Open Access Article
Open Access ArticlePreparation of a platinum nanoparticle catalyst located near photocatalyst titanium oxide and its catalytic activity to convert benzyl alcohols to the corresponding ethers†
Yuki Wadaa,
Toshiki Akiyama a,
Kazuo Harada
a,
Kazuo Harada a,
Tetsuo Honma
a,
Tetsuo Honma b,
Hiroshi Naka‡
b,
Hiroshi Naka‡
 c,
Susumu Saito
c,
Susumu Saito cd and
Mitsuiro Arisawa
cd and
Mitsuiro Arisawa *a
*a
aGraduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamada-oka, Suita, Osaka 565-0871, Japan. E-mail: arisaw@phs.osaka-u.ac.jp
bJapan Synchrotron Radiation Research Institute, 1-1-1 Kouto, Sayo-cho, Sayo-gun, Hyogo 679-5198, Japan
cResearch Center for Materials Science, Nagoya University, Chikusa, Nagoya 464-8602, Japan
dGraduate School of Science, Nagoya University, Chikusa, Nagoya 464-8602, Japan
First published on 23rd June 2021
Abstract
A novel platinum nanoparticle catalyst closely located near the surface of titanium oxide, PtNP/TiO2, has been prepared. This catalyst has both the properties of a photocatalyst and a metal nanoparticle catalyst, and acquired environmentally friendly catalytic activity, which cannot be achieved by just one of these catalysts, to afford ethers from benzyl alcohols under the wavelength of 420 nm.
The electrolysis of water by a heterogeneous metal oxide semiconductor (MOS) photocatalyst, called the Honda–Fujishima effect,1 received considerable attention because it enables the conversion of solar energy. As a result, photoreactions by heterogeneous photocatalysts based on MOS have been reported until now, including numerous practical applications such as carbon dioxide reduction2 and pollutant removal.3
Research on photocatalytic reactions using visible light is progressing steadily. Among them, TiO2 has been extensively studied due to its high catalytic activity, low cost, non-toxicity, and long-term stability.4,5
Titanium oxide has a very strong oxidizing power, but it does not have a strong reducing power.6 By using a transition metal such as platinum on titanium oxide as a support, its reducing power can be increased, and the amount of hydrogen generated in the electrolysis of water can be improved eight times compared to the case of titanium oxide alone.7
Furthermore, when MOS contacts the metal, a potential barrier called Schottky barrier is formed at the interface. The basic characteristic of this Schottky barrier lies in the Schottky barrier height (Φ), which represents the difference between the CB of the MOS distorted by the contact with a metal and the Fermi level (EF) of the metal. Although it has been studied for almost half a century, how to determine the barrier is still not well understood. Due to the formation of the Schottky barrier height, the energy required to move the electrons from the valence band of MOS to the conductor changes from Eg to Φ, and the energy becomes smaller and it changes to a longer wavelength. As a result, a photoreaction with titanium oxide using light with a wavelength longer than 387 nm has also been reported (Scheme 1)8
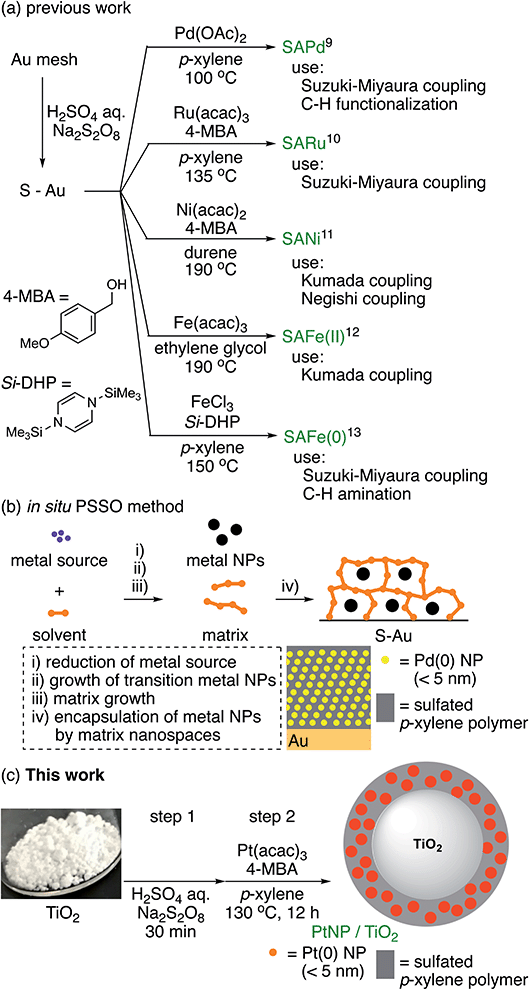
On the other hand, we have recently developed a sulfur-modified Au-supported Pd NP catalyst (SAPd) that is applicable in Suzuki–Miyaura coupling9a,9b and C–H functionalization9c (Scheme 2a). It was constructed by approximately 10 layers of self-assembled Pd(0) NPs (mean size: <5 nm) supported on a sulfur-modified Au surface. We speculated that the self-assembled Pd NPs, which were encapsulated in a sulfated p-xylene polymer matrix,9d were formed using in situ metal NP and nanospace simultaneous organization (PSSO), as illustrated in Scheme 2b: (i) the reduction of a high-valence metal source, (ii) growth of transition metal NPs, (iii) growth of a matrix with appropriately sized nanopores, and (iv) encapsulation of the metal NPs in these nanopores. To prepare SARu,10 SANi,11 SAFe(II),12 and SAFe(0)13 (Scheme 2a), the PSSO method involves the in situ reduction of a noble metal precursor to produce in situ metal NPs.
In this research project, we decided to create a novel metal NP catalyst having metal NPs near the surface of TiO2 by substituting the gold-supported SAPd for gold with a titanium oxide (TiO2) photocatalyst. Alternatively, by substituting the solid gold support for a photocatalyst, we thought that we could create a novel metal NPs catalyst with an unprecedented reactivity by combining the properties of both photocatalyst and metal nanoparticle catalyst (Scheme 2c).
Preparation and properties of catalysts
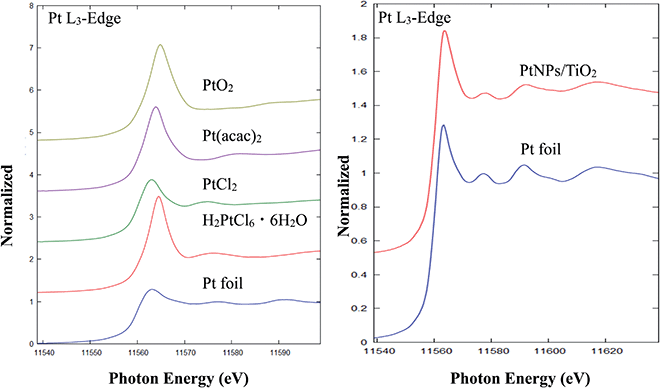
The preparation of PtNPs/TiO2 using TiO2 as the supported solid was carried out. As the result of examining numerous preparation conditions, since TiO2 is a powder, p-xylene, which is a liquid at room temperature, was found to be more suitable than 1,2,4,5-tetramethylbenzene (melting point: 179.2 °C). A solution containing TiO2 (500 mg), Pt(acac)2 (60 mg) and 4-methoxybenzyl alcohol (1.0 mL) in p-xylene (17 mL) was slowly stirred at 50 rpm to obtain a gray solid (Scheme 3). At this time, the amount of platinum supported on TiO2, based on inductively coupled plasma-mass spectrometry (ICP-MS), was 0.00044 wt%.Next, to investigate the state of Pt supported on the surface of PtNPs/TiO2, the Pt-L3 orbital of PtNP/TiO2 and Pt standard samples were measured via the X-ray absorption near edge structure (XANES) analysis. That is, as a result of comparing the spectra of PtNP/TiO2 with those of the standard samples, Pt foil, H2PtCl6·6H2O, PtCl2, Pt(acac)2, and PtO2, Pt on PtNP/TiO2 was closest to the Pt foil, and it was found that the Pt supported on was Pt(0) such as an organometallic catalyst, or Pt(0) formed into a bulk state by a metal bond (Fig. 1, left). It was also found that platinum supported on all PtNPs/TiO2 is Pt(0) regardless of the amount of platinum supported (Fig. 1, right).
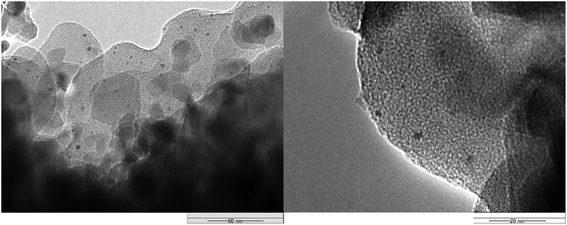
Furthermore, we observed PtNPs/TiO2 via transmission electron microscopy (TEM) (Fig. 2). As a result, it was clarified that a polymer layer was present on the surface of titanium oxide similar to SAPd. Similar to SAPd, this polymer layer is considered to form a matrix of p-xylene and SO42− derived as (p-xylene)m·(SO4)n polymer and Pt(0)NPs. This polymer stabilizes Pt(0)NPs at around 3–4 nm and prevents aggregation.
Catalytic activity
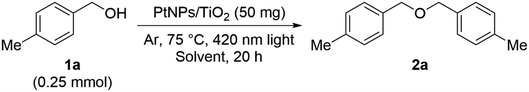
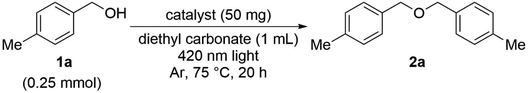
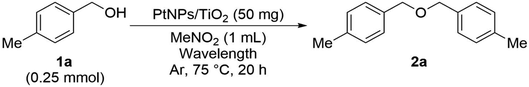
As the result of investigating the catalytic activity of the as-prepared PtNPs/TiO2, it was found that this catalyst can be used as a catalyst for the etherification reaction (Table 1). Therefore, the reaction solvent aiming to improve the yield of this reaction was investigated. When using the hydrocarbon solvent toluene, 2a was obtained in moderate yield (entry 1). On the other hand, when ethylene glycol or DMF, which are highly polar solvents, were used, the reaction did not proceed at all (entries 2 and 3). Probably, ethylene glycol is oxidized and decomposed into an aldehyde, and DMF is decomposed due to the generation of radicals, so that the desired reaction could not proceed. When nitromethane and diethyl carbonate, which are frequently used in electrolytic synthesis, were used, the yields were improved to 82% and 89%, respectively.14 The use of a stable aprotic high-dielectric solvent under these conditions resulted in an improved yield.Next, we confirmed the essential elements required for this etherification reaction (Table 2). When TiO2 was examined without the PtNP catalyst, the reaction did not proceed at all (entries 1 and 2). When sulfur-modified titanium oxide was used, the yield was 7% and a slight progress of the reaction could be confirmed, but the result was far below 89% (entry 3). The reaction did not proceed even in entry 4, where the PtNP catalyst and SAPt with Au as the supported solid were used, and the reaction did not substantially proceed even when PdNPs/TiO2 in which PtNPs were replaced with PdNPs were used (entry 5). In addition, the reaction hardly proceeded without the light irradiation (entry 6). In addition, when this reaction was examined in air, more benzaldehyde as a by-product was obtained than in the argon atmosphere, so the reaction was examined in an argon atmosphere. Therefore, it was found that both PtNPs/TiO2 and light are indispensable for this reaction to take place.
Next, the wavelength of the emitted light was examined (Table 3). When we performed the control experiments irradiated at a wavelength of 365 nm, which is higher in energy than the bandgap energy of titanium oxide, the yield of 2a decreased to 22%. Since it was confirmed that the aldehyde compound was produced in a yield of 45% or more under these experimental conditions, it was considered that the alcohol was oxidized by the holes formed in titanium oxide. Furthermore, when we performed the control experiments irradiated with light at 400 nm wavelength, the yield of 2a further decreased to 35%, and when the wavelength was extended to 470 nm, the yield of 2a dropped significantly, the reaction hardly proceeded, and the starting material 1a was recovered. Therefore, light with a wavelength of 420 nm is optimal for this reaction, and we decided to use this light in subsequent experiments.
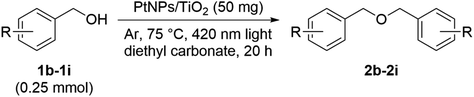
The generality of this reaction was examined under the optimum conditions obtained above. First, an unsubstituted benzyl alcohol 1b, substrates 1c and 1g–1i with an alkyl group as a substituent, substrates 1d and 1e with a halogen atom, and substrate 1f with a nitro group, which is a strong electron-withdrawing group, were examined. When unsubstituted 1b and 4-tBu derivative 1c were used, corresponding ethers 2b and 2c were obtained (Table 4). It is considered that the yield of 2i was higher than that of 2g and 2h due to the presence of three electron-donating groups. In addition, the reaction hardly proceeded on the substrates 1d and 1e with a halogen atom each, and the reaction did not proceed at all on the substrates 1f that had a nitro group. From these results, it was found that an electron donating group, such as methyl group is imperative for this reaction to proceed with good yield.
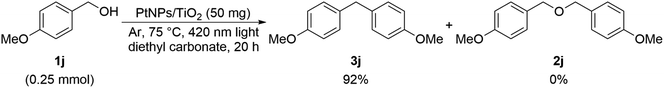
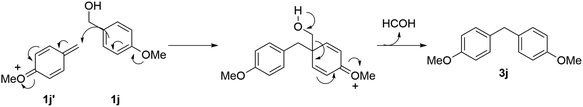
Subsequently, the experiment was continued using the 4-OMe derivative 1j. As a result, the corresponding ether 2j was not obtained at all, instead a diphenylmethane compound 3j was obtained (Scheme 4).15
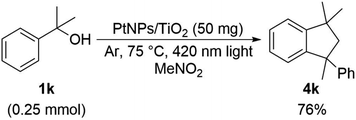
Furthermore, the experiment was continued using a substrate having a substituent at the benzyl position. When 2-phenyl-2-propanol 1k, which is a tertiary alcohol, and 1-phenyl-1-ethanol 1l and 1-p-tolyl-1-ethanol 1m, which are secondary alcohols, were used as substrates and diethyl carbonate was used as a solvent, reaction was resulted in a complex mixture. When nitromethane was used as the solvent, 1,1,3-trimethyl-3-phenylindane 4k, in which the substrate was dimerized, was obtained only in the case of 1k (Scheme 5), but the reaction was resulted in a complex mixture again with secondary alcohols (Scheme 6).
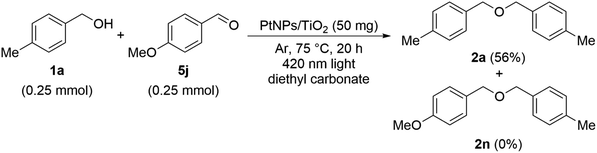
The experimental results obtained so far suggest that the reaction mechanism and products differ depending on the substrate. First, in order to investigate the reaction mechanism, the following reaction was examined using 4-methylbenzyl alcohol 1a and 4-methoxybenzaldehyde 5j. As a result, although the reactivity was lowered, dibenzyl ether derivative 2a was obtained in a yield of 56% same as before, and compound 2l in which the methyl and methoxy forms were coupled was not obtained at all. Therefore, it was clarified that this reaction does not pass through aldehyde 5j as a reaction intermediate (Scheme 7).
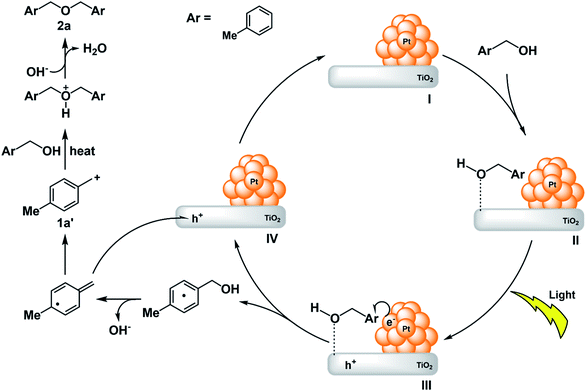
Since the existence of TiO2 and PtNPs as well as irradiation with wavelength of 420 nm were essential (Tables 2 and 3), the following reaction mechanism was estimated (Fig. 3). First, the irradiation of light causes electrons to move from titanium oxide to Pt. Alternatively, electrons move from platinum to titanium oxide. In the latter case, it is considered to take place due to the surface plasmon resonance of Pt. Here, it is assumed that electrons have moved from titanium oxide to Pt. 4-Methylbenzyl alcohol 1a, which is a substrate, approaches the surface of titanium oxide, and electrons on Pt, generated by irradiation with light, move onto the benzene ring of the substrate. Then, the hydroxyl group is desorbed, electrons are deprived of the holes formed on titanium oxide, and simultaneously, a benzyl cation intermediate 1a′ is generated, and another 4-methylbenzyl alcohol 1a nucleophilically attacks the benzyl cation intermediate 1a′ to afford ether 2a.
When the substrate is 4-methoxybenzyl alcohol 1j, it is considered that the ipso-position Friedel–Crafts reaction between the benzyl cation intermediate 1j′ and another 4-methoxybenzyl alcohol 1j proceeded to give 3j. In this case, formic acid is considered to be eliminated (Scheme 8).
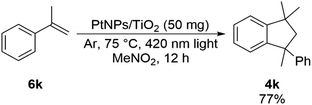
As shown in Scheme 5, 1,1,3-trimethyl-3-phenylindane 4k was obtained when 1k of tertiary alcohol was used as a substrate. Since α-methylstyrene 6k was considered as an intermediate in the reaction mechanism of this compound, it was confirmed that this reaction proceeds using 6k as a substrate. As a result, 1, 1, 3-trimethyl-3-phenylindane 4k was obtained in 77% yield (Scheme 9), and it was suggested that α-methylstyrene 6k or an equivalent thereof was used as an intermediate.
In summary, we have prepared Pt nanoparticle catalysts closely located on a semiconductor photocatalyst titanium oxide, PtNPs/TiO2, and found its unprecedented catalytic activity on the etherification reaction under the irradiation of 420 nm. All compositions of PtNPs, TiO2 and irradiation of 420 nm are indispensable in this reaction. The as-prepared PtNPs/TiO2 can be handled stably even in air.
Author contributions
Y. W., T. A., H. N., S. S., and M. A. contributed to the chemical reactions. Y. W., K. H. and M. A. contributed to the analysis of leached Pt in the reaction mixture. Y. W., T. A, T. H. and M. A. contributed to the analysis using XAFS. Y. W. and M. A. contributed to writing of the manuscript, and M. A. is the corresponding author.Conflicts of interest
There is no conflict of interest to declare.Note added after first publication
This article replaces the version published on 23 Jun 2021, which contained errors in Scheme 2 and Figure 1. The correct versions are now shown.Acknowledgements
We acknowledge Professor Jun-ya Hasegawa, and Mr. Shuhei Shimoda (Institute for Catalysis, Hokkaido University, Japan) for TEM experiments. This study was partially supported by a Grant-in-Aid from JSPS KAKENHI for Precisely Designed Catalysts with Customized Scaffolding (Grant No. JP 18H04206a and JP 15KT0063), by Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under Grant Number JP20am0101084, by Cooperative Research Program of “Network Joint Research Center for Materials and Devices” from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), and Nagase Science Technology Foundation.References
- A. Fujishima and K. Honda, Electrochemical photolysis of water at a semiconductor electrode, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- S. N. Habisreutinger, L. Schmidt-Mende and J. K. Stolarczyk, Photocatalytic reduction of CO2 on TiO2 and other semiconductors, Angew. Chem., Int. Ed., 2013, 52, 7372–7408 CrossRef CAS.
- C. Chen, W. Ma and J. Zhao, Semiconductor-mediated photodegradation of pollutants under visible-light irradiation, Chem. Soc. Rev., 2010, 39, 4206–4219 RSC.
- (a) K. Hashimoto, H. Irie and A. Fujishima, TiO2 photocatalysis: a historical overview and future prospects, Jpn. J. Appl. Phys., 2005, 44, 8269 CrossRef CAS; (b) K. Nakata and A. Fujishima, TiO2 photocatalysis: design and applications, J. Photochem. Photobiol., C, 2012, 13, 169 CrossRef CAS.
- (a) M. Addamo, V. Augugliaro, A. D. Paola, E. García-López, V. Loddo, G. Marcì, R. Molinari, L. Palmisano and M. Schiavello, Preparation, characterization, and photoactivity of polycrystalline nanostructured TiO2 catalysts, J. Phys. Chem. B, 2004, 108, 3303–3310 CrossRef CAS; (b) J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Understanding TiO2 photocatalysis: mechanisms and materials, Chem. Rev., 2014, 114(19), 9919–9986 CrossRef CAS PubMed; (c) V. Etacheri, C. D. Valentin, J. Schneider, D. Bahnemann and S. C. Pillai, Visible-light activation of TiO2 photocatalysts: advances in theory and experiments, J. Photochem. Photobiol., C, 2015, 25, 1–29 CrossRef CAS; (d) H. Choi, M. Carboni, Y. K. Kim, C. H. Jung, S. Y. Moon, M. M. Koebel and J. Y. Park, Synthesis of high surface area TiO2 aerogel support with Pt nanoparticle catalyst and CO oxidation study, Catal. Lett., 2018, 148, 1504–1513 CrossRef CAS; (e) R. Fiorenza, M. Condorelli, L. D'Urso, G. Compagnini, M. Bellardita, L. Palmisano and S. Scirè, Catalytic and photothermo-catalytic applications of TiO2–CoOx composites, J. Photocatal., 2020, 1, 3–15 CrossRef.
- M. Dahl, Y. Liu and Y. Yin, Composite titanium dioxide nanomaterials, Chem. Rev., 2014, 114, 9853–9889 CrossRef CAS PubMed.
- D. Duonghong, E. Borgarello and M. Gratzel, Dynamics of light-induced water cleavage in colloidal systems, J. Am. Chem. Soc., 1981, 103, 4685–4690 CrossRef CAS.
- Y. Shiraishi, D. Tsukamoto, Y. Sugano, A. Shiro, S. Ichikawa, S. Tanaka and T. Hirai, Platinum nanoparticles supported on anatase titanium dioxide as highly active catalysts for aerobic oxidation under visible light irradiation, ACS Catal., 2012, 2, 1984–1992 CrossRef CAS.
- (a) N. Hoshiya, S. Shuto and M. Arisawa, The actual active species of sulfur-modified gold-supported palladium as a highly effective palladium reservoir in the Suzuki–Miyaura coupling, Adv. Synth. Catal., 2011, 353, 743–748 CrossRef CAS; (b) M. Al-Amin, S. Arai, N. Hoshiya, T. Honma, Y. Tamenori, T. Sato, M. Yokoyama, A. Ishii, M. Takeuchi, T. Maruko, S. Shuto and M. Arisawa, Development of second generation gold-supported palladium material with low-leaching and recyclable characteristics in aromatic amination, J. Org. Chem., 2013, 78, 7575–7581 CrossRef CAS PubMed; (c) K. Takagi, M. Al-Amin, N. Hoshiya, J. Wouters, H. Sugimoto, Y. Shiro, H. Fukuda, S. Shuto and M. Arisawa, Palladium-nanoparticle-catalyzed 1,7-palladium migration involving C–H activation, followed by intramolecular amination: regioselective synthesis of N1-arylbenzotriazoles and an evaluation of their inhibitory activity toward indoleamine 2,3-dioxygenase, J. Org. Chem., 2014, 79, 6366–6371 CrossRef CAS PubMed; (d) M. Arisawa, M. Al-Amin, T. Honma, Y. Tamenori, S. Arai, N. Hoshiya, T. Sato, M. Yokoyama, A. Ishii, M. Takeguchi, T. Miyazaki, M. Takeuchi, T. Maruko and S. Shuto, Formation of self-assembled multi-layer stable palladium nanoparticle for ligand-free coupling reactions, RSC Adv., 2015, 5, 676–683 RSC and references cited therein..
- T. Akiyama, T. Taniguchi, N. Saito, R. Doi, T. Honma, Y. Tamenori, Y. Ohki, N. Takahashi, H. Fujioka, Y. Sato and M. Arisawa, Ligand-free Suzuki–Miyaura coupling using ruthenium(0) nanoparticles and a continuously irradiating microwave system, Green Chem., 2017, 19, 3357–3369 RSC.
- N. Hoshiya, K. Fujiki, T. Taniguchi, T. Honma, Y. Tamenori, M. Xiao, N. Saito, M. Yokoyama, A. Ishii, H. Fujioka, S. Shuto, Y. Sato and M. Arisawa, Self-assembled multilayer-stabilized nickel nanoparticle catalyst for ligand-free cross-coupling reactions: in situ metal nanoparticle and nanospace simultaneous organization, Adv. Synth. Catal., 2016, 358, 2449–2459 CrossRef CAS.
- T. Akiyama, Y. Wada, K. Jenkinson, T. Honma, K. Tsuruta, Y. Tamenori, H. Haneoka, T. Takehara, T. Suzuki, K. Murai, H. Fujioka, Y. Sato, A. E. H. Wheatley and M. Arisawa, Reusable immobilized iron(II) nanoparticle precatalysts for ligand-free Kumada coupling, ACS Appl. Nano Mater., 2018, 1, 6950–6958 CrossRef CAS.
- T. Akiyama, Y. Wada, M. Yamada, Y. Shio, T. Honma, S. Shimoda, K. Tsuruta, Y. Tamenori, H. Haneoka, T. Suzuki, K. Harada, H. Tsurugi, K. Mashima, J.-y. Hasegawa, Y. Sato and M. Arisawa, Self-assembled multilayer iron(0) nanoparticle catalyst for ligand-free carbon–carbon/carbon–nitrogen bond-forming reactions, Org. Lett., 2020, 22, 7244–7249 CrossRef CAS PubMed.
- Although we repeatedly used the catalyst, we have not succeeded in its repeatedly use until now.
- When we used the 2j as the starting material under the optimized conditions, we couldn’t get the 3j as the product and just recovered 2j.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra00988e |
| ‡ Current address: Graduate School of Pharmaceutical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan. |
| This journal is © The Royal Society of Chemistry 2021 |