Open Access Article
Open Access ArticleTagetes erecta as an organic precursor: synthesis of highly fluorescent CQDs for the micromolar tracing of ferric ions in human blood serum†
Pinky Sagar a,
Gopal Krishna Guptab,
Monika Srivastava
a,
Gopal Krishna Guptab,
Monika Srivastava c,
Amit Srivastavab and
S. K. Srivastava
c,
Amit Srivastavab and
S. K. Srivastava *a
*a
aDepartment of Physics, Institute of Science, Banaras Hindu University, Varanasi, India 221005. E-mail: sanjay_itbhu@yahoo.com
bDepartment of Physics, TDPG College, VBS Purvanchal University, Jaunpur, India 222001
cSchool of Materials Science and Technology, IIT (BHU) Varanasi, India 221005
First published on 3rd June 2021
Abstract
The present article illustrates the green synthesis of novel carbon quantum dots (CQDs) from biomass viz. Tagetes erecta (TE), and subsequently fabrication of a metal ion probe for the sensing of Fe3+ in real samples. TE-derived CQDs (TE-CQDs) have been synthesized by a facile, eco-friendly, bottom-up hydrothermal approach using TE as a carbon source. The successful synthesis and proper phase formation of the envisaged material has been confirmed by various characterization techniques (Raman, XRD, XPS, TEM, and EDS). Notably, the green synthesized TE-CQDs show biocompatibility, good solubility in aqueous media, and non-toxicity. The as-synthesized TE-CQDs show an intense photoluminescence peak at 425 nm and exhibit excitation dependent photoluminescence behavior. The proposed TE-CQD-based probe offers a remarkable fluorescence (FL) quenching for Fe3+ with high selectivity (Kq ∼ 10.022 × 1013 M−1 s−1) and a sensitive/rapid response in a linear concentration range 0–90 μM (regression coefficient R2 ∼ 0.99) for the detection of Fe3+. The limit of detection (LOD) of the probe for Fe3+ has been found as 0.37 μM in the standard solution. It has further been applied for the detection of Fe3+ in real samples (human blood serum) and displays good performance with LOD ∼ 0.36 μM. The proposed TE-CQD-based ion sensing probe has potential prospects to be used effectively in biological studies and clinical diagnosis.
1. Introduction
Iron is one of the most abundant elements in the earth's crust and essential for numerous metabolic activities of humans including DNA synthesis, facilitation of electron transfer, and transportation of oxygen.1–4 Deficiency of iron causes the serious disease anemia.5 However, over-accumulation of iron leads to heart damage, Parkinson's disease, liver, endocrine organ failure, etc.4 Arumugham T. et al. and others have documented that an excess of iron produces free radicals throughout the redox cycling process in the presence of H2O2 and oxygen via Fenton reaction and turn severely affects the tissues, DNA, protein, and lipids by oxidative reactions.1,4,6,7 Fe3+ and Fe2+ have been observed as two oxidation states of iron and are dynamically tweaked to one another. Subsequently, the precise and quantitative information of the trace level of Fe3+ is very essential as it plays a vital role in complex physiological and metabolic processes.4 However, sensing of Fe3+ has been performed by many researchers, but in HBS based on CQDs probe through FL technique, have rarely been reported5 so far.CQDs, known as carbon dots/carbon nanoparticles have been an emerging class of innovative fluorescent materials due to their outstanding properties like tiny size (less than 10 nm), low toxicity, excellent emission tenability, chemical stability, temperature stability, high carrier mobility, biocompatibility, resistivity to photobleaching, cost-effectiveness, etc.8–13 Moreover, owing to their environment friendliness in comparison to other chemical dyes and semiconductor QDs, superiority in photostability, ability in up and down-conversion, non-blinking FL emission, CQDs have been preferable for the sensing applications due to their FL quenching or enhancement occurrence. A wide spectrum of approaches has been developed for the synthesis of CQDs such as plasma treatment,14 chemical ablation,15 arc discharge,16 acidic oxidation, electrochemical oxidation, hydrothermal carbonization,12,17 microwave irradiation,18 combustions (pyrolysis).19 However, some reported methods involves tedious steps and mark harmfulness to the ecosystem such as polamine functionalization,20 poly coupling reactions,21 thiol–yne click reactions,22 photo initiated raft,23 thiol–ene click reaction,24 azide–alkyne click reaction.25 Li et al. have synthesized CQDs via hydrothermal approach using citric acid as a precursor followed by the addition of CTAB. It leads to cytotoxicity26 and makes it non-suitable for biosensing applications.27 The hydrothermal synthesis of CQDs has been one of the easy and outstanding approaches, and widely popular among the researchers due to its mild circumstances, large-scale production, deprived of strong acids, and purity of samples.12 CQDs, as-synthesized through this approach has been extensively employed for different applications such as biosensors, therapeutic purposes, drug discovery, energy storage device, and so forth.4,28,29
Beyond troubling ambiguity with the selection of appropriate synthesis route, a true and big issue has been the preference of environment friendly and cost-effective precursor also. To find a way around, diverse precursors including green/biowaste/sustainable sources such as banana juice, coffee grounds, cellulose,3,30 Catharanthus roseus,7 pistachio shells,31 waste paper,32 ethylenediamine,33 rice husk,34 watermelon juice,35 gelatine,36 bamboo,37 etc. have been used as rich carbon source replacing hazardous chemicals.38,39 Serendipitously, we have found TE as an emerging precursor to synthesize the CQDs having excellent FL and biocompatibility. Notably, TE contains thiophenes, triterpenoids, flavonoids, carotenoids, oxycarotenoid, and xanthophylls chemical constituents which have worth therapeutic values such as non-cytotoxicity, antioxidant, antibacterial, antifungal, wound healing, etc.40 The earlier studies suggest that the extraction of flower petals has been a rich source of pharmaceutically important ethanol and ethyl acetate, used in anti-cancer drugs against H460 lung cancer, CaCO2 colon cancer lines, MCF-7 breast cancer lines.41,42 Apart from the far-reaching range of the applications of TE, a recent report reveals the phytoremediation of lateritic soil polluted by heavy metal.43 Maji et al. have studied the antibacterial properties and interaction of human serum albumin of TE leaves.44
Innumerable sensing probes using carbon-based materials for the detection of metal ions using electron paramagnetic resonance,45 solution-gated graphene transistors (SGGT),46 coupled plasma mass spectroscopy,47 atomic absorption/emission spectroscopy,48 colorimetry,49 electrochemical detection,50 etc., have grabbed attention. But they are pricey, need complex instrument operation and sample pre-treatment along with long interval procedures.46,49 However, the FL technique has emerged as one of the most pronounced techniques owing to its high accuracy, superficial handling, and no pre-cure of an analyte, which makes it a favourable option for several sensing applications.51 Mohammed et al. have engineered an FL nanosensor based on B,N co-doped carbon nanodots for the successive and reliable determination of Fe3+. The engineered sensor has shown its ability to detect Fe3+, however, it also binds with Fe2+ simultaneously, which marks it inferior for selective detection.9 Fan et al. designed an SGGT based on the functionalization with CQDs for the detection of Fe3+.46 Qian et al. have reported dual fluorescent sensors for the sensing of Fe3+ and Ag+, which makes it vague for Fe3+.52 Among these methods, a sensitive, specific, simple operative and accurately gauging of Fe3+ status in real samples based on FL technique has hardly been discussed.3,7,34 Henceforth, eco-friendly synthesis of materials with excellent optical properties and their FL based sensing applications for the rapid analysis of noteworthy biological samples has remained an emerging topic.
In the present study, a novel CQDs has been synthesized from an eco-friendly and sustainable TE as a carbon source for the very first time opting for a facile bottom-up “hydrothermal method” as a simplistic alternate for the pure, less than 10 nm-sized and large scale production. Besides the process does not involve any concentrated acids or strong reducing agents and surface passivation reagents. Furthermore, TE-CQDs have been characterized by the XRD, TEM, and Raman techniques and subsequently confirm the formation of CQDs with a good abundance of 4–6 nm sized particles. Moreover, the photophysical properties of TE-CQDs have also been investigated through UV-Visible, photoluminescence, and FT-IR spectroscopy. FT-IR studies reveal the presence of many active sites on the surface of TE-CQDs such as COOH, NH, and OH functional groups. The nanometer-sized TE-CQDs exhibit blue FL with UV-Visible light (λ = 365 nm). The FL quenching behaviour has also been investigated through the gradual addition of Fe3+ in DI water. The quenching process has been analyzed with Stern–Volmer (S–V) plot, modified S–V plot, and time-resolved photoluminescence (TRPL) spectra, which unveil the miscellaneous quenching i.e. static as well as dynamic quenching. However, the high value of the quenching constant (Kq ∼ 10.022 × 1013 M−1 s−1) indicates the selective and sensitive detection of Fe3+. The lower LOD has been found as 0.37 μM in the standard solution under an optimized condition with linearity range 0–90 μM and regression coefficient R2 = 0.99. Additionally, to validate the practical application, a similar experiment with HBS has been performed and elucidated an in-depth mechanism of FL quenching phenomenon in the existence of Fe3+. Henceforth, the present study infers that the as-synthesized TE-CQDs have noteworthy sensing application competence and virtuous commercial prospective.
2. Experimental section
2.1. Reagents
TE was collected from the botanical garden of the BHU campus, Varanasi, India. Other chemical reagents were procured from Sigma Aldrich. For the dilution of the reagents required during synthesis, DI water was used. All the sensing measurements were done with DI water. The metal salts L-cysteine, D-glucose, glycine, creatinine, ascorbic acid, uric acid, and Tris HCl buffer were purchased from HiMedia, Merck, and Sigma Aldrich. Different kinds of metal salts were also prepared in DI water for optical sensing. For the real sample sensing, HBS samples were collected from Prakash Pathology, Varanasi, India.2.2. Apparatus
The photophysical properties of TE-CQDs were characterized by UV-Visible absorption spectrophotometer (PerkinElmer, USA), photoluminescence spectrophotometer (PerkinElmer, USA), and time-resolved photoluminescence spectrophotometer (TRPL, FLS920 Edinburgh, UK) using DI water as a solvent in quartz cuvettes of 1 cm optical path length. Raman spectroscopy was carried out on a Raman spectrometer (Renishaw, UK) equipped with a diode pump solid-state laser as a constant power source of 5 mW mm−2. Fourier transform-infrared spectroscopy (FT-IR) was done on FT-IR spectrophotometer Varian Excalibur 3000, Palo Alto, CA using KBr as pellets ranging from wavenumber 400 cm−1 to 4000 cm−1. XPS was recorded on XPS spectrophotometer, AMICUS, UK. All the measurements were repeated in triplicates for the determination of the elemental composition (at%). Transmission electron microscopy (TEM) and Energy Dispersive X-ray (EDX) observations were recorded on FEI Technai G2 F20-Twin (Swiss Republic) at an accelerating voltage of 300 kV on a carbon-coated copper grid. X-ray powder diffraction (XRD) pattern was recorded on Miniflex 600 X-Ray-Diffractometer (Cu-Kα radiation, λ = 1.54056 Å, and 3° min−1 scan rate) ranging 2θ from 5° to 70°.2.3. Synthesis of TE-CQDs from TE
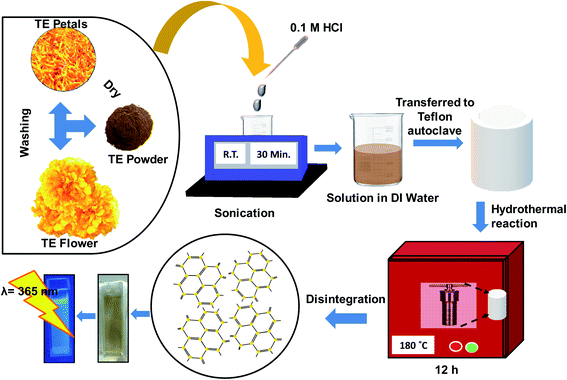
TE-CQDs were synthesized by one hand facile hydrothermal method using TE as a main reaction precursor. TE petals were washed thoroughly with DI water and allowed to dry at room temperature. Further, the TE petals were ground well in a pestle with the help of mortar. Next, 1.8719 g of TE powder was dissolved in 50 mL of 0.1 M HCl followed by ultrasonication for 30 min at room temperature. The as-received homogenous mixture was transferred to the stainless steel autoclave with Teflon vessel (100 mL capacity) and allowed to heat at 180 °C for 12 h as shown in Fig. 1. Further, it was kept to be cooled naturally to room temperature, the yellowish colloidal solution containing TE-CQDs was collected in a clean vessel and centrifuged further at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min. The centrifuged solution was filtered to discard non-soluble/residual particles and kept safe for further characterizations and sensing measurements.
000 rpm for 30 min. The centrifuged solution was filtered to discard non-soluble/residual particles and kept safe for further characterizations and sensing measurements.
2.4. Sensing procedure of Fe3+
To study the interaction of different metal ions/proteins, freshly prepared metal ion solutions have been separately added to a quartz cuvette containing TE-CQDs. Further, the variation in the FL intensity has been recorded with the peak wavelength ∼425 nm at excitation wavelength 320 nm. After that interference study has been performed by adding 100 times higher concentration of different metal ions/proteins to the colloidal solution of TE-CQDs. Then, FL titration experiment based on quenching phenomena has been carried out by adding Fe3+ solution to the TE-CQDs.2.5. Sensing procedure of HBS Fe3+
Briefly, 20 μL of each HBS samples were taken into Eppendorf centrifuge tubes and diluted with 1 M Tris HCl buffer solution (pH = 7.4), separately and kept inside the refrigerator (4 °C) until use. However, the diluted solutions were used for the sensing of iron in HBS at room temperature.3. Results and discussion
3.1. Characterizations of TE-CQDs
TE-CQDs in colloidal form, exhibit outstanding properties rendering their applications in various fields such as bio-sensing, optoelectronics, etc.53,54 The UV-Visible spectrum has been recorded in quartz cuvettes (path length 1 cm) with the scanning wavelength range ∼190 to 800 nm. The optical UV-Visible absorption spectrum of as-synthesized TE-CQDs reveals the appearance of an absorption band at 261 nm and an appendage owing to n–π* transition in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O39 and π–π* transition in the aromatic compounds55,56 (Fig. 2a). The aqueous solution of as-synthesized TE-CQDs shows excellent blue FL, as depicted in Fig. 2a. To confirm this, the photoluminescence emission spectrum at different excitation wavelengths has been recorded in the region 240 nm to 370 nm, as shown in Fig. S1.† There has been a redshift which is a natural characteristic of a colloidal synthesis and may be attributed to the polydispersity of the QDs. However, as the excitation wavelength is increased, the FL intensity decreases and unveil the excitation-dependent properties of TE-CQDs33,57 (Fig. S2a†). Further, the FL excitation spectra of TE-CQDs has been noted for λem ∼ 425 nm with an obvious spectroscopic overlapping for λex ∼ 320 nm (Fig. S2b†).
O39 and π–π* transition in the aromatic compounds55,56 (Fig. 2a). The aqueous solution of as-synthesized TE-CQDs shows excellent blue FL, as depicted in Fig. 2a. To confirm this, the photoluminescence emission spectrum at different excitation wavelengths has been recorded in the region 240 nm to 370 nm, as shown in Fig. S1.† There has been a redshift which is a natural characteristic of a colloidal synthesis and may be attributed to the polydispersity of the QDs. However, as the excitation wavelength is increased, the FL intensity decreases and unveil the excitation-dependent properties of TE-CQDs33,57 (Fig. S2a†). Further, the FL excitation spectra of TE-CQDs has been noted for λem ∼ 425 nm with an obvious spectroscopic overlapping for λex ∼ 320 nm (Fig. S2b†).
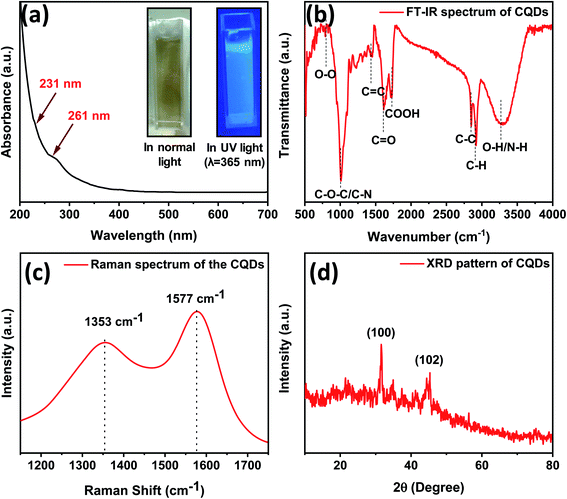 | ||
| Fig. 2 (a) UV-Visible spectrum, inset: optical images of colloidal solution of TE-CQDs, and (b) FT-IR spectrum, (c) Raman spectrum and (d) XRD pattern of as-synthesized TE-CQDs. | ||
As shown in Fig. 2b, to ascertain the chemical moieties present over the surface of the as-synthesized TE-CQDs, FT-IR analysis has been performed. Furthermore, FT-IR of a precursor (Fig. S3†) has also been carried out, as an indicator to optimize the synthesis criteria for TE-CQDs. The FT-IR spectra of TE-CQDs and its precursor exhibits absorption band at ∼3322 cm−1, 2930 cm−1, 2849 cm−1, 1734 cm−1, 1707 cm−1, 1567 cm−1, 1015 cm−1, and 831 cm−1 ascribes to O–H/N–H stretching, C–H bending, C–C/CH2, COOH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O–C/C–N and O–O functional groups,31,39,58 respectively. The presence of symmetric and asymmetric stretching of CH2 reveals that the carbon quantum dots primarily consist of hydrocarbons. Furthermore, the existence of carboxyl and carbonyl functional groups (C
C, C–O–C/C–N and O–O functional groups,31,39,58 respectively. The presence of symmetric and asymmetric stretching of CH2 reveals that the carbon quantum dots primarily consist of hydrocarbons. Furthermore, the existence of carboxyl and carbonyl functional groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) significantly indicates that the as-synthesized TE-CQDs possess graphitic structure. The absorption bands at 3305 cm−1, 1734 cm−1, and 1567 cm−1 correspond to its hydrophilic nature and excellent stability in water.58,59
C) significantly indicates that the as-synthesized TE-CQDs possess graphitic structure. The absorption bands at 3305 cm−1, 1734 cm−1, and 1567 cm−1 correspond to its hydrophilic nature and excellent stability in water.58,59
Raman spectroscopy has been a well-known and effective technique to confirm the crystal quality and several layers in the as-synthesized 2D materials. Fig. 2c depicts the Raman spectra of the as-synthesized TE-CQDs. It consists of two characteristic peaks at 1353 cm−1 and 1577 cm−1 which correspond to the graphitic growth (G) and defect (D) bands.60 The G-band attributes to E2g (in-plane vibration of sp2 bonded atoms) whereas the D-band owes to A1g (out of plane vibration), for as-synthesized TE-CQDs. The Raman mode E2g shows comparatively low intensity which might be due to the Rayleigh scattering or selection rules of the geometry whereas the high intensity of A1g mode unveils the presence of oxygen-rich groups over the surface of TE-CQDs,61 and are in good consistency with the FT-IR study.
The XRD pattern of as-synthesized TE-CQDs reveals a graphitic structure with good crystallinity. The XRD pattern exhibits two peaks at ∼31° and ∼45° corresponding to reflection planes (100) and (102) indicating the formation of graphitic TE-CQDs.33,62 as can be seen in Fig. 2d. Furthermore, the peak at ∼23° may be due to the presence of an oxygen-containing functional group and is in accord with other research studies.31
XPS measurement has been carried out to elaborate the surface chemical compositions of the as-synthesized TE-CQDs sample. Fig. 3a depicts the presence of C1s, N1s, and O1s peaks correspond to compositions having an atomic percentage of ∼82%, ∼4%, and ∼14%, respectively. C1s spectrum (Fig. 3b) reveals the presence of four distinct functional groups i.e. C–C (283.8 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (284.5 eV), C–O (285.6 eV) and C
C (284.5 eV), C–O (285.6 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (287.3 eV).63,64 The N1s (Fig. 3c) peak corresponds to two different functional groups C–N–C (399.80) and N–H (401.69 eV).9,64 The O1s spectrum (Fig. 3d) describes two peaks corresponding to C
O (287.3 eV).63,64 The N1s (Fig. 3c) peak corresponds to two different functional groups C–N–C (399.80) and N–H (401.69 eV).9,64 The O1s spectrum (Fig. 3d) describes two peaks corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (530.81 eV) and C–OH/C–O–C (532.31 eV).9,26,64 Hence, XPS reveals the XPS measurement reveals the presence of various functional groups present over the surface of TE-CQDs which has been well corroborated through FT-IR study too.
O (530.81 eV) and C–OH/C–O–C (532.31 eV).9,26,64 Hence, XPS reveals the XPS measurement reveals the presence of various functional groups present over the surface of TE-CQDs which has been well corroborated through FT-IR study too.
