Open Access Article
Open Access ArticleIn situ formation of 2-thiobarbituric acid incorporated g-C3N4 for enhanced visible-light-driven photocatalytic performance†
Tingting Chen‡
a,
Shan Hu‡ab,
Quanfeng Xinga,
Xiaofeng Yuc,
Jinming Chenc,
Xiaolong Lic,
Xiuquan Xu *c and
Bo Zhangab
*c and
Bo Zhangab
aSchool of Environment and Safety Engineering, Jiangsu University, Zhenjiang 212013, PR China
bJiangsu Province Synergistic Innovation Center of Modern Agricultural Equipment and Technology, Zhenjiang 212013, PR China
cSchool of Pharmacy, Jiangsu University, Zhenjiang 212013, PR China. E-mail: xxq781026@ujs.edu.cn
First published on 14th June 2021
Abstract
Embedding heterocycles into the skeleton of g-C3N4 has been proved to be a simple and efficient strategy for improving light response and the separation of photo-excited charges. Herein, 2-thiobarbituric acid incorporated g-C3N4 (TBA/CN) with good photocatalytic efficiency for Rh B degradation and H2 production was successfully achieved via a facile thermal copolymerization approach. The incorporation of aromatics and S atoms into the skeleton of g-C3N4 was identified via systematic characterizations. This unique structure contributed to the narrowed band-gap, extended delocalization of lone pair electrons and changed electron transition pathway, which led to the enhanced visible light utilization, accelerated charge migration and prolonged electron lifetime, subsequently resulting in the significant boost of photocatalytic activity. The optimal TBA/CN-3 sample yielded the largest Rh B degradation rate constant k value of 0.0273 min−1 and simultaneously highest rate of H2 evolution of 0.438 mmol g−1 h−1, which were almost 3.5 and 3.8 folds as fast as that of the pristine CN, respectively. Finally, the photocatalytic mechanism was proposed for the detailed elucidation of the process of Rh B degradation coupled with H2 production.
1. Introduction
In recent decades, emerging global energy dilemma and environmental pollution have become the two urgent threats to human existence and social development.1,2 Semiconductor photocatalysis has widely proven to be one of the most encouraging and prospective alternative techniques for effectively easing up the energy crisis and environmental issues due to its incorporated merits of high efficiency, energy-saving ability and eco-friendliness.3–5 Numerous photocatalysts have been constructed and successfully employed for H2 evolution, pollutant degradation and the conversion of CO2 to CO and CH4. However, the practical application of traditional photocatalysts, such as TiO2 and WO3, was mostly suppressed by the limitations of toxicity, high cost, low efficiency and insufficient visible light utilization.6–8Recently, graphitic carbon nitride (g-C3N4, CN), a metal-free semiconductor polymer, has received unprecedented attention due to its unique traits of visible-light-driven, tunable electronic structure, physical–chemical stability, good-durability, inexpensive nature and facile synthesis, which are perfect fit for the H2 production, pollutant degradation, and CO2 reduction.9–11 Nevertheless, the practical applicability of CN is still restricted by its inherent bottlenecks of limited visible light harvesting and ultrafast recombination rate of photogenerated charge carriers.12,13 To overcome the above-mentioned defects, considerable efforts have been devoted to boost the photocatalytic property of CN, including porous morphology formation,14 heteroatom doping,15 heterostructure construction,16 aromatic ring integration17 and dye sensitization.18
Among these, embedding heteroaromatic molecules into the skeleton of CN has been confirmed to be an effective strategy, which resulted in an extended π-conjugated system and new localized internal field formation, could expand the visible light response range and facilitate the separation and transportation of charges, leading to an increased photocatalytic efficiency.17,19,20 To date, numerous small molecules containing aromatic rings, such as tris(p-fluorophenyl)phosphine,21 thiophene,22 benzene,23 pyridine24 and salicylic acid,25 have been copolymerized successfully into the framework of CN, which not only resulted in the facilitation of visible-light utilization and increasing charge separation efficiency, but also modulated the redox band potentials, making the effective use of photo-excited charge carriers.
Motivated by the above-mentioned discussion, in this study, 2-thiobarbituric acid incorporated CN was synthesized via a thermal copolymerization approach. Fascinatingly, the as-synthesized TBA/CN composites endowed improved visible-light utilization and accelerative separation efficiency of photo-excited charge carriers, resulting in a substantially boosted photocatalytic performance towards the degradation of Rh B and H2 evolution. Furthermore, the possible mechanism for the outstanding improved photocatalytic property was deeply interpreted.
2. Experimental section
2.1. Photocatalyst preparation and characterization
Analytical pure grade urea and 2-thiobarbituric acid purchased from Aladdin Chemical Reagent Co., Ltd (Shanghai, China) were used as precursors. The modified CN composites were synthesized via a direct annealing route. In detail, the mixture of 20 g urea and desired amounts (0.05, 0.1, 0.2 and 0.3 g) of 2-thiobarbituric acid was completely dissolved in 5 mL ultrapure water via magnetic stirring. After drying at 60 °C overnight, the obtained residues were placed into a covered crucible and heated at 520 °C for 120 min at an increasing rate of 5 °C min−1. When restoring to room temperature, the resulting composites were collected and labeled as TBA/CN-1, TBA/CN-2, TBA/CN-3 and TBA/CN-4 correspondingly.For comparison, pristine g-C3N4 was also obtained via the direct calcination of urea under the same procedure, and the final product was named as CN.
The crystal and molecular structures, the surface morphologies and elemental composition and the light response behaviors of the as-synthesized composites were systematically characterized via X-ray diffraction (XRD), Fourier transformed infrared (FT-IR) spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), UV-Vis diffuse reflectance spectroscopy (UV-Vis DRS), X-ray photoelectron spectroscopy (XPS), photoluminescence (PL) spectroscopy and time-resolved photoluminescence (TRPL) spectroscopy.
The photoelectrochemical behaviors of these samples, including photocurrent (PC) response and electrochemical impedance spectra (EIS), were recorded on a CHI 760E electrochemical workstation.
2.2. Photocatalytic performance measurements
The visible-light driven photocatalytic properties of the as-synthesized samples were assessed by Rh B degradation coupled with H2 production. A 300 W Xe lamp (λ > 420 nm) was chosen as the radiation source and ultrapure water was employed throughout the experiments.In a typical degradation experiment, 50 mg of the photocatalyst was fully mixed with 100 mL of Rh B (10 mg L−1) by ultrasonic treatment for 5 min. Before exposure to light, the reaction system achieved adsorption–desorption equilibrium by magnetic stirring for 30 min in darkness. At designated intervals, 3.0 mL suspension was collected and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. Finally, the concentration of Rh B in the supernatant was estimated using a Unico UV-2800A spectrophotometer at the characteristic wavelength of 552 nm.
000 rpm for 10 min. Finally, the concentration of Rh B in the supernatant was estimated using a Unico UV-2800A spectrophotometer at the characteristic wavelength of 552 nm.
In the photocatalytic H2 generation test, 30 mg of sample deposited with 1.0 wt% Pt was fully suspended into 50 mL ultrapure water containing 10% (vol/vol) triethanolamine (TEOA), where TEOA was used as the hole scavenger and Pt was used as the co-catalyst. H2 evolution during the photocatalytic reaction was calculated using an online gas chromatograph (Agilent, GC-8890) equipped with a thermal conductivity detector and high-purity nitrogen was used as the carrier gas.
3. Results and discussion
3.1. Material characterization
The possible reaction route for the TBA/CN framework is shown in Fig. 1. Here, the crystal and molecular structures of the as-synthesized composites were first identified by the XRD and FT-IR analyses. As depicted in Fig. 2a, all the samples possessed two typical diffraction peaks of graphitic materials at around 12.9° and 27.5°, corresponding to the (100) and (002) crystal facets (JCPDS 87-1526),26 which suggested that the original crystal structure of CN was largely intact after the introduction of small amounts of TBA. Specifically, the front peak was related to the in-plane repeating motifs of tri-s-triazine, while the subsequent peak was attributed to the repeated interfacial stacking of the conjugated aromatic system in the CN nanosheets, respectively.27,28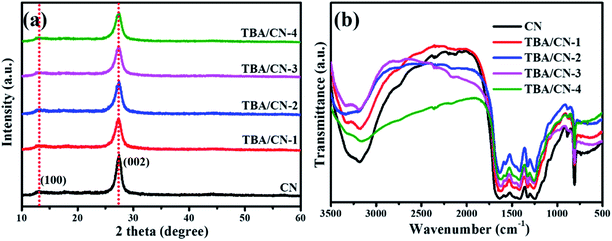 | ||
| Fig. 2 XRD patterns (a) and FT-IR spectra (b) for pristine CN, TBA/CN-1, TBA/CN-2, TBA/CN-3 and TBA/CN-4. | ||
Moreover, the obtained TBA/CN samples exhibited essentially similar FT-IR feature to that of pristine CN. In detail, the wide band located in the region between 3000 and 3400 cm−1 was derived from the terminal N–H and surface-adsorbed O–H stretching vibrations.29 Series of characteristic signals in the fingerprint region from 1150 to 1700 cm−1 were related to the different types of aromatic C–N or C–C stretching vibrations in the tri-s-triazine ring and heptazine heterocyclic ring units.30,31 Finally, the sharp peak at 809 cm−1 was attributed to the unique breathing vibration of the heptazine units.32 In addition, no evident absorption peaks associated with the bonds of the S atom were detected in the FT-IR spectra mainly due to its low modification level and relatively weak absorption.33
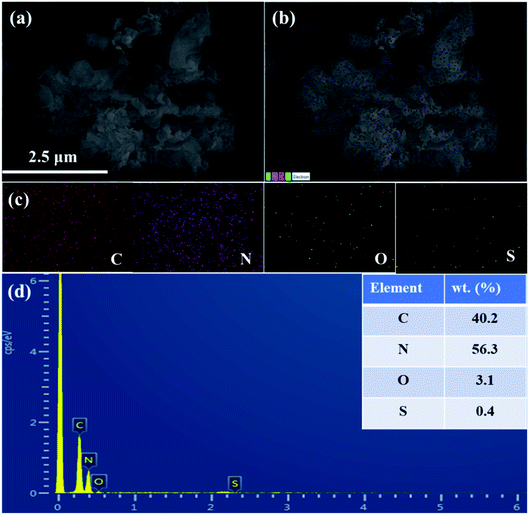
The morphology of TBA/CN-3 was observed by SEM and TEM. Fig. 3a indicated that TBA/CN-3 displayed an aggregated multilayer porous two-dimensional structure, which was in good accordance with the typical urea-derived CN structure.34 The corresponding elemental mapping (Fig. 3b and c) clearly reveal that C, N, O, and S elements were evenly dispersed throughout the TBA/CN-3 samples, demonstrating that TBA was successfully integrated into the CN skeleton. This result could be further confirmed by EDS, exhibiting the presence of C, N, O and S in the TBA/CN-3 sample. The representative TEM image of TBA/CN-3, as given in Fig. 4, also elucidated the ultrathin nanosheet-like structure equipped with abundant nanosized pores, which was largely due to the gas templating effects of urea and TBA.35 The ultrathin nanosheet morphology and enriched nanoholes on the surface of TBA/CN-3 would provide more active sites and might lead to significant acceleration of the photo-excited charge separation and migration, subsequently resulting in much improved photoactivity.36,37
The porous feature of TBA/CN-3 was further identified by N2 adsorption–desorption isotherms. As depicted in Fig. 5a, the isotherms of TBA/CN-3 as well as CN exhibited typical type IV curves featured with H3 hysteresis loops, suggesting their unaltered mesoporous structures.38 The BJH pore size distribution plots in Fig. 5b demonstrated that both TBA/CN-3 and pure CN had porous structures with average pore sizes of about 4.0 nm, which was quite consistent with the results of TEM. The almost similar parameters of BET specific surface area, pore volume and average pore size further confirmed that the structural integrity of CN remained intact even after the introduction of TBA. Thus, the enhancement of photocatalytic efficiency was mainly due to the incorporation of TBA rather than the surface morphology change of CN.
 | ||
| Fig. 5 N2 adsorption–desorption isotherms of CN, TBA/CN-3 (a) and the corresponding pore-size distribution curves (b). | ||
The XPS analysis was carried out to gain insight into the surface composition and valence states of different elements in CN and TBA/CN-3 samples, and the results are depicted in Fig. 6. From the XPS survey spectra (Fig. 6a), the peaks of C 1s, N 1s and O 1s could be clearly detected in both the samples, while the S 2p peak was only presented in the TBA/CN-3 sample. The weak absorption peaks of S 2p in the survey spectrum of TBA/CN-3 are mainly due to the small introduced content. The C 1s high-resolution XPS spectrum of TBA/CN-3 (Fig. 6b) could be well fitted into three peaks centered at 284.88, 286.60 and 288.13 eV, which correspond to the different chemical environments of carbon including C–C, C–NH2 and N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in the heterocycle, respectively.39,40 In addition, with respect to the C 1s high-resolution XPS spectrum of CN provided in Fig. S1b,† no difference between TBA/CN-3 and CN was detected, indicating the occurrence of little change in the basic skeleton structure of CN. Similarly, the N 1s high-resolution XPS spectrum of TBA/CN-3 (Fig. 6c) was also consistent with that of the pristine CN (Fig. S1c†). Specifically, the three peaks with bond energies of 398.44, 399.80 and 400.94 eV were assigned to C–N
N in the heterocycle, respectively.39,40 In addition, with respect to the C 1s high-resolution XPS spectrum of CN provided in Fig. S1b,† no difference between TBA/CN-3 and CN was detected, indicating the occurrence of little change in the basic skeleton structure of CN. Similarly, the N 1s high-resolution XPS spectrum of TBA/CN-3 (Fig. 6c) was also consistent with that of the pristine CN (Fig. S1c†). Specifically, the three peaks with bond energies of 398.44, 399.80 and 400.94 eV were assigned to C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, (C)3–N and terminal C–N–H amino functional groups, respectively.41 In terms of the S 2p XPS spectrum (Fig. 6d), TBA/CN-3 presented three peaks at 164.05, 165.89 and 169.29 eV, corresponding to the C–S bond, S
C, (C)3–N and terminal C–N–H amino functional groups, respectively.41 In terms of the S 2p XPS spectrum (Fig. 6d), TBA/CN-3 presented three peaks at 164.05, 165.89 and 169.29 eV, corresponding to the C–S bond, S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and oxidized S (S–Ox) generated during annealing.42,43 The S atom with extra electrons introduced into the CN skeleton could lead to a significant electronic polarization effect, which would be highly conducive to the migration and conductivity of the charge carriers.44
C bond and oxidized S (S–Ox) generated during annealing.42,43 The S atom with extra electrons introduced into the CN skeleton could lead to a significant electronic polarization effect, which would be highly conducive to the migration and conductivity of the charge carriers.44
 | ||
| Fig. 6 Survey XPS spectra (a) and high-resolution XPS spectra of C 1s (b), N 1s (c) and S 2p (d) for TBA/CN-3. | ||
The optical behaviors of the as-synthesized samples were evaluated by UV-Vis DRS and the corresponding band gaps were obtained according to the method of Kubelka–Munk.45 As presented in Fig. 7, pristine CN had a fundamental absorption edge at about 440 nm, with the calculated value of band gap being 2.70 eV. In comparison, the TBA/CN samples displayed a noticeable gradual red-shift and stronger response in the visible light region with the increase in TBA.
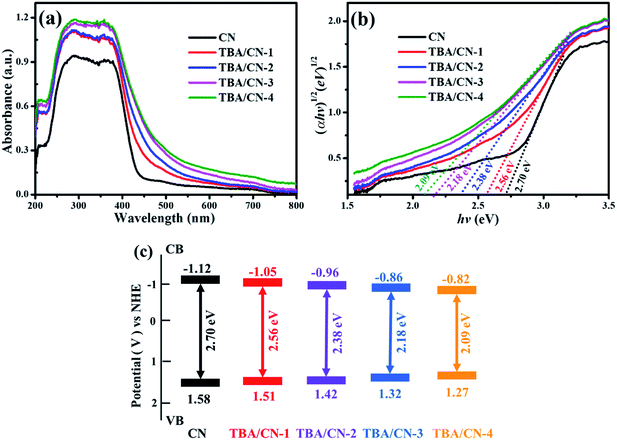 | ||
| Fig. 7 UV-Vis DRS (a), the plot of (αhv)1/2 versus the energy of light (hv) spectra (b) and the band edge potentials (c) of the as-prepared samples. | ||
The electronic band structure of VB and CB for the as-synthesized samples could be calculated according to the following formula:46
| EVB = X − Ee + 1/2Eg |
| ECB = EVB − Eg |
Therefore, the EVB values of CN and TBA/CN samples were calculated to be 1.58, 1.51, 1.42, 1.32 and 1.27 eV, while the ECB values were −1.12, −1.05, −0.96, −0.86 and −0.82 eV, respectively (Fig. 7c). Hence, it can be clearly deduced that the TBA modification could narrow the band gap of CN, which resulted in more visible light harvest, consequently elevating the generation of photo-excited charges and thus improving the photocatalytic properties.
The separation efficiency and migration behaviors of the photo-excited charges over CN and TBA/CN samples were compared via the PL analysis. Fig. 8a presents the particular strong emission peak of pristine CN at 448 nm, suggesting the immense recombination of photo-excited charges.47 Compared with CN, the peak intensities of TBA/CN samples significantly reduced and gradually redshifted to 456, 477, 490 and 492 nm for TBA/CN-1, TBA/CN-2, TBA/CN-3 and TBA/CN-4 correspondingly, which were consistent with the results of the UV-Vis DRS analysis. TBA/CN-3 possessed the weakest PL peak intensity, which indicated that the recombination of the photoinduced carriers was most effectively restrained.48 As shown in Fig. 8b and c, based on Gaussian fitting, the PL emission of CN could be well deconvoluted into three peaks centered at 436.1, 459.6 and 500.6 nm, corresponding to the electron transition pathways of σ*–LP (lone pair state), π*–p, and π*–LP.49,50 However, these three peaks of TBA/CN-3 clearly redshifted to 471.7, 508.5 and 574.2 nm in comparison to those of CN, suggesting that the electronic transition pathway has changed. Moreover, the improved ratio of peaks B and C of TBA/CN-3 revealed that the corresponding electronic transition types of π*–p, and π*–LP dominated, which was mainly due to the increase in the sp2 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and S atoms with more valence electrons by the incorporation of TBA.51
C bonds and S atoms with more valence electrons by the incorporation of TBA.51
 | ||
| Fig. 8 PL spectra of the as-prepared samples (a). Schematic of the electron transition form of CN (b). The Gaussian fitting of the PL spectra of CN (c) and TBA/CN-3 (d). | ||
Therefore, these results implied that the loading of TBA with abundant aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and S heteroatoms could change the electron transition path, thus preventing the charge recombination, which would result in an enhancement of the photocatalytic performance.
C bonds and S heteroatoms could change the electron transition path, thus preventing the charge recombination, which would result in an enhancement of the photocatalytic performance.
3.2. Photocatalytic property
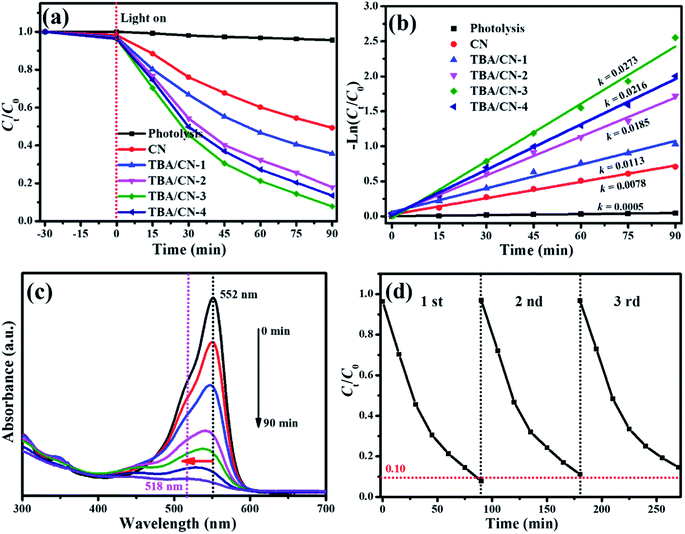
The photodecomposition of Rh B was first carried out to evaluate the photocatalytic activity of the as-synthesized samples. As presented in Fig. 9a, a negligible change of Rh B after 90 min visible light exposure demonstrated that it was very stable under the experimental conditions. After adding the photocatalyst, the degradation efficiency significantly enhanced, and the TBA/CN samples showed better degradation effect than the pristine CN. As expected, TBA/CN-3 showed the optimal photocatalytic efficiency and about 92.0% of Rh B could be removed after 90 min of visible light illumination. However, the TBA/CN-4 sample with the narrowest band gap of 2.09 eV exhibited reduced degradation activity, which can be possibly due to the formation of recombination centers and the reduction of charge separation efficiency caused by excessive TBA incorporation.52 The corresponding degradation kinetics was calculated by the following pseudo-first-order equation: ,53 and the results are shown in Fig. 9b. The largest rate constant k value of 0.0273 min−1 for TBA/CN-3 was obtained, which was almost 3.5-fold as that of the pristine CN. Fig. 9c depicts the UV-Vis absorption change of Rh B in a given reaction time over TBA/CN-3. With prolonged reaction time, the UV-Vis absorption intensity of Rh B markedly declined, and the characteristic absorption wavelength at 552 nm shifted to 518 nm, indicating the complete disruption of the conjugated aromatic system and chromogenic groups.
,53 and the results are shown in Fig. 9b. The largest rate constant k value of 0.0273 min−1 for TBA/CN-3 was obtained, which was almost 3.5-fold as that of the pristine CN. Fig. 9c depicts the UV-Vis absorption change of Rh B in a given reaction time over TBA/CN-3. With prolonged reaction time, the UV-Vis absorption intensity of Rh B markedly declined, and the characteristic absorption wavelength at 552 nm shifted to 518 nm, indicating the complete disruption of the conjugated aromatic system and chromogenic groups.
Thereafter, the recycling experiments were performed to evaluate the practical applications of TBA/CN-3. As shown in Fig. 9d, it was noticeable that there was only a slight decline in the photocatalytic degradation efficiency over TBA/CN-3 after three consecutive recycles, representing excellent photostability and reusability.
To reveal the dominant oxidative species involved in the photodecomposition of Rh B, active radical capturing experiments were carried out, in which p-benzoquinone (BQ), isopropanol (IPA) and ammonium oxalate (AO) were introduced as the quenchers of superoxide radicals (˙O2−), hydroxyl radicals (˙OH) and photo-excited holes (h+), respectively.54,55 As shown in Fig. 10a, the degradation efficiency of Rh B over TBA/CN-3 significantly reduced from 92.0% to 40.5% with the addition of BQ, reduced to 47.9% with the addition of AO and slightly reduced to 75.4% with the addition of IPA. These results indicate that ˙O2− and h+ played the decisive roles with ˙OH being partially involved in the photodegradation of Rh B. To further confirm the above-mentioned results, ESR experiments were conducted to detect the signals of ˙O2− and ˙OH during the photocatalytic process. It could be found from Fig. 10b and c that strong signals typical of ˙O2− and ˙OH were detected in TBA/CN-3, but the signals were relatively weak in CN. It signified that the TBA modification could observably benefit the yield of ˙O2− and ˙OH radicals in the photocatalytic degradation system.
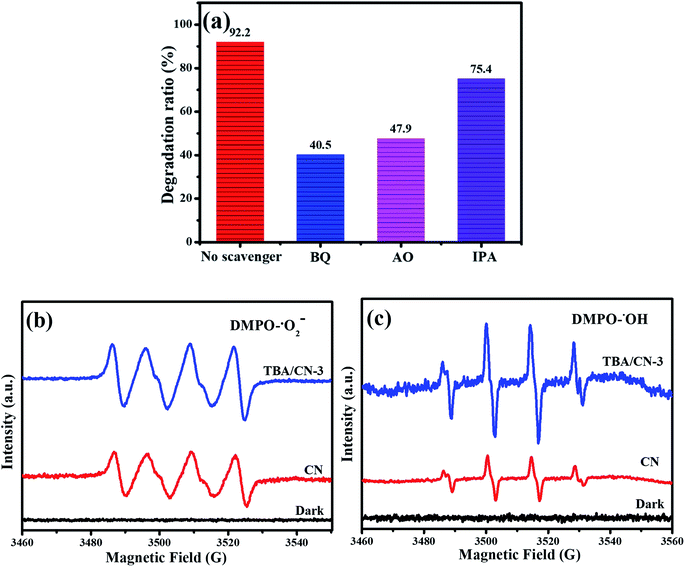 | ||
| Fig. 10 Effect of different scavengers on the photodegradation of Rh B (a), ESR spectra of DMPO-˙O2− (b) and DMPO-˙OH (c) over TBA/CN-3. | ||
The photocatalytic H2 evolution efficiency of the five as-synthesized samples was determined and the results are illustrated in Fig. 11. It can be seen in Fig. 11a that all the samples achieved stable near-linear generation of H2 with marked difference in the evolution rate. The pristine CN exhibited smallest photocatalytic H2 evolution rate of about 0.115 mmol g−1 h−1 (Fig. 11b), while the TBA/CN samples exhibited gradually enhanced rates with the increase in the concentration of the introduced TBA. The TBA/CN-3 sample possessed optimal H2 evolution rate of 0.438 mmol g−1 h−1, which was almost 3.8 times than that of CN. The enhanced H2 evolution of TBA/CN-3 was largely due to the stronger visible light harvesting and faster photo-exited charge transfer with lower recombination.
The photocatalytic H2 generation experiments were also repeated three times to ensure the durability of TBA/CN-3. As shown in Fig. 11c, the TBA/CN-3 sample displayed a steady H2 evolution without any apparent decline over three cycles (for 12 h in total), indicating a good photocatalytic stability and reproducibility. Furthermore, it can be seen in Fig. 11d that almost identical XRD patterns of TBA/CN-3 before and after the photocatalytic experiments further confirmed its durability.
3.3. Plausible mechanisms of the enhanced photocatalytic properties
In order to reveal the enhanced photocatalytic properties of TBA/CN-3, TRPL, PC value and EIS measurements were carried out. TRPL decay spectra in Fig. 12a illustrate that TBA/CN-3 presented an evident longer average lifetime of 7.44 ns compared to pure g-C3N4 (5.29 ns), suggesting the highly efficient migration of the charge carriers.56,57 The prolonged lifetime of photo-excited charge was possibly attributable to the established unique electron transfer pathway of N–C–S–C–N (or N–C–C–C–N) via the introduction of TBA (Fig. 1). Thereafter, the PC value and EIS behavior of TBA/CN-3 further consolidated the observed results. As clearly depicted in Fig. 12b, the PC density of TBA/CN-3 was more robust than that of CN, suggesting dramatical enhancement of the photo-excited charge carrier migration.58 Similarly, TBA/CN-3 exhibited a much reduced EIS Nyquist plot radius than that of pure CN, further confirming the smaller electron transfer resistance and faster charge transfer.59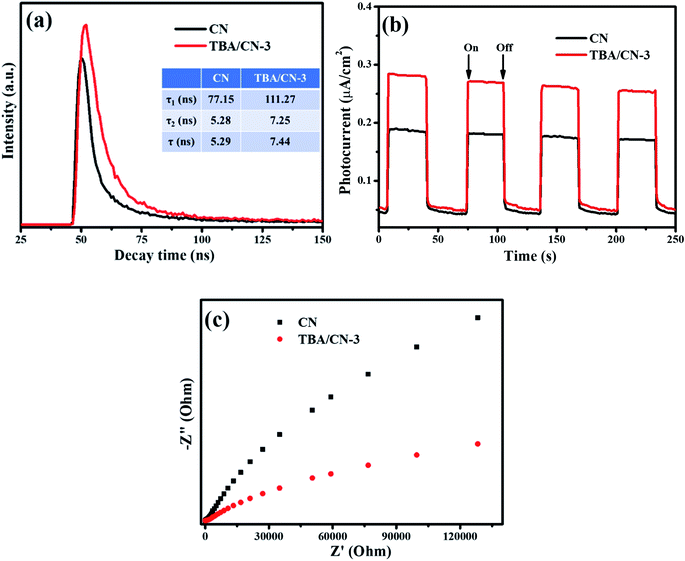 | ||
| Fig. 12 Comparison of the TRPL decay spectra (a), photocurrent response (b) and EIS Nyquist curves between CN and TBA/CN-3 (c). | ||
In light of the above results, the possible mechanism of the enhanced photocatalytic Rh B degradation and H2 evolution over TBA/CN composites is schematically predicted in Fig. 13. When TBA is incorporated into the skeleton of CN, extended delocalization of the lone pair electrons and unique electron transfer pathways would lead to enhanced visible light absorption, prolonged electron lifetime and facilitated charge migration. Irradiated by visible light, TBA/CN-3 was immediately activated, resulting in the production of photo-excited electrons and holes. The electrons on VB could effortlessly transfer along the bandgap to the CB of TBA/CN-3. Because of the prolonged lifetime, the stored electrons in the CB could have more opportunities to generate ˙O2− by reducing dissolved O2, which was benefited from the more negative potential (−0.86 V vs. NHE) of TBA/CN-3 than that of O2/˙O2− (−0.33 V vs. NHE).60 Furthermore, these ˙O2− could be further partially converted to ˙OH through complex chain reactions. Moreover, these accumulated electrons could also easily transfer to the Pt co-catalyst and have sufficient reduction capability to product H2 by water splitting on the surface of TBA/CN-3. Because of the lower VB potential (+1.32 V vs. NHE) compared to the standard redox potential of H2O/˙OH (+1.99 V vs. NHE) and OH−/˙OH (+2.80 V vs. NHE),61 the retained holes were not able to oxidize H2O or OH− to yield ˙OH, but could directly oxidize Rh B or react with TEOA to form TEOA+. As a result, the electrons in the CB and the holes on the VB could be separated efficiently, and these holes and electrons with high strong redox capacity strongly contributed to the enhancement of the photocatalytic activity of TBA/CN-3.
 | ||
| Fig. 13 Possible mechanisms on the photocatalytic degradation of Rh B with simultaneous H2 production over TBA/CN-3 under visible light irradiation. | ||
4. Conclusion
Overall, the TBA modified g-C3N4 was synthesized by copolymerizing the mixture of urea and TBA. The systematic characterization results proved that the structural units of TBA were well incorporated into the skeleton of g-C3N4 nanosheets. The PL, TRPL and photoelectrochemical experimental results suggested that the TBA/CN samples possessed prolonged lifetime of the photo-excited carriers, enhanced carrier transfer efficiency and reduced photocurrent resistance, which were in favor of the Rh B degradation and H2 evolution. The optimized TBA/CN exhibited 3.5 and 3.8-folds improved visible-light-driven Rh B degradation and H2 evolution activities compared to the pristine CN, respectively. This research may provide a new insight into the rational design of g-C3N4-based photocatalysts for efficiently visible light capture and conversion.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors genuinely gratitude the financial support from the Agricultural Science and Technology Innovation Foundation of Jiangsu Province (CX-(2019)-2042), Natural Science Research Foundation for Jiangsu Colleges (13KJB350001), Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX19-0567) and Development Fund of Synergistic Innovation Canter of Modern Agricultural Equipment and Technology (2019-018).References
- H. F. Qin, Y. H. Zuo, J. T. Jin, W. L. Wang, Y. L. Xu and L. F. Cui, et al., ZnO nanorod arrays grown on g-C3N4 micro-sheets for enhanced visible light photocatalytic H2evolution, RSC Adv., 2019, 9, 24483–24488 RSC.
- H. Yi, D. L. Huang, L. Qin, G. M. Zeng, C. Lai and M. Cheng, et al., Selective prepared carbon nanomaterials for advanced photocatalytic application in environmental pollutant treatment and hydrogen production, Appl. Catal., B, 2018, 239, 408–424 CrossRef CAS.
- G. J. Guan, E. Y. Ye, M. L. You and Z. B. Li, Hybridized 2D nanomaterials toward highly efficient photocatalysis for degrading pollutants: current status and future perspectives, Small, 2020, 16, 1907087 CrossRef CAS PubMed.
- N. Fajrina and M. Tahir, A critical review in strategies to improve photocatalytic water splitting towards hydrogen production, Int. J. Hydrogen Energy, 2019, 44, 540–577 CrossRef CAS.
- C. M. Li, H. H. Wu, Y. H. Du, S. B. Xi, H. J. Dong and S. H. Wang, et al., Mesoporous 3D/2D NiCoP/g-C3N4 Heterostructure with Dual Co-N and Ni-N Bonding States for Boosting Photocatalytic H2 Production Activity and Stability, ACS Sustainable Chem. Eng., 2020, 8, 12934–12943 CrossRef CAS.
- K. Nakata and A. Fujishima, TiO2 photocatalysis: design and applications, J. Photochem. Photobiol., C, 2012, 13, 169–189 CrossRef CAS.
- A. Y. Meng, L. Y. Zhang, B. Cheng and J. G. Yu, Dual Cocatalysts in TiO2 Photocatalysis, Adv. Mater., 2019, 31, 1807660 CrossRef PubMed.
- J. K. Jia, C. Y. Jiang, X. R. Zhang, P. J. Li, J. X. Xiong and Z. Zhang, et al., Urea-modified carbon quantum dots as electron mediator decorated g-C3N4/WO3 with enhanced visible-light photocatalytic activity and mechanism insight, Appl. Surf. Sci., 2019, 495, 143524 CrossRef CAS.
- J. R. Huang, X. F. Yang, S. Hussain, J. N. Tao, G. W. Liu and G. J. Qiao, Cooperative enhancement solar hydrogen generation of reformed g-C3N4/TiO2 mesocrystals composites, J. Nanoelectron. Optoelectron., 2020, 15, 46–53 CrossRef CAS.
- B. T. Xu, M. B. Ahmed, J. L. Zhou, A. Altaee, G. Xu and M. H. Wu, Graphitic carbon nitride based nanocomposites for the photocatalysis of organic contaminants under visible irradiation: progress, limitations and future directions, Sci. Total Environ., 2018, 633, 546–559 CrossRef CAS PubMed.
- C. Prasad, H. Tang, Q. Q. Liu, I. Bahadur, S. Karlapudi and Y. J. Jiang, A latest overview on photocatalytic application of g-C3N4 based nanostructured materials for hydrogen production, Int. J. Hydrogen Energy, 2020, 45, 337–379 CrossRef CAS.
- S. Zhang, P. C. Gu, R. Ma, C. T. Luo, T. Wen and G. X. Zhao, et al., Recent developments in fabrication and structure regulation of visible-light-driven g-C3N4-based photocatalysts towards water purification: a critical review, Catal. Today, 2019, 335, 65–77 CrossRef CAS.
- X. Q. Xu, S. M. Wang, X. F. Yu, J. Dawa, D. L. Gui and R. H. Tang, Biosynthesis of Ag deposited phosphorus and sulfur co-doped g-C3N4 with enhanced photocatalytic inactivation performance under visible light, Appl. Surf. Sci., 2020, 501, 144245 CrossRef CAS.
- L. Q. Jing, Y. G. Xu, M. J. Zhou, J. J. Deng, W. Wei and M. Xie, et al., Novel broad-spectrum-driven oxygen-linked band and porous defect co-modified orange carbon nitride for photodegradation of bisphenol A and 2-mercaptobenzothiazole, J. Hazard. Mater., 2020, 396, 122659 CrossRef CAS PubMed.
- X. Q. Xu, S. M. Wang, T. J. Hu, X. F. Yu, J. P. Wang and C. Jia, Fabrication of Mn/O co-doped g-C3N4: excellent charge separation and transfer for enhancing photocatalytic activity under visible light irradiation, Dyes Pigm., 2020, 175, 108107 CrossRef CAS.
- H. S. Li, L. L. Cai, X. Wang and H. X. Shi, Fabrication of AgCl/Ag3PO4/graphitic carbon nitride heterojunctions for enhanced visible light photocatalytic decomposition of methylene blue, methylparaben and E. coli, RSC Adv., 2021, 11, 6383–6394 RSC.
- D. K. Chauhan, S. Jain, V. R. Battula and K. Kailasam, Organic motifs functionalization via covalent linkage in carbon nitride: an exemplification in photocatalysis, Carbon, 2019, 152, 40–58 CrossRef CAS.
- Y. F. Liu, S. F. Kang, L. F. Cui and Z. Ma, Boosting near-infrared-driven photocatalytic H2 evolution using protoporphyrin-sensitized g-C3N4, J. Photochem. Photobiol., A, 2020, 396, 112517 CrossRef CAS.
- Y. A. Zhu, C. B. Xiong, S. Q. Song, Z. G. Le and S. J. Jiang, Coordination-driven synthesis of perfected π-conjugated graphitic carbon nitride with efficient charge transfer for oxygen activation and gas purification, J. Colloid Interface Sci., 2019, 538, 237–247 CrossRef CAS PubMed.
- D. Vidyasagar, S. G. Ghugal, S. S. Umare and M. Banavoth, Extended π-conjugative n-p type homostructural graphitic carbon nitride for photodegradation and charge-storage applications, Sci. Rep., 2019, 9, 7186 CrossRef PubMed.
- K. L. Chen, J. Q. Yan, D. D. Sun, S. S. Zhang, Y. Y. Zhao and J. H. Huang, Electron-donating tris(p-fluorophenyl)phosphine-modified g-C3N4 for photocatalytic hydrogen evolution and p-chlorophenol degradation, Int. J. Hydrogen Energy, 2021, 46, 1976–1988 CrossRef CAS.
- F. Y. Ge, S. Q. Huang, J. Yan, L. Q. Jing, F. Chen and M. Xie, et al., Sulfur promoted n-π* electron transitions in thiophene-doped g-C3N4 for enhanced photocatalytic activity, Chin. J. Catal., 2021, 42, 450–459 CrossRef CAS.
- J. Liu, Y. Yu, R. L. Qi, C. Y. Cao, X. Y. Liu and Y. J. Zheng, et al., Enhanced electron separation on in-plane benzene-ring doped g-C3N4 nanosheets for visible light photocatalytic hydrogen evolution, Appl. Catal., B, 2019, 244, 459–464 CrossRef CAS.
- W. Yang, X. Y. Shan, Y. Chen and Y. H. Gao, Enhanced photocatalytic performance of C3N4 via doping with π-deficient conjugated pyridine ring and BiOCl composite heterogeneous materials, Diamond Relat. Mater., 2020, 108, 107926 CrossRef CAS.
- C. Y. Zhou, D. L. Huang, P. Xu, G. M. Zeng, J. H. Huang and T. Z. Shi, et al., Efficient visible light driven degradation of sulfamethazine and tetracycline by salicylic acid modified polymeric carbon nitride via charge transfer, Chem. Eng. J., 2019, 370, 1077–1086 CrossRef CAS.
- X. Y. Wei, X. P. Xu, X. F. Yang, J. Y. Li and Z. G. Liu, Visible light degradation of reactive black-42 by novel Sr/Ag-TiO2@g-C3N4 photocatalyst: RSM optimization, reaction kinetics and pathways, Spectrochim. Acta, Part A, 2020, 228, 117870 CrossRef CAS PubMed.
- J. R. Bai, P. Zhou, P. Xu, Y. Y. Deng and Q. F. Zhou, Synergy of dopants and porous structures in graphitic carbon nitride for efficient photocatalytic H2 evolution, Ceram. Int., 2021, 47, 4043–4048 CrossRef CAS.
- P. She, J. Li, H. G. Bao, X. Q. Xu and Z. Hong, Green synthesis of Ag nanoparticles decorated phosphorus doped g-C3N4with enhanced visible-light-driven bactericidal activity, J. Photochem. Photobiol., A, 2019, 384, 112028 CrossRef CAS.
- H. Y. Ji, Y. M. Fan, J. Yan, Y. G. Xu, X. J. She and J. M. Gu, et al., Construction of SnO2/graphene-like g-C3N4 with enhanced visible light photocatalytic activity, RSC Adv., 2017, 7, 36101–36111 RSC.
- T. T. Zhu, Y. H. Song, H. Y. Ji, Y. G. Xu, Y. X. Song and J. X. Xia, et al., Synthesis of g-C3N4/Ag3VO4 composites with enhanced photocatalytic activity under visible light irradiation, Chem. Eng. J., 2015, 271, 96–105 CrossRef CAS.
- G. R. Jia, Y. Wang, X. Q. Cui, Z. X. Yang, L. L. Liu and H. Y. Zhang, et al., Asymmetric embedded benzene ring enhances charge transfer of carbon nitride for photocatalytic hydrogen generation, Appl. Catal., B, 2019, 258, 117959 CrossRef CAS.
- Z. Zhu, P. W. Huo, Z. Y. Lu, Y. S. Yan, Z. Liu and W. D. Shi, et al., Fabrication of magnetically recoverable photocatalysts using g-C3N4 for effective separation of charge carriers through like-Z-scheme mechanism with Fe3O4 mediator, Chem. Eng. J., 2018, 331, 615–625 CrossRef CAS.
- Q. Liang, M. Zhang, C. H. Liu, S. Xu and Z. Y. Li, Sulfur-doped graphitic carbon nitride decorated with zinc phthalocyanines towards highly stable and efficient photocatalysis, Appl. Catal., A, 2016, 519, 107–115 CrossRef CAS.
- J. R. Bai, C. C. Yin, H. Y. Xu, G. Chen, Z. J. Ni and Z. L. Wang, et al., Facile urea-assisted precursor pre-treatment to fabricate porous g-C3N4 nanosheets for remarkably enhanced visible-light-driven hydrogen evolution, J. Colloid Interface Sci., 2018, 532, 280–286 CrossRef CAS PubMed.
- M. Y. Ding, W. Wang, Y. J. Zhou, C. H. Lu, Y. R. Ni and Z. Z. Xu, Facile in situ synthesis of 2D porous g-C3N4 and g-C3N4/P25(N) heterojunction with enhanced quantum effect for efficient photocatalytic application, J. Alloys Compd., 2015, 635, 34–40 CrossRef CAS.
- G. M. Ba, T. T. Huo, Q. H. Deng, H. P. Li and W. G. Hou, Mechanochemical synthesis of nitrogen-deficient mesopore-rich polymeric carbon nitride with highly enhanced photocatalytic performance, ACS Sustainable Chem. Eng., 2020, 8, 18606–18615 CrossRef CAS.
- L. Shi, L. Q. Yang, W. Zhou, Y. Y. Liu, L. S. Yin and X. Hai, et al., Photoassisted construction of holey defective g-C3N4 photocatalysts for efficient visible-light-driven H2O2 production, Small, 2018, 14, 1703142 CrossRef PubMed.
- Y. J. Zhou, J. Z. Li, C. Y. Liu, P. W. Huo and H. Q. Wang, Construction of 3D porous g-C3N4/AgBr/rGO composite for excellent visible light photocatalytic activity, Appl. Surf. Sci., 2018, 458, 586–596 CrossRef CAS.
- H. Tang, R. Wang, C. X. Zhao, Z. P. Chen, X. F. Yang and D. Bukhvalov, et al., Oxamide-modified g-C3N4 nanostructures: tailoring surface topography for high-performance visible light photocatalysis, Chem. Eng. J., 2019, 374, 1064–1075 CrossRef CAS.
- H. P. Li, Z. W. Liang, Q. H. Deng and W. G. Hou, Band structure engineering of polymeric carbon nitride with oxygen/carbon codoping for efficient charge separation and photocatalytic performance, J. Colloid Interface Sci., 2020, 564, 333–343 CrossRef CAS PubMed.
- H. N. Che, C. X. Li, P. J. Zhou, C. B. Liu, H. J. Dong and C. M. Li, Band structure engineering and efficient injection rich-pi-electrons into ultrathin g-C3N4 for boosting photocatalytic H2 production, Appl. Surf. Sci., 2020, 505, 144564 CrossRef CAS.
- Y. C. Lu, J. Chen, A. J. Wang, N. Bao, J. J. Feng and W. P. Wang, et al., Facile synthesis of oxygen and sulfur co-doped graphitic carbon nitride fluorescent quantum dots and their application for mercury(II) detection and bioimaging, J. Mater. Chem. C, 2015, 3, 73–78 RSC.
- H. Q. Lv, Y. Huang, R. T. Koodali, G. M. Liu, Y. B. Zeng and Q. G. Meng, et al., Synthesis of sulfur-doped 2D graphitic carbon nitride nanosheets for efficient photocatalytic degradation of phenol and hydrogen evolution, ACS Appl. Mater. Interfaces, 2020, 12, 12656–12667 CrossRef PubMed.
- C. C. Hu, W. Z. Hung, M. S. Wang and P. J. Lu, Phosphorus and sulfur codoped g-C3N4 as an efficient metal-free photocatalyst, Carbon, 2018, 127, 374–383 CrossRef CAS.
- Z. Chen, T. T. Fan, M. Y. Shao, X. Yu, Q. L. Wu and J. H. Li, et al., Simultaneously enhanced photon absorption and charge transport on a distorted graphitic carbon nitride toward visible light photocatalytic activity, Appl. Catal., B, 2019, 242, 40–50 CrossRef.
- Y. Z. Hong, C. S. Li, G. Y. Zhang, Y. D. Meng, B. X. Yin and Y. Zhao, et al., Efficient and stable Nb2O5 modified g-C3N4 photocatalyst for removal of antibiotic pollutant, Chem. Eng. J., 2016, 299, 74–84 CrossRef CAS.
- Y. Z. Hong, Z. Y. Fang, B. X. Yin, B. F. Luo, Y. Zhao and W. D. Shi, et al., A visible-light-driven heterojunction for enhanced photocatalytic water splitting over Ta2O5 modified g-C3N4 photocatalyst, Int. J. Hydrogen Energy, 2017, 42, 6738–6745 CrossRef CAS.
- L. Ge, C. C. Han, X. L. Xiao, L. L. Guo and Y. J. Li, Enhanced visible light photocatalytic hydrogen evolution of sulfur-doped polymeric g-C3N4 photocatalysts, Mater. Res. Bull., 2013, 48, 3919–3925 CrossRef CAS.
- Y. Q. Yang, H. F. Jin, C. Zhang, H. H. Gan, F. T. Yi and H. Q. Wang, Nitrogen-deficient modified P-Cl co-doped graphitic carbon nitride with enhanced photocatalytic performance, J. Alloys Compd., 2020, 821, 153439 CrossRef CAS.
- Y. W. Yuan, L. L. Zhang, J. Xing, M. I. B. Utama, X. Lu and K. Z. Du, et al., High-yield synthesis and optical properties of g-C3N4, Nanoscale, 2015, 7, 12343–12350 RSC.
- P. Y. Lin, J. Shen, X. H. Yu, Q. Q. Liu, D. S. Li and H. Tang, Construction of Ti3C2 MXene/O-doped g-C3N4 2D-2D Schottky-junction for enhanced photocatalytic hydrogen evolution, Ceram. Int., 2019, 45, 24656–24663 CrossRef CAS.
- G. Q. Zhang, Y. S. Xu, C. X. He, P. X. Zhang and H. M. Mi, Oxygen-doped crystalline carbon nitride with greatly extended visible-light-responsive range for photocatalytic H2 generation, Appl. Catal., B, 2021, 283, 119636 CrossRef CAS.
- F. Li, P. Zhu, S. M. Wang, X. Q. Xu, Z. J. Zhou and C. D. Wu, One-pot construction of Cu and O co-doped porous g-C3N4 with enhanced photocatalytic performance towards the degradation of levofloxacin, RSC Adv., 2019, 9, 20633–20642 RSC.
- Y. Z. Hong, C. S. Li, B. X. Yin, D. Li, Z. Y. Zhang and B. D. Mao, et al., Promoting visible-light-induced photocatalytic degradation of tetracycline by an efficient and stable beta-Bi2O3@g-C3N4 core/shell nanocomposite, Chem. Eng. J., 2018, 338, 137–146 CrossRef CAS.
- D. Li, F. F. Shi, D. L. Jiang, M. Chen and W. D. Shi, CdIn2S4/g-C3N4 heterojunction photocatalysts: enhanced photocatalytic performance and charge transfer mechanism, RSC Adv., 2017, 7, 231–237 RSC.
- Y. X. Wang, L. Rao, P. F. Wang, Y. Guo, X. Guo and L. X. Zhang, Porous oxygen-doped carbon nitride: supramolecular preassembly technology and photocatalytic degradation of organic pollutants under low-intensity light irradiation, Environ. Sci. Pollut. Res., 2019, 26, 15710–15723 CrossRef CAS PubMed.
- L. Luo, J. N. Ma, H. X. Zhu and J. W. Tang, Embedded carbon in a carbon nitride hollow sphere for enhanced charge separation and photocatalytic water splitting, Nanoscale, 2020, 12, 7339–7346 RSC.
- T. J. Chen, C. J. Song, M. S. Fan, Y. Z. Hong, B. Hu and L. B. Yu, et al., In situ fabrication of CuS/g-C3N4 nanocomposites with enhanced photocatalytic H2 production activity via photoinduced interfacial charge transfer, Int. J. Hydrogen Energy, 2017, 42, 12210–12219 CrossRef CAS.
- Q. Y. Chen, H. L. Dou, S. H. Zheng, X. Rao and Y. P. Zhang, Photocatalytic H2 evolution and MB degradation over nickel-doped graphitic carbon nitride microwires under visible light irradiation, J. Photochem. Photobiol., A, 2019, 382, 111931 CrossRef CAS.
- T. Y. Wang, W. Quan, D. L. Jiang, L. L. Chen, D. Li and S. C. Meng, et al., Synthesis of redox-mediator-free direct Z-scheme AgI/WO3 nanocomposite photocatalysts for the degradation of tetracycline with enhanced photocatalytic activity, Chem. Eng. J., 2016, 300, 280–290 CrossRef CAS.
- H. Guo, N. Jiang, H. J. Wang, N. Lu, K. F. Shang and J. Li, et al., Degradation of antibiotic chloramphenicol in water by pulsed discharge plasma combined with TiO2/WO3 composites: mechanism and degradation pathway, J. Hazard. Mater., 2019, 371, 666–676 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra02121d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |