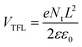Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A novel 3-methylthiophene additive to boost the performance and stability of perovskite solar cells
Sadeer M. Majeeda,
Duha S. Ahmeda and
Mustafa K. A. Mohammed *b
*b
aDepartment of Applied Science, University of Technology, Baghdad 100001, Iraq
bDijlah University College, Al-Masafi Street, Al-Dora, Baghdad 00964, Iraq. E-mail: mustafa_kareem97@yahoo.com; Tel: +9647719047121
First published on 10th March 2021
Abstract
Perovskite solar cells (PSCs) have emerged as a practical candidate for new-generation photovoltaic devices to meet global energy demands. Recently, researchers' attempts have been focused on the crucial issues related to PSCs, i.e., stability and performance. In this research, MAPbI3-based PSCs were prepared via a two-step deposition process. To boost the power conversion efficiency (PCE) of the prepared PSCs, an additive engineering approach was employed. A novel 3-methylthiophene (MTP) organic molecule was added to the methylammonium iodide (MAI)/isopropanol (IPA) solution precursor. The additive improved the crystallinity of the perovskite layer, which indicates a more desirable film with lower surface defects and larger particle size. Modified PSCs reduced carries recombination rate at the interfacial of perovskite/hole transport layer (HTL), and the charge transport process is facilitated due to a desirable delocalized π-electron system of the MTP additive. The PCE of PSCs in the presence of MTP additive improved from 12.32% to 16.93% for pristine devices. Importantly, MTP-based PSCs showed higher ambient air stability due to the hydrophobic structure of MTP compared to pristine PSCs.
1. Introduction
In recent years, global warming concerns, and consequently global climate changes,1 have attracted the attention of many governments worldwide. Simultaneously, with an increase in the Earth's population, global energy demands (GEDs) have increased. These days, fossil fuels, which increase heat-trapping greenhouse gas levels in the Earth's atmosphere, are known as the primary source to meet GEDs. To solve this issue, researchers have suggested green and renewable energy sources, for instance, solar energy, wind energy, and hydro-energy. Typically, solar cell technology has provided a green energy source to energy demands.2–5 Recently, next-generation solar cell technologies have emerged to develop efficient and low-cost solar cells. Organic–inorganic PSCs are the most promising technology to facilitate efficient and low-cost solar cell development.6–8Unique optoelectronic characteristics of MAPbI3 films, for instance, high absorption in a broad spectrum, low exciton energy, tunable bandgap, long-length carrier diffusion, and the low recombination rate, make them favorable for photovoltaic (PV) applications.9–11 To date, different strategies have been used to boost the PV parameters of PSCs. Electron transport layer (ETL) and HTL modification,12,13 interfacial engineering at the interface of perovskite layer and charge transport layers,14,15 grain boundary passivation of perovskite film,16 additive engineering,17 and composition engineering of perovskite structure18 are some of the efficient methods to enhance the performance of PSCs.
However, the efficiency of PSCs with fast progress rose to a competitive value with silicon solar cells, but they suffer from intrinsic short-term stability. When the perovskite layer is exposed to humidity, light irradiation, or heat, the perovskite film starts to degrade. A routine degradation process in well-known MAPbI3 (CH3NH3PbI3) is CH3NH3PbI3 → CH3NH2↑ + HI↑ + PbI2. Encapsulation of PSCs is suggested to protect the perovskite layer from humidity and oxygen. Lee et al.19 used a thin-film encapsulation (TFE) method with a periodical structure of organic (poly(1,3,5-trimethyl-1,3,5-trivinylcyclotrisiloxane)) and inorganic (Al2O3) layers. They found that the TFE method could improve the stability of PSCs specifically. The encapsulated device maintained 97% of its initial PCE. However, this method increases the cost of the fabrication process of PSCs. In addition, the encapsulation process has its challenges.20–22 Besides, improving the crystallinity properties of perovskite layers can also address the stability of PSCs.10 Saidaminov et al.23 expressed that the origin of the lattice strain is due to the ionic size mismatch between the A cation and lead halide in the perovskite structure. They proved that Cd incorporation could remove strain lattice in the perovskite structure without introducing traps and boost ambient air stability of corresponding PSCs. Boosting the hydrophobic behavior of the perovskite layer is an alternative technique to address the poor humidity stability of PSCs.24 Shu et al.25 introduced phenyltrimethylammonium bromide (PTABr) to modify and boost the surface hydrophobicity of the perovskite. After the addition of PTABr to the perovskite film, unencapsulated PSCs retained 86% of their original PCE after keeping for two months in air conditions.
In this study, MAPbI3 PSCs were fabricated by a two-step deposition process. To boost the PV properties of the fabricated PSCs, a novel 3-methylthiophene (MTP) molecule was inserted into the MAI/IPA precursor solution. The suggested approach showed that the MTP additive enlarges the crystal grain size of the MAPbI3 layer and consequently suppresses the grain boundaries in the perovskite layer. It was also found that the PV parameters of MTP-treated devices show more improvement compared to devices without additives. The modified PSCs with the MTP additive showed more stable behavior in humid conditions, indicating the improved hydrophobic behavior of the MTP -based perovskite layer.
2. Experimental details
2.1. Materials
Chlorobenzene (CB), MAI, and lead iodide (PbI2) were provided from Merck. Fluorine tin oxide (FTO, 15 Ω sq−1) was provided from Solaronix. 4-Tertbutylpyridine (TBP), phenyl-C61-butyric acid methyl ester (PCBM), ethanol (40%), and bis(trifluoromethane)sulfonimide lithium salt (Li-TFSI, 99.95%) were obtained from Alfa Aesar. Note that all additional chemicals were obtained from Sigma-Aldrich.2.2. Solution preparation
Compact TiO2 (c-TiO2) was prepared by adding 2.5 ml of ethanol and 35 μl HCl (2 M) to 350 μl of titanium(IV) tetra isopropoxide (in 2.5 ml ethanol) under stirring at 0 °C for 15 min. To prepare mesoporous TiO2 (mp-TiO2), TiO2 paste was dissolved in ethanol at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio and stirred for one day in ambient air. PbI2 solution was prepared by dissolving 600 mg of PbI2 powder in 950 μl of N,N-dimethylformamide and 50 μl of dimethyl sulfoxide and stirred at 65 °C overnight. The MAI solution was prepared by dispersing 40 mg ml−1 of MAI powder in IPA and stirring for 20 min at room temperature (RT). For MAI/MTP mixture, an MTP additive was added to the MAI/IPA solution at different volume ratios (2.5%, 5%, and 7.5%) and mixed by stirring for 5 min at RT. The HTL solution was obtained by mixing 17.5 μl Li-TFSI in acetonitrile (520 mg ml−1) and 28.8 μl TBP to a 60 mM Spiro-OMeTAD in CB solvent.
5 ratio and stirred for one day in ambient air. PbI2 solution was prepared by dissolving 600 mg of PbI2 powder in 950 μl of N,N-dimethylformamide and 50 μl of dimethyl sulfoxide and stirred at 65 °C overnight. The MAI solution was prepared by dispersing 40 mg ml−1 of MAI powder in IPA and stirring for 20 min at room temperature (RT). For MAI/MTP mixture, an MTP additive was added to the MAI/IPA solution at different volume ratios (2.5%, 5%, and 7.5%) and mixed by stirring for 5 min at RT. The HTL solution was obtained by mixing 17.5 μl Li-TFSI in acetonitrile (520 mg ml−1) and 28.8 μl TBP to a 60 mM Spiro-OMeTAD in CB solvent.
2.3. Device fabrication
The cleaning process of FTO substrates was done as per a general routine in the literature.26 The c-TiO2 was deposited on FTO at 2000 rpm for 30 s, followed by annealing at 500 °C for 25 min. Then, mp-TiO2 layers were deposited at 4000 rpm for 60 s and baked at 450 °C for 40 min. For perovskite films, 1.2 M PbI2 was spin-coated at 3500 rpm, followed by drying at room temperature for 1 min and annealing at 100 °C for 3 min. A 40 mg ml−1 MAI solution was then loaded on the PbI2 layer for 5 s, followed by spin coating at 3000 rpm for 30 s. After that, intermediate perovskite films were annealed at 100 °C for 10 min to complete the perovskite layer fabrication. The HTL solution was spin-coated at 4000 rpm for 30 s on top of the perovskite layer. Finally, a 100 nm Au electrode layer was evaporated on the HTL.2.4. Characterization
Ultraviolet-visible spectroscopy (UV-vis, Ocean Optics) and steady-state photoluminescence (PL) spectroscopy (Agilent Cary Eclipse Fluorescence) were employed to study the optical features of perovskite films. The structural properties of perovskites were investigated by X-ray diffraction (Shimadzu). The morphology of perovskites was investigated using scanning electron microscopy (SEM, VEGA3). Current density–voltage (J–V) characteristics of PSCs were studied using a Keithley instrument (Model 2400, AM 1.5G one Sun) under 100 mW cm−2 illumination. The devices were measured with a 2 mm × 4 mm mask. A contact angle test of the perovskite layers was characterized, utilizing Phoenix 300. The incident photon to-current efficiency (IPCE) spectra were acquired by an IPCE system (Newport, USA).3. Results and discussion
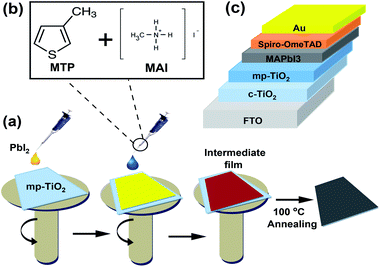
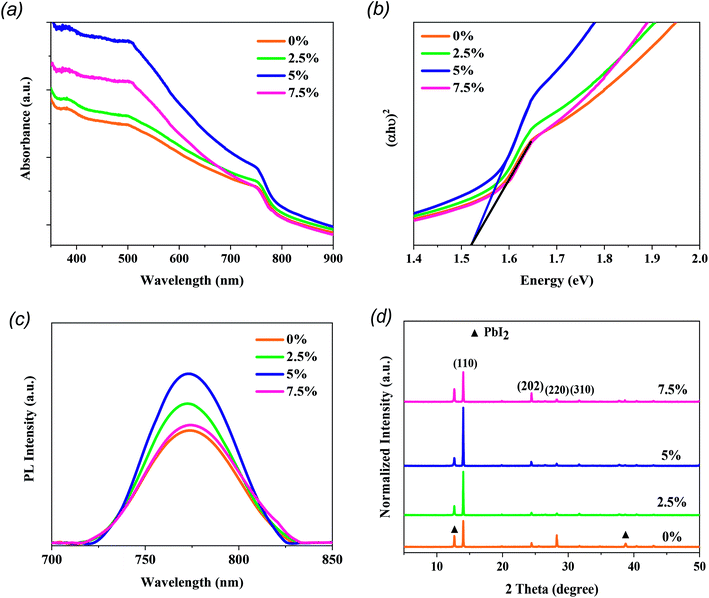
For mesoporous PSCs, MAPbI3 perovskite films were fabricated via a two-step deposition process (see Fig. 1a). MTP additives in different contents (2.5%, 5%, and 7.5%) were used in the MAI precursor solution to assist the charge extraction process from perovskite and prevent the degradation of the corresponding devices. Fig. 1b demonstrates the chemical structures of MTP additive and MAI. The mesoporous PSC devices designed in this study were structured as FTO/c-TiO2/mp-TiO2/MAPbI3/Spiro-OMeTAD/Au (see Fig. 1c). A solution of dense yellow PbI2 was deposited onto mp-TiO2 ETL, followed by spin-coating MAI/MTP mixture. The as-prepared MAPbI3 layers were annealed to complete the crystallization process. Spin-coating of the Spiro-OMeTAD HTL and evaporation of the Au electrode completes the device architecture. The PCE of the subsequent PSCs was found to vary with the MTP amount.The impacts of the MTP additives on the optical and structural properties of the MAPbI3 photoactive layers were carefully studied by a range of measurements. As shown in Fig. 2a, the UV-vis absorption spectra reveal that the MAPbI3 layer with 5% MTP shows an optimum absorbance compared to the pure and 2.5% MTP-treated perovskite layers. Nevertheless, an excess amount of MTP (7.5%) leads to a lower absorbance due to lower crystallinity of the MAPbI3 layer (will be discussed later) and an inferior PSC performance. The enhanced UV-vis spectrum of MAPbI3 is in favor of a boosted Jsc when used in solar cells.27 The optical bandgap of pure and MTP treated perovskite films was measured by Tauc plot28–30 and is depicted in Fig. 2b. The bandgap (Eg) of the MAPbI3 layers is the same as that of the ref. 3 and 5, according to UV-vis measurements. As seen in Tauc plots, the absorption spectra of MAPbI3 films show an identical bandgap of 1.52 eV, implying that the well-crystallized MAPbI3 films are deemed a direct Eg semiconductor. The steady-state PL spectra of pure and MAPbI3-containing various contents of MTP additives are presented in Fig. 2c. PL plots reveal identical PL peak position (773 nm) even when the concentration of MTP in MAI solutions increases, indicating that the MTP treatment and varying its content does not significantly alter the Eg of the fabricated MAPbI3 layers.31 Moreover, the PL intensity of the perovskite film with 5% MTP was improved, while that of 2.5% and 7.5% MTP merely enhanced. This indicates that the photoluminescence quenching effect is significantly improved. This means that the defect states of MAPbI3 with 5% MTP was suppressed, leading to the increased fill factor (FF) and efficiency of the PSC by reducing the recombination rate.32 The UV-vis spectra of MAPbI3 films demonstrate consistent characteristics of the PL measurements, where the perovskite with 5% MTP has the highest light-harvesting and charge extraction abilities compared with other MAPbI3 films.
The XRD patterns of MAPbI3 perovskites treated with MTP are illustrated in Fig. 2d. Note that all MAPbI3 layers are tetragonal crystals with reflection peaks at 14.03°, 24.41°, 28.25°, and 31.7° related to the (110), (202), (220), and (310) diffraction planes, respectively.33 By comparison, the perovskite film with 5% MTP additive reveals intensified peaks compared to the other films, indicating a highly crystalline MAPbI3 structure. Also, a high reflection peak of PbI2 located at 12.4° can be observed in the pure MAPbI3 layer, suggesting the presence of residual PbI2 and more decomposition of untreated MAPbI3 film during the fabrication process. With MTP treatment, the peak of PbI2 is significantly quenched, especially for the perovskite with 5% MTP additive. This implies that the more stabilized MAPbI3 can be achieved with MTP treatment.34 However, when the amount of MTP increases to 7.5%, MAPbI3 exhibits a higher signal of PbI2, and the crystallinity of MAPbI3 reduces. As stated in the UV-vis and PL results, excess amount of MTP has a diverse effect on the crystallinity and reduces the light absorption and charge extraction properties. No shift in the peaks nor new peaks are shown in the XRD results, indicating that the MTP treatment does not change the structure of the MAPbI3 layers. Combining the XRD results with the above findings from UV-vis and PL experiments, a conclusion can be drawn that the impact of MTP is essential. At optimized content (5%), an enhancement of crystallinity is observed, giving rise to the light-harvesting efficiency with low recombination rate in MTP treated film compared to the pristine film.
