Open Access Article
Open Access ArticleCanola protein thermal denaturation improved emulsion-templated oleogelation and its cake-baking application†
Yan Ran Tang and
Supratim Ghosh *
*
Department of Food and Bioproduct Sciences, University of Saskatchewan, 51 Campus Drive, Saskatoon, SK S7N5A8, Canada. E-mail: supratim.ghosh@usask.ca; Tel: +1-306-966-2555
First published on 19th July 2021
Abstract
The stability and viscoelasticity of an oil-in-water emulsion formed with canola proteins could be significantly improved by heat-induced protein thermal denaturation followed by aggregation at the oil droplet surface. This phenomenon was used to develop emulsion-templated oleogels with improved rheology and used in cake baking. Canola oil (50 wt%)-in-water emulsions stabilized by 1 and 4 wt% canola protein isolates (CPI), prepared by high-pressure homogenization, were dried at 60 °C in a vacuum oven followed by shearing to create the oleogels. Before drying, the emulsions were heated (90 °C for 30 min) to induce protein denaturation. The oleogel from 4 wt% CPI heated emulsions (HE) exhibited the lowest oil loss, highest gel strength, firmness and stickiness compared to all other oleogels. Cake batter prepared with shortening showed the lowest specific gravity, highest viscosity and storage modulus compared to CPI oleogels. Confocal micrographs of shortening cake batters showed smaller air bubbles entrapped in the continuous fat phase. In comparison, the oleogel cake batters showed dispersion of larger air bubbles, oil droplets, and protein aggregates. The oleogel cake showed a darker colour compared to the shortening cake due to the dark colour of CPI. Interestingly, oleogel cakes showed lower hardness, higher cohesiveness and springiness than the shortening cake, which was attributed to the higher cake volume of the former due to the formation of larger air channels stabilized by canola proteins. In conclusion, CPI stabilized emulsion-templated oleogels could be used as a potential shortening replacer in cake and other baking applications.
1. Introduction
Oleogelation is an approach where liquid oil is fabricated into a gel-like structure without traditional saturated or trans-fat. It is seen as an alternative to structure formation in unsaturated fatty acid-rich oils to meet the zero-trans and low-saturated fat challenge.1,2 In oleogels, the traditional fat crystal network of saturated fat-rich oils is replaced by molecular or polymeric gelators. There are two approaches to oleogel development: direct and indirect oleogelation.3–5 Direct oleogelation involves directly mixing the liquid oil with oleogelators such as waxes or phytosterols at their melting temperature. Upon cooling, the oleogelators crystallize or form structures that entrap the liquid oil within the matrix, forming a self-supporting gel. Indirect approaches such as foam and emulsion-templated oleogelation are used for biopolymeric oleogelators, such as proteins and polysaccharides. The biopolymeric oleogelators are first used to stabilize foam or emulsion so that their hydrophobic moieties are exposed. Subsequently, the aqueous phase of the system is removed through drying, thereby forming the oleogel. In the foam-templated approach, the liquid oil is added to the dried foam to develop oleogels. In the emulsion-templated approach, the dried emulsions are sheared to form homogeneous oleogels.6 However, the disintegration of the oil droplets structure during drying and shearing remains a challenge for the emulsion-templated oleogelation. Recent studies of emulsion-templated oleogelation utilized protein–polysaccharides complexes as oleogelators to improve their stability. For example, sodium caseinate (SC) and alginate (ALG) complex,7 gelatin–tannic acid-flaxseed gum complexe,8 soy protein and κ-carrageenan complex.9 Wijaya et al.7 reported the formation of stable oleogels with high gel strength and high oil binding capacity using an SC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ALG ratio of 12
ALG ratio of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at pH 7, which was ascribed to the thickening effect of the SC
1 at pH 7, which was ascribed to the thickening effect of the SC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ALG mixtures at the interface entrapping the oil within the network. Tavernier et al.9 observed no oil leakage in their soy protein–κ carrageenan stabilized emulsion-templated oleogel after several months of storage. However, they reported a higher gel strength of the oleogel stabilized solely with soy protein than the soy protein–κ carrageenan complex, attributed to a different protein conformation at the interface when it was complexed with κ carrageenan. Other than the complexes, polysaccharides, such as hydroxypropyl methylcellulose (HPMC),10 regenerated cellulose (RC) and carboxymethyl cellulose (CMC)11 were also used as oleogelators in the emulsion-templated approach. Meng et al.10 used guar gum, gum Arabic and xanthan gum as thickening agents to form emulsion-templated oleogels with higher gel strength and lower oil loss using HPMC as an emulsifier. Shear thinning behaviour was also commonly observed among the various oleogels.10–12 Patel13 investigated thixotropy recovery of oleogels which gave an insight on the repeated applicability of oleogels as structured oil in food. The emulsion-templated oleogels structured with HPMC, cellulose and gelatin had shown significant thixotropic recovery with the highest recovery of 90% observed for HPMC and methylcellulose-based oleogels.8,11,14 Emulsion-templated oleogel structured with gelatin and xanthan gum exhibited partial thixotropic recovery even at high temperature (80 °C).15 One common factor in most emulsion-templated oleogel development was the importance of fabricating strong interfacial layers in emulsions so that the oil droplets' structure is stable against coalescence during drying and shearing. In this respect, the plant proteins' ability to undergo thermal denaturation followed by aggregation at the oil droplet surface, thereby improving the stability of the interface, has not been investigated. The present work is focused on the thermal denaturation of canola proteins at the oil droplet surface in improving the stability of the emulsion-templated oleogels.
ALG mixtures at the interface entrapping the oil within the network. Tavernier et al.9 observed no oil leakage in their soy protein–κ carrageenan stabilized emulsion-templated oleogel after several months of storage. However, they reported a higher gel strength of the oleogel stabilized solely with soy protein than the soy protein–κ carrageenan complex, attributed to a different protein conformation at the interface when it was complexed with κ carrageenan. Other than the complexes, polysaccharides, such as hydroxypropyl methylcellulose (HPMC),10 regenerated cellulose (RC) and carboxymethyl cellulose (CMC)11 were also used as oleogelators in the emulsion-templated approach. Meng et al.10 used guar gum, gum Arabic and xanthan gum as thickening agents to form emulsion-templated oleogels with higher gel strength and lower oil loss using HPMC as an emulsifier. Shear thinning behaviour was also commonly observed among the various oleogels.10–12 Patel13 investigated thixotropy recovery of oleogels which gave an insight on the repeated applicability of oleogels as structured oil in food. The emulsion-templated oleogels structured with HPMC, cellulose and gelatin had shown significant thixotropic recovery with the highest recovery of 90% observed for HPMC and methylcellulose-based oleogels.8,11,14 Emulsion-templated oleogel structured with gelatin and xanthan gum exhibited partial thixotropic recovery even at high temperature (80 °C).15 One common factor in most emulsion-templated oleogel development was the importance of fabricating strong interfacial layers in emulsions so that the oil droplets' structure is stable against coalescence during drying and shearing. In this respect, the plant proteins' ability to undergo thermal denaturation followed by aggregation at the oil droplet surface, thereby improving the stability of the interface, has not been investigated. The present work is focused on the thermal denaturation of canola proteins at the oil droplet surface in improving the stability of the emulsion-templated oleogels.
Cakes are one of the popular bakery products in the market. The main ingredients required for cake baking are flour, sugar, fat, emulsifiers, leavening agent and water.16 After mixing, cake batter can be considered as a complex mixture of air and oil-in-water emulsion with dissolved and dispersed dry ingredients in the continuous aqueous phase. Air incorporation was seen as one of the most critical aspects in making a high-volume cake with desirable texture. Among the ingredients, fat assists in the air bubble's entrapment during mixing and leaven the product during baking.17 Shortening, which is formulated with blends of highly saturated fats, and monoglycerides are commonly used in the commercial production of cake. Apart from air entrapment, shortening also tenderizes the crumb, retains moisture in the product, and enhances the mouthfeel.16 Despite the wide applications of shortening in bakery products, the health concern of using shortening had been rising due to the reported link of highly saturated fat to cardiovascular diseases.18 Therefore, researchers have been developing shortening replacers in baking with inulin, vegetable oils and oleogels.19–21 The oleogels utilized in cake baking were mainly wax-based20,22,23 and phytosterols-based oleogels24 prepared using direct oleogelation. Application of emulsion-templated oleogel as shortening replacer in a cake was reported by Patel et al.25 and Luo et al.26 using methylcellulose and tea polyphenol–palmitate particles plus citrus pectin as emulsion stabilizers and oleogelators, respectively. The sensory hedonic scores for overall cake quality reported by Luo et al.26 were 21.5–27.6 for the oleogel cakes and 32.0 for the butter cake. It was proposed that the parameters that scored the lower score in the oleogel cakes were texture, crust, and crumb colour, while the flavour and odour scores were comparable to the butter cake. The higher hardness of the oleogel cakes was attributed to a lower air bubble incorporation in the batters and the cross-linked network of the citrus pectin in the batter. Patel et al.27 compared the texture of cakes made with butter, margarine, liquid oil and oleogels, over 3 days. Oleogel cakes showed a comparable result to the shortening cake and significant improvement compared to the liquid oil cake in the increase of hardness and chewiness over time, attributed to the physical barrier formed by the oleogel that reduced the cross-linking of the gluten network over time.28
The objectives of the present work was to utilize heat-denatured canola protein isolate (CPI) as an oleogelator through the emulsion-templated approach and then investigate the oleogel-based cake batter, structure and texture in comparison to traditional shortening. Canola proteins are known for their heat-induced gelling properties and were widely explored in the development of hydrogels.29–33 The gelling mechanism of canola proteins relies on the protein denaturation without splitting of disulphide bonds at its denaturation temperature, forming a particulate fractal structure supporting by the hydrophobic interactions, hydrogen bonds and disulphide bonds.30 However, canola proteins have never been used to create gels in hydrophobic oil phases. In our previous study, CPI was used to form a thick interfacial layer at the oil–water interface, which stabilized concentrated oil-in-water emulsions against coalescence during regular storage and heat treatment.34 The heat treatment of CPI-stabilized emulsions induced strong gelation due to protein aggregation at the oil droplet surface and the continuous phase. Therefore, we hypothesized that heat-denatured CPI's ability to form a dense and stable interfacial network stabilizing the oil droplets could be successfully utilized in the development of CPI-stabilized emulsion-templated oleogels. The replacement of highly saturated shortening by the CPI-based oleogel is expected to significantly improve the health beneficial aspect of baking fat.
2. Materials and method
2.1 Materials
Canola protein isolate (CPI: 94.5% d.b. protein, 1.6% moisture, 0.7% ash, 1.0% lipid, and 2.2% carbohydrate) was isolated from cold-pressed canola meal (donated by Pleasant Valley Oil Mills, Alberta, Canada) according to the method described by Tang and Ghosh.34 The ingredients used in cake baking, such as canola oil (Great Value brand), vegetable oil shortening (Crisco brand, composed of soybean oil, hydrogenated palm oil, modified palm oil, mono and diglycerides, TBHQ and citric acid), granulated sugar (Redpath, ON, Canada), baking powder (Magic baking powder, Kraft General Foods Canada Inc.), table salt (Windsor, QC, Canada), all-purpose flour (Robinhood Original All-Purpose Flour, ON, Canada) were purchased from a local grocery store (Walmart, Saskatoon, Canada). Milli-Q™ water (Millipore Corporation, MA, USA) was used to prepare all the solutions. All other chemicals were purchased from Sigma Aldrich (Oakville, ON, Canada).2.2 Oleogels preparation with the emulsion-templated approach
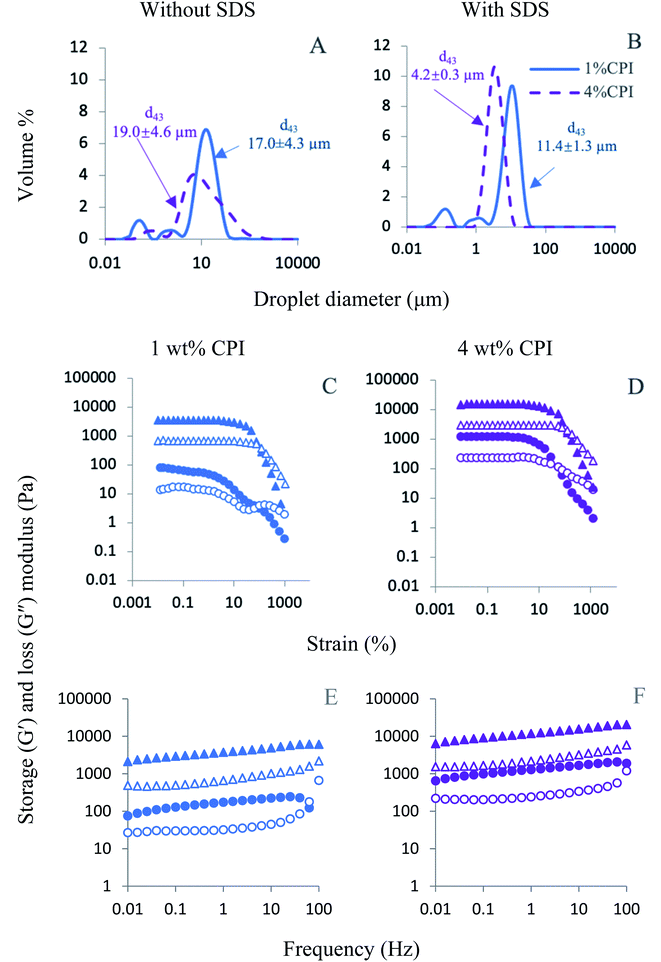
Oleogel was prepared with an emulsion-templated approach. Oil-in-water (O/W) emulsions containing 50 wt% canola oil were prepared with 1 and 4 wt% CPI via six passes of high-pressure homogenization at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi (Emulsiflex C3, Avestin Inc, Ottawa, ON, Canada). In our previous study, 1, 2, 3 and 4 wt% CPI was used to stabilize 50 wt% oil-in-water emulsions. The droplet size were measured using a static laser diffraction particle size analyzer (Mastersizer 2000, Malvern Instrument, Montreal, QC, Canada).34 They were stable against coalescence at all concentrations with 4 wt% CPI emulsions showed the smallest droplet size and the highest gel strength.30 In the present study, only 1 and 4 wt% CPI-stabilized oil-in-water emulsions were used as a template for oleogelation. The emulsions were heated at 90 °C for 30 min to ensure complete protein denaturation and cooled down to room temperature before drying. To confirm protein denaturation, thermal behaviour of CPI was determined using a Differential Scanning Calorimeter (DSC) (Fig. S1, ESI†). From the DSC thermogram, it can be confirmed that holding the emulsion at 90 °C for 30 min, would completely denature CPI. The rheological properties of the heated emulsions were analyzed according to Tang et al.35 The heated (HE) and unheated emulsions (UE) were then dried at 0.4 atm vacuum (Vacuum Oven 9630, National Appliance Company, Portland, OR, USA) at 60 °C to constant weight. Finally, the oleogels were formed by shearing the dried emulsions using a 250 W kitchen hand blender (Oster®, Canada) until a homogenous texture was obtained.
000 psi (Emulsiflex C3, Avestin Inc, Ottawa, ON, Canada). In our previous study, 1, 2, 3 and 4 wt% CPI was used to stabilize 50 wt% oil-in-water emulsions. The droplet size were measured using a static laser diffraction particle size analyzer (Mastersizer 2000, Malvern Instrument, Montreal, QC, Canada).34 They were stable against coalescence at all concentrations with 4 wt% CPI emulsions showed the smallest droplet size and the highest gel strength.30 In the present study, only 1 and 4 wt% CPI-stabilized oil-in-water emulsions were used as a template for oleogelation. The emulsions were heated at 90 °C for 30 min to ensure complete protein denaturation and cooled down to room temperature before drying. To confirm protein denaturation, thermal behaviour of CPI was determined using a Differential Scanning Calorimeter (DSC) (Fig. S1, ESI†). From the DSC thermogram, it can be confirmed that holding the emulsion at 90 °C for 30 min, would completely denature CPI. The rheological properties of the heated emulsions were analyzed according to Tang et al.35 The heated (HE) and unheated emulsions (UE) were then dried at 0.4 atm vacuum (Vacuum Oven 9630, National Appliance Company, Portland, OR, USA) at 60 °C to constant weight. Finally, the oleogels were formed by shearing the dried emulsions using a 250 W kitchen hand blender (Oster®, Canada) until a homogenous texture was obtained.
2.3 Oil binding capacity
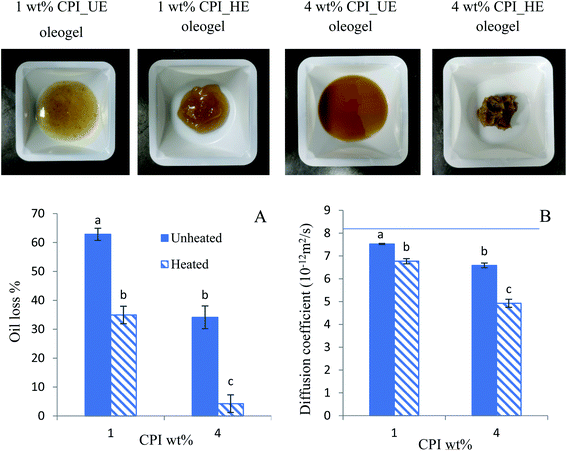
The oil binding capacity of the oleogels was evaluated according to Marangoni et al.36 with slight modifications. The oleogel samples (approximately 2.5 g) were transferred to a 50 mL centrifuge tube and centrifuged in DuPont Sorvall Instruments Model RC-5C centrifuge at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min. After centrifugation, the released free oil at the surface was decanted, and the weight of the centrifuge tube was measured. The percentage oil loss was calculated by eqn (1):
000 rpm for 15 min. After centrifugation, the released free oil at the surface was decanted, and the weight of the centrifuge tube was measured. The percentage oil loss was calculated by eqn (1):
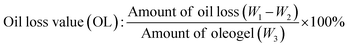 | (1) |
2.4 Measurement of oil diffusion using PFG-NMR
The oil binding capacity of the oleogels in terms of oil diffusion coefficient was measured using a pulse field gradient (PFG) NMR with stimulated echo pulse sequence.7 The NMR measurements were performed at 23 °C with a Bruker Avance III spectrometer (magnetic field strength 14.1 T, corresponding to a 1H frequency of 600.17 MHz), using a standard Bruker 5 mm BBO probe with Z-gradient. The samples were filled into the standard glass NMR tube (5 mm OD) without any deuterated solvents. The 1H Diffusion-Ordered Spectroscopy (DOSY) data were acquired using the stimulated echo pulse (STE) sequence with bipolar gradient pulses. The detailed experimental parameters were as following: diffusion delay (Δ) of 200 ms, diffusion gradient length (δ) of 6 ms, 15 linear gradient increments with gradient strength (G) from 0.96 to 45.74 g cm−1, 16 scans for each spectrum with relaxation delay 12 s. The combination of Δ and δ was optimized to provide at least 95% signal attenuation at the strongest gradient field. The DOSY data were analyzed using the Bruker Topspin software (version 3.6). The diffusion coefficient, D, was calculated from the equation below| I = I0 exp[−γ2δ2(Gz)(Δ − δ/3) × D] | (2) |
2.5 Rheological properties of oleogels
The rheological properties of the emulsions and oleogels were measured using a rheometer (AR G2, TA instruments, Montreal, QC, Canada) with a 40 mm cross-hatched parallel plate geometry.37 The samples were loaded onto the Peltier plate using a spatula. All the measurements were performed at 25 °C with a geometry gap of 1000 μm. For the determination of viscoelasticity, oscillatory strain sweep and frequency sweep were performed. The oscillatory strain sweep was applied at the constant frequency of 1 Hz and an increasing strain from 0.01% to 1000%. The frequency sweep was performed at a constant 0.1% strain within the linear viscoelasticity region, increasing frequency from 0.01 Hz to 100 Hz. The reversibility in the viscoelastic behaviour of the oleogel was also determined by repeating the strain sweep measurements using ascending, descending and second ascending pathways. The viscosity was measured by rotational shear using a cross-hatched parallel plate geometry as a function of increasing shear rate from 0.01 s−1 to 200 s−1. To evaluate the structure recovery (thixotropy) at a different shear rate, the samples were subjected to a 3-interval thixotropy test where the samples were sheared at 0.1 s−1 for 600 s, followed by 10 s−1 for 600 s and again at 0.1 s−1 for 600 s. The viscosity, storage modulus (G′) and loss modulus (G′′) of the samples were recorded with the TRIOS software version 4.5.0.42498 (TA Instruments, Montreal, QC, Canada).2.6 Texture analysis of oleogels
The hardness and cohesiveness of the oleogels were measured using a texture analyzer (TA-Plus texture analyzer, Texture Technologies Corp., South Hamilton, MA, USA) fitted with a conical spreadability test probe (TA-425 TTC spreadability RIG) according to Mohanan et al.37 All the measurements were taken in compression mode with a penetration depth of 65 mm, test speed of 3 mm s−1 and post-test speed of 10 mm s−1. Before the measurement, the samples were loaded and pressed gently using a plastic spatula into the female cone to reduce air incorporation as much as possible. Subsequently, the female cone was fixed on the bottom platform of the texture analyzer. The male cone was to penetrate the sample and returned to the initial position after 65 mm penetration. A graph of force (g) versus time (s) was plotted during the measurement with the Exponent software version 6.1.16.0 (Stable Micro System, Surrey, United Kingdom). The hardness and cohesiveness were obtained from the maximum positive and negative force of the graph.2.7 Preparation and characterizations of cakes
2.8 Statistical analysis
All experiments were conducted in triplicate. The results were reported as mean ± standard deviation. The results were analyzed using an independent t-test and analysis of variance (ANOVA) with a 95% confidence level where p < 0.05 indicates a significant difference. The statistical analysis was done using SPSS software (v25, IBM, Armonk, NY, USA).3. Results and discussion
3.1 Emulsions characterization
3.2 Visual observation of oleogels
The oleogels were formed by drying the emulsions. The unheated emulsion (UE) oleogels exhibited liquid like texture, most likely attributed to the destruction of the oil droplets due to shearing, leading to the release of liquid oil (Fig. 2, top row). The heated emulsions (HE) oleogels exhibited gel-like structure after shearing, where it can be observed that the 4 wt% CPI HE oleogel was much firmer than the 1 wt% CPI HE oleogel (Fig. 2, top row).3.3 Oleogel oil binding capacity
3.4 Rheological properties of oleogels
![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) n), where τ is the applied stress and
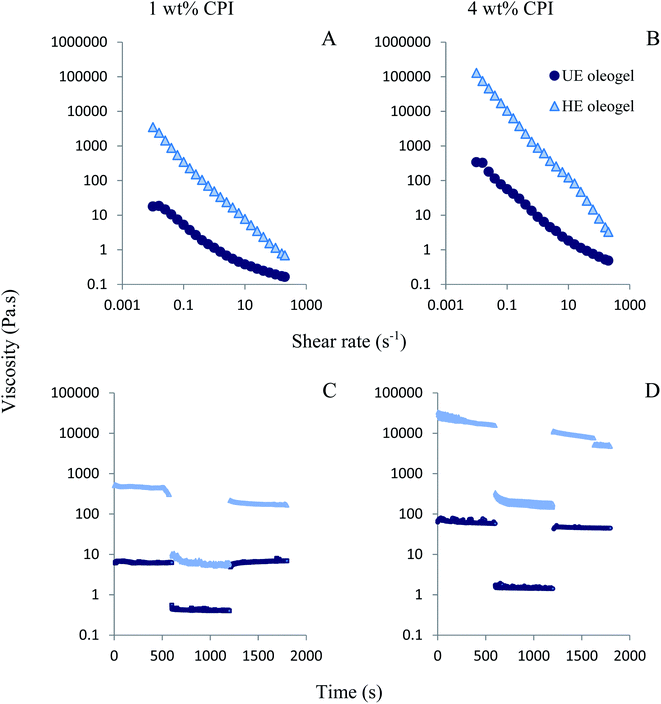
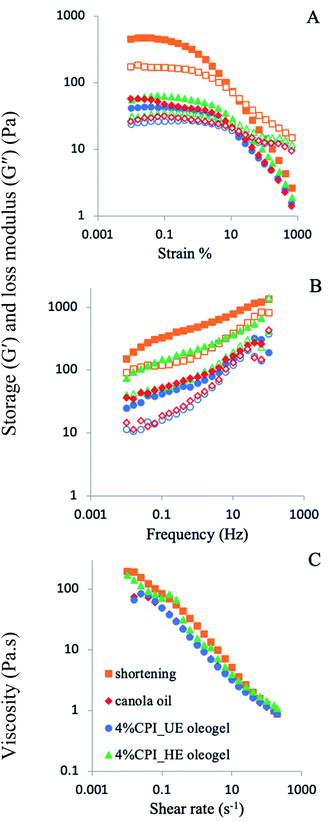
n), where τ is the applied stress and ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) is the shear rate. The values of the consistency coefficient (K) and flow behaviour index (n) was calculated and shown in Fig. S4 (ESI†). Consistency coefficient (K) is a measure of apparent viscosity at 1 s−1 shear rate, which followed the similar trend observed in Fig. 3A. Higher values of K was obtained for the oleogels from heated emulsions (HE oleogel), compared to unheated emulsions (UE oleogel). Increase in CPI concentration from 1 to 4 wt% also led to an increase in the values of K. The flow behaviour index (n) is a measure of pseudoplasticity, where lower values of n for HE oleogels compared to UE oleogels indicate higher degree of pseudoplasticity, which could be due to more structural breakdown in the former.
is the shear rate. The values of the consistency coefficient (K) and flow behaviour index (n) was calculated and shown in Fig. S4 (ESI†). Consistency coefficient (K) is a measure of apparent viscosity at 1 s−1 shear rate, which followed the similar trend observed in Fig. 3A. Higher values of K was obtained for the oleogels from heated emulsions (HE oleogel), compared to unheated emulsions (UE oleogel). Increase in CPI concentration from 1 to 4 wt% also led to an increase in the values of K. The flow behaviour index (n) is a measure of pseudoplasticity, where lower values of n for HE oleogels compared to UE oleogels indicate higher degree of pseudoplasticity, which could be due to more structural breakdown in the former.
Another critical property that affects the functionality of oleogels in food products is their thixotropic recovery.13 In thixotropic materials, structural strength or viscosity decreased at a higher shear rate, still, the material should recover its structure, at least partially, when the shear rate is lowered. To gain insight into the oleogel structural recovery, a three-interval thixotropy test (3-ITT) was performed according to Tavernier et al.49 In this test, the viscosity was measured as a function of three time-intervals (600 s) under low, high and again low values of constant shear rates (0.1, 10, 0.1 s−1). The 3-ITT results are shown in Fig. 3C and D. The structural recovery ratio was calculated by comparing the apparent viscosity at the end of the first time-interval (0.1 s−1) and at the end of the last time-interval (0.1 s−1). The UE oleogels showed high structural recovery values 93% and 94% for 1 wt% (Fig. 3C) and 4 wt% CPI oleogels (Fig. 3D), respectively. The high structural recovery of the UE oleogels could be resulted from the lack of stable oil droplets in the structure and due to the presence of free liquid oil (higher oil loss, Fig. 2A). Therefore, these values might not be a good indicator of the structural recovery in the UE oleogels as there was not a great extent of packed oil droplet structure to begin with (visual observation in Fig. 2, top row).49 In contrast to the UE oleogels, the HE oleogels had a lower structural recovery with values 68% and 60% for 1 wt% (Fig. 3C) and 4 wt% CPI (Fig. 3D), respectively. Previously, thixotropic recovery ranged from around 17% to above 75% was reported for various emulsion-templated oleogels. For instance, Jiang et al.11 reported above 75% recovery for their regenerated cellulose and carboxymethyl cellulose-stabilized oleogels. Tavernier et al.49 investigated the effect of candelilla wax concentration in the emulsion-templated oleogels stabilized by 2.5 wt% soy protein. Though the overall structural recovery was lower than the other literature values, the authors reported an increase in structural recovery from 11% to 17% as the wax concentration increased from 1 to 5%. Luo et al.26 also showed good recovery of the structure in the thixotropy test of tea polyphenol palmitate particles plus citrus pectin stabilized oleogels; however, no exact values were reported. In the present case, thixotropic recovery of the HE oleogels decreased when the protein concentration increased from 1 to 4 wt%, which could be attributed to the inability of the oleogel to recover the strong protein network structure in 4 wt% CPI oleogel compared to the near-complete recovery of the weaker protein network structure in 1 wt% CPI oleogel.
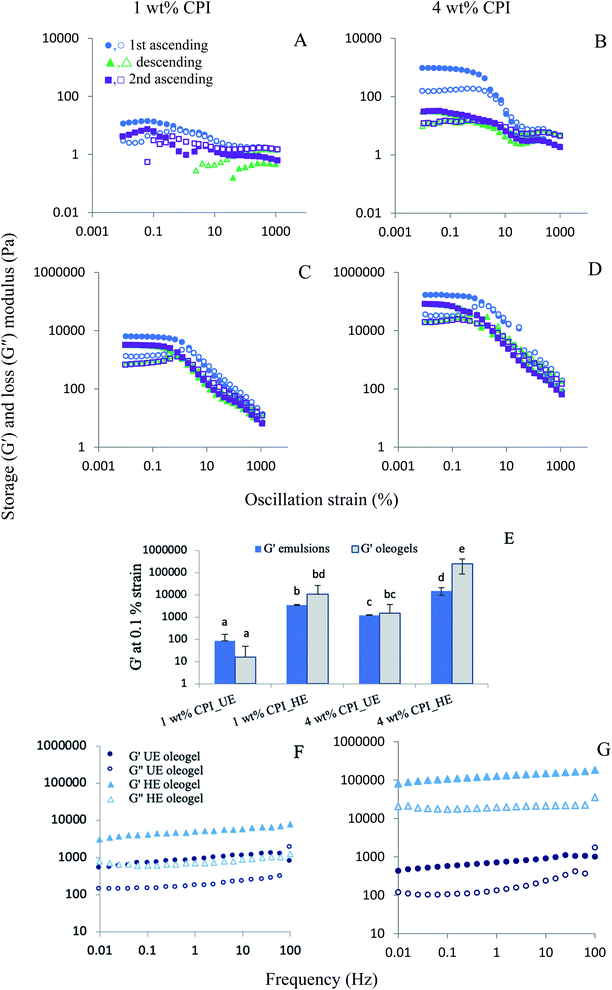
Emulsion-templated oleogels with gel strength ranged from 104 to 106 Pa was reported by various researchers. A common trend was the increase in G′ with an increase in the oleogelator concentration. In the present case, a maximum G′ of about 2.5 × 105 Pa was observed for the 4 wt% CPI HE oleogels. According to Wijaya et al.,7 the highest G′ within the LVR was about 105 Pa for the oleogels stabilized by sodium caseinate–alginate complexes. Tavernier et al.9 reported the highest G′ between 105 Pa to 106 Pa in their emulsion-templated oleogels stabilized with 2.5% soy protein. The variability of the oleogel gel strengths among different studies could be attributed to various factors such as emulsion preparation and stabilization, oleogelator type and concentration, drying and shearing methods.
To directly compare the gel strength of the oleogels and the corresponding emulsions from the present study, their G′ values at 0.1% strain were plotted in Fig. 4E. For the unheated samples, emulsions' G′ values were not significantly different than the corresponding oleogels (p > 0.05). In contrast, for the 4 wt% CPI heated samples, the oleogels showed a significantly higher G′ than the corresponding emulsions (p < 0.05). Its G′ value was also the highest (∼2.5 × 105 Pa) among all the oleogels and emulsions. The higher G′ of the oleogels than the emulsions could result from the removal of water from the continuous phase during drying, leading to the close-packed structure and stronger network formation by the protein-coated oil droplets and the aggregated proteins in the continuous phase.7 Here, the strength of the protein interfacial layer and its ability to fully cover the oil droplets are critical for oleogel viscoelasticity because only the 4 wt% CPI oleogels with heat-denatured protein-covered oil droplets showed an increased gel strength compared to the emulsions.
In the frequency sweep viscoelasticity study, all oleogels showed a higher G′ than G′′ at all frequencies up to 100 Hz (Fig. 4E and F). At 100 Hz, a crossover of G′ and G′′ was observed in the UE oleogels, indicating a gel breakdown at high-frequency shear. The HE oleogels exhibited strong gel-like behaviour with higher G′ and G′′ than the UE oleogels at all frequencies without any crossover, with the 4 wt% CPI HE oleogels showed the highest G′ at about 105 Pa.
3.5 Texture analysis of oleogels
The texture parameters (firmness and stickiness) of the oleogels are shown in Fig. 5. A graph of force versus time was plotted during the measurement, and the positive maximum peak height was taken as the firmness while the maximum negative peak height was taken as stickiness.50 Firmness represents the total amount of force required during the shearing process. In contrast, stickiness represents the force necessary to overcome the adhesive forces between the sample and the surface of the probe with which the sample comes in contact.51 Overall, the 4 wt% CPI HE oleogel showed the highest firmness (47.5 ± 16.7 N) and stickiness (34.8 ± 15.8 N). The firmness of the 4 wt% CPI HE oleogel is comparable to the commercial shortening (46.5 ± 1.03 N) reported by Mohanan et al.37 using a similar experimental setup. For the 1 wt% CPI oleogels, there was no significant difference in firmness and stickiness between the UE and HE samples (p > 0.05). For the 4 wt% CPI oleogels, both the firmness and stickiness of the HE sample were higher than that of the UE sample (p < 0.05). The increase in firmness and stickiness in the heated emulsions oleogels could be ascribed to the strengthened protein network around the oil droplets from the heat denaturation and subsequent aggregation. Upon removing the water from the emulsions, the protein–protein network further strengthened around the oil droplets due to fewer protein–water interactions through hydrogen bonds.52 This can be seen from the one order of magnitude increase in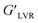 of the emulsions before and after heat treatment (Fig. 1C and D) and again in the corresponding oleogels (Fig. 4E). The increase in gel strength of the oleogels also improved their firmness. The stickiness of the oleogels could be due to the change in hydrophobic interactions or hydrogen bonding from the proteins. Yang et al.30 carried out the gel dissociation test to understand the interactions involved in the canola protein hydrogel. They had reported a sticky texture after the gel was immersed in urea for 48 h to disrupt the hydrogen bonds among the proteins.
of the emulsions before and after heat treatment (Fig. 1C and D) and again in the corresponding oleogels (Fig. 4E). The increase in gel strength of the oleogels also improved their firmness. The stickiness of the oleogels could be due to the change in hydrophobic interactions or hydrogen bonding from the proteins. Yang et al.30 carried out the gel dissociation test to understand the interactions involved in the canola protein hydrogel. They had reported a sticky texture after the gel was immersed in urea for 48 h to disrupt the hydrogen bonds among the proteins.
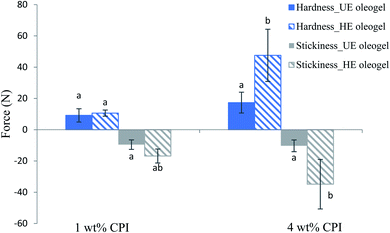 | ||
| Fig. 5 Texture parameters (firmness and stickiness) of the oleogels from different emulsion templates. Different letters indicate significant difference within the same parameters (p < 0.05). | ||
3.6 Characterization of cake batters prepared with different types of baking fat
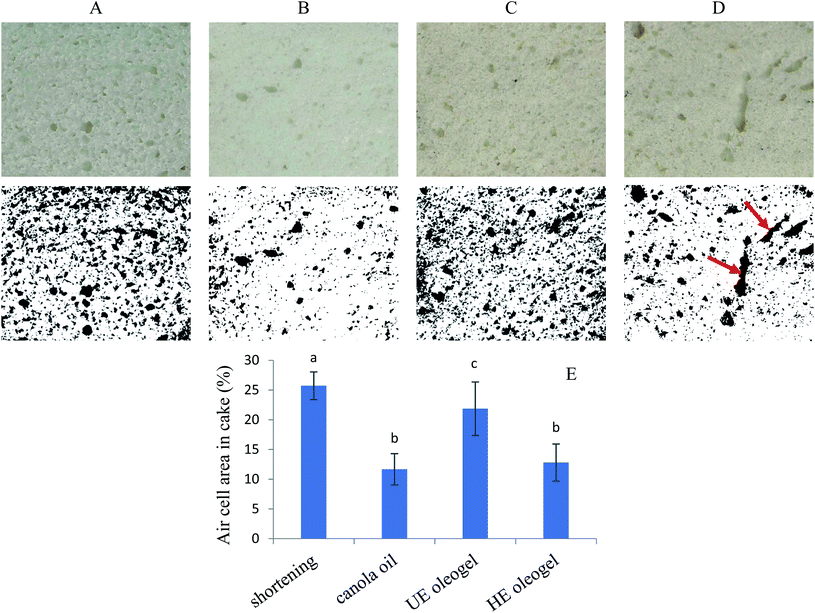
Since the 4 wt% CPI emulsion oleogels exhibited more desirable characteristics (higher gel strength and lower oil loss), both the UE and HE oleogels made from 4 wt% CPI emulsions were used as baking fat in cake preparation. Shortening and canola oil was also used as a control baking fat. The image of the cake batters after mixing are shown in Table 1. The cake batter prepared from the oleogels appeared to be darker than that of the shortening and canola oil cake batters.Since fat enhances the batter aeration for leavening of cake during baking, the specific gravity of the cake batter was determined to understand the extent of aeration due to shortening replacement with oleogels.16 The specific gravity of the shortening cake batter (1.01 ± 0.02) was the lowest of all; however, only the specific gravity of HE oleogel cake batter (1.09 ± 0.03) was significantly different than all the others (p < 0.05) (Table 1). The mechanism of air bubble stabilization by shortening is different from canola oil and oleogels. The shortening cake batter showed numerous small air bubbles entrapped in the continuous fat phase (Fig. 7). The solid crystals and the monoglyceride emulsifiers present in the shortening helped to stabilize a large number of air bubbles.17,56 Oleogels were able to incorporate a lower amount and larger air bubbles compared to shortening. Some air bubbles were also seen to be stabilized by oil droplets (see arrows in Fig. 7B and C); however, oil droplets were not as efficient as fat crystals and monoglycerides. Hence, HE oleogel with a higher oil droplet stability was not capable of stabilizing a large number of air bubbles (Fig. 7D). A higher specific gravity of the oleogel cake batter than the shortening cake had also been reported by Amoah et al.20 and Patel et al.25
3.7 Evaluation of cake properties prepared with different types of baking fat
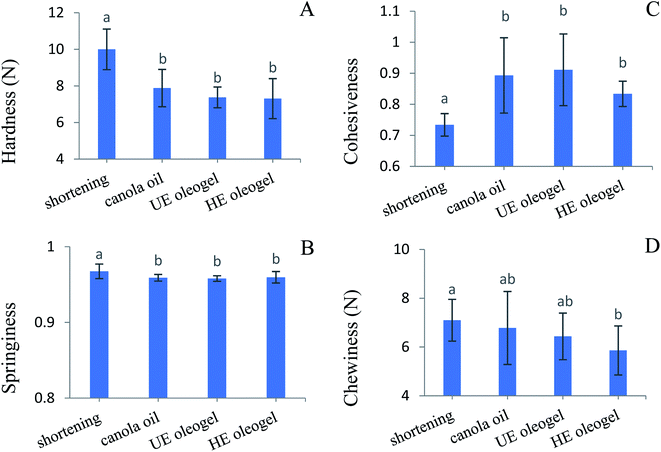 | ||
| Fig. 8 Texture parameters (A) hardness, (B) springiness, (C) cohesiveness, (D) chewiness of cakes prepared with different types of fats. Different letters indicate significant difference (p < 0.05). | ||
Springiness (Fig. 8B) indicates a cake's ability to recover to its initial height between the two compression cycles. Although it is significantly different, the shortening cake's springiness (0.97 ± 0.01) was almost similar to the canola oil and oleogel cakes (0.96 ± 0.004). The cohesiveness (Fig. 8C) of the cake was calculated from the ratio of the second peak area to the first peak area in the force versus time graph. It gives an indication of the crumbliness or perceptions of denseness in the sensory studies.61 The cohesiveness of the shortening cake was lower than that of the canola oil and oleogel cakes (p < 0.05), suggesting less crumbliness for the shortening cake. Chewiness (Fig. 8D) is a function of hardness, springiness and cohesiveness. The shortening, canola oil and UE oleogel cakes exhibited higher chewiness with values ranged from 6.44 ± 0.96 N to 7.09 ± 0.86 N. In comparison, the HE oleogel cake showed a significantly lower chewiness (5.86 ± 1.00 N) than the shortening cake (p < 0.05), but it was similar to the canola oil and UE-oleogel cakes (p > 0.05).
To understand the origin of higher cake volume in the HE oleogel cake, the internal cellular structure of the cakes was recorded, and the cell area percentages were calculated by analyzing the binary images (Fig. 9). The shortening cake showed the highest cell area percentage, which could be attributed to stabilization of numerous air bubbles by the fat crystals and monoglycerides present in the shortening batter microstructure (Fig. 7A). In contrast, the lowest cell area percentage of canola oil and HE oleogel cake can be attributed to a lower amount air bubbles poorly stabilized by oil droplets and proteins, as observed in the batter microstructure (Fig. 7B–D). Although a higher air cell area percentage often resulted in a higher specific volume,20,62 this correlation was not observed in this study. The shortening cake showed a lower specific volume despite having a higher cell area percentage, while the HE oleogel cake showed a higher specific volume despite a lower cell area percentage. Such difference could be partially attributed to the different batter microstructure and the different mechanism of air bubble stabilization in shortening and oleogel cakes (Fig. 7). According to Matsakidou et al.,63 continuous bubble channels are essential in maintaining the cake volume during cooling. During cake baking, the starch granules gelatinized and the protein aggregated, the batter transformed into a solid foam leading to the cake structure formation.16 Simultaneously, the high temperature in the cake also caused the air cells to expand, ruptured and coalesced. These big bubbles could rapidly float to the surface and disappear; however, if the cake structure was set before the bubble dispersion, the big air bubbles would form tunnels in the cakes.17 As shown in Fig. 9D, continuous channels (shown with arrows) were observed in the HE oleogel cake, possibly due to the stabilization effect of denatured aggregated canola proteins, contributing to the higher specific volume despite a lower total cell area percentage. Higher specific volume of HE oleogel cake led to lower hardness than the shortening cake.
4. Conclusions
In conclusion, protein isolate extracted from cold-pressed canola meal was successfully utilized to develop emulsion-templated oleogels. Heated and unheated 1 and 4 wt% CPI-stabilized 50 wt% oil-in-water emulsions were vacuum dried to remove water, followed by shearing to create a homogeneous texture of the oleogels. Heated emulsions exhibited better structure formation in the oleogels with significantly lower oil loss and higher gel strength than the oleogels developed from unheated emulsions, which were ascribed to heat-induced canola protein denaturation and aggregation at the oil droplet surface and in the continuous phase. Among all, the 4 wt% CPI HE oleogel exhibited superior characteristics in terms of gel-like structure, lowest oil loss, higher viscosity and viscoelasticity, and higher firmness and cohesiveness. Therefore, complete replacement of highly saturated shortening with the 4 wt% CPI UE and HE oleogels in cake baking was investigated. Despite having a higher specific gravity of the batter and the lower air cell area of the cake crumb, the HE oleogel cake exhibited higher cake specific volume than the shortening cake due to the formation of larger air channels in the cake stabilized by the presence of aggregated canola proteins. Both UE and HE oleogels cakes exhibited lower hardness, higher springiness and cohesiveness than the shortening cake, which was attributed to higher cake volume in the presence of canola protein-stabilized oleogels. Overall, the present study showed the potential of heat-denatured CPI in forming stable oleogels via the emulsion-templated method and the ability of the CPI oleogels to replace conventional highly saturated shortening in cake baking. Successful application such oleogel to replace saturated fat in baking would significantly improve the health beneficial aspects of processed food.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This research was supported by SaskCanola Dr Roger Rimmer Graduate Student Scholarship, NSERC Discovery Grant, Canada Foundation for Innovation (CFI) Leaders Opportunity Fund and Saskatchewan Science Foundation.References
- A. G. Marangoni, J. Am. Oil Chem. Soc., 2012, 89, 749–780 CrossRef CAS.
- M. A. Rogers, Food Res. Int., 2009, 42, 747–753 CrossRef CAS.
- J. Daniel and R. Rajasekharan, J. Am. Oil Chem. Soc., 2003, 80, 417–421 CrossRef CAS.
- M. A. Rogers, A. J. Wright and A. G. Marangoni, Soft Matter, 2008, 4, 1483–1490 RSC.
- X. Yan, Y. Cui, Q. He, K. Wang and J. Li, Chem. Mater., 2008, 20, 1522–1526 CrossRef CAS.
- I. Demirkesen and B. Mert, Crit. Rev. Food Sci. Nutr., 2020, 60, 2460–2479 CrossRef CAS PubMed.
- W. Wijaya, Q.-Q. Sun, L. Vermeir, K. Dewettinck, A. R. Patel and P. Van der Meeren, Food Struct., 2019, 21, 100112 CrossRef.
- C. Qiu, Y. Huang, A. Li, D. Ma and Y. Wang, J. Agric. Food Chem., 2018, 66, 13243–13252 CrossRef CAS PubMed.
- I. Tavernier, A. R. Patel, P. Van der Meeren and K. Dewettinck, Food Hydrocolloids, 2017, 65, 107–120 CrossRef CAS.
- Z. Meng, K. Qi, Y. Guo, Y. Wang and Y. Liu, Food Chem., 2018, 246, 137–149 CrossRef CAS PubMed.
- Y. Jiang, L. Liu, B. Wang, X. Sui, Y. Zhong, L. Zhang, Z. Mao and H. Xu, Food Hydrocolloids, 2018, 77, 460–464 CrossRef CAS.
- A. R. Patel, D. Schatteman, A. Lesaffer and K. Dewettinck, RSC Adv., 2013, 3, 22900–22903 RSC.
- A. R. Patel, Mater. Chem. Phys., 2017, 195, 268–274 CrossRef CAS.
- A. R. Patel, N. Cludts, M. D. Bin Sintang, B. Lewille, A. Lesaffer and K. Dewettinck, ChemPhysChem, 2014, 15, 3435–3439 CrossRef CAS PubMed.
- A. R. Patel, P. S. Rajarethinem, N. Cludts, B. Lewille, W. H. De Vos, A. Lesaffer and K. Dewettinck, Langmuir, 2015, 31, 2065–2073 CrossRef CAS PubMed.
- E. Wilderjans, A. Luyts, K. Brijs and J. A. Delcour, Trends Food Sci. Technol., 2013, 30, 6–15 CrossRef CAS.
- J. Zhou, J. M. Faubion and C. E. Walker, LWT--Food Sci. Technol., 2011, 44, 1802–1808 CrossRef CAS.
- P. W. Siri-Tarino, S. Chiu, N. Bergeron and R. M. Krauss, Annu. Rev. Nutr., 2015, 35, 517–543 CrossRef CAS PubMed.
- J. Rodríguez-García, A. Puig, A. Salvador and I. Hernando, J. Food Sci., 2012, 77, C189–C197 CrossRef PubMed.
- C. Amoah, J. Lim, S. Jeong and S. Lee, LWT--Food Sci. Technol., 2017, 86, 430–437 CrossRef.
- M. Sowmya, T. Jeyarani, R. Jyotsna and D. Indrani, Food Hydrocolloids, 2009, 23, 1827–1836 CrossRef CAS.
- H. Pehlivanoglu, G. Ozulku, R. M. Yildirim, M. Demirci, O. S. Toker and O. Sagdic, J. Food Process. Preserv., 2018, 42, e13621 CrossRef.
- I. Demirkesen and B. Mert, J. Am. Oil Chem. Soc., 2019, 96, 545–554 CrossRef CAS.
- S. A. Willett and C. C. Akoh, J. Food Sci., 2019, 84, 1390–1399 CrossRef CAS PubMed.
- A. R. Patel, P. S. Rajarethinem, A. Grędowska, O. Turhan, A. Lesaffer, W. H. De Vos, D. Van de Walle and K. Dewettinck, Food Funct., 2014, 5, 645–652 RSC.
- S.-Z. Luo, X.-F. Hu, Y.-J. Jia, L.-H. Pan, Z. Zheng, Y.-Y. Zhao, D.-D. Mu, X.-Y. Zhong and S.-T. Jiang, Food Hydrocolloids, 2019, 95, 76–87 CrossRef CAS.
- A. R. Patel, N. Cludts, M. D. B. Sintang, A. Lesaffer and K. Dewettinck, Food Funct., 2014, 5, 2833–2841 RSC.
- E. B. Bennion and G. Bamford, The technology of cake making, Springer Science & Business Media, 1997 Search PubMed.
- J. H. Kim, N. V. Varankovich, A. K. Stone and M. T. Nickerson, Food Res. Int., 2016, 89, 408–414 CrossRef CAS PubMed.
- C. Yang, Y. Wang, T. Vasanthan and L. Chen, Food Hydrocolloids, 2014, 40, 225–236 CrossRef CAS.
- R. He, H.-Y. He, D. Chao, X. Ju and R. Aluko, Food Bioprocess Technol., 2014, 7, 1344–1353 CrossRef CAS.
- L. W. Léger and S. D. Arntfield, J. Am. Oil Chem. Soc., 1993, 70, 853–861 CrossRef.
- Y. L. Folawiyo and R. K. O. Apenten, J. Sci. Food Agric., 1996, 70, 241–246 CrossRef CAS.
- Y. R. Tang and S. Ghosh, Food Hydrocolloids, 2020, 106399 Search PubMed.
- H.-N. Liang and C.-h. Tang, LWT--Food Sci. Technol., 2014, 58, 463–469 CrossRef CAS.
- A. G. Marangoni and N. Garti, Edible oleogels: structure and health implications, Elsevier, 2018 Search PubMed.
- A. Mohanan, Y. R. Tang, M. T. Nickerson and S. Ghosh, RSC Adv., 2020, 10, 14892–14905 RSC.
- AACC, Approved Methods of Analysis, 11th edn, Method 90–10.01, 1999 Search PubMed.
- Y. Zou, J. Guo, S.-W. Yin, J.-M. Wang and X.-Q. Yang, J. Agric. Food Chem., 2015, 63, 7405–7414 CrossRef CAS PubMed.
- M. A. Gularte, M. Gómez and C. M. Rosell, Food Bioprocess Technol., 2012, 5, 3142–3150 CrossRef CAS.
- A. Mohanan, M. T. Nickerson and S. Ghosh, J. Am. Oil Chem. Soc., 2020, 97, 1319–1333 CrossRef CAS.
- Z. Shao, A. S. Negi and C. O. Osuji, Soft Matter, 2013, 9, 5492–5500 RSC.
- R. C. Kramb and C. F. Zukoski, J. Phys.: Condens. Matter, 2010, 23, 035102 CrossRef PubMed.
- J. H. Kim, N. V. Varankovich and M. T. Nickerson, Food Res. Int., 2016, 81, 31–38 CrossRef CAS.
- A. I. Blake and A. G. Marangoni, J. Am. Oil Chem. Soc., 2014, 91, 885–903 CrossRef CAS.
- P. V. Gadkari, N. Longmore, M. Reaney and S. Ghosh, Colloid Interface Sci. Commun., 2019, 30, 100180 CrossRef CAS.
- A. I. Romoscanu, A. Fenollosa, S. Acquistapace, D. Gunes, T. Martins-Deuchande, P. Clausen, R. Mezzenga, M. Nydén, K. Zick and E. Hughes, Langmuir, 2010, 26, 6184–6192 CrossRef CAS PubMed.
- C. Zhang, Z. Jin, B. Zeng, W. Wang, G. Palui and H. Mattoussi, J. Phys. Chem. B, 2020, 124(22), 4631–4650 CrossRef CAS PubMed.
- I. Tavernier, C. D. Doan, P. Van der Meeren, B. Heyman and K. Dewettinck, Eur. J. Lipid Sci. Technol., 2018, 120, 1700393 CrossRef.
- S. Bayarri, I. Carbonell and E. Costell, J. Dairy Sci., 2012, 95, 6926–6936 CrossRef CAS PubMed.
- E. Yılmaz and M. Öğütcü, RSC Adv., 2015, 5, 50259–50267 RSC.
- E. Scholten, Curr. Opin. Food Sci., 2019, 27, 36–42 CrossRef.
- B. Brooker, Food Struct., 1993, 12, 2 Search PubMed.
- S. S. Sahi and J. M. Alava, J. Sci. Food Agric., 2003, 83, 1419–1429 CrossRef CAS.
- N. Hesso, C. Garnier, C. Loisel, S. Chevallier, B. Bouchet and A. Le-Bail, Food Struct., 2015, 5, 31–41 CrossRef.
- S. M. Lakshminarayan, V. Rathinam and L. KrishnaRau, J. Sci. Food Agric., 2006, 86, 706–712 CrossRef CAS.
- L. Xu and L. Diosady, Food Res. Int., 2002, 35, 23–30 CrossRef CAS.
- E. Wilderjans, B. Pareyt, H. Goesaert, K. Brijs and J. A. Delcour, Food Chem., 2008, 110, 909–915 CrossRef CAS PubMed.
- C. E. Stauffer, Emulsifiers, Eagan Press Handbook Series, AACC, St. Paul, MN, 1999, pp. 47–66 Search PubMed.
- J. Zhou, J. M. Faubion and C. E. Walker, LWT--Food Sci. Technol., 2011, 44, 1802–1808 CrossRef CAS.
- M. A. Brandt, E. Z. Skinner and J. A. Coleman, J. Food Sci., 1963, 28, 404–409 CrossRef.
- S. Mironeasa, G. G. Codină and M. A. Oroian, Bull. UASVM Food Sci. Technol., 2016, 73, 2344 Search PubMed.
- A. Matsakidou, G. Blekas and A. Paraskevopoulou, LWT--Food Sci. Technol., 2010, 43, 949–957 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra02250d |
| This journal is © The Royal Society of Chemistry 2021 |