Open Access Article
Open Access Article3-Methyl-1,2,3-triazolium-1N-dinitromethylylide and the strategy of zwitterionic dinitromethyl groups in energetic materials design†
Dominique R. Wozniak a,
Matthias Zellerb,
Edward F. C. Byrdc and
Davin G. Piercey
a,
Matthias Zellerb,
Edward F. C. Byrdc and
Davin G. Piercey *d
*d
aDepartment of Materials Engineering, Purdue Energetics Research Center, Purdue University, 205 Gates Road West Lafayette, IN 47904, USA
bDepartment of Chemistry, Purdue University, West Lafayette, Indiana 47907, USA
cU.S. Army Research Laboratory, Aberdeen Proving Ground, Maryland 21005, USA
dDepartment of Materials Engineering, Department of Mechanical Engineering, Purdue Energetics Research Center, Purdue University, 205 Gates Road West Lafayette, IN 47904, USA. E-mail: dpiercey@purdue.edu
First published on 14th May 2021
Abstract
3-Methyl-1,2,3-triazolium-1N-dinitromethylylide, an exemplary zwitterionic energetic molecule, is the first fully-studied energetic material making use of the zwitterionic dinitromethyl functional group. This compound has impact and friction sensitivities of 8 J and 144–160 N respectively with a detonation velocity of 8162 m s−1.
Introduction
In the design of energetic materials, there is a specific interest in imparting increased performance while avoiding the generally-expected accompanying increase in sensitivity.1–8 A combination of oxygen balance, high heat of formation, and high density is often sought after in the pursuit of high performance materials. It is of equal importance to also incorporate methods of stabilization in these energetic compounds; for example, vicinal C-amines and C-nitro groups set up strong hydrogen bonding networks which can impart stability9,10 and highly nitrogenous backbones can be stabilized by removing electron density on alternate nitrogen atoms.11 More recently, it has been shown that zwitterionic energetic materials are able to combine high performance through increased density while also increasing stability through the electrostatic interactions within the same molecule.12–17N-oxides are widely used in energetic materials due to their ability to increase performance and stability of a compound.18,19 Introduction of N-oxides often leads to increased stability of heterocyclic backbones while also helping to increase oxygen balance.15,18 LLM-105 is an exemplary compound of this class, which while structurally similar to TATB, is thermally stable up to 361 °C and is 10–15% more powerful.20 The effect of introducing an N-oxide has also been examined in tetrazoles, where the density and performance of the N-oxide nitrotetrazole salts increased when compared to the nitrotetrazole, while sensitivities decreased.17 TKX-50 is another N-oxide containing energetic material with exceptional performance and stability, outperforming HMX and nearing CL-20 performance while maintaining a lower mechanical sensitivity.21 A similar trend was also observed with 1,2,3,4-tetrazines. The fully unsaturated 1,2,3,4-tetrazine is extremely unstable and mostly exists in annulated systems18,22 stabilized by addition of N-oxides to the 1 and 3 positions.22,23
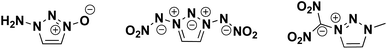
Similar to N-oxides, N-nitroimides are also used to impart energy content to energetic materials.24 These functional groups are able to form chains of alternating positive and negative charges, and therefore may be able stabilize a molecule by removing electron density on alternate atoms when the backbone is selected properly (Fig. 1).11,25 Unfortunately, heterocyclic structures containing the N-nitroimide functional group have not been as extensively studied as N-oxides, and many display unfavorable sensitivities to mechanical stimuli.11,26,27
Dinitromethyl groups are often used in energetic materials to increase the performance as a result of their high oxygen content and generally high densities.28 Energetic materials that include a dinitromethyl functional group are often high performing and are also an efficient method of creating energetic salts once the acidic proton is removed.12 Due to these properties, dinitromethyl groups were seen as potential new building blocks for zwitterionic molecules, which could potentially both impart energy while helping increase stability through electrostatic interactions.
For this work, we hypothesized that addition of a dinitromethyl group onto a positively charged heterocycle would produce a zwitterionic energetic material, with a possible energetic utility comparable to N-oxides or N-nitroimides. Studies done by Semenov and Shevelev, where they studied the method of generation of a dinitromethylylides and sulfonium dinitroylides, along with their study of N-dinitromethylazoles were the inspiration for this work.29–31 They developed a general method for the production of heterocyclic nitrogen dinitromethylylides but did not provide any energetic characterization.30,31 Outside of this work, very few zwitterionic dinitromethyl containing compounds have been synthesized and fully characterized and to the best of our knowledge there is no full energetic characterization of a material containing this unique functional group. In this work we report the first full energetic characterization of an azolium dinitromethylylide for preliminary investigation of the utility of this functional group in energetic materials design.
Results and discussion
Synthesis
1-Methyl-1,2,3-triazole was alkylated using excess chloroacetone in acetonitrile with potassium iodide catalyst (Scheme 1). Nitration of the produced 1-acetonyl-3-methyl-1,2,3-triazolium chloride using 98% H2SO4 and 100% HNO3 led to the obtaining of 3-methyl-1,2,3-triazolium-1N-dinitromethylylide as a solid after purification by extraction with ethyl acetate (Scheme 2). The nitration was carried out similar to that described by Shreeve et al.32NMR
1H and 13C NMR spectra were collected for the product. The proton spectra of the precursor 1-methyl-1,2,3-triazole showed the N–CH3 peak at 4.06 ppm and the two heterocyclic hydrogens at 8.03 and 7.71 ppm. These peaks shifted downfield in the product to 4.47, 9.27 and 9.00 ppm respectively. The carbon spectra shows a similar shift, with the N–CH3 peak moving from 36.10 ppm in the precursor to 41.20 ppm in the product. The heterocyclic carbon peaks shift from 133.42 and 125.60 ppm to 135.88 and 132.77 ppm respectively. The C–(NO2)2 peak appears at 130.20 ppm. Respective spectra can be found in the ESI.†X-ray structure
Colorless crystals of compound 1 were obtained through slow evaporation from ethyl acetate over several days at room temperature (Fig. 2). Compound 1 crystallizes in the orthorhombic space group Cmca with a density of 1.757 g cm−3.The dinitro groups are planar with each other and perpendicular to the ring. The bond lengths in the ring of compound 1 are halfway between a single and double bond. The N1–C4 bond measures 1.406 Å which is shorter than a single bond, probably caused by the zwitterionic nature of the bond. This is similar in length to the N–N bonds in the 1,3-di(nitroimide)-1,2,3-triazolate anion, which ranges from 1.40–1.44 Å.11 When comparing the bond lengths of the triazole ring of compound 1 to that of the potassium salt of 1,3-bis(nitroimido)-1,2,3-triazole, the C1–N1 and C2–N3 bond lengths are shorter in compound 1 (1.351 and 1.351 Å compared to 1.355 and 1.357 Å respectively).11 Conversely, the lengths of the N1–N2 and N2–N3 in compound 1 are longer than those in the potassium salt of 1,3-bis(nitroimido0-1,2,3-triazole (1.334 and 1.313 Å and 1.323 and 1.322 Å respectively). The bond length of the dinitromethyl group of 1 is slighter shorter than found in the zwitterionic 5-iminio-4,5-dihydro-1,3,4-oxadiazol-2-yl)dinitromethanide, 1.406 Å and 1.443 Å respectively.33 This bond is similar the N–N–NO2 bond found in the 1,3-bis(nitroimido)-1,2,3-triazolate salts (Fig. 1), which ranged from 1.40–1.44 Å.11 It is also longer than the N–O bond in 1-amino-1,2,3-triazole-3-oxide (Fig. 1), which measures 1.304 Å.18 It is interesting to note that despite similar bond lengths in the zwitterion compared to the 1,3-bis(nitroimido)-1,2,3-triazolate salts, the thermal stability of our compound was lower.
Explosive properties
Conclusions
In conclusion, a new zwitterionic energetic material was produced containing a unique azolium dinitromethylylide functional group and it was characterized for the first time as an energetic material. Compound 1 possesses mechanical stabilities comparable to that of RDX and HMX and energetic performances higher than TNT. These properties Bode well for the consideration of the zwitterionic azolium dinitromethylylide functional group in the toolkit of functional groups for energetic materials design.Experimental section
General methods
All reagents and solvents were used as received (Sigma-Aldrich, Fluka, Acros Organics, Fisher Scientific Co LLC) if not stated otherwise. Melting and decomposition points were measured with a TA Instruments Q20 DSC using heating rates of 5 °C min−1. 1H and 13C NMR spectra were measured with a Bruker AV-III-800 (5 mm QCI Z-gradient cryoprobe). All chemical shifts are quoted in ppm relative to TMS (1H, 13C). Infrared spectra were measured with a PerkinElmer Spectrum Two FT-IR spectrometer. Transmittance values are described as “strong” (s), “medium” (m) and “weak” (w). Low resolution mass spectra were measured with an Agilent 1260 Infinity II Quaternary LC instrument. Elemental analysis data was collected using a vario EL cube. High resolution mass spectra were measured with a Thermo Fisher Scientific LTQ Orbitrap with APCI probe. Sensitivity data were determined using a BAM friction tester (Reichel & Partner Gmbh) and BAM Drophammer (OZM research).Caution! The described compounds are energetic materials with sensitivity to various stimuli. While we encountered no issues in the handling of these materials, proper protective measures (face shield, ear protection, body armor, Kevlar gloves, and earthened equipment) should be used at all times.
Sensitivities
For initial safety testing, impact and friction sensitivities were determined for 1. Impact sensitivity was performed according to STANAG 4489 and modified according to instruction38 on an OZM drophammer by the BAM method.39 Friction sensitivity was carried out in accordance with STANAG 2287 (ref. 40) and modified according to instruction38 using a BAM friction tester. 1 had impact and friction sensitivities of 8 J and 144–160 N respectively. These sensitivities classify 1 as a “sensitive” material.41Computational methods
Enthalpy of formation and density were calculated using the Byrd and Rice method.35–37 The calculated densities were within 1.6% of densities calculated by X-ray crystallography. The molecular geometries were optimized using the B3LYP spin-restricted Kohn–Sham density functional theory (KS-DFT)42–45 with the 6-31G** Pople Gaussian basis set,46–48 using the Gaussian09 program package.49 Computational densities were obtained by dividing the mass of the molecule by the volume calculated from the volume contained within the B3LYP/6-31G** 0.001 electron boh−3 isosurface of the electron density, and subsequently modified by electrostatic parameters generated from charge distributions of said isosurface. Crystal densities obtained from X-ray crystallography were used when computing detonation performances. For the prediction of the heat of formation, the B3LYP/6-311++G(2df,2p) energy is computed from the B3LYP/6-31G* optimized geometry to obtain the gas phase heat of formation. Finally, the heat of sublimation was calculated from the molecular surface area (determined from the volume contained within the 0.001 electron boh−3 isosurface of the electron density) and pertinent electrostatic parameters.The calculations of the detonation parameters for compounds 1 were performed using the EXPLO6.05 software package.50,51 The software utilizes the Becker–Kistiakowsky–Wilson's equation of state (CFEOS) for solid carbon.52 In order to calculate the equilibrium composition of the detonation products the program utilizes the modified White, Johnson, and Dantzig's free energy minimization technique. EXPLO6.05 allows for calculation of detonation parameters at the CJ point. BKWN parameters (α, β, κ, θ) were used in the following BKW equation, with Xi representing the mol fraction of the i-th gaseous product and ki being the molar co-volume of said i-th gaseous product:53
The detonation properties calculated using EXPLO6.05 software program using densities determined through X-ray crystallographic methods are summarized in the ESI in Table S2† and compared to HMX and RDX.
X-ray structure
Single crystals of the investigated compound were coated with a trace of Fomblin oil and were transferred to the goniometer head of a Bruker Quest diffractometer with kappa geometry, a Cu Kα wavelength (λ = 1.54178 Å) I-μ-S microsource X-ray tube, laterally graded multilayer (Goebel) mirror for monochromatization, a Photon III C14 area detector. The instrument is equipped with an Oxford Cryosystems low temperature device and examination and data collection were performed at 150 K. Data were collected, reflections were indexed and processed, and the files scaled and corrected for absorption using APEX3 (ref. 54) and SADABS.55 The space groups were assigned using XPREP within the SHELXTL suite of programs56,57 and solved by direct methods using ShelXS57 and refined by full matrix least squares against F2 with all reflections using Shelxl2018 (ref. 58) using the graphical interface Shelxle.59 H atoms attached to carbon were positioned geometrically and constrained to ride on their parent atoms. C–H bond distances were constrained to 0.95 Å for alkene C–H moieties, and to 0.98 Å for CH3 moieties, respectively. Methyl CH3 atoms were allowed to rotate but not to tip to best fit the experimental electron density. Uiso(H) values were set to a multiple of Ueq(C) with 1.5 for CH3, NH3+ and OH, and 1.2 for C–H, CH2, B–H, N–H and NH2 units, respectively. Room temperature unit cell data were obtained from a 180° phi scan between 6.09 and 29.42° in θ by full integration of all data based on 357 reflections.Complete crystallographic data, in CIF format, have been deposited with the Cambridge Crystallographic Data Centre. CCDC 2049360 contain the ESI crystallographic data for this paper.†
Preparation of 3-methyl-1,2,3-triazolium-1N-dinitromethylylide (1)
1 g of 1-methyl-1,2,3-triazole (0.012 mol) was dissolved in acetonitrile. In a separate beaker, 0.1 g KI (0.60 mmol) was dissolved in as little ethanol as possible. To the solution of 1-methyl-1,2,3-triazole, 5 mL of chloroacetone (0.060 mol) was added while stirring in the dark. The KI solution was immediately added and stirred while covered in the dark overnight. The resulting solution was evaporated to dryness to yield 2.076 g of product. This product was cooled in beaker to 0 °C and 24 mL of cooled 98% H2SO4 was added. The resulting solution was cooled to −5 °C and 12 mL of 100% HNO3 was added slowly, keeping the temperature of the solution below 0 °C. The solution turned from an orange color to a light yellow color. The solution was allowed to stir overnight in an ice bath. It was then poured into an ice/ammonium bicarbonate solution while vigorously stirring. White solids formed. Resulting solid was extracted with ethyl acetate and evaporated to dryness yielding 1 (100 mg, 4.5%). 1H NMR (DMSO-d6): δ (ppm) = 9.27 (s, 1H, C–H), 9.00 (s, 1H, C–H), 4.47 (s, 3H, C–H3); 13C NMR (DMSO-d6): δ (ppm) = 135.88 (1C), 132.77 (1C), 130.20 (1C), 41.20 (CH3); HR-MS (QTOF+) m/z: expected: 188.0414 (M + H)+ found: 188.0413 (M + H)+; IR (cm−1): ṽ = 3180 (w), 3161 (w), 1536 (w), 1500 (m), 1452 (m), 1432 (m), 1418 (m), 1406 (m), 1358 (w), 1324 (w), 1269 (m), 1240 (m), 1215 (s), 1142 (s), 1111 (s), 1085 (s), 1024 (m), 977 (m), 821 (m), 788 (s), 757 (s), 735 (s), 680 (m), 641 (w), 620 (w), 485 (m); anal. calcd for C4H5N5O4: C, 25.68; N, 37.43; H, 2.69. Found: C, 23.66; N, 33.88; H, 2.48; DSC (5 °C min−1): 150 °C (dec); BAM friction: 144–160 N; BAM impact: 8 J.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support of this work by The Army Research Office (ARO) under grant number W911NF-18-1-0463 is acknowledged. Financial support of our laboratory by Purdue University and The Office of Naval Research (ONR) also is acknowledged. The National Science Foundation is acknowledged through the Major Research Instrumentation Program under Grant No. CHE 1625543 for the single crystal X-ray diffractometer. We are indebted to and thank Ms. Shannon Creegan, Mr Matthew Gettings, Mr Joseph Yount, and Mr Timothy Manship for many helpful and inspired discussions in support of our work.References
- N. E. Leonov, M. S. Klenov, O. V. Anikin, A. M. Churakov, Y. A. Strelenko, A. A. Voronin, D. B. Lempert, N. V. Muravyev, I. V. Fedyanin, S. E. Semenov and V. A. Tartakovsky, ChemistrySelect, 2020, 5, 12243–12249 CrossRef CAS.
- I. L. Dalinger, K. Y. Suponitsky, T. K. Shkineva, D. B. Lempert and A. B. Sheremetev, J. Mater. Chem. A, 2018, 6, 14780–14786 RSC.
- Y. Tang, D. Kumar and J. M. Shreeve, J. Am. Chem. Soc., 2017, 139, 13684–13687 CrossRef CAS PubMed.
- W. Huang, Y. Tang, G. H. Imler, D. A. Parrish and J. M. Shreeve, J. Am. Chem. Soc., 2020, 142, 3652–3657 CrossRef CAS PubMed.
- T. M. Klapötke, D. G. Piercey and J. Stierstorfer, Dalton Trans., 2012, 41, 9451–9459 RSC.
- T. A. D. Nguyen, J. M. Veauthier, G. F. Angles-Tamayo, D. E. Chavez, E. Lapsheva, T. W. Myers, T. R. Nelson and E. J. Schelter, J. Am. Chem. Soc., 2020, 142, 4842–4851 CrossRef CAS PubMed.
- L. M. Barton, J. T. Edwards, E. C. Johnson, E. J. Bukowski, R. C. Sausa, E. F. C. Byrd, J. A. Orlicki, J. J. Sabatini and P. S. Baran, J. Am. Chem. Soc., 2019, 141, 12531–12535 CrossRef CAS PubMed.
- M. L. Gettings, M. T. Thoenen, E. F. C. Byrd, J. J. Sabatini, M. Zeller and D. G. Piercey, Chem.–Eur. J., 2020, 26, 14530–14535 CrossRef CAS PubMed.
- P. Pagoria, Propellants, Explos. Pyrotech., 2016, 41, 452–469 CrossRef CAS.
- T. M. Klapötke, D. G. Piercey and J. Stierstorfer, Propellants, Explos. Pyrotech., 2011, 36, 160–167 CrossRef.
- T. M. Klapötke, C. Petermayer, D. G. Piercey and J. Stierstorfer, J. Am. Chem. Soc., 2012, 134, 20827–20836 CrossRef PubMed.
- T. Liu, S. Liao, S. Song, K. Wang, Y. Jin and Q. Zhang, Chem. Commun., 2019, 56, 209–212 RSC.
- A. A. Voronin, A. M. Churakov, M. S. Klenov, Y. A. Strelenko, I. V. Fedyanin and V. A. Tartakovsky, Eur. J. Org Chem., 2017, 2017, 4963–4971 CrossRef CAS.
- A. A. Voronin, V. P. Zelenov, A. M. Churakov, Y. A. Strelenko, I. V. Fedyanin and V. A. Tartakovsky, Tetrahedron, 2014, 70, 3018–3022 CrossRef CAS.
- V. P. Zelenov, I. V Fedyanin, D. V Khakimov, T. S. Pivina and N. D. Zelinsky, Izv. Akad. Nauk, Ser. Khim., 2017, 66, 1240–1249 CAS.
- T. M. Klapötke, D. G. Piercey and J. Stierstorfer, Chem.–Eur. J., 2011, 17, 13068–13077 CrossRef PubMed.
- M. Göbel, K. Karaghiosoff, T. M. Klapötke, D. G. Piercey and J. Stierstorfer, J. Am. Chem. Soc., 2011, 133, 2790 CrossRef.
- D. G. Piercey, D. E. Chavez, S. Heimsch, C. Kirst, T. M. Klapötke and J. Stierstorfer, Propellants, Explos. Pyrotech., 2015, 40, 491–497 CrossRef CAS.
- M. S. Klenov, A. A. Guskov, O. V. Anikin, A. M. Churakov, Y. A. Strelenko, I. V. Fedyanin, K. A. Lyssenko and V. A. Tartakovsky, Angew. Chem. Int. Ed., 2016, 55, 11472–11475 CrossRef CAS PubMed.
- P. Pagoria, M. X. Zhang, N. Zuckerman, G. Lee, A. Mitchell, A. DeHope, A. Gash, C. Coon and P. Gallagher, Propellants, Explos. Pyrotech., 2018, 43, 15–27 CrossRef CAS.
- N. Fischer, D. Fischer, T. M. Klapötke, D. G. Piercey and J. Stierstorfer, J. Mater. Chem., 2012, 22, 20418–20422 RSC.
- A. Y. Tyurin, A. M. Churakov, Y. A. Strelenko, M. O. Ratnikov and V. A. Tartakovsky, Russ. Chem. Bull., 2006, 55, 1648–1653 CrossRef CAS.
- A. M. Churakov and V. A. Tartakovsky, Chem. Rev., 2004, 104, 2601–2616 CrossRef CAS PubMed.
- A. A. Dippold, M. Feller and T. M. Klapötke, Cent. Eur. J. Energ. Mater., 2011, 8, 261–278 CAS.
- V. A. Tartakovsky, Mater. Res. Soc. Symp. Proc. Vol., 1996, 418, 15–24 CrossRef CAS.
- T. P. Kofman, G. Y. Kartseva and M. B. Shcherbinin, Russ. J. Org. Chem., 2002, 38, 1343–1350 CrossRef CAS.
- A. I. Glushkov, O. P. Shitov and V. A. Tartakovsky, Russ. Chem. Bull., Int. Ed., 2003, 52, 467–470 CrossRef CAS.
- Y. Zhou, H. Gao and J. M. Shreeve, Energ. Mater. Front., 2020, 1, 2–15 CrossRef.
- S. A. Shevelev, V. V. Semenov and A. A. Fainzil’berg, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1976, 25, 1906–1909 CrossRef.
- V. V. Semenov, S. A. Shevelev and L. G. Mel’nikova, Mendeleev Commun., 1993, 3, 58–60 CrossRef.
- B. A. T. Semenov, V. Viktor, S. A. Shevelev, A. B. Bruskin and M. I. Kanishchev, Russ. Chem. Bull., 2009, 58, 2077–2096 CrossRef.
- Q. Yu, G. H. Imler, D. A. Parrish and J. M. Shreeve, Chem.–Eur. J., 2017, 23, 17682–17686 CrossRef CAS PubMed.
- T. Liu, S. Liao, S. Song, K. Wang, Y. Jin and Q. Zhang, Chem. Commun., 2019, 56, 209–212 RSC.
- S. A. Ugrak, B. I. Vinogradov, V. M. Dalinger and I. L. Shevelev, Russ. Chem. Bull., 1995, 44, 2087–2092 CrossRef.
- B. M. Rice and E. F. C. Byrd, J. Comput. Chem., 2013, 34, 2146–2151 CrossRef CAS PubMed.
- B. M. Rice, J. J. Hare and E. F. C. Byrd, J. Phys. Chem. A, 2007, 111, 10874–10879 CrossRef CAS PubMed.
- E. F. C. Byrd and B. M. Rice, J. Phys. Chem. A, 2009, 113, 345–352 CrossRef CAS PubMed.
- WIWEB-Standardarbeitsanweisung 4-5.1.03, Ermittlung Der Explosionsgerfährlichkeit Oder Der Reibeempfindlichkeir Mit Dem Reibeapparat, Erding, November 8, 2002.
- http://www.bam.de.
- NATO, Standardization Agreement (STANAG) on Explosives, Friction Sensitivity Tests, no. 4487, 1st edn, Brussels, August 22, 2002 Search PubMed.
- Note, “impact: insensitive > 40 J, less sensitive ≥ 35 J, sensitive ≥ 4 J, very sensitive ≤ 3 J; friction: insensitive > 360 N, less sensitive = 360 N, sensitive < 360 N and > 80 N, very sensitive ≤ 80 N, extremely sensitive ≤ 10 N.” UN recommendations on..
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed.
- S. H. Vosko, L. Wilk and M. Nusair, Can. J. Phys., 1980, 58, 1200–1211 CrossRef CAS.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, J. Chem. Phys., 1980, 72, 650–654 CrossRef CAS.
- M. J. Frisch, J. A. Pople and J. S. Binkley, J. Chem. Phys., 1984, 80, 3265–3269 CrossRef CAS.
- S. P. von Ragué, T. Clark, A. J. Kos, G. W. Spitznagel, C. Rohde, D. Arad, K. N. Houk and N. G. Rondan, J. Am. Chem. Soc., 1984, 106, 6467–6475 CrossRef.
- M. J. Frisch. G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheesseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, Gaussian 09, Wallingford CT, 2009 Search PubMed.
- Software released by M. Sućeska, Zagreb. Croat, 2018.
- M. Sućeska, Mater. Sci. Forum, 2004, 465–466, 325–330 Search PubMed.
- M. Sućeska, EXPLO6.04 Program, Zagreb, Coatia, 2017 Search PubMed.
- (a) M. Sućeska, Mater. Sci. Forum, 2009, 465, 325 Search PubMed; (b) M. Sućeska, Propellants, Explos., Pyrotech., 1991, 16, 197 CrossRef; (c) M. Sućeska, Propellants, Explos., Pyrotech., 1999, 24, 280 CrossRef; (d) M. L. Hobbs and M. R. Baer, Proc. of the 10th Symp. (International) onDetonation, 1993 Search PubMed.
- U. Bruker, Apex3 v2019.1-0, SAINT V8.40A, 2019, Bruker AXS Inc.: Madison (WI) Search PubMed.
- L. Krause, R. Herbst-Irmer, G. M. Sheldrick and D. Stalke, J. Appl. Crystallogr., 2015, 48, 3–10 CrossRef CAS PubMed.
- W. U. a SHELXTL suite of programs, Bruker Advanced X-ray Solutions, Bruker AXS Inc., Madison, version 6.14, 2000–2003 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- I. Uson and G. M. Sheldrick, Acta Crystallogr., Sect. D: Struct. Biol., 2018, 74, 106–116 CrossRef CAS PubMed.
- C. B. Hübschle, G. M. Sheldrick and B. Dittrich, J. Appl. Crystallogr., 2011, 44, 1281–1284 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: NMR, impact, friction, X-ray diffraction. CCDC 2049360. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra00953b |
| This journal is © The Royal Society of Chemistry 2021 |