To further check the strengthening impact of MTP on perovskites, FESEM was employed to follow the MAPbI3 morphology change. Upon MTP modification, MAPbI3 layers show a considerably larger grain size than that of the pristine MAPbI3 layer (Fig. 3a–c), which is probably because the MTP additive may influence the crystal nucleation and crystallization kinetics of MAPbI3. Besides, the grain boundaries are suppressed from the non-uniform surface of the untreated perovskite layer with an increase in the MTP volume ratio, showing compact films with larger grain size and hence improved MAPbI3 crystallinity. It is well-known that humidity can permeate within the grain grooves to degrade the MAPbI3 layer.35 Therefore, increasing the grain size is an efficient strategy to stabilize the perovskite film. On the contrary, a perovskite film with smaller grains and some pinholes on its surface appeared in the 7.5% MTP treated film (Fig. 3d), indicating that a perovskite film with more grain boundaries, such as this film, is not desirable for photovoltaic application. In other words, excess MTP (7.5%) hinders the nucleation and development of a uniform MAPbI3 layer, resulting in smaller crystallite dimensions with many flaws on the surface. The variation of MAPbI3 layer morphologies in top-view FESEM images is consistent with XRD results with the variation of MTP amounts as well.
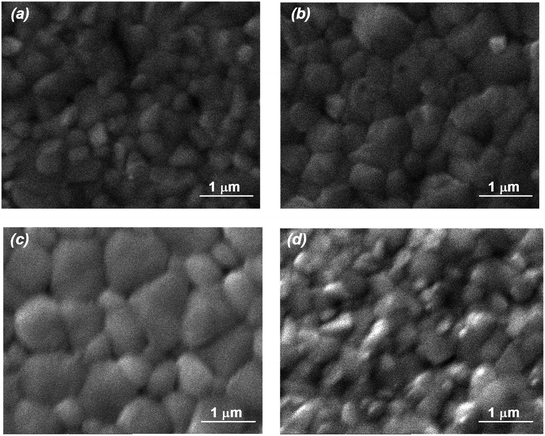 | ||
| Fig. 3 Surface characterization of MAPbI3 films using top-view FESEM. (a) Pristine film. (b) Treated with 2.5% MTP. (c) Treated with 5% MTP. (d) Treated with 7.5% MTP. | ||
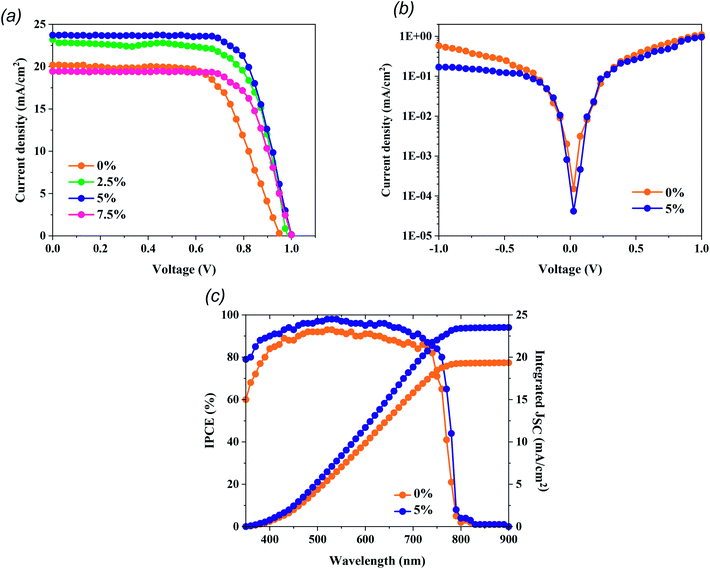
To probe the PV performance based on our treatment approach, we constructed n–i–p mesoporous PSCs employing MAPbI3 films as the photoactive materials. As presented in Fig. 4a and Table 1, the MAPbI3-based PSCs exhibited increased PV parameters after MTP treatment. J–V measurements revealed that cell performance improves with MTP amount until the volume ratio = 5%. The best PSC was fabricated with 5% MTP modification and yielded a PCE of 16.93%, a Jsc of 23.72 mA cm−2, a Voc of 1.00 V, and an FF of 71.4%. We attribute this enhancement to the better crystallization process of perovskite, passivation of the grain boundaries, and lower charge recombination due to the MTP treatment, which is consistent with the trend shown in XRD, PL, and FESEM results. As described in the MAPbI3 characteristics, the perovskite film with appropriate MTP additive depicts improved light-harvesting, contributing to generating more photo-induced electrons and holes. The MTP treated perovskite showed fewer boundaries and defects. Therefore, the number of carrier recombination sites is significantly suppressed. For too much MTP additive (7.5%), the PV performance of PSCs suppresses to a lower PCE of 13.65%. As exhibited in Fig. 4b, the dark current density of the PSC with MTP-modifying MAPbI3 is lower than that of PSC with pristine MAPbI3, signifying that the leakage current of PSC with MTP treatment is decreased, resulting in the retardation of charge recombination and suppressed defects of perovskite.36 The corresponding IPCE spectra of champion PSCs with pristine and optimized MAPbI3 films were also recorded and are illustrated in Fig. 4c. As seen, the integrated current densities from the pristine and treated PSCs are 19.3 mA cm−2 and 23.4 mA cm−2, respectively, which corroborate with the Jsc values from the J–V sweep measurement. Furthermore, the device with the MTP additive shows higher IPCE than the pristine one, which agrees well with the highest Jsc. These findings are again consistent with the features of MTP treatment, which contribute to better crystallized MAPbI3 and effective charge injection.
| Device | Voc (V) | Jsc (mA cm−2) | FF (%) | PCE (%) | |
|---|---|---|---|---|---|
| 0% | Average | 0.939 | 19.22 | 62.36 | 11.25 |
| Best | 0.962 | 20.19 | 63.50 | 12.32 | |
| 2.5% | Average | 0.964 | 21.95 | 67.40 | 14.37 |
| Best | 0.987 | 23.20 | 68.20 | 15.61 | |
| 5% | Average | 0.990 | 23.03 | 70.02 | 16.00 |
| Best | 1.000 | 23.72 | 71.40 | 16.93 | |
| 7.5% | Average | 0.985 | 19.19 | 69.13 | 13.06 |
| Best | 1.000 | 19.44 | 70.2 | 13.65 |
With regard to PSC reproducibility, 10 cells were constructed and J–V measurements were carried out under AM 1.5G illumination conditions. The statistical distribution of PV parameters is presented in Fig. 5 and it is clear that the cells modified with 5% MTP reveal excellent reproducibility and a higher average PCE (16.0%) than those with pristine cells (11.25%). Additionally, the interval statistical distribution of 5% MTP-modified cells is narrower than that of the unmodified cells, which indicates good reliability of MTP function and agrees well with the findings from the champion PSC.
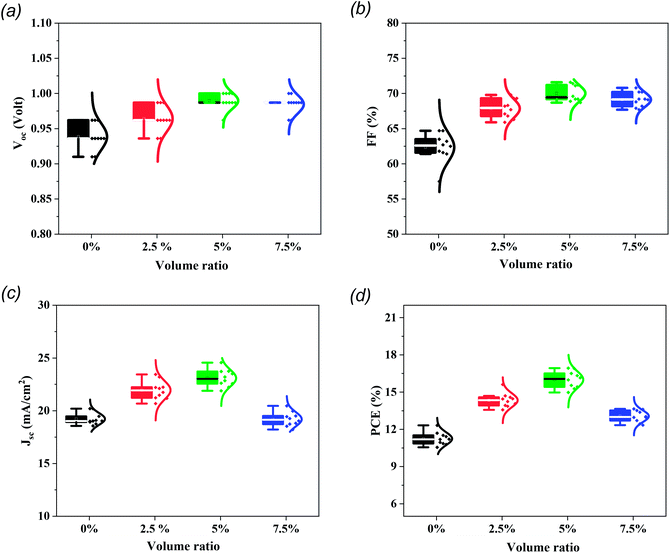 | ||
| Fig. 5 The statistical distribution of (a) Voc, (b) FF, (c) Jsc, and (d) PCE of PSCs with various volume ratios of MTP additive in perovskite precursor. For each group, 10 devices were monitored. | ||
As shown above, additive engineering significantly increases the PCE of PSCs, and the PSCs based on 5% MTP achieve most outstanding performance. Thus, we performed additional measurements on devices based on pure MAPbI3 and 5% MTP treated MAPbI3 perovskite films. To evaluate the trap-state density (Nt) of perovskites by space-charge-limited current (SCLC) measurement, we prepared the electron-only structures with the configuration of FTO/c-TiO2/mp-TiO2/MAPbI3/PCBM/Au, and measured the dark current–voltage curves, as depicted in Fig. 6a and b. According to the below formula, Nt can be calculated by the trap filled limit voltage (VTFL).
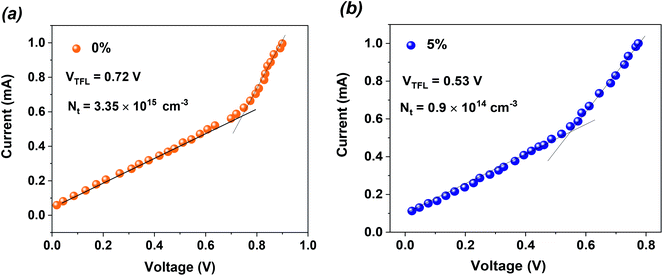 | ||
| Fig. 6 Dark current–voltage curves of the electron-only devices for (a) pure MAPbI3 and (b) MTP-treated MAPbI3 displaying VTFL kink point behavior. | ||
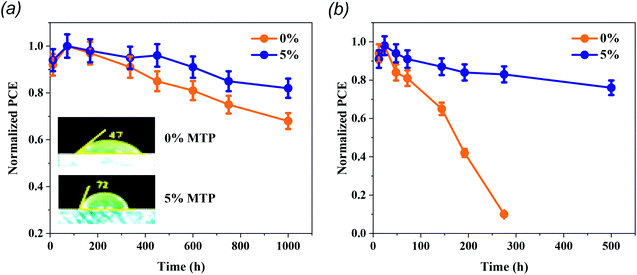
The pinholes centered at the surface and internal grain boundaries in the MAPbI3 layer always impede the charge transfer and become recombination sites, reducing the carrier lifetime. In this context, these sites are inclined to become the adsorption centers for moisture and O2, therefore causing the suppression of PCE and the stability of PSCs.37 High-quality perovskite film is substantial to resist erosion by moisture and to stabilize the PSCs. In order to investigate the hydrophobicity of pristine and 5% MTP-treated MAPbI3 layers, water-contact angles were measured (inset of Fig. 7a). By comparing the water-contact angles on MAPbI3 layers, the contact angle of the pristine and MTP modified MAPbI3 is 47° and 72°, respectively, indicating that the hydrophobicity of MAPbI3 film greatly enhanced after MTP modification. In addition, previous reports have shown that there is a strong relationship between the wetting capability of water on MAPbI3 and PSC stability, particularly the contribution to the erosion resistance capability and the overall stability of PSC in moisture conditions.12,38
To elucidate the operational stability of our enhanced MAPbI3-based solar cells, we performed a series of measurements on unencapsulated PSCs at room temperature. As demonstrated in Fig. 7a, the stability of MTP-treated PSCs reveals a higher stabilized PCE. The MTP based PSCs maintain 83% of their initial PCE after storing in a dry box (<20% RH) for 1000 h, whereas pristine PSCs maintain only 64% of their original PCE. Most importantly, we investigated the effect of aging time on unencapsulated PSCs in ambient conditions with RH of 30–50% (Fig. 7b). The pristine PSCs exhibit a fast decline of PCE (degrades below 10% in less than 300 h). In contrast, the MTP-treated PSCs show a long lifetime, attaining over 80% of their original PCE after 500 h, which further verifies that the MTP treatment strategy brought about significant enhancements in the long-term stability of devices. The more stable behavior of the treated PSCs is due to the improved hydrophobic behavior of the perovskite films after the addition of MTP additive, as shown in the inset of Fig. 7a.
It is worth noting that the achieved findings revealed that the design of PSCs employing the MTP treatment in ambient air could enhance the PCE and stability of the MAPbI3-based solar cells, which is a step forward in PSC commercialization. Based on the comparison of the results listed in Table 2, it can be argued that the most beneficial approach for MTP usage in PSCs is incorporating it into the MAI solution, as reported here. As shown, the MTP additive was compared with ethylammonium chloride (EACl), 1,8-diiodooctane (DIO), guanidinium thiocyanate (GuSCN), and urea (NH2CONH2) additives.
| Author | Fabrication conditions | Fabricating method | Additive treatment | Perovskite | PCE improvement | Operational stability |
|---|---|---|---|---|---|---|
| Muhammad et al.39 | Not reported | One-step | EACl | MAPbI3 | 15% | ≈12% loss after 1000 h test in 30% relative humidity in air without encapsulation |
| Vincent et al.40 | Ambient air | One-step | DIO | MAPbI3 | 60% | Not reported |
| Nian et al.41 | Nitrogen glovebox | One-step | GuSCN | MAPbI3 | 7% | ≈10% loss after 350 h test in ambient humidity in air without encapsulation |
| Ying et al.42 | Not reported | One-step | NH2CONH2 | MAPbI3 | 11% | Not reported |
| This work | Ambient air | Sequential deposition | MTP | MAPbI3 | 38% | ≈20% loss after 500 h test in 20% relative humidity in dry box without encapsulation |
4. Conclusion
In this work, we have introduced an efficient method for simultaneous passivation of the grain boundaries and recombination processes in a single cation MAPbI3 film using MTP additives to boost the PCE and stability of PSCs. By adding MTP to the MAPbI3 harvester layer, we obtained a PCE of 16.93% in mesoporous devices, which is higher than that of untreated devices (12.32%). Without any encapsulation, it was found that MTP-based cells show superior stability performance compared with pristine cells after storage in a humid environment for 500 h. Our findings reveal that 5% of MTP additive can enhance the MAPbI3 layer merits, including crystallinity, light absorption, morphology and suppressed residual PbI2 while decreasing the recombination rate. Passivating the surfaces and grain boundaries through MTP treatment, the MAPbI3 film efficiently prevents the decomposition pathway at the corresponding interfaces. This study will provide insights into the role of MTP additive engineering in the enhancement of PCE and the stability of PSCs.Conflicts of interest
The authors declare that they have no conflict of interest.References
- G. Benelli, A. B. B. Wilke, J. R. Bloomquist, N. Desneux and J. C. Beier, Overexposing mosquitoes to insecticides under global warming: A public health concern?, Sci. Total Environ., 2020, 143069 Search PubMed.
- M. D. Humadi, H. T. Hussein, M. S. Mohamed, M. K. A. Mohammed and E. Kayahan, A facile approach to improve the performance and stability of perovskite solar cells via FA/MA precursor temperature controlling in sequential deposition fabrication, Opt. Mater., 2021, 112, 110794 CrossRef CAS.
- M. K. A. Mohammed, A. K. Al-Mousoi, M. S. Mehde and A. M. Al-Gebori, Engineered electronic properties of the spin-coated MAPI for hole-transport free perovskite solar cell (HT-free PSC): Spinning time and PSC performance relationship, Chem. Phys. Lett., 2020, 754, 137718 CrossRef CAS.
- M. K. A. Mohammed, Studying the Structural, Morphological, Optical, and Electrical Properties of CdS/PbS Thin Films for Photovoltaic Applications, Plasmonics, 2020, 15, 1989–1996 CrossRef CAS.
- A. K. Al-Mousoi and M. K. A. Mohammed, Engineered surface properties of MAPI using different antisolvents for hole transport layer-free perovskite solar cell (HTL-free PSC), J. Sol-Gel Sci. Technol., 2020, 96, 659–668 CrossRef CAS.
- L. Meng, J. You and Y. Yang, Addressing the stability issue of perovskite solar cells for commercial applications, Nat. Commun., 2018, 9, 1–4 CrossRef CAS PubMed.
- M. K. A. Mohammed, High-performance hole conductor-free perovskite solar cell using a carbon nanotube counter electrode, RSC Adv., 2020, 10, 35831–35839 RSC.
- H. T. Hussein, R. S. Zamel, M. S. Mohamed and M. K. A. Mohammed, High-performance fully-ambient air processed perovskite solar cells using solvent additive, J. Phys. Chem. Solids, 2021, 149, 109792 CrossRef CAS.
- M. K. A. Mohammed, 21.4% efficiency of perovskite solar cells using BMImI additive in the lead iodide precursor based on carbon nanotubes/TiO2 electron transfer layer, Ceram. Int., 2020, 46, 27647–27654 CrossRef.
- J. Y. Kim, J.-W. Lee, H. S. Jung, H. Shin and N.-G. Park, High-Efficiency Perovskite Solar Cells, Chem. Rev., 2020, 120, 7867–7918 CrossRef.
- D. S. Ahmed, M. K. A. Mohammed and S. M. Majeed, Green Synthesis of Eco-Friendly Graphene Quantum Dots for Highly Efficient Perovskite Solar Cells, ACS Appl. Energy Mater., 2020, 3(11), 10863–10871 CrossRef CAS.
- C. Zhen, T. Wu, R. Chen, L. Wang, G. Liu and H.-M. Cheng, Strategies for Modifying TiO2 Based Electron Transport Layers to Boost Perovskite Solar Cells, ACS Sustainable Chem. Eng., 2019, 7, 4586–4618 CrossRef.
- J. Zhang, H. Luo, W. Xie, X. Lin, X. Hou, J. Zhou, S. Huang, W. Ou-Yang, Z. Sun and X. Chen, Efficient and ultraviolet durable planar perovskite solar cells via a ferrocenecarboxylic acid modified nickel oxide hole transport layer, Nanoscale, 2018, 10, 5617–5625 RSC.
- M. K. A. Mohammed, M. Dehghanipour, U. Younis, A. E. Shalan, P. Sakthivel, G. Ravi, P. H. Bhoite and J. Pospisil, Improvement of the interfacial contact between zinc oxide and a mixed cation perovskite using carbon nanotubes for ambient-air-processed perovskite solar cells, New J. Chem., 2020, 44, 19802–19811 RSC.
- X. Gu, Y. Wang, T. Zhang, D. Liu, R. Zhang, P. Zhang, J. Wu, Z. D. Chen and S. Li, Enhanced electronic transport in Fe 3þ-doped TiO2 for high efficiency perovskite solar cells, J. Mater. Chem. C, 2017, 5, 10754–10760 RSC.
- Z. Liu, F. Cao, M. Wang, M. Wang and L. Li, Observing Defect Passivation of the Grain Boundary with 2-Aminoterephthalic Acid for Efficient and Stable Perovskite Solar Cells, Angew. Chem., 2020, 132, 4190–4196 CrossRef.
- D. S. Ahmed and M. K. A. Mohammed, Novel mixed solution of ethanol/MACl for improving the crystallinity of air processed triple cation perovskite solar cells, Sol. Energy, 2020, 207, 1240–1246 CrossRef CAS.
- M. Saliba, T. Matsui, K. Domanski, J.-Y. Seo, A. Ummadisingu, S. M. Zakeeruddin, J.-P. Correa-Baena, W. R. Tress, A. Abate, A. Hagfeldt and M. Grätzel, Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance, Science, 2016, 354, 206–209 CrossRef.
- Y. I. Lee, N. J. Jeon, B. J. Kim, H. Shim, T. Y. Yang, S. I. Seok, J. Seo and S. G. Im, A Low-Temperature Thin-Film Encapsulation for Enhanced Stability of a Highly Efficient Perovskite Solar Cell, Adv. Energy Mater., 2018, 8, 1701928 CrossRef.
- A. Uddin, M. B. Upama, H. Yi and L. Duan, Encapsulation of Organic and Perovskite Solar Cells: A Review, Coatings, 2019, 9, 65 CrossRef.
- F. Corsini and G. Griffini, Recent progress in encapsulation strategies to enhance the stability of organometal halide perovskite solar cells, JPhys Energy, 2020, 2, 031002 CrossRef.
- S. Ma, Y. Bai, H. Wang, H. Zai, J. Wu, L. Li, S. Xiang, N. Liu, L. Liu and C. Zhu, 1000 h Operational Lifetime Perovskite Solar Cells by Ambient Melting Encapsulation, Adv. Energy Mater., 2020, 10, 1902472 CrossRef CAS.
- M. I. Saidaminov, J. Kim, A. Jain, R. Quintero-Bermudez, H. Tan, G. Long, F. Tan, A. Johnston, Y. Zhao and O. Voznyy, Suppression of atomic vacancies via incorporation of isovalent small ions to increase the stability of halide perovskite solar cells in ambient air, Nat. Energy, 2018, 3, 648–654 CrossRef.
- G. Xu, S. Wang, P. Bi, H. Chen, M. Zhang, R. Xue, X. Hao, Z. Wang, Y. Li and Y. Li, Hydrophilic Fullerene Derivative Doping in Active Layer and Electron Transport Layer for Enhancing Oxygen Stability of Perovskite Solar Cells, Sol. RRL, 2020, 4, 1900249 CrossRef.
- H. Shu, J. Xia, H. Yang, J. Luo, Z. Wan, H. A. Malik, F. Han, X. Yao and C. Jia, Self-Assembled Hydrophobic Molecule-Based Surface Modification: A Strategy to Improve Efficiency and Stability of Perovskite Solar Cells, ACS Sustainable Chem. Eng., 2020, 8, 10859–10869 Search PubMed.
- J. Zhou, J. Wu, N. Li, X. Li, Y.-Z. Zheng and X. Tao, Efficient all-air processed mixed cation carbon-based perovskite solar cells with ultra-high stability, J. Mater. Chem. A, 2019, 7, 17594–17603 RSC.
- J. Li, T. Jiu, S. Chen, L. Liu, Q. Yao, F. Bi, C. Zhao, Z. Wang, M. Zhao, G. Zhang, Y. Xue, F. Lu and Y. Li, Graphdiyne as a Host Active Material for Perovskite Solar Cell Application, Nano Lett., 2018, 18, 6941–6947 CrossRef.
- M. K. A. Mohammed, Carbon nanotubes loaded ZnO/Ag ternary nanohybrid with improved visible light photocatalytic activity and stability, Optik, 2020, 217, 164867 CrossRef.
- M. K. A. Mohammed, Sol-gel synthesis of Au-doped TiO2 supported SWCNT nanohybrid with visible-light-driven photocatalytic for high degradation performance toward methylene blue dye, Optik, 2020, 223, 165607 CrossRef.
- D. S. Ahmed and M. K. A. Mohammed, Studying the bactericidal ability and biocompatibility of gold and gold oxide nanoparticles decorating on multi-wall carbon nanotubes, Chem. Pap., 2020, 74, 4033–4046 CrossRef.
- H. Li, R. Zhang, Y. Li, Y. Li, H. Liu, J. Shi, H. Zhang, H. Wu, Y. Luo, D. Li, Y. Li and Q. Meng, Graphdiyne-Based Bulk Heterojunction for Efficient and Moisture-Stable Planar Perovskite Solar Cells, Adv. Energy Mater., 2018, 8, 1802012 CrossRef.
- X. Dong, D. Chen, J. Zhou, Y. Z. Zheng and X. Tao, High crystallization of a multiple cation perovskite absorber for low-temperature stable ZnO solar cells with high-efficiency of over 20%, Nanoscale, 2018, 10, 7218–7227 RSC.
- Y.-C. Wang, X. Li, L. Zhu, X. Liu, W. Zhang and J. Fang, Efficient and Hysteresis-Free Perovskite Solar Cells Based on a Solution Processable Polar Fullerene Electron Transport Layer, Adv. Energy Mater., 2017, 7, 1701144 CrossRef.
- T. Singh and T. Miyasaka, Stabilizing the Efficiency Beyond 20% with a Mixed Cation Perovskite Solar Cell Fabricated in Ambient Air under Controlled Humidity, Adv. Energy Mater., 2018, 8, 1700677 CrossRef.
- M. K. A. Mohammed, G. Sarusi, P. Sakthivel, G. Ravi and U. Younis, Improved stability of ambient air-processed methylammonium lead iodide using carbon nanotubes for perovskite solar cells, Mater. Res. Bull., 2021, 137, 111182 CrossRef CAS.
- Z. Jianjun, T. Tong, Z. Liuyang, L. Xiaohe, Z. Haiyuan and Y. Jiaguo, Enhanced Performance of Planar Perovskite Solar Cell by Graphene Quantum Dot Modification, ACS Sustainable Chem. Eng., 2018, 6, 8631–8640 CrossRef.
- R. Teimouri, Z. Heydari, M. P. Ghaziani, M. Madani, H. Abdy, M. Kolahdouz and E. Asl- Soleimani, Synthesizing Li doped TiO2 electron transport layers for highly efficient planar perovskite solar cell, Superlattices Microstruct., 2020, 145, 106627 CrossRef CAS.
- Y. Cho, A. M. Soufiani, J. S. Yun, J. Kim, D. S. Lee, J. Seidel, X. Deng, M. A. Green, S. Huang and A. W. Y. Ho-Baillie, Mixed 3D–2D Passivation Treatment for Mixed-Cation Lead Mixed-Halide Perovskite Solar Cells for Higher Efficiency and Better Stability, Adv. Energy Mater., 2018, 8, 1703392 CrossRef.
- M. Mateen, Z. Arain, X. Liu, A. Iqbal, Y. Ren, X. Zhang, C. Liu, Q. Chen, S. Ma, Y. Ding, M. Cai and S. Dai, Boosting optoelectronic performance of MAPbI3 perovskite solar cells via ethylammonium chloride additive engineering, Sci. China Mater., 2020, 63, 2477–2486 CrossRef.
- V. O. Eze, Y. Seike and T. Mori, Synergistic effect of additive and solvent vapor annealing on the enhancement of MAPbI3 perovskite solar cells fabricated in ambient air, ACS Appl. Mater. Interfaces, 2020, 12, 46837–46845 CrossRef CAS.
- N. Cheng, W. Li, M. Zhang, H. Wu, S. Sun, Z. Zhao, Z. Xiao, Z. Sun, W. Zi and L. Fang, Enhance the performance and stability of methylammonium lead iodide perovskite solar cells with guanidinium thiocyanate additive, Curr. Appl. Phys., 2019, 19, 25–30 CrossRef.
- Y. Li, L. Li, A. S. Yerramilli, Y. Chen, D. Fang, Y. Shend and T. L. Alford, Enhanced power conversion efficiency and preferential orientation of the MAPbI3 perovskite solar cells by introduction of urea as additive, Org. Electron., 2019, 73, 130–136 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |