Open Access Article
Open Access ArticleSynchronous oil/water separation and wastewater treatment on a copper-oxide-coated mesh†
Yahua Liu a,
Peng Xua,
Wenna Gea,
Chenguang Lua,
Yunlai Lia,
Shichao Niu
a,
Peng Xua,
Wenna Gea,
Chenguang Lua,
Yunlai Lia,
Shichao Niu b,
Junqiu Zhangb and
Shile Feng
b,
Junqiu Zhangb and
Shile Feng *a
*a
aKey Laboratory for Precision & Non-traditional Machining Technology of Ministry of Education, Dalian University of Technology, Dalian 116024, China. E-mail: fengshile@dlut.edu.cn
bKey Laboratory of Bionic Engineering, Ministry of Education, Jilin University, Changchun 130022, China
First published on 17th May 2021
Abstract
Despite remarkable progress in oil/water separation and wastewater treatment, the ability to carry out the two processes in a synchronous manner has remained difficult. Here, synchronous oil/water separation and wastewater treatment were proposed on mesh surfaces coated with copper-oxide particles, which possess superwetting and catalytic properties. The superwetting performance generates additional pressure to achieve the permselectivity of the designed mesh, on which the oil phase is selectively repelled while the water phase passes though easily. Moreover, the catalytic performance of the copper oxide forms reactive oxygen species to purify the water during oil/water separation process. We show that the oil/water separation and catalytic degradation efficiencies for organic pollutants can reach more than 99% by adjusting the content of copper oxide on the mesh surfaces. Such a unique design for integrating multifunctionality on single mesh surfaces strongly underpins the synchronization of oil/water separation and wastewater treatment, which will provide a new insight for separating pure water from industrial oil/water mixtures.
1. Introduction
Industrial oily wastewater and oil spill accidents bring about extensive water pollution, which have a serious impact on the environment and human health.1 Despite various conventional methods including flotation, absorbing, filtration and centrifugation for water extraction from oily wastewater, unsatisfactory efficiency, additional energy consumption and complicated operation are always present in the process.2–5 To address these drawbacks, functional super-wetting/anti-wetting materials including metals,6 metal oxides and polymers have been introduced,7–12 achieving high-efficiency and low-cost oil/water separation via their antipodal wettability performance for water and oil. On the one hand, oil-repellent surfaces with superhydrophilic and underwater superoleophobic properties can repel the oil completely while allowing the water phase to pass through, allowing the separation of water and oil.13,14 On the other hand, water-repellent materials with superhydrophobic/superoleophilic properties can selectively block the water from being drawn out, allowing an efficient oil/water separation.15–18Apart from oil, organic pollutants existing in oily wastewater is another major factor to affect water quality. Specifically, many of these emerging pollutants are non-biodegrade, immobile and toxic in nature, such as industry dyes, pharmaceuticals and their metabolites, disinfection by-products, etc.19 Recently, extensive efforts have been directed towards addressing organic pollution in water,20,21 among which catalytic degradation is considered as a promising approach.22,23 For example, peroxymonosulfate (PMS), when catalytically activated by copper oxide, can generate multiple reactive oxygen species which exhibit strong degradation capacities to organic pollutions.24
Despite commendable progress,25–29 one needs to assure the synchronous oil/water separation and wastewater treatment, which in practical realizations raises the issue: efficient oil/water separation and wastewater treatment in one step. To meet these requirements, in this research, an integrated multifunctional copper-oxide-coated mesh (IMCM) was designed via facile immersing and burning methods. By leverages the superwetting property and catalytic performance of copper oxide, the designed surface manifests synchronously high-efficiency oil/water separation and organic pollutants purification in water with peroxymonosulfate (see Video S1†). This research may promote the practical application of meshes in oil/water separation.
2. Experimental section
2.1. Surface preparation
Stainless Steel Meshes (SSM) of 500 mesh used in this work were purchased from the local market (Hebei Anping Metal Wire Manufacturing Co. Ltd., China). SSM was cut into pieces with a size 2 × 2 cm2 and ultrasonically rinsed in acetone, anhydrous ethanol and deionized water in sequence for 10 min to remove undesired contamination and impurities and dried in air at ∼40 °C. Then the cleaned SSM was immersed into CuCl2·2H2O (Tianjin Damao Chemical Reagent Factory, China) solution (solvent is ethanol) with a mass fraction (w) of 1%, 3%, 5% and 7% for 2 min. After that, the mesh surface was burned at 1000 °C for 30 s to be coated with copper oxide (Fig. 1(a)). The immersing and burning processes were repeated for three cycles.2.2. Characterization of IMCM
The micro/nanostructures and chemical composition of IMCM surface were characterized by scanning electron microscopy (SEM, SUPRA 55 SAPPHIRE, Germany) equipped with an energy dispersive spectrometer (EDS). The contact angles of water and oil under water on the IMCM surface were measured by optical contact angle meter system (OCA25 system, Dataphysics GmbH, Germany) and determined by five individual measurements. The organics remnants in water after separation were detected by an ultraviolet-visible spectrometer (Lambda 750S, America). X-ray Diffractometer (D8 ADVANCE, Bruker Corporation) was used to observe the crystal structure of the surface.2.3. Oil/water separation and catalytic degradation of organic pollutants
The oil/water mixtures in the experiment were composed of oil and water (with PMS of 3 mmol L−1) with volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The water and oil were dyed to blue and red color with methylene blue (MB, 5 mg L−1) and Sudan III (5 mg L−1), respectively. Here, MB and Rhodamine B were chosen as organic pollutants to evaluate the catalytic degradation efficiency of IMCM. The device (Fig. S1†) for oil/water separation was designed by fixing the IMCM surface between two glass tubes, which can synchronously achieve oil/water separation and catalytic degradation of organic pollutants. The oil/water mixture was poured into the upper glass tube and the whole separation proceeded by gravity, which was recorded by a digital single lens reflex camera (Canon, EOS 80D, Japan). The separation efficiency (Es) can be defined as Es = maw/mow. Here maw and mow are the mass of water after separation and the mass of water in the original oil/water mixtures, respectively. The flux (F) of the mesh is depicted as F = V/St. Here V, S, and t represent the volume of water, the effective area of the mesh and the whole separating time, respectively. The catalytic degradation efficiency (Ec) is calculated as Ec = (C0 − C)/C0. Here C0 and C are the concentration of organic pollutants before and after catalytic degradation.
1. The water and oil were dyed to blue and red color with methylene blue (MB, 5 mg L−1) and Sudan III (5 mg L−1), respectively. Here, MB and Rhodamine B were chosen as organic pollutants to evaluate the catalytic degradation efficiency of IMCM. The device (Fig. S1†) for oil/water separation was designed by fixing the IMCM surface between two glass tubes, which can synchronously achieve oil/water separation and catalytic degradation of organic pollutants. The oil/water mixture was poured into the upper glass tube and the whole separation proceeded by gravity, which was recorded by a digital single lens reflex camera (Canon, EOS 80D, Japan). The separation efficiency (Es) can be defined as Es = maw/mow. Here maw and mow are the mass of water after separation and the mass of water in the original oil/water mixtures, respectively. The flux (F) of the mesh is depicted as F = V/St. Here V, S, and t represent the volume of water, the effective area of the mesh and the whole separating time, respectively. The catalytic degradation efficiency (Ec) is calculated as Ec = (C0 − C)/C0. Here C0 and C are the concentration of organic pollutants before and after catalytic degradation.
3. Results and discussion
3.1. Fabrication of IMCM
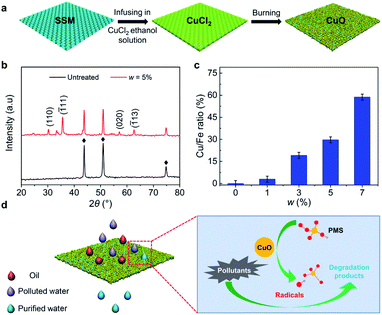
Materials with superwetting and catalytic performances are the foundation for synchronous oil/water separation and organic pollutants purification in one step. Fortunately, copper oxide is a paragon that synergizes these two properties. In this regard, we fabricated an integrated multifunctional copper-oxide-coated mesh (IMCM) via immersing SSM into CuCl2·2H2O solution for 2 min and burning at 1000 °C for 30 s (Fig. 1(a)). The mass fraction (w) of CuCl2·2H2O solution are 0%, 1%, 3%, 5%, 7%, respectively. Note that IMCM with w = 0% means that the SSM is only cleaned but without any further treatment for comparison. The chemical reactions in the preparation are as follows:| Cu2+ + 2OH− ≜ Cu(OH)2 | (1) |
| Cu(OH)2 ≜ CuO + H2O | (2) |
To confirm the chemical components of the fabricated surface, we performed X-ray diffraction (XRD) analyses on IMCM with w of 5% (Fig. 1(b)). For untreated SSM, the peaks marked with asterisk can be indexed to austenite (FeCr0·29Ni0·16C0.06), which is the major component of the SSM. For IMCM, the new characteristic diffraction peaks corresponding to the reflection of (110), (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11), (020) and (
11), (020) and (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 13) crystal planes of monoclinic CuO phase (JCPDS 04-009-2287) are appeared, which indicates that CuO is successfully coated on designed surface. In addition, the EDS measurement shows that Cu and O elements are existed on IMCM and the ratio of Cu/Fe element can be controlled from 3% to 58% by mediating the value of w (Fig. 1(c) and Fig. S2†). The morphologies of IMCM with different w were shown in Fig. S3.† For cleaned mesh without further treatment (w = 0%), the surface is smooth (Fig. S3(a)†). With the increase of w, nanoparticles are formed on the surface and the size of particles increases gradually with formed overlapping and stacking structures (Fig. S3(b–e)).† Further SEM images show that the nanoparticles also distribute uniformly on the cross section of IMCM (Fig. S4†). Combination of the results shown in EDS and SEM measurements, we obtain that the nanoparticles are CuO, which successful grow on IMCM.
13) crystal planes of monoclinic CuO phase (JCPDS 04-009-2287) are appeared, which indicates that CuO is successfully coated on designed surface. In addition, the EDS measurement shows that Cu and O elements are existed on IMCM and the ratio of Cu/Fe element can be controlled from 3% to 58% by mediating the value of w (Fig. 1(c) and Fig. S2†). The morphologies of IMCM with different w were shown in Fig. S3.† For cleaned mesh without further treatment (w = 0%), the surface is smooth (Fig. S3(a)†). With the increase of w, nanoparticles are formed on the surface and the size of particles increases gradually with formed overlapping and stacking structures (Fig. S3(b–e)).† Further SEM images show that the nanoparticles also distribute uniformly on the cross section of IMCM (Fig. S4†). Combination of the results shown in EDS and SEM measurements, we obtain that the nanoparticles are CuO, which successful grow on IMCM.
