Open Access Article
Open Access ArticleFluorinated MIL-101 for carbon capture utilisation and storage: uptake and diffusion studies under relevant industrial conditions†
Paola A. Sáenz Cavazosa,
Mariana L. Díaz-Ramírez b,
Elwin Hunter-Sellarsa,
Sean R. McIntyrea,
Enrique Limab,
Ilich A. Ibarra
b,
Elwin Hunter-Sellarsa,
Sean R. McIntyrea,
Enrique Limab,
Ilich A. Ibarra *b and
Daryl R. Williams*a
*b and
Daryl R. Williams*a
aSurfaces and Particle Engineering Laboratory (SPEL), Department of Chemical Engineering, Imperial College London, South Kensington Campus, London SW7 2AZ, UK. E-mail: d.r.williams@imperial.ac.uk
bLaboratorio de Fisicoquímica y Reactividad de Superficies (LaFReS), Instituto de Investigaciones en Materiales, Universidad Nacional Autónoma de México, Circuito Exterior s/n, CU, Coyoacán, 04510 Ciudad de México, Mexico. E-mail: argel@unam.mx
First published on 12th April 2021
Abstract
Carbon capture utilisation and storage (CCUS) using solid sorbents such as zeolites, activated carbon and Metal–Organic Frameworks (MOFs) could facilitate the reduction of anthropogenic CO2 concentration. Developing efficient and stable adsorbents for CO2 capture as well as understanding their transport diffusion limitations for CO2 utilisation plays a crucial role in CCUS technology development. However, experimental data available on CO2 capture and diffusion under relevant industrial conditions is very limited, particularly for MOFs. In this study we explore the use of a gravimetric Dynamic Vapour Sorption (DVS) instrument to measure low concentration CO2 uptake and adsorption kinetics on a novel partially fluorinated MIL-101(Cr) saturated with different water vapour concentrations, at ambient pressure and temperature. Results show that up to water P/P0 = 0.15 the total CO2 uptake of the modified material improves and that the introduction of small amounts of water enhances the diffusion of CO2. MIL-101(Cr)-4F(1%) proved to be a stable material under moist conditions compared to other industrial MOFs, allowing facile regeneration under relevant industrial conditions.
Introduction
It is well known that clean energy sources and carbon capture utilisation and storage (CCUS) are vital for climate change mitigation and limiting global warming. However, due to a variety of technical, economic and commercial challenges CCUS has not been fully deployed to the required scale.1,2 According to the Global Carbon Capture and Storage (CCS) Institute there are 51 large-scale CCS facilities around the world, of which, only 19 are fully operating and an estimated 2000 more are needed to achieve global climate targets.2Large-scale projects that utilize CO2 capture with solid state adsorbents are few compared to the widely used liquid absorption technologies.1 One of the main reasons for this difference is the enormous variety of available potential adsorbent materials, which makes the screening process for CCS sorbents tremendously challenging. Particularly in the case of Metal–Organic Frameworks (MOFs) which have an inherent adsorption tunability due to their unique chemical structure. MOFs ability to incorporate open metal sites (OMSs), Lewis basic sites (LBSs), covalently bound polar functional groups, framework flexibility, and hydrophobicity offers thousands of ‘tailor design’ possibilities to enhance adsorption capacity, selectivity, and stability.1,3
While high-throughput simulation and modelling techniques have been shown to greatly accelerate the pace of identifying promising candidates for CO2 capture, their evaluation criteria usually concerns a single adsorption or material property at a time.4–7 For instance, Wilmer et al. established correlations between CO2/N2 separation ability based solely on MOFs structural and chemical characteristics without considering the other additional components present in industrial flue gases, like water vapour, oxygen and acidic gases (i.e. H2S, NOx, SOx).7 Going one step further, Li et al. identified potential MOFs for Post-combustion Carbon Capture (PCC) in the presence of high relative humidity (RH) 80% based on their CO2/H2O selectivity.5 Though understanding MOFs performance at high RH's is important, the amount of water usually present in the flue gas is around 4–18% RH.8–10 In addition, and very interestingly, various studies have confirmed that the presence of low amounts of water can sometimes enhance the CO2 uptake of several materials.11–15 From a cost perspective, Huck et al. proposed a new approach for material evaluation based on parasitic energy which is a metric that predicts the total energy penalty imposed on a CCS power plant using porous adsorbents based on a thermodynamic analysis.4 What all these approaches fail to consider is several relevant industrial conditions simultaneously, due mainly to the lack of experimental data needed to develop such complex models.
Additionally, some MOFs can capture and convert CO2 at the same time. MOF's high surface area and porous structure provide favourable catalytic efficiencies and high reaction yields, with some of these bifunctional materials allowing a direct transition from CCS to CCUS.8,16 Understanding transport diffusion limitations in MOFs could positively influence the catalyst effectiveness and facilitate the design of adsorption processes.17 Nevertheless, this information requirement adds another level of complexity to the adsorbent screening process because the data available on mass-transfer resistance and diffusion limitations for MOFs is still scarce.1,3 Fast and accurate characterisation techniques that provide crucial experimental data on solid sorbents, when linked together with molecular simulation and process-scale modelling are particularly desirable to move on to the next technology readiness level.1,3,16
In this study we investigate the use of a gravimetric Dynamic Vapour Sorption (DVS) instrument as a rapid characterisation technique for novel adsorbent materials that permits several relevant industrial conditions to be investigated, allowing a range of equilibrium and kinetics descriptors of the adsorption process to be determined. In order to highlight the tunability and bifunctionality of MOFs we chose MIL-101(Cr)-4F(1%) as our material for this case study. This material is the result of the successful partial fluorination of MIL-101(Cr); which we achieved recently.18 MIL-101(Cr)-4F(1%) showed an increased hydrophobicity and a higher selectivity towards CO2 in anhydrous conditions when compared to its pristine form MIL-101(Cr).18 Furthermore, MIL-101(Cr) based composites have shown outstanding catalytic activity (96.5% yield) in the conversion of terminal alkynes into propiolic acids with CO2, as well as in several other carbon conversion reactions.3,19,20
The DVS instrument was used to measure and analyse adsorption and co-adsorption of low concentration CO2 and H2O on the enhanced material and its pristine form at ambient pressure and temperature (298 K and 1 bar), attempting to replicate real industrial conditions. To further understand the CO2 diffusion limitations in the presence of water vapour of these materials, we used the gravimetric kinetic data collected and determined the CO2 diffusion coefficient as a function of water vapour concentration. To the best of our knowledge, this study is the first time CO2 experiments under the presence of water vapour are reported on this material. The data obtained in this study on MIL-101(Cr)-4F(1%) and MIL-101(Cr) provides a broader understanding of novel materials capabilities for carbon capture and conversion under pertinent industrial conditions.
Experimental
Materials
MIL-101(Cr) and MIL-101(Cr)-4F(1%) were synthesised as described in previous literature.18 Samples were activated at 453 K under vacuum (10−3 bar) for 2 h before every experiment to ensure any contaminants or excess moisture were removed. Commercial MOF samples of HKUST-1 and MIL-53(Al) were purchased from BASF and activated using the same process mentioned above.Sorption measurements
All sorption measurements were carried out gravimetrically using a DVS Resolution (Surface Measurement Systems, UK) using an electronic microbalance with a resolution of ±0.01 μg and a symmetric measurement configuration. Meaning that both, the sample pan, and the reference pan are exposed to the same gas at the same temperature and ambient pressure, thus minimising buoyancy effects. The balance itself is fitted inside a stainless-steel housing unit which is mounted above the gas/vapor adsorption manifold. The entire balance manifold system is located inside a temperature enclosure that maintains a temperature uniformity of <0.1 K. A series of mass flow controls allows gas mixtures containing air, CO2 and H2O to be prepared which flow over the adsorbent sample. The relative humidity and CO2 concentration within the gas stream are measured in real time with capacitance and speed of sound sensors respectively.The equipment was leak checked before each experiment by measuring the system output gas flowrate with an independent mass flow meter (Agilent Technologies, USA) and comparing it to the system input flowrate, ensuring both values were always the same. After sample activation, 10–30 mg of sample was placed inside the sample pan for measuring water and CO2 isotherms at 298 K and 1 bar. Sufficient time was allowed for the samples to reach mass equilibrium at each of the user selected gas phase concentrations, until no significant mass change was detected (<0.00075% min−1 dm/dt). The equipment recorded the change in mass versus time using the DVS software. A total flow rate of 200 cm3 min−1 of dry air was used as carrier gas. We also performed single component experiments using pure N2 as a carrier gas and no significant difference in water or CO2 uptake was observed. In order to better simulate “real world” conditions dry air containing ∼78% N2 and ∼21% O2 was used throughout all experiments.
Co-adsorption measurements
Co-adsorption measurements were carried out using the dual-solvent mode of the DVS Resolution. Following activation, samples were exposed to a selected range of partial pressure of water vapour to simulate flue gas composition (P/P0,water = 0, 0.05, 0.1, 0.15 and 0.2), and while this pressure was held constant, a step increase in CO2 concentration to 0.05 bar was introduced, followed by desorption of CO2 and finally desorption of water vapour.Considering that we were also interested in the kinetic CO2 sorption profiles from the co-adsorption measurements to extract CO2 diffusion coefficients. Smaller sample masses (5–20 mg) widely dispersed in the sample pan were used to avoid bed resistances and heat transfer limitations.21 All steps were carried out at constant temperature and total pressure (298 K and 1 bar). Enough time was allowed for the samples to reach equilibrium with the gas phase until no significant mass change was detected (0.00075% min−1 dm/dt). Dry air with a total flow rate of 400 cm3 min−1 was used as carrier gas. This methodology has been previously used in other studies to measure multi-component sorption.22,23
Particle size
A sieve analysis using test sieves of different aperture 250, 180 and 90 μm (Endecotts Ltd, UK) and an automatic sieve shaker from the same manufacturer allowed to determine the average particle size of the materials studied. The ASTM standard test method for particle size distribution of catalytic materials by sieving was applied.24Results and discussion
Water isotherms
The shape of the water isotherm of the functionalised material at 295 K and 1 bar (see Fig. 1) corresponds to that of a mesoporous solid with a Type V isotherm, where multilayer adsorption is observed at low partial pressures 0 to 0.2 P/P0, followed by a clear hysteresis loop between 0.2 and 0.6 P/P0 that can be associated with capillary condensation taking place in the mesopores. The maximum water uptake for MIL-101(Cr)-4F(1%) was 49.1 mmol g−1, which is lower than that reported for the pristine material under the same conditions (55.5 mmol g−1).25 This data supports an overall augmented hydrophobicity that can be attributed to the incorporation of fluorine atoms in this material.18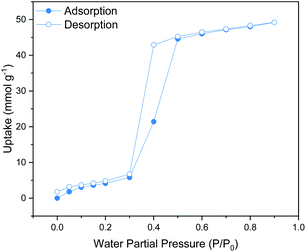 | ||
| Fig. 1 Water (H2O) adsorption–desorption isotherms at 298 K and 1 bar of MIL-101(Cr)-4F(1%) from P/P0 = 0 to 0.9. Solid circles represent adsorption and open circles represent desorption. | ||
CO2 isotherms
CO2 adsorption isotherms of MIL-101(Cr) and MIL-101(Cr)-4F(1%) were obtained at ambient pressure and temperature (298 K and 1 bar). Both isotherms present a linear behaviour (see Fig. 2). The CO2 uptake of the two materials is very similar up to 0.6 bar, whereas at higher partial pressures the pristine material shows a slightly increased CO2 uptake. This can be attributed to the lower pore volume of MIL-101(Cr)-4F(1%) compared to MIL-101(Cr), 1.19 cm3 g−1 and 1.32 cm3 g−1 respectively.18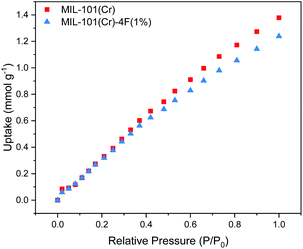 | ||
| Fig. 2 Carbon dioxide (CO2) adsorption isotherms at 298 K and 1 bar of MIL-101(Cr) and MIL-101(Cr)-4F(1%) relative CO2 partial pressures range from P/P0 = 0 to 1.0. | ||
Given that the pressure region we are interested in for PCC applications is between 0 and 0.2 bar, we can say that both materials have comparable CO2 uptakes within this region. However, MIL-101(Cr)-4F(1%) has the advantage of being more hydrophobic, making it less vulnerable when exposed to moist environments and a better candidate than MIL-101(Cr) under real industrial capture conditions.26,27
Co-adsorption of water and CO2
Encouraged by the results from a previous work,18 where we showed that CO2 moves easier through MIL-101(Cr) than through MIL-101(Cr)-4F(1%) due to a less acidic nature of Cr(III) sites on the pristine material, and considering that the CO2 level present in flue gases is regularly between 4–14% in volume,28 we decided to explore the effects of low concentration CO2 sorption and diffusion under humid conditions on both materials. We hypothesised that the introduction of small amounts of water in the framework of MIL-101(Cr) and MIL-101(Cr)-4F(1%) would promote the transport of CO2 through the materials. This is because water molecules would occupy the coordinatively unsaturated sites (CUS) resulting from the activation of the material. In small amounts, the water molecules would be well-ordered inside the MOF cavities providing a smoother path for incoming CO2 molecules and would also lower the strength of the interactions of the framework with the CO2.Five different RH concentrations were studied 0, 10, 15 and 20%, which correspond to water partial pressures of 0, 0.05, 0.10, 0.15 and 0.20 P/P0 respectively. For all RH's the CO2 concentration was kept constant at 5%, which corresponds to a CO2 pressure of 0.05 bar in ambient conditions. The data obtained from a typical co-adsorption experiment consists of four steps, two for adsorption and two for desorption (see Fig. 3). CO2 uptake was calculated by subtracting the mass at the end the second step from the mass at the end of the first step. The results from the co-adsorption experiments are presented in Fig. 4. For dry materials, i.e. P/P0 = 0, the partially fluorinated material has a very slight lower CO2 uptake than its pristine form (0.086 vs. 0.092 mmol g−1, respectively) which agrees with our previous findings from the single component CO2 isotherms. However, the materials behave very differently when water vapour is present. For MIL-101(Cr) the presence of water is detrimental for its performance, with CO2 capacity decreasing at higher water partial pressures, up to the highest value studied (P/P0 = 0.2), where its CO2 uptake decreases by around 18% compared to the dry material.
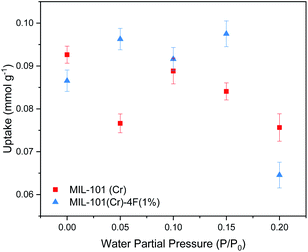 | ||
| Fig. 4 Carbon dioxide (CO2) adsorption capacities of MIL-101(Cr) and MIL-101(Cr)-4F(1%) of CO2 P/P0 = 0.05 at different water partial pressures (0, 0.05, 0.1, 0.15 and 0.2 P/P0) at 298 K and 1 bar. | ||
MIL-101(Cr) shows no direct proportionality between the CO2 adsorption capacity and the quantity of water present on the sample, which agrees with previous findings.29 On the other hand, MIL-101(Cr)-4F(1%) shows a CO2 uptake enhancement at low and moderate water loadings, reaching a maximum uptake of 0.097 mmol g−1 at P/P0 = 0.15. Nevertheless, at P/P0 = 0.2 bar the fluorinated material shows a 22% lower CO2 uptake compared to the dry material. This behaviour can be attributed to the loss of terminal water molecules in the Cr–O cluster during the activation of the material, complete loss of these water molecules means that some favoured CO2 adsorption sites may be lost as well. However, with increasing RH values, some of these adsorption sites are recovered and therefore, the uptake improves. There is a value of RH where the presence of H2O molecules has no longer a positive effect, and rather, these molecules compete for adsorption sites with CO2. This phenomenon has been previously observed in other MOFs with CUS, such as Cu-BTC30 and UiO-66.31 It is important to mention that while the CO2 uptake of the materials studied is not exceptional under real industrial conditions, they are both very stable when exposed to moist environments, compared to other commercial materials such as HKUST-1 or MIL-53(Al). Specifically, under the same conditions, where their CO2 uptake at water P/P0 = 0.2 decreases by 99% and 80% respectively compared to their dry form (see Fig. S2 ESI†). Further CO2 enhancement on the fluorinated material could be achieved by the incorporation of amine groups such as alkylamines, polyamines or multiunit amines via wet impregnation methods. This has shown to improve MOFs CO2 adsorption capacity and regeneration stability in dilute CO2 concentrations due to the high amine–CO2 reaction equilibrium constants, but is not in the scope of this study.32,33
Following adsorption, we studied the desorption of CO2 at different RHs. Desorption under these conditions is extremely relevant to the adsorbent regeneration step during temperature and pressure swing adsorption processes.34 Decreasing the energy requirements for this stage is crucial for the cost reduction and feasibility of CCS technologies.1,34 We observed that both materials, when dry, they were able to desorb ∼50% of the CO2. Fig. 5 shows the results once water is introduced. Since we are using a gravimetric technique, we measured the mass at the end of the last step for every experiment and compared it to the water adsorption isotherm. The results indicate that there is some water being re-absorbed or that some CO2 remains within the framework. If the latter is considered, we can say that at P/P0 = 0.05 the percent of CO2 desorbed increased to 90.32% and 71.80% for MIL-101(Cr) and MIL-101(Cr)-4F(1%) respectively. This could be because the water is promoting the transport of CO2 in the desorption step as well. As mentioned before the less acidic nature of Cr(III) allows for easier movement of the CO2 molecules through MIL-101(Cr) and thus the difference between the pristine form and the fluorinated one. Fig. 5 clearly indicates a stable amount of CO2 desorbed in MIL-101(Cr)-4F(1%) throughout all the RH studied, whereas for the pristine material, a decreasing trend can be observed. This could be related to the more hydrophobic nature of the material incorporated with fluorine atoms and could potentially translate in a reduction of the energy needed to desorb the remaining CO2. Thus, giving rise to MIL-101(Cr)-4F(1%) as a promising candidate for PCC.
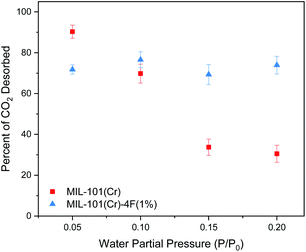 | ||
| Fig. 5 Percent of carbon dioxide (CO2) desorbed on MIL-101(Cr) and MIL-101(Cr)-4F(1%) with different water partial pressures (0, 0.05, 0.1, 0.15 and 0.2 P/P0) at 298 K and 1 bar. | ||
Additionally, ensuring the material is stable during various adsorption–desorption cycles and in the presence of other contaminants such as acidic gases could further help decrease costs.1 Remarkably, MIL-101(Cr)-4F(1%) has shown high SO2 capture under humid conditions and an outstanding cycling performance up to 50 cycles with facile regeneration.35 For industrial scale applications it is also important to consider the competition for adsorption sites with other acidic gases present in the flue gas, however this is not in the scope of the present study.
Determination of diffusion coefficients
Adsorption efficiency is greatly influenced by both equilibrium and kinetics.36 Therefore, determining parameters such as diffusivity is of great interest.Moreover, understanding diffusion limitations of MOFs is important for future catalytic applications in CCUS. In this study we used the dynamic gravimetric uptake curves from the co-adsorption experiments to determine adsorption kinetics and extract the CO2 diffusion coefficients of MIL-101(Cr) and MIL-101(Cr)-4F(1%) at a range of different water vapour concentrations.
The gravimetric instrument settings during the adsorption–desorption experiments were selected in order to minimize heat transfer and external mass transfer resistances.21 Firstly, we verified that the uptake experiments were slow enough so as to not mistake mass transfer with instrument response time. Fig. 6 shows the gravimetric measurement of 0.05 bar CO2 in MIL-101(Cr)-4F(1%) for different sample masses. The curves have a timescale in the order of 250 minutes and according to the manufacturer the system response time is <1 minute.37 Therefore, mass transfer kinetics can be measured accurately by assuming drag and buoyancy effects reach steady state almost immediately.21 Then, extra-crystalline diffusion and heat transfer control can also be avoided by changing the sample mass and obtaining consistent results (see Fig. 6).21,38 Finally, small amounts of sample were used to avoid bed resistances and heat transfer limitations.21
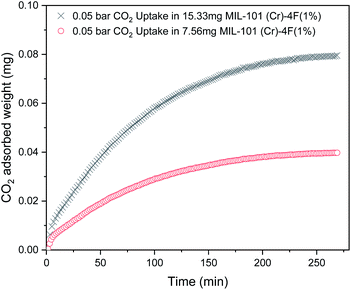 | ||
| Fig. 6 Carbon dioxide (CO2) gravimetric experiment uptakes for 15.33 mg and 7.56 mg MIL-101(Cr)-4F(1%) at 298 K and 0.05 bar. | ||
We assumed spherical particles for both materials and determined the CO2 diffusion coefficients in the presence of different water vapour concentrations using the following model (see eqn (1)).21,39 This model is derived from Fick's second law of diffusion and it is a good approximation for slow diffusing systems.
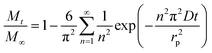 | (1) |
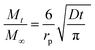 | (2) |
In order to determine the particle radius, the particle size distribution of MIL-101(Cr) and MIL-101(Cr)-4F(1%) was estimated using test sieves. Tables S1 and S2 ESI† show the results for each material. The average particle size can be found by plotting the cumulative percentage data against the sieve aperture and determining the size corresponding to 50%. The average particle radius of MIL-101(Cr) and MIL-101(Cr)-4F(1%) was 104 μm and 96 μm respectively. Table 1 shows the diffusion coefficients of MIL-101(Cr) and MIL-101(Cr)-4F(1%) at different water vapour loadings. The values obtained in this study fall in the range of experimental diffusion coefficients found for pure CO2 in MOFs or porous solids.40,41 The diffusion coefficient of both materials studied in dry conditions, is in the order of 8.6 × 10−10 and 5.0 × 10−10 cm2 s−1 for MIL-101(Cr) and MIL-101(Cr)-4F(1%), respectively. This means CO2 is able to move faster through the pristine material, conceding with what was reported in our previous study.18
| Water partial pressure P/P0 | MIL-101(Cr) | MIL-101(Cr)-4F(1%) | ||
|---|---|---|---|---|
| D/rp2 | D (cm2 s−1) | D/rp2 | D (cm2 s−1) | |
| 0 | 8.0 × 10−6 | 8.6 × 10−10 ± 2 × 10−11 | 5.4 × 10−6 | 5.0 × 10−10 ± 1 × 10−11 |
| 0.05 | 2.7 × 10−4 | 2.9 × 10−8 ± 1 × 10−11 | 1.5 × 10−4 | 1.3 × 10−8 ± 2 × 10−11 |
| 0.1 | 2.6 × 10−4 | 2.8 × 10−8 ± 1 × 10−11 | 1.5 × 10−4 | 1.3 × 10−8 ± 2 × 10−11 |
| 0.15 | 2.7 × 10−4 | 2.8 × 10−8 ± 1 × 10−11 | 1.7 × 10−4 | 1.5 × 10−8 ± 1 × 10−11 |
| 0.20 | 2.3 × 10−4 | 2.4 × 10−8 ± 1 × 10−11 | 5.9 × 10−5 | 5.3 × 10−9 ± 1 × 10−11 |
A small increase in water i.e., at P/P0 = 0.05 results in an easier transport of CO2, because water molecules adsorb on the most attractive sites for adsorption (i.e., open metal sites) providing a more homogeneous surface and consequently allowing CO2 molecules to move more freely. This phenomena is clearly observed with the diffusion coefficient values increasing in two orders of magnitude, 2.9 × 10−8 for MIL-101(Cr) and 1.3 × 10−8, for MIL-101(Cr)-4F(1%). For P/P0 between 0.05 and 0.15 the diffusion coefficients of the two materials remain almost unchanged, while for high water partial pressures i.e., at P/P0 = 0.2 the diffusion coefficient drops. Very interestingly, the decrease observed is more prominent on the fluorinated material compared to the pristine form.
Where the diffusion coefficient drops from 1.5 × 10−8 to 5.3 × 10−9. This could be related to the dramatic loss that we also observe with the CO2 uptake in MIL-101(Cr)-4F(1%) at the highest water partial pressure studied (P/P0 = 0.2) (see Fig. 4). However, the lowest diffusion coefficient for MIL-101(Cr)-4F(1%) does not coincide with the partial pressure that has the highest CO2 uptake.
This indicates that in order promote the interaction between the CO2 molecules and the porous material an optimum rate of transport must be achieved. This means, not too slow that the trapped molecules can block and thus impeding other molecules to pass, but not so fast that the molecules are not able to interact with the framework. A trade-off between uptake and kinetics should ultimately be considered depending on the specific process application.
Conclusions
Understanding the effects of different flue gas composition and conditions in the adsorption and kinetics of solid sorbents for CO2 capture is of great importance for CCUS technology to advance.In this study a single experimental technique (Dynamic Vapour Sorption, DVS) was used to determine CO2 uptake and adsorption kinetics of low concentration CO2 with different water vapour concentrations (0.0, 0.05, 0.10, 0.15 and 0.20) on MIL-101(Cr)-4F(1%) and its pristine form MIL-101(Cr). All experiments were carried out at ambient pressure and temperature to resemble economically feasible industrial conditions. Our results show that at low and moderate water loadings the total CO2 uptake capacity of MIL-101(Cr)-4F(1%) improved, with the best uptake (0.097 CO2 mmol g−1) at P/P0 = 0.15. However, higher partial pressures seem to inhibit CO2 uptake. As for the pristine material, the highest water loading decreases it's overall CO2 capacity by 18% compared to its dry form. Both materials present a stable behaviour in moist environments when compared to other commercial adsorbents with higher CO2 capacity (HKUST-1 and MIL-53(Al)) under the same conditions. Desorption results of CO2 with different water loadings at ambient pressure and temperature suggest that the fluorinated material would have a minimum energy penalty during the regeneration step.
CO2 diffusion coefficients at different water partial pressures were extracted from the mass uptake curves of the co-adsorption experiments. For both materials, CO2 diffusion occurs faster when water is introduced; this is because water is responsible for providing a more homogeneous surface and permits an easier movement for CO2. For water concentrations from P/P0 = 0.05 to 0.15 the CO2 diffusion coefficients of both materials remain stable, however, at the maximum water partial pressure studied P/P0 = 0.2 a drop in the diffusion coefficient in the fluorinated material can be observed, coinciding with a drop in CO2 uptake. This data suggests that certain water vapour concentrations of up to 0.15 P/P0 can promote CO2 diffusion which coincidentally corresponds to the water concentrations of most industrial importance for CCS. Above these conditions, a compromise between uptake and transport kinetics should be considered.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank PAPIIT UNAM (IN202820), México for financial support. M. L. D.-R. thanks CONACyT, México for the PhD grant number 276862. U. Winnberg and G. Ibarra-Winnberg for scientific input. P. A. S.-C would like to thank CONACyT-SENER for the PhD grant in Energy and Sustainability.References
- M. Bui, C. S. Adjiman, A. Bardow, E. J. Anthony, A. Boston, S. Brown, P. S. Fennell, S. Fuss, A. Galindo, L. A. Hackett, J. P. Hallett, H. J. Herzog, G. Jackson, J. Kemper, S. Krevor, G. C. Maitland, M. Matuszewski, I. S. Metcalfe, C. Petit, G. Puxty, J. Reimer, D. M. Reiner, E. S. Rubin, S. A. Scott, N. Shah, B. Smit, J. P. M. Trusler, P. Webley, J. Wilcox and N. Mac Dowell, Energy Environ. Sci., 2018, 11, 1062–1176 RSC.
- G. C. Institute, Global Status of CCS: Brief for Policymakers, 2020, https://www.globalccsinstitute.com/resources/publications-reports-research/ Search PubMed.
- M. Ding, R. W. Flaig, H.-L. Jiang and O. M. Yaghi, Chem. Soc. Rev., 2019, 48, 2783–2828 RSC.
- J. M. Huck, L.-C. Lin, A. H. Berger, M. N. Shahrak, R. L. Martin, A. S. Bhown, M. Haranczyk, K. Reuter and B. Smit, Energy Environ. Sci., 2014, 7, 4132–4146 RSC.
- S. Li, Y. G. Chung and R. Q. Snurr, Langmuir, 2016, 32, 10368–10376 CrossRef CAS PubMed.
- M. Tong, Y. Lan, Q. Yang and C. Zhong, Green Energy Environ., 2018, 3, 107–119 CrossRef.
- C. E. Wilmer, O. K. Farha, Y.-S. Bae, J. T. Hupp and R. Q. Snurr, Energy Environ. Sci., 2012, 5, 9849–9856 RSC.
- L. Liang, C. Liu, F. Jiang, Q. Chen, L. Zhang, H. Xue, H.-L. Jiang, J. Qian, D. Yuan and M. Hong, Nat. Commun., 2017, 8, 1233 CrossRef PubMed.
- C. Song, W. Pan, S. T. Srimat, J. Zheng, Y. Li, Y.-H. Wang, B.-Q. Xu and Q.-M. Zhu, in Studies in Surface Science and Catalysis, ed. S.-E. Park, J.-S. Chang and K.-W. Lee, Elsevier, 2004, vol 153, pp. 315–322 Search PubMed.
- R. Zevenhoven and P. Kilpinen, Control of pollutants in flue gases and fuel gases, Finland, 2001 Search PubMed.
- G. A. Gonzalez-Martinez, T. Jurado-Vazquez, D. Solis-Ibarra, B. Vargas, E. Sanchez-Gonzalez, A. Martinez, R. Vargas, E. Gonzalez-Zamora and I. A. Ibarra, Dalton Trans., 2018, 47, 9459–9465 RSC.
- V. B. López-Cervantes, E. Sánchez-González, T. Jurado-Vázquez, A. Tejeda-Cruz, E. González-Zamora and I. A. Ibarra, Polyhedron, 2018, 155, 163–169 CrossRef.
- R. A. Peralta, B. Alcántar-Vázquez, M. Sánchez-Serratos, E. González-Zamora and I. A. Ibarra, Inorg. Chem. Front., 2015, 2, 898–903 RSC.
- M. Sagastuy-Brena, P. G. M. Mileo, E. Sanchez-Gonzalez, J. E. Reynolds, T. Jurado-Vazquez, J. Balmaseda, E. Gonzalez-Zamora, S. Devautour-Vinot, S. M. Humphrey, G. Maurin and I. A. Ibarra, Dalton Trans., 2018, 47, 15827–15834 RSC.
- A. Zárate, R. A. Peralta, P. A. Bayliss, R. Howie, M. Sánchez-Serratos, P. Carmona-Monroy, D. Solis-Ibarra, E. González-Zamora and I. A. Ibarra, RSC Adv., 2016, 6, 9978–9983 RSC.
- J. R. Fernández, S. Garcia and E. S. Sanz-Pérez, Ind. Eng. Chem. Res., 2020, 59, 6767–6772 CrossRef.
- H. S. Fogler, Elements of chemical reaction engineering, Prentice Hall, Boston, 5th edn, 2016 Search PubMed.
- M. L. Díaz-Ramírez, E. Sánchez-González, J. R. Álvarez, G. A. González-Martínez, S. Horike, K. Kadota, K. Sumida, E. González-Zamora, M.-A. Springuel-Huet, A. Gutiérrez-Alejandre, V. Jancik, S. Furukawa, S. Kitagawa, I. A. Ibarra and E. Lima, J. Mater. Chem. A, 2019, 7, 15101–15112 RSC.
- M. Ding and H.-L. Jiang, ACS Catal., 2018, 8, 3194–3201 CrossRef CAS.
- X.-H. Liu, J.-G. Ma, Z. Niu, G.-M. Yang and P. Cheng, Angew. Chem., Int. Ed, 2015, 54, 988–991 CrossRef CAS PubMed.
- J.-Y. Wang, E. Mangano, S. Brandani and D. M. Ruthven, Adsorption, 2021, 27, 295–318 CrossRef CAS.
- E. Hunter-Sellars, J. J. Tee, I. P. Parkin and D. R. Williams, Microporous Mesoporous Mater., 2020, 298, 110090 CrossRef CAS.
- E. Hunter-Sellars, P. A. Saenz-Cavazos, A. R. Houghton, S. R. McIntyre, I. P. Parkin and D. R. Williams, Adv. Funct. Mater., 2020, 2008357 Search PubMed.
- ASTM International, D4513-11(2017) Standard Test Method for Particle Size Distribution of Catalytic Materials by Sieving, ASTM International, West Conshohocken, PA, 2017, DOI:10.1520/D4513-11R17.
- H. W. B. Teo, A. Chakraborty and S. Kayal, Appl. Therm. Eng., 2017, 120, 453–462 CrossRef CAS.
- C. A. Trickett, A. Helal, B. A. Al-Maythalony, Z. H. Yamani, K. E. Cordova and O. M. Yaghi, Nat. Rev. Mater., 2017, 2, 17045 CrossRef CAS.
- S. Mukherjee, S. Sharma and S. K. Ghosh, APL Mater., 2019, 7, 050701 CrossRef.
- D. Y. C. Leung, G. Caramanna and M. M. Maroto-Valer, Renewable Sustainable Energy Rev., 2014, 39, 426–443 CrossRef CAS.
- N. Chanut, S. Bourrelly, B. Kuchta, C. Serre, J. S. Chang, P. A. Wright and P. L. Llewellyn, ChemSusChem, 2017, 10, 1543–1553 CrossRef CAS PubMed.
- A. Ö. Yazaydın, A. I. Benin, S. A. Faheem, P. Jakubczak, J. J. Low, R. R. Willis and R. Q. Snurr, Chem. Mater., 2009, 21, 1425–1430 CrossRef.
- A. D. Wiersum, E. Soubeyrand-Lenoir, Q. Yang, B. Moulin, V. Guillerm, M. B. Yahia, S. Bourrelly, A. Vimont, S. Miller, C. Vagner, M. Daturi, G. Clet, C. Serre, G. Maurin and P. L. Llewellyn, Chem.–Asian J., 2011, 6, 3270–3280 CrossRef CAS PubMed.
- Y. Liu, J. Shi, J. Chen, Q. Ye, H. Pan, Z. Shao and Y. Shi, Microporous Mesoporous Mater., 2010, 134, 16–21 CrossRef CAS.
- X. Su, L. Bromberg, V. Martis, F. Simeon, A. Huq and T. A. Hatton, ACS Appl. Mater. Interfaces, 2017, 9, 11299–11306 CrossRef CAS PubMed.
- Y. Lara, L. M. Romeo, P. Lisbona, S. Espatolero and A. I. Escudero, Energy Technol., 2018, 6, 1649–1659 CrossRef CAS.
- E. Martínez-Ahumada, M. L. Díaz-Ramírez, H. A. Lara-García, D. R. Williams, V. Martis, V. Jancik, E. Lima and I. A. Ibarra, J. Mater. Chem. A, 2020, 8, 11515–11520 RSC.
- B. Shirani and M. Eic, Can. J. Chem. Eng., 2016, 94, 2023–2034 CrossRef CAS.
- S. M. Systems, DVS Resolution Brochure, https://www.surfacemeasurementsystems.com/wp-content/uploads/2014/05/DVS-Resolution-brochure-v1.0.pdf, 2020 Search PubMed.
- B. Shirani, X. Han and M. Eic, Sep. Purif. Technol., 2020, 230, 115831 CrossRef CAS.
- J. Crank and E. P. J. Crank, The Mathematics of Diffusion, Clarendon Press, 1979 Search PubMed.
- Z. Zhao, Z. Li and Y. S. Lin, Ind. Eng. Chem. Res., 2009, 48, 10015–10020 CrossRef CAS.
- T. M. Tovar, J. Zhao, W. T. Nunn, H. F. Barton, G. W. Peterson, G. N. Parsons and M. D. LeVan, J. Am. Chem. Soc., 2016, 138, 11449–11452 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Figures and additional characterization. See DOI: 10.1039/d1ra01118a |
| This journal is © The Royal Society of Chemistry 2021 |