Open Access Article
Open Access ArticleSpirocyclic derivatives as antioxidants: a review†
Karen Acosta-Quiroga‡
a,
Cristian Rojas-Peña‡a,
Luz Stella Nerio a,
Margarita Gutiérrez
a,
Margarita Gutiérrez *b and
Efraín Polo-Cuadrado
*b and
Efraín Polo-Cuadrado *b
*b
aUniversidad de la Amazonia, Programa de Química, Cl. 17 Diagonal 17 con, Cra. 3F, Florencia 180001, Colombia
bLaboratorio Síntesis Orgánica y Actividad Biológica, Instituto de Química de Recursos Naturales, Universidad de Talca, Casilla 747, Talca 3460000, Chile. E-mail: mgutierrez@utalca.cl; epolo@utalca.cl
First published on 21st June 2021
Abstract
In recent years, spiro compounds have attracted significant interest in medicinal chemistry due to their numerous biological activities attributed primarily to their versatility and structural similarity to important pharmacophore centers. Currently, the development of drugs with potential antioxidant activities is of great importance since numerous investigations have shown that oxidative stress is involved in the development and progression of numerous diseases such as cancer, senile cataracts, kidney failure, diabetes, high blood pressure, cirrhosis, and neurodegenerative diseases, among others. This article provides an overview of the synthesis and various antioxidant activities found in naturally occurring and synthetic spiro compounds. Among the antioxidant activities reviewed are DPPH, ABTS, FRAP, anti-LPO, superoxide, xanthine oxidase, peroxide, hydroxyl, and nitric oxide tests, among others. Molecules that presented best results for these tests were spiro compounds G14, C12, D41, C18, C15, D5, D11, E1, and C14. In general, most active compounds are characterized for having at least one oxygen atom; an important number of them (around 35%) are phenolic compounds, and in molecules where this functional group was absent, aryl ethers and nitrogen-containing functional groups such as amine and amides could be found. Recent advances in the antioxidant activity profiles of spiro compounds have shown that they have a significant position in discovering drugs with potential antioxidant activities.
Introduction
Spiro compounds, molecules containing two rings with only one shared atom, are an essential class of synthetic or naturally occurring substances. Their good balance between conformational restriction and flexibility makes them free from absorption and permeability issues, characteristic of conformationally more flexible linear molecules, and more flexible compared to aromatic heterocycles, adapting to many biological targets attractive for new drug discovery projects.1,2 Although spirocyclic systems are unusual scaffolds for bioactive molecules, recent progress on the isolation and characterization of new compounds from natural products and new synthetic routes to spiro building blocks have facilitated their incorporation into more molecules with pharmacological applications, such as antidiabetic, anticancer, anti-Alzheimer's, and in some cases, they have been successfully developed as commercial drugs.2–8 It has also been found that an important number of spiro compounds act as antioxidants (AO), substances that may prevent damage caused by reactive oxygen species (ROS) and reactive nitrogen species (RNS). ROS and RNS are generated in living cells, either naturally or due to some biological dysfunction such as stress. These compounds have essential roles in cell signaling, but many of these compounds are harmful to cells and their significant components, including DNA, enzymes, cell membrane lipids, and proteins.9 Thus, AOs are helpful as scavengers of free radicals, inhibiting oxidative degradation, such as protein oxidation, lipid oxidation, DNA oxidation, and glycoxidation, and processes associated with the development of neurodegenerative diseases, metabolic syndromes, atherosclerosis, infections, and cancer. In this way, the isolation and synthesis of molecules with AO properties for exogenous supplementation are promising ways of combating the undesirable effects of ROS and RNS-induced oxidative damage.In order to characterize the AO properties of a compound or extracts, two parameters should be assessed: AO activity (rate constant of a reaction between an AO and an oxidant) and AO capacity (amount of a particular free radical captured by an AO sample). There is no universal method for measuring AO activity/capacity in a very accurate and quantitative way because these results are highly affected by the reactive species employed in the assay. The properties of the assay system can significantly influence the effectiveness of an AO, and hence, the results may vary with different methods.10 Therefore, this property can be monitored by a wide variety of assays involving different mechanisms; in vitro methods can work through hydrogen atom transfer (HAT) or electron transfer (ET).11 Most AO assays for spirocyclic compounds are based on ET reactions, including 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assay, Trolox Equivalent AO Capacity (TEAC) assay, ferric reducing AO power assay (FRAP) assay, cupric-reducing AO capacity (CUPRAC) assay, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay, superoxide anion (SOA) radical scavenging assay and hydroxyl radical scavenging assay, these ET-based methods detect the ability of a potential AO to transfer one electron to reduce any compound, including metals, carbonyls, or radicals.12 AO molecules can react either by multiple mechanisms or by a predominant mechanism, and the identification of these makes it possible to determine the possible uses for a molecule.11
In this review, the AO properties of spirocyclic compounds of natural and synthetic origin, assessed through “in vitro” and “in vivo” methods, are presented based on comparisons with substances used as positive controls in each case. For some compounds, to increase attention on their biological potential, information about other pharmacological properties associated with oxidative damage is presented. Articles used for this review were chosen through a search, up to December 2020, in the SciFinder and PubMed databases, using “spiro antioxidant” and “spiro antioxidative” as keywords. The ring combinations (where A = any atom) for molecules whose AO activity is described in this review are presented in Table 1.
[2.4.0] Spirocyclic system (A)
[2.4.0] Spirocyclic system (A); compounds of synthetic origin
Heterocyclic spiropyran compounds are of great biological and medicinal interest; they have been reported as inhibitors of the growth of human colon adenocarcinoma and the HL-60 p leukemia cell line and the production of the HIV-1 p-24 virus,13,14 in addition to acting as strong vasopressin antagonists.15In 2011, the synthesis of spiro [indole-3,2-oxirane]-30-benzoyl-2-(1H)-fluorinated compounds (A1–A3) was reported, and their AO activities were determined through nitric oxide (NO) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) tests.
Compounds A1–A3 had an inhibition percentage at 100 μM of 80.21, 90.12, 85.12%, respectively in the NO test, while for the DPPH test, inhibition percentages were obtained at 100 μM of 82.39, 91.32, and 86.32% correspondingly. In Scheme 1, the synthesis reported for compounds A1–A3 is shown.16
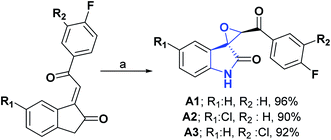 | ||
| Scheme 1 Structures, reagents and conditions for the synthesis of spiro heterocycles [2.4.0] A1–A3. (a) H2O2 (30%), NaOH, CTAB, H2O, PTC, ultrasonic irradiation, (50% power, 1.0 A), rt, 12 min. | ||
The synthesis and AO activity, using the DPPH test, of a new spirocyclopropanoxyindole [2.4.0] compound (A4) has been reported.17 This compound showed moderately good activity, with an inhibition percentage at 1 mg mL−1 of 63.90%, compared to vitamin C as standard, with 82.30% inhibition at the same concentration. Scheme 2 shows the general procedure for the synthesis of compound A4.
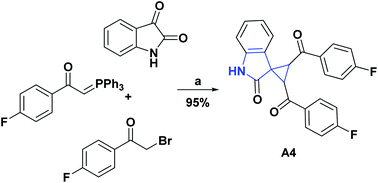 | ||
| Scheme 2 Structures, reagents and conditions for the synthesis of spiro heterocycle [2.4.0] A4. (a) Et3N, H2O, ultrasonic irradiation, 60 W, rt, 20 min. | ||
[2.7.0] Spirocyclic system (B)
[2.7.0] Spirocyclic system (B); compounds of natural origin
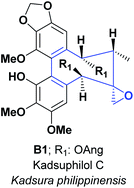
Various substances obtained from nature are considered rich sources of AO. They are accountable for minimizing the cell damage that may lead to heart diseases, cancer, Alzheimer's, and other diseases. The only spirocyclic combinations of three and eight-membered rings are represented in natural products. The spiro-compound B1: Kadsuphilol C, was isolated from the leaves and stems of Kadsura philippinensis.This compound was screened for radical scavenging activity through DPPH assay, compared with well-known AO, vitamin C, and vitamin E. B1 exhibited more potent activity than vitamins C and E at several concentrations (6.25, 12.50, 25.00, 50, and 100 μM). The data were 47.40% and 51.40% for B1, compared with 19.20% and 36.70% for vitamin C, 1.30% and 12.90% for vitamin E at 6.25 and 50 μM, respectively (see Fig. 1).18
[4.4.0] Spirocyclic system (C)
[4.4.0] Spirocyclic system (C); compounds of natural origin
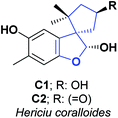
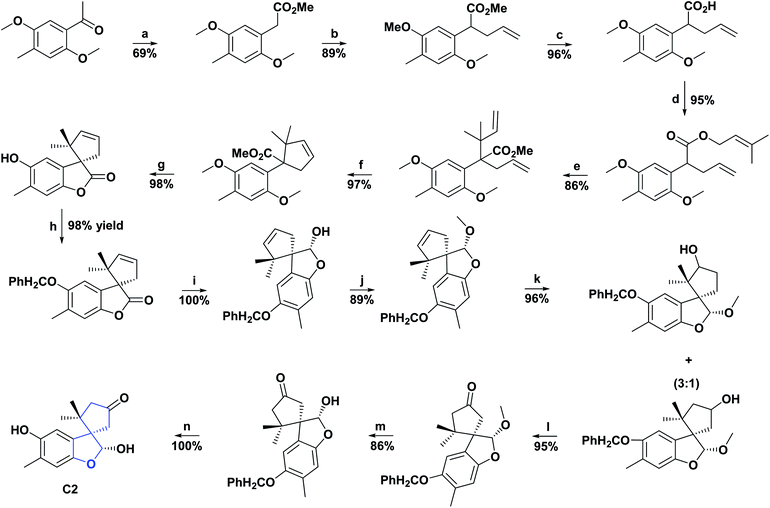
Hericium coralloide is a basidiomycete mushroom that belongs to the Hericiaceae family; it has a strong resemblance to white coral, is edible, used as a traditional medicine in Korea and China and distributed in Europe, North America, and Asia. The most famous representative species of the Hericiaceae family is H. erinaceus, which is used in alternative medicine, and is processed into food supplements.19 Numerous investigations have reported various activities in H. erinaceus, such as cytotoxic, neuroprotective, and antibacterial activities.20,21In contrast, there are only a few reports regarding the activities of H. coralloide. Studies have demonstrated that H. coralloides produce inducers of neurotrophic factors and brain-derived nerve growth factors known as corallocins A–C. Furthermore, H. coralloides ethyl acetate extract has been reported to have high AO activity “in vitro” with IC50 of 0.93, 1.84, 1.59, and 0.60 mg mL−1 against DPPH, hydroxyl radicals (HOR), ABTS and superoxide anion (SOA), respectively. Two spirobenzofurans (C1 and C2) were isolated from the culture broth of H. coralloides22 and their AO capacities were assessed using ABTS and DPPH scanning test methods.
These compounds presented AO activity in a dose-dependent manner. Both compounds showed potent AO activity in the ABTS radical scavenging assay with IC50 values of 66.00 and 29.60 μM, respectively, and significant AO activity with IC50 of 121.00 and 90.80 μM, correspondingly in the DPPH radical scavenging test. C1 and C2 were compared with the positive controls Trolox and butylated hydroxyanisole, presented in the ABTS test IC50 values of 25.20 and 18.50 μM and for the DPPH assay 55.70 and 78.30 μM, respectively (see Fig. 2). The total synthesis of compound C2 was described in 2005, and the general procedure is shown in Scheme 3.23
The genus Yucca (Agavaceae) comprises several species characterized by the presence of steroidal saponins; one of the best-known species is Yucca schidigerao,24,25 also called Yucca, found mainly in the southwestern United States and Mexico. Studies carried out on the phenolic extract of yucca roots have found that it has potent AO activity and great antiplatelet activity.25,26
Cassava bark studies have reported that “in vitro” tests of phenolic compounds exert an AO effect on different reactive oxygen species (ROS), produced in resting blood platelets and blood platelets activated by thrombin.
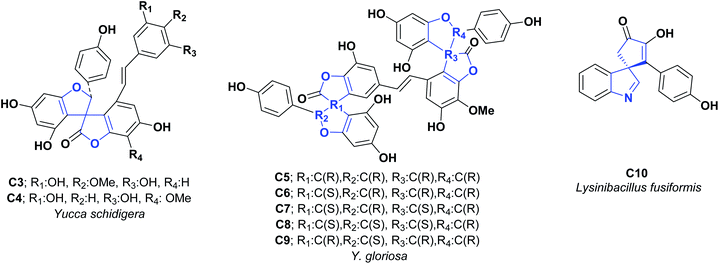
From the methanolic extract of the Yucca schidigerao bark, two new phenolic constituents with unusual spiro structures were isolated, called yuccaols D (C3) and E (C4). To determine their AO properties, the trolox equivalent AO capacity test was developed, obtaining TEAC values of 1.42 and 1.85 mM for compounds C3 and C4, respectively; these results indicate a better activity than the positive quercetin control, which offered a value of 2.60 mM (see Fig. 3).27
Yucca gloriosa is a member of the Asparagaceae family, which mainly grows in Eastern Georgia and has a rich source of steroidal saponins. The fraction of saponins found in its leaves has been used as the primary raw material for the synthesis of steroid hormones.28 An investigation in 2006 of the bark of Y. gloriosa revealed two very unusual phenolic constituents called gloriosaols A (C5) and B (C6). In 2007 three new phenolic derivatives of gloriosaols C (C7), D (C8), and E (C9) were isolated. The AO activities of compounds C5–C9 were evaluated using the TEAC test; the values were 5.55, 3.00, 5.60, 4.91, and 4.91 mM, respectively, compared to the positive quercetin control with a value of 2.60 mM (see Fig. 3).29 It is important to mention that no information was found related to the synthesis of compounds C3–C9.
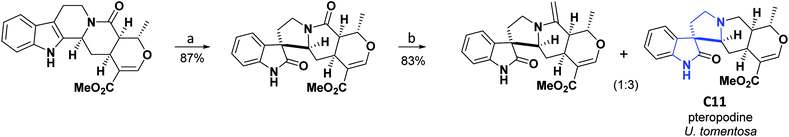
Uncaria tomentosa is a tropical medicinal vine in the Rubiaceae family, widely distributed in the Amazon rainforest and other areas of South and Central America. U. tomentosa is a powerful complementary herb for the treatment of most parasites; it has also shown a wide variety of biological activities that include cardiovascular, anticancer, AO, antimicrobial, and anti-inflammatory effects.30–35 The compound spiro [4.4.0] (C11) called pteropodine was isolated from U. tomentosa, and its AO activity was evaluated using the DPPH assay. Compound C11 presented a radical inhibition percentage of 98.00 percent at a concentration of 250 μg mL−1.36 Total synthesis of pteropodine (C11) was published in 1990, and the general procedure is shown in Scheme 4.37
[4.4.0] Spirocyclic system (C); compounds of synthetic origin
Isatin or 1H-indole-2,3-dione is a metabolic by-product of epinephrine found in mammalian tissues and fluids. This molecule has excellent synthetic versatility, which has generated a growing interest in the development of new derivative compounds.38 For this reason, there have been several reports of studies on a wide range of biological activities of isatin-derived molecules, such as analgesic, anti-inflammatory, antipyretic, and cytotoxic activities.39–41 Moreover, thanks to its lactam ring, isatin and its derivatives are responsible for having free radical scavenging activity due to their N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O residues.42
O residues.42
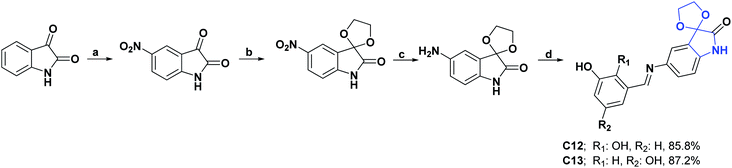
Schiff bases are another important class of organic compounds with pharmacological activities, such as antibacterial, antifungal, antimalarial, anti-inflammatory, and antipyretic.43,44 Therefore, due to the interest that has arisen in the mentioned compounds, these two pharmacophore blocks have been unified in two new Schiff bases derived from spiroisatin (C12 and C13), and their AO capacities were determined using DPPH cationic radical scavenging tests, CUPRAC and ABTS.45 Results obtained for C12 and C13 showed IC50 values of 4.49 and 18.65 μM in the DPPH test, 0.39 and 0.86 μM in the ABTS test, and A050 values of 0.42 and 1.35 μM in the CUPRAC test, respectively.