3.2. Superwetting and catalytic performance of IMCM
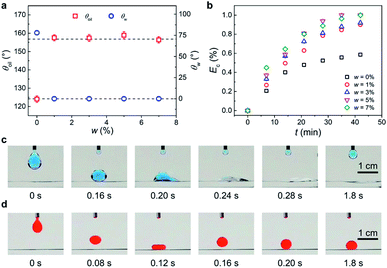
To examine the superwetting performance of IMCM, we measured its water contact angle (θw) and underwater oil contact angle (θoil) on the surfaces which were treated with w of 0%, 1%, 3%, 5%, 7%, respectively. Here the oil is dichloroethane. For w = 0%, the mesh manifests a θw of 78° ± 2.0° and underwater θoil of 124° ± 1.8° (Fig. S5(a and b)†). For w of 1%, 3%, 5%, 7%, superhydrophilicity and underwater superoleophobicity are achieved with an average θw and θoil of ∼0° and 157°, respectively (Fig. 2(a) and S5(c), S5(d)).† We also tested the dynamic wettability of the mesh treated with various value of w (Fig. S6†). It is clear to see that for w < 5%, θSA > 20° and for w ≥ 5%, θSA < 10°. Therefore, we obtain that IMCM treated with w ≥ 5% manifests superoleophobicity and low-adhesion underwater. The superhydrophilicity and underwater superoleophobicity also applied to other types of oil, as exemplified in Fig. S7.† We demonstrate that the excellent superwetting performance of IMCM is mainly determined by the coated hydrophilic chemical component of CuO and could be enhanced by surface micro/nano structures, which guarantees the water to pass through the mesh while the oil to be repelled (Fig. 1(d)).To validate the catalytic performance of the surface, the IMCM surface was immersed in a mixed solution containing 5 mg L−1 MB−1 and 3 mmol L−1 PMS, with deionized water as the solvent. The catalytic degradation efficiency (Ec) is calculated as Ec = (C0 − C)/C0, where C0 and C are the concentration of MB before and after catalytic degradation, respectively. As shown in Fig. 2(b), Ec increases gradually and then maintains unchanged over time. Note that, the IMCM surface with w = 5% achieves the optimal catalytic degradation performance, which degrades almost all the organic pollutions in water in the shortest time. As has been studied in previous research, the lower content of CuO limits the surface catalytic efficiency for w < 5%. While for w > 5%, redundant CuO may scavenge some generated radicals and thus reduces the catalytic efficiency.30,31 Therefore, IMCM surface (w = 5%) with the optimal catalytic efficiency is selected for further exploration.
To verify the superwetting and catalytic performance, we visualize the dynamic process of droplets on IMCM with w = 5%. Here the water droplet including PMS and oil droplet (dichloroethane) are dyed to blue and red color by MB and Sudan III, which are chosen as organic pollutants. During this test, the IMCM was pre-wetted by water because it is not superhydrophilic without pre-wetting under oil (liquid paraffin) environment (Fig. S8†). For water droplet in oil medium, as shown in Fig. 2(c), the water spreads and passes through IMCM rapidly in less than 1.8 s. Strikingly, the water droplet becomes transparent gradually due to the degradation of MB by multiple reactive oxygen species which is generated in catalytically activating process. This result sharply contrasts with the observation of the dynamics process of oil droplet in water medium (Fig. 2(d)), where the oil droplet manifests a ball shape and very low interface adhesion for a thin water film generated between the oil and IMCM surface (Fig. S5(d)).† The dynamic behaviors of water and oil droplets indicate that IMCM possesses excellent superhydrophilicity, underwater superoleophobicity and catalytic degradation performance of organic pollutants, which is consistent with that shown in Fig. 2(a) and (b).
3.3. Oil/water separation performance
In the oil/water separation process, we pour the oil/water mixture with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
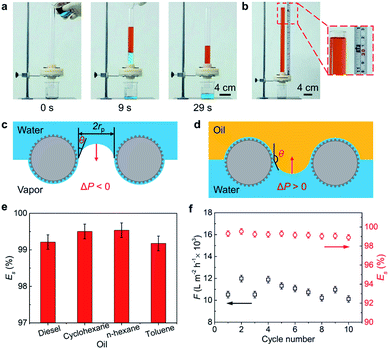
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 into the upper glass tube of a separating unit which is composed by fixing the IMCM between two glass tubes (Fig. S1†). As shown in Fig. 3(a), the water passes through the IMCM easily, while the oil is completely blocked (see Video S2†). Moreover, the maximum height of blocked n-hexane column (hmax) can reach up to ∼31 cm (Fig. 3(b)), corresponding to a maximum hydrostatic pressure of ∼2 kPa. This critical pressure is called breakthrough pressure (ΔP), which is defined as the maximum pressure applied on the surface before the liquid permeates into the surface pore structure.
1 into the upper glass tube of a separating unit which is composed by fixing the IMCM between two glass tubes (Fig. S1†). As shown in Fig. 3(a), the water passes through the IMCM easily, while the oil is completely blocked (see Video S2†). Moreover, the maximum height of blocked n-hexane column (hmax) can reach up to ∼31 cm (Fig. 3(b)), corresponding to a maximum hydrostatic pressure of ∼2 kPa. This critical pressure is called breakthrough pressure (ΔP), which is defined as the maximum pressure applied on the surface before the liquid permeates into the surface pore structure.
The breakthrough pressure is determined by both the surface wetting properties and structures. For pores with a cylindrical geometry, ΔP can be depicted as: ΔP = −2γ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/r, with γ, r and θ represent the surface tension of the liquid, the radius of the pores, and the intrinsic contact angle of the liquid on a flat surface, respectively.32 In the absence of external forces, liquid can pass through pore structures with ΔP < 0, while be blocked with ΔP > 0.33 In our experiment, superhydrophilic surface with θw of ∼0° is formed on designed IMCM surface, which causes a negative ΔP to promote water to pass through the surface spontaneously (Fig. 3(c)). While due to underwater superoleophobicity with average θoil of ∼157°, the ΔP of different kinds of oils is always positive (Table S1†) that forms a blocking effect for oil (Fig. 3(d)). The water anti-blocking and oil blocking effect provide a guarantee for the efficient oil/water separation.
θ/r, with γ, r and θ represent the surface tension of the liquid, the radius of the pores, and the intrinsic contact angle of the liquid on a flat surface, respectively.32 In the absence of external forces, liquid can pass through pore structures with ΔP < 0, while be blocked with ΔP > 0.33 In our experiment, superhydrophilic surface with θw of ∼0° is formed on designed IMCM surface, which causes a negative ΔP to promote water to pass through the surface spontaneously (Fig. 3(c)). While due to underwater superoleophobicity with average θoil of ∼157°, the ΔP of different kinds of oils is always positive (Table S1†) that forms a blocking effect for oil (Fig. 3(d)). The water anti-blocking and oil blocking effect provide a guarantee for the efficient oil/water separation.
We further explored the separation efficiency (Es) in oil/water separating process on IMCM surface. It is clear that the Es always exceeds 99% for different kinds of oil/water mixtures, as shown in Fig. 3(e). Not only separation efficiency, but also flux (F) of water is an important index for evaluating oil/water separation ability. On the fabricated surface, the F of water can reach up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L m−2 h−1. Note that, Es and F are both little changed in 10 cycles on the IMCM surface, indicating an efficient and stable oil/water separation performance (Fig. 3(f)). This result is attributed to the outstanding self-cleaning ability of IMCM, e.g., the oil attached to the surface is easily removed due to the repelling effect of a thin water film generated between oil and IMCM surface (Fig. S5(d) and S9†).
000 L m−2 h−1. Note that, Es and F are both little changed in 10 cycles on the IMCM surface, indicating an efficient and stable oil/water separation performance (Fig. 3(f)). This result is attributed to the outstanding self-cleaning ability of IMCM, e.g., the oil attached to the surface is easily removed due to the repelling effect of a thin water film generated between oil and IMCM surface (Fig. S5(d) and S9†).
3.4. Synchronous oil/water separation and wastewater treatment
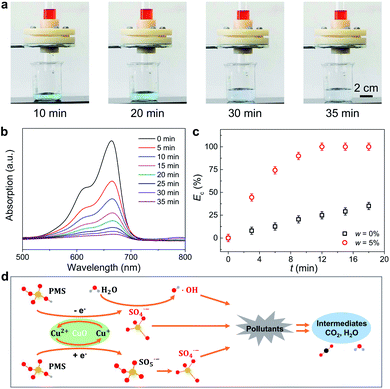
Achieving synchronous oil/water separation and wastewater treatment is a great challenge for the time delay of catalytic performance. To address this drawback, a porous filter paper was added on the IMCM surface to slow down the passing velocity of oil/water mixtures. Here, the water (with PMS of 3 mmol L−1) contains 5 mg L−1 MB−1 as organic pollutants to evaluate the catalytic degradation efficiency of the IMCM while the oil is n-hexane. For IMCM surfaces treated with series of w, the water and oil can be separated completely. Further observation reveals that the water separated by IMCM with w = 0% always stays blue with a slightly lighter in 35 min, due to the degradation ability of PMS (Fig. S10†). In contrast, the water separated by IMCM with w = 5% fades gradually and finally becomes transparent (Fig. 4(a)), which is attributed to the gradual catalytic degradation of MB in water, as evidenced by the peak height of UV-vis absorbance spectrum of MB (Fig. 4(b)). This result indicates that IMCM surface with w = 5% exhibits excellent synchronous oil/water separation and wastewater treatment performance. We also measured the catalytic degradation of IMCM to other organic pollutants, e.g., rhodamine B. It is clear that IMCM with w = 5% manifests an excellent Ec of almost 100% in 12 min, while IMCM with w = 0% manifests an Ec of 25% (Fig. 4(c)). This further indicates that the catalytic degradation performance of IMCM with w = 5% is robust for various kinds of organic pollutants.The catalytic degradation performance of IMCM differed depending on whether or not CuO is present. In our experiment, CuO acts as a catalyst, which can activate PMS to generate multiple reactive oxygen species for high-efficiency degradation of organic pollutions (Fig. 4(d)). The reaction equations are depicted as:
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Cu2+ + HSO5− → Cu2+ + HSO5− → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Cu+ + SO5˙− + H+ Cu+ + SO5˙− + H+
| (3) |
| 2SO5˙− → 2SO4˙− + O2 | (4) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Cu+ + HSO5− → Cu2+ + SO4˙− + OH− Cu+ + HSO5− → Cu2+ + SO4˙− + OH−
| (5) |
| SO4˙− + H2O → ˙OH + SO42− + H+ | (6) |
| SO4˙− + ˙OH + pollutants → intermediates + CO2 + H2O + SO42− | (7) |
First, the Cu2+ is reduced to Cu+ in the presence of PMS and the PMS is activated to SO5˙− and SO4˙− (eqn (3) and (4)).34,35 Subsequently, the Cu+ will react with the PMS to produce SO4˙− (eqn (5)).21 Besides, some SO4˙− radicals will react with H2O to generate ˙OH (eqn (6)).36 In these processes, the Cu2+ and Cu+ act as catalyst via converting to each other with electron transfer. Finally, the generated reactive oxygen species (SO4˙−, ˙OH) can degrade the organic pollutions into intermediates, CO2 and H2O (eqn (7)) and achieve wastewater treatment.37
4. Conclusion
In this research, we report a unique strategy for synchronous oil/water separation and wastewater treatment in one step on integrated multifunctional copper-oxide-coated mesh (IMCM) surfaces. The fabricated IMCM surface exhibits oil/water separation and catalytic degradation efficiencies of more than 99%. We show that such excellent capacities are underpinned by the superwetting and catalytic properties of copper oxide: the superhydrophilic ability generates additional pressure to facilitate the penetration of water while blocking the oil, endowing an efficient oil/water separation; in addition, the copper oxide forms reactive oxygen species to degrade organic pollution during water penetration process. This synchronization strategy will enhance the pure water separation efficiency from industrial oily sewage.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We are grateful for the support from National Natural Science Foundation of China (52075071, 51605073, 52005075), the Fundamental Research Funds for the Central Universities (DUT18RC(3)048, DUT19RC(3)055), Liao Ning Revitalization Talents Program (XLYC1807092) and Opening Project of the Key Laboratory of Bionic Engineering (Ministry of Education), Jilin University (KF20200002). Y. L. and S. F. acknowledge support from the Star Ocean Outstanding Talents Program.Notes and references
- B. Jiang, H. Zhang, L. Zhang, Y. Sun, L. Xu, Z. Sun, W. Gu, Z. Chen and H. Yang, Ind. Eng. Chem. Res., 2017, 56, 11817–11826 CrossRef CAS.
- O. Abdelwahab, N. K. Amin and E. S. Z. El-Ashtoukhy, J. Hazard. Mater., 2009, 163, 711–716 CrossRef CAS PubMed.
- X. Bai, Z. Zhao, H. Yang and J. Li, Sep. Purif. Technol., 2019, 221, 294–302 CrossRef CAS.
- G. Zhang, Y. Li, A. Gao, Q. Zhang, J. Cui, S. Zhao, X. Zhan and Y. Yan, Chem. Eng. J., 2019, 369, 576–587 CrossRef CAS.
- M. Ge, C. Cao, J. Huang, X. Zhang, Y. Tang, X. Zhou, K. Zhang, Z. Chen and Y. Lai, Nanoscale Horiz., 2018, 3, 235–260 RSC.
- S. Feng, Y. Xing, S. Deng, W. Shang, D. Li, M. Zhang, Y. Hou and Y. Zheng, Adv. Mater. Interfaces, 2018, 5, 1701193 CrossRef.
- I. Sadeghi, N. Govinna, P. Cebe and A. Asatekin, ACS Appl. Polym. Mater., 2019, 1, 765–776 CrossRef CAS.
- N. Govinna, I. Sadeghi, A. Asatekin and P. Cebe, J. Polym. Sci., Part B: Polym. Phys., 2019, 57, 312–322 CrossRef CAS.
- L. Tang, Z. Zeng, G. Wang, L. Shen, L. Zhu, Y. Zhang and Q. Xue, ACS Appl. Mater. Interfaces, 2019, 11, 18865–18875 CrossRef CAS PubMed.
- M. Wang, Z. Xu, Y. Guo, Y. Hou, P. Li and Q. J. Niu, J. Membr. Sci., 2020, 611, 118409 CrossRef CAS.
- L. Feng, Z. Zhang, Z. Mai, Y. Ma, B. Liu, L. Jiang and D. Zhu, Angew. Chem., Int. Ed., 2004, 43, 2012–2014 CrossRef CAS PubMed.
- L. Qiu, Y. Sun and Z. Guo, J. Mater. Chem. A, 2020, 8, 16831–16853 RSC.
- J. Li, L. Yan, H. Li, W. Li, F. Zha and Z. Lei, J. Mater. Chem. A, 2015, 3, 14696–14702 RSC.
- M. Liu, S. Wang and L. Jiang, Nat. Rev. Mater., 2017, 2(7), 17036 CrossRef CAS.
- Y. Si, Q. Fu, X. Wang, J. Zhu, J. Yu, G. Sun and B. Ding, ACS Nano, 2015, 9, 3791–3799 CrossRef CAS PubMed.
- J. Ge, L. A. Shi, Y. C. Wang, H. Y. Zhao, H. Bin Yao, Y. B. Zhu, Y. Zhang, H. W. Zhu, H. A. Wu and S. H. Yu, Nat. Nanotechnol., 2017, 12, 434–440 CrossRef CAS PubMed.
- W. Zhang, Z. Shi, F. Zhang, X. Liu, J. Jin and L. Jiang, Adv. Mater., 2013, 25, 2071–2076 CrossRef CAS PubMed.
- J. Yuan, X. Liu, O. Akbulut, J. Hu, S. L. Suib, J. Kong and F. Stellacci, Nat. Nanotechnol., 2008, 3, 332–336 CrossRef CAS PubMed.
- W. Da Oh, Z. Dong and T. T. Lim, Appl. Catal., B, 2016, 194, 169–201 CrossRef.
- N. H. Tran, T. Urase, H. H. Ngo, J. Hu and S. L. Ong, Bioresour. Technol., 2013, 146, 721–731 CrossRef CAS PubMed.
- M. Bilal, M. Adeel, T. Rasheed and H. M. N. Iqbal, J. Mater. Res. Technol., 2019, 8, 2359–2371 CrossRef CAS.
- S. Rojas and P. Horcajada, Chem. Rev., 2020, 120, 8378–8415 CrossRef CAS PubMed.
- H. Meng, C. Nie, W. Li, X. Duan, B. Lai, Z. Ao, S. Wang and T. An, J. Hazard. Mater., 2020, 399, 123043 CrossRef CAS PubMed.
- F. Mushtaq, X. Chen, H. Torlakcik, B. J. Nelson and S. Pané, Nano Res., 2020, 13, 2183–2191 CrossRef CAS.
- J. Wang and S. Wang, Chem. Eng. J., 2018, 334, 1502–1517 CrossRef CAS.
- Z. Lei, J. Feng, Y. Yang, J. Shen, W. Zhang and C. Wang, J. Hazard. Mater., 2020, 382, 121057 CrossRef CAS PubMed.
- Y. Zhu, D. Wang, L. Jiang and J. Jin, NPG Asia Mater., 2014, 6, e101 CrossRef CAS.
- J. Y. Huang, S. H. Li, M. Z. Ge, L. N. Wang, T. L. Xing, G. Q. Chen, X. F. Liu, S. S. Al-Deyab, K. Q. Zhang, T. Chen and Y. K. Lai, J. Mater. Chem. A, 2015, 3, 2825–2832 RSC.
- J. Song, S. Huang, Y. Lu, X. Bu, J. E. Mates, A. Ghosh, R. Ganguly, C. J. Carmalt, I. P. Parkin, W. Xu and C. M. Megaridis, ACS Appl. Mater. Interfaces, 2014, 6, 19858–19865 CrossRef CAS PubMed.
- J. Yan, J. Li, J. Peng, H. Zhang, Y. Zhang and B. Lai, Chem. Eng. J., 2019, 359, 1097–1110 CrossRef CAS.
- B. C. Gilbert and J. K. Stell, J. Chem. Soc., Faraday Trans., 1990, 86, 3261–3266 RSC.
- M. A. Gondal, M. S. Sadullah, M. A. Dastageer, G. H. McKinley, D. Panchanathan and K. K. Varanasi, ACS Appl. Mater. Interfaces, 2014, 6, 13422–13429 CrossRef CAS PubMed.
- C. Chen, D. Weng, A. Mahmood, S. Chen and J. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 11006–11027 CrossRef CAS PubMed.
- M. Xu, J. Li, Y. Yan, X. Zhao, J. Yan, Y. Zhang, B. Lai, X. Chen and L. Song, Chem. Eng. J., 2019, 369, 403–413 CrossRef CAS.
- Z. Yang, D. Dai, Y. Yao, L. Chen, Q. Liu and L. Luo, Chem. Eng. J., 2017, 322, 546–555 CrossRef CAS.
- Z. Li, D. Liu, Y. Zhao, S. Li, X. Wei, F. Meng, W. Huang and Z. Lei, Chemosphere, 2019, 233, 549–558 CrossRef CAS PubMed.
- J. Zou, J. Ma, L. Chen, X. Li, Y. Guan, P. Xie and C. Pan, Environ. Sci. Technol., 2013, 47, 11685–11691 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra02334a |
| This journal is © The Royal Society of Chemistry 2021 |