To get an insight into the structural morphology of the as-synthesized TE-CQDs, TEM investigations have been carried out. Fig. 4 represents the TEM micrograph of an as-synthesized sample which reveals the formation of spherical particles with an average diameter of 4.5–6.0 nm and predicts a good abundance of ∼5 nm sized particles (Fig. 4a and c) and soluble in water. The lattice spacing (2.2 Å) has been observed through the Fig. 4d and directs the graphitic plane (100) in the as-synthesized TE-CQDs.65 Also, the EDX profile infer a high wt% of the carbon in the as-synthesized sample, as shown in Fig. S4.†
 | ||
| Fig. 4 (a) and (b) TEM images, (c) particle size profile, (d) HRTEM image indicating lattice fringes of as-synthesized TE-CQDs. | ||
3.2. Sensitive and selective sensing of Fe3+
Functional groups containing oxygen-rich compounds over the surface of the as-synthesized TE-CQDs render its potential prospect in metal ion detection as it is easier to interact with metal cations for the formation of complex compounds. Henceforth, the as-synthesized TE-CQDs can be successfully applied for the sensing of anions, cations, and other hazardous chemicals by its turn-off FL effect as a probe. In selectivity experiment it has been found that our synthesized TE-CQDs are highly selective to Fe3+. FL intensity has been quenched to 70% upon addition of Fe3+ while in case of metal ion/protein solutions (Zn2+, Mg2+, Na+, K+, Co2+, Sn2+, Cu2+, Mn2+, Ni2+, Cd2+, Pb2+, Fe2+, Cr2+, L-cysteine, ascorbic acid, uric acid, glutathione, glycine, and creatinine), no obvious changes have been observed.Furthermore, in the interference analysis of different metal ion/protein solutions, a separate fluorescent-based interference experiment has been carried out. In the process, the concentration (∼0.2 mM) of different metal ions/proteins has been kept nearly 100 times higher than the Fe3+ solution. The study has been carried out with excitation wavelength ∼320 nm and an intense peak at ∼425 nm. With the addition of various metal ion/protein solutions, it has been observed that the FL intensity of as-synthesized TE-CQDs gets quenched nearly to ∼10% only, while with Fe3+, there has been complete disappearance of the FL. Fig. S5† depicts the optical representation leading to the quenching process of the FL of as-synthesized TE-CQDs with the addition of Fe3+. The relative FL intensity plot has been shown in Fig. 5 and the respective QP has been accessed through the equation:
 | (1) |
Furthermore, to check the response of various metal ions/proteins on the FL intensity of TE-CQDs in presence of Fe3+, an interference has been performed on TE-CQDs solution containing Fe3+. There is no significant change that depicts a highly selective response of the proposed sensor towards the detection of Fe3+. To check the sensitivity, an emission-based FL titration experiment has been performed. With a gradual addition of different concentrations (from 0 μM to 200 μM) of Fe3+ solution, the FL intensity of TE-CQDs quenches at 200 μM and further gets saturated (Fig. 6a). Notably, FL quenching by Fe3+ can be seen by naked eyes in presence of UV lamp (λ = 365 nm) as shown in Fig. S5.†
3.3. Sensing of Fe3+ by TE-CQDs in standard solution
To understand characteristics of the FL quenching of as-synthesized TE-CQDs sample, with the gradual addition of Fe3+, an S–V plot dealing with the variation of F0/F with the concentration of Fe3+ (Q) has been drawn (Fig. 6b) by employing the equation:4
 | (2) |
So for a better understanding of the complex quenching phenomenon, a modified S–V plot has been (Fig. S6†) drawn following the equation:66
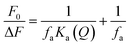 | (3) |
Furthermore, to analyze the dynamic and static quenching processes, a quite sensitive technique TRPL spectroscopy, with and without successive addition of the quencher at excitation wavelength 320 nm assuming FL intensity peak at ∼425 nm has been performed and shown in Fig. 6c. The average lifetime of as-synthesized TE-CQDs decays from ∼2.83 ns to ∼1.24 ns (inset of Fig. 6c). The TRPL decay investigation has been done using suitable software and the data has been found best fitted with the third-order exponential decay following the equation:66
 | (4) |
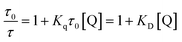 | (5) |
3.4. Sensing of Fe3+ by TE-CQDs in HBS
To check the feasibility of the present framework for the sensing of Fe3+ in real samples, different concentration levels of HBS have been added to the solution of TE-CQDs and analysed through the method as discussed earlier. It has been found that the FL quenching occurs similarly to the standard solution. The FL emission spectra of the HBS after standard addition along with the calibration graph, S–V plot (Fig. 7a and b), and modified S–V plot (Fig. S7†) have been analysed for quenching of TE-CQDs FL by Fe3+. Moreover, the linearity of the proposed probe pertaining KS–V as 7.5 × 103 M−1, 8.7 × 103 M−1 with regression coefficient R2 = 0.99 in both cases (standard solution and HBS) (Fig. S8a and S8b†) offers remarkable attention. It also suggests that there has been no singular difference between the standard solution and HBS Fe3+ sensing. A similar analysis has been made with the HBS sample 2 and displayed in ESI (Fig. S9†). On the other hand, the matrix interference has been found negligible at 100-time higher concentration of the HBS sample. To check the stability of the method, similar measurements have been done almost after a week to a month, and no substantial significant change has occurred. A brief discussion regarding the sensing of iron ions in the respective samples has been elaborated in Table S2.† | ||
| Fig. 7 (a) FL emission spectra with various concentration of Fe3+ in HBS sample 1 and (b) S–V plot showing exponential quenching of FL intensity of TE-CQDs. | ||
3.5. The mechanism for FL quenching
It is evident from FL emission spectra and TRPL spectra that the FL intensity has quenched remarkably with the addition of the quencher in the solution of TE-CQDs and decay lifetime also gets changed substantially. Accordingly, there has been a clear indication of FL quenching due to both types of quenching processes-collisional, and static. This could be due to the presence of various functional groups over the surface of the as-synthesized TE-CQDs. FT-IR measurements reveal the presence of COOH, OH, N–H, CH2 electron-rich groups in the colloidal solution of TE-CQDs, and can efficiently attach with Fe3+.5,67 It has also been known that Fe3+ has significant potential to quench the FL by energy or electron transfer from an excited state to its 3d orbital owing to its paramagnetic behaviour.3,68 A schematic illustration has been shown in Fig. 8 for both types of quenching. The complex formed in the excited state and ground state when get de-excited gives FL and No-FL,66 respectively. Hence, FL of as-synthesized TE-CQDs gets quenched with the gradual addition of Fe3+. | ||
| Fig. 8 Schematic illustration of FL quenching effect-based sensing of Fe3+ by the TE-CQDs as sensor. | ||
3.6. Limit of detection
The LOD has been calculated based on the FL titration measurements. Fig. 9 represents the variation of normalized FL intensity of TE-CQDs and log[concentration of quencher].69 The present study possesses a good LOD as 0.37 μM and 0.36 μM in DI water (Fig. 9a) and HBS sample 1 (Fig. 9b), respectively, and have found to be comparable to other reported works (Table 1) and permissible in the frame of the guidelines of USEPA. | ||
| Fig. 9 Normalized FL intensity vs. log[concentration of quencher] plot for the detection of the LOD of the metal ion sensor in (a) standard solution and (b) HBS sample 1. | ||
| Sensing probe | Linearity range (μM) | Limit of detection (μM) | KS–V (M−1) | Matrix | References |
|---|---|---|---|---|---|
| Eu3+:CDs@ZIF-8 | 0–6 | 0.89 | — | Standard solution | 70 |
| CDs | 8–80 | 3.8 | — | Standard solution | 71 |
| NCQDs | 0–70 | 0.50 | — | Tap water, river water | 72 |
| CDs | 33–133 | 0.53 | 1.62 × 103 | Tap water, groundwater | 73 |
| Phe-CDs | 5–500 | 0.72 | — | Tap water | 74 |
| CQDs@OMS | 25–750 | — | — | Standard solution | 75 |
| TE-CQDs | 0–90 | 0.37 | 7.5 × 103 | Standard solution | Present work |
| TE-CQDs | 0–70 | 0.36 | 8.7 × 103 | Human blood serum | Present work |
4. Conclusion
In summary, TE-CQDs have been successfully synthesized from TE as a natural precursor via hydrothermal treatment, without using any strong reducing additive and tedious post-cure, as a facile and cost-effective approach and subsequently studied the bio-sensing behavior for Fe(III). The as-synthesized TE-CQDs have been characterized by Raman, XRD, TEM, EDX, and XPS for their structural and elemental characterizations. Further, the photophysical properties of TE-CQDs have been investigated by UV-Visible and FL spectroscopy. The synthesized TE-CQDs are highly selective and exhibit remarkable sensing behavior to Fe3+. Quenching constant Ka has a value ∼4.24 × 103 M−1 which is almost 6.5% of the collision quenching phenomena derived constant and suggests a defined way of quenching by the quencher towards the TE-CQDs FL intensity, as unveiled by modified S–V plot. TRPL studies suggest the high selectivity (Kq ∼ 10.022 × 1013 M−1 s−1) and sensitivity of the TE-CQDs towards the Fe3+ sensing. The developed probe has good LOD ∼ 0.37 μM and 0.36 μM in standard solution (linearity range ∼ 0–90 μM) and HBS (linearity range ∼0–70 μM), respectively. To the best of our knowledge, the present study inspects the synthesis of TE-derived CQDs, which is a cost-effective, eco-friendly approach for the very first time. Also, our findings unveil that the as-synthesized TE-CQDs can be used as an efficient, reliable, and feasible biosensor for sensing Fe3+ in biological samples.Conflicts of interest
Authors have no conflicts to declare.Acknowledgements
Pinky Sagar is thankful to DST, New Delhi for providing INSPIRE Fellowship (DST/INSPIRE/03/2018/000041). Dr Monika Srivastava acknowledges DST, New Delhi (SR/WOS-A/CS-52/2018) for WOS fellowship. The authors are thankful to the Bio-Physics Lab, Department of Physics, BHU Varanasi for the availability of different kinds of characterization facilities including FT-IR, UV-Visible, photoluminescence, and TRPL. Authors also convey their thanks to CIF, IIT BHU Varanasi for the access to characterization services.References
- G. M. Rodgers and J. A. Gilreath, Acta Haematol., 2019, 142, 13–20 CrossRef CAS PubMed.
- S. Gómez-Ramírez, E. Bisbe, A. Shander, D. R. Spahn and M. Muñoz, Acta Haematol., 2019, 142, 21–29 CrossRef PubMed.
- B. Xue, Y. Yang, R. Tang, Y. Sun, S. Sun, X. Cao, P. Li, Z. Zhang and X. Li, Cellulose, 2020, 27, 729–742 CrossRef CAS.
- G. Kalaiyarasan, J. Joseph and P. Kumar, ACS Omega, 2020, 5, 22278–22288 CrossRef PubMed.
- S. H. K. Yap, K. K. Chan, G. Zhang, S. C. Tjin and K.-T. Yong, ACS Appl. Mater. Interfaces, 2019, 11, 28546–28553 CrossRef CAS PubMed.
- S. Dev and J. L. Babitt, Hemodial. Int., 2017, 21, S6–S20 CrossRef PubMed.
- T. Arumugham, M. Alagumuthu, R. G. Amimodu, S. Munusamy and S. K. Iyer, Sustainable Mater. Technol., 2020, 23, e00138 CrossRef CAS.
- F. Liu, S. Zhu, D. Li, G. Chen and S.-H. Ho, iScience, 2020, 23, 101174 CrossRef CAS PubMed.
- L. J. Mohammed and K. M. Omer, Sci. Rep., 2020, 10, 1–12 CrossRef PubMed.
- G. Kalaiyarasan, M. Veerapandian, G. JebaMercy, K. Balamurugan and J. Joseph, ACS Biomater. Sci. Eng., 2019, 5, 3089–3099 CrossRef CAS PubMed.
- S. Y. Lim, W. Shen and Z. Gao, Chem. Soc. Rev., 2015, 44, 362–381 RSC.
- Y. Wang and A. Hu, J. Mater. Chem. C, 2014, 2, 6921–6939 RSC.
- Y. Xiong, J. Schneider, E. V. Ushakova and A. L. Rogach, Nano Today, 2018, 23, 124–139 CrossRef CAS.
- M. Sabet and K. Mahdavi, Appl. Surf. Sci., 2019, 463, 283–291 CrossRef CAS.
- S. Duraisamy, T. Suppan, K. Mohanta, M. Krishnamoorthy and B. G. Priyadarshini, Nanotechnology, 2020, 31, 235401 CrossRef CAS PubMed.
- R. Das, R. Bandyopadhyay and P. Pramanik, Mater. Today Chem., 2018, 8, 96–109 CrossRef CAS.
- S. Panda, B. Paital and S. Mohapatra, Colloids Surf., A, 2020, 124445 CrossRef CAS.
- Y. Choi, N. Thongsai, A. Chae, S. Jo, E. B. Kang, P. Paoprasert, S. Y. Park and I. In, J. Ind. Eng. Chem., 2017, 47, 329–335 CrossRef CAS.
- X. Li, J. Chang, F. Xu, X. Wang, Y. Lang, Z. Gao, D. Wu and K. Jiang, Res. Chem. Intermed., 2015, 41, 813–819 CrossRef CAS.
- Y. Dong, R. Wang, G. Li, C. Chen, Y. Chi and G. Chen, Anal. Chem., 2012, 84, 6220–6224 CrossRef CAS PubMed.
- W.-B. Wu, Y.-C. Wong, Z.-K. Tan and J. Wu, Catal. Sci. Technol., 2018, 8, 4257–4263 RSC.
- I. Kaminska, W. Qi, A. Barras, J. Sobczak, J. Niedziolka-Jonsson, P. Woisel, J. Lyskawa, W. Laure, M. Opallo and M. Li, Chem.–Eur. J., 2013, 19, 8673–8678 CrossRef CAS PubMed.
- J. Chen, M. Liu, Q. Huang, L. Huang, H. Huang, F. Deng, Y. Wen, J. Tian, X. Zhang and Y. Wei, Chem. Eng. J., 2018, 337, 82–90 CrossRef CAS.
- Y. Liu, W. Hou, H. Sun, C. Cui, L. Zhang, Y. Jiang, Y. Wu, Y. Wang, J. Li and B. S. Sumerlin, Chem. Sci., 2017, 8, 6182–6187 RSC.
- R. Jiang, M. Liu, T. Chen, H. Huang, Q. Huang, J. Tian, Y. Wen, Q.-y. Cao, X. Zhang and Y. Wei, Dyes Pigm., 2018, 148, 52–60 CrossRef CAS.
- T. Li, L. Xie, R. Long, C. Tong, Y. Guo, X. Tong, S. Shi and Q. Lin, Microchim. Acta, 2019, 186, 791 CrossRef CAS PubMed.
- Y. Yan, C. Zhang, W. Gu, C. Ding, X. Li and Y. Xian, J. Phys. Chem. C, 2016, 120, 12170–12177 CrossRef CAS.
- J. B. Essner, J. A. Kist, L. Polo-Parada and G. A. Baker, Chem. Mater., 2018, 30, 1878–1887 CrossRef CAS.
- H.-c. Tan, W.-h. Zhao, Q. Qiu, R. Zhang, Y.-y. Zuo and L.-j. Yang, Fullerenes, Nanotubes, Carbon Nanostruct., 2017, 25, 417–422 CrossRef CAS.
- G. Yang, X. Wan, Y. Su, X. Zeng and J. Tang, J. Mater. Chem. A, 2016, 4, 12841–12849 RSC.
- F. Abdolrezaei and M. Sabet, Luminescence, 2020, 35, 684–693 CrossRef CAS PubMed.
- R.-C. Wang, J.-T. Lu and Y.-C. Lin, J. Alloys Compd., 2020, 813, 152201 CrossRef CAS.
- J. Zheng, Y. Xie, Y. Wei, Y. Yang, X. Liu, Y. Chen and B. Xu, Nanomaterials, 2020, 10, 82 CrossRef CAS PubMed.
- W. Wang, Z. Wang, J. Liu, Y. Peng, X. Yu, W. Wang, Z. Zhang and L. Sun, Ind. Eng. Chem. Res., 2018, 57, 9144–9150 CrossRef CAS.
- M. Lu, Y. Duan, Y. Song, J. Tan and L. Zhou, J. Mol. Liq., 2018, 269, 766–774 CrossRef CAS.
- Q. Liang, W. Ma, Y. Shi, Z. Li and X. Yang, Carbon, 2013, 60, 421–428 CrossRef CAS.
- T. Wang, X. Liu, C. Ma, Z. Zhu, Y. Liu, Z. Liu, M. Wei, X. Zhao, H. Dong and P. Huo, J. Alloys Compd., 2018, 752, 106–114 CrossRef CAS.
- H. Xu, L. Xie and M. Hakkarainen, ACS Sustainable Chem. Eng., 2017, 5, 5360–5367 CrossRef CAS.
- N. Chaudhary, P. K. Gupta, S. Eremin and P. R. Solanki, J. Environ. Chem. Eng., 2020, 8, 103720 CrossRef CAS.
- A. A. Safar, A. O. Ghafoor and D. Dastan, Pol. J. Environ. Stud., 2020, 29, 2317–2326 CrossRef CAS.
- O. Vallisuta, V. Nukoolkarn, A. Mitrevej, N. Sarisuta, P. Leelapornpisid, A. Phrutivorapongkul and N. Sinchaipanid, Exp. Ther. Med., 2014, 7, 246–250 CrossRef PubMed.
- J. Shetty, H. Harikiran and J. Fernandes, J. Pharm. Res., 2009, 2, 1035–1038 Search PubMed.
- T. M. Madanan, I. K. Shah, G. K. Varghese and R. K. Kaushal, Environ. Chem. Ecotoxicol., 2021, 3, 17–22 CrossRef.
- A. Maji, M. Beg, S. Das, M. N. Aktara, S. Nayim, A. Patra, M. M. Islam and M. Hossain, Process Biochem., 2020, 97, 191–200 CrossRef CAS.
- S. Suzen, H. Gurer-Orhan and L. Saso, Molecules, 2017, 22, 181 CrossRef PubMed.
- Q. Fan, J. Li, J. Wang, Z. Yang, T. Shen, Y. Guo, L. Wang, M. S. Irshad, T. Mei and X. Wang, J. Mater. Chem. C, 2020, 8, 4685–4689 RSC.
- K. K. Jinadasa, P. Herbello-Hermelo, E. Peña-Vázquez, P. Bermejo-Barrera and A. Moreda-Piñeiro, Talanta, 2021, 224, 121841 CrossRef CAS PubMed.
- B. B. Yıldırmaz, A. Gölcü, B. T. Zaman, N. A. Kasa, E. G. Bakırdere and S. Bakırdere, Chem. Pap., 2021, 1–8 Search PubMed.
- J. H. Yoe and A. L. Jones, Ind. Eng. Chem., Anal. Ed., 1944, 16, 111–115 CrossRef CAS.
- S. Li, C. Zhang, S. Wang, Q. Liu, H. Feng, X. Ma and J. Guo, Analyst, 2018, 143, 4230–4246 RSC.
- K. P. Carter, A. M. Young and A. E. Palmer, Chem. Rev., 2014, 114, 4564–4601 CrossRef CAS PubMed.
- Z. Qian, J. Ma, X. Shan, H. Feng, L. Shao and J. Chen, Chem.–Eur. J., 2014, 20, 2254–2263 CrossRef CAS PubMed.
- R. Wang, K.-Q. Lu, Z.-R. Tang and Y.-J. Xu, J. Mater. Chem. A, 2017, 5, 3717–3734 RSC.
- K. Dave and V. G. Gomes, Nano Energy, 2019, 104093 Search PubMed.
- S. Yang, W. Yue, D. Huang, C. Chen, H. Lin and X. Yang, RSC Adv., 2012, 2, 8827–8832 RSC.
- X. Feng and Y. Zhang, RSC Adv., 2019, 9, 33789–33793 RSC.
- G. Li, M. Pei and P. Liu, Mater. Sci. Eng. C, 2020, 110653 CrossRef CAS PubMed.
- S. K. Srivastava, P. Sagar, M. Srivastava and R. Prakash, Anal. Methods, 2020, 12, 3014–3024 RSC.
- V. Sharma, A. K. Saini and S. M. Mobin, J. Mater. Chem. B, 2016, 4, 2466–2476 RSC.
- S. Muthulingam, I.-H. Lee and P. Uthirakumar, J. Colloid Interface Sci., 2015, 455, 101–109 CrossRef CAS PubMed.
- Y.-L. Zhang, L. Wang, H.-C. Zhang, Y. Liu, H.-Y. Wang, Z.-H. Kang and S.-T. Lee, RSC Adv., 2013, 3, 3733–3738 RSC.
- S. Bansal, J. Singh, U. Kumari, I. P. Kaur, R. P. Barnwal, R. Kumar, S. Singh, G. Singh and M. Chatterjee, Int. J. Nanomed., 2019, 14, 809 CrossRef PubMed.
- H. Wu, L.-F. Pang, M.-J. Fu, X.-F. Guo and H. Wang, J. Pharm. Biomed. Anal., 2020, 180, 113052 CrossRef CAS PubMed.
- J. Zhu, H. Chu, T. Wang, C. Wang and Y. Wei, Microchem. J., 2020, 158, 105142 CrossRef CAS.
- L. Chunduri, A. Kurdekar, S. Patnaik, B. V. Dev, T. M. Rattan and V. Kamisetti, Mater. Focus, 2016, 5, 55–61 CrossRef CAS.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer Science & Business Media, 2013 Search PubMed.
- M. A. Issa, Z. Z. Abidin, S. Sobri, S. A. Rashid, M. A. Mahdi and N. A. Ibrahim, Sci. Rep., 2020, 10, 11710 CrossRef CAS PubMed.
- J. Li, Q. Wang, Z. Guo, H. Ma, Y. Zhang, B. Wang, D. Bin and Q. Wei, Sci. Rep., 2016, 6, 1–8 CrossRef CAS PubMed.
- M. Shortreed, R. Kopelman, M. Kuhn and B. Hoyland, Anal. Chem., 1996, 68, 1414–1418 CrossRef CAS PubMed.
- X. Guo, Q. Pan, X. Song, Q. Guo, S. Zhou, J. Qiu and G. Dong, J. Am. Ceram. Soc., 2021, 104, 886–895 CrossRef CAS.
- Y. Chen, X. Sun, W. Pan, G. Yu and J. Wang, Front. Chem., 2020, 7, 911 CrossRef PubMed.
- J. Zhu, H. Chu, T. Wang, C. Wang and Y. Wei, Microchem. J., 2020, 105142 CrossRef CAS.
- A. M. Senol and E. Bozkurt, Microchem. J., 2020, 159, 105357 CrossRef CAS.
- Z.-F. Pu, Q.-L. Wen, Y.-J. Yang, X.-M. Cui, J. Ling, P. Liu and Q.-E. Cao, Spectrochim. Acta, Part A, 2020, 229, 117944 CrossRef CAS PubMed.
- Y. Dong, J. Ma, C. Liu and Y. Bao, Ceram. Int., 2020, 46, 11115–11123 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Comprises photoluminescence spectra of TE-CQDs at various excitation wavelengths, the optical excitation spectrum of TE-CQDs, images of TE-CQDs in presence and absence of Fe3+, FT-IR spectrum of TE, binding affinity constant values against different interference, modified S–V plot for the sensing of Fe3+ in standard solution and HBS sample 1, linearity plot of the probe, FL quenching analysis for the HBS sample 2, TRPL study table and a brief study of the present work. See DOI: 10.1039/d1ra01571k |
| This journal is © The Royal Society of Chemistry 2021 |