3, 125.0, 122.8, 122.5, 109.8, 109.6, 67.0, 64.5, 64.2, 60.4, 57.9, 57.3, 55.8, 51.8, 51.5, 50.9, 38.6, 38.2, 14.5, 14.3; HRMS (ESI) calcd for C21H23N2O3 m/z 351.1703 [M + H]+, found 351.1711.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.3); 1H NMR (400 MHz, CDCl3): δ 8.28 (s, 1H), 7.51 (d, J = 7.4 Hz, 1H), 7.44 (d, J = 7.3 Hz, 2H), 7.37–7.31 (m, 1H), 7.29 (d, J = 7.6 Hz, 1H), 7.25–7.16 (m, 2H), 7.06 (td, J = 7.6, 0.9 Hz, 1H), 6.87 (d, J = 7.7 Hz, 1H), 4.06–3.98 (m, 2H), 3.78–3.70 (m, 2H), 3.69 (s, 3H), 2.76 (dd, J = 13.7, 9.3 Hz, 1H), 2.17 (dd, J = 13.7, 3.6 Hz, 1H), 1.01 (d, J = 6.5 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 180.8, 175.0, 140.6, 139.3, 133.3, 128.5, 128.3, 128.1, 127.0, 124.2, 122.9, 109.3, 66.1, 62.3, 57.3, 51.8, 51.5, 38.3, 12.9; HRMS (ESI) calcd for C21H23N2O3 m/z 351.1703 [M + H]+, found 351.1711.
8.3); 1H NMR (400 MHz, CDCl3): δ 8.28 (s, 1H), 7.51 (d, J = 7.4 Hz, 1H), 7.44 (d, J = 7.3 Hz, 2H), 7.37–7.31 (m, 1H), 7.29 (d, J = 7.6 Hz, 1H), 7.25–7.16 (m, 2H), 7.06 (td, J = 7.6, 0.9 Hz, 1H), 6.87 (d, J = 7.7 Hz, 1H), 4.06–3.98 (m, 2H), 3.78–3.70 (m, 2H), 3.69 (s, 3H), 2.76 (dd, J = 13.7, 9.3 Hz, 1H), 2.17 (dd, J = 13.7, 3.6 Hz, 1H), 1.01 (d, J = 6.5 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 180.8, 175.0, 140.6, 139.3, 133.3, 128.5, 128.3, 128.1, 127.0, 124.2, 122.9, 109.3, 66.1, 62.3, 57.3, 51.8, 51.5, 38.3, 12.9; HRMS (ESI) calcd for C21H23N2O3 m/z 351.1703 [M + H]+, found 351.1711.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3): δ 8.58 and 8.41 (s, 1H), 7.48–7.42 (m, 3H), 7.31 (t, J = 7.4 Hz, 2H), 7.26–7.15 (m, 2H), 7.05 (td, J = 7.6, 0.8 Hz, 1H), 6.86 (d, J = 7.6 Hz, 1H), 4.09 (d, J = 14.2 Hz, 1H), 3.99 (dd, J = 9.1, 3.3 Hz, 1H), 3.88 (d, J = 14.2 Hz, 1H), 3.68 (s, 3H), 2.75 (dd, J = 13.6, 9.1 Hz, 1H), 2.16 (dd, J = 13.6, 3.3 Hz, 1H), 1.78–1.65 (m, 1H), 1.63–1.50 (m, 2H), 0.59 (t, J = 7.5 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 181.3, 175.1, 140.4, 139.8, 134.4, 128.4, 128.3, 127.9, 126.9, 124.0, 122.9, 109.4, 73.0, 63.1, 57.1, 52.0, 51.5, 40.0, 21.2, 11.1; HRMS (ESI) calcd for C22H25N2O3 m/z 365.1860 [M + H]+, found 365.1866.
1); 1H NMR (400 MHz, CDCl3): δ 8.58 and 8.41 (s, 1H), 7.48–7.42 (m, 3H), 7.31 (t, J = 7.4 Hz, 2H), 7.26–7.15 (m, 2H), 7.05 (td, J = 7.6, 0.8 Hz, 1H), 6.86 (d, J = 7.6 Hz, 1H), 4.09 (d, J = 14.2 Hz, 1H), 3.99 (dd, J = 9.1, 3.3 Hz, 1H), 3.88 (d, J = 14.2 Hz, 1H), 3.68 (s, 3H), 2.75 (dd, J = 13.6, 9.1 Hz, 1H), 2.16 (dd, J = 13.6, 3.3 Hz, 1H), 1.78–1.65 (m, 1H), 1.63–1.50 (m, 2H), 0.59 (t, J = 7.5 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 181.3, 175.1, 140.4, 139.8, 134.4, 128.4, 128.3, 127.9, 126.9, 124.0, 122.9, 109.4, 73.0, 63.1, 57.1, 52.0, 51.5, 40.0, 21.2, 11.1; HRMS (ESI) calcd for C22H25N2O3 m/z 365.1860 [M + H]+, found 365.1866.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.2); 1H NMR (400 MHz, CDCl3): δ 8.74 (d, J = 48.5 Hz, 1H), 7.80 (d, J = 7.4 Hz, 1H), 7.39 (d, J = 7.2 Hz, 2H), 7.32 (t, J = 10.1 Hz, 2H), 7.27–7.19 (m, 2H), 7.12–7.06 (m, 1H), 6.91 (d, J = 7.7 Hz, 1H), 4.01 (d, J = 14.3 Hz, 1H), 3.80 (d, J = 14.3 Hz, 1H), 3.69 (dd, J = 10.2, 6.3 Hz, 1H), 3.47 (s, 3H), 3.11 (dd, J = 10.1, 3.7 Hz, 1H), 2.66 (dd, J = 13.3, 10.5 Hz, 1H), 2.09 (dd, J = 13.3, 6.0 Hz, 1H), 1.69–1.54 (m, 2H), 0.51 (t, J = 7.5 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 181.6, 174.0, 140.3, 138.0, 132.3, 129.3, 128.2, 127.9, 127.1, 126.5, 122.8, 109.7, 73.0, 64.8, 56.6, 56.1, 51.8, 40.1, 23.3, 9.8; HRMS (ESI) calcd for C22H25N2O3 m/z 365.1860 [M + H]+, found 365.1866.
6.2); 1H NMR (400 MHz, CDCl3): δ 8.74 (d, J = 48.5 Hz, 1H), 7.80 (d, J = 7.4 Hz, 1H), 7.39 (d, J = 7.2 Hz, 2H), 7.32 (t, J = 10.1 Hz, 2H), 7.27–7.19 (m, 2H), 7.12–7.06 (m, 1H), 6.91 (d, J = 7.7 Hz, 1H), 4.01 (d, J = 14.3 Hz, 1H), 3.80 (d, J = 14.3 Hz, 1H), 3.69 (dd, J = 10.2, 6.3 Hz, 1H), 3.47 (s, 3H), 3.11 (dd, J = 10.1, 3.7 Hz, 1H), 2.66 (dd, J = 13.3, 10.5 Hz, 1H), 2.09 (dd, J = 13.3, 6.0 Hz, 1H), 1.69–1.54 (m, 2H), 0.51 (t, J = 7.5 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 181.6, 174.0, 140.3, 138.0, 132.3, 129.3, 128.2, 127.9, 127.1, 126.5, 122.8, 109.7, 73.0, 64.8, 56.6, 56.1, 51.8, 40.1, 23.3, 9.8; HRMS (ESI) calcd for C22H25N2O3 m/z 365.1860 [M + H]+, found 365.1866.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. S. thank FONDECYT (Project #11200555) and Instituto de Investigación Interdisciplinaria, Vicerrectoría Académica, Universidad de Talca, Talca, Chile for financial support for this work. L. S. S. and F. M. N. are grateful to FONDECYT (Project #1180084), and A. P. S. is thankful to DoP, Ministry of Chemicals & Fertilizers, Govt. of India, New Delhi, for the award of NIPER fellowship. NIPERH Research Communication No. NIPER-H/2021/151.References
- (a) N. Ye, H. Chen, E. A. Wold, P. Y. Shi and J. Zhou, ACS Infect. Dis., 2016, 2, 382–392 CrossRef CAS PubMed; (b) B. Zhou, Y. Yang, J. Shi, Z. Luo and Y. Li, J. Org. Chem., 2013, 78, 2897–2907 CrossRef CAS PubMed; (c) C. Li, C. Chan, A. C. Heimann and S. J. Danishefsky, Angew. Chem., Int. Ed., 2007, 46, 1444–1447 CrossRef CAS PubMed; (d) J. D. White, Y. Li and D. C. Ihle, J. Org. Chem., 2010, 75, 3569–3577 CrossRef CAS PubMed.
- (a) G. J. Mei and F. Shi, Chem. Commun., 2018, 54, 6607–6621 RSC; (b) M. Pelay-Gimeno, A. Glas, O. Koch and T. N. Grossmann, Angew. Chem., Int. Ed., 2015, 54, 8896–8927 CrossRef CAS PubMed; (c) G. Bhaskar, Y. Arun, C. Balachandran, C. Saikumar and P. T. Perumal, Eur. J. Med. Chem., 2012, 51, 79–91 CrossRef CAS PubMed.
- (a) A. Barakat, M. Shahidul Islam, H. Mansur Ghawas, A. Mohammed Al-Majid, F. F. El-Senduny, F. A. Badria, Y. A. M. M. Elshaier and H. A. Ghabbour, RSC Adv., 2018, 8, 14335–14346 RSC; (b) B. Yu, Y.-C. Zheng, X.-J. Shi, P.-P. Qi and H.-M. Liu, Anti-Cancer Agents Med. Chem., 2016, 16, 1315–1324 CrossRef CAS PubMed; (c) L. Chen, J. Xie, H. Song, Y. Liu, Y. Gu, L. Wang and Q. Wang, J. Agric. Food Chem., 2016, 64, 6508–6516 CrossRef CAS PubMed; (d) K. R. Senwar, P. Sharma, T. S. Reddy, M. K. Jeengar, V. L. Nayak, V. G. M. Naidu, A. Kamal and N. Shankaraiah, Eur. J. Med. Chem., 2015, 102, 413–424 CrossRef CAS PubMed.
- Y.-T. Yang, J.-F. Zhu, G. C. Liao, H.-J. Xu and B. Yu, Curr. Med. Chem., 2018, 25, 2233–2244 CrossRef CAS PubMed.
- M. A. El-Hashash and S. A. Rizk, J. Heterocycl. Chem., 2017, 54, 1776–1784 CrossRef CAS.
- R. S. Kumar, P. Antonisamy, A. I. Almansour, N. Arumugam, G. Periyasami, M. Altaf, H. R. Kim and K. B. Kwon, Eur. J. Med. Chem., 2018, 152, 417–423 CrossRef CAS PubMed.
- L. M. Zhou, R. U. Qu and G. F. Yang, Expert Opin. Drug Discovery, 2020, 15, 603–625 CrossRef CAS PubMed.
- M. G. Kulkarni, A. P. Dhondge, S. W. Chavhan, A. S. Borhade, Y. B. Shaikh, D. R. Birhade, M. P. Desai and N. R. Dhatrak, Beilstein J. Org. Chem., 2010, 6, 876–879 CrossRef CAS PubMed.
- H. Lee, S. H. Baek, J. H. Lee, C. Kim, J. H. Ko, S. G. Lee, A. Chinnathambi, S. A. Alharbi, W. M. Yang, J. Y. Um, G. Sethi and K. S. Ahn, Int. J. Mol. Sci., 2017, 18, 1095 CrossRef PubMed.
- P. R. Sebahar, H. Osada, T. Usui and R. M. Williams, Tetrahedron, 2002, 58, 6311–6322 CrossRef CAS.
- L. L. Chen, J. X. Song, J. H. Lu, Z. W. Yuan, L. F. Liu, S. S. K. Durairajan and M. Li, J. Neuroimmune Pharmacol., 2014, 9, 380–387 CrossRef PubMed.
- T. Mukaiyama, K. Ogata, I. Sato and Y. Hayashi, Chem.–Eur. J., 2014, 20, 13583–13588 CrossRef CAS PubMed.
- (a) H. Zinnes and J. Shavel Jr, J. Org. Chem., 1966, 1966, 1765–1771 CrossRef PubMed; (b) A. C. Peterson and J. M. Cook, Tetrahedron Lett., 1994, 35, 2651–2654 CrossRef CAS; (c) C. Pellegrini, C. Strässler, M. Weber and H. J. Borschberg, Tetrahedron: Asymmetry, 1994, 5, 1979–1992 CrossRef CAS; (d) J. Shavel and H. Zinnes, J. Am. Chem. Soc., 1962, 84, 1320–1321 CrossRef CAS; (e) M. Sathish, F. M. Nachtigall and L. S. Santos, RSC Adv., 2020, 10, 38672–38677 RSC; (f) J. Xu, L. Liang, H. Zheng, Y. R. Chi and R. Tong, Nat. Commun., 2019, 10, 4754 CrossRef PubMed.
- (a) D. Bora, R. Tokala, S. E. John, B. Prasanth and N. Shankaraiah, Org. Biomol. Chem., 2020, 18, 2307–2311 RSC; (b) R. Tokala, D. Bora, S. Sana, F. M. Nachtigall, L. S. Santos and N. Shankaraiah, J. Org. Chem., 2019, 84, 5504–5513 CrossRef CAS PubMed; (c) P. Sharma, P. K. Niggula, N. H. Krishna, D. Prasanna, B. Sridhar and N. Shankaraiah, Org. Chem. Front., 2016, 3, 1503–1508 RSC; (d) S. Nekkanti, K. Veeramani, P. K. Niggula and N. Shankaraiah, Green Chem., 2016, 18, 3439–3447 RSC.
- K. Huthmacher and D. Most, Cyanuric Acid and Cyanuric Chloride Ullmann's Encyclopedia of Industrial Chemistry, 2000, Wiley-VCH, Weinheim, DOI:10.1002/14356007.a08191.
- A. Hu, A. Chen, B. Xie and N. Yu, Preparation of omeprazole intermediate 2-chloromethyl-3,5-dimethyl-4-methoxypyridine, 2019, CN 110317164 Search PubMed.
- S. Gaspa, M. Carraro, L. Pisano, A. Porcheddu and L. D. Luca, Eur. J. Org. Chem., 2019, 2019, 3544–3552 CrossRef CAS.
- Y. Jing, C. G. Daniliuc and A. Studer, Org. Lett., 2014, 16, 4932–4935 CrossRef CAS PubMed.
- K. L. Manasa, Y. Tangella, G. Ramu and B. N. Babu, ChemistrySelect, 2017, 2, 9162–9167 CrossRef CAS.
- H. Veisi, Synthesis, 2010, 2631–2635 CrossRef CAS.
- (a) J. Ye, Y. Lin, Q. Liu, D. Xu, F. Wu, B. Liu, Y. Gao and H. Chen, Org. Lett., 2018, 20, 5457–5460 CrossRef CAS PubMed; (b) J. Ye, J. Wu, T. Lv, G. Wu, Y. Gao and H. Chen, Angew. Chem., Int. Ed., 2017, 56, 14968–14972 CrossRef CAS PubMed; (c) C. Yan, Y. Liu and Q. Wang, RSC Adv., 2014, 4, 60075–60078 RSC.
- (a) Y. Q. Huang, H. J. Song, Y. X. Liu and Q. M. Wang, Chem.–Eur. J., 2018, 24, 2065–2069 CrossRef CAS PubMed; (b) Y. Lin, J. Ye, W. Zhang, Y. Gao and H. Chen, Adv. Synth. Catal., 2019, 361, 432–435 CrossRef CAS.
- (a) L. Shen, E. J. Park, T. P. Kondratyuk, D. Guendisch, L. Marler, J. M. Pezzuto, A. D. Wright and D. Sun, Bioorg. Med. Chem., 2011, 19, 6182–6195 CrossRef CAS PubMed; (b) L. Chen, Y. Hao, H. Song, Y. Liu, Y. Li, J. Zhang and Q. Wang, J. Agric. Food Chem., 2020, 68, 10618–10625 CrossRef CAS PubMed; (c) J. Dong, T. Z. Meng, X. X. Shi, W. H. Zou and X. Lu, Tetrahedron: Asymmetry, 2013, 24, 883–893 CrossRef CAS; (d) C. L. Hansen, J. W. Clausen, R. G. Ohm, E. Ascic, S. T. Le Quement, D. Tanner and T. E. Nielsen, J. Org. Chem., 2013, 78, 12545–12565 CrossRef CAS PubMed; (e) P. D. Bailey and S. P. Hollinshead, Heterocycles, 1987, 26, 389–399 CrossRef CAS.
- (a) S. Jaegli, J. Dufour, H. L. Wei, T. Piou, X. H. Duan, J. P. Vors, L. Neuville and J. Zhu, Org. Lett., 2010, 12, 4498–4501 CrossRef CAS PubMed; (b) T. Oishi, M. Nagai, T. Onuma, H. Moriyama, K. Tsutae, M. Ochiai and Y. Ban, Chem. Pharm. Bull., 1969, 17, 2306–2313 CrossRef CAS; (c) H. Li, Z. Wang and L. Zu, RSC Adv., 2015, 5, 60962–60965 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra02381k |
| ‡ M. Sathish and A. P. Sakla contributed equally. |
| This journal is © The Royal Society of Chemistry 2021 |