The AO capacities of these compounds were compared with quercetin (positive control), which showed IC50 values of 8.69, 15.49, and A050 of 18.47 μM in the DPPH, ABTS, and CUPRAC tests, respectively. At the end of this study, the authors determined that these compounds showed better AO activity in all tests than the positive control. The synthesis of compounds C12 and C13 is shown in Scheme 5.45
Pyrrolizidines and pyrrolothiazoles make up another important group of nitrogenous heterocycles that also stand out for their wide range of pharmacological activities such as antimycobacterial, hepatoprotective, antibiotic, antidiabetic, anticonvulsant, and anti-acetylcholinesterase (AChE).46–52
The generation of molecules with these structural residues is, therefore, of great interest. Indenoquinoxalines and their derivatives also exhibit a spectrum of biological activities such as antimicrobial, antifungal, anticancer, and anthelmintic properties.53 This led in 2017 and 2018 to the unifying of these pharmacophoric nuclei into two new spiro-indenoquinoxaline pyrrolizines (C14 and C15),54 and two spiro-indenoquinoxaline pyrrolothiazoles (C16–C17),53 whose AO capacities were evaluated through the DPPH, NO, and SOA tests.
The IC50 values obtained for compounds C14–C17 were, respectively, 2.96, 3.56, 3.09 and 3.11 μg mL−1 for the DPPH assay, 1.34, 3.56, 2.58 and 3.26 μg mL−1 in the NO test and 4.00, 3.80, 4.29 and 4.34 μg mL−1 in the SOA test. For all compounds and tests, the AO activity was better than the positive control (BHT), which presented IC50 values of 7.93, 7.19, and 9.30 μg mL−1 in the DPPH, NO, and SOA tests. The general procedure for obtaining compounds C14–C17 is shown in Scheme 6.53,54
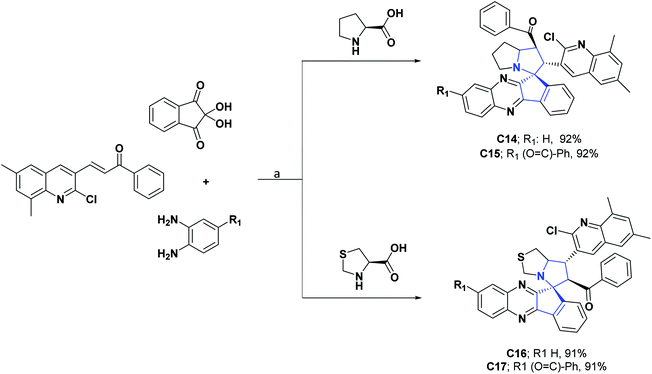 | ||
| Scheme 6 Structures, reagents and conditions for the synthesis of spiro heterocycles [4.4.0] C14–C17. (a) MeOH, L-proline, reflux 1.5 h for C14, 1 h for C15, 2 h for C16, 3 h for C17. | ||
Like the molecules above, spirooxindoles are compounds of great pharmacological interest due to their wide variety of biological activities, including anti-inflammatory, antidiabetic, antitumor, antiviral, antimalarial, antifungal, antitubercular, and AChE inhibitors.55–61 Many of these activities have been mainly related to the well-defined three-dimensional structure of spiroxyindoles and their conformational limitations generated by spirocarbon causing structural stiffness. However, spirooxindoles are undoubtedly the group of spiro molecules of synthetic origin whose AO activities have been most studied. Therefore, the synthesis and study of the AO activity of spiro compounds [4.4.0] derived from oxindoles (C18–C36) have been reported.
Compounds C18–C21 are hybrid double spiros containing heteroatoms; their AO activities were evaluated by DPPH and LPO tests.62 IC50 values reported for these compounds (C18–C21) are, respectively, 14.66, 15.03, 15.14, and 14.88 μM in the DPPH assay, while 13.75, 14.20, 16.00, and 16.74 μM in the LPO. All compounds presented good AO activity, in comparison with the positive controls, vitamin C, quercetin, and BHA, which repeatedly presented IC50 values of 14.99, 14.74, and 14.76 μM in the DPPH test and 18.34, 13.91, and 24.00 μM in the LPO assay.62
The authors emphasized that the good AO activity presented by the compounds could be due to the methoxy group in the ortho position of quinoline, which serves as a source of electrons, which could improve the stabilization of the oxygen radical resulting from DPPH. Also, they highlighted that the AO properties of the compounds coincided with vitamin C, which has a cyclic ester (lactone), and that this is because oxindoles have a very close structural similarity since they contain a cyclic amide (lactam) that makes them good candidates for exhibiting AO properties. Syntheses of compounds C18–C21 are shown in Scheme 7.62
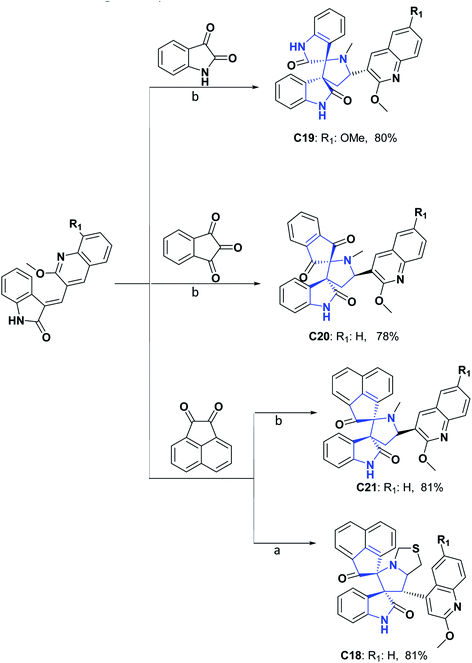 | ||
| Scheme 7 Structures, reagents and conditions for the synthesis of spiro heterocycles [4.4.0] C18–C21. (a) MeOH, thiazolidine-4-carboxylic acid, reflux; (b) MeOH, methylglycine, reflux. | ||
DPPH, ABTS, and anti-liposomal peroxidation tests were assessed for spiroxindoles C22–C26.63,64 The results obtained showed that these compounds have EC50 values of 0.98, 1.30, 13.00, 8.33 and 4.19 mM, respectively, in the DPPH assay, EC50 of 0.98, 1.02, 0.99, 1.16 and 1.55 mM in the ABTS test and EC50 of 0.04, 0.03, 0.11, 0.12 and 0.12 mM in the anti-LPO test. Additionally, compounds C22–C26 were compared with vitamin C and tocopherol as positive controls that respectively presented EC50 values of 0.36 and 0.35 mM in the DPPH test and 1.20 and 1.20 mM in the ABTS test. In the case of the anti-LP test, the molecules were only compared with tocopherol, which presented an EC50 value of 0.15 mM. Syntheses of compounds C22–C26 are shown in Scheme 8.63,64
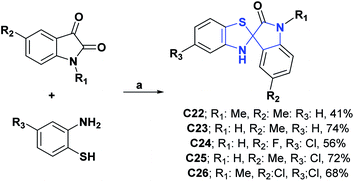 | ||
| Scheme 8 Structure, reagents, and conditions for the synthesis of spiro heterocycles [4.4.0] C22–C26. (a) EtOH, reflux, 5 h for C22 and C23, 3–6 h for C24–C26. | ||
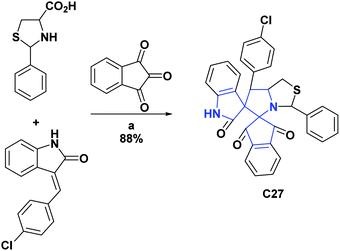
Likewise, the AO activities of oxindoles C27–C30 were determined by the DPPH radical scavenging test. For compounds C27 and C28, IC50 values were determined, which were respectively 32.50 μM and 0.44 μg mL−1. Additionally, these compounds (C27 and C28) were compared, respectively, with BHA that presented an IC50 of 58.60 μM and BHT that presented an IC50 of 5.37 μg mL−1. General procedures for obtaining compounds C27 and C28 are shown in Schemes 9 and 10.65,66
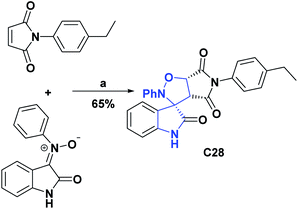 | ||
| Scheme 10 Structures, reagents, and conditions for the synthesis of spiro heterocycle [4.4.0] C28. (a) CH3CN, reflux, 1.5 h. | ||
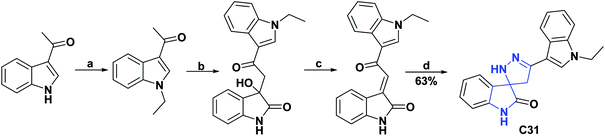
In compounds C29 and C30, only the percentage of DPPH radical scavenging at 450 μM was determined and presented values of 77.45 and 77.78%.67 Compounds C29 and C30 were better as compared to vitamin C as a positive control, which at the equivalent concentration presented a value of 55.20%. Compound C31 at a concentration of 20 μg L−1 showed a percentage of DPPH radical uptake of 65.55% and was compared with vitamin C, which at an equivalent concentration showed a percentage of uptake of 99.67%.68 General procedures for obtaining compounds C29–C31 are shown in Schemes 11 and 12.67,68
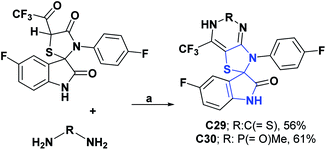 | ||
| Scheme 11 Structures, reagents, and conditions for the synthesis of spiro heterocycles [4.4.0] C29 and C30. (a) EtOH, reflux, 10 h. | ||
In contrast, the activity of spiroxyindole C32 was studied “in vitro” in the spontaneous LPO model in liver homogenate. The intensity of the lipid oxidation processes was evaluated by accumulating compounds that reacted with thiobarbituric acid (TBA reagents). The decrease in the content of TBA reagents in the liver sample indicated the presence of the AO activity of the studied compound. Thus, compound C32 at 50 μM had a TBA reagent content of 8.45 mmol g−1 of tissue and was compared to an intact sample that had a TBA reagent content of 0.37 mmol g−1 of tissue. The synthesis of compound C32 is shown in Scheme 13.69
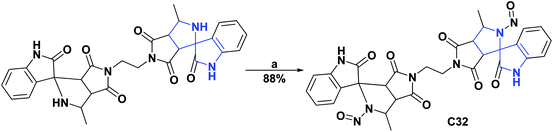 | ||
| Scheme 13 Structures, reagents, and conditions for the synthesis of spiro heterocycle [4.4.0] C32. (a) AcOH, 10 °C, NaNO2, after 12 h; water was added. | ||
It is important to remember that oxidation processes do not only affect biological organisms; an example of this is the oxidation reactions that predominantly occur in lubricating oils in service, which are responsible for various lubrication problems, including an increase in the total acid number, the formation of varnishes, sludge and sediment and the depletion of additives. The AO activities of compounds C33,70 and C34 (ref. 71) were studied according to the standard method ASTMD-943 and according to the change in the total acid number (TAN) that they generated in the mother sample. Compounds C33 and C34 at 200 ppm showed TAN values of 2.31–140.72 and 1.57–61.50 mg KOH per g of sample × 102, respectively, in the test range of 24–96 h. The compounds were compared with vitamin C as a positive control that at the same concentration and in the same time interval showed TAN values of 10.14 to 94.63 mg KOH per g sample × 102. The general syntheses of compounds C33 and C34 are shown in Schemes 14 and 15.70,71
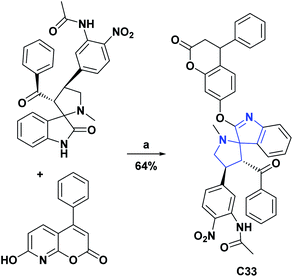 | ||
| Scheme 14 Structures, reagents, and conditions for the synthesis of spiro heterocycle [4.4.0] C33. (a) POCl3, rt. | ||
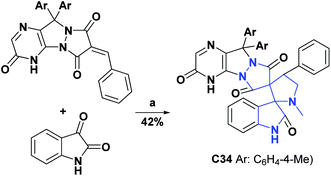 | ||
Scheme 15 Structures, reagents, and conditions for the synthesis of spiro heterocycle [4.4.0] C34. (a) MeOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in boiling, methylglycine, MCDC. 1) in boiling, methylglycine, MCDC. | ||
Compound C35, an epiroselenide derived from oxindole, was evaluated for its AO activity through the peroxynitrite (PN) inhibition test. Compound C35 had an IC50 of 12.83 μM, compared to the Ebselen positive control that showed an IC50 of 0.92 μM. The process of obtaining compound C35 is shown in Scheme 16.72 Finally, the AO activity of spiroxyindole C36 was subjected to the DPPH test, in which it showed inhibition percentages of 24.05, 33.30, 44.25, 52.75, 62.80, and 75.00 percent at concentrations of 10, 20, 40, 60, 80, and 100 μg mL−1 respectively, in comparison with vitamin C used as a positive control, and presented inhibition percentages of 45.00, 53.10, 51.70, 76.00, 82.45 and 95.00 at the same concentrations, respectively. The synthesis of the molecule C36 is shown in Scheme 17.73
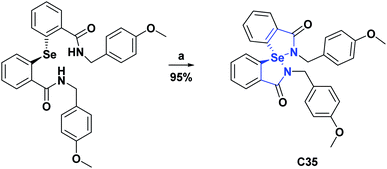 | ||
| Scheme 16 Structure, reagents, and conditions for the synthesis of spiro heterocycle [4.4.0] C35. (a) H2O2. | ||
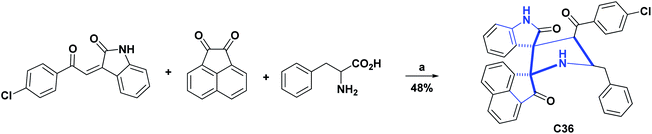 | ||
| Scheme 17 Structures, reagents and conditions for the synthesis of spiro heterocycle [4.4.0] C36. (a) MeOH, reflux, 30 min. | ||
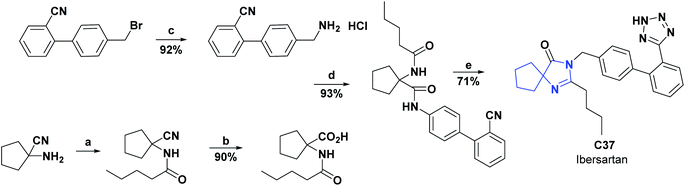
C37, known as Irbesartan, is an angiotensin receptor that has been studied in rats and found to be a possible drug suitable for inhibiting oxidative stress in atherosclerosis, myocardial infarction, atrial fibrillation, diabetes, and diabetic nephropathy, used frequently for the treatment of hypertension (see Scheme 18).74 The AO capacity of C37 was evaluated in Wistar rats, to which small doses of intragastric Irbesartan were administered for 28 days, and then in blood serum and liver tissue, the changes in indicators such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and malondialdehyde (MDA) were measured.
The activities of SOD (U mL−1), GSH-Px (U mL−1), CAT (U mL−1) and MDA (nmol mL−1) at 28 days were 210.73 ± 2.62, 1312.63 ± 87.89, 11.19 ± 4.29 and 8.81 ± 0.86, respectively, as compared to their controls with 206.96 ± 3.46, 1101.70 ± 66.54, 5.68 ± 1.62 and 10.39 ± 2.32.
AO capacity of C37 was also studied through the oxidation of erythrocytes induced by 2,2-[azobis(2-amidinopropane)] hydrochloride (AAPH) and H2O2, in addition to radical scavenging (ABTS). The inhibition time of C37 in the hemolysis induced by AAPH at a concentration of 100 μM was 77 minutes of the 217 minutes of the hemolysis time, while for trolox as a positive control at the same concentration it presented an inhibition time of 160 minutes with a hemolysis time of 290 minutes.74
The inhibition rate of C37 in H2O2-induced hemolysis was at a concentration of 100 μM of 85.75% at a hemolysis rate of 28.95%, as compared to trolox at the same concentration with a hemolysis rate of 30.14% and an inhibition rate of 94.21%. Finally, the IC50 value for the ABTS assay was 7.60 μM, compared to trolox with an IC50 of 11.80 μM.74 The results of the different experiments show that irbesartan can effectively scavenge ABTS radicals, and inhibit AAPH, H2O2-induced hemolysis of erythrocytes. Irbesartan can also increase GSH-Px, SOD, CAT activities and decrease MDA content in rats, showing that C37 is a good AO.74 In 2007, Korrapati et al. reported a method for obtaining compound C37, and the general procedure is shown in Scheme 18.75
Table 2 summarizes the AO test results for compounds C12–D37 and the positive controls against which they were contrasted.
| Antioxidant assay | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry/positive control (units) | DPPH | ABTS | LPO | NO | SOA | CUPRAC | TAN | PN |
| a N.A, not applicable. N.F., not found. % inh, inhibition percentage. | ||||||||
| C1245/quercetin (IC50 μM) | 4.49/8.69 | 0.39/15.49 | N.A | N.A | N.A | 0.42/18.47 (A050) | N.A | N.A |
| C1345/quercetin (IC50 μM) | 18.65/8.69 | 0.86/15.49 | N.A | N.A | N.A | 1.35/18.47 (A050) | N.A | N.A |
| C1454/BHT (IC50 μg mL−1) | 2.96/7.93 | N.A | N.A | 1.34/7.19 | 4.00/9.30 | N.A | N.A | N.A |
| C1554/BHT (IC50 μg mL−1) | 3.56/7.93 | N.A | N.A | 3.56/7.19 | 3.80/9.30 | N.A | N.A | N.A |
| C1653/BHT (IC50 μg mL−1) | 3.09/7.93 | N.A | N.A | 2.58/7.19 | 4.29/9.30 | N.A | N.A | N.A |
| C1753/BHT (IC50 μg mL−1) | 3.11/7.93 | N.A | N.A | 3.26/7.19 | 4.34/9.30 | N.A | N.A | N.A |
| C1862/vitamin C (IC50 μM) | 14.65/14.99 | N.A | 13.75/18.34 | N.A | N.A | N.A | N.A | N.A |
| C1962/vitamin C (IC50 μM) | 15.03/14.99 | N.A | 14.20/18.34 | N.A | N.A | N.A | N.A | N.A |
| C2062/vitamin C (IC50 μM) | 15.14/14.99 | N.A | 16.00/18.34 | N.A | N.A | N.A | N.A | N.A |
| C2162/vitamin C (IC50 μM) | 14.88/14.99 | N.A | 16.74/18.34 | N.A | N.A | N.A | N.A | N.A |
| C2263,64/tocopherol (EC50 mM) | 0.98/0.35 | 0.98/1.20 | 0.04/0.15 | N.A | N.A | N.A | N.A | N.A |
| C2363,64/tocopherol (EC50 mM) | 1.30/0.35 | 1.02/1.20 | 0.03/0.15 | N.A | N.A | N.A | N.A | N.A |
| C2454/tocopherol (EC50 mM) | 13.00/0.35 | 0.99/1.20 | 0.11/0.15 | N.A | N.A | N.A | N.A | N.A |
| C2554/tocopherol (EC50 mM) | 8.33/0.35 | 1.16/1.20 | 0.12/0.15 | N.A | N.A | N.A | N.A | N.A |
| C2654/tocopherol (EC50 mM) | 4.19/0.35 | 1.55/1.20 | 0.12/0.15 | N.A | N.A | N.A | N.A | N.A |
| C2765,66/BHA (IC50 μM) | 32.50/58.60 | N.A | N.A | N.A | N.A | N.A | N.A | N.A |
| C2865,66/BHT (IC50 μg mL−1) | 0.44/5.37 | N.A | N.A | N.A | N.A | N.A | N.A | N.A |
| C2967/vitamin C % inh (400 μM) | 77.45/55.2% | N.A | N.A | N.A | N.A | N.A | N.A | N.A |
| C3067/vitamin C % inh (400 μM) | 77.78/55.2% | N.A | N.A | N.A | N.A | N.A | N.A | N.A |
| C3168/vitamin C % inh (20 μg L−1) | 65.55/99.6% | N.A | N.A | N.A | N.A | N.A | N.A | N.A |
| C3269/intact sample (TBA mmol per g tissue) | N.A | N.A | 8.52/0.37 | N.A | N.A | N.A | N.A | N.A |
| C3370/vitamin C (mg KOH per g sample to 24 h) | N.A | N.A | N.A | N.A | N.A | N.A | 2.31/10.14 | N.A |
| C3471/vitamin C (mg KOH per g sample to 24 h) | N.A | N.A | N.A | N.A | N.A | N.A | 1.57/10.14 | N.A |
| C3572/ebselen (IC50 μM) | N.A | N.A | N.A | N.A | N.A | N.A | N.A | 12.30/0.92 |
| C3673/vitamin C % inh (100 μg L−1) | 75.0/95.0% | N.A | N.A | N.A | N.A | N.A | N.A | N.A |
| C3774/trolox (IC50 μM) | N.A | 7.60/11.80 | N.A | N.A | N.A | N.A | N.A | N.A |
[4.5.0] Spirocyclic system (D)
[4.5.0] Spirocyclic system (D); compounds of natural origin
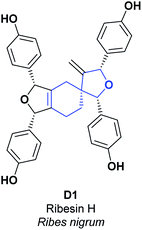
Ribes nigrum is a small perennial shrub, commonly known as black currant, which belongs to the Grossulariaceae family and is distributed mainly in Northern Europe and Asia.76,77 The black currant is commonly cultivated to obtain its red fruits, which are of particular interest in the generation of food products (juices, jams, and syrups), due to their flavor and their relatively high amount of vitamin C. Hence, they have exceptional AO activity as compared to other fruits and vegetables.78 The leaves of R. nigrum have been used in traditional medicine for the treatment of rheumatic disease in Europe, and are recognized because of their AO and anti-inflammatory effects.79 In R. nigrum leaves, several prodelphinidins, procyanidins, and proanthocyanidins have been found. According to several studies, they prevent aging of the skin and heart disease, in addition to scavenging free oxygen radicals and inhibiting lipid peroxidation (LPO) induced by UV radiation.80The spiro compound [4.5.0] D1, called Ribesin H, was isolated from R. nigrum leaves; its AO activity was assessed using SOA and DPPH tests. During the SOA test, compound D1 presented better results for concentration (EC50 3.26 μM) as compared to the butylated hydroxyanisole (BHA) used as a positive control (EC50 17.02 μM). Additionally, in the DPPH test, compound D1 showed an EC50 value of 50 μM, in contrast to the BHA, which presented an EC50 value of 26.71 μM (see Fig. 4).76 Total synthesis for the compound D1 was not found.
The rhizome of Acorus tatarinowii is a well-known traditional Chinese medicine that plays an essential role in clinical therapy due to its high pharmacological activity, low toxicity, infrequent complications, and outstanding performance in the treatment of disorders related to the central nervous system.81 Several studies have supported its usefulness in pharmacological actions related to the central nervous system, such as epilepsy, cerebrovascular diseases, and senile dementia, including Alzheimer's disease.82 Various studies have reported its sedative, antidepressant, digestive, analgesic, diuretic, and antifungal effects.83–86
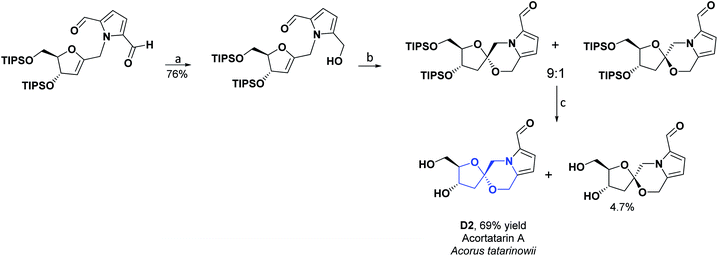
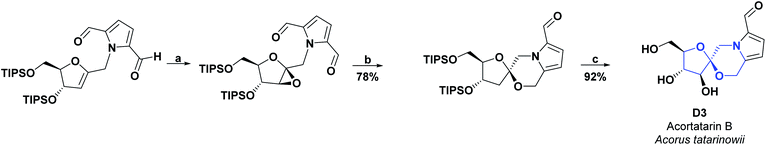
Two new spiro alkaloids that have a naturally unusual morpholine ring in their structure, called Acortatarins A (D2) and B (D3), were isolated in 2011 from the rhizome of Acorus tatarinowii. The AO capacities of compounds D2 and D3 were determined, according to the percentage of ROS production inhibition induced by glucose in mesangial cells. Compound D2, at a concentration of 50 μM, inhibited ROS generation by approximately 50%, while compound D3 at the same value of concentration inhibited ROS generation by around 30%.87 In 2015, compound D2 was found in the EtOH extract of the dried mycelium of the edible medicinal mushroom Xylaria nigripes. The AO capacity was reckoned by preventing oxidative stress-induced cytotoxicity of rat A7r5 vascular smooth muscle cells. At a concentration of 25 μM, compound D2 generated 94% cell viability as compared to the hydrated catechin used as a positive control, which at the same concentration caused 97.1% cell viability.88 In 2017, a synthesis route was proposed for compounds D2 and D3. Researchers of that study estimated the AO activity of these two compounds according to the inhibition of glucose-induced ROS production in mesangial cells. Compound D2 had an IC50 of 4.60 μM, while compound D3 had an IC50 of 11.00 μM.89 The total synthesis of compounds D2 and D3 is shown in Schemes 19 and 20, respectively.90
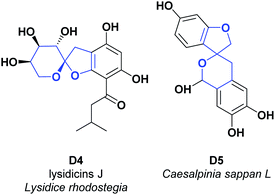
Lysidice rhodostegia is a traditional Chinese shrub plant that belongs to the Leguminosae family's genus Lysidice. Provinces in China such as Guangdong, Guangxi, and Yunnan91 distribute the L. rhodostegia. Its roots have been used in Chinese alternative medicine to treat pain, fractures, and bleeding. Previous investigations related to this plant have led to the isolation of an array of structurally diverse compounds, including phloroglucinols, flavonoids, stilbenes, and triterpenoids, some of which have displayed potent vasodilatory and antioxidative activities.92
A new acylfloroglucinol, was isolated from the roots of Lysidice rhodostegia, called lysidicins J (D4). The AO activity of the mentioned compound was determined by the content of malondialdehyde (detected by the thiobarbituric acid method), produced during microsomal LPO induced by ferrous cysteine. Compound D4 showed AO activity with inhibition rates of 100% at a concentration of 0.10 μM. However, its AO capacity disappeared at lower concentrations (0.01 and 0.001 μM). We compared this compound with vitamin E as a positive control, which presented an EC50 of 33.40 μM (see Fig. 5).93
Caesalpinia sappan L. is a tree species belonging to the Leguminosae family, commonly known as Brazilian wood or sappan. C. sappan L. is distributed in Southeast Asia, and its dried heartwood has been used as a traditional ingredient in foods or beverages and has a wide variety of medicinal properties.94 In Indonesia, its material has long been used in folk medicine to treat tuberculosis, diarrhea, dysentery, skin infections, anemia, detoxification, syphilis treatment, an antiseptic to stop bleeding, and pain due to blood circulation disorders.95
The investigation of the chemical components of C. sappan wood resulted in the isolation of several structural types of phenolic components, including xanthones, coumarins, chalcones, flavones, homoisoflavonoids, and brasilin.96 Scientific studies validated most of C. sappan L.'s folkloric uses as an AO, including its antibacterial, anti-inflammatory, anti-photoaging, hypoglycemic vasorelaxant, hepatoprotective, iron-chelating agent, and anti-acne activities.96
The compound spiro [4.5.0] (D5) was isolated from the bark of C. sappan L. and the AO activity was tested via xanthine oxidase (XO), SOA, and HOR tests. The results showed IC50 values > 50, <50, and <300 μg mL−1, respectively. The target compound was contrasted with α-tocopherol, β-carotene, and BHT, which presented IC50 values > 200 μg mL−1 in all the tests (see Fig. 5).97
Lysinibacillus is a rod-shaped, Gram-positive mesophilic bacterium. Under harsh conditions, this bacterium can form inactive spores that can resist heat, chemicals, and ultraviolet light. Organisms of this genus were previously considered members of the genus Bacillus. However, its taxonomic status, the group 2 rRNA of the genus Bacillus, was changed to the genus Lysinibacillus in 2007.98 Lysinibacillus is found mainly in soil and has been isolated from plant tissues, fermented plant seed products, and even pufferfish liver specimens. Studies have reported that isolated chemical compounds of the genus Lysinibacillus have antimicrobial activities, and they have also been shown to be potential biological control agents for diseases affecting cocoa.99
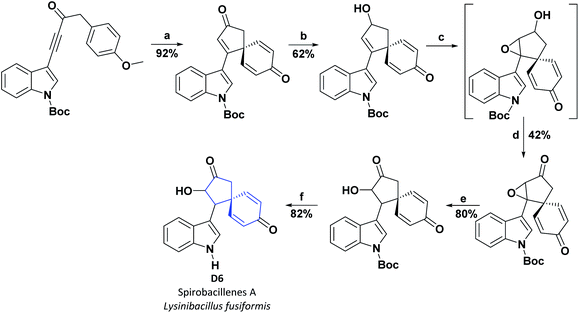
Two previously unreported spiro-cyclopentenones, designated spirobacillenes A (D6) and B (C10), were isolated from the broth culture of Lysinibacillus fusiformis, and inhibition action against NO and ROS production in the LPS-induced RAW 264.70 macrophage cell line was tested. Compound D6 presented an IC50 of 39 and 43 μM for NO and ROS, respectively, while compound C10 did not display significant AO activities (see Fig. 3).100 The synthetic process for obtaining D6 is shown in Scheme 21.101
The sea sponge Suberea mollis belongs to the order Verongida and the family Aplysinellidae. Marine sponges of the order Verongida, including the genus Suberea, possess an unusual chemical structure due to the extensive amount of sterols and the lack of classic terpenes and brominated compounds associated with tyrosine.102 Some Suberea show diverse bioactivities, including antibacterial, antiviral, enzyme inhibition, and cytotoxic activities.103–106
Suberea mollis extract has been reported to have a potent protective effect against liver damage induced by carbon tetrachloride, and it also showed vigorous scavenging activity against free radicals in the DPPH assay.107
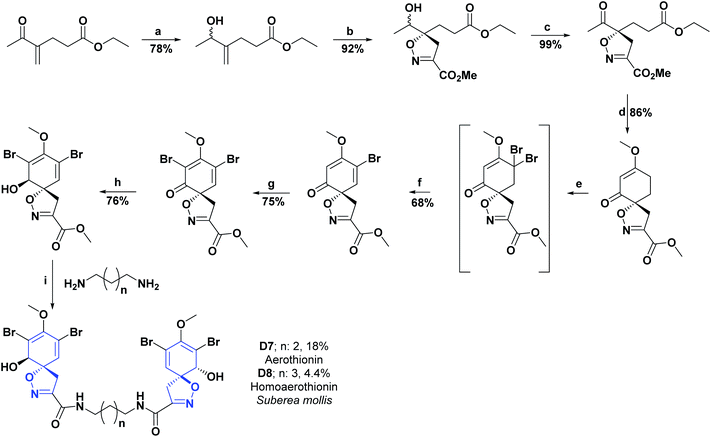
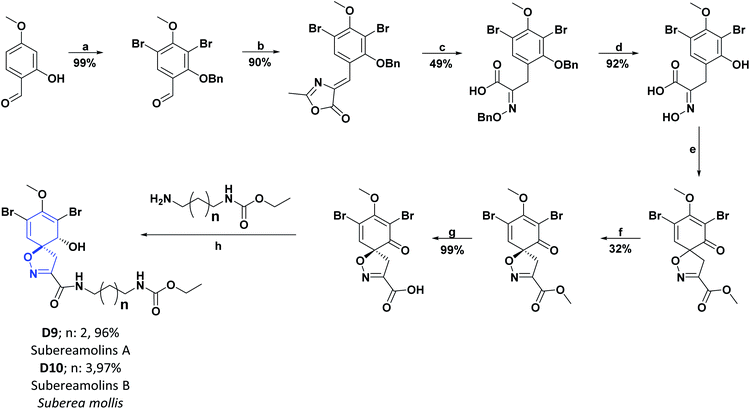
Researchers who studied the red sea sponge Suberea mollis, isolated new compounds known as aerothionin (D7), homoaerothionine (D8), and two new alkaloids derived from bromotyrosine: subereamolins A (D9) and B (D10). The AO activities were examined for the isolated compounds using a chemical assay based on DPPH on a TLC plate. The results obtained were qualitative and were evaluated according to the discoloration observed on the chromatographic scale. In addition, the compounds D7–D10 showed slight activity and were compared with vitamin E as a positive control, which showed intense discoloration on the TLC plate.102 Schemes 22 and 23 show the syntheses of compounds D7–D8 and D9–D10, respectively.108–110
[4.5.0] Spirocyclic system (D); compounds of synthetic origin
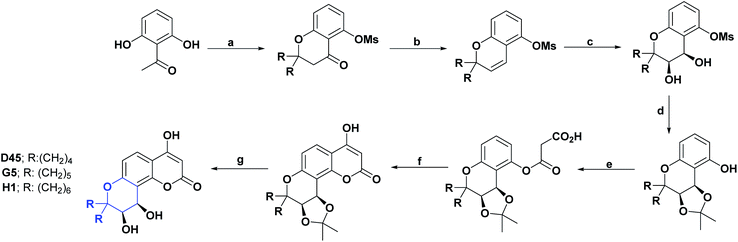
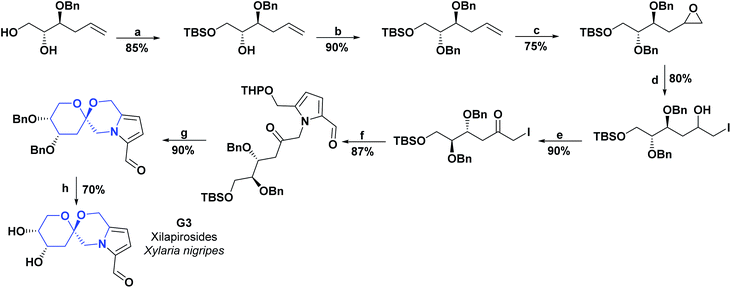
As with spirooxindoles [4.4.0], a wide variety of spirooxindoles [4.5.0] (D11–D31) were synthesized, and their AO properties have been studied. Compounds D11–D13 were synthesized in 2018 and, and their AO activities were evaluated through DPPH and peroxide reactive (POR) tests. Compounds D11–D13 showed IC50 values of 20.13, 31.87, and 25.16 μg mL−1, respectively, in the DPPH assay and IC50 values of 23.27, 25.37, and 31.82 μg mL−1 in the POR assay. All compounds were compared with vitamin C as a positive control, showing IC50 values of 19.16 and 20.66 μg mL−1, for the DPPH and POR assays, respectively.111 The synthesis of compounds D11–D13 is shown in Scheme 24.111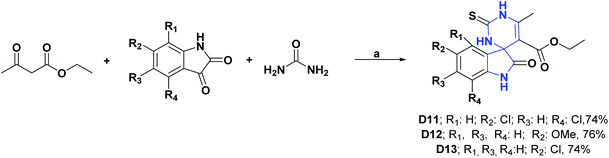 | ||
| Scheme 24 Structures, reagents and conditions for the synthesis of spiro heterocycles [4.5.0] D11–D13. (a) Fe3O4-NPs, EtOH, reflux. | ||
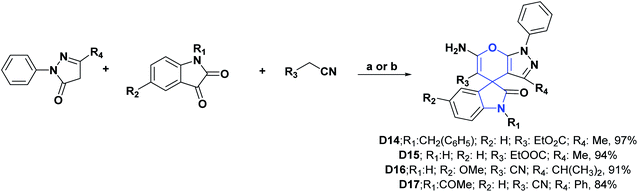
Spirooxindoles [4.5.0] D14 and D15 were studied in order to evaluate their AO capacities utilizing the DPPH, ABTS, and NO tests. The authors found that compounds D14 and D15 respectively presented IC50 values of 619.45 and 539.53 μg mL−1 in the DPPH assay, 1597.07 and 1010.20 μg mL−1 in the ABTS assay, and 891.09 and 671.95 μg mL−1 in the NO assay. Vitamin C was used as a positive control, showing IC50 values of 409.75, 550.00, and 544.21 μg mL−1 in the DPPH, ABTS, and NO tests, respectively.112
In contrast to compounds D14 and D15, the AO activities of compounds D16 and D17 were evaluated by the FRAP assay. Compound D16 presented an IC50 value of 405 μg mL−1, while compound D17 presented an IC50 value of 310 μg mL−1. Both compounds (D16 and D17) were compared with Trolox as a positive control, showing an IC50 value of 180 μg mL−1.113 Compounds D14–D17 were generally synthesized as shown in Scheme 25.112,113
Similarly, the AO activity of iodine-substituted spiropyrrolidine oxindole (D18) was studied by the NO test. Compound D18, in concentrations of 6 μg mL−1 and 10 μg mL−1 respectively presented NO radical uptake of 71.43 and 2.80%, where the percentage of radical uptake decreased with concentration. The authors mentioned that this attribute was probably due to the saturation of the carrier molecule to transport the largest number of drug molecules to the active site of the receptor.114 The synthesis of compound D18 is shown in Scheme 26.114
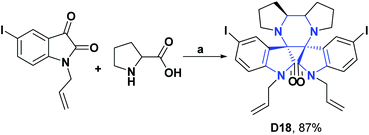 | ||
| Scheme 26 Structures, reagents and conditions for the synthesis of spiro heterocycle [4.5.0] D18. (a) toluene–MeOH, reflux, 2 h. | ||
Compounds D19–D30 were studied in order to assess their AO activities using the DPPH assay. In the case of D19–D22, they presented IC50 values of 29.76, 28.07, 11.60, and 12.19 μg mL−1, respectively, and were compared with vitamin C as a positive control that showed an IC50 of 3.89 μg mL−1.115–117 The syntheses of compounds D19–D22 are shown in Schemes 27–29, respectively.118
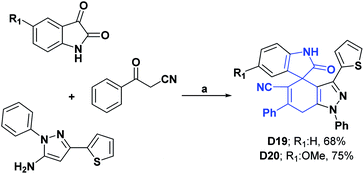 | ||
| Scheme 27 Structures, reagents and conditions for the synthesis of spiro heterocycles [4.5.0] D19 and D20. (a) AcOH–H2O, stirring, 120 °C, 8–11 h. | ||
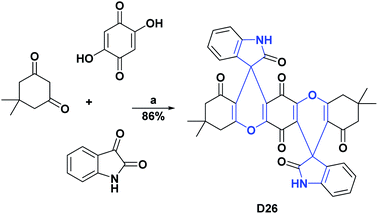
Likewise, compounds D23–D25 showed, respectively, IC50 values of 7.34, 10.06, 10.84, μM and were compared with vitamin C as a positive control that presented an IC50 of 3.45 μM.119 Compound D26 exhibited an IC50 of 0.16 mg mL−1, and it was compared with the positive control quercetin that showed an IC50 of 0.55 mg mL−1. The general processes for the syntheses of compounds D23–D26 are presented in Schemes 30 and 31.120
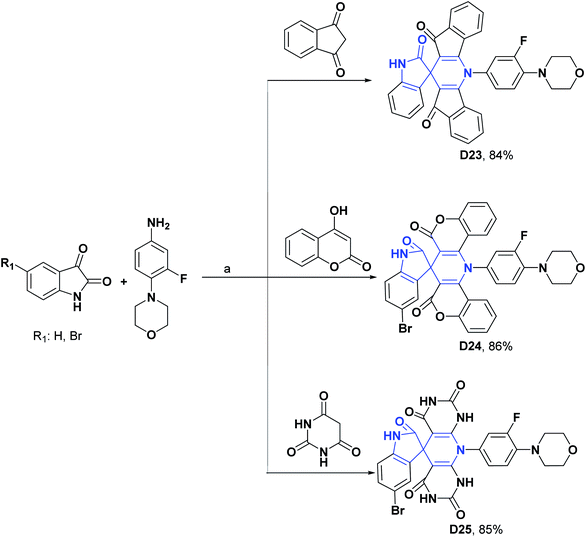 | ||
| Scheme 30 Structures, reagents and conditions for the synthesis of spiro heterocycles [4.5.0] D23–D25. (a) ZnCl2 + urea, 80 °C, 67 min for D23, 80 min for D24, and 75 min for D25. | ||
Compounds D27 and D28 at a concentration of 400 μg mL−1 presented percentages of DPPH radical scavenging of 55 and 58% and were compared with BHA as a positive control that at the same concentration manifested a percentage of scavenging of 99%.121
Finally, compounds D29 and D30 at a concentration of 1 mg mL−1 presented DPPH free radical scavenging percentages of 55.53% and 96.2%, respectively.122,123 The syntheses of compounds D27–D30 are shown in Schemes 32–34.
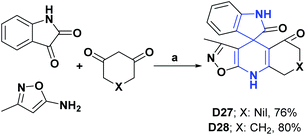 | ||
| Scheme 32 Structures, reagents and conditions for the synthesis of spiro heterocycles [4.5.0] D27 and D28. (a) CSA, ultrasonic irradiation, (low power), 70 °C, 30 min, EtOH. | ||
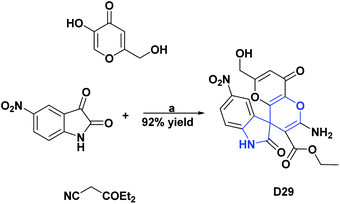 | ||
| Scheme 33 Structures, reagents and conditions for the synthesis of spiro heterocycle [4.5.0] D29. (a) H2O, SBA-15-DABCO, reflux, 2 h. | ||
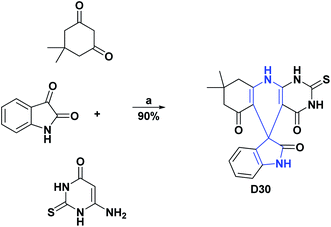 | ||
| Scheme 34 Structures, reagents and conditions for the synthesis of spiro heterocycles [4.5.0] D30. (a) EtOH–H2O, SBA-15-PhSO3H, stirring, 80 °C, 5 h. | ||
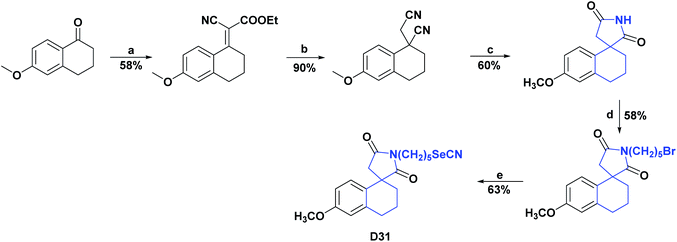
The synthesis of compound D31, a spiro [4.5.0] derived from tetralin-1,3′-pyrrolidine, was reported, and the general process is shown in Scheme 35. It was evaluated for its AO capacity by the cadmium depletion test in mice, superoxide dismutase, which consisted of intoxication for twenty days by Cd in mice, resulting in a depletion of the AO enzymes glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) of 49.54%, 67.30%, and 43.30%, respectively, compared to animals under normal conditions.
The results of the AO assay showed that in mice treated with D31, the amount of depleted AO enzymes was restored to similar values (200 nmol GSH per mg of protein, 50 units of SOD per mg of protein, and 13 units CAT per mg protein) to those presented by the mice under normal conditions (160 nmol GSH per mg protein, 70 SOD units per mg protein and 14 CAT units per mg protein). The authors attributed the good AO results of the studied compound to the fact that it manages to form a Cd–Se complex that regulates depleted enzymes and at the same time hinders the presence of free Cd in the medium.124
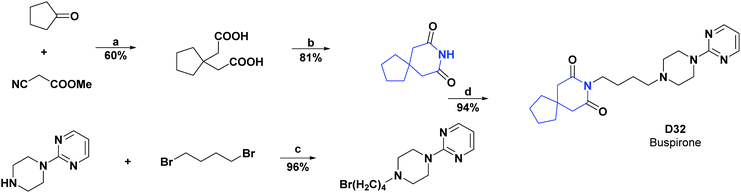
Compound D32, commonly known as buspirone, is a drug used as an anxiolytic agent. It is believed to be involved in the functioning of the neurotransmitter serotonin in the brain, particularly by acting as a partial agonist of the presynaptic 5-HT1A (dorsal raphe) and postsynaptic 5-HT1A (cortex, hippocampus) receptor.137 Besides, buspirone has a metabolite 1-pyrimidinyl-piperazine (1-PP) that can contribute to the treatment of neurodegenerative diseases.138
In 2009, to justify oxidative stress as a possible mechanism for pilocarpine-induced seizures, a study was carried out to determine whether buspirone (D32) is capable of exerting an anticonvulsant effect and altering the oxidative stress observed after pilocarpine-induced seizures. The effect of buspirone on oxidative stress was studied in the hippocampus of adult male Wistar rats (250–280 g) after seizures and pilocarpine-induced status epilepticus. Oxidative stress was evaluated in terms of the inhibition of lipoperoxidation, decreased amount of nitrite, and increased activity of SOD and CAT. It was found that in the hippocampus of the group of rats that were previously treated with buspirone there was a significant reduction in the level of lipid peroxidation (60.00%) and the content of nitrites (44.00%), as well as an increase in activities of SOD (47.00%) and CAT (40.00%) as compared to rats that were not treated with buspirone.139 In 2009 the synthesis of buspirone (D32) was reported, and the general procedure is shown in Scheme 36.140
Table 3 summarizes the AO test results of compounds D33–D51 and the positive controls against which they were contrasted (See Schemes 37–48).
| Antioxidant assay | |||||||
|---|---|---|---|---|---|---|---|
| Entry/positive control (units) | Scheme | DPPH | ABTS | FRAP | LPO | XO | POR |
| a N. A, not applicable. N. F, not found. % inh, inhibition percentage. | |||||||
| D33125/vitamin C (IC50 mg mL−1) | 37 | 0.17/0.15 | N.A | N.A | N.A | N.A | N.A |
| D34125/vitamin C (IC50 mg mL−1) | 37 | 0.16/0.15 | N.A | N.A | N.A | N.A | N.A |
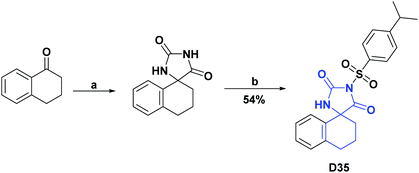
| D35126/trolox % inh (10 μM) | 38 | 82.30/82.45% | N.A | N.A | N.A | N.A | N.A |
| D36127/BHT (IC50 mg mL−1) | 39 | 0.90/0.72 | 2.31/0.93 | N.A | N.A | N.A | N.A |
| D37127/BHT (IC50 mg mL−1) | 39 | 0.50/0.72 | 0.43/0.93 | N.A | N.A | N.A | N.A |
| D38128/α-tocopherol (EC50 mM) | 40 | 0.28/0.28 | 0.88/1.02 | 2.66/2.75 | 0.12/0.13 | N.A | N.A |
| D39128/α-tocopherol (EC50 mM) | 40 | 0.29/0.28 | 0.96/1.02 | 2.33/2.75 | 0.12/0.13 | N.A | N.A |
| D40128/α-tocopherol (EC50 mM) | 40 | 1.19/0.28 | 1.61/1.02 | 0.135/2.75 | 0.25/0.13 | N.A | N.A |
| D41128/α-tocopherol (EC50 mM) | 40 | 0.97/0.28 | 1.63/1.02 | 1.34/2.75 | 0.25/0.13 | N.A | N.A |
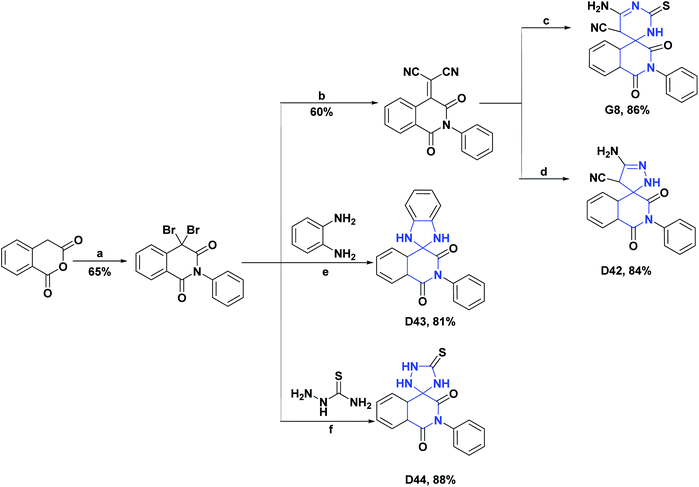
| D42129/vitamin C % inh (2 mM) | 41 | N.A | 79.60/88.90% | N.A | N.A | N.A | N.A |
| D43129/vitamin C % inh (2 mM) | 41 | N.A | 66.70/88.90% | N.A | N.A | N.A | N.A |
| D44129/vitamin C % inh (2 mM) | 41 | N.A | 25.90/88.90% | N.A | N.A | N.A | N.A |
| D45130/4-hydroxycoumarin (IC50 μM) | 42 | 97.80/124.10 | N.A | N.A | N.A | % inh: 63.50/19.30% | N.A |
| D46131/BHA % inh (100 μg mL−1) | 43 | 92.52/93.60% | N.A | N.A | N.A | N.A | N.A |
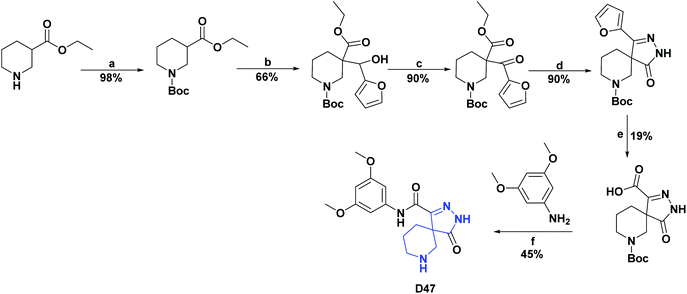
| D47132/vitamin C (% inh) | 44 | 81.92/82.33% | N.A | N.A | N.A | N.A | N.A |
| D48133/vitamin C (IC50 μM) | 45 | 48.49/20.05 | N.A | N.A | N.A | N.A | N.A |
| D49134/vitamin C % inh | 46 | 88.80/99.10% | N.A | N.A | N.A | N.A | N.A |
| D50135/vitamin C % inh (2000 μM) | 47 | 60.00%/N·F | N.A | N.A | N.A | N.A | N.A |
| D51136/vitamin C (IC50 μg mL−1) | 48 | 8.81/145.4 | N.A | N.A | N.A | N.A | 25.12/73.13 |
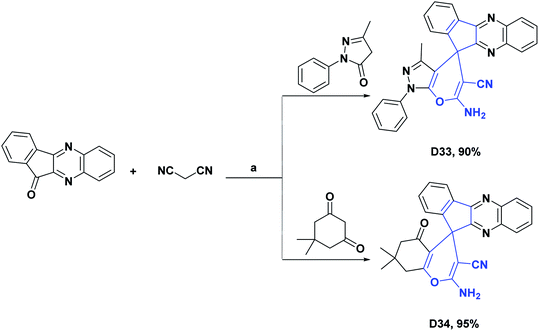 | ||
| Scheme 37 Structures, reagents and conditions for the synthesis of spiro heterocycles [4.5.0] D33 and D34. (a) APVPB, H2O, reflux, 5 h. | ||
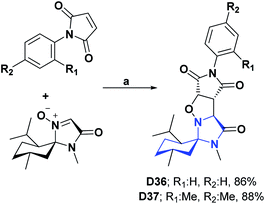 | ||
| Scheme 39 Structures, reagents and conditions for the synthesis of spiro heterocycles [4.5.0] D36 and D37. (a) Toluene, stirring, 110 °C, 48 h. | ||
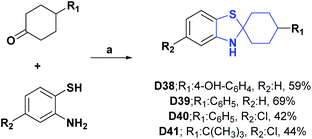 | ||
| Scheme 40 Structures, reagents and conditions for the synthesis of spiro heterocycles [4.5.0] D38–D41. (a) EtOH, reflux, 8 h. | ||
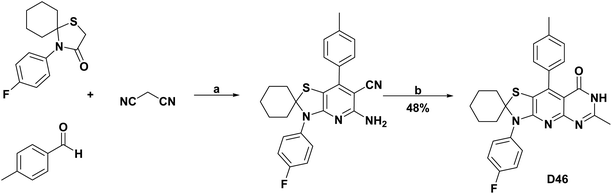 | ||
| Scheme 43 Structures, reagents and conditions for the synthesis of spiro heterocycle [4.5.0] D46. (a) AcNH4, AcOH, reflux, 24 h. (b) AcOH, AC2O, reflux, 9 h. | ||
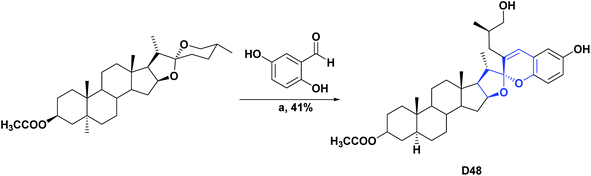 | ||
| Scheme 45 Structures, reagents and conditions for the synthesis of spiro heterocycle [4.5.0] D48. (a) BF3OEt2·DCM, 24 h. | ||
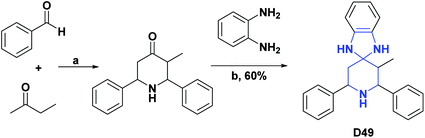 | ||
| Scheme 46 Structures, reagents and conditions for the synthesis of spiro heterocycle [4.5.0] D49. (a) AcNH4, EtOH; (b) benzene, anhydrous K2CO3, reflux, 24 h. | ||
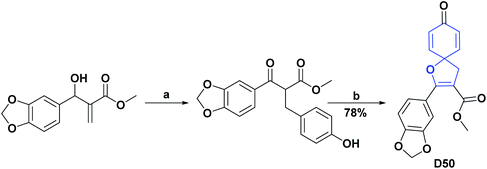 | ||
| Scheme 47 Structures, reagents and conditions for the synthesis of spiro heterocycle [4.5.0] D50. (a) 4-Iodophenol, Et3N, Nájera's catalyst,141 DMF, 110 °C, 3 h. (b) PIFA, anhydrous CH3CN, −10 °C, N2, 10–15 min. | ||
[4.6.0] Spirocyclic system (E)
[4.6.0] Spirocyclic system (E); compound of synthetic origin
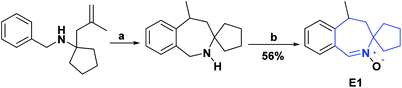
Nitrone spin traps, α-phenyl-N-tert-butylnitrone (PBN), and structurally related compounds react covalently with short-lived free radicals, such as OH, and are effective against aging ischemic strokes, and in other activities related to oxidative stress. In 2008, a new condensed spirocycle nitrone derivative [4.6.0] (E1) was synthesized, characterized, and reported. This compound was shown to inhibit LPO and reduce HOR generated during autoxidation with 6-hydroxydopamine, in addition to inhibiting protein carbonylation (PCO). The IC50 (μM) calculated in this study for compound E1 was 36.00 ± 6.20 for LPO, 53.00 ± 3.90 for PCO, and 7.00 ± 1.20 for OH, presenting a much higher activity as compared to PBN positive control, with IC50 (μM) of 8500 ± 400, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 ± 182 and 782 ± 39, respectively.142 The synthesis of compound E1 is presented in Scheme 49.143
300 ± 182 and 782 ± 39, respectively.142 The synthesis of compound E1 is presented in Scheme 49.143
In 2017, the synthesis and DPPH radical scavenging activity of 18 spirooxindole derivatives, containing oxazepine moiety, was published. Most of these molecules had AO capacity; however, at 200 μg mL−1, compounds E2–E6 showed DPPH percentage inhibitions of 78.92, 78.43, 81.50, 81.72, and 73.83%, respectively, values close to that of the positive control, vitamin C, whose inhibition was 98.7%. The synthesis of compounds E2–E6 is shown in Scheme 50.144
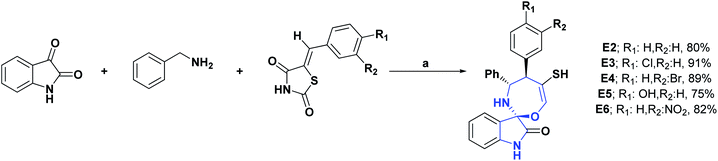 | ||
| Scheme 50 Structures, reagents and conditions for the synthesis of spiro heterocycles [4.6.0] E2–E6. (a) Et3N, THF, reflux, 20 min. | ||
[4.7.0] Spirocyclic system (F)
[4.7.0] Spirocyclic system (F); compounds of synthetic origin
Heterocycles containing the 1,4-thiazepine moiety are important targets in synthetic and medicinal chemistry because this fragment is a key moiety in many natural and synthetic biologically active agents.145 Six novel spiro (imidazo [4′,5′:4,5′]benzo[1,2-e][1,4]thiazepine)-9,3′-indolines (F1–F5) were reported. Studies demonstrate that these compounds scavenge the free radical DPPH. The IC50 values of all the compounds F1–F5 ranged from 14.00 to 50.00 μM, with AO activity and were compared with standard vitamin C (IC50: 8.64 μM). In the series, compounds F2 and F3 possessing chlorine and bromine groups as substitutions on the benzene ring showed better activity against DPPH free radicals (IC50: 20.75 and 14.46 μM, respectively). The authors associate this phenomenon with the increased lipophilicity of molecules, which is due to the substitution of an electronegative atom such as chlorine/bromine at the aromatic rings C5 position. This suggests that C5-substitution with different halides increases the AO activity of spiro(imidazo[4′,5′:4,5′]benzo[1,2-e][1,4]thiazepine)-9,3′-indolines. The synthesis of compounds F1–F5 is shown in Scheme 51.146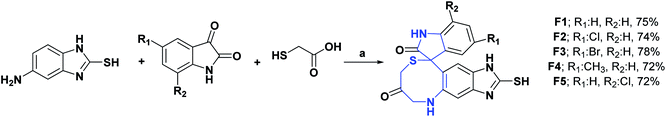 | ||
| Scheme 51 Structures, reagents and conditions for the synthesis of spiro heterocycles [4.6.0] F1–F5. (a) MeCN-PTSA, reflux, 80 °C, 24 h. | ||
[5.5.0] Spirocyclic system (G)
[5.5.0] Spirocyclic system (G); compounds of natural origin
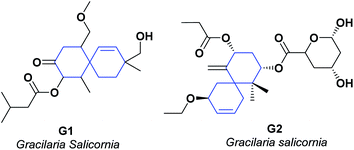
Scientific researchers have reported on the chemistry of specialized metabolites of red algae of the genus Gracilaria. The different investigations on organic extracts of “Gracilaria sp.” have shown that their secondary metabolites have significant anti-inflammatory and AO potential.147–150 Two new metabolites G1 and G2,151 were isolated from an ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (EtOAc
methanol (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH) extract of Gracilaria salicornia.
MeOH) extract of Gracilaria salicornia.
The ability of the isolated compounds to scavenge DPPH and ABTS radicals was also determined. Compounds G1 and G2 presented IC50 (mM) of 1.61 ± 0.03 and 1.13 ± 0.01, respectively, in stabilizing the DPPH. In the stabilization of the radical ABTS, they presented IC50 (mM) 1.72 ± 0.04 and 1.24 ± 0.02, respectively. Both compounds showed better results than α-tocopherol, which displayed an IC50 (mM) of 1.42 ± 0.04 for the DPPH test and 1.79 ± 0.05 for the ABTS test (see Fig. 6).151
Xylaria nigripes, also known as Wu Ling Shen, belongs to the Xylariaceae family of fungi; it is used in traditional Chinese medicine and grows in nature around abandoned nests of termites, Odontotermes formosanus.152,153 This medicinal mushroom contains many bioactive molecules such as steroids, glycosides, alkaloids, and amino acids.154 Xylaria nigripes is traditionally used to treat depression, trauma, sleep disorders, and also as a nerve tonic.155 Various “in vivo” and “in vitro” investigations have shown that Xylaria nigripes has biological activities such as antitumor,156 prevention of spatial memory impairment,157 anti-inflammatory,158 immunomodulatory, hepatoprotective,159 antidepressant activity in epileptic patients,152 improvements of sensitivity to insulin, neuroprotective,160 and AO activities.153,161
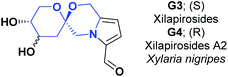
In 2015, a spirocyclic pyrrole alkaloid known as xilapirosides G3 was isolated for the first time from the EtOH extract of X. nigripes. A new analog of compound G3 was also synthesized, named by the authors as xilapirosides A2 (G4). The AO capacity for both compounds was evaluated at different concentrations (25, 50, and 100 μM) to prevent cytotoxicity induced by the oxidative stress of A7r5 rat vascular smooth muscle cells. tert-Butyl hydroperoxide (tBHP) was used as a soluble source of peroxide radicals (POR) and caused 40.40% vascular smooth muscle cell death at a concentration of 200 mM. It is reasonable to say that the cytotoxicity induced by oxidative stress was notably attenuated when the vascular smooth muscle cells were pretreated with the spiro-alkaloids G3 and G4. Compound G3 presented cell viabilities of 64.70, 71.52, 95.36%, and G4 presented cell viabilities of 74.90, 90.30, and 97.10%. Both compounds were compared with hydrated catechin (positive control) that showed 97.10% and 103.90% of cell viability at concentrations of 50 and 100 μM (see Fig. 7).88
The total synthesis of compound G3 is shown in Scheme 52.
[5.5.0] Spirocyclic system (G); compounds of synthetic origin
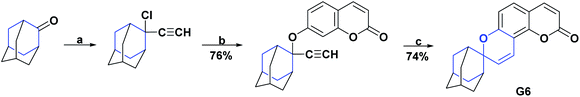
Coumarin rings are important secondary metabolites of plants and have been extensively studied for their wide variety of biological activities. The recorded activities of coumarins and their derivatives are, among others, antitumor, anti-inflammatory, AO, analgesic, and anti-HIV.162–164 Also, they have been reported to be helpful in the treatment of asthma and have been used against lymphedema.165–167 Among the most representative compounds of coumarins, the derivatives of 4-hydroxycoumarin stand out, which have been proven to be powerful pharmacological centers, such as the drugs warfarin and acenocoumarol, used for more than 20 years in anticoagulant therapy.168Two new compounds derived from spiro of 4-hydroxypyranocoumarin (G5 and G6)130,168 were synthesized, and their AO activities were evaluated by the DPPH test. The spiro G5 presented an IC50 value of 161.40 μM as compared with the positive controls 4-hydroxycoumarin, 7-hydroxycoumarin, and BHT with IC50 >124.10, 400, and 83.80 μM, respectively. The process for obtaining compound G5 is described in a general way in Scheme 42.130
In the DPPH test, compound G6 presented an inhibition percentage of 41.00% at a concentration of 400 μM; also, this compound was evaluated by the HOR scavenging test, where it presented an inhibition percentage of 93.00% as compared to 7-hydroxycoumarin, which at the same concentration showed a percentage of inhibition of 60.00%. The general synthesis for obtaining compound G6 is shown in Scheme 53.
Like coumarins, quinolines are important secondary metabolites of plants that have a large body of research supporting a wide variety of biological activities such as antimalarial, anticancer (including breast cancer), antibiotic, anti-HIV, potent liver X receptor agonist, a potent competitive inhibitor of nucleotide mimicking virus hepatitis C NS3 helicase, Pim-1 kinase inhibitor, and vascular endothelial growth factor receptor 2 inhibitor.169–177
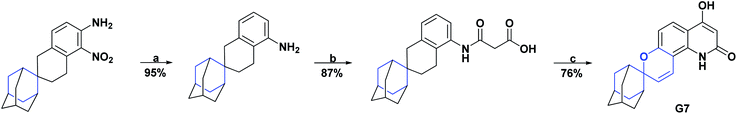
The synthesis of two spiroquinoline derivatives, G7 and G8, has been reported.129,178 The AO activity of compound G7 was evaluated by the free radical scavenging DPPH assay, showing an IC50 value of 20.65 μM and was compared with vitamin C as a positive control that presented a value of 43.9 μM. The synthesis of compound G7 is shown in Scheme 54.178
The AO activity of compound G8 was evaluated by the erythrocyte hemolysis test and the ABTS radical stabilization test. In the erythrocyte hemolysis test with compound G8, a hemolysis percentage of 5.00% was obtained, compared to vitamin C, with which a hemolysis percentage of 3.93% was obtained. In the ABTS test, the spiro compound exhibited a percent inhibition of 81.50%, while vitamin C exhibited a percent inhibition of 88.90% for the same test. The synthesis of compound G8 is shown in Scheme 41.129
Chromone scaffold molecules are substructures that possess a wide variety of biological activities, including AO, antifungal, antiviral, antimicrobial, antiallergic, anti-inflammatory, antiproliferative, and antitumor activity.162,179–181 Chromones represent an attractive source of compounds of pharmacological interest due to their low toxicity. Furthermore, several spirochromanone derivatives have been reported to exhibit a wide range of biological activities: acetyl CoA carboxylase inhibitor,182 antiarrhythmic activity,183 delta-opioid receptor agonists for the treatment of pain.184
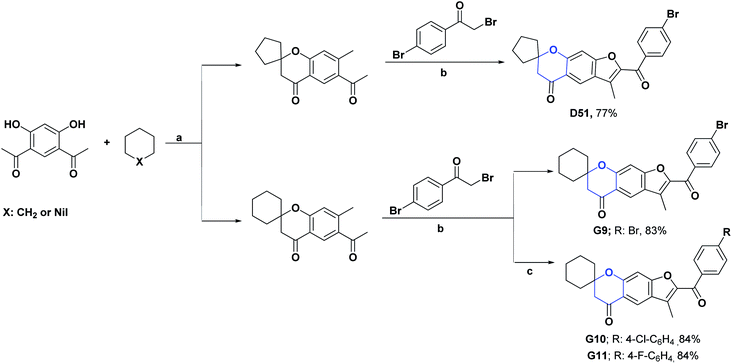
A series of 2′-substituted-3′-methylpyro [cyclohexane-1,7′-furo [3,2-g] chroman]-5′(7′H)-one spiros (G9–G11) have been synthesized. Their AO activities were studied by the DPPH radical scavenging test and the hydrogen POR test. In the DPPH assay, the compounds G9–G11 showed IC50 values (μg mL−1) of 2.64, 3.93, and 11.03, respectively. In the POR test, IC50 of (μg mL−1) 83.14, 96.65, and 32.11, respectively, were obtained. The compounds were compared with vitamin C as a positive control, which presented an IC50 of 145.4 μg mL−1 in the DPPH assay and 77.13 μg mL−1 in the hydrogen POR assay.
It is important to highlight that in the DPPH test, all compounds presented IC50 values lower than vitamin C, with compounds G9 and G10 giving the best results. In the hydrogen POR test, only compound G11 showed lower IC50 values than the positive control.136 In Scheme 48 the syntheses of compounds G9–G11 are presented.
Spirochroman compounds [5.5.0] G12 and G13 were reported in 2015 and 2016.185,186 The AO activities of compounds G12 and G13 were determined by the DPPH radical trapping test. Spiro compounds G12 and G13 presented excellent AO activity with IC50 values of 0.50 and 1.25 μM, respectively, compared to vitamin C (positive control), which presented an IC50 of 8.64 μM. Schemes 55 and 56 present the synthesis of compounds G12 and G13, respectively.
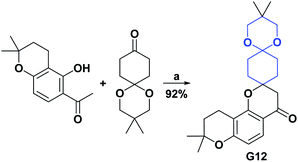 | ||
| Scheme 55 Structures, reagents and conditions for the synthesis of spiro heterocycle [5.5.0] G12. (a) Pyrrolidine, EtOH, microwave, 4 min. | ||
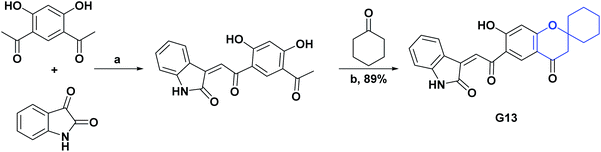 | ||
| Scheme 56 Structures, reagents and conditions for the synthesis of spiro heterocycle [5.5.0] G13. (a) KOH, microwave; (b) pyrroline-[bmim]Cl·FeCl3, microwave, 60 °C, 5 min. | ||
The functionalized heterocycle compounds are of great interest for the design and development of drugs. Many of the fused pyridine compounds have been reported to be valuable compounds that possess a wide variety of biological properties such as anticonvulsant, antibacterial, antipsychotic, antifungal, antileishmanial, antidiabetic activities, antimicrobial, AO, anticancer, antiviral, antihypertensive, antimalarial, anti-inflammatory, and antitumor.187–189
Through a multicomponent reaction, a functional spiropyridine compound [5.5.0] (G14) was synthesized. The AO activity of compound G14 was evaluated by the DPPH radical scavenging test. This compound presented excellent AO capacity with an IC50 value of 0.07 μM. Spiro compound G14 was compared with vitamin C as a positive control, which had an IC50 for the DPPH assay of 0.05 μM. The synthesis of compound G14 is shown in Scheme 57.189
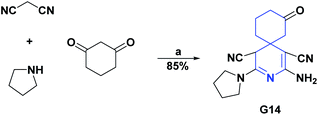 | ||
| Scheme 57 Structures, reagents and conditions for the synthesis of spiro heterocycle [5.5.0] G14. (a) EtOH, reflux, 18 h. | ||
[5.6.0] Spirocyclic system (H)
[5.6.0] Spirocyclic system (H); compounds of synthetic origin
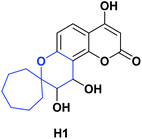
Compound H1 is a spiro [5.6.0], which, like compounds G5 and G6, belongs to the hydroxycoumarin family. In 2008, Panteleon et al. synthesized and evaluated the AO capacity of compound H1 using the DPPH test, finding that said compound had an IC50 of 157.70 μM. These results were compared with the positive controls 4-hydroxycoumarin, 7-hydroxycoumarin, and BHT with IC50 > 124.10, 400, and 83.80 μM respectively (see Fig. 8). The synthesis of compound H1 is shown in Scheme 42.130Conclusions
The syntheses and AO activities of many spiro compounds of natural and synthetic origin have been reviewed. Table 1s of ESI† summarizes some features of the 119 spiro compounds reviewed; it is shown that the most abundant spiro heterocycles with information about AO properties were [4.5.0], followed by [4.4.0], and most of the spiro [4.4.0] compounds studied had better results than the positive controls used in the respective assays. In general, the revised compounds demonstrated some AO activities that were mainly limited to the DPPH, ABTS, FRAP, anti-LPO, superoxide, xanthine oxidase, peroxide, hydroxyl, and nitric oxide tests. The molecules that presented the best results for these tests were the spiro compounds G14, C12, D41, C18, C15, D5, D11, E1, and C14. The most active compounds were characterized for having at least one oxygen atom; a number of them (around 35%) are phenolic compounds, and in the molecules where this functional group was absent, aryl ethers and nitrogen functional groups such as amine and amides could be found. It is important to note that spirocyclic compounds of synthetic origin have better AO activity in most of the reviewed tests as compared to compounds of natural origin. However, the discovery of the latter has increased in recent years. Therefore, they are likely to inspire new pathways for the design of drugs based on AO properties and the development of new synthetic libraries. Thus, in terms of their development, spiro heterocycles will continue to be an essential structural scaffold in medicinal chemistry.Abbreviations
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| APVPB | Acetic acid-functionalized poly (4-vinylpyridinium) bromide |
| Ac2O | Acetic anhydride |
| AChE | Acetylcholinesterase |
| AO | Antioxidant (s) |
| BF3OEt2 | Boron trifluoride etherate |
| BHA | Butylated hydroxyanisole |
| BHT | Butylhydroxytoluene |
| bmim | 1-Butyl-3-methylimidazolium |
| t-BOC | tert-Butyloxycarbonyl protecting group |
| CAN | Ceric ammonium nitrate |
| CAT | Catalase |
| CSA | Camphorsulfonic acid |
| CTAB | Cetyltrimethylammonium bromide |
| CUPRAC | Cupric ion reducing antioxidant capacity assay |
| DABCO | 1,4-Diazabicyclo[2.2.2]octane |
| DCC | Dicyclohexylcarbodiimide |
| DCM | Dichloromethane |
| DIPEA | N,N-Diisopropylethylamine |
| DMAP | 4-Dimethylaminopyridine |
| DMF | Dimethylformamide |
| DMP | Dess–Martin periodinane |
| DMSO | Dimethyl sulfoxide |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EC50 | Half maximal effective concentration |
| EtOH | Ethanol |
| GSH | Glutathione |
| GTBSA | Glycoluril tetrakis(butane-1-sulfonic acid) |
| HATU | Hexafluorophosphate azabenzotriazole tetramethyl uronium |
| HOBT | 1-Hydroxybenzotriazole |
| HOR | Hydroxyl radicals |
| IC50 | Half maximal inhibitory concentration |
| LHMDS | Lithium bis(trimethylsilyl)amide |
| LPO | Lipid peroxidation |
| MCDC | The multicomponent 1,3-dipolar cycloaddition |
| m-CPBA | meta-Chloroperoxybenzoic acid |
| MeOH | Methanol |
| mg | Milligram |
| mL | Millilitre |
| mM | Millimolar |
| NaHMDS | Sodium hexamethyldisilazane |
| NBS | N-Bromosuccinimide |
| Nil | Null |
| NO | Nitric oxide |
| NPs | Nanoparticles |
| PBN | α-Phenyl-N-tert-butylnitrone |
| PCO | Protein carbonylation |
| PIFA | [Bis(trifluoroacetoxy)iodo]benzene |
| PN | peroxynitrite |
| POR | Peroxide reactive |
| PTC | Phase transfer catalyst |
| PTSA | p-Toluenesulfonic acid |
| ROS | Reactive oxygen species |
| rt | Room temperature |
| SOA | Superoxide anion |
| SOD | Superoxide dismutase |
| T3P | Propylphosphonic anhydride |
| TAN | Total acid number |
| TBA | Acid thiobarbituric |
| TBAB | Tetrabutylammonium bromide |
| TBAF | Tetrabutylammonium fluoride |
| tBHP | tert-Butyl hydroperoxide |
| TEAC | Trolox equivalent antioxidant capacity |
| TFA | Trifluoroacetic acid |
| THF | Tetrahydrofuran |
| TLC | Thin-layer chromatography |
| TPP | Triphenylphosphine |
| μM | Micromolar |
| XO | Xanthine oxidase |
Conflicts of interest
There are no conflicts to declare.References
- Y. Zheng, C. M. Tice and S. B. Singh, Bioorg. Med. Chem. Lett., 2014, 24, 3673–3682 CrossRef CAS PubMed.
- Y. J. Zheng and C. M. Tice, Expert Opin. Drug Discovery, 2016, 11, 831–834 CrossRef PubMed.
- M. Krasavin, A. Lukin, D. Bagnyukova, N. Zhurilo, I. Zahanich, S. Zozulya, J. Ihalainen, M. M. Forsberg, M. Lehtonen, J. Rautio, D. Moore and I. G. Tikhonova, Bioorg. Med. Chem., 2016, 24, 5481–5494 CrossRef CAS PubMed.
- D. Goyard, B. Kónya, A. S. Chajistamatiou, E. D. Chrysina, J. Leroy, S. Balzarin, M. Tournier, D. Tousch, P. Petit, C. Duret, P. Maurel, L. Somsák, T. Docsa, P. Gergely, J.-P. Praly, J. Azay-Milhau and S. Vidal, Eur. J. Med. Chem., 2016, 108, 444–454 CrossRef CAS PubMed.
- L. H. Ramdani, O. Talhi, N. Taibi, L. Delort, C. Decombat, A. Silva, K. Bachari, M. P. Vasson and F. Caldefie-Chezet, Anticancer Res., 2016, 36, 6399–6408 CrossRef CAS PubMed.
- Ismail, B. Kuthati, G. Thalari, V. Bommarapu, C. Mulakayala, S. K. Chitta and N. Mulakayala, Bioorg. Med. Chem. Lett., 2017, 27, 1446–1450 CrossRef CAS PubMed.
- Y. Laras, N. Pietrancosta, T. Tomita, T. Iwatsubo and J. L. Kraus, J. Enzyme Inhib. Med. Chem., 2008, 23, 996–1001 CrossRef CAS PubMed.
- J. Gálvez, S. Polo, B. Insuasty, M. Gutiérrez, D. Cáceres, J. H. Alzate-Morales, P. De-la-Torre and J. Quiroga, Comput. Biol. Chem., 2018, 74, 218–229 CrossRef PubMed.
- M. Valko, D. Leibfritz, J. Moncol, M. T. D. Cronin, M. Mazur and J. Telser, Int. J. Biochem. Cell Biol., 2007, 39, 44–84 CrossRef CAS PubMed.
- D. J. Charles, Antioxidant properties of spices, herbs and other sources, 2013 Search PubMed.
- S. Picco, L. Villegas, F. Tonelli, M. Merlo, J. Rigau, D. Diaz and M. Masuelli, in Itechopen, 2016, p. 13 Search PubMed.
- R. Apak, M. Özyürek, K. Güçlü and E. Çapanoʇlu, J. Agric. Food Chem., 2016, 64, 1046–1070 CrossRef CAS PubMed.
- S. Tanaka, Y. Honmura, S. Uesugi, E. Fukushi, K. Tanaka, H. Maeda, K. I. Kimura, T. Nehira and M. Hashimoto, J. Org. Chem., 2017, 82, 5574–5582 CrossRef CAS PubMed.
- N. Murakami, Y. Ye, M. Kawanishi, S. Aoki, N. Kudo, M. Yoshida, E. E. Nakayama, T. Shioda and M. Kobayashi, Bioorg. Med. Chem. Lett., 2002, 12, 2807–2810 CrossRef CAS.
- R. E. Schwartz, C. F. Hirsch, J. M. Sigmund and D. J. Pettibone, US Pat., US4803217A, 1986 Search PubMed.
- A. Dandia, R. Singh and S. Bhaskaran, Ultrason. Sonochem., 2011, 18, 1113–1117 CrossRef CAS PubMed.
- M. Pourshab, S. Asghari, M. Tajbakhsh and A. Khalilpour, Chem. Biodiversity, 2019, 16, e1900087 Search PubMed.
- Y. C. Shen, Y. Bin Cheng, T. W. Lan, C. C. Liaw, S. S. Liou, Y. H. Kuo and A. T. Khalil, J. Nat. Prod., 2007, 70, 1139–1145 CrossRef CAS PubMed.
- C. Zhang, C. Li, W. Ye and M. Yang, Mitochondrial DNA, 2017, 2, 385–386 CrossRef PubMed.
- K. H. Kim, S. U. Choi, H. J. Noh, O. Zee and K. R. Lee, Nat. Prod. Sci., 2014, 20, 76–79 CAS.
- M. Hazekawa, A. Kataoka, K. Hayakawa, T. Uchimasu, R. Furuta, K. Irie, Y. Akitake, M. Yoshida, T. Fujioka, N. Egashira, R. Oishi, K. Mishima, K. Mishima, T. Uchida, K. Iwasaki and M. Fujiwara, J. Health Sci., 2010, 56, 296–303 Search PubMed.
- J. K. E. Woo and I. L. B. Yun, J. Antibiot., 2018, 299, 822–825 Search PubMed.
- A. Srikrishna and B. Vasantha Lakshmi, Tetrahedron Lett., 2005, 46, 7029–7031 CrossRef CAS.
- C. Bassarello, G. Bifulco, P. Montoro, A. Skhirtladze, E. Kemertelidze and S. Piacente, Tetrahedron, 2006, 63, 148–154 CrossRef.
- S. Piacente, C. Pizza and W. Oleszek, Phytochem. Rev., 2005, 4, 177–190 CrossRef CAS.
- P. R. Cheeke, in Saponins in Food, Feedstuffs and Medicinal Plants, Springer Netherlands, 2000, pp. 241–254 Search PubMed.
- S. Piacente, P. Montoro, W. Oleszek and C. Pizza, J. Nat. Prod., 2004, 67, 882–885 CrossRef CAS PubMed.
- A. Skhirtladze, A. Plaza, P. Montoro, M. Benidze, E. Kemertelidze, C. Pizza and S. Piacente, Biochem. Syst. Ecol., 2006, 34, 809–814 CrossRef CAS.
- C. Bassarello, G. Bifulco, P. Montoro, A. Skhirtladze, M. Benidze, E. Kemertelidze, C. Pizza and S. Piacente, J. Agric. Food Chem., 2007, 55, 6636–6642 CrossRef CAS PubMed.
- C. M. Gurrola-Díaz, P. M. E. García-López, K. Gulewicz, R. Pilarski and S. Dihlmann, Phytomedicine, 2011, 18, 683–690 CrossRef PubMed.
- R. A. Ccahuana-Vasquez, S. S. Ferreira dos Santos, C. Y. Koga-Ito and A. O. Cardoso Jorge, Braz. Oral Res., 2007, 21, 46–50 CrossRef PubMed.
- R. Rojas-Duran, G. González-Aspajo, C. Ruiz-Martel, G. Bourdy, V. H. Doroteo-Ortega, J. Alban-Castillo, G. Robert, P. Auberger and E. Deharo, J. Ethnopharmacol., 2012, 143, 801–804 CrossRef CAS PubMed.
- B. C. Azevedo, M. Roxo, M. C. Borges, H. Peixoto, E. J. Crevelin, B. W. Bertoni, S. H. T. Contini, A. A. Lopes, S. C. França, A. M. S. Pereira and M. Wink, Molecules, 2019, 24, 3299 CrossRef CAS PubMed.
- G. E. Batiha, A. M. Beshbishy, D. S. Tayebwa, H. M. Shaheen, N. Yokoyama and I. Igarashi, Jpn. J. Vet. Parasitol., 2018, 17, 1–13 Search PubMed.
- G. E. S. Batiha, A. M. Beshbishy, L. Wasef, Y. H. A. Elewa, M. E. A. El-Hack, A. E. Taha, A. A. Al-Sagheer, H. P. Devkota and V. Tufarelli, Appl. Sci., 2020, 10, 2668 CrossRef.
- R. Paniagua-Pérez, E. Madrigal-Bujaidar, D. Molina-Jasso, S. Reyes-Cadena, I. Álvarez-González, L. Sánchez-Chapul and J. Pérez-Gallaga, Basic Clin. Pharmacol. Toxicol., 2009, 104, 222–227 CrossRef PubMed.
- S. F. Martin and M. Mortimore, Tetrahedron Lett., 1990, 31, 4557–4560 CrossRef CAS.
- T. B. S. Giorno, B. V. Da Silva, A. D. C. Pinto and P. D. Fernandes, Life Sci., 2016, 151, 189–198 CrossRef CAS PubMed.
- F. Wang, Y. Fang, T. Zhu, M. Zhang, A. Lin, Q. Gu and W. Zhu, Tetrahedron, 2008, 64, 7986–7991 CrossRef CAS.
- M. E. Matheus, F. de A. Violante, S. J. Garden, A. C. Pinto and P. D. Fernandes, Eur. J. Pharmacol., 2007, 556, 200–206 CrossRef CAS PubMed.
- G. S. M. Figueiredo, R. S. Zardo, B. V. Silva, F. A. Violante, A. C. Pinto and P. D. Fernandes, Pharmacol. Biochem. Behav., 2013, 103, 431–439 CrossRef CAS PubMed.
- P. Pakravan, S. Kashanian, M. M. Khodaei and F. J. Harding, Pharmacol. Rep., 2013, 65, 313–335 CrossRef CAS PubMed.
- S. Ghosh, N. Roy, T. S. Singh and N. Chattopadhyay, Spectrochim. Acta, Part A, 2018, 188, 252–257 CrossRef CAS PubMed.
- L. Xia, Y. F. Xia, L. R. Huang, X. Xiao, H. Y. Lou, T. J. Liu, W. D. Pan and H. Luo, Eur. J. Med. Chem., 2015, 97, 83–93 CrossRef CAS PubMed.
- F. Sonmez, Z. Gunesli, B. Z. Kurt, I. Gazioglu, D. Avci and M. Kucukislamoglu, Mol. Diversity, 2019, 23, 829–844 CrossRef CAS PubMed.
- S. M. Rajesh, S. Perumal, J. C. Menéndez, P. Yogeeswari and D. Sriram, Medchemcomm, 2011, 2, 626–630 RSC.
- G. Wu, L. Ouyang, J. Liu, S. Zeng, W. Huang, B. Han, F. Wu, G. He and M. Xiang, Mol. Diversity, 2013, 17, 271–283 CrossRef CAS PubMed.
- A. A. Shvets, Y. V. Nelyubina, K. A. Lyssenko and S. V. Kurbatov, Russ. Chem. Bull., 2012, 61, 1659–1662 CrossRef CAS.
- P. Meffre, Amino Acids, 1999, 16, 251–272 CrossRef CAS PubMed.
- T. D. Aicher, B. Balkan, P. A. Bell, L. J. Brand, S. H. Cheon, R. O. Deems, J. B. Fell, W. S. Fillers, J. D. Fraser, J. Gao, D. C. Knorr, G. G. Kahle, C. L. Leone, J. Nadelson, R. Simpson and H. C. Smith, J. Med. Chem., 1998, 41, 4556–4566 CrossRef CAS PubMed.
- G. Trapani, M. Franco, A. Latrofa, G. Genchi, G. Siro Brigiani, M. Mazzoccoli, M. Persichella, M. Serra, G. Biggio and G. Liso, Eur. J. Med. Chem., 1994, 29, 197–204 CrossRef CAS.
- S. Sivakumar, R. Ranjith Kumar, M. A. Ali and T. S. Choon, Eur. J. Med. Chem., 2013, 65, 240–248 CrossRef CAS PubMed.
- K. S. Mani, B. Murugesapandian, W. Kaminsky and S. P. Rajendran, Tetrahedron Lett., 2018, 59, 2859–2950 CrossRef.
- K. S. Mani, W. Kaminsky and S. P. Rajendran, New J. Chem., 2017, 42, 301–310 RSC.
- A. Kamal, K. S. Babu, Y. Poornachandra, B. Nagaraju, S. M. Ali Hussaini, S. P. Shaik, C. Ganesh Kumar and A. Alarifi, Arabian J. Chem., 2019, 12, 3546–3554 CrossRef CAS.
- C. Marti and E. M. Carreira, Eur. J. Org. Chem., 2003, 2003, 2209–2219 CrossRef.
- S. Yu, D. Qin, S. Shangary, J. Chen, G. Wang, K. Ding, D. McEachern, S. Qiu, Z. Nikolovska-Coleska, R. Miller, S. Kang, D. Yang and S. Wang, J. Med. Chem., 2009, 52, 7970–7973 CrossRef CAS PubMed.
- T. Sasaki, S. Eguchi and Y. Hirako, Tetrahedron, 1976, 32, 437–440 CrossRef CAS.
- B. K. S. Yeung, B. Zou, M. Rottmann, S. B. Lakshminarayana, S. H. Ang, S. Y. Leong, J. Tan, J. Wong, S. Keller-Maerki, C. Fischli, A. Goh, E. K. Schmitt, P. Krastel, E. Francotte, K. Kuhen, D. Plouffe, K. Henson, T. Wagner, E. A. Winzeler, F. Petersen, R. Brun, V. Dartois, T. T. Diagana and T. H. Keller, J. Med. Chem., 2010, 53, 5155–5164 CrossRef CAS PubMed.
- A. Thangamani, Eur. J. Med. Chem., 2010, 45, 6120–6126 CrossRef CAS PubMed.
- L. M. Zhou, R. Y. Qu and G. F. Yang, Expert Opin. Drug Discovery, 2020, 15, 603–625 CrossRef CAS PubMed.
- S. Mathusalini, T. Arasakumar, K. Lakshmi, C.-H. Lin, P. S. Mohan, M. G. Ramnath and R. Thirugnanasampandan, New J. Chem., 2016, 40, 5164–5169 RSC.
- N. Karalı, Ö. Güzel, N. Özso, S. Ozbey and A. Salman, Eur. J. Med. Chem., 2010, 45, 1068–1077 CrossRef PubMed.
- G. Ermut, N. Karalı, N. Ösoy and A. Can, J. Enzyme Inhib. Med. Chem., 2014, 29, 457–468 CrossRef CAS PubMed.
- G. Periyasami, K. Ponmurugan, N. Arumugam and R. Sureshkumar, BMC Chem., 2019, 13, 1–11 CrossRef CAS PubMed.
- M. Kaur, B. Singh, B. Singh and A. Arjuna, J. Heterocycl. Chem., 2016, 54, 1348–1354 CrossRef.
- T. E. Ali and R. M. Abdel-rahman, J. Sulfur Chem., 2014, 35, 37–41 Search PubMed.
- H. M. Abo-salem, A. Nassrallah, A. A. F. Soliman and M. S. Ebied, Molecules, 2020, 25, 1124 CrossRef CAS PubMed.
- Y. I. Syumka, A. B. Kravchenko, V. P. Chernykh and L. A. Shemchuk, Visn. Farm., 2018, 95, 5–13 CrossRef.
- S. K. Attia, A. T. Elgendy and S. A. Rizk, J. Mol. Struct., 2019, 1184, 583–592 CrossRef CAS.
- M. A. El-hashash and S. A. Rizk, J. Heterocycl. Chem., 2016, 54, 1776–1784 CrossRef.
- D. S. Lamani, D. Bhowmick and G. Mugesh, Molecules, 2015, 20, 12959–12978 CrossRef PubMed.
- A. I. Almansour, N. Arumugam, R. S. Kumar, R. Raju, K. Ponmurugan, N. A. AlDhabi and D. Premnath, J. Infect. Public Health., 2020, 13, 2001–2008 CrossRef PubMed.
- M. X. Wang, X. Z. Zhao, C. Y. Gai, H. M. Liu, Y. P. Xu and J. Li, Chem. Res. Chin. Univ., 2011, 27, 75–79 CAS.
- K. V. V. P. Rao, R. Dandala, V. K. Handa, I. V. S. Rao, A. Rani and A. Naidu, Synth. Commun., 2007, 37, 2897–2905 CrossRef CAS.
- T. Sasaki, W. Li, S. Zaike, Y. Asada, Q. Li, F. Ma, Q. Zhang and K. Koike, Phytochemistry, 2013, 95, 333–340 CrossRef CAS PubMed.
- J. Zheng, B. Yang, S. Tuomasjukka, S. Ou and H. Kallio, J. Agric. Food Chem., 2009, 57, 2977–2987 CrossRef CAS PubMed.
- M. L. Ruiz Del Castillo, G. Dobson, R. Brennan and S. Gordon, J. Agric. Food Chem., 2004, 52, 948–952 CrossRef CAS PubMed.
- N. Garbacki, M. Kinet, B. Nusgens, D. Desmecht and J. Damas, J. Inflamm., 2005, 2, 9 CrossRef CAS PubMed.
- N. Garbacki, M. Tits, L. Angenot and J. Damas, BMC Pharmacol., 2004, 4, 25 CrossRef PubMed.
- C. Deng, N. Li and X. Zhang, J. Chromatogr. A, 2004, 1059, 149–155 CrossRef CAS PubMed.
- W. P. Liao, L. Chen, Y. H. Yi, W. W. Sun, M. M. Gao, T. Su and S. Q. Yang, Epilepsia, 2005, 46, 21–24 CrossRef PubMed.
- T. Han, P. Han, W. Peng and X. R. Wang, Pharm. Biol., 2013, 51, 589–594 CrossRef PubMed.
- H. Liu, Z. Song, D. G. Liao, T. Y. Zhang, F. Liu, K. Zhuang, K. Luo, L. Yang, J. He and J. P. Lei, Phyther. Res., 2015, 29, 996–1003 CrossRef CAS PubMed.
- Z. J. Wang, Y. Y. Zhu, X. Yi, Z. S. Zhou, Y. J. He, Y. Zhou, Z. H. Qi, D. N. Jin, L. X. Zhao and X. D. Luo, J. Ethnopharmacol., 2020, 261, 113–119 Search PubMed.
- L. Yan, Z. Liu, L. Xu, Y. Qian, P. Song and M. Wei, BMC Complementary Med. Ther., 2020, 20, 268 CrossRef PubMed.
- X. Tong, L. Zhou, Y. Wang, C. Xia, Y. Wang, M. Liang, F. Hou and Y. Cheng, Org. Lett., 2010, 13, 4478 CrossRef.
- M. Li, J. Xiong, Y. Huang, L. Wang, Y. Tang, G. Yang, X. Liu, B. Wei, H. Fan, Y. Zhao, W. Zhai and J. Hu, Tetrahedron, 2015, 71, 5285–5295 CrossRef CAS.
- A. L. Verano and D. S. Tan, Chem. Sci., 2017, 8, 3687–3693 RSC.
- J. M. Wurst, A. L. Verano and D. S. Tan, Org. Lett., 2012, 14, 4442–4445 CrossRef CAS PubMed.
- S. Gao, G. M. Fu, L. H. Fan, S. S. Yu and D. Q. Yu, J. Integr. Plant Biol., 2005, 47, 759–763 CrossRef CAS.
- Y. C. Hu, X. F. Wu, S. Gao, S. S. Yu, Y. Liu, J. Qu, J. Liu and Y. B. Liu, Org. Lett., 2006, 8, 2269–2272 CrossRef CAS PubMed.
- X. Wu, Y. Wang, S. Yu, N. Jiang, J. Ma, R. Tan and Y. Hu, Tetrahedron, 2011, 67, 8155–8159 CrossRef CAS.
- R. Safitri, L. Reniarti, M. Madihah, L. Delia, M. R. A. Syamsunarno and R. Panigoro, KnE life sci., 2017, 3, 497 CrossRef.
- R. Safitri, I. Indrawati, M. R. A. A. Syamsunarno, M. Ghozali, B. A. Gani and R. Panigoro, Asian J. Pharm. Clin. Res., 2018, 11, 444–447 CrossRef.
- N. P. Nirmal, M. S. Rajput, R. G. S. V. Prasad and M. Ahmad, Asian Pac. J. Trop. Med., 2015, 8, 421–430 CrossRef CAS PubMed.
- R. Safitri, P. Tarigan, H. Joachim and R. J. Rumampuk, BioFactors, 2003, 19, 71–77 CrossRef CAS PubMed.
- R. L. Melnick, C. Suárez, B. A. Bailey, P. A. Backman and P. Los Rios, Biol. Control, 2011, 57, 236–245 CrossRef.
- S. Mahendran, S. Saravanan, P. Vijayabaskar, K. T. K. Anandapandian and T. Shankar, Int. J. Recent Sci. Res., 2013, 4, 501–505 Search PubMed.
- H. B. Park, Y. J. Kim, J. K. Lee, K. R. Lee and H. C. Kwon, Org. Lett., 2012, 14, 5002–5005 CrossRef CAS PubMed.
- W. P. Unsworth, J. D. Cuthbertson and R. J. K. Taylor, Org. Lett., 2013, 15, 3306–3309 CrossRef CAS PubMed.
- J. M. Badr, L. A. Shaala, M. I. Abou-Shoer, M. K. Tawfik and A. A. M. Habib, J. Nat. Prod., 2008, 71, 1472–1474 CrossRef CAS PubMed.
- F. Bibi, M. Yasir, A. Al-Sofyani, M. I. Naseer and E. I. Azhar, Saudi J. Biol. Sci., 2020, 27, 1139–1147 CrossRef CAS PubMed.
- S. P. Gunasekera and S. S. Cross, J. Nat. Prod., 1992, 55, 509–512 CrossRef CAS PubMed.
- M. Tsuda, Y. Sakuma and J. Kobayashi, J. Nat. Prod., 2001, 64, 980–982 CrossRef CAS PubMed.
- B. F. Bowden, B. J. McCool and R. H. Willis, J. Org. Chem., 2004, 69, 7791–7793 CrossRef CAS PubMed.
- A. T. Abbas, N. A. El-Shitany, L. A. Shaala, S. S. Ali, E. I. Azhar, U. A. Abdel-Dayem and D. T. A. Youssef, J. Evidence-Based Complementary Altern. Med., 2014, 2014, 1–9 CrossRef PubMed.
- P. Das and A. T. Hamme, Eur. J. Org. Chem., 2015, 2015, 5159–5166 CrossRef CAS.
- S. Nishiyama and S. Yamamura, Bull. Chem. Soc. Jpn., 1985, 58, 3453–3456 CrossRef CAS.
- J. W. Shearman, R. M. Myers, J. D. Brenton and S. V. Ley, Org. Biomol. Chem., 2011, 9, 62–65 RSC.
- S. Maddela, A. Makula, M. D. Galigniana, D. G. T. Parambi, F. Federicci, G. Mazaira, O. M. Hendawy, S. Dev, G. E. Mathew and B. Mathew, Arch. Pharm., 2018, 351, 1800174 CrossRef PubMed.
- A. Dandia, D. Saini and S. Bhaskaran, Med. Chem. Res., 2014, 23, 725–734 CrossRef CAS.
- R. Shrestha, K. Sharma, Y. Rok and L. Y. Wee, Mol. Diversity, 2016, 20, 847–858 CrossRef CAS PubMed.
- S. K. Das, J. Adv. Pharm. Technol. Res., 2013, 4, 198–205 CrossRef CAS PubMed.
- M. Moghaddam-manesh, D. Ghazanfari and E. Sheikhhossein, ChemistrySelect, 2019, 4, 9247–9251 CrossRef CAS.
- S. T. Al-rashood, A. R. Hamed, G. S. Hassan, M. Hamad, A. A. Almehizia, A. Alharbi, M. M. Al-, W. M. Eldehna, A. A. Almehizia, A. Alharbi, M. M. Al-sanea and W. M. Eldehna, J. Enzyme Inhib. Med. Chem., 2020, 35, 831–839 CrossRef CAS PubMed.
- M. Moghaddam-manesh, E. Sheikhhosseini and D. Ghazanfari, Bioorg. Chem., 2020, 98, 103751 CrossRef CAS PubMed.
- W. M. Eldehna, D. H. El-Naggar, A. R. Hamed, H. S. Ibrahim, H. A. Ghabbour and H. A. Abdel-Aziz, J. Enzyme Inhib. Med. Chem., 2018, 33, 309–318 CrossRef CAS PubMed.
- V. B. Nishtala, D. Gandamalla and N. R. Yellu, Synth. Commun., 2019, 40, 2671–2682 CrossRef.
- M. Zarei, H. Sepehrmansourie, M. A. Zolfigol, R. Karamian and S. H. M. Farida, New J. Chem., 2018, 42, 14308–14317 RSC.
- E. Pelit, J. Chem., 2017, 2017, 1–9 CrossRef.
- R. Baharfar and R. Azimi, J. Chem. Sci., 2015, 127, 1389–1395 CrossRef CAS.
- R. Azimi and R. Baharfar, Can. J. Chem., 2014, 92, 1163–1168 CrossRef CAS.
- U. Hossain Sk, A. K. Sharma, S. Ghosh and S. Bhattacharya, Eur. J. Med. Chem., 2010, 45, 3265–3273 CrossRef PubMed.
- A. R. Moosavi-zare, M. A. Zolfigol, E. Noroozizadeh, M. Zarei, R. Karamian and M. Asadbegy, J. Mol. Catal. A. Chem., 2016, 425, 217–228 CrossRef CAS.
- Z. Iqbal, G. Morahan, M. Arooj, A. N. Sobolev and S. Hameed, Eur. J. Med. Chem., 2019, 168, 154–175 CrossRef CAS PubMed.
- S. Ghannay, S. Bakari, A. Ghabi, A. Kadri, M. Msaddek and K. Aouadi, Bioorg. Med. Chem. Lett., 2017, 27, 2302–2307 CrossRef CAS PubMed.
- G. Cİhan-üstündağ, N. Özsoy, E. Öztaş, N. Karali and G. Çapan, Marmara Pharm. J., 2017, 21, 978–986 CrossRef.
- M. M. Youssef and M. A. Amin, Molecules, 2010, 15, 8827–8840 CrossRef CAS PubMed.
- V. Panteleon, I. K. Kostakis, P. Marakos, N. Pouli and I. Andreadou, Bioorg. Med. Chem. Lett., 2008, 18, 5781–5784 CrossRef CAS PubMed.
- E. M. Flefel and H. H. Sayed, Med. Chem. Res., 2014, 23, 2515–2527 CrossRef CAS.
- R. Srinivasan, B. Narayana, B. Kunhanna, C. Govindaraju and D. Raj, Indian J. Chem., 2018, 57, 1391–1408 Search PubMed.
- M. A. Ramos-enríquez, O. N. Medina-campos, J. Pedraza-chaverri and M. A. Iglesias-arteaga, Steroids, 2015, 98, 132–137 CrossRef PubMed.
- E. Balachandravinayagam, M. Natarajan and S. Ganesan, Int. J. Pharm., Chem. Biol. Sci., 2014, 4, 620–627 Search PubMed.
- R. C. Gomes, R. P. Sakata, W. P. Almeida and F. Coelho, Med. Chem., 2019, 15, 373–382 CrossRef CAS PubMed.
- D. Ashok, E. V. L. Madhuri, M. Sarasija, S. S. Kanth, M. Vijjulatha, M. D. Alaparthi and S. R. Sagurthi, RSC Adv., 2017, 7, 25710–25724 RSC.
- P. Blier, R. Bergeron and C. de Montigny, Neuropsychopharmacology, 1997, 16, 333–338 CrossRef CAS PubMed.
- S. Caccia, I. Conti, G. Vigano and S. Garattini, Pharmacology, 1986, 33, 46–51 CrossRef CAS PubMed.
- R. L. M. de Freitas, Í. M. de S. Santos, G. F. de Souza, A. da R. Tomé, G. B. Saldanha and R. M. de Freitas, Brain Res. Bull., 2010, 81, 505–509 CrossRef PubMed.
- J. Mou, Z. M. Zong and X. Y. Wei, Org. Prep. Proced. Int., 2008, 40, 391–394 CrossRef CAS.
- D. A. Alonso, C. Nájera and M. C. Pacheco, Org. Lett., 2000, 2, 1823–1826 CrossRef CAS PubMed.
- R. Soto-Otero, E. Méndez-Álvarez, S. Sánchez-Iglesias, F. I. Zubkov, L. G. Voskressensky, A. V Varlamov, M. de Candia and C. Altomare, Biochem. Pharmacol., 2008, 75, 1526–1537 CrossRef CAS PubMed.
- A. Varlamov, V. Kouznetsov, F. Zubkov, A. Chernyshev, G. Alexandrov, A. Palma, L. Vargas and S. Salas, Synthesis, 2001, 2001, 0849–0854 CrossRef.
- M. Zahedifar, B. Pouramiri, F. Ezzati Ghadi, R. Razavi and A. Ramzani Ghara, Mol. Diversity, 2019, 2019, 1–15 Search PubMed.
- A. M. Venkatesan, A. Agarwal, T. Abe, H. Ushirogochi, I. Yamamura, M. Ado, T. Tsuyoshi, O. Dos Santos, Y. Gu, F. W. Sum, Z. Li, G. Francisco, Y. I. Lin, P. J. Petersen, Y. Yang, T. Kumagai, W. J. Weiss, D. M. Shlaes, J. R. Knox and T. S. Mansour, J. Med. Chem., 2006, 49, 4623–4637 CrossRef CAS PubMed.
- R. Anisetti and M. S. Reddy, J. Sulfur Chem., 2012, 33, 363–372 CrossRef CAS.
- L. D. S. Chaves, L. A. D. Nicolau, R. O. Silva, F. C. N. Barros, A. L. P. Freitas, K. S. Aragão, R. D. A. Ribeiro, M. H. L. P. Souza, A. L. D. R. Barbosa and J. V. R. Medeiros, Immunopharmacol. Immunotoxicol., 2013, 35, 93–100 CrossRef CAS PubMed.
- A. Nabil-Adam, M. A. Shreadah, N. M. Abd El-Moneam and S. A. El-Assar, Recent Pat. Biotechnol., 2020, 14, 203–228 CrossRef CAS PubMed.
- J. P. L. De Castro, L. E. C. Costa, M. P. Pinheiro, T. Dos Santos Francisco, P. H. M. De Vasconcelos, L. M. Funari, R. M. Daudt, G. R. C. Dos Santos, N. S. M. Cardozo and A. L. P. Freitas, Polimeros, 2018, 28, 178–186 CrossRef.
- B. W. S. Souza, M. A. Cerqueira, A. I. Bourbon, A. C. Pinheiro, J. T. Martins, J. A. Teixeira, M. A. Coimbra and A. A. Vicente, Food Hydrocoll, 2012, 27, 287–292 CrossRef CAS.
- K. Chakraborty and T. Antony, Nat. Prod. Lett., 2019, 2019, 770–781 Search PubMed.
- W. F. Peng, X. Wang, Z. Hong, G. X. Zhu, B. M. Li, Z. Li, M. P. Ding, Z. Geng, Z. Jin, L. Miao, L. W. Wu and S. K. Zhan, Seizure, 2015, 29, 26–33 CrossRef PubMed.
- R. D. Divate, P. M. Wang, C. C. Wang, S. T. Chou, C. T. Chang and Y. C. Chung, Int. J. Immunopathol. Pharmacol., 2017, 30, 105–112 CrossRef CAS PubMed.
- F. Wang, S. Han, S. Hu, Y. Xue, J. Wang, H. Xu, L. Chen, G. Zhang and Y. Zhang, Molecules, 2014, 19, 1250–1257 CrossRef PubMed.
- Y. Lin, X. Y. Wang, R. Ye, W. H. Hu, S. C. Sun, H. J. Jiao, X. H. Song, Z. Z. Yuan, Y. Y. Zheng, G. Q. Zheng and J. C. He, J. Ethnopharmacol., 2013, 145, 320–327 CrossRef PubMed.
- Y. P. Ma, D. B. Mao, L. J. Geng, W. Y. Zhang, Z. Wang and C. P. Xu, Chem. Biochem. Eng. Q., 2013, 27, 177–184 CAS.
- Z. Zhao, Y. Li, H. Chen, L. Huang, F. Zhao, Q. Yu, Z. Xiang and Z. Zhao, Int. J. Clin. Exp. Med., 2014, 7, 356–362 Search PubMed.
- H.-J. Ko, A. Song, M.-N. Lai and L.-T. Ng, J. Ethnopharmacol., 2011, 138, 762–768 CrossRef PubMed.
- A. Song, H. J. Ko, M. N. Lai and L. T. Ng, Immunopharmacol. Immunotoxicol., 2011, 33, 454–460 CrossRef CAS PubMed.
- J. Xiong, Y. Huang, X.-Y. Wu, X.-H. Liu, H. Fan, W. Wang, Y. Zhao, G.-X. Yang, H.-Y. Zhang and J.-F. Hu, Helv. Chim. Acta, 2016, 99, 83–89 CrossRef CAS.
- G. Wu, Wei Sheng Wu Xue Bao, 2001, 41, 363–366 CAS.
- M. Ghate, R. A. Kusanur and M. V. Kulkarni, Eur. J. Med. Chem., 2005, 40, 882–887 CrossRef CAS PubMed.
- M. Salem, M. Marzouk and A. El-Kazak, Molecules, 2016, 21, 249 CrossRef PubMed.
- M. Zhu, L. Ma, J. Wen, B. Dong, Y. Wang, Z. Wang, J. Zhou, G. Zhang, J. Wang, Y. Guo, C. Liang, S. Cen and Y. Wang, Eur. J. Med. Chem., 2020, 186, 111900 CrossRef CAS PubMed.
- F. Borges, F. Roleira, N. Milhazes, L. Santana and E. Uriarte, Curr. Med. Chem., 2005, 12, 887–916 CrossRef CAS PubMed.
- Z. M. Nofal, M. I. El-Zahar and S. S. Abd El-Karim, Molecules, 2000, 5, 99–113 CrossRef CAS.
- F. G. Medina, J. G. Marrero, M. Macías-Alonso, M. C. González, I. Córdova-Guerrero, A. G. Teissier García and S. Osegueda-Robles, Nat. Prod. Rep., 2015, 32, 1472–1507 RSC.
- V. Panteleon, P. Marakos, N. Pouli and E. Mikros, J. Pharm. Pharmacol., 2003, 55, 1029–1039 CrossRef CAS PubMed.
- A. Kumar, K. Srivastava, S. Raja Kumar, S. K. Puri and P. M. S. Chauhan, Bioorg. Med. Chem. Lett., 2010, 20, 7059–7063 CrossRef CAS PubMed.
- N. C. Desai, G. M. Kotadiya and A. R. Trivedi, Bioorg. Med. Chem. Lett., 2014, 24, 3126–3130 CrossRef CAS PubMed.
- V. R. Solomon, C. Hu and H. Lee, Bioorg. Med. Chem., 2010, 18, 1563–1572 CrossRef CAS PubMed.
- A. Mahamoud, J. Chevalier, A. Davin-Regli, J. Barbe and J.-M. Pages, Curr. Drug Targets, 2006, 7, 843–847 CrossRef CAS PubMed.
- L. Strekowski, V. A. Honkan, A. Czarny, M. T. Cegla, R. L. Wydra, S. E. Patterson, J. L. Mokrosz and R. F. Schinazi, J. Med. Chem., 1991, 34, 1739–1746 CrossRef CAS PubMed.
- J. W. Ullrich, R. Morris, R. C. Bernotas, J. M. Travins, J. Jetter, R. Unwalla, E. Quinet, P. Nambi, I. Feingold, C. Huselton, C. Enroth, A. Wilhelmsson, A. Goos-Nilsson and J. Wrobel, Bioorg. Med. Chem. Lett., 2010, 20, 2903–2907 CrossRef CAS PubMed.
- G. Maga, S. Gemma, C. Fattorusso, G. A. Locatelli, S. Butini, M. Persico, G. Kukreja, M. P. Romano, L. Chiasserini, L. Savini, E. Novellino, V. Nacci, S. Spadari and G. Campiani, Biochemistry, 2005, 44, 9637–9644 CrossRef CAS PubMed.
- F. Sliman, M. Blairvacq, E. Durieu, L. Meijer, J. Rodrigo and D. Desmaële, Bioorg. Med. Chem. Lett., 2010, 20, 2801–2805 CrossRef CAS PubMed.
- Y. Yang, L. Shi, Y. Zhou, H. Q. Li, Z. W. Zhu and H. L. Zhu, Bioorg. Med. Chem. Lett., 2010, 20, 6653–6656 CrossRef CAS PubMed.
- V. Panteleon, I. K. Kostakis, P. Marakos, N. Pouli and I. Andreadou, Chem. Pharm. Bull., 2009, 57, 446–452 CrossRef CAS PubMed.
- J. Nawrot-Modranka, E. Nawrot and J. Graczyk, Eur. J. Med. Chem., 2006, 41, 1301–1309 CrossRef CAS PubMed.
- B. dui Wang, Z. Y. Yang and T. rong Li, Bioorg. Med. Chem., 2006, 14, 6012–6021 CrossRef PubMed.
- L. Pisco, M. Kordian, K. Peseke, H. Feist, D. Michalik, E. Estrada, J. Carvalho, G. Hamilton, D. Rando and J. Quincoces, Eur. J. Med. Chem., 2006, 41, 401–407 CrossRef CAS PubMed.
- M. C. P. Nilesh N Gajera, Der Chem. Sin., 2012, 3, 80–90 Search PubMed.
- J. M. Elliott, H. G. Selnick, D. A. Claremon, J. J. Baldwin, S. A. Buhrow, J. W. Butcher, C. N. Habecker, S. W. King, J. J. Lynch, B. T. Phillips, G. S. Ponticello, E. M. Radzilowski, D. C. Remy, R. B. Stein, J. I. White and M. B. Young, J. Med. Chem., 1992, 35, 3973–3976 CrossRef CAS PubMed.
- B. Le Bourdonnec, R. T. Windh, L. K. Leister, Q. J. Zhou, C. W. Ajello, M. Gu, G. H. Chu, P. A. Tuthill, W. M. Barker, M. Koblish, D. D. Wiant, T. M. Graczyk, S. Belanger, J. A. Cassel, M. S. Feschenko, B. L. Brogdon, S. A. Smith, M. J. Derelanko, S. Kutz, P. J. Little, R. N. Dehaven, D. L. DeHaven-Hudkins and R. E. Dolle, J. Med. Chem., 2009, 52, 5685–5702 CrossRef CAS PubMed.
- D. Ashok, D. Mohan, G. Aamate and V. Kumar, Med. Chem. Res., 2016, 25, 2882–2894 CrossRef CAS.
- D. Ashok, S. Gundu and V. Kumar, Russ. J. Gen. Chem., 2015, 85, 708–717 CrossRef CAS.
- R. Naresh Kumar, G. Jitender Dev, N. Ravikumar, D. Krishna Swaroop, B. Debanjan, G. Bharath, B. Narsaiah, S. Nishant Jain and A. Gangagni Rao, Bioorg. Med. Chem. Lett., 2016, 26, 2927–2930 CrossRef CAS PubMed.
- J. Klenc, E. Raux, S. Barnes, S. Sullivan, B. Duszynska, A. J. Bojarski and L. Strekowski, J. Heterocycl. Chem., 2009, 46, 1259–1265 CrossRef.
- M. Lavanya, I. V. Asharani and D. Thirumalai, Chem. Biol. drug desing, 2019, 93, 464–472 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra01170g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |