Open Access Article
Open Access ArticleUnraveling the surface properties of PMMA/azobenzene blends as coating films with photoreversible surface polarity†
Shameer Hisham a,
Norazilawati Muhamad Sarih
a,
Norazilawati Muhamad Sarih *a,
Hairul Anuar Tajuddina,
Zul Hazrin Zainal Abidinb and
Zanariah Abdullaha
*a,
Hairul Anuar Tajuddina,
Zul Hazrin Zainal Abidinb and
Zanariah Abdullaha
aDepartment of Chemistry, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia. E-mail: nmsarih@um.edu.my; Fax: +603-79674193; Tel: +603-79674240
bCentre for Ionics University of Malaya, Department of Physics, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia
First published on 26th April 2021
Abstract
Various reports demonstrated that azobenzene derivatives are the chromophore of choice in photoresponsive surfaces showing reversible surface polarity. Hitherto the surface study of coating films based on polymer/azobenzene blends using contact angle measurements remained unexplored. To provide insight into the surface polarity of polymer/dye blend films, poly(methyl methacrylate) (PMMA) blends containing photoresponsive 4-hydroxy-4′-methylazobenzene (AZO1) and 4,4′-dimethylazobenzene (AZO2) as coating films on clear glass substrates are investigated in this work. Contact angle measurements were carried out to unravel the role of substituents in the surface polarity and the orientation of chromophores in the coating matrices before and after UV light (λmax = 365 nm) irradiation. Changes in water contact angles measured on the PMMA/azobenzene coating films indicated that the surface polarity is reversible as the chromophores underwent reversible trans–cis isomerisation. It has been revealed that the repeated trans–cis isomerisation led to the random reorientation and arrangement of chromophores in PMMA/AZO1 coating films. Then, to indicate the possibility of the disruption of interfacial interactions due to the repeated trans–cis isomerisation processes, as a proof of concept experiment, it is shown that the commercial acrylic-based pressure-sensitive sticker which adhered strongly to the PMMA/AZO1(13) coating film is peeled off from the coating surface after being subjected to a cycle of UV light irradiation for 12 hours, followed by dark conditions for another 12 hours within 14 days. The proof of concept study will lead to more development of smart photoresponsive coating films using simple polymer/dye blends.
1 Introduction
Long-lasting stickers on various surfaces indicate the successful advancement of adhesive technology. Among the commonly used adhesives are acrylic-based pressure-sensitive adhesives. Acrylic pressure-sensitive adhesives have been applied in a myriad of products including adhesive mounting tapes, labels, medical pads, and protective and decorative films, among others due to their instantaneous adhesiveness in addition to their ease of adhesive work.1 However, those adhesives when removed may often lead to residues remaining on walls and surfaces which damage and cause hideous appearance of surfaces. The main factor to achieve strong adhesiveness is the maximum interaction between the adhesive and the surface. Therefore, it is proposed that the reversible polarity of the surface can cause gradual reduction of interactions, and hence the adhesive can peel off by itself.Numerous reports have indicated that azobenzenes are the chromophore of choice in photoresponsive materials owing to their efficient trans–cis isomerisation processes as well as exceptional photostability even after prolonged irradiation.2 Isomerisation of trans-azobenzene into the metastable cis-isomer will typically bring about a change in its molecular geometry and thus increase its molecular dipole from zero in the trans-isomer to about 3.0 D in the cis-isomer.3 Typically, photoresponsive surfaces showing reversible wettability and polarity utilise azobenzene-functionalised molecules, side-chain polymers and even macrocycles where they are then formed into self-assembled monolayers (SAMs),4–17 Langmuir–Blodgett films,18,19 liquid crystalline films,20,21 polymer thin films22–28 and nanoparticles.29 Detailed surface studies including contact angle measurements, X-ray photoelectron spectroscopy (XPS) and atomic force microscopy (AFM) were carried out to elucidate the photochemical effects of azobenzene chromophores on the wettability, morphology and polarity of the surfaces. However, to the best of our knowledge, the surface study of coating films based on simple polymer/azobenzene dye blends using contact angle measurements has never been reported.
Thus, this work aimed to provide insight into the substituent effects of azobenzene chromophores on the (i) polarity of the coating blend surface, (ii) the orientation of chromophores and (iii) photochemistry of chromophores in the coating matrices before and after UV light (λmax = 365 nm) irradiation using contact angle measurements. Based on a previous report,20 the orientation of azobenzene moieties in liquid-crystalline films greatly influenced the surface polarity. Another investigation30 demonstrated that repeated irradiation of well-ordered azobenzene-containing SAMs with UV and visible light led to the random reorientation and arrangement of azobenzene chromophores.
In this work, 4-hydroxy-4′-methylazobenzene (AZO1) and 4,4′-dimethylazobenzene (AZO2) were blended into poly(methyl methacrylate) (PMMA) and subsequently coated onto clear glass substrates. The coating film surfaces were then characterised using contact angle measurements. Since the repeated irradiation of azobenzene chromophores could trigger the rearrangement of chromophores, it is hypothesised that the interaction sites on the surface could be altered as well. Therefore, as a proof of concept, commercial acrylic-based pressure-sensitive stickers were adhered onto both PMMA and PMMA/AZO1 coating films. Subsequently, each coating film underwent repeated irradiation with UV light for 12 hours, followed by dark conditions for another 12 hours for 14 days. If the hypothesis is true, then it is expected that the repeated changes in surface polarity could disrupt the adhesive/coating interactions, and therefore the sticker should peel off.
2 Experimental
2.1 General information
All chemicals and reagents were obtained from Merck unless otherwise stated. The melting point of AZO1,2 was recorded on a Mel-Temp II melting point apparatus (Laboratory Devices Inc., Holliston, MA, USA). NMR spectra were obtained using Bruker Avance III (400 MHz) (Bruker BioSpin AG, Fällanden, Switzerland) NMR spectrometers with tetramethylsilane as the internal standard. All chemical shifts are reported in ppm. FTIR spectra were measured on a Perkin-Elmer ATR-400 series FTIR spectrometer (PerkinElmer, Shelton, CT, USA). Absorption spectra were measured with a Cary 60 UV-Visible spectrometer. For the preparation of coating films, PMMA (average Mw = 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) and xylene (reagent grade) were obtained from Sigma-Aldrich (Darmstadt, Germany).
000 g mol−1) and xylene (reagent grade) were obtained from Sigma-Aldrich (Darmstadt, Germany).
2.2 Synthesis
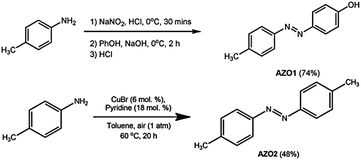
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHCl3 = 1
CHCl3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 afforded AZO1 as an orange crystalline solid (74%), mp 155–158 °C (Lit: 143–144 °C31); UV (EtOAc) λmax: 348 nm, εmax: 2.63 × 104 L mol−1 cm−1; FTIR (νmax, cm−1): 3025 (br, O–H), 1581 (aromatic C
1 afforded AZO1 as an orange crystalline solid (74%), mp 155–158 °C (Lit: 143–144 °C31); UV (EtOAc) λmax: 348 nm, εmax: 2.63 × 104 L mol−1 cm−1; FTIR (νmax, cm−1): 3025 (br, O–H), 1581 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) C), 1504 (N
C), 1504 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) N), 1274 (C–O); 1H NMR (400 MHz, CDCl3) δ = 2.35 (s, 3H, –CH3), 5.76 (bs, 1H, –OH), 6.85 (d, J = 7.56 Hz, 2H, Ar-H), 7.22 (d, J = 7.72 Hz, 2H, Ar-H), 7.71 (d, J = 7.44 Hz, 2H, Ar-H), 7.77 (d, J = 7.56 Hz, 2H, Ar-H); 13C NMR (100 MHz, CDCl3) δ = 20.44, 114.76, 121.50, 123.77, 128.70, 139.93, 146.03, 149.67, 157.14.
N), 1274 (C–O); 1H NMR (400 MHz, CDCl3) δ = 2.35 (s, 3H, –CH3), 5.76 (bs, 1H, –OH), 6.85 (d, J = 7.56 Hz, 2H, Ar-H), 7.22 (d, J = 7.72 Hz, 2H, Ar-H), 7.71 (d, J = 7.44 Hz, 2H, Ar-H), 7.77 (d, J = 7.56 Hz, 2H, Ar-H); 13C NMR (100 MHz, CDCl3) δ = 20.44, 114.76, 121.50, 123.77, 128.70, 139.93, 146.03, 149.67, 157.14.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) C). 1H NMR (400 MHz, CDCl3) δ = 2.35 (s, 6H, –CH3), 7.22 (d, J = 7.60 Hz, 4H, Ar-H), 7.73 (d, J = 7.80 Hz, 4H, Ar-H). 13C NMR (100 MHz, CDCl3) δ = 21.49, 122.74, 129.72, 141.21, 150.85.
C). 1H NMR (400 MHz, CDCl3) δ = 2.35 (s, 6H, –CH3), 7.22 (d, J = 7.60 Hz, 4H, Ar-H), 7.73 (d, J = 7.80 Hz, 4H, Ar-H). 13C NMR (100 MHz, CDCl3) δ = 21.49, 122.74, 129.72, 141.21, 150.85.2.3 Preparation of PMMA/azobenzene blends as coating films
PMMA120K and AZO1,2 were blended together by dissolving them in xylene at 60 °C until a homogeneous solution was formed. The compositions of the coating films are shown in Table 1. Clear glass substrates were cleaned with detergent, followed by rinsing with distilled water. Then, they were sonicated in distilled water for 15 minutes, and then in EtOH. Prior to the application of coating solution, the substrates were dried by purging under the flow of N2 gas to remove residual EtOH. The coating films were prepared by the doctor blade method and dried overnight in open-air. The dry film thickness for all coating films on glass substrates was measured using a micrometer screw gauge and was found to be in the range of 30 to 40 μm.| Coating | Binder | Dye | Dye content (wt%) | |
|---|---|---|---|---|
| Binder solution | Dry film | |||
| PMMA | PMMA120K (20 wt% solution in xylene) | — | 0 | 0 |
| PMMA/AZO1(5) | AZO1 | 1 | 5 | |
| PMMA/AZO1(13) | 3 | 13 | ||
| PMMA/AZO1(33) | 10 | 33 | ||
| PMMA/AZO1(50) | 20 | 50 | ||
| PMMA/AZO2(5) | AZO2 | 1 | 5 | |
| PMMA/AZO2(13) | 3 | 13 | ||
2.4 Differential scanning calorimetry (DSC)
DSC measurements were performed using a TA Instruments DSC Q20 differential scanning calorimeter (TA Instruments, Inc., New Castle, DE, USA) at a nitrogen flow rate of 20 mL min−1. The samples (5–10 mg) were first heated to 150 °C and held at that temperature for 1 minute to erase the thermal history (1st scan). Then, they were cooled to −50 °C and then reheated to 200 °C at a rate of 10 °C min−1 (2nd scan). Glass transition temperature, indicated as Tg, was determined from the second heating scans. Tg was defined as the inflection point of the heat flow curve.2.5 Contact angle measurements
Static contact angle measurements were conducted using a Krüss G23 goniometer (A.Krüss Optronic, Hamburg, Germany) with deionised water (drop volume = 3 μL) as the contact liquid under ambient conditions. All data reported are the average of at least three individual measurements.The trans–cis photoisomerisation kinetics of azobenzene-doped PMMA coatings were studied by first irradiating the coatings with UV-A light (365 nm, 20 W) using a Toki T8/20 W UV lamp (Toki Corporation, Tokyo, Japan). The contact angles of samples were then measured with respect to the irradiation time until a plateau of contact angles was observed, signifying that the samples reached a photostationary state (PSS). Upon reaching the PSS, the coating films were then kept under dark conditions for the thermal cis–trans isomerisation kinetic studies where the contact angles were recorded with respect to the relaxation time until a plateau of contact angles was noticed. For the isomerisation kinetic studies, the contact angles were measured at 10–60 minute intervals. In addition, all measurements were carried out within 60 seconds to minimise the exposure of chromophores to ambient light. The experimental setup is shown in Fig. S7.† The relationship between contact angles and time was then correlated using the equation
| θ(t) = (θ0 − θPSS)exp(−kt) + θPSS | (1) |
2.6 Proof of concept sticker peel-off test
Commercial acrylic-based pressure-sensitive stickers were applied onto PMMA and PMMA/AZO1(13) coating films. Then, the backside of the coating films was irradiated first with UV-A light (365 nm, 20 W) for 12 hours, then kept in the dark for another 12 hours to simulate day and night using a Toki T8/20 W UV lamp (Toki Corporation, Tokyo, Japan). This process was repeated for 14 consecutive days. The extent of sticker peeling off was calculated by recording the area of the sticker that has peeled off from the surface. The part where the sticker remained unpeeled onto the surface appeared to be darker than other parts of the surface.3 Results and discussion
Azobenzene derivatives AZO1 and AZO2 were chosen as photoresponsive chromophores for this work to study the effects of functional groups on the physical and optical properties of PMMA coating films. They were synthesised using established methods, producing moderate to good yields. AZO1 contains one methyl group and one –OH group, whereas AZO2 consists of only methyl groups. The –OH groups in AZO1 molecules may improve its solubility in the PMMA matrix possibly due to the formation of intermolecular C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H hydrogen bonds. Therefore, it is expected that AZO1 showed better homogeneity in the PMMA matrix compared to AZO2. It is well-known that the presence of strong electron donors such as –OH groups could also influence the speed of trans–cis isomerisation of azobenzene derivatives. Hence, the performance of AZO1 and AZO2 dyes in trans–cis isomerisation in PMMA can be compared.
O⋯H hydrogen bonds. Therefore, it is expected that AZO1 showed better homogeneity in the PMMA matrix compared to AZO2. It is well-known that the presence of strong electron donors such as –OH groups could also influence the speed of trans–cis isomerisation of azobenzene derivatives. Hence, the performance of AZO1 and AZO2 dyes in trans–cis isomerisation in PMMA can be compared.
3.1 Physical characterisation of PMMA/azobenzene blends as coating films
Fig. 1 shows the prepared coating films. Increasing the weight percentage of AZO1 dyes beyond 33% resulted in inhomogeneity of the mixture, as evidenced by the significantly rough surface of the PMMA/AZO1(50) coating (Fig. 1(e)), in comparison to the other coating films. Consequently, this leads to the composition being inapplicable for coating systems. Hence, only coating films with 5, 13 and 33 wt% of AZO1 dyes are further characterised.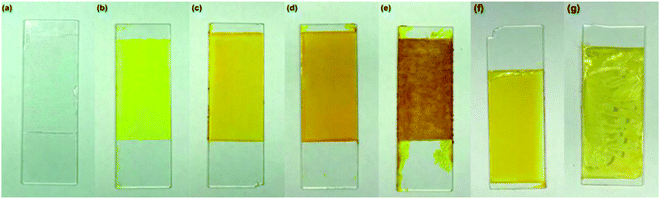 | ||
| Fig. 1 (a) PMMA, (b) PMMA/AZO1(5), (c) PMMA/AZO1(13), (d) PMMA/AZO1(33), (e) PMMA/AZO1(50), (f) PMMA/AZO2(5) and (g) PMMA/AZO2(13) coating films. | ||
It can be seen that increasing the content of AZO2 dyes beyond 5 wt% triggered the crystallisation of AZO2 in PMMA films, as shown in Fig. 1(g). This is possibly due to the poor solubility of AZO2 dyes in PMMA matrices.34 Therefore, only coating films with 5 wt% of AZO2 dyes are further characterised.
By changing one of the methyl groups in AZO2 to –OH, the solubility of azobenzene dyes in PMMA matrices is enhanced, evidenced by the higher content of AZO1 dyes able to be blended homogeneously in PMMA coating films. This is possibly due to the intermolecular C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H hydrogen bonding between PMMA and AZO1. Another way to circumvent the crystallisation of dyes in the polymer matrix is by synthesising azobenzene derivatives containing long alkyl chains or branches in order to increase the solubility in the polymer matrix.34
O⋯H hydrogen bonding between PMMA and AZO1. Another way to circumvent the crystallisation of dyes in the polymer matrix is by synthesising azobenzene derivatives containing long alkyl chains or branches in order to increase the solubility in the polymer matrix.34
It can also be observed that PMMA/AZO2 coating films have poor adhesion onto glass substrates as some parts of the film peeled off (Fig. 1(f) and (g)). In contrast, the presence of –OH groups in AZO1 have enabled better adhesion of films onto glass substrates possibly by intermolecular hydrogen bonding interaction between the glass and coating film.
In order to investigate the role of –OH groups in increasing the homogeneity of AZO1 in PMMA, DSC studies were carried out on PMMA/AZO1(5) and PMMA/AZO2(5) coating films for comparison study. DSC studies can provide sufficient information about the compatibility of polymer blends. From Fig. 2, the Tg of pure PMMA is 68.30 °C. The Tg of the PMMA coating film is indeed lower than that of the pure commercial grade PMMA granules used (Tg = 105 °C). It has been reported that the selection of solvents can influence the Tg of a polymer by altering the packing density and morphology of the polymer.35 Moreover, it has been observed that the Tg of a polymer film can change with film thickness and the type of substrate that the film is cast on.36 Thus, it is possible that the lower value of the observed Tg for the PMMA coating film is probably due to a combination of these reasons.
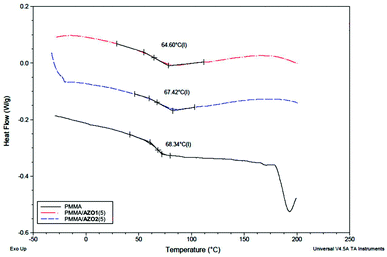 | ||
| Fig. 2 DSC thermograms of PMMA (black solid line), PMMA/AZO1(5) (red dash dot line) and PMMA/AZO2(5) (blue dash line). | ||
From the DSC thermograms, it is shown that only a single Tg is present in both PMMA/AZO1(5) and PMMA/AZO2(5) DSC thermograms. Typically, the DSC curve will indicate only a single Tg transition if two components are fully miscible with each other.37 Therefore, the results suggested that the AZO1 and AZO2 dyes (5 wt% dry film content) were miscible in the PMMA matrices. It is expected that the addition of a low-molecular weight component would decrease the Tg of the bulk polymer, where the small amount of small-molecule additives could change the molecular arrangement and crystallinity of the bulk polymer. Thus, a careful observation indicated that the addition of AZO1 and AZO2 dyes into PMMA decreased the Tg of PMMA from 68.30 °C to 64.55 and 67.05 °C, respectively. The addition of AZO1 dyes decreased the Tg further than that of AZO2 dyes. The larger Tg deviation of the PMMA/AZO1(5) film in comparison to that of the PMMA/AZO2(5) film is a possible sign of intermolecular C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H hydrogen bonding interactions between PMMA and AZO1. Previous studies have also indicated that the intermolecular hydrogen bonding interactions between two components in polymer blends caused a decrease in the Tg of the bulk material.37,38 Thus, it is probable that the presence of –OH groups in AZO1 enhanced its solubility in the PMMA matrix due to intermolecular hydrogen bonding interactions.
O⋯H hydrogen bonding interactions between PMMA and AZO1. Previous studies have also indicated that the intermolecular hydrogen bonding interactions between two components in polymer blends caused a decrease in the Tg of the bulk material.37,38 Thus, it is probable that the presence of –OH groups in AZO1 enhanced its solubility in the PMMA matrix due to intermolecular hydrogen bonding interactions.
3.2 Contact angle measurements
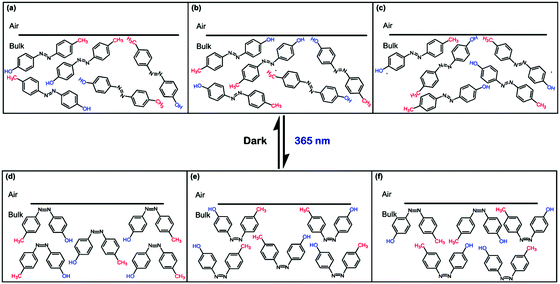
Upon a closer look at the structure of AZO1, it can be deduced that the dye itself is asymmetric; that is, there are two different substituents at the 4- and 4′-positions, which are –OH and –CH3. Therefore, AZO1 chromophores can have a few possible arrangements on the coating surface, as illustrated in Fig. 3.
From Fig. 3(a), trans-AZO1 molecules orient in a way that –CH3 groups are saturated on the coating surface while in Fig. 3(b), the –OH groups are saturated on the coating surface. trans-AZO1 molecules can also arrange in a random manner, as shown in Fig. 3(c). Then, when irradiated with UV-A, cis-AZO1 can also have different possibilities of arrangements on the coating surface, as depicted in Fig. 3(d–f).
Well-ordered surfaces containing alkyl groups can give water contact angle values from 74 to 142° depending on surface composition, alkyl chain length and surface roughness.41–46 However, water contact angle values of surfaces containing hydroxyl (–OH) groups could be from 22 to 44°.45,47,48 Furthermore, surfaces containing –COOH groups can provide water contact angle values from 30° to as low as 15° depending on surface compositions.45,47,49–51
If trans-AZO1 can have well-ordered arrangements in a way that –CH3 groups saturate the coating surface (Fig. 3(a)), then it is anticipated that the static water contact angle value increases to more than 70° (hydrophobic surface). Conversely, if –OH groups saturate the coating surface (Fig. 3(b)), then it is predicted that the static water contact angle value should decrease to below 40° (hydrophilic surface). To unravel which scenario is the best to describe the arrangement of AZO1 in the coating surface, water contact angle measurements are taken for the blank PMMA coating as well as the dyed coatings and suitable comparisons can be made.
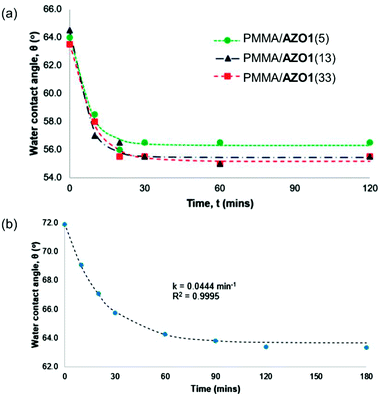
The water contact angle values of the PMMA/AZO1 coating films after a few UV-A irradiation cycles are shown in Fig. 4(a). It is noted that water contact angles of the blank PMMA coating were relatively constant before and after UV-A exposure. The average contact angle value of 63° has indicated that the native PMMA surface is relatively hydrophilic (<90°). However, when AZO1 chromophores were added, the water contact angle values of the coating films remained nearly the same regardless of the dye concentration – indicating that the coating surfaces were neither saturated with hydrophobic –CH3 groups nor hydrophilic –OH groups from the dye molecules. This can only mean that trans-AZO1 molecules are suggested to have random arrangements on the coating surface (Fig. 3(c)).
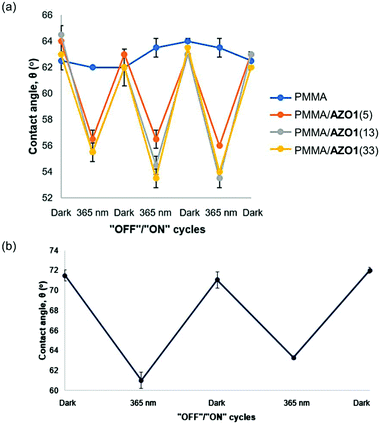 | ||
| Fig. 4 Changes in contact angle values for (a) various PMMA/AZO1 and (b) PMMA/AZO2(5) coating films upon UV light irradiation cycles. | ||
After exposure to UV-A irradiation, the water contact angle of the samples dropped in the range of 55.5°–56.5°, which has clearly shown that the trans–cis photoisomerisation of AZO1 has occurred in the coating films. Moreover, this indicated that the cis-isomer is more polar, and the changes in the dipole moment of the azo molecules contributed to an overall reduction in the contact angle values.52 It can also be shown that increasing the content of AZO1 chromophores in coating films from 13 wt% to 33 wt% did not give any significant decrease in the water contact angle values. This possibly showed that saturation of the surface with chromophores has occurred, most likely due to the segregation of chromophores to the coating film surface.39 Moreover, crystallisation of chromophores due to phase separation of chromophores from the coating film may also happen at higher concentrations as indicated in Fig. 1, further signifying the segregation processes of chromophores.
It is also suggested that cis-AZO1 molecules can have random arrangements on the coating surfaces as well (Fig. 3(f)). A combination of dipole moments of cis-AZO1 and –OH groups on the surfaces led to a reduction in static water contact angle values. As a result, the coating films become more hydrophilic after UV-A exposure. Subsequently, when these coating films were kept in the dark, the contact angles reverted, signifying that the thermal cis–trans isomerisation process has occurred and this has confirmed the reversibility of AZO1 isomerisation. The reversibility of trans–cis isomerisation processes is confirmed as the contact angle values changed accordingly upon UV irradiation cycles.
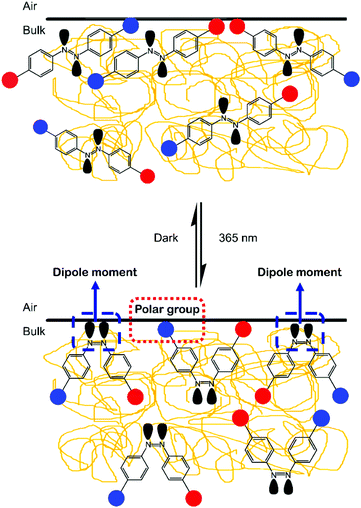
Since the AZO1 chromophores were found to have random arrangements in the PMMA matrices, it is proposed that the reversible trans–cis isomerization processes could alter the interaction sites on the coating film surface. This is illustrated in Fig. 5. Initially, the interaction sites of PMMA/AZO1 coating films mainly consist of hydrophilic –OH groups. Then, when trans → cis photoisomerization has occurred, there are new interaction sites available, in the form of dipole moments from the lone pair electrons in the azo N atoms. When cis-AZO1 thermally relaxed, these additional interactions disappeared.
To prove our proposed theory, contact angle measurements on the PMMA/AZO2(5) coating film were conducted as well. From Fig. 4(b), it was observed that initially, the contact angle was 71.5°, which indicated that the PMMA surface became less polar. In this case, only –CH3 groups are present in AZO2 molecules, therefore eliminating the various probable arrangements of molecules as discussed previously. Thus, the presence of –CH3 groups on the surface caused the surface to be more hydrophobic. By comparing the contact angle values of PMMA/AZO1 coating films (63.0°–64.5°), it is clear that AZO1 molecules do have random arrangements in PMMA coating films, contributing to the lower contact angle values than the PMMA/AZO2(5) coating film.
Upon irradiation with UV light, the PMMA/AZO2(5) surface became more hydrophilic as a result of the formation of cis-AZO2, in which its higher dipole moment increased the polarity of the surface. Moreover, it was shown that the change in contact angle values Δθ for the PMMA/AZO2(5) coating film was higher (Δθ = 10.5°) than that of PMMA/AZO1(5) (Δθ = 7.5°). Again, the reversibility of trans–cis isomerisation processes is confirmed as the contact angle values changed accordingly upon UV irradiation cycles.
| Coating | kE→Z (min−1) | t1/2 (min) | R2 |
|---|---|---|---|
| PMMA/AZO1(5) | 0.1434 | 4.8337 | 0.9838 |
| PMMA/AZO1(13) | 0.1603 | 4.3241 | 0.9884 |
| PMMA/AZO1(33) | 0.1181 | 5.8692 | 0.9921 |
| PMMA/AZO2(5) | 0.0444 | 15.6114 | 0.9995 |
It can also be seen that the PSS is reached for all coating films at about the 30th minute mark. In other words, 30 minutes of UV light irradiation should provide a sufficient time for the dyes to convert into the cis-isomers. It is also worth mentioning that the formation of a plateau or maximum contact angle when the amount of AZO1 dyes increased beyond 13 wt% possibly implied that the chromophores are saturated on the surfaces, as discussed earlier.
The rate of isomerisation of AZO2 in PMMA is markedly slower than that of AZO1, with AZO2 reaching the PSS only after 2 hours (Fig. 6(b)). In addition, its half-life of 15.6 min is also significantly slower than that of AZO1 (4.3 to 5.9 minutes). This has clearly indicated that the presence of a strong electron donor on one side of the azobenzene molecule increases the photoisomerisation rate.
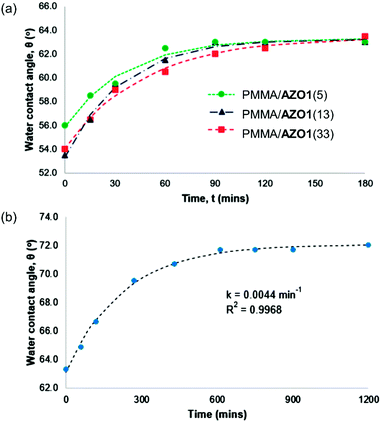 | ||
| Fig. 7 Thermal cis → trans isomerisation of (a) various PMMA/AZO1 and (b) PMMA/AZO2(5) coating films. | ||
| Coating | kZ→E (min−1) | t1/2 (min) | R2 |
|---|---|---|---|
| PMMA/AZO1(5) | 0.0280 | 24.7553 | 0.9818 |
| PMMA/AZO1(15) | 0.0299 | 23.1822 | 0.9939 |
| PMMA/AZO1(33) | 0.0211 | 32.8506 | 0.9929 |
| PMMA/AZO2(5) | 0.0044 | 157.2832 | 0.9968 |
In contrast, AZO2 chromophores showed a significantly lower thermal isomerisation rate, where the molecules fully reverted after 10 hours, with a half-life of 157.3 minutes (Fig. 7(b)). Meanwhile, the half-life of cis-AZO1 in PMMA coating films ranged from 23.2 to 32.9 minutes. These indicate that the thermal cis → trans isomerisation process is fast, albeit considerably slower than pseudostilbene-type molecules (lifetimes of their cis-isomers are usually in the order of milliseconds/seconds).3,53 In contrast, for the case of azobenzene-type molecules (in this respect, AZO2), the lifetimes of their cis-isomers could be in the order of hours or even days.3,53 As a consequence, this specific property of azobenzene-type molecules may prove to be disadvantageous for the current work as it will take a significantly longer time for the cis-isomer to thermally revert to the more thermodynamically stable trans-isomer. However, rapid regeneration of the trans-isomer can be done easily and efficiently by irradiation of the cis-isomer with blue or white light.34,54,55
3.3 Proof of concept sticker peel-off test
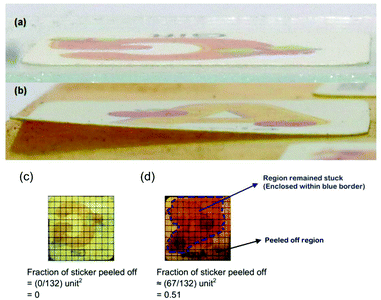
From the results of static contact angle measurements, we showed that the polarity of surfaces can be tuned. The random orientations of AZO1 chromophores in the coating film may bring about different interaction sites on the surface. Therefore, it is interesting to study whether interfacial interactions between the coating film surface and any adhesive can be altered when subjected to UV light irradiation. Thus, as a proof of concept, commercial acrylic-based pressure-sensitive stickers were applied on top of PMMA and PMMA/AZO1(13) coating films. Subsequently, both coating films were subjected to a cycle of UV light irradiation for 12 hours and then kept in the dark for another 12 hours for 14 consecutive days. The PMMA/AZO1(13) coating was chosen for the sticker peel-off test since it showed the best photoswitching performance, whereas the PMMA/AZO2(5) coating film was not chosen due to the slower isomerisation rate of AZO2 in comparison to AZO1. Fig. 8 shows the conditions of the stickers on the coating films after 14 irradiation cycles.The extent of sticker peeling off from coating film surfaces was determined by recording the area that the sticker has peeled off. Fig. 8(c) and (d) show the extent of sticker peeling off from the coating surfaces. The darker regions represent the area of the sticker still sticking onto the surface, whereas the lighter regions represent the area of the sticker already peeled off. It is clearly noticed that the sticker remained unpeeled on the PMMA coating film after 14 days. In contrast, the sticker has peeled nearly half of itself off from the PMMA/AZO1(13) coating film surface within 14 days.
Despite the limitations of the study, it is proposed that the repeated excitation of azobenzene chromophores in the coating film caused substantial changes in the sticker/coating interactions (Fig. 9). Initially, the sticker/coating interactions consisted of weak van der Waals forces and hydrogen bonding interactions (sticker and –OH moieties in AZO1). Subsequently, when the chromophores are excited upon UV light irradiation to form the cis-isomers, there are additional dipole–dipole interactions between the coating and sticker surfaces. However, these additional interactions are lost once the chromophores reverted to the trans-isomer. The repetition of trans–cis isomerisation has caused random reorientation and arrangements of the chromophores.30 As a result, there is a considerable amount of alterations to sticker/coating interactions and consequently, the sticker peeled itself off slowly from the coating film surface. Furthermore, the proposed peeling-off mechanism is in agreement with the results of static contact angle measurements.
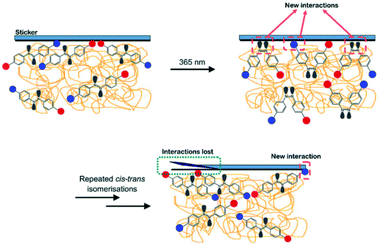 | ||
| Fig. 9 Proposed sticker peeling-off mechanism. Red circles represent –CH3 groups and blue circles represent –OH groups. Nonbonding lone pair electrons are shown in grey. | ||
4 Conclusions
In this work, simple PMMA/azobenzene blends were applied as coating films onto clear glass substrates. We showed that both AZO1 and AZO2 chromophores are photoreversible in PMMA matrices upon repeated UV light irradiation, reflected by the changes in contact angles. The lower contact angles of PMMA/AZO1 coating films in comparison to the PMMA/AZO2 coating film indicated that the AZO1 chromophores are oriented and distributed randomly in the coating matrix. The –OH group in AZO1 also increased the rate of isomerisation in comparison to AZO2. Since the reorientation of AZO1 chromophores brought about changes in the interaction sites on the coating film surface, it was proved that the repetition of trans–cis isomerisation processes caused the commercial acrylic-based pressure-sensitive sticker that was initially adhered to the PMMA/AZO1(13) coating film to peel off from the coating surface possibly due to the disruption of the adhesive/coating interactions. It is expected that these findings would further catalyse the development of photoresponsive self-cleaning coating films as well as having the potential to address problems associated with illegal sticker advertisements.Conflicts of interest
The authors declare that there is no conflict of interests.Acknowledgements
This work was supported by the University of Malaya under Frontier Research Grant (FG042-17AFR) and Impact-Oriented Interdisciplinary Research Grant (IIRG006A-19IISS). S. Hisham acknowledges funding from the Ministry of Education Malaysia and the SLAB fellowship scheme from the University of Malaya.References
- Z. Czech and M. Wesolowska, Eur. Polym. J., 2007, 43, 3604–3612 CrossRef CAS.
- H. D. Bandara and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809–1825 RSC.
- K. G. Yager and C. J. Barrett, J. Photochem. Photobiol., A, 2006, 182, 250–261 CrossRef CAS.
- L. Siewierski, W. Brittain, S. Petrash and M. Foster, Langmuir, 1996, 12, 5838–5844 CrossRef CAS.
- K. Tamada, J. Nagasawa, F. Nakanishi, K. Abe, T. Ishida, M. Hara and W. Knoll, Langmuir, 1998, 14, 3264–3271 CrossRef CAS.
- S. Evans, S. Johnson, H. Ringsdorf, L. Williams and H. Wolf, Langmuir, 1998, 14, 6436–6440 CrossRef CAS.
- S.-K. Oh, M. Nakagawa and K. Ichimura, J. Mater. Chem., 2002, 12, 2262–2269 RSC.
- F. Hamelmann, U. Heinzmann, U. Siemeling, F. Bretthauer and J. V. der Brüggen, Appl. Surf. Sci., 2004, 222, 1–5 CrossRef CAS.
- S. Sortino, S. Petralia and S. Conoci, J. Mater. Chem., 2004, 14, 811–813 RSC.
- Y. Wen, W. Yi, L. Meng, M. Feng, G. Jiang, W. Yuan, Y. Zhang, H. Gao, L. Jiang and Y. Song, J. Phys. Chem. B, 2005, 109, 14465–14468 CrossRef CAS PubMed.
- N. Delorme, J.-F. Bardeau, A. Bulou and F. Poncin-Epaillard, Langmuir, 2005, 21, 12278–12282 CrossRef CAS PubMed.
- X. Zhang, Y. Wen, Y. Li, G. Li, S. Du, H. Guo, L. Yang, L. Jiang, H. Gao and Y. Song, J. Phys. Chem. C, 2008, 112, 8288–8293 CrossRef CAS.
- M. Han, D. Ishikawa, T. Honda, E. Ito and M. Hara, Chem. Commun., 2010, 46, 3598–3600 RSC.
- M. Han, T. Honda, D. Ishikawa, E. Ito, M. Hara and Y. Norikane, J. Mater. Chem., 2011, 21, 4696–4702 RSC.
- M. Min, G. S. Bang, H. Lee and B.-C. Yu, Chem. Commun., 2010, 46, 5232–5234 RSC.
- X. Pei, A. Fernandes, B. Mathy, X. Laloyaux, B. Nysten, O. Riant and A. M. Jonas, Langmuir, 2011, 27, 9403–9412 CrossRef CAS PubMed.
- O. Nachtigall, C. Kördel, L. H. Urner and R. Haag, Angew. Chem., Int. Ed., 2014, 53, 9669–9673 CrossRef CAS PubMed.
- C. L. Feng, J. Jin, Y. J. Zhang, Y. L. Song, L. Y. Xie, G. R. Qu, Y. Xu and L. Jiang, Surf. Interface Anal., 2001, 32, 121–124 CrossRef CAS.
- C. L. Feng, Y. J. Zhang, J. Jin, Y. L. Song, L. Y. Xie, G. R. Qu, L. Jiang and D. B. Zhu, Langmuir, 2001, 17, 4593–4597 CrossRef CAS.
- S. Pan, M. Ni, B. Mu, Q. Li, X. Y. Hu, C. Lin, D. Chen and L. Wang, Adv. Funct. Mater., 2015, 25, 3571–3580 CrossRef CAS.
- C. Li, F. Cheng, J.-a. Lv, Y. Zhao, M. Liu, L. Jiang and Y. Yu, Soft Matter, 2012, 8, 3730–3733 RSC.
- W. Jiang, G. Wang, Y. He, X. Wang, Y. An, Y. Song and L. Jiang, Chem. Commun., 2005, 3550–3552 RSC.
- W. Feng, H. Kun and M.-X. Wan, Chin. Phys., 2005, 14, 306 CrossRef.
- H. Ge, G. Wang, Y. He, X. Wang, Y. Song, L. Jiang and D. Zhu, ChemPhysChem, 2006, 7, 575–578 CrossRef CAS PubMed.
- M. Y. Paik, S. Krishnan, F. You, X. Li, A. Hexemer, Y. Ando, S. H. Kang, D. A. Fischer, E. J. Kramer and C. K. Ober, Langmuir, 2007, 23, 5110–5119 CrossRef CAS PubMed.
- L. Ding and T. P. Russell, Macromolecules, 2007, 40, 2267–2270 CrossRef CAS.
- N. M. Ahmad, X. Lu and C. J. Barrett, J. Mater. Chem., 2010, 20, 244–247 RSC.
- Y. Huang, H. Kang, G. Li, C. Wang, Y. Huang and R. Liu, RSC Adv., 2013, 3, 15909–15916 RSC.
- S. Pan, R. Guo and W. Xu, Soft Matter, 2014, 10, 9187–9192 RSC.
- M. Kaneta, T. Honda, K. Onda and M. Han, New J. Chem., 2017, 41, 1827–1833 RSC.
- K. Haghbeen and E. W. Tan, J. Org. Chem., 1998, 63, 4503–4505 CrossRef CAS.
- C. Zhang and N. Jiao, Angew. Chem., 2010, 122, 6310–6313 CrossRef.
- G. Srinivasa, K. Abiraj and D. C. Gowda, Tetrahedron Lett., 2003, 44, 5835–5837 CrossRef CAS.
- C. Pakula, C. Hanisch, V. Zaporojtchenko, T. Strunskus, C. Bornholdt, D. Zargarani, R. Herges and F. Faupel, J. Mater. Sci., 2011, 46, 2488–2494 CrossRef CAS.
- S. P. Jeong, L. A. Renna, C. J. Boyle, H. S. Kwak, E. Harder, W. Damm and D. Venkataraman, Sci. Rep., 2017, 7, 1–12 CrossRef PubMed.
- L. Singh, P. J. Ludovice and C. L. Henderson, in Advances in Resist Technology and Processing XX, International Society for Optics and Photonics, 2003, vol. 5039, pp. 1008–1018 Search PubMed.
- S. W. Kuo, C. F. Huang and F. C. Chang, J. Polym. Sci., Part B: Polym. Phys., 2001, 39, 1348–1359 CrossRef CAS.
- M. Poutanen, O. Ikkala and A. Priimagi, Macromolecules, 2016, 49, 4095–4101 CrossRef CAS.
- L. R. Hutchings, N. M. Sarih and R. L. Thompson, Polym. Chem., 2011, 2, 851–861 RSC.
- S. J. Hardman, N. Muhamad-Sarih, H. J. Riggs, R. L. Thompson, J. Rigby, W. N. Bergius and L. R. Hutchings, Macromolecules, 2011, 44, 6461–6470 CrossRef CAS.
- A. C. Henry, T. J. Tutt, M. Galloway, Y. Y. Davidson, C. S. McWhorter, S. A. Soper and R. L. McCarley, Anal. Chem., 2000, 72, 5331–5337 CrossRef CAS PubMed.
- X. Song, J. Zhai, Y. Wang and L. Jiang, J. Colloid Interface Sci., 2006, 298, 267–273 CrossRef CAS PubMed.
- A. Shanmugharaj, J. Yoon, W. Yang and S. H. Ryu, J. Colloid Interface Sci., 2013, 401, 148–154 CrossRef CAS PubMed.
- M. Taborelli, L. Eng, P. Descouts, J. Ranieri, R. Bellamkonda and P. Aebischer, J. Biomed. Mater. Res., 1995, 29, 707–714 CrossRef CAS PubMed.
- Y. Arima and H. Iwata, Biomaterials, 2007, 28, 3074–3082 CrossRef CAS PubMed.
- A. Schmohl, A. Khan and P. Hess, Superlattices Microstruct., 2004, 36, 113–121 CrossRef CAS.
- J. H. Lee, H. W. Jung, I.-K. Kang and H. B. Lee, Biomaterials, 1994, 15, 705–711 CrossRef CAS PubMed.
- R. Jayasekara, I. Harding, I. Bowater, G. Christie and G. T. Lonergan, Polym. Test., 2004, 23, 17–27 CrossRef CAS.
- Y. An, M. Chen, Q. Xue and W. Liu, J. Colloid Interface Sci., 2007, 311, 507–513 CrossRef CAS PubMed.
- G. Toworfe, R. Composto, I. Shapiro and P. Ducheyne, Biomaterials, 2006, 27, 631–642 CrossRef CAS PubMed.
- Y. Sasai, N. Matsuzaki, S.-i. Kondo and M. Kuzuya, Surf. Coat. Technol., 2008, 202, 5724–5727 CrossRef CAS.
- H. S. Lim, J. T. Han, D. Kwak, M. Jin and K. Cho, J. Am. Chem. Soc., 2006, 128, 14458–14459 CrossRef CAS PubMed.
- C. J. Barrett, J.-i. Mamiya, K. G. Yager and T. Ikeda, Soft Matter, 2007, 3, 1249–1261 RSC.
- A. A. Beharry, O. Sadovski and G. A. Woolley, J. Am. Chem. Soc., 2011, 133, 19684–19687 CrossRef CAS PubMed.
- M. Dong, A. Babalhavaeji, S. Samanta, A. A. Beharry and G. A. Woolley, Acc. Chem. Res., 2015, 48, 2662–2670 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The results of UV-visible, 1H and 13C-NMR spectra of AZO1 and AZO2. See DOI: 10.1039/d1ra01192h |
| This journal is © The Royal Society of Chemistry 2021 |