Open Access Article
Open Access ArticleClinically oriented Alzheimer's biosensors: expanding the horizons towards point-of-care diagnostics and beyond
Bayu Tri Murti
ab,
Athika Darumas Putri
bc,
Yi-June Huang
de,
Shih-Min Wei
de,
Chih-Wei Peng
ef and
Po-Kang Yang
 *deg
*deg
aGraduate Institute of Biomedical Materials and Tissue Engineering, College of Biomedical Engineering, Taipei Medical University, Taipei, Taiwan
bSemarang College of Pharmaceutical Sciences (STIFAR), Semarang City, Indonesia
cDepartment of Pharmaceutical Sciences, School of Pharmacy, College of Pharmacy, Taipei Medical University, Taipei, Taiwan
dGraduate Institute of Nanomedicine and Medical Engineering, College of Biomedical Engineering, Taipei Medical University, Taipei, Taiwan. E-mail: yangpk@tmu.edu.tw
eInternational Ph.D. Program in Biomedical Engineering, College of Biomedical Engineering, Taipei Medical University, Taipei, Taiwan
fSchool of Biomedical Engineering, College of Biomedical Engineering, Taipei Medical University, Taipei, Taiwan
gDepartment of Biomedical Sciences and Engineering, National Central University, Chung-li, Taiwan
First published on 8th June 2021
Abstract
The development of minimally invasive and easy-to-use sensor devices is of current interest for ultrasensitive detection and signal recognition of Alzheimer's disease (AD) biomarkers. Over the years, tremendous effort has been made on diagnostic platforms specifically targeting neurological markers for AD in order to replace the conventional, laborious, and invasive sampling-based approaches. However, the sophistication of analytical outcomes, marker inaccessibility, and material validity strongly limit the current strategies towards effectively predicting AD. Recently, with the promising progress in biosensor technology, the realization of a clinically applicable sensing platform has become a potential option to enable early diagnosis of AD and other neurodegenerative diseases. In this review, various types of biosensors, which include electrochemical, fluorescent, plasmonic, photoelectrochemical, and field-effect transistor (FET)-based sensor configurations, with better clinical applicability and analytical performance towards AD are highlighted. Moreover, the feasibility of these sensors to achieve point-of-care (POC) diagnosis is also discussed. Furthermore, by grafting nanoscale materials into biosensor architecture, the remarkable enhancement in durability, functionality, and analytical outcome of sensor devices is presented. Finally, future perspectives on further translational and commercialization pathways of clinically driven biosensor devices for AD are discussed and summarized.
1. Introduction
The prevalence of Alzheimer's disease (AD), 60–70% of dementia's total occurrence, has been increasing extensively worldwide. In 2016, 43.8 million people suffered from dementia, and these statistical figures are expected to even reach 100 million by 2030–2050.1,2 AD corresponds to an extremely harmful and irreversible neurodegenerative disorder which can gradually cause serious brain problems.3 Currently, the common approach to characterize AD is by various pathological markers, which include amyloid-β (Aβ) plaques, tau proteins, vascular damage, loss of synapses, damaged neuronal cells, formation of dystrophic neurites, noticeable gliosis, etc.4–6To overcome AD, there are two major strategies being actively applied. The first strategy is to develop new biological and chemical entities for therapeutic usages. For instance, a great deal of effort has been reported to develop chemotherapeutic drugs and their delivery vehicles to effectively eliminate or target the disease biomarkers. However, serious limitations have been found from AD pathophysiology, lacking consensus on crystal-clear comprehension, and heterogeneity of responsible hallmarks.7,8 More importantly, this strategy also restricts the development of highly selective medicines to clinically eradicate the biomarkers. In contrast, the second strategy is to establish biomarkers for early-stage detection and progression monitoring via engineered nanomedical approaches. Biosensor is a typical example, leading to the early prevention of the disease and avoid a more severe stage. This strategy has been widely accepted as an appropriate toolbox to overcome AD, which is coherent with the rapid observation on drug discovery and development.
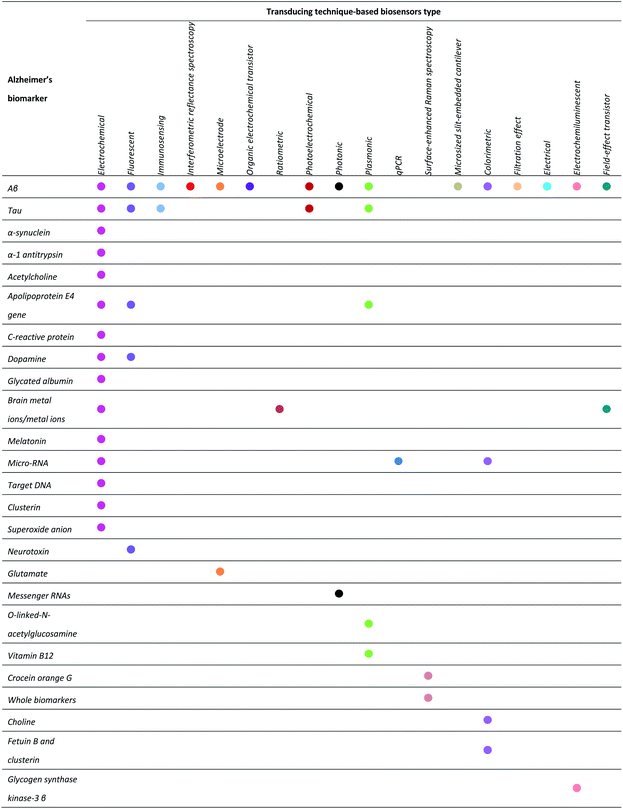
Recently, biosensor technology has been largely explored in the various fields of neurodegenerative diseases.9,10 Biosensors, as first terminology coined by Clark and Lyons in 1962,11 is described by International Union of Pure and Applied Chemistry (IUPAC) as “a device that uses specific biochemical reactions mediated by isolated enzymes, immunosystems, tissues, organelles or whole cells to detect chemical compounds usually by electrical, thermal or optical signals”.12–14 Generally, biosensors consist of three parts. The first part is the biorecognition element, including antibody, peptide, enzyme, microbe, cell receptor, and DNA/RNA aptamer. Second, the transducer will convert the biological event to a measurable signal. Third, a signal processor will translate the signal into user-friendly display such as computerized graphics, diagrams, spectra, etc. The analytical performance of biosensors depends on sensitivity, specificity, selectivity, and accuracy. To reach an optimum level of sensor detection, various types of biosensors have been reported, such as electrochemical-, field-effect transistor-, plasmonic-, immuno-, fluorescent-, optical sensors, etc.15–19 (Table 1). Nevertheless, some critical issues still remain unsolved from the recent findings. Particularly, how to enable device to effectively detect biomarkers at an early stage of the AD, sample invasiveness, and device miniaturization have become an urgent matter.
Today, biosensors have been addressed as the point-of-care (POC) diagnostics strategy for AD. It works as a screening platform instead of a conclusive diagnostic method. Tremendous studies have been conducted in this field.10,20–23 Most of the constructed devices were applied to either blood or human cerebrospinal fluid (CSF) samples, while several reports employed minimally invasive samples, such as nasal secretions, saliva, etc.24–26 The mainstream development is now directed towards POC device in which testable at the time and place of patient care, capable as a self-testing, as well as complying the ASSURED guideline: Affordable, Sensitive, Specific, User-friendly, Rapid and Robust, Equipment-free, and Delivered.27,28
In this review, we attempt to summarize forward-looking developments of promising detection strategies towards AD, especially for clinically driven biosensors design. The most recent development of biomarkers for AD, biosensor design and state-of-the-art biosensing strategies (i.e., in vitro and in vivo) are presented. Moreover, the importance of in silico study to support the experimental findings is also briefly discussed (Fig. 1(A)). Finally, a perspective on the regulation of the biosensor development and commercialization pathways is also provided as future remarks. This aims to signify the emerging role of biosensor in current diagnosis for AD and to address their advancements towards future clinical applications.
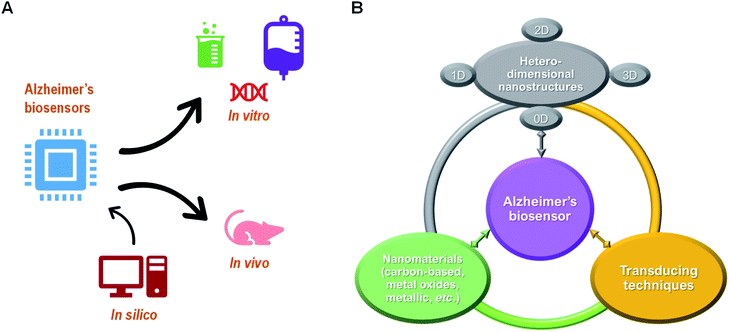 | ||
| Fig. 1 (A) Clinical application of biosensor for AD and (B) their core elements towards modern diagnostic approaches. | ||
2. State-of-the-art on clinically oriented biosensor for AD
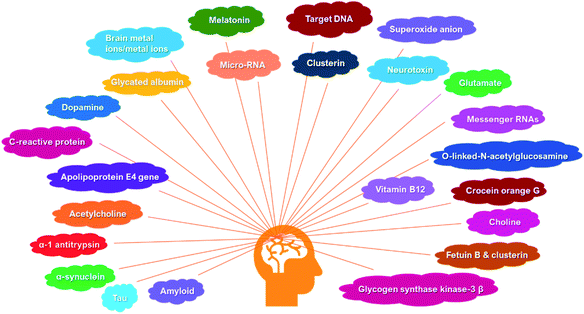
Currently, the decisive cause of AD remains unknown. Many hypotheses have been associated with the Alzheimer's etiology including amyloid cascade hypothesis, tau propagation hypothesis, neurotransmitter hypothesis, calcium homeostasis hypothesis, mitochondrial cascade, neurovascular hypothesis, metal ion hypothesis, inflammatory hypothesis, exercise hypothesis, virus hypothesis, diabetes hypothesis, lymphatic system hypothesis, etc.3 Among them, regardless of its recent failure in clinical trials, amyloid hypothesis has been regarded as the mainstream concept underlying AD research in the past two decades.29 The solid consensus in this field will be beneficial in escalating the successful therapeutic interventions as well as in AD diagnosis.In the scope of Alzheimer's diagnosis, there were notable reports on biosensor encompassing early detection and disease monitoring. Several biomarkers have recently been utilized such as amyloid, tau, apolipoprotein E4 (ApoE4) gene, α-synuclein, α-1 antitrypsin, acetylcholine, etc. The overview of these biomarkers is illustrated in Fig. 2. Motivated by the clinical need for biosensor-based diagnosis, the surveyed biosensors are divided into two main categories, i.e., in vitro and in vivo biosensing systems. As for the in vitro, the biosensor device could be applied to test the samples in which the biochemical reactions occur outside of living body. The samples include human based-biofluids or artificial samples, cell culture, tissue excluding the living organism, and physiologically relevant pre-clinical analytes.30,31 As for the in vivo methodologies, the biosensor device is simply operated inside the body or may be implanted into a living system.32 Further, an insight into the biosensor architecture potentially used as POC diagnosis of AD is also briefly presented.
2.1. Biomarkers as crucial target for biosensor system
Biomarkers basically belong to proteins, enzymes, biological metals, genes, small biomolecules, and metabolites which have been largely employed as the bioanalyte in AD biosensor development (Fig. 2).33 Aβ, tau protein, and phosphorylated tau are shown to be predominant markers for the AD.33,34 Additionally, most of them are known to be accumulated in CSF and neuroimaging samples.35 To date, scientists and clinicians have been working on developing advance tools of AD diagnosis by targeting the various type of biomarkers.36,37 For example, the progress on biosensors based on specific Aβ detection,10 and aptamer-based biosensors for AD have been reported.38 However, it should be noted that a clinically oriented biomarker with appropriate biosensor design for AD has not yet been properly addressed. In the following, we will stress our focus to provide recent developments on biosensors for AD based on biomarker sources employed in pre-clinical and clinical settings.In clinical setting, many diagnostic methods have been applied to detect Aβ. Traditionally, neuroimaging (e.g., positron emission tomography, magnetic resonance imaging, and near infrared fluorescence) is the foremost clinical assessment tool for Aβ deposition in vivo. Its imaging quality depends on several factors such as the instrument resolution, the ability of contrast agent to surpass blood–brain barrier (BBB), its specificity towards Aβ diffuse, core, and intermediate plaques species.50–52 In general, the use of neurological imaging has been limited within community owing to the high analytical cost and operational, and a poor understanding of amyloid burden relationship with cognitive dysfunction.51,53–56 Instead, immunoassay method (i.e., enzyme-linked immunosorbent assay (ELISA)) has been a plausible choice for the clinical diagnosis of Aβ. ELISA is the gold standard immunoassay for CSF Aβ (42) detection and is the most frequently used diagnostic method in AD.36,52,57,58 Tremendous studies have reported the ELISA application in detecting Aβ levels in CSF, human plasma, and serum samples.59–64 Numerous lab-based ELISA kits have now been available in the market with the detection range of 10 to 1000 pg mL−1 and proficient of discriminating AD patients with healthy control with good sensitivity (80%) and moderate specificity (83.3%).52,65 In spite of time-consuming and laborious issues of traditional ELISA, the alternative ELISA kits have been widely developed for amyloid detection, which include paper-based ELISA63 and digital ELISA.66,67 Both of them are cost-effective, easy to operate, and portable and thus suitable for POC diagnostic approach.52,65
In addition to amyloid-based diagnosis, biomarker for AD is not only relied on Aβ molecules (i.e., monomer), but also on their elongated forms68 so-called Aβ oligomers (AβO) and fibrils. AβO has been regarded as the most neurotoxic entities and meticulously associated with the AD severity than that of insoluble Aβ aggregates,69 specifically in the early phase of disease initiation. Their concentrations were observed to be up to 70-fold higher in AD as compared to non-demented controls.70,71 The relationship between the Aβ assemblies and their toxicity was previously elucidated based upon the peptides binding with the fluorescent probes.72–74 Due to the higher toxicity of AβO over amyloid fibrils, the detection of oligomeric amyloid is advantageous to precisely represent the progression of AD, particularly for its early stage.75,76 Recently, an electrochemical biosensor design was reported to detect AβO in an in vitro environment using thiolated cellular prion protein peptide as bioreceptor.71
Antibody is favourable entity to detect amyloid biomarkers owing to its high affinity to the designated antigen.38,77 Recently, scientist employed another recognition element such as aptamer to selectively detect AD biomarkers.38,78 Aptamer has offered many advantages to biosensor design, such as higher affinity (as compared to classical antibody), less expensive, non-in vivo production, reproducible system, smaller molecular size, and availability for wide range of analytes.38 In general, aptamer is defined as short sequence of nucleotides (DNA or RNA) that primarily designed to mimic antibody function. Aptamers are three-dimensional bioreceptors with molecular size significantly smaller than conventional protein antibody.38 This bio-entity is produced by systematic evolution of ligands by exponential enrichment (SELEX) resulting in prominent-affinity and selectivity entities towards analytes. Meanwhile, aptamer is also a fascinating choice in clinically oriented biosensors due to their specificity, biocompatibility, non-toxicity, as well as non-immunogenic features.38 As for the current biosensors of AD, aptamers are tenaciously used as an alternative of antibody setting especially for bioreceptor in detection strategy. For instance, aptamer is useful to overcome the Debye–Hückel screening effect in field-effect transistor (FET)-based biosensor (vide infra), which commonly hinder the electrical signal from the target molecules under high concentrations of such ions.79 FET, integrated with proper biorecognition elements such as aptamer or antibody, is a unique and useful sensing device to detect biomolecular targets80–82 offering many advantages such as real-time, highly sensitive, specific, and label-free transduction of biochemical signals.83,84 In principle, the sensing mechanism of an FET device involves structural and functional integration of biorecognition element in which the selective interaction of bioreceptors and analyte produces changes in biophysical and biochemical signal. The signal is then transduced and amplified via a field-effect towards the signal display.83,85,86 Aptamer is highly beneficial in this typical device since the biosensing is carried out under the physiological fluids87 such as human CSF and blood. However, aptamer also possesses several limitations while embedded in biosensors, which include lack of high-quality aptamers for clinically important targets and non-specific binding of aptamer-surrounding environment.88 To overcome these limitations, an appropriate biosensor design with functional biomarker is needed, such as the compliment of nanomaterial, sandwich-type aptasensor, antibody complex, and polymer inclusion.89 Additionally, a major concern has been given upon the failure of amyloid in latest clinical trials in which scientist started to reconsider that predominant role of amyloid in AD pathogenesis.90,91
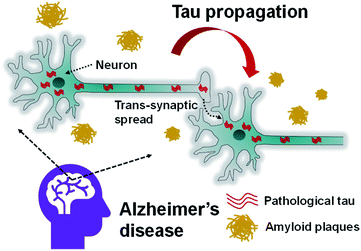 | ||
| Fig. 3 Schematic depiction of cell-to-cell tau spreading in AD brain via neuronal trans-synaptic transmission. Adapted from ref. 98, open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/). | ||
2.2. In vitro biosensing system
A versatile biosensing device, such as a commercial glucometer or alcohol meter, merely initiated with the assay at a non-natural sample and animal biofluids. In other words, the biochemical reaction, as a result of analyte and biorecognition element interaction, occurs outside of a living organism under the in vitro environment, such as a culture dish, a test tube, a microtiter plate, etc.104 Nowadays, significant advances from nanotechnology and microfabrication process have shed light on the development of in vitro biosensors. Take microfluidic device as an example, the devices fundamentally combine miniaturized features of computing chip with living cells and tissues mimicking the human biology.105 Additionally, organ-on-chips device have also been well-established simply to impersonate the more complex organ systems, since the excellent capability of the biosensors and microfluidic system.106,107 These advances make the in vitro environment to be one of the plausible choices for biosensor development.In vitro biosensor, as part of in vitro diagnosis (IVDs), refers to the assays or tests that are carried out on samples such as blood or tissue withdrawn from the human body. By regulation, the IVDs is defined by U.S. FDA as the specific subcategory of medical devices comprising “those reagents, instruments and systems intended for the diagnosis of disease or other conditions”. Relevant diagnostics are then suitable with this category, which include a determination of the state of health, cure, mitigation, treatment, and prevention.108 Moreover, IVDs may also be used in precision and personalized medicine to identify the patients benefitting from a particular therapies and treatment.108,109 In 2016, the global diagnostics market was counted as US$40–45 billion with POC diagnostics supplying US$12–13 billion. The annual growth rate of IVDs is forecasted to be 5%.110,111 The rapidly growing market of IVDs emphasize the prominence of this technology, not only for academia-based research but also in industry. Recently, the global pandemic of COVID-19 also fuels the research and development in pursuit of IVDs technology, especially for cardiovascular diseases, neurological diseases, liver dysfunction, pneumonia, etc.
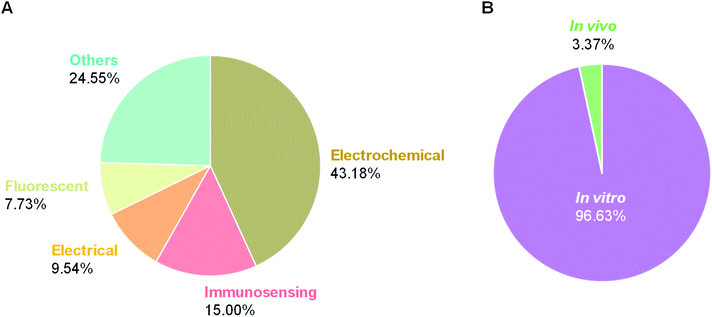
For the sensing techniques driven to in vitro analysis, several efforts have been conducted for biosensor design targeting AD.112 For example, a state-of-the-art soft material fabricated with DNA origami has been introduced as artificial peptide nano-network biosensor to tackle the pathological peptides aggregates in neurodegenerative disease, by imitating pathogenesis process.31 Particularly, periphery platelet enables itself to secrete amyloid proteins and initiate their cross-linking to establish a surface peptide molecular-based system. This platform could sophistically discriminate the AD patients from healthy volunteers by detecting potential neurodegenerative activity of platelet with the <1 pg mL−1 detection limit and 3.3–3300 pg mL−1 dynamic range, which is superior to ELISA method. This biosensor is potentially used towards the label-free and early screening (IVDs) of AD biomarkers circulating in minimally invasive blood sample. Table 1 and Fig. 4(A) show the distribution status of state-of-the-art biosensors for AD, the cutting-edge usage of biosensors in anchoring from forefront analyte recognition towards signal amplification and data acquisition.113 To effectively diagnose AD, various techniques have also been developed towards specific biomarker and sensing strategies, such as fluorescent biosensors, immunosensors, electrochemical biosensors, field-effect transistor-type biosensors etc. (Table 1 and Fig. 4(A)).114 Among them, it is noted that electrochemical biosensor has attracted prodigious attentions (i.e., 43.18% in Fig. 4(A)). More interestingly, in vitro platform occupies the major portion of biosensor development for AD, counting over 95% (Fig. 4(B)).114 The reason could be due to the intricate procedure of in vivo biosensor which commonly need to be implanted into the living animal model. Meanwhile, in vivo biosensors also need to concern with the biocompatibility, systemic toxicity, biodegradability of the materials used, that make in vivo biosensors more complex to achieve in comparison with in vitro ones.115–117
Several types of working modes have been well-established in electrochemical sensors via amperometry,131 voltammetry,132 impedimetric,133 conductometry,13 and interdigitated microelectrode techniques.24 The advantages of this sensing platform mainly rely on two aspects i.e., capability of device miniaturization and cost-effective instrumentation.134 More importantly, some commercialized biosensors have become popular owing to these merits (i.e., POC glucose sensors and alcohol monitoring device).134,135 Meanwhile, it is also noted that electrochemical biosensors have been utilized for routine analysis paving the way for POC diagnostics of AD.10,22,23
As for the diagnosis of AD, an electrochemical biosensor platform was recently reported to detect AβO in an in vitro environment using thiolated cellular prion protein peptide as bioreceptor.71 Moreover, Sun et al. also employed composite hetero-structured nanomaterials and three-dimensional (3D) hydrogel to achieve 0.1 pM detection limit in 0.1–10 nM linear working range, complying the clinical diagnostic concentration of amyloids in human plasma and CSF (5.5–195 pM). This 3D-hydrogel biosensor composed of graphene oxide (GO)/Au nanoparticles (Au NPs) provided significantly larger surface area as compared to solid electrode allowing rapid penetration of target biomolecules towards bioreceptor binding moiety. The purpose of utilizing GO in hydrogel is to contribute towards the tunable conductivity and bionic structure of the electrode.
Additionally, a differential pulse voltammetry (DPV)-based electrochemical biosensor was employed by Negahdary and Heli to detect Aβ (42) in an artificial CSF and spiked serum samples.136 This strategy embedded Aβ (42)-binding peptides on the microporous Au nanostructure, and successfully achieved attomolar (aM) level of detection limit i.e., 0.2 pg mL−1 (44.3 aM), with a linear working range of 3–7000 pg mL−1. Authors further extended the similar work with the use of different biorecognition element i.e., 107-mer thiol-modified RNA aptamer.129 However, this study represented the slightly higher LOD (i.e., 0.4 pg mL−1 or 88.6 aM) than that of antibody setting. The results indicate the better compatibility of antibody with the electrochemical-based strategy, in particular for amyloid detection, as compared to aptamer. Indeed, owing to the high surface area during immobilization process, the addition of Au nanomaterials in those studies are another key medium towards the ultrasensitive biosensors device.129
Another electrochemical biosensing platform was designed to detect Aβ in its oligomer by using brain samples of normal mice and with AD.137 The label- and antibody-free biosensors were fabricated for the AβO detection employing cellular prion protein as a receptor for AβO yielding detection limit at picomolar level (10−4 pM). In contrast to the classical ELISA method, this platform used PrPC receptor to capture the amyloid target as opposed of antibody suffered from time-consuming procedure and lengthy incubation. Additionally, an electrochemical strategy was developed to measure AβO in vitro acquiring a few μM LOD by dual transducing techniques, where CV and UV-Vis spectrophotometry in human blood serum and artificial CSF.138 This approach utilized the competitive nature of Zn ions and AβO releasing ferrocene from its Zn zeolitic imidazole framework which then subsequently detected by the transducers. This study protocol provided a decent feasibility of using artificial sample comprising biomarker of AD.
Actually, instead of amyloid-based marker, melatonin could also be detected by electrochemical method with the prime marker source from rat's liver extracts (spiked with melatonin).132 Melatonin abnormal circulation have been correlated to several diseases such as AD, type II diabetes mellitus, and several types of cancers.132,139 This melatonin-targeted immunosensor was reported as easy-to-use device for rapid quantification of melatonin reaching micromolar LOD, which can be further potentially used as a POC device in the future.132 Other sensing techniques were also successfully employed in developing electrochemical biosensor of AD with animal-sample tests, which include microelectrode array (MEA),140 ratiometric photoacoustic nanoprobe,141 and electrochemiluminescence.142
As widely understood, tau protein is also an excellent candidate for human sample-based biosensor for AD. An electrochemical biosensor was recently developed to detect tau-381 based on aptamer–antibody sandwich assay in human serum from patients with AD.143 DPV technique was used herein as quantification method resulting in LOD of 0.42 pM with 0.5 pM to 100 pM dynamic range. This biosensor combines the advantages of prominent affinity of aptamer with the signal amplification of the Au NPs yielding ultrasensitive detection means for tau protein in real human serum. Meanwhile, an electrochemical sensor based on a single bioreceptor, aptamer, immobilized in carboxyl graphene nanomaterials, thionin, and Au NPs modified glassy-carbon electrode was subsequently introduced.144 However, the LOD in this work was found to be slightly higher (0.70 pM) than their previous report (0.42 pM). Nevertheless, both reports emphasize the capability of electrochemical based sensors as an early screening of AD, particularly for clinically relevant sample (i.e., real blood from AD patients). Further, it is noted that other biomarkers were also useful in human sample-based biosensor, such as ApoE4 gene from four genomic DNA samples extracted from human blood.145
Despite technologically convenience and advantage offered by electrochemical method, the biomarker detection using this technique still exists several limitations and challenges that need to be overcome. For instance, the as-designed device usually lacks selectivity since the reference electrode limits the charge carriers,146,147 sensor instability over prolonged storage time, limited design for multianalytes detection, and lack of regenerative sensor devices.148 The further development should aim at addressing these issues and simultaneously maintain the forefront characteristics of electrochemical biosensors such as high sensitivity, device miniaturization capability, and relatively cost-effective in mass production.
As for the fluorescent-based AD biosensors, series of biomarkers have been employed as an in vitro setting such as amyloid,159–161 dopamine,162–170 neurotoxin,171 tau,172 and ApoE4 DNA.173 The major targeted analyte in this type of biosensor is AβO considered as the main culprit for AD initiation and progression. By quantifying AβO in CSF and plasma, it is found to be beneficial for determining the disease severity.174,175 In 2017, Jiang et al. designed a fluorescent biosensor to detect AβO in artificial CSF by employing Fe3O4 NPs and BaYF5:Yb, Er upconversion NPs (UCNPs) as vastly sensitive labels, incorporated with the AβO aptamer and its complementary oligonucleotide.159 The sensing performance of such a biosensor was shown by the 0.2–15 nM of linear working range and 36 pM of LOD. The addition of uniquely UCNPs and Fe3O4 NPs with aptamer can contribute to the picomolar sensitivity of the platform, particularly under artificial physiological surface. More studies are required to confirm the merit of this biosensor design to detect AβO in the relevant clinical samples i.e., CSF and blood plasma.
Similar target was also detected by a quench body technique (denoted as Q-body) embedded to a fluorescent sensor in an in vitro environment.160 Q-body is a new kind of strategy to detect a broad range of biomolecules employing fluorescence quenching of the dye(s) attached to the antibody fragment.176 Previously, Aβ-derived diffusible ligand (ADDL) was sensed by the double-labelled Fab type Q-bodies (the heavy and light chains) with a higher sensitivity than the Aβ peptides suggesting the promising usage of Fab type Q-bodies as a notorious bioimaging tool.160 It should be noted that fluorescent-based technique also possesses several drawbacks, particularly in detecting the biomarkers of AD. On the one hand, label-based fluorescent biosensing measurement is often time consuming, cost-intensive, and possibly blockade the active binding sites in recognizing the targeted analytes. On the other hand, fluorescent-based technique would potentially affect the affinity-based interaction of bioreceptor and the biomarkers.177 Nonetheless, this method has been widely used as “gold standard” in clinical setting particularly for monitoring of early stage of Aβ nucleation owing to its robust staining properties and method's conveniences.178,179
In terms of clinical perspectives, fluorescent technique has also been reported as proficient transducers to sense AD biomarkers in human samples.180 Rajasekhar et al. reported a near-infrared (NIR) fluorescent-based biosensor constructed to detect amyloid aggregates in human brain tissue sample.181 The authors used coumarin–quinoline (CQ) conjugate as the fluorescent probe exhibiting ∼100-fold fluorescence power in vitro once bound to Aβ aggregates with augmented quantum yield. CQ showed non-toxic properties for neuronal cells and excellent permeability against BBB. CQ probe also demonstrated unequivocal selectivity towards the target protein as compared to other toxic protein aggregates such as tau, α-synuclein, and islet amyloid polypeptide in human brain tissue. This was currently regarded as a reliable method in distinguishing AD from tau pathology and mixed dementia. Uniquely, this study also reported in silico approach i.e., density functional theory (DFT), molecular docking, and molecular dynamics, to elucidate the binding characteristics of ThT (control) and CQ within Aβ (42) fibril and further define the binding constant as compared to experimental data. Molecular structure of CQ and selective staining of Aβ plaques in human brain tissue are represented by Fig. 5(A) while the staining on the same plaque without NFTs of tau and the neuritic component (stained only with Tau phos Ser396/Ser404 (PHF1) antibody) are depicted by part (B) and (C), respectively. Nevertheless, the overlaid image indicated there was no colocalization of PHF1 and CQ compound (Fig. 4(D)). In this study, in silico study is beneficial to elucidate the lowest energy configuration of ligand (ThT or CQ) and protein (Aβ (42)) binding (Fig. 5(E)), to characterize the chemical bonding status, which is responsible for a binding motif (ligand–protein) and regarded as a validation of the experimental finding.181–183
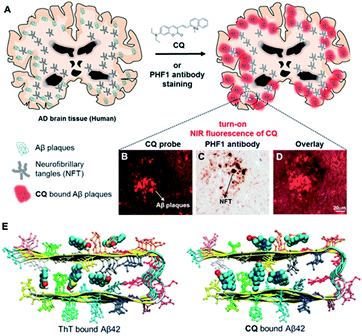 | ||
| Fig. 5 (A) Chemical structure of CQ and selective staining of Aβ plaques in human brain tissue. (B) CQ stains the plaques in the Alzheimer's brain tissue while NFTs of tau were not detected. (C) The neuritic component in the same plaque is only stained with Tau phos Ser396/Ser404 (PHF1) antibody. (D) The overlaid image demonstrated no colocalization of PHF1 and CQ compound. (E) Binding sites for ThT and CQ within Aβ (42) fibril (12 mer assembly) observed by in silico studies. Reproduced from ref. 181 with permission. Copyright (2017) Elsevier. | ||
Zhao et al. also reported a fluorescent-based aptasensor to detect amyloid markers by an employment of double stranded DNA (dsDNA)/GO as the fluorescent probe yielding LOD down to 0.1 nM with a linear detectable range from 0.1 nM to 40 nM.184 This technique could discriminate the sample from AD patients and healthy persons indicating its acceptable selectivity. Moreover, it is noted that GO has effectively participated in reducing the non-specific adsorption due to its large surface area which provide the enormous covalent conjugation with Aβ (40) oligomers-targeting aptamer and therefore, improved the sensitivity and specificity of the device.184 The role of nanomaterials in recent biosensor architecture is crucial to provide a high-performance sensing device in which for now and in the future, these will likely become a promising platform in disease screening as well as POC diagnosis of AD. Additionally, some recent studies also provide detailed explanation and strategies of fluorescent-based based biosensor for AD under in vitro environment,185–188 due to their sensitivity and generally non-invasive.
A biosensing platform based on LSPR on 2D-photonic crystal (2D-PC) and Au-coated 2D-PC was constructed to detect AD-linked DNA oligonucleotide associated with ApoE4 gene sequence in vitro.194 ApoE gene is known as a gene which responsible for Alzheimer's progression.195 The use of this modified LSPR in this study was able to enhance specificity of ApoE gene detection resulting in a promising proof-of-concept for the miniaturized and wearable biosensors in numerous diagnostic and defence applications.194 An interesting part of LSPR is the capability to detect analyte down to single nanoparticle as well as probing tremendously small volumes down to very low LOD achievement and cost-effective device in sensing avenue. However, despite the tremendous merits of LSPR assay in biosensing fields, their practical and clinical applications are still limited.196 This may gain further concerns, specifically when this transducing method is being translated towards clinical diagnosis.
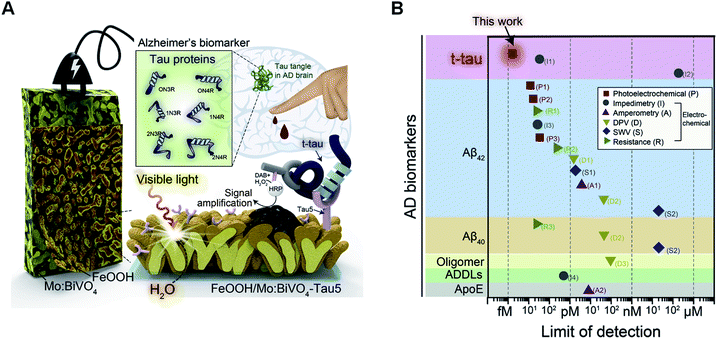 | ||
| Fig. 6 (A) Representation of water oxidation-coupled, FeOOH/Mo:BiVO4-based PEC sensing platform for the detection of femtomolar levels of tau. (B) LOD of Alzheimer's biomarker-targeting sensing platforms reported in literature. Reproduced from ref. 127 with permission. Copyright (2020) Elsevier. | ||
In addition to the great attempts made by using 1D nanomaterials in diagnosis platform for AD, two dimensional (2D) nanomaterials serve as highly promising materials for FET biosensors owing to their fruitful structural and electronic properties,199,204 such as large surface-to-volume ratio, high electrical conductivity, fast electron transfer kinetic reaction, and easy functionalization.205 For instance, a graphene-based FET (G-FET) was fabricated to monitor the Aβ aggregation on the basis of ganglioside GM1-enriched supported lipid bilayer (GM1*-SLB/G-FET) (Fig. 7(A)).206 The as-fabricated G-FET device is capable of detecting and monitoring the early nucleation phase of amyloid formation. It shows a larger potential as a promising biomimetic sensor to investigate membrane-related protein functions and interaction kinetics, as compared to ThT assay. Indeed, concurrent detections of the Aβ40 aggregation by both GM1*-SLB/G-FET and ThT assays may benefit in future diagnosis of AD (Fig. 7(B)).206 However, the ability of device to monitor the amyloid in the real sample (CSF or plasma) remains challenging with the presence of other interference proteins. Another vast obstacle of FET in clinical biosensing measurement relies on the high concentration of salts/buffers in clinical sample-induced Debye–Hückel screening effect.79
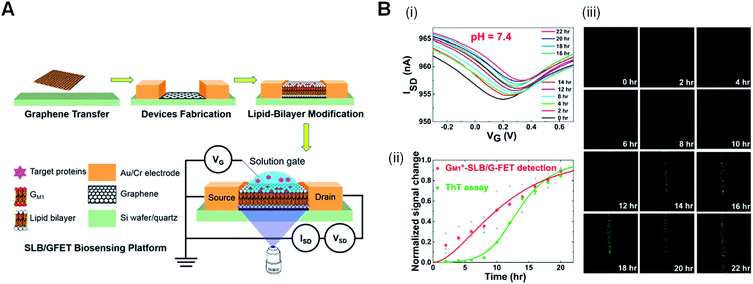 | ||
| Fig. 7 (A) Schematic illustration of preparing a SLB/G-FET device with a solution-gate electrode. (B) Simultaneous detections of the Aβ (40) aggregation by both GM1*-SLB/G-FET and ThT assay. (i) The gradual aggregation of the negatively charged Aβ (40) induced a positive doping to the device. (ii) ThT assay on the similar GM1*-SLB/G-FET device where the fluorescence images were attained by collecting 450–550 nm emission from the ThT dye excited at 405 nm. (iii) Comparison of the observed signals by ThT assay (green dots) and GM1*-SLB/G-FET (red dots) during the Aβ (40) aggregation. Reproduced from ref. 206 with permission. Copyright (2020) American Chemical Society. | ||
Debye–Hückel effect is the discussion about the correlation of Debye length and unambiguous selective detection of macromolecules. Debye length (λD) corresponds to the distance measured from FET-biosensor surface and electrolytic buffer solution (e.g., phosphate-buffered saline) describing the screening of surface charges by ions in an electrolyte solution. Debye length is described as the eqn (1) below,
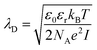 | (1) |
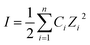 | (2) |
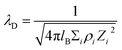 | (3) |
| λD = 0.32(I)−1/2 | (4) |
From these equations, the most important parameter is ionic strength (I) which directly determine the λD. The selection of biorecognition element is also crucial for FET biosensor, particularly for the needs to be applied to relatively high-salt biological samples, such as blood, CSF, sweat, etc. This effect may lead to the clearance of electrical signal produced by target–receptor binding affinity.198 Also, it is suggested that the dimensional size of recognition elements need to be below λD in order to omit the Debye–Hückel screening effect. Instead, another general approach is by diluting the sample into several orders of concentration to increase λD.
Towards next-stage of pre-clinical based-in vitro biosensors, the use of animal biofluid is the crucial steps of biosensor translational efforts, particularly for AD. As the frequent usage in electrochemical transducer, a recent immunosensing approach was developed to detect Aβ (40) fragment in brain tissue lysates prepared from AD-induced rats.133 The devoted impedimetric micro-immunosensing assay used monoclonal Aβ (40) antibodies which were immobilized on a disc-shaped microelectrode surface connected to an impedimetric signal transducer resulting in 4.81 pg mL−1 (∼1.11 pM) LOD with a dynamic range of 1–104 pg mL−1 (∼0.23–24.02 pM). The constructed platform yielded a lower LOD as compared with the designated conventional ELISA method. On the other hand, the amyloid protein together with Cu2+ ions were capable of being detected in plasma and hippocampus of rats with normal and AD by a single ratiometric platform with biological processes association and direct involvement of Cu2+ ions in Aβ (42) aggregation.208 This dual detection mode was achieved by detection of Cu2+ ions through neurokinin B and the Cu2+ releasing from a complex while the ions tend to bind the Aβ (42) protein in the solution. The detection limit was observed as low as 0.04 μM for Cu2+ and 0.5 ng mL−1 (∼1.108 μM) for Aβ (42).
In vitro measurement could also be carried out by using various transducing methods, such as interferometric reflectance spectroscopy,209 organic electrochemical transistor,210 surface-enhanced Raman spectroscopy,211,212 and quantitative polymerase chain reaction.213 These transducers can provide clinically relevant-LOD concentration, simplicity, and reasonable cost of production to an extent by depending on the targets in analyte–bioreceptor interaction type and their native environment. However, further studies are still required to confirm the selectivity and specificity of the device towards real clinical samples.
2.3. In vivo biosensing system
In vivo biosensors have been treated as one of the future technologies of personalized healthcare. An implantable biosensor in human body can deliver essential healthcare information of the patients by continuous monitoring basis which are beneficial for reducing prolonged clinical procedures. This type of sensing platform is particularly important for those who need continuous healthcare monitoring. A small fluctuation will be easily captured and further provide the “health status” of one which further designate the following necessary treatment or therapy. For the life-threatening disease such as cancers, cardiovascular, and nondegenerative disease, the in vivo biosensor may serve as “baseline”-associated device for the prevention of further serious problems or complex outcomes. Another scenario is to continuously monitor chemotherapeutic medicine which can yield the guesswork out of dosing by displaying an individualized report on the pharmacokinetics. Despite many reports in in vivo biosensors towards this direction, only few reports addressed preclinical studies or further approved for human implantation.1162.4. Current in vivo strategies of biosensors for AD
The use of electrochemistry to measure electroactive neurotransmitters such as dopamine, serotonin, norepinephrine, and their metabolites,163–170 in whole animals is pioneered by Ralph Adams and his colleagues during the early 1970s214 in which the dysregulation of these species can lead to AD.215–217 Since then, the field has emerged as one of the important facets in microsensor218 and real-time biological events monitoring, particularly for living tissue environment167,219–221 and single-cell analysis.164,222,223Indeed, conventional electrochemical techniques, i.e., voltammetric, impedimetric, amperometry, and potentiometric, preserve a modest strategy towards Aβ detection in vivo.224,225 Voltammetric method tends to be the most frequently used herein. The sensor merely measures current–potential relationship in which the potential represents as fingerprint-like electrochemical parameter for the determined species while the current is directly proportional to the species. The voltammetric family includes differential pulse voltammetry (DPV), cyclic voltammetry (CV), stripping voltammetry, AC voltammetry, linear sweep voltammetry (LSV), polarography, etc. Among in vitro and in vivo biosensing techniques, DPV seems to be favourable in measuring amyloid biomarkers, particularly for quantitative analysis. Whilst, CV has been widely employed for chemical modifications due to its capability to quantify the redox behaviour of deposited nanomaterials in a triangular shape.
Microelectrode, a micro/nanoscale dimension of chemical sensors, has been widely developed as in vivo biosensing strategy to directly measure the analyte concentration inside the brain based on potential changes across chemically selective membranes at their tip.167 Ding et al. developed an Au-microelectrode biosensor to detect Aβ level from CSF of live mice in situ.225 They governed hemin and Cu2+ ion that typically bind to Aβ to induce strong coordination of Cu2+, Aβ, and hemin. The deposited silver NPs onto Au-microelectrode respond to dynamic alterations of the Aβ, which is subsequently turned into an amplified selective signal. The CSF was sampled from cisterna magna of the mice vigilantly using microneedle to avoid the blood vessels damage. EIS and LSV methods were employed to characterize the probes-deposition and to quantify Aβ level, respectively. The detection limit was shown at 0.2 pM along with wide linear range from 1 pM to 50 nM. Similar concept of electrochemical application was extended using silk fibroin material by Liu et al. on the development of POC device for Aβ detection based on blood sample.226 In addition, a thorough discussion on state-of-the-art of microelectrode-based in vivo neurochemicals sensing has been discussed in a review by Xu et al.227
A microelectrode principle was further employed by Peng et al. using carbon fibre to monitor the superoxide anion radical (O2˙−) which directly correlate with production of reactive oxygen species-induced Aβ.224 They engineered ionic-liquid polymer with carbon nanotubes to mask the immobilized oxide dismutase (i.e., catalysing O2˙− into peroxide species) from the enzyme leakage thereby, achieving better sensitivity (Fig. 8). In vivo experiment was carried out by implanting the microelectrode into live rats' corpus striatum. The catalytic processes were examined using CV and amperometric methods. This study was able to exhibit as low as 0.42 μM of LOD with a decent linear relationship towards O2˙− from 1.0 to 228.0 μM. The results also manifested the use of functionalized ionic liquid polymer (PIL) to preserve electrocatalytic activity, augment the stability, and serve as potential low-toxic matrix to support the substrate binding (e.g., enzyme or probe).
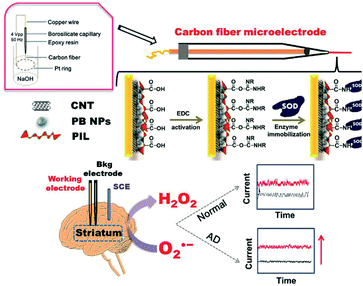 | ||
| Fig. 8 Schematic illustration demonstrating the fabrication of superoxide dismutase/functionalized ionic liquid polymer/Prussian blue/carbon nanotubes/carbon fiber microelectrode (SOD/PIL/PB/CNT/CFME) sensor for the quantification of O2˙−. Reproduced from ref. 224 with permission. Copyright (2019) Elsevier. | ||
For the in vivo biosensor, the need for miniaturized working electrode is important to minimize the possible adverse effect of tissue damage during implantation and to increase the spatial resolution towards probe discrete brain regions as well as the sampling rates.218 Furthermore, translating the in vivo application into real human application remains another critical challenge. There are various biological aspects to be considered, which may differ in expected outcomes with the in vivo, such as uncontrolled inorganic material degradation of the implant in a complex biofluid. Other assessments are required, including systemic toxicity, immune response, irritation, that have satisfactorily been discussed by Gray and coworkers.115
3. A perspective on device's translational and commercialization pathways
Current development of biosensors has been paving the way on translating the sensing functionality to the personal healthcare device. Several types of biosensor products have successfully been distributed globally, including the blood-glucose, uric acid, cholesterol, and tropical disease diagnostics, which further improve the health core-facility down to suburban settings or the remote endemic. More importantly, the integrated application to non-invasively monitor the disease could facilitate better progress and plans for future medication. The presence of this particular technology should possibly never shirk the low cost and uncomplicated processing of a device. In accordance with this, the instrument-less biosensor, like a paper-based diagnostic tool, seems to be promising commercialization thereof.228,229 Concurrently, there is a new trend in wearable sensors which have been reaching the public market since 2014 and expected to substantially increase by the year of progress.229 Compared to the POC, this type of marketable sensor is predicted to supplement the market along with the highly needs for the updated smart device. However, several obstacles related to their capability prior to public commercialization need to be addressed. The instability of the device is the bottleneck that seems perpetual. The device must cope with the dynamic biofluid, entangled constituents present in the biofluid, and biorecognition capability during the period of use.230–233 The robust transmission of the generated signal to translate the detection into readable or report-mode is also considered. The ideal wearable sensor may not be seen as yet, but the promising concept has been one-by-one adequately delivered and fenced with full delicacy to improve the personalized healthcare in the future.4. Conclusions and outlook
Designing suitable and reliable biosensor for AD have continually attracted great interests with the noteworthy growth in recent years. The key factors of these biosensor designs are based on the progress by corresponding biomarkers, sample sources, nanomaterials used as well as advanced transducing techniques. Particularly, it is worth to note that electrochemical biosensor is the most common technique to produce biosensor along with Aβ peptide and tau protein as frequent targets for diagnosing AD. The excellent sensitivity, easy-to-use, simple fabrication, and good selectivity which could be reached through this device architecture. Instead, the closeness and convenience to the end-users are other remarkable benefits of the electrochemical sensing inspired from the success story of glucose monitoring devices which are widely available as POC testing in market. On the other hand, plasmonic detection and fluorescent biosensor are also taken into account as promising strategies for diagnosing AD. Numerous types of other biosensors may serve as alternative methods towards POC diagnostics of the AD. Moreover, theoretical chemistry has admittedly validated and supported the experimental results by its central contributions towards determining the optimized geometry and binding motif of attributed molecular interactions (i.e., via molecular dynamics and molecular docking).Non-invasiveness of the sampling method and device sensitivity are the critical points in the successful development of biosensor for AD. Complying the POC diagnostics, further research could be conducted towards development of ultra-performance biosensors architecture by using biocompatible nanomaterials (e.g., polyethylene glycol, chitosan, biopolymer, etc.), minimally-invasive samples (e.g., blood, saliva), and attempting to reach single molecule analyte as ultimate goal of the device construction. However, from the physician viewpoint, the urgent need in current diagnostic device is not only devoted on the ultrasensitivity feature, but also the wide dynamic or linear range covering the clinical concentration of AD biomarkers. Molecular electronics and self-powered biosensors appear to be the future of bioelectronics. Currently, miniaturization of the system entering the mobile world (i.e., biosensors based on mobile phone; Android or IOS) and self-powered device are vastly considerable in revealing advanced, smart, and early POC- and even beyond, eHealth-, based diagnosis platform of the disease.
Author contributions
B. T. M., A. D. P. developed the main conceptualization and wrote the original manuscript in consultation with Y. J. H., C. W. P., and P. K. Y. S. M. W., Y. J. H., C. W. P., and P. K. Y. assisted in writing, reviewing, and editing of the manuscript. All the authors contributed to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
B. T. M. and A. D. P. acknowledge the Taipei Medical University, Taiwan for providing the funding support of this project. This study was supported by grants from the Ministry of Science and Technology, under Grant No. 108-2636-E-038-003, 109-2636-E-038-004. This work was also financially supported by the Higher Education Sprout Project by the Ministry of Education in Taiwan by Grant No. DP2-109-21121-01-N-02-03, DP2-110-21121-01-N-02-03, and in part by grants from Taipei Medical University (TMU 108-AE1-B40).References
- R. Brookmeyer, E. Johnson, K. Ziegler-Graham and H. M. Arrighi, Alzheimer's Dement., 2007, 3, 186–191 CrossRef PubMed.
- E. Nichols, C. E. I. Szoeke, S. E. Vollset, N. Abbasi, F. Abd-Allah, J. Abdela, M. T. E. Aichour, R. O. Akinyemi, F. Alahdab, S. W. Asgedom, A. Awasthi, S. L. Barker-Collo, B. T. Baune, Y. Béjot, A. B. Belachew, D. A. Bennett, B. Biadgo, A. Bijani, M. S. Bin Sayeed, C. Brayne, D. O. Carpenter, F. Carvalho, F. Catalá-López, E. Cerin, J.-Y. J. Choi, A. K. Dang, M. G. Degefa, S. Djalalinia, M. Dubey, E. E. Duken, D. Edvardsson, M. Endres, S. Eskandarieh, A. Faro, F. Farzadfar, S.-M. Fereshtehnejad, E. Fernandes, I. Filip, F. Fischer, A. K. Gebre, D. Geremew, M. Ghasemi-Kasman, E. V. Gnedovskaya, R. Gupta, V. Hachinski, T. B. Hagos, S. Hamidi, G. J. Hankey, J. M. Haro, S. I. Hay, S. S. N. Irvani, R. P. Jha, J. B. Jonas, R. Kalani, A. Karch, A. Kasaeian, Y. S. Khader, I. A. Khalil, E. A. Khan, T. Khanna, T. A. M. Khoja, J. Khubchandani, A. Kisa, K. Kissimova-Skarbek, M. Kivimäki, A. Koyanagi, K. J. Krohn, G. Logroscino, S. Lorkowski, M. Majdan, R. Malekzadeh, W. März, J. Massano, G. Mengistu, A. Meretoja, M. Mohammadi, M. Mohammadi-Khanaposhtani, A. H. Mokdad, S. Mondello, G. Moradi, G. Nagel, M. Naghavi, G. Naik, L. H. Nguyen, T. H. Nguyen, Y. L. Nirayo, M. R. Nixon, R. Ofori-Asenso, F. A. Ogbo, A. T. Olagunju, M. O. Owolabi, S. Panda-Jonas, V. M. d. A. Passos, D. M. Pereira, G. D. Pinilla-Monsalve, M. A. Piradov, C. D. Pond, H. Poustchi, M. Qorbani, A. Radfar, R. C. Reiner Jr, S. R. Robinson, G. Roshandel, A. Rostami, T. C. Russ, P. S. Sachdev, H. Safari, S. Safiri, R. Sahathevan, Y. Salimi, M. Satpathy, M. Sawhney, M. Saylan, S. G. Sepanlou, A. Shafieesabet, M. A. Shaikh, M. A. Sahraian, M. Shigematsu, R. Shiri, I. Shiue, J. P. Silva, M. Smith, S. Sobhani, D. J. Stein, R. Tabarés-Seisdedos, M. R. Tovani-Palone, B. X. Tran, T. T. Tran, A. T. Tsegay, I. Ullah, N. Venketasubramanian, V. Vlassov, Y.-P. Wang, J. Weiss, R. Westerman, T. Wijeratne, G. M. A. Wyper, Y. Yano, E. M. Yimer, N. Yonemoto, M. Yousefifard, Z. Zaidi, Z. Zare, T. Vos, V. L. Feigin and C. J. L. Murray, Lancet Neurol., 2019, 18, 88–106 CrossRef.
- P.-P. Liu, Y. Xie, X.-Y. Meng and J.-S. Kang, Signal Transduction Targeted Ther., 2019, 4, 29 CrossRef PubMed.
- A. Serrano-Pozo, M. P. Frosch, E. Masliah and B. T. Hyman, Cold Spring Harbor Perspect. Med., 2011, 1, 1–23 Search PubMed.
- A. L. Calderon-Garcidueñas and C. Duyckaerts, in Handbook of Clinical Neurology, ed. G. G. Kovacs and I. Alafuzoff, Elsevier, 2018, vol. 145, pp. 325–337 Search PubMed.
- H. Zetterberg and S. C. Burnham, Mol. Brain, 2019, 12, 1–7 CrossRef CAS PubMed.
- K. Blennow and H. Zetterberg, J. Intern. Med., 2018, 284, 643–663 CrossRef CAS PubMed.
- M. Canevelli, I. Bacigalupo, G. Gervasi, E. Lacorte, M. Massari, F. Mayer, N. Vanacore and M. Cesari, Front. Aging Neurosci., 2019, 11, 282 CrossRef CAS PubMed.
- H. V. S. Ganesh, A. M. Chow and K. Kerman, TrAC, Trends Anal. Chem., 2016, 79, 363–370 CrossRef CAS.
- A. Kaushik, R. D. Jayant, S. Tiwari, A. Vashist and M. Nair, Biosens. Bioelectron., 2016, 80, 273–287 CrossRef CAS PubMed.
- L. C. Clark Jr and C. Lyons, Ann. N. Y. Acad. Sci., 1962, 102, 29–45 CrossRef CAS PubMed.
- B. Nagel, H. Dellweg and L. Gierasch, Pure Appl. Chem., 1992, 64, 143–168 CAS.
- D. R. Thévenot, K. Toth, R. A. Durst and G. S. Wilson, Biosens. Bioelectron., 2001, 16, 121–131 CrossRef.
- IUPAC, Compendium of chemical terminology (the “Gold Book”), Blackwell Scientific Publications, Oxford, 2nd edn, 1997 Search PubMed.
- L. Li, L. Yang, S. Zhang, Y. Sun, F. Li, T. Qin, X. Liu, Y. Zhou and S. Alwarappan, J. Mater. Chem. C, 2020, 8, 14723–14731 RSC.
- H. Zheng, S. Zhang, X. Liu, Y. Zhou and S. Alwarappan, Biosens. Bioelectron., 2020, 162, 112234 CrossRef CAS PubMed.
- R. Gupta, M. Ozhukil Valappil, A. Sakthivel, A. Mathur, C. S. Pundir, K. Murugavel, J. Narang and S. Alwarappan, J. Electrochem. Soc., 2020, 167, 107501 CrossRef CAS.
- A. Sakthivel, A. Chandrasekaran, M. Sadasivam, P. Manickam and S. Alwarappan, J. Electrochem. Soc., 2021, 168, 017507 CrossRef CAS.
- L. Yang, L. Li, F. Li, H. Zheng, T. Li, X. Liu, J. Zhu, Y. Zhou and S. Alwarappan, Anal. Bioanal. Chem., 2021, 413, 193–203 CrossRef CAS PubMed.
- O. Tokel, F. Inci and U. Demirci, Chem. Rev., 2014, 114, 5728–5752 CrossRef CAS PubMed.
- H. Kim, J. U. Lee, S. Song, S. Kim and S. J. Sim, Biosens. Bioelectron., 2018, 101, 96–102 CrossRef CAS PubMed.
- C. Toyos-Rodríguez, F. J. García-Alonso and A. de la Escosura-Muñiz, Sensors, 2020, 20, 4748 CrossRef PubMed.
- M. Edyta, Curr. Med. Chem., 2020, 27, 1–25 CrossRef.
- Y. H. Kim, S.-M. Lee, S. Cho, J.-H. Kang, Y.-K. Minn, H. Park and S. H. Choi, Sci. Rep., 2019, 9, 4966 CrossRef PubMed.
- Y. Kim, Y. K. Yoo, H. Y. Kim, J. H. Roh, J. Kim, S. Baek, J. C. Lee, H. J. Kim, M.-S. Chae, D. Jeong, D. Park, S. Lee, H. Jang, K. Kim, J. H. Lee, B. H. Byun, S. Y. Park, J. H. Ha, K. C. Lee, W. W. Cho, J.-S. Kim, J.-Y. Koh, S. M. Lim and K. S. Hwang, Sci. Adv., 2019, 5, eaav1388 CrossRef CAS PubMed.
- S.-J. Yoo, G. Son, J. Bae, S. Y. Kim, Y. K. Yoo, D. Park, S. Y. Baek, K.-A. Chang, Y.-H. Suh, Y.-B. Lee, K. S. Hwang, Y. Kim and C. Moon, Sci. Rep., 2020, 10, 11234 CrossRef PubMed.
- A. St John and C. P. Price, Clin. Biochem. Rev., 2014, 35, 155–167 Search PubMed.
- D. Mabey, R. W. Peeling, A. Ustianowski and M. D. Perkins, Nat. Rev. Microbiol., 2004, 2, 231–240 CrossRef CAS PubMed.
- F. Kametani and M. Hasegawa, Front. Neurosci., 2018, 12, 25 CrossRef PubMed.
- H. Kim, J. U. Lee, S. Kim, S. Song and S. J. Sim, ACS Sens., 2019, 4, 595–602 CrossRef CAS PubMed.
- K. Zhang, Q. Yang, Z. Fan, J. Zhao and H. Li, Biosens. Bioelectron., 2019, 145, 111701 CrossRef CAS PubMed.
- H. E. Koschwanez and W. M. Reichert, Biomaterials, 2007, 28, 3687–3703 CrossRef CAS PubMed.
- K. Blennow, H. Hampel, M. Weiner and H. Zetterberg, Nat. Rev. Neurol., 2010, 6, 131–144 CrossRef CAS PubMed.
- N. R. Barthélemy, Y. Li, N. Joseph-Mathurin, B. A. Gordon, J. Hassenstab, T. L. S. Benzinger, V. Buckles, A. M. Fagan, R. J. Perrin, A. M. Goate, J. C. Morris, C. M. Karch, C. Xiong, R. Allegri, P. C. Mendez, S. B. Berman, T. Ikeuchi, H. Mori, H. Shimada, M. Shoji, K. Suzuki, J. Noble, M. Farlow, J. Chhatwal, N. R. Graff-Radford, S. Salloway, P. R. Schofield, C. L. Masters, R. N. Martins, A. O'Connor, N. C. Fox, J. Levin, M. Jucker, A. Gabelle, S. Lehmann, C. Sato, R. J. Bateman, E. McDade and the Dominantly Inherited Alzheimer Network, Nat. Med., 2020, 26, 398–407 CrossRef PubMed.
- L. J. Thal, K. Kantarci, E. M. Reiman, W. E. Klunk, M. W. Weiner, H. Zetterberg, D. Galasko, D. Pratico, S. Griffin, D. Schenk and E. Siemers, Alzheimer Dis. Assoc. Disord., 2006, 20, 6–15 CrossRef PubMed.
- C. Humpel, Trends Biotechnol., 2011, 29, 26–32 CrossRef CAS PubMed.
- S. E. O'Bryant, V. Gupta, K. Henriksen, M. Edwards, A. Jeromin, S. Lista, C. Bazenet, H. Soares, S. Lovestone, H. Hampel, T. Montine, K. Blennow, T. Foroud, M. Carrillo, N. Graff-Radford, C. Laske, M. Breteler, L. Shaw, J. Q. Trojanowski, N. Schupf, R. A. Rissman, A. M. Fagan, P. Oberoi, R. Umek, M. W. Weiner, P. Grammas, H. Posner and R. Martins, Alzheimer's Dement., 2015, 11, 549–560 CrossRef PubMed.
- B. Shui, D. Tao, A. Florea, J. Cheng, Q. Zhao, Y. Gu, W. Li, N. Jaffrezic-Renault, Y. Mei and Z. Guo, Biochimie, 2018, 147, 13–24 CrossRef CAS PubMed.
- J. Hardy and D. J. Selkoe, Science, 2002, 297, 353–356 CrossRef CAS PubMed.
- J. L. Molinuevo, S. Ayton, R. Batrla, M. M. Bednar, T. Bittner, J. Cummings, A. M. Fagan, H. Hampel, M. M. Mielke, A. Mikulskis, S. O'Bryant, P. Scheltens, J. Sevigny, L. M. Shaw, H. D. Soares, G. Tong, J. Q. Trojanowski, H. Zetterberg and K. Blennow, Acta Neuropathol., 2018, 136, 821–853 CrossRef CAS PubMed.
- A. T. Petkova, Y. Ishii, J. J. Balbach, O. N. Antzutkin, R. D. Leapman, F. Delaglio and R. Tycko, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 16742–16747 CrossRef CAS PubMed.
- S. R. Chowdhury, S. N. Balaji, S. Mondal, N. Meher, V. Trivedi and P. K. Iyer, ACS Appl. Bio Mater., 2018, 1, 403–413 CrossRef CAS.
- S. Mondal, Y. Vashi, P. Ghosh, D. Roy, M. Barthakur, S. Kumar and P. K. Iyer, ACS Chem. Neurosci., 2020, 11, 3277–3287 CrossRef CAS PubMed.
- K. Blennow and H. Hampel, Lancet Neurol., 2003, 2, 605–613 CrossRef CAS PubMed.
- H. Hampel, A. Mitchell, K. Blennow, R. A. Frank, S. Brettschneider, L. Weller and H. J. Moller, J. Neural Transm., 2004, 111, 247–272 CrossRef CAS PubMed.
- A. Nabers, L. Perna, J. Lange, U. Mons, J. Schartner, J. Güldenhaupt, K.-U. Saum, S. Janelidze, B. Holleczek, D. Rujescu, O. Hansson, K. Gerwert and H. Brenner, EMBO Mol. Med., 2018, 10, 1–11 CrossRef PubMed.
- R. Mercan, B. Bıtık, M. E. Tezcan, A. Kaya, A. Tufan, M. A. Ozturk, S. Haznedaroglu and B. Goker, ISRN Rheumatol., 2014, 2014, 354648 Search PubMed.
- X. Hadoux, F. Hui, J. K. H. Lim, C. L. Masters, A. Pébay, S. Chevalier, J. Ha, S. Loi, C. J. Fowler, C. Rowe, V. L. Villemagne, E. N. Taylor, C. Fluke, J.-P. Soucy, F. Lesage, J.-P. Sylvestre, P. Rosa-Neto, S. Mathotaarachchi, S. Gauthier, Z. S. Nasreddine, J. D. Arbour, M.-A. Rhéaume, S. Beaulieu, M. Dirani, C. T. O. Nguyen, B. V. Bui, R. Williamson, J. G. Crowston and P. van Wijngaarden, Nat. Commun., 2019, 10, 4227 CrossRef PubMed.
- H. Hampel, S. E. O'Bryant, J. L. Molinuevo, H. Zetterberg, C. L. Masters, S. Lista, S. J. Kiddle, R. Batrla and K. Blennow, Nat. Rev. Neurol., 2018, 14, 639–652 CrossRef PubMed.
- H. Matsuda, Y. Shigemoto and N. Sato, Jpn. J. Radiol., 2019, 37, 735–749 CrossRef PubMed.
- F. Márquez and M. A. Yassa, Mol. Neurodegener., 2019, 14, 21 CrossRef PubMed.
- A. Jamerlan, S. S. A. An and J. Hulme, TrAC, Trends Anal. Chem., 2020, 129, 115919 CrossRef CAS.
- J. M. Wardlaw, E. E. Smith, G. J. Biessels, C. Cordonnier, F. Fazekas, R. Frayne, R. I. Lindley, J. T. O'Brien, F. Barkhof, O. R. Benavente, S. E. Black, C. Brayne, M. Breteler, H. Chabriat, C. DeCarli, F.-E. de Leeuw, F. Doubal, M. Duering, N. C. Fox, S. Greenberg, V. Hachinski, I. Kilimann, V. Mok, R. v. Oostenbrugge, L. Pantoni, O. Speck, B. C. M. Stephan, S. Teipel, A. Viswanathan, D. Werring, C. Chen, C. Smith, M. van Buchem, B. Norrving, P. B. Gorelick and M. Dichgans, Lancet Neurol., 2013, 12, 822–838 CrossRef PubMed.
- J. T. O'Brien and K. Herholz, BMC Med., 2015, 13, 163 CrossRef PubMed.
- P. Scheltens, K. Blennow, M. M. B. Breteler, B. de Strooper, G. B. Frisoni, S. Salloway and W. M. Van der Flier, Lancet, 2016, 388, 505–517 CrossRef CAS.
- G. Chételat, J. Arbizu, H. Barthel, V. Garibotto, I. Law, S. Morbelli, E. van de Giessen, F. Agosta, F. Barkhof, D. J. Brooks, M. C. Carrillo, B. Dubois, A. M. Fjell, G. B. Frisoni, O. Hansson, K. Herholz, B. F. Hutton, C. R. Jack Jr, A. A. Lammertsma, S. M. Landau, S. Minoshima, F. Nobili, A. Nordberg, R. Ossenkoppele, W. J. G. Oyen, D. Perani, G. D. Rabinovici, P. Scheltens, V. L. Villemagne, H. Zetterberg and A. Drzezga, Lancet Neurol., 2020, 19, 951–962 CrossRef.
- P. Obrocki, A. Khatun, D. Ness, K. Senkevich, J. Hanrieder, F. Capraro, N. Mattsson, U. Andreasson, E. Portelius, N. J. Ashton, K. Blennow, M. Schöll, R. W. Paterson, J. M. Schott and H. Zetterberg, Alzheimer's Res. Ther., 2020, 12, 20 CrossRef PubMed.
- J. Zhao, W. Chang, L. Liu, X. Xing, C. Zhang, H. Meng, S. C. B. Gopinath, T. Lakshmipriya, Y. Chen and Y. Liu, J. Immunol. Methods, 2021, 489, 112942 CrossRef CAS PubMed.
- H. Vanderstichele, E. V. Kerschaver, C. Hesse, P. Davidsson, M.-A. Buyse, N. Andreasen, L. Minthon, A. Wallin, K. Blennow and E. Vanmechelen, Amyloid, 2000, 7, 245–258 CrossRef CAS PubMed.
- R. Mayeux, L. S. Honig, M.-X. Tang, J. Manly, Y. Stern, N. Schupf and P. D. Mehta, Neurology, 2003, 61, 1185–1190 CrossRef CAS PubMed.
- M. Thambisetty, R. Tripaldi, J. Riddoch-Contreras, A. Hye, Y. An, J. Campbell, J. Sojkova, A. Kinsey, S. Lynham, Y. Zhou, L. Ferrucci, D. F. Wong, S. Lovestone and S. M. Resnick, J. Alzheimer's Dis., 2010, 22, 1099–1109 CAS.
- D. Brambilla, R. Verpillot, B. Le Droumaguet, J. Nicolas, M. Taverna, J. Kóňa, B. Lettiero, S. H. Hashemi, L. De Kimpe, M. Canovi, M. Gobbi, V. Nicolas, W. Scheper, S. M. Moghimi, I. Tvaroška, P. Couvreur and K. Andrieux, ACS Nano, 2012, 6, 5897–5908 CrossRef CAS PubMed.
- W.-H. Sung, J.-T. Hung, Y.-J. Lu and C.-M. Cheng, Diagnostics, 2020, 10, 272 CrossRef CAS PubMed.
- W. Xia, T. Yang, G. Shankar, I. M. Smith, Y. Shen, D. M. Walsh and D. J. Selkoe, Arch. Neurol., 2009, 66, 190–199 CrossRef PubMed.
- T.-Y. Wei, Y. Fu, K.-H. Chang, K.-J. Lin, Y.-J. Lu and C.-M. Cheng, Trends Biotechnol., 2018, 36, 290–303 CrossRef CAS PubMed.
- L. Song, D. R. Lachno, D. Hanlon, A. Shepro, A. Jeromin, D. Gemani, J. A. Talbot, M. M. Racke, J. L. Dage and R. A. Dean, Alzheimer's Res. Ther., 2016, 8, 58 CrossRef PubMed.
- G. C. O'Connell, M. L. Alder, A. R. Webel and S. M. Moore, Bioanalysis, 2019, 11, 2087–2094 CrossRef PubMed.
- M. Sakono and T. Zako, FEBS J., 2010, 277, 1348–1358 CrossRef CAS PubMed.
- J. Bieschke, M. Herbst, T. Wiglenda, R. P. Friedrich, A. Boeddrich, F. Schiele, D. Kleckers, J. M. Lopez del Amo, B. A. Grüning, Q. Wang, M. R. Schmidt, R. Lurz, R. Anwyl, S. Schnoegl, M. Fändrich, R. F. Frank, B. Reif, S. Günther, D. M. Walsh and E. E. Wanker, Nat. Chem. Biol., 2011, 8, 93–101 CrossRef PubMed.
- Y. Gong, L. Chang, K. L. Viola, P. N. Lacor, M. P. Lambert, C. E. Finch, G. A. Krafft and W. L. Klein, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 10417–10422 CrossRef CAS PubMed.
- L. Sun, Y. Zhong, J. Gui, X. Wang, X. Zhuang and J. Weng, Int. J. Nanomed., 2018, 13, 843–856 CrossRef CAS PubMed.
- W.-h. Wu, Q. Liu, X. Sun, J.-s. Yu, D.-s. Zhao, Y.-p. Yu, J.-j. Luo, J. Hu, Z.-w. Yu, Y.-f. Zhao and Y.-m. Li, Biochem. Biophys. Res. Commun., 2013, 439, 321–326 CrossRef CAS PubMed.
- M. J. Guerrero-Muñoz, D. L. Castillo-Carranza, U. Sengupta, M. A. White and R. Kayed, ACS Chem. Neurosci., 2013, 4, 1520–1523 CrossRef PubMed.
- U. Sengupta, A. N. Nilson and R. Kayed, EBioMedicine, 2016, 6, 42–49 CrossRef PubMed.
- E. Y. Hayden and D. B. Teplow, Alzheimer's Res. Ther., 2013, 5, 60 CrossRef PubMed.
- E. N. Cline, M. A. Bicca, K. L. Viola and W. L. Klein, J. Alzheimer's Dis., 2018, 64, S567–S610 CAS.
- S. Scarano, S. Lisi, C. Ravelet, E. Peyrin and M. Minunni, Anal. Chim. Acta, 2016, 940, 21–37 CrossRef CAS PubMed.
- Z. Li, M. A. Mohamed, A. M. Vinu Mohan, Z. Zhu, V. Sharma, G. K. Mishra and R. K. Mishra, Sensors, 2019, 19, 5435 CrossRef CAS PubMed.
- V. Kesler, B. Murmann and H. T. Soh, ACS Nano, 2020, 14, 16194–16201 CrossRef CAS PubMed.
- Y. K. Yoo, J. Kim, G. Kim, Y. S. Kim, H. Y. Kim, S. Lee, W. W. Cho, S. Kim, S.-M. Lee, B. C. Lee, J. H. Lee and K. S. Hwang, Sci. Rep., 2017, 7, 8882 CrossRef PubMed.
- H. Park, H. Lee, S. H. Jeong, E. Lee, W. Lee, N. Liu, D. S. Yoon, S. Kim and S. W. Lee, Anal. Chem., 2019, 91, 8252–8258 CrossRef CAS PubMed.
- S. Hideshima, H. Hayashi, H. Hinou, S. Nambuya, S. Kuroiwa, T. Nakanishi, T. Momma, S.-I. Nishimura, Y. Sakoda and T. Osaka, Sci. Rep., 2019, 9, 11616 CrossRef PubMed.
- X. Dai, R. Vo, H.-H. Hsu, P. Deng, Y. Zhang and X. Jiang, Nano Lett., 2019, 19, 6658–6664 CrossRef CAS PubMed.
- D. Sadighbayan, M. Hasanzadeh and E. Ghafar-Zadeh, TrAC, Trends Anal. Chem., 2020, 133, 116067 CrossRef CAS PubMed.
- Y. Cui, Q. Wei, H. Park and C. M. Lieber, Science, 2001, 293, 1289–1292 CrossRef CAS PubMed.
- G. Zheng, F. Patolsky, Y. Cui, W. U. Wang and C. M. Lieber, Nat. Biotechnol., 2005, 23, 1294–1301 CrossRef CAS PubMed.
- N. Nakatsuka, K.-A. Yang, J. M. Abendroth, K. M. Cheung, X. Xu, H. Yang, C. Zhao, B. Zhu, Y. S. Rim, Y. Yang, P. S. Weiss, M. N. Stojanović and A. M. Andrews, Science, 2018, 362, 319–324 CrossRef CAS PubMed.
- W. Zhou, P.-J. Jimmy Huang, J. Ding and J. Liu, Analyst, 2014, 139, 2627–2640 RSC.
- S. Song, L. Wang, J. Li, C. Fan and J. Zhao, TrAC, Trends Anal. Chem., 2008, 27, 108–117 CrossRef CAS.
- A. J. Doig, M. P. del Castillo-Frias, O. Berthoumieu, B. Tarus, J. Nasica-Labouze, F. Sterpone, P. H. Nguyen, N. M. Hooper, P. Faller and P. Derreumaux, ACS Chem. Neurosci., 2017, 8, 1435–1437 CrossRef CAS PubMed.
- S. Makin, Nature, 2018, 559, S4–S7 CrossRef CAS PubMed.
- H. Zetterberg and B. B. Bendlin, Mol. Psychiatry, 2021, 26, 296–308 CrossRef PubMed.
- M. A. Busche and B. T. Hyman, Nat. Neurosci., 2020, 23, 1183–1193 CrossRef CAS PubMed.
- C. A. Brunello, M. Merezhko, R.-L. Uronen and H. J. Huttunen, Cell. Mol. Life Sci., 2020, 77, 1721–1744 CrossRef CAS PubMed.
- S. Palmqvist, P. S. Insel, E. Stomrud, S. Janelidze, H. Zetterberg, B. Brix, U. Eichenlaub, J. L. Dage, X. Chai, K. Blennow, N. Mattsson and O. Hansson, EMBO Mol. Med., 2019, 11, e11170 CAS.
- H. I. L. Jacobs, T. Hedden, A. P. Schultz, J. Sepulcre, R. D. Perea, R. E. Amariglio, K. V. Papp, D. M. Rentz, R. A. Sperling and K. A. Johnson, Nat. Neurosci., 2018, 21, 424–431 CrossRef CAS PubMed.
- B. J. Hanseeuw, R. A. Betensky, H. I. L. Jacobs, A. P. Schultz, J. Sepulcre, J. A. Becker, D. M. O. Cosio, M. Farrell, Y. T. Quiroz, E. C. Mormino, R. F. Buckley, K. V. Papp, R. A. Amariglio, I. Dewachter, A. Ivanoiu, W. Huijbers, T. Hedden, G. A. Marshall, J. P. Chhatwal, D. M. Rentz, R. A. Sperling and K. Johnson, JAMA Neurol., 2019, 76, 915–924 CrossRef PubMed.
- J. Neitzel, R. Nuttall and C. Sorg, Front. Neurol., 2018, 9, 26 CrossRef PubMed.
- R. E. Bennett, S. L. DeVos, S. Dujardin, B. Corjuc, R. Gor, J. Gonzalez, A. D. Roe, M. P. Frosch, R. Pitstick, G. A. Carlson and B. T. Hyman, Am. J. Pathol., 2017, 187, 1601–1612 CrossRef CAS PubMed.
- F. P. Chong, K. Y. Ng, R. Y. Koh and S. M. Chye, Cell. Mol. Neurobiol., 2018, 38, 965–980 CrossRef CAS PubMed.
- N. N. Naseri, H. Wang, J. Guo, M. Sharma and W. Luo, Neurosci. Lett., 2019, 705, 183–194 CrossRef CAS PubMed.
- S. Lisi, S. Scarano, S. Fedeli, E. Pascale, S. Cicchi, C. Ravelet, E. Peyrin and M. Minunni, Biosens. Bioelectron., 2017, 93, 289–292 CrossRef CAS PubMed.
- S. Lisi, E. Fiore, S. Scarano, E. Pascale, Y. Boehman, F. Ducongé, S. Chierici, M. Minunni, E. Peyrin and C. Ravelet, Anal. Chim. Acta, 2018, 1038, 173–181 CrossRef CAS PubMed.
- B. D. Malhotra and M. A. Ali, in Nanomaterials for Biosensors, ed. B. D. Malhotra and M. A. Ali, William Andrew Publishing, United States, 2018, pp. 183–219 Search PubMed.
- A. Dove, Science, 2018, 359, 1287–1289 CAS.
- A. Kalmykov, C. Huang, J. Bliley, D. Shiwarski, J. Tashman, A. Abdullah, S. K. Rastogi, S. Shukla, E. Mataev, A. W. Feinberg, K. J. Hsia and T. Cohen-Karni, Sci. Adv., 2019, 5, 1–11 Search PubMed.
- E. Ferrari, C. Palma, S. Vesentini, P. Occhetta and M. Rasponi, Biosensors, 2020, 10, 110 CrossRef CAS PubMed.
- Ž. Težak, M. V. Kondratovich and E. Mansfield, Pers. Med., 2010, 7, 517–530 CrossRef PubMed.
- T. H. Kim, S. Lee and X. Chen, Expert Rev. Mol. Diagn., 2013, 13, 257–269 CrossRef CAS PubMed.
- C. Morel, L. McClure, S. Edwards, V. Goodfellow, D. Sandberg and J. Thomas, Ensuring innovation in diagnostics for bacterial infection: implications for policy, World Health Organization, Copenhagen (Denmark), 2016 Search PubMed.
- Z. Li and H. C. Shum, in Nanotechnology and Microfluidics, ed. X. Jiang, C. Bai and M. Liu, Wiley-VCH Verlag GmbH & Co. KGaA, Germany, 2020, pp. 83–107 Search PubMed.
- L. C. Brazaca, I. Sampaio, V. Zucolotto and B. C. Janegitz, Talanta, 2020, 210, 120644 CrossRef CAS PubMed.
- Scopus, Document search, https://www.scopus.com/, accessed December 09, 2020 Search PubMed.
- PubMed, Alzheimer biosensor, https://pubmed.ncbi.nlm.nih.gov/, accessed February 16, 2020 Search PubMed.
- M. Gray, J. Meehan, C. Ward, S. P. Langdon, I. H. Kunkler, A. Murray and D. Argyle, Vet. J., 2018, 239, 21–29 CrossRef CAS PubMed.
- G. Rong, S. R. Corrie and H. A. Clark, ACS Sens., 2017, 2, 327–338 CrossRef CAS PubMed.
- S. Vaddiraju, I. Tomazos, D. J. Burgess, F. C. Jain and F. Papadimitrakopoulos, Biosens. Bioelectron., 2010, 25, 1553–1565 CrossRef CAS PubMed.
- L. Lin, Y. Liu, L. Tang and J. Li, Analyst, 2011, 136, 4732–4737 RSC.
- M. Pohanka, V. Adam and R. Kizek, Sensors, 2013, 13, 11498–11506 CrossRef PubMed.
- J. V. Rushworth, A. Ahmed, H. H. Griffiths, N. M. Pollock, N. M. Hooper and P. A. Millner, Biosens. Bioelectron., 2014, 56, 83–90 CrossRef CAS PubMed.
- L. Liu, Q. He, F. Zhao, N. Xia, H. Liu, S. Li, R. Liu and H. Zhang, Biosens. Bioelectron., 2014, 51, 208–212 CrossRef CAS PubMed.
- L. Liu, N. Xia, M. Jiang, N. Huang, S. Guo, S. Li and S. Zhang, J. Electroanal. Chem., 2015, 754, 40–45 CrossRef CAS.
- Y. Yu, X. Sun, D. Tang, C. Li, L. Zhang, D. Nie, X. Yin and G. Shi, Biosens. Bioelectron., 2015, 68, 115–121 CrossRef CAS PubMed.
- K. Kurzątkowska, A. Jankowska, A. Wysłouch-Cieszyńska, L. Zhukova, M. Puchalska, W. Dehaen, H. Radecka and J. Radecki, J. Electroanal. Chem., 2016, 767, 76–83 CrossRef.
- F. S. Diba, S. Kim and H. J. Lee, Catal. Today, 2017, 295, 41–47 CrossRef CAS.
- E. Pérez-Ruiz, D. Decrop, K. Ven, L. Tripodi, K. Leirs, J. Rosseels, M. van de Wouwer, N. Geukens, A. De Vos, E. Vanmechelen, J. Winderickx, J. Lammertyn and D. Spasic, Anal. Chim. Acta, 2018, 1015, 74–81 CrossRef PubMed.
- K. Kim and C. B. Park, Biosens. Bioelectron., 2020, 154, 112075 CrossRef CAS PubMed.
- C.-C. Wu, B.-C. Ku, C.-H. Ko, C.-C. Chiu, G.-J. Wang, Y.-H. Yang and S.-J. Wu, Electrochim. Acta, 2014, 134, 249–257 CrossRef CAS.
- M. Negahdary and H. Heli, Talanta, 2019, 198, 510–517 CrossRef CAS PubMed.
- I.-H. Cho, D. H. Kim and S. Park, Biomater. Res., 2020, 24, 6 CrossRef CAS PubMed.
- O. C. Bodur, S. Dinç, M. Özmen and F. Arslan, Biotechnol. Appl. Biochem., 2021, 68, 20–29 CrossRef CAS PubMed.
- L. C. Brazaca, C. B. Bramorski, J. Cancino-Bernardi, S. da Silveira Cruz-Machado, R. P. Markus, B. C. Janegitz and V. Zucolotto, Colloids Surf., B, 2018, 171, 94–100 CrossRef CAS PubMed.
- N. Zakaria, M. Z. Ramli, K. Ramasamy, L. S. Meng, C. Y. Yean, K. K. Banga Singh, Z. M. Zain and K.-F. Low, Anal. Biochem., 2018, 555, 12–21 CrossRef CAS PubMed.
- D. G. Rackus, M. H. Shamsi and A. R. Wheeler, Chem. Soc. Rev., 2015, 44, 5320–5340 RSC.
- H. Teymourian, A. Barfidokht and J. Wang, Chem. Soc. Rev., 2020, 49, 7671–7709 RSC.
- M. Negahdary and H. Heli, Microchim. Acta, 2019, 186, 766 CrossRef CAS PubMed.
- J. Qin, D. G. Jo, M. Cho and Y. Lee, Biosens. Bioelectron., 2018, 113, 82–87 CrossRef CAS PubMed.
- J. Qin, M. Cho and Y. Lee, ACS Appl. Mater. Interfaces, 2019, 11, 11743–11748 CrossRef CAS PubMed.
- P. C. Daniel, M. F. Analia and I. B. Luis, Curr. Neuropharmacol., 2010, 8, 218–227 CrossRef PubMed.
- H. C. Hunsberger, S. E. Setti, R. T. Heslin, J. E. Quintero, G. A. Gerhardt and M. N. Reed, J. Visualized Exp., 2017, 55418 Search PubMed.
- S. Wang, G. Yu, Y. Ma, Z. Yang, Y. Liu, J. Wang and X. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 1917–1923 CrossRef CAS PubMed.
- H. Liu, X. Zhou, Q. Shen and D. Xing, Theranostics, 2018, 8, 2289–2299 CrossRef CAS PubMed.
- B. Shui, D. Tao, J. Cheng, Y. Mei, N. Jaffrezic-Renault and Z. Guo, Analyst, 2018, 143, 3549–3554 RSC.
- D. Tao, B. Shui, Y. Gu, J. Cheng, W. Zhang, N. Jaffrezic-Renault, S. Song and Z. Guo, Biosensors, 2019, 9, 84 CrossRef CAS PubMed.
- H. Lu, L. Wu, J. Wang, Z. Wang, X. Yi, J. Wang and N. Wang, Microchim. Acta, 2018, 185, 549 CrossRef PubMed.
- D. Grieshaber, R. MacKenzie, J. Vörös and E. Reimhult, Sensors, 2008, 8, 1400–1458 CrossRef CAS PubMed.
- A. C. Pereira, M. G. F. Sales and L. R. Rodrigues, in Advanced Biosensors for Health Care Applications, ed. Inamuddin, R. Khan, A. Mohammad and A. M. Asiri, Elsevier, 2019, pp. 71–103 Search PubMed.
- S. Vigneshvar, C. C. Sudhakumari, B. Senthilkumaran and H. Prakash, Front. Bioeng. Biotechnol., 2016, 4, 11 CrossRef CAS.
- J. W. Lichtman and J.-A. Conchello, Nat. Methods, 2005, 2, 910–919 CrossRef CAS PubMed.
- H. Zhu, S. O. Isikman, O. Mudanyali, A. Greenbaum and A. Ozcan, Lab Chip, 2013, 13, 51–67 RSC.
- N. Tomita, Y. Mori, H. Kanda and T. Notomi, Nat. Protoc., 2008, 3, 877–882 CrossRef CAS PubMed.
- A. R. Miller, G. L. Davis, Z. M. Oden, M. R. Razavi, A. Fateh, M. Ghazanfari, F. Abdolrahimi, S. Poorazar, F. Sakhaie, R. J. Olsen, A. R. Bahrmand, M. C. Pierce, E. A. Graviss and R. Richards-Kortum, PLoS One, 2010, 5, e11890 CrossRef PubMed.
- K. G. McIntosh, M. J. Cusack, A. Vershinin, Z. W. Chen, E. A. Zimmerman, E. S. Molho, D. Celmins and P. J. Parsons, J. Toxicol. Environ. Health, Part A, 2012, 75, 1253–1268 CrossRef CAS PubMed.
- U. Obahiagbon, J. T. Smith, M. Zhu, B. A. Katchman, H. Arafa, K. S. Anderson and J. M. Blain Christen, Biosens. Bioelectron., 2018, 117, 153–160 CrossRef CAS PubMed.
- D. C. Christodouleas, B. Kaur and P. Chorti, ACS Cent. Sci., 2018, 4, 1600–1616 CrossRef CAS PubMed.
- Q. Yang, J. Li, X. Wang, H. Peng, H. Xiong and L. Chen, Biosens. Bioelectron., 2018, 112, 54–71 CrossRef CAS PubMed.
- S. Knowlton, A. Joshi, P. Syrrist, A. F. Coskun and S. Tasoglu, Lab Chip, 2017, 17, 2839–2851 RSC.
- H. Wei, Y. Peng, Z. Bai, Z. Rong and S. Wang, Analyst, 2021, 146, 558–564 RSC.
- L.-F. Jiang, B.-C. Chen, B. Chen, X.-J. Li, H.-L. Liao, H.-M. Huang, Z.-J. Guo, W.-Y. Zhang and L. Wu, Talanta, 2017, 170, 350–357 CrossRef CAS PubMed.
- J. Dong, R. Fujita, T. Zako and H. Ueda, Anal. Biochem., 2018, 550, 61–67 CrossRef CAS PubMed.
- N. Akhtar, S. K. Metkar, A. Girigoswami and K. Girigoswami, Mater. Sci. Eng., C, 2017, 78, 960–968 CrossRef CAS PubMed.
- S. Govindaraju, S. R. Ankireddy, B. Viswanath, J. Kim and K. Yun, Sci. Rep., 2017, 7, 40298 CrossRef CAS PubMed.
- T. K. Chen, G. Luo and A. G. Ewing, Anal. Chem., 1994, 66, 3031–3035 CrossRef CAS PubMed.
- K. D. Kozminski, D. A. Gutman, V. Davila, D. Sulzer and A. G. Ewing, Anal. Chem., 1998, 70, 3123–3130 CrossRef CAS PubMed.
- S. Alwarappan, K. S. A. Butcher and D. K. Y. Wong, Sens. Actuators, B, 2007, 128, 299–305 CrossRef CAS.
- S. R. Ali, Y. Ma, R. R. Parajuli, Y. Balogun, W. Y. C. Lai and H. He, Anal. Chem., 2007, 79, 2583–2587 CrossRef CAS PubMed.
- R. M. Wightman, Science, 2006, 311, 1570–1574 CrossRef CAS PubMed.
- A. Liu, M. D. Wei, I. Honma and H. Zhou, Adv. Funct. Mater., 2006, 16, 371–376 CrossRef CAS.
- I. Streeter, G. G. Wildgoose, L. Shao and R. G. Compton, Sens. Actuators, B, 2008, 133, 462–466 CrossRef CAS.
- M. Senel, E. Dervisevic, S. Alhassen, M. Dervisevic, A. Alachkar, V. J. Cadarso and N. H. Voelcker, Anal. Chem., 2020, 92, 12347–12355 CrossRef CAS PubMed.
- R. Singh, S. Kashayap, V. Singh, A. M. Kayastha, H. Mishra, P. S. Saxena, A. Srivastava and R. K. Singh, Biosens. Bioelectron., 2018, 101, 103–109 CrossRef CAS PubMed.
- A. Huang, L. Zhang, W. Li, Z. Ma, S. Shuo and T. Yao, R. Soc. Open Sci., 2018, 5, 171808 CrossRef PubMed.
- A. Mars, M. Hamami, L. Bechnak, D. Patra and N. Raouafi, Anal. Chim. Acta, 2018, 1036, 141–146 CrossRef CAS PubMed.
- L. Wang-Dietrich, S. A. Funke, K. Kühbach, K. Wang, A. Besmehn, S. Willbold, Y. Cinar, O. Bannach, E. Birkmann and D. Willbold, J. Alzheimer's Dis., 2013, 34, 985–994 CAS.
- M. J. Wang, S. Yi, J.-y. Han, S. Y. Park, J.-W. Jang, I. K. Chun, S. E. Kim, B. S. Lee, G. J. Kim, J. S. Yu, K. Lim, S. M. Kang, Y. H. Park, Y. C. Youn, S. S. A. An and S. Kim, Alzheimer's Res. Ther., 2017, 9, 98 CrossRef PubMed.
- R. Abe, H. Ohashi, I. Iijima, M. Ihara, H. Takagi, T. Hohsaka and H. Ueda, J. Am. Chem. Soc., 2011, 133, 17386–17394 CrossRef CAS PubMed.
- M. J. Schöning and A. Poghossian, Label-free biosensing: advanced materials, devices and applications, Springer, 2018 Search PubMed.
- M. Biancalana and S. Koide, Biochim. Biophys. Acta, 2010, 1804, 1405–1412 CrossRef CAS PubMed.
- C. Xue, T. Y. Lin, D. Chang and Z. Guo, R. Soc. Open Sci., 2017, 4, 160696 CrossRef PubMed.
- M. Ameri, Z. Shabaninejad, A. Movahedpour, A. Sahebkar, S. Mohammadi, S. Hosseindoost, M. S. Ebrahimi, A. Savardashtaki, M. Karimipour and H. Mirzaei, Int. J. Biol. Macromol., 2020, 162, 1100–1108 CrossRef CAS PubMed.
- K. Rajasekhar, N. Narayanaswamy, N. A. Murugan, K. Viccaro, H.-G. Lee, K. Shah and T. Govindaraju, Biosens. Bioelectron., 2017, 98, 54–61 CrossRef CAS PubMed.
- A. D. Putri, B. T. Murti, S. Kanchi, M. I. Sabela, K. Bisetty, A. Tiwari, Inamuddin and A. M. Asiri, Sci. Rep., 2019, 9, 7873 CrossRef PubMed.
- S. Gupta, U. Jain, B. T. Murti, A. D. Putri, A. Tiwari and N. Chauhan, Sci. Rep., 2020, 10, 21217 CrossRef CAS PubMed.
- Y. Zhao, X. Li, Y. Yang, S. Si, C. Deng and H. Wu, Biosens. Bioelectron., 2020, 149, 111840 CrossRef CAS PubMed.
- K. Tainaka, R. Sakaguchi, H. Hayashi, S. Nakano, F. F. Liew and T. Morii, Sensors, 2010, 10, 1355–1376 CrossRef CAS PubMed.
- S. Okumoto, A. Jones and W. B. Frommer, Annu. Rev. Plant Biol., 2012, 63, 663–706 CrossRef CAS PubMed.
- P. Zheng and N. Wu, Chem.–Asian J., 2017, 12, 2343–2353 CrossRef CAS PubMed.
- H. Deng, S. Yan, Y. Huang, C. Lei and Z. Nie, TrAC, Trends Anal. Chem., 2020, 122, 115757 CrossRef CAS.
- M. Soler, C. S. Huertas and L. M. Lechuga, Expert Rev. Mol. Diagn., 2019, 19, 71–81 CrossRef CAS PubMed.
- X. Li, H. Wang, J. Long, G. Pan, T. He, O. Anichtchik, R. Belshaw, D. Albani, P. Edison, E. K. Green and J. Scott, Sci. Rep., 2018, 8, 17394 CrossRef PubMed.
- M. Altuna-Azkargorta and M. Mendioroz-Iriarte, Neurología, 2020, 1–7 Search PubMed.
- C. Chen, X. Hou and J. Si, Nanotechnology, 2019, 30, 275501 CrossRef CAS PubMed.
- P. C. Ray, Chem. Rev., 2010, 110, 5332–5365 CrossRef CAS PubMed.
- H. Su, X. R. Cheng, T. Endo and K. Kerman, Biosens. Bioelectron., 2018, 103, 158–162 CrossRef CAS PubMed.
- M. Safieh, A. D. Korczyn and D. M. Michaelson, BMC Med., 2019, 17, 64 CrossRef PubMed.
- E. Petryayeva and U. J. Krull, Anal. Chim. Acta, 2011, 706, 8–24 CrossRef CAS PubMed.
- Z. Farka, T. Juřík, D. Kovář, L. Trnková and P. Skládal, Chem. Rev., 2017, 117, 9973–10042 CrossRef CAS PubMed.
- K.-I. Chen, B.-R. Li and Y.-T. Chen, Nano Today, 2011, 6, 131–154 CrossRef CAS.
- S. Mao, J. Chang, H. Pu, G. Lu, Q. He, H. Zhang and J. Chen, Chem. Soc. Rev., 2017, 46, 6872–6904 RSC.
- X. Duan, Y. Huang, Y. Cui, J. Wang and C. M. Lieber, Nature, 2001, 409, 66–69 CrossRef CAS PubMed.
- Y. Cui and C. M. Lieber, Science, 2001, 291, 851–853 CrossRef CAS PubMed.
- Y. Kutovyi, H. Hlukhova, N. Boichuk, M. Menger, A. Offenhäusser and S. Vitusevich, Biosens. Bioelectron., 2020, 154, 112053 CrossRef CAS PubMed.
- A. Anand, C.-H. Chi, S. Banerjee, M.-Y. Chou, F.-G. Tseng, C.-Y. Pan and Y.-T. Chen, Small, 2018, 14, 1–10 CrossRef PubMed.
- P.-K. Yang and C.-P. Lee, in Monolayers of Emerging Nanomaterials, IntechOpen, 2019 Search PubMed.
- S. Su, Q. Sun, X. Gu, Y. Xu, J. Shen, D. Zhu, J. Chao, C. Fan and L. Wang, TrAC, Trends Anal. Chem., 2019, 119, 115610 CrossRef CAS.
- C.-J. Kuo, H.-C. Chiang, C.-A. Tseng, C.-F. Chang, R. K. Ulaganathan, T.-T. Ling, Y.-J. Chang, C.-C. Chen, Y.-R. Chen and Y.-T. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 12311–12316 CrossRef CAS PubMed.
- E. Stern, R. Wagner, F. J. Sigworth, R. Breaker, T. M. Fahmy and M. A. Reed, Nano Lett., 2007, 7, 3405–3409 CrossRef CAS PubMed.
- Y. Yu, P. Wang, X. Zhu, Q. Peng, Y. Zhou, T. Yin, Y. Liang and X. Yin, Analyst, 2018, 143, 323–331 RSC.
- M. Amouzadeh Tabrizi, J. Ferré-Borrull and L. F. Marsal, Biosens. Bioelectron., 2019, 137, 279–286 CrossRef CAS PubMed.
- S. Wustoni, S. Wang, J. R. Alvarez, T. C. Hidalgo, S. P. Nunes and S. Inal, Biosens. Bioelectron., 2019, 143, 111561 CrossRef CAS PubMed.
- L. Zhang, W. Lian, P. Li, H. Ma, X. Han, B. Zhao and Z. Chen, Biosens. Bioelectron., 2020, 148, 111816 CrossRef CAS PubMed.
- C. Carlomagno, M. Cabinio, S. Picciolini, A. Gualerzi, F. Baglio and M. Bedoni, J. Biophotonics, 2020, 13, 1–11 CrossRef PubMed.
- W. Choi, S. Y. Yeom, J. Kim, S. Jung, S. Jung, T. S. Shim, S. K. Kim, J. Y. Kang, S. H. Lee, I.-J. Cho, J. Choi and N. Choi, Biosens. Bioelectron., 2018, 101, 235–244 CrossRef CAS PubMed.
- R. N. Adams, Anal. Chem., 1976, 48, 1126A–1138A CrossRef CAS PubMed.
- R. A. Sweet, V. L. Nimgaonkar, M. I. Kamboh, O. L. Lopez, F. Zhang and S. T. DeKosky, Arch. Neurol., 1998, 55, 1335–1340 CrossRef CAS PubMed.
- G. Koch, Z. Esposito, C. Codecà, F. Mori, H. Kusayanagi, F. Monteleone, F. Di Lorenzo, G. Bernardi and A. Martorana, Clin. Neurophysiol., 2011, 122, 703–707 CrossRef CAS PubMed.
- Y. Xu, J. Yan, P. Zhou, J. Li, H. Gao, Y. Xia and Q. Wang, Prog. Neurobiol., 2012, 97, 1–13 CrossRef CAS PubMed.
- E. S. Bucher and R. M. Wightman, Annu. Rev. Anal. Chem., 2015, 8, 239–261 CrossRef CAS PubMed.
- P. T. Kissinger, J. B. Hart and R. N. Adams, Brain Res., 1973, 55, 209–213 CrossRef CAS PubMed.
- M. A. Makos, D. M. Omiatek, A. G. Ewing and M. L. Heien, Langmuir, 2010, 26, 10386–10391 CrossRef CAS PubMed.
- Y. Wang and A. Ewing, ChemBioChem, 2021, 22, 807–813 CrossRef CAS PubMed.
- X. Li, S. Majdi, J. Dunevall, H. Fathali and A. G. Ewing, Angew. Chem., Int. Ed., 2015, 54, 11978–11982 CrossRef CAS PubMed.
- N. T. N. Phan, X. Li and A. G. Ewing, Nat. Rev. Chem., 2017, 1, 0048 CrossRef CAS.
- Q. Peng, X. Yan, X. Shi, S. Ou, H. Gu, X. Yin, G. Shi and Y. Yu, Biosens. Bioelectron., 2019, 144, 111665 CrossRef CAS PubMed.
- S. Ding, Y. Xu, Q. Liu, H. Gu, A. Zhu and G. Shi, Analyst, 2020, 145, 2331–2338 RSC.
- T.-C. Liu, Y.-C. Lee, C.-Y. Ko, R.-S. Liu, C.-C. Ke, Y.-C. Lo, P.-S. Hong, C.-Y. Chu, C.-W. Chang, P.-W. Wu, Y.-Y. Chen and S.-Y. Chen, Theranostics, 2018, 8, 4210–4225 CrossRef CAS PubMed.
- C. Xu, F. Wu, P. Yu and L. Mao, ACS Sens., 2019, 4, 3102–3118 CrossRef CAS PubMed.
- K. Mahato, P. K. Maurya and P. Chandra, 3 Biotech, 2018, 8, 149 CrossRef PubMed.
- K. Kim, M.-J. Kim, D. W. Kim, S. Y. Kim, S. Park and C. B. Park, Nat. Commun., 2020, 11, 119 CrossRef CAS PubMed.
- S. Chen and M. H. Shamsi, J. Micromech. Microeng., 2017, 27, 083001 CrossRef.
- P. Kassal, M. D. Steinberg and I. M. Steinberg, Sens. Actuators, B, 2018, 266, 228–245 CrossRef CAS.
- S. K. Metkar and K. Girigoswami, Biocatal. Agric. Biotechnol., 2019, 17, 271–283 CrossRef.
- T. Wadhera, D. Kakkar, G. Wadhwa and B. Raj, J. Electron. Mater., 2019, 48, 7635–7646 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |