Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalytic activity of Co-nanocrystal-doped tungsten carbide arising from an internal magnetic field†
M. Morishita *a,
A. Nozakia,
H. Yamamotoa,
N. Fukumuroa,
M. Morib,
K. Arakib,
F. Sakamotob,
A. Nakamurab and
H. Yanagitac
*a,
A. Nozakia,
H. Yamamotoa,
N. Fukumuroa,
M. Morib,
K. Arakib,
F. Sakamotob,
A. Nakamurab and
H. Yanagitac
aDepartment of Chemical Engineering and Materials Science, University of Hyogo, 2167 Shosha, Himeji 671-2280, Japan. E-mail: morisita@eng.u-hyogo.ac.jp
bGraduate Student of University of Hyogo, 2167 Shosha, Himeji 671-2280, Japan
cSanalloy Industry Co., Ltd, 290-44 Takahashi, Fukusaki-cho, Kanzaki 679-2216, Japan
First published on 14th April 2021
Abstract
Pt is an excellent and widely used hydrogen evolution reaction (HER) catalyst. However, it is a rare and expensive metal, and alternative catalysts are being sought to facilitate the hydrogen economy. As tungsten carbide (WC) has a Pt-like occupied density of states, it is expected to exhibit catalytic activity. However, unlike Pt, excellent catalytic activity has not yet been observed for mono WC. One of the intrinsic differences between WC and Pt is in their magnetic properties; WC is non-magnetic, whereas Pt exhibits high magnetic susceptibility. In this study, the WC lattice was doped with ferromagnetic Co nanocrystals to introduce an ordered-spin atomic configuration. The catalytic activity of the Co-doped WC was ∼30% higher than that of Pt nanoparticles for the HER during the hydrolysis of ammonia borane (NH3BH3), which is currently attracting attention as a hydrogen fuel source. Measurements of the magnetisation, enthalpy of adsorption, and activation energy indicated that the synergistic effect of the WC matrix promoting hydrolytic cleavage of NH3BH3 and the ferromagnetic Co crystals interacting with the nucleus spin of the protons was responsible for the enhanced catalytic activity. This study presents a new catalyst design strategy based on the concept of an internal magnetic field. The WC–Co material presented here is expected to have a wide range of applications as an HER catalyst.
Introduction
The development of catalysts for the hydrogen evolution reaction (HER) is critical for producing hydrogen fuel as a substitute to fossil fuels to reduce global CO2 emissions. Tungsten carbide (WC) has 10 valence electrons, 5d6 from W and 2p4 from C, similar to Pt (5d10). Since 1973, when Levy and Boudart1 proposed that WC exhibited singular catalytic activity similar to Pt, this topic has been investigated by many researchers. Bennett et al.2 measured the valence band spectrum of WC using X-ray photoelectron spectroscopy (XPS) and compared it with the spectra of W and Pt. Near the Fermi level (EF), the electronic density of states (DOS) of WC more closely resembled that of Pt3 than W.4 Houston5 compared the DOS of W, WC, and Pt using soft X-ray appearance potential spectroscopy (SXAPA). Contrary to the results of previous studies,3,4 the width of the unoccupied portion of the 5d band of W in WC was larger than in pure W.5 Another study6 showed that the differences between the DOS measured by SXAPA5 and predicted using a rigid band model1,2 resulted in different crystal structures of body centred cubic (bcc) W, face centred cubic (fcc) Pt, and hexagonal WC. Mattheiss and Hamann7 investigated the DOS of the bulk and (0001) surface of WC using a relativistic linear augmented-plane wave method.8 The DOS near the EF of the (0001) surface associated with the catalytic properties was larger than in the bulk. Recently, the electronic states of Pt were updated9 using the projector-augmented-plane wave method implemented in the VASP code8 using the generalised gradient approximation (Perdew–Burke–Ernzerhof version) (GGA-PBE),10 which clarified that EF is located at the top of the 5d DOS, consistent with its highest catalytic activity of all elements.Esposite et al.11 showed that a monolayer film of Pt deposited on a bulk WC substrate exhibited a similar electrochemical hydrogen overpotential, ηH2, to that of Pt in an aqueous H2SO4 solution, indicating that the surface electronic and chemical properties of monolayer Pt on a bulk WC substrate are significantly similar to those of bulk Pt. Zhang et al.12 synthesised a Fe3C/Co3C/WC/C carbide composite prepared by combining hydrothermal synthesis with resin impregnation and pyrolysis. Linear-sweep voltammetry of the Fe3C/Co3C/WC/C in an aqueous KOH solution exhibited similar behaviour to that of a Pt/C electrode, indicating that it is a candidate cathode material for polymer electrolyte membrane fuel cells (PEMFCs).13 In addition, a 7.5 wt% Pt/W2C catalyst showed a current density 2–3 times higher than that of a commercial 20 wt% Pt/C for electrolysis in H2SO4 aqueous solution due to the synergistic effect of Pt and WC.13 Good catalytic activity for the hydrogen evolution reaction (HER) via electrolysis of the H2SO4 aqueous solution was observed using a powder microelectrode composed of 16.20 wt% Pt/WC14 or a bimetallic carbide composed of Mo2C and WC.15 Zheng et al. demonstrated that PEMFCs using a Nafion membrane containing WC nanoparticles exhibited enhanced power density and durability over 100 h of use.16
The catalytic activities of WC and its composites have only been observed under conditions of an applied external voltage,11–16 and the predicted intrinsic catalytic activity of mono WC under voltage-free operation has not yet been verified. Although Levy and Boudart observed that WC catalysed the reduction of WO3 with hydrogen gas in the presence of water, and the isomerisation of 2,2-dimethylpropane to 2-methybutane, the rates were merely 0.37% and 0.01%, respectively, in comparison with the performance of Pt.1
Bennett et al.2 noted that one of the intrinsic differences between WC and Pt is their magnetic properties; WC is non-magnetic, whereas Pt exhibits high magnetic susceptibility. Cerri et al.17 studied the magnetic properties of polycrystalline Gd and found that hydrogen chemisorption induced a disordering of the electronic spin polarisation on the surface. An FTIR spectroscopy study confirmed that antiferromagnetic LaFeO3 accelerated the nucleus spin conversion of ortho liquid hydrogen (composed of two antiparallel nucleus spins) to para liquid hydrogen (composed of two parallel nucleus spins) to reach equilibrium.18 Furthermore, Galces-Pineda et al.19 observed that an external magnetic field accelerated oxygen evolution during the electrolysis of water.
An application of HER catalysts is the generation of hydrogen fuel from NH3BH3. In its stable crystal form, NH3BH3 contains 19.6 wt% hydrogen,20 and is being investigated for efficient transportation of hydrogen-based fuel for portable fuel-cell systems. Previous studies investigated the HER by hydrolysis over 10 wt% Co21 or 2 wt% Pt22 (both supported by Al2O3) and found that the HER in the latter was significantly faster than that in the former. A similar HER in NH3BH3(aq) catalysed by Ni nanoparticles (NPs) supported by a zeolite molecular sieve was observed.23
In this study, the WC lattice was doped with ferromagnetic Co nanocrystals (WC–Cocarbide)24 to introduce an ordered-spin configuration as an internal magnetic field to avoid the need for an external applied voltage. The catalytic performance of WC–Cocarbide for the HER in NH3BH3(aq) was compared with that of Pt nanoparticles (PT_NPs), commercial mono WC, and a bcc W–Co solid solution (W–Coalloy) in the spin glass state. The present study aims to investigate the (1) effect of an internal magnetic field instead of an external voltage on the catalytic activity; (2) kinetics of the HER; and (3) practical applications of the catalyst.
Materials and methods
The W–Coalloy powder consisting of a solid solution of Co-supersaturated bcc W was prepared using a hydrothermal synthesis method that we have described previously.24 The precursor materials were 99% ammonium tungstate pentahydrate (5(NH4)2O·12WO3·5H2O; Kanto Chemical Co. Inc., Japan) and 99% cobalt acetate tetrahydrate (Co(C2H3O2)2·4H2O; Kojundo Chemical Laboratory Co. Ltd, Japan), which were mixed to achieve a molar ratio of W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co = 80
Co = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. Briefly, W–Coalloy was carburised at 1173 K with a gas with a composition of 23 vol% CO2, 32 vol% H2, and 45 vol% Ar to form WC containing Co nanocrystals (WC–Cocarbide). In this study, CO2 was used as the carburisation gas instead of CO to increase the safety of the process. Since CO2 barely carburises the W–Coalloy based on the thermochemical equation, CO2 was converted into CO in situ in the furnace using 50
20. Briefly, W–Coalloy was carburised at 1173 K with a gas with a composition of 23 vol% CO2, 32 vol% H2, and 45 vol% Ar to form WC containing Co nanocrystals (WC–Cocarbide). In this study, CO2 was used as the carburisation gas instead of CO to increase the safety of the process. Since CO2 barely carburises the W–Coalloy based on the thermochemical equation, CO2 was converted into CO in situ in the furnace using 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mol% Fe–Al powder, where the Al thermally reduces CO2 into CO. Although 10.8 ks was shown to be a sufficient processing time for carburisation using CO,24 a total of 32.4 ks was required when CO2 was used. During this carburisation procedure, the samples were ground at each 10.8 ks interval.
50 mol% Fe–Al powder, where the Al thermally reduces CO2 into CO. Although 10.8 ks was shown to be a sufficient processing time for carburisation using CO,24 a total of 32.4 ks was required when CO2 was used. During this carburisation procedure, the samples were ground at each 10.8 ks interval.
The chemical composition and structure of WC–Cocarbide were confirmed by X-ray diffraction (XRD; Rigaku, Ultima IV) and electron probe microanalysis (EPMA; JOEL, JXA-8900) operated at a 15 kV accelerating voltage. The nanostructure of the Co crystal was observed by high-resolution transmission microscopy (HRTEM; JOEL, JEM-2100) operated at an accelerating voltage of 200 kV. The metallic state of the surface of the Co crystal was confirmed via XPS (ULVAC-PHI Inc., PHI5000) using monochromatic X-rays (Al Kα, 1486.6 eV). The specific surface area (SSA) of all samples was measured using the Brunauer–Emmett–Teller method (Shimadzu, TriStar II 3020) with the Kr physisorption isotherm obtained at 77 K.
A standard catalyst containing 1 wt% Pt_NPs on a carrier of Al2O3 particles (Catalysis Society of Japan)25 was used to obtain comparative data for the catalytic reaction. The SSA of the Pt was 3.40 m2 gcat−1 and the total SSA of the catalyst, including the Al2O3, was 176 m2 gcat−1.25 A commercial mono WC particle (Kojundo Chemical Laboratory Co. Ltd, Japan; 99%) was also used as a reference sample.
The WC–Cocarbide, W–Coalloy, Pt_NPs, and WC samples were flushed in a H2 atmosphere at 473 K for 2 h. Then, 20 mg of each sample was placed in a glass test tube with 1 ml of H2O. In addition, 0.5 mmol of NH3BH3(cr) was dissolved in 1.5 ml of H2O to form an aqueous solution of NH3BH3(aq) that was mixed with each of the aforementioned samples to evolve hydrogen by hydrolysis of the solution. Four repeat measurements of the hydrogen evolution volume (HEV) were performed for the WC–Cocarbide, W–Coalloy, and Pt_NPs samples, whereas only one measurement was considered sufficient for WC as it showed no catalytic activity.
The HER rate for each sample was evaluated by determining the slope of the HEV curve as a function of time using the least squares method. The average HER rates of the four repeat measurements are presented here. The 1-sigma error (68% confidence interval)26 was calculated by dividing the standard deviation (σ) of the four measurements by the average value.
To determine Ea, the HEV over WC–Cocarbide was measured at 308, 318, 328, and 338 K using a solution prepared with 1.0 mmol NH3BH3(cr) and 1.5 ml of H2O. A higher solution concentration than that used in the previous measurements was selected to achieve a longer reaction time and hence, more accurate Arrhenius plots. Three HER measurements were performed at each temperature and the average HER rate is presented. The 1-sigma error was evaluated by dividing 1σ of the three measurements by the average value at each temperature. The 1-sigma error was evaluated from the standard deviation of the 12 total measurements.
To determine the value of M of WC–Cocarbide, the magnetic hysteresis loop was measured using a SQUID instrument (QD, MPMS) under a magnetic field increased to a maximum of 3 × 104 Oe. Temperatures of 308 and 4 K were used to investigate the maximum value of M,27,28 respectively.
The standard enthalpies of adsorption of H2(g), ΔadH°m, for WC–Cocarbide, WC, and a commercial 99.9% Pt powder (Kojundo Chemical Laboratory Co. Ltd, Saitama) were measured at 423 K using a Calvet-type microcalorimetre (SETRAM, C80) in a H2 atmosphere.
Results and discussion
Hydrogen evolution over WC–Co
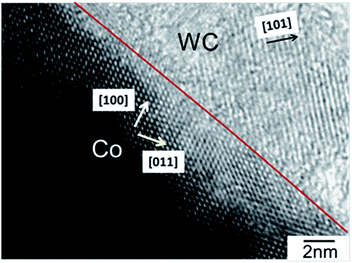
Fig. 1 shows a representative bright-field TEM image of a WC–Cocarbide sample. Co crystals with the fcc structure, [100] orientation, and diameter of 60 nm were observed in the WC matrix with [101] orientation. The atomic configuration was the same as that observed in our previous study.24 The SSA values of the WC–Cocarbide, W–Coalloy, and Pt_NPs samples are shown in Table 1. The WC–Cocarbide exhibited an SSA ∼ 60% smaller than that of the Pt_NPs, whereas WC–Cocarbide exhibited a smaller SSA than that of W–Coalloy.| Sample | SSA (m2 gcat−1) | NHER (H2 mol min−1 m−2) | N (NH3BH3(aq) mmol−1) | V (H2O mL−1) | T (K) |
|---|---|---|---|---|---|
| WC–Cocarbide | 1.35 | 3.76 ± 0.37 | 0.5 | 2.5 | 308 |
| WC–Cocarbide | 1.35 | 7.63 ± 0.32 | 1.0 | 2.5 | 308 |
| WC–Cocarbide | 1.35 | 12.14 ± 2.42 | 1.0 | 2.5 | 318 |
| WC–Cocarbide | 1.35 | 26.92 ± 3.13 | 1.0 | 2.5 | 328 |
| WC–Cocarbide | 1.35 | 46.91 ± 7.37 | 1.0 | 2.5 | 338 |
| W–Coalloy | 2.23 | 0.45 ± 0.11 | 0.5 | 2.5 | 308 |
| Pt_NPs | 3.40 | 2.90 ± 1.04 | 0.5 | 2.5 | 308 |
| Comm. WC | 1.03 | 0 | 0.5 | 2.5 | 308 |
| Comm. Pt | 0.12 | — | — | — | — |
Fig. S1 (see ESI†) shows the XRD results. Only peaks related to the WC matrix and Co crystals were observed. Fig. S2 (see ESI†) shows the Co 2p XPS spectrum of WC–Cocarbide. The peak at 778.4 eV was assigned to the metallic state Co0. Fig. S3 (see ESI†) shows representative EPMA/SEM images of W Lα, C Kα, and Co Kα for the WC–Cocarbide particle. Because W, C, and Co were distributed homogeneously, HRTEM observation (Fig. 1) was necessary to distinguish the Co crystals from the WC matrix.
Fig. 2 shows the change in the average HEV over time (t) for hydrolysis of the NH3BH3(aq) over WC–Cocarbide, W–Coalloy, Pt_NPs, and commercial WC samples. The slope of the HEV(t) curve for WC–Cocarbide was steeper than that for Pt_NPs, indicating that WC–Cocarbide exhibited singular catalytic activity, whereas W–Coalloy exhibited less activity.
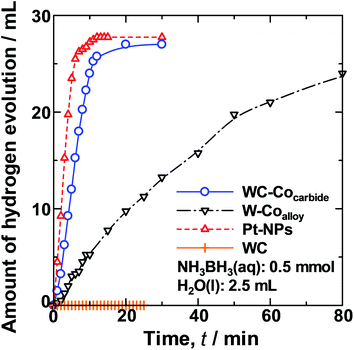 | ||
| Fig. 2 Comparison of the HEV over time from hydrolysis of a NH3BH3(aq) solution (0.5 mmol, 2.5 ml) at 308 K using the various catalyst samples. | ||
The normalised HER rates per unit area, NHER (H2 mol min−1 m−2), determined by normalising the HEV by the SSA values are shown in Table 1, along with their error values. The NHER of WC–Cocarbide was ∼30% higher than that of Pt_NPs, indicating that it exhibits singular catalytic activity similar to Pt. The NHER of W–Coalloy was only approximately 10% of that of WC–Cocarbide.
Activation energy
To clarify the mechanism of the singular catalytic activity of WC–Cocarbide, its Ea value was investigated. Fig. 3 shows the HEV as a function of time during the hydrolysis of NH3BH3(aq) over WC–Cocarbide at 308, 318, 328, and 338 K. Comparing the data measured at 308 K to the corresponding curve in Fig. 2, the HEV at the same time was consistent with doubling the NH3BH3 concentration. Hence, a large number of reaction sites were available for studying the HER. The slopes of the HEV(t) curves increased with increasing temperature, indicating that the hydrolysis was a result of a thermally activated process.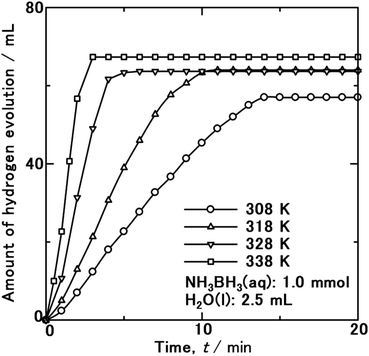 | ||
| Fig. 3 HEV from hydrolysis of NH3BH3 aqueous solution (1 mmol, 2.5 ml) using WC–Cocarbide as a catalyst at various temperatures. | ||
The Ea is defined by the Arrhenius equation:
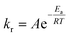 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kr vs. T−1). The ln
kr vs. T−1). The ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kr values decreased linearly as a function of T−1, where Ea was calculated from the slope of this curve. The Ea and A values for the HER over WC–Cocarbide were 54.0 ± 13.0 kJ mol−1 and 1.01 × 1010 ± 2.44 × 109 mol m−2 min−1, respectively, where the uncertainty was evaluated as the error propagation26 of the uncertainties of the NHER data at each temperature listed in Table 1. This Ea value was larger than the literature value for Pt NPs (2 wt% Pt on an Al2O3 support)22 and similar to that of Co NPs (10 wt% Co on an Al2O3 support),21 indicating that the hydrogen was released from the surface of the Co nanocrystals in the WC matrix.
kr values decreased linearly as a function of T−1, where Ea was calculated from the slope of this curve. The Ea and A values for the HER over WC–Cocarbide were 54.0 ± 13.0 kJ mol−1 and 1.01 × 1010 ± 2.44 × 109 mol m−2 min−1, respectively, where the uncertainty was evaluated as the error propagation26 of the uncertainties of the NHER data at each temperature listed in Table 1. This Ea value was larger than the literature value for Pt NPs (2 wt% Pt on an Al2O3 support)22 and similar to that of Co NPs (10 wt% Co on an Al2O3 support),21 indicating that the hydrogen was released from the surface of the Co nanocrystals in the WC matrix.
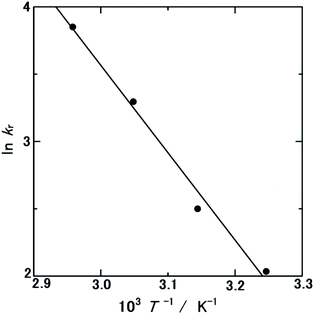 | ||
Fig. 4 ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kr versus T−1 plot calculated from Table S1.† kr versus T−1 plot calculated from Table S1.† | ||
The catalytic activity of WC–Cocarbide was evaluated considering the hydrogen overpotential (ηH2). The ηH2of Pt is 0 V by definition, whereas for Co it is in the range of −0.25 to −0.47 V in 1 M H2SO4 aqueous solution at 303 K under a current density of 0.3–10 mA cm−2.29 Chandra and Xu22 determined Ea (21 kJ mol−1) of the HER in NH3BH3(aq) over Pt NPs.22 The difference in the activation energy, ΔEa, between WC–Cocarbide and Pt NPs22 was 33 kJ mol−1, which corresponds to −0.34 V, consistent with the ηH2 of Co.29 This analysis indicated that the hydrogen release sites were the Co nanocrystals in the WC matrix. In general, ηH2 is defined as the complex energies consisting of the elementary processes: (I) alignment of H+(aq) on the electrode; (II) accepting electrons to form the radical hydrogen atom H(rad.); (III) bonding two H(rad.) to form a H2 molecule; (IV) convergence of the radicals to form hydrogen gas H2(g); and (V) desorption of H2(g) from the electrode. In this study, the separation of these elementary processes of ηH2 was impossible as Ea was determined only from a simple Arrhenius plot. Further computational simulations are necessary to clarify this point. The A value is discussed later with respect to the thermodynamic cycle.
Magnetic behaviour
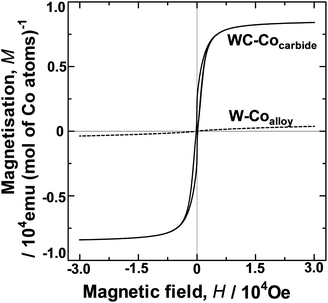
The HER over the W–Coalloy powder was not enhanced as observed for WC–Cocarbide (Fig. 2), indicating that H2(g) is not readily evolved over the Co atoms substituted into the bcc W lattice. However, the HER was enhanced in the case of the WC–Cocarbide sample with Co atoms configured in the nanocrystal domain. The most likely difference between these configurations is related to their magnetic properties. Hence, M was investigated to clarify the different HER results. Fig. 5 shows M as a function of the magnetic field (H) for WC–Cocarbide and W–Coalloy at 4 K. WC–Cocarbide showed a clear hysteresis loop, indicating that it is a ferromagnetic material. Assuming that the value of M for WC–Cocarbide resulted from the Co crystals, the maximum saturated value, MS, was 8.41 × 103 emu (mol Co atoms)−1. However, the value of M for W–Coalloy was close to zero, indicating that it had a spin glass state.30 Similarly, the M (H) data were measured at 308 K, yielding similar results to those at 4 K for both samples.The magnetic moment β of a Co atom in the Co nanocrystals in WC–Cocarbide at 4 K and 308 K were 1.50 and 1.42 μB per Co atom, respectively, where μB is the unit of the Bohr magneton. In the elemental Co crystal, the value of β for a Co atom is 1.7 μB.27 Hence, the Co nanocrystals had a β value close to that of elemental Co. In contrast, the β value of a Co atom substituted into the bcc W lattice in the W–Coalloy measured at 4 K and 308 K was 6.79 × 10−2 and 7.48 × 10−3 μB, respectively. As described in our previous study,24 the XRD pattern of W–Coalloy showed only the peaks of W.24 The composition of W–Coalloy corresponds to the two phase equilibria region of the W phase and the intermediate phase of Co7W6 in the phase diagram of the W–Co binary system.31 Co7W6 has complicated long-range periodic structure.32 Formation of the complicated long-range periodic structures such as the sigma phase in the heat-resistant alloys takes a lot of time to accomplish their atomic configuration.33 Hence, the equilibrium phase of Co7W6 was not formed during reduction with H2 gas. The Co atoms are concluded to be supersaturated in the W lattice as a nonequilibrium state. Consequently, the spin configuration among isolated Co atoms was random (i.e., a spin glass state30). Hence, the spin-ordered state of the Co in WC–Cocarbide appears to be one of the factors determining the singular catalytic activity.
Cerri et al.17 found that hydrogen chemisorption induced a disordering of electronic spin polarisation on the surface of ferromagnetic Gd, resulting in a change in M, and the Curie temperature, TC. It is likely that hydrogen chemisorption relaxes the spin configuration in the system. In aqueous solutions, the spin configuration of the protons is relevant. Here, the protons are likely to be absorbed on the ferromagnetic Co nanocrystals, such that their nucleus spin configurations are aligned to be antiparallel to relax the spin polarisation of the surface.
Thermodynamic cycle
The following equations show the thermodynamic equilibria among the species associated with the HER from hydrolysis of NH3BH3(cr), where the standard enthalpies of formation, ΔfH°m, at 298.15 K of the standard substances of NH3BH3(cr),34 H2O(l),35 ammonia (NH3(aq)),35 orthoboric acid (B(OH)3(aq)),35 ammonium (NH4+(aq)),35 metaboric acid (BO2−(aq)),36 and H2(g)35 are summarised in Table S1.† Eqn (2) shows the hydration reaction of NH3BH3(cr), where the thermodynamic value is unknown. Eqn (3) shows the HER of the hydrolysis of NH3BH3(aq). Eqn (4), rewritten as the sum of eqn (2) and (3), indicates the HER from the initial substance of NH3BH3(cr). Eqn (5) shows the formation of NH4+(aq). Eqn (6) shows the formation of BO2−(aq). Finally, eqn (7), rewritten as the sum of eqn (4)–(6), shows the final state of the hydrolysis.| NH3BH3(cr) = NH3BH3(aq) | (2) |
| NH3BH3(aq) + 3H2O(l) = NH3(aq) + B(OH)3(aq) + 3H2(g) | (3) |
| NH3BH3(cr) + 3H2O(l) = NH3(aq) + B(OH)3(aq) + 3H2(g) | (4) |
| ΔrH°/kJ = 3 × ΔfH°m(H2(g)) + ΔfH°m(NH3(aq)) + ΔfH°m(B(OH)3(aq)) − ΔfH°m(NH3BH3(cr)) − 3 × ΔfH°m(H2(g)) = −118.494 ± 5.921 |
| NH3(aq) + H+(aq) = NH4+(aq), ΔrH°/kJ = −52.090 ± 0.411 | (5) |
| B(OH)3(aq) = BO2−(aq) + H2O(l) + H+(aq), ΔrH°/kJ = 14.600 ± 0.980 | (6) |
| NH3BH3(cr) + 2H2O(l) = BO2−(aq) + NH4+(aq) + 3H2(g), ΔrH°/kJ = −155.983 ± 6.016 | (7) |
Since eqn (5)–(7) are spontaneous reactions, the HER is given by eqn (4). As the standard entropy, S°m, of NH3BH3(cr) has not yet been measured, the standard entropy of reaction, ΔrS°, and the standard Gibbs energy of reaction, ΔrG°, are unknown. However, ΔrG° is more negative than ΔrH° as the HER increases ΔrS°. Therefore, when a driving energy is applied corresponding to the hydrogen overpotential of Co, the HER reaches equilibrium, as defined by eqn (7) via eqn (4).
In previous studies, Co NPs (10 wt% Co on an Al2O3 support)21 had an HER rate 10 times lower than that of Pt NPs (2 wt% Pt on an Al2O3 support).22 However, in the present study, the HER rate of WC–Cocarbide was 30% higher than that of Pt_NPs, even if Ea corresponds to the ηH2 of Co. The excellent catalytic activity was a result of the high A value, as determined from the Arrhenius plot (Fig. 4). The WC matrix seems to facilitate the release of H+(aq) from NH3BH3(aq) and contribute to increasing A (i.e., the number of H+(aq) collisions). In accordance with the electron theory, the Pt-like high DOS near the EF of the surface of WC7 can induce adsorption of NH3BH3 molecules, which can promote the decomposition of B–N bonds to form NH3(aq), B(OH)3(aq), and H2(g) via highly unstable BH3(aq) close to the equilibrium states (see eqn (3)). Furthermore, considering the thermodynamic hierarchy, as shown in Table S1,† the ΔfG°m of WB37 is smaller than that of WC,38 indicating that the chemical bonding between the W and B atoms is more stable than that between the W and C atoms. In addition, it is well known that natural tungsten ore consists of ammonium tungstate,39 which is the starting material used in the present study, indicating the high affinity between tungsten and ammonia. Therefore, W in the WC matrix has a driving force for attracting NH3BH3(aq), resulting in preferential adsorption. Although a previous study20 investigated the hydrolysis kinetics of NH3BH3 using first principles calculations based on the transition state theory and estimated the atomic distance of a B–N bond, the interaction between the WC matrix and NH3BH3(aq) should be further investigated by first principles and molecular dynamics calculations. The BH3(aq) molecule can release three protons while bonding with three OH− ions to form B(OH)3. As shown in Table 1, when the amount of NH3BH3(aq) was doubled, NHER also doubled, indicating that there were sufficient HER reaction sites. Hence, the WC matrix played a crucial role in adsorbing NH3BH3 molecules and decomposing B–N bonds, followed by supplying protons to the Co crystals.
Specific enthalpy of hydrogen adsorption
The ΔadH°m values of WC–Cocarbide, and commercial WC and Pt powders at 423 K were −22.42 ± 0.90, −21.87 ± 0.87, and −109.72 ± 4.39 J m−2, respectively. We found that the ΔadH°m of WC was less exothermic than that of Pt, implying that the desorption of H2(g) from the surface of WC was not difficult. Previous studies have investigated the catalytic activity of WC13 and related carbide composites11,12 under an external voltage. When an external voltage is applied, the H+(aq) ions align on the surface of WC, followed by hydrogen evolution. However, when no external voltage is applied, such an alignment of H+(aq) is unlikely to be significant. In contrast, on the surface of WC–Cocarbide, the H+(aq) ions seem to align as a result of the magnetic field of the Co nanocrystals, followed by hydrogen evolution. The ΔadH°m of Pt is extremely high, indicating that hydrogen was stored in its lattice. Pt simultaneously achieves the adsorption, storage, and desorption of hydrogen species, although the specific mechanism is still unknown.Mechanism of a singular catalytic behaviour of WC–Cocarbide
Fig. 6 shows a schematic of the catalytic HER over WC–Cocarbide, which involves the following steps. (1) The NH3BH3 molecules are absorbed on the WC matrix by the attractive interaction of W–B bonds; (2) the B–N bonds in NH3BH3(aq) are broken to form stable NH3(aq) and unstable BH3(aq); (3) the B atoms in BH3(aq) with a significantly short lifetime release three quasi-stable protons and coordinate three OH−(aq) ions from the surrounding H2O molecules to form stable B(OH)3 molecules, wherein the H2O releases three protons; (4) sufficient amounts of protons from BH3(aq) and H2O are adsorbed on the ferromagnetic Co nanocrystals in the WC matrix, resulting in antiparallel alignment of the nucleus spin configurations of the two H+(aq) to relax the spin polarisation of the system; (5) hydrogen molecules are evolved as the protons accept electrons, e−, such that Ea is consistent with ηH2 of Co, wherein Co is protected by galvanic protection;40 (6) one e− is released during the decomposition of BH3, along with a proton, while the other e− is released from OH−(aq) during coordination to form B(OH)3(aq), i.e. the origin of charge transfer is the B atom adsorbed on the W atom, wherein the charge is transferred to Co via the WC matrix; (7) the WC matrix decomposing NH3BH3(aq) and generating H+(aq) applies a driving energy corresponding to the necessary hydrogen overpotential, wherein the WC matrix supplies excess protons to the Co sites.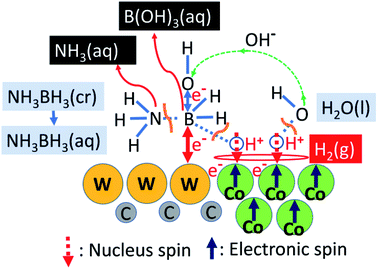 | ||
| Fig. 6 Schematic of hydrogen evolution from the hydrolysis of NH3BH3(aq) over the WC–Cocarbide catalyst. | ||
The relative volume ratio of the WC matrix vs. Co nanocrystals is estimated to be 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 from the densities of pristine WC and Co. When the relative SSA ratio of the WC matrix vs. Co nanocrystals is hypothetically equal to the relative volume ratio, the SSA of the Co nanocrystals is 0.15 m2 gcat−1. Assuming the Co nanocrystals only contribute HER, NHER is evaluated as 33.84 (H2 mol min−1 m−2) which is twelve times faster than that of Pt_NPs (=2.90 (H2 mol min−1 m−2)). Such an extremely fast NHER is never caused by the single Co nanocrystals. Hence, the Pt-like catalytic activity is attributed to the synergistic effect of the WC matrix and ferromagnetic Co nanocrystals.
13 from the densities of pristine WC and Co. When the relative SSA ratio of the WC matrix vs. Co nanocrystals is hypothetically equal to the relative volume ratio, the SSA of the Co nanocrystals is 0.15 m2 gcat−1. Assuming the Co nanocrystals only contribute HER, NHER is evaluated as 33.84 (H2 mol min−1 m−2) which is twelve times faster than that of Pt_NPs (=2.90 (H2 mol min−1 m−2)). Such an extremely fast NHER is never caused by the single Co nanocrystals. Hence, the Pt-like catalytic activity is attributed to the synergistic effect of the WC matrix and ferromagnetic Co nanocrystals.
Previous studies have determined the Ea values for the HER in NH3BH3(aq) of 21 kJ mol−1 over Pt NPs22 and 62 kJ mol−1 over Co NPs,21 and suggested that the rate determining step (RDS) was the cleavage of the B–N bonds, as unstable BH3 reacts with H2O to form H2. In addition, Wang et al.23 determined an Ea value of 42.7 kJ mol−1 for the HER in NH3BH3(aq) catalysed by Ni NPs, and suggested that the RDS was the cleavage of the O–H bond in H2O (Ea = 493 kJ mol−1), as the equilibrium bonding energy was more endothermic than that of the B–N (Ea = 117 kJ mol−1) and B–H (430 kJ mol−1) bonds. The ΔEa between Co NPs21 and Pt NPs22 and that between Ni NPs23 and Pt NPs22 were 41 and 21.7 kJ mol−1, corresponding to −0.43 and −0.22 V, respectively, thereby demonstrating consistency with the ηH2 of Co and Ni.29 These Ea results support the mechanism depicted in Fig. 6.
Another definition of ηH2 in electrochemistry is the HER current density, i0, determined by extrapolating the cathodic Tafel line to the reference hydrogen electrode.41 Fig. S4† shows the correlation between magnetic susceptibility, X,42 and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i0
i0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 of the transition metals of the fourth, fifth, and sixth periods (rows) of the periodic table of the elements. In the sixth period, the log
41 of the transition metals of the fourth, fifth, and sixth periods (rows) of the periodic table of the elements. In the sixth period, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i0 value of Pt was large, consistent with the high HER catalytic activity, while X was large, corresponding to a high log
i0 value of Pt was large, consistent with the high HER catalytic activity, while X was large, corresponding to a high log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i0. Similarly, in the fifth period, the log
i0. Similarly, in the fifth period, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i0 values and X values of Rh and Pd were large. In the fourth period, the log
i0 values and X values of Rh and Pd were large. In the fourth period, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i0 values of Fe, Co, and Ni were high. As these metals are ferromagnetic with spontaneous magnetisation, their X values are not relevant. However, their spin-ordered ferromagnetic state likely increased their log
i0 values of Fe, Co, and Ni were high. As these metals are ferromagnetic with spontaneous magnetisation, their X values are not relevant. However, their spin-ordered ferromagnetic state likely increased their log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i0 values. In contrast, the diamagnetic transition metals of Au and Hg in the sixth period, Ag and Cd in the fifth period, and Cu and Zn in the fourth period with negative X values showed small log
i0 values. In contrast, the diamagnetic transition metals of Au and Hg in the sixth period, Ag and Cd in the fifth period, and Cu and Zn in the fourth period with negative X values showed small log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i0 values consistent with their lower HER catalytic activities. Therefore, we conclude that the magnetic properties of the transition metals are related to the HER catalytic activity. Materials with high X values are likely to interact readily with the nucleus spin of H+(aq). Ferromagnetism seems to induce alignment of the nucleus spin of H+(aq) on the ferromagnetic metal surface, as discussed with regard to the contribution of the Co nanocrystals in this study. After formation of two radical H atoms by donation of electrons, the spin conversion of the two H atoms should form the 1σg molecular orbital of a H2 molecule. The magnetic properties of metals are likely to contribute to the spin conversion. After H2 molecules are formed, they are released from the surface of the metals with a high positive X value or ferromagnetism because H2 is diamagnetic with a negative X value (−19.7 × 10−9 m3 kg−1).
i0 values consistent with their lower HER catalytic activities. Therefore, we conclude that the magnetic properties of the transition metals are related to the HER catalytic activity. Materials with high X values are likely to interact readily with the nucleus spin of H+(aq). Ferromagnetism seems to induce alignment of the nucleus spin of H+(aq) on the ferromagnetic metal surface, as discussed with regard to the contribution of the Co nanocrystals in this study. After formation of two radical H atoms by donation of electrons, the spin conversion of the two H atoms should form the 1σg molecular orbital of a H2 molecule. The magnetic properties of metals are likely to contribute to the spin conversion. After H2 molecules are formed, they are released from the surface of the metals with a high positive X value or ferromagnetism because H2 is diamagnetic with a negative X value (−19.7 × 10−9 m3 kg−1).
Recently, it was shown that an external magnetic field enhanced the oxygen evolution reaction (OER) during electrolysis of a KOH aqueous solution.19 A Pt plate was used as the cathode and ferrite NiZnFe4Ox (deposited on Ni foam by direct magnetic interaction between the materials) was used as the anode. An external magnetic field of 0.45 T was applied to the anode using a rare-earth permanent magnet, which resulted in the OER current almost doubling (from 24 to 40 mA cm−2 at ≥1.65 V).19 They hypothesised that the external voltage accelerated the spin conversion to form the paramagnetic triplet of O2(g). Li et al.43,44 prepared a series of metal–organic frameworks (MOFs) composed of the Fe–Ni binary43 and W–Co–Fe ternary system44 as electrocatalysts. Their superior catalytic properties appear to result from the magnetic elements of Fe,43,44 Ni43, and Co.43,44 These findings are highly relevant to the present study.
There have been many investigations of the electronic states of HER.29 However, the interaction between the nucleus spin of H+(aq) and electronic spin of metals has not yet been investigated. Thomas et al.45,46 calculated the energy eigenvalues of the electrons and nuclei of hydrogen atoms (protons) by considering the wave functions of both protons and electrons, and describing the zero-point motion of the protons by a Slater-type wave function. This approach could be applied to the HER catalytic reaction.
As described above with respect to the thermodynamics, the WC matrix appears to facilitate the release of H+(aq) from NH3BH3(aq) and contribute to increasing A (i.e., the number of collisions between H+(aq) ions). However, H2(g) appears to be barely formed by such collisions. The alignment of H+(aq) on the catalyst surface and the donation of an electron to the ion are necessary. When an external electric voltage is applied to WC, these requirements are likely to be satisfied, resulting in the catalytic activity observed in previous studies.11–16 The internal magnetic field is considered sufficient to induce catalytic activity without requiring an external voltage.
Pt seems to easily adsorb NH3BH3(aq) with the highest DOS at the EF of all elements, and easily evolves H2(g) from its surface with the smallest ηH2 of any known element. A study of the interaction between Pt with a high magnetic susceptibility and protons with a nucleus spin appears to be a necessary future work to consider the effect of the magnetic field from the earth on the spin alignment of the protons.
Pt catalysts have low reusability in HER from HCOOH due to CO poisoning. WC–Cocarbide is advantageous for avoiding CO poisoning due to that it is prepared by carburizing with CO via CO2. Reusability of WC–Cocarbide should be further investigated.
Conclusion
The catalytic activities of WC and its composites had previously only been observed under the conditions of an applied external voltage, and the predicted intrinsic catalytic activity of mono WC without an external voltage had not been verified. We hypothesised that the introduction of an internal magnetic field would provide conditions similar to those provided by the external voltage to enhance the catalytic performance of WC. To this end, the WC lattice was doped with ferromagnetic Co nanocrystals to introduce an ordered-spin configuration as an internal magnetic field. The internal magnetic field successfully increased the HER rate during hydrolysis of NH3BH3 to a value even higher than that of the Pt nanoparticles. The enhanced catalytic activity was attributed to the synergistic effect of the WC matrix promoting hydrolytic cleavage of NH3BH3 and the ferromagnetic Co crystals interacting with the nucleus spin of the protons. Our new strategy for enhancing the catalytic performance by introducing an internal magnetic field is expected to provide new opportunities for developing novel catalysts, such as those for hydrolysis of NH3BH3 and the electrodes of fuel cells.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by an academic research grant project provided by the Hyogo Science and Technology Association.Notes and references
- R. B. Levy and M. Boudart, Science, 1973, 181, 547 CrossRef CAS PubMed.
- L. H. Bennett, J. R. Cuthill, A. J. McAlister and N. E. Erickson, Science, 1974, 184, 563 CrossRef CAS PubMed.
- J. B. Ketterson, F. M. Mueller and L. R. Windmiller, Phys. Rev., 1969, 186, 656 CrossRef CAS.
- L. F. Matteiss, Phys. Rev., 1965, 139, 1893 CrossRef.
- J. E. Houston, G. E. Laramore and R. L. Park, Science, 1974, 185, 258 CrossRef CAS PubMed.
- L. H. Bennett, J. R. Cuthill, A. J. McAlister and N. E. Erickson, Science, 1975, 187, 858 CrossRef CAS PubMed.
- L. F. Mattheiss and D. R. Hamann, Phys. Rev. B: Condens. Matter Mater. Phys., 1984, 15, 1731 CrossRef.
- P. Blaha, K. Schwarz, F. Tran, R. Laskowski, G. K. H. Madsen and L. D. Marks, J. Chem. Phys., 2020, 152, 074101 CrossRef CAS PubMed.
- X. Zhang, B. Grabowski, C. Freysoldt, F. Körmann and J. Neugebauer, Phys. Rev. B, 2017, 95, 165126 CrossRef.
- P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef PubMed.
- D. V. Esposite, S. T. Hunt, A. L. Stottlemyer, K. D. Dobson, B. E. McCandless, R. W. Birkmire and J. G. Chen, Angew. Chem., Int. Ed., 2010, 49, 9859 CrossRef PubMed.
- J. Zhang, J. Chen, Y. Jiang, F. Zhou, G. Wang, R. Wang and R. Wang, Appl. Surf. Sci., 2016, 389, 157 CrossRef CAS.
- D. J. Ham, R. Ganesan and J. S. Lee, Int. J. Hydrogen Energy, 2008, 33, 6865 CrossRef CAS.
- C. Ma, J. Sheng, N. Brandon, C. Zhang and G. Li, Int. J. Hydrogen Energy, 2007, 32, 2824 CrossRef CAS.
- P. Xiao, X. Ge, H. Wang, A. Liu, A. Fisher and X. Wang, Adv. Funct. Mater., 2015, 25, 1520 CrossRef CAS.
- W. Zheng, L. Wang, F. Deng, S. A. Stephen, A. Gilles, A. K. Prasad, S. G. Advani, Y. Yan and D. G. Vlachos, Nat. Commun., 2017, 8, 418 CrossRef PubMed.
- A. Cerri, D. Mauri and M. Landolt, Phys. Rev. B: Condens. Matter Mater. Phys., 1983, 27, 6526 CrossRef CAS.
- T. Das, S. Kweon, J. Choi, S. Y. Kim and I. Oh, Int. J. Hydrogen Energy, 2015, 40, 383 CrossRef CAS.
- F. A. Garces-Pineda, M. Blasco-Ahicart, D. Nieto-Castro, N. Lopez and J. R. Galan-Mascalos, Nat. Energy, 2019, 4, 519 CrossRef CAS.
- T. Banu, T. Debnath, T. Ash and A. K. Das, J. Chem. Phys., 2015, 143, 194305 CrossRef PubMed.
- Q. Xu and M. Chandra, J. Power Sources, 2006, 163, 364 CrossRef CAS.
- M. Chandra and Q. Xu, J. Power Sources, 2007, 168, 135 CrossRef CAS.
- C. Wang, J. Tuninetti, Z. Wang, C. Zheng, R. Ciganda, L. Salmon, S. Moya, J. Ruiz and D. Astruc, J. Am. Chem. Soc., 2017, 139, 11610 CrossRef CAS PubMed.
- M. Morishita, H. Yamamoto, T. Okahira, M. Yoshioka, N. Fukumuro, H. Matsuda, M. Ikebe, M. Iwasaki, H. Yanagida and H. Nishimaki, J. Am. Ceram. Soc., 2012, 95, 3797 CrossRef CAS.
- K. Nakai and K. Nakamura, Proceedings of the 22nd reference catalyst forum, 2001, p. 31 Search PubMed.
- J. R. Taylor, An Introduction to Error Analysis: The Study of Uncertainty in Physical Measurement, Oxford University Press, Oxford, 1982, p. 57 Search PubMed.
- S. Chikazumi, Physics of Ferromagnetism Vol. 1: Magnetic Properties of Matter, Shokabo, Tokyo, 22nd edn, 2007, pp. 7, 11, 47, 184 Search PubMed.
- C. Kittel, Introduction to Solid State Physics, John Wiley & Sons. Inc., New York, 6th edn, 1986, p. 426 Search PubMed.
- H. Ezaki, M. Morinaga and S. Watanabe, Electrochim. Acta, 1993, 38, 557 CrossRef CAS.
- V. Cannela and J. A. Mydosh, Phys. Rev. B: Solid State, 1972, 6, 4220 CrossRef.
- S. V. Nagender Naidu, A. M. Sriramamurthy and P. Rama Rao, Co–W, in Binary Alloy Phase Diagrams, ed. T. B. Massalski, ASM International: The Materials Information Society, Materials Park, OH, 2nd edn, 1990, pp. 1257–1259 Search PubMed.
- P. Villars and L. D. Calvert, Co7W6, in Peason's Handbook of Crystallographic Data for Intermetallic Phases, ed. P. Villars and L. D. Calvert, ASM, Meterials Park, OH, 1985, vol. 2, p. 1845 Search PubMed.
- M. Morinaga, N. Yukawa and H. Ezaki, Solid Solubilities in Transition Metal-Based f.c.c. Alloys, Philos. Mag. A, 1985, 51, 223 CrossRef CAS.
- Y. K. Shaulov, G. O. Shmyreva and S. Tubyanskaya, Zh. Fiz. Khim., 1966, 40, 122 CAS.
- H. Gamsjäger, J. Bugajski, T. Gajda, R. J. Lemire and W. Preis, Chemical Thermodynamics of Nickel, in Chemical Thermodynamics, Nuclear Energy Agency, Organization for Economic Co-operation, Development, Elsevier Science Publisher B.V., Amsterdam, 2005, vol. 6, p. 43 Search PubMed.
- D. D. Wagman, W. H. Evans, V. B. Parker, R. H. Schumm, I. Halow, S. M. Bailey, K. L. Churney and R. L. Nuttall, J. Phys. Chem., 1982, 11, 123 Search PubMed.
- H. Yamamoto, M. Morishita and S. Hiramatsu, Metall. Mater. Trans. B, 2017, 48, 1703 CrossRef CAS.
- I. Barlin, Thermochemical Data of Pure Substance, VCH Verlag, part 1, 1989, p. 1642 Search PubMed.
- T. Azakami, Y. Awakura and M. Itagaki, Extractive Metallurgy, Japan Inst. Met., Maruzen, Tokyo, 1999, p. 167 Search PubMed.
- M. Morishita, K. Koyama, M. Murase and Y. Mori, ISIJ Int., 1996, 36, 714 CrossRef CAS.
- H. Kita and K. Uozaki, Introduction to Electrochemistry, Gihoudo, Tokyo, 1987, p. 239 Search PubMed.
- Metals Data Book, ed. S. Nagasaki, Japan Inst. Met., Maruzen, Tokyo, 1993, pp. 18–19 Search PubMed.
- Y. Li, M. Lu, Y. Wu, Q. Ji, H. Xu, J. Gao, G. Qian and Q. Zhang, J. Mater. Chem. A, 2020, 8, 18215 RSC.
- Y. Li, T. Zhao, M. Lu, Y. Wu, Y. Xie, H. Xu, J. Gao, J. Yao, G. Qian and Q. Zhang, Small, 2019, 15, 1901940 CrossRef CAS PubMed.
- I. L. Thomas, Phys. Rev., 1969, 185, 90 CrossRef CAS.
- I. L. Thomas and H. W. Joy, Phys. Rev. A, 1970, 2, 1200 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra01181b |
| This journal is © The Royal Society of Chemistry 2021 |