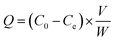Open Access Article
Open Access ArticlePreparation of surface molecularly imprinted polymer and its application for the selective extraction of teicoplanin from water†
Hao Zhou a,
Kanlin Penga,
Yijuan Suab,
Xuqin Songac,
Jingli Qiua,
Renping Xionga and
Limin He
a,
Kanlin Penga,
Yijuan Suab,
Xuqin Songac,
Jingli Qiua,
Renping Xionga and
Limin He *a
*a
aNational Reference Laboratory of Veterinary Drug Residues (SCAU), College of Veterinary Medicine, South China Agricultural University, Guangzhou, 510642, China. E-mail: hlm1922@sohu.com
bDepartment of Ecology, College of Natural Resources and Environment, South China Agricultural University, Guangzhou, 510642, China. E-mail: syj@scau.edu.cn; Fax: +86 20 85284896; Tel: +86 20 85280234
cCollege of Animal Science, Guizhou University, Guiyang, 550025, China
First published on 13th April 2021
Abstract
In this study, a new surface molecularly imprinted polymer (SMIP) of teicoplanin (TEC) was prepared in an aqueous solution using amino-modified silica gel as a carrier. The molar ratio of the template molecule, functional monomer and cross-linker in the optimized synthesis system was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40. The structure and morphology of SMIP were characterized by Fourier-transform infrared spectra and scanning electron microscopy, respectively. It was shown that the silica gel modified with different active groups; the type and structure of functional monomers have a great influence on the specificity of SMIP. The SMIPs synthesized from a series of methacrylic acid and its hydroxylalkyl esters as functional monomers have good specificity for TEC. The results of static adsorption experiments showed that the adsorption capacity of SMIP was 6.5 times that of non-molecularly imprinted polymer, which were 152.6 mg g−1 and 23.6 mg g−1, respectively, indicating that SMIP had a larger affinity for TEC. Finally, the SMIP was successfully used as a dispersive solid-phase extraction adsorption material to selectively extract and enrich TEC from the water sample. The limit of detection of the proposed liquid chromatographic method for TEC was 5 μg L−1.
40. The structure and morphology of SMIP were characterized by Fourier-transform infrared spectra and scanning electron microscopy, respectively. It was shown that the silica gel modified with different active groups; the type and structure of functional monomers have a great influence on the specificity of SMIP. The SMIPs synthesized from a series of methacrylic acid and its hydroxylalkyl esters as functional monomers have good specificity for TEC. The results of static adsorption experiments showed that the adsorption capacity of SMIP was 6.5 times that of non-molecularly imprinted polymer, which were 152.6 mg g−1 and 23.6 mg g−1, respectively, indicating that SMIP had a larger affinity for TEC. Finally, the SMIP was successfully used as a dispersive solid-phase extraction adsorption material to selectively extract and enrich TEC from the water sample. The limit of detection of the proposed liquid chromatographic method for TEC was 5 μg L−1.
1. Introduction
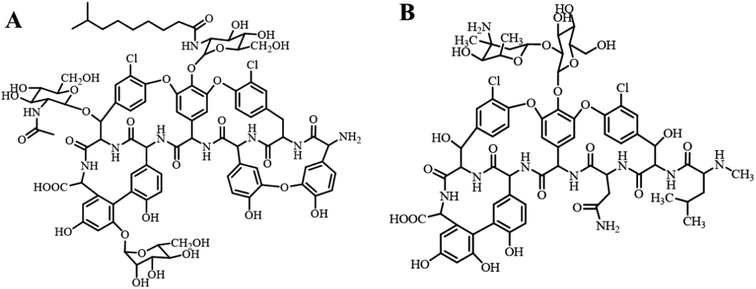
Teicoplanin (TEC) is a natural glycopeptide antibiotic (GPA) produced by soil-dwelling filamentous actinomycetes.1 It is mainly composed of five compounds with similar structures (A2-1, A2-2, A2-3, A2-4 and A2-5), A2-2 (Fig. 1). TEC has excellent antibacterial activity against Gram-positive bacteria. It interferes with the formation of the peptidoglycan skeleton by binding to D-Ala-D-Ala of the membrane-bound lipid II of peptidoglycan,2,3 which is the main component of the cell wall of Gram-positive bacteria. Therefore, TEC is mainly used for treating various diseases caused by Gram-positive bacterial infections such as methicillin-resistant Staphylococcus aureus (MRSA) and susceptible enterococcus. Especially for MRSA infections, TEC can be used as an alternative treatment for vancomycin (VCM, Fig. 1), which is the last-line defense against serious infections caused by staphylococci, enterococci and other Gram-positive bacteria.4,5 For elderly people, newborns, patients with renal insufficiency and other people who cannot use VCM, TEC can be considered as the first choice for the treatment of multi-drug resistant Staphylococcus aureus and enterococcus infections. Taking into account the cross-resistance of antibiotics, GPAs including TEC and VCM have been banned in the veterinary field by the European Union (EU), China and some other countries.6,7 The application of GPAs, and even abuse in food animals will eventually have a negative effect on the eco-environment, especially the environmental water, which might affect environmental safety and the health of humans and animals. Therefore, it is necessary and important to develop new methods for selective extraction, separation and enrichment of GPAs from environmental samples.Molecular imprinting technology (MIT) is a late 20th-century emergence of highly selective separation technology. The idea of this technique was derived from the specific recognition of antigen–antibody. The molecularly imprinted polymer (MIP) prepared on the basis of MIT is characterized by its high selectivity to the target molecule, and has been widely applied in different fields such as sample pretreatment,8 chiral resolution,9 chemical/biological sensing,10 catalytic degradation11 and controlled release of drugs.12 Tan et al.13 selected the glycosyl moiety of TEC as the template, 4-vinylphenylboronic acid and methyl methacrylate (MMA) as the functional monomers for preparing MIP via precipitation polymerization, and applied it to the extraction of TEC in biological samples. The average recoveries of TEC in urine, milk and plasma samples ranged from 75.2% to 92.4%. However, because some template molecules were embedded in the interior of the imprinted microspheres, for the MIP prepared using precipitation polymerization, there are some limitations such as difficulty in the elution of the template molecule, low adsorption capacity and slow mass transfer. The surface molecular imprinting technique can overcome these shortcomings. In this technique, the polymer is grafted onto the surface of a solid substrate to obtain the surface molecularly imprinted polymer (SMIP).
In the surface-imprinting technique, the carrier material plays a supporting role in grafting the polymer onto its surface. Many materials can be used as the carrier including magnetic Fe3O4 microspheres,14 silica gel,15,16 quantum dots (QD),17 polystyrene,18 carbon nanotube19 and nano-TiO2.20 They usually have a large specific surface area, controllable morphology and particle size, low preparation cost and strong surface reaction ability. Silica gel particle is the most widely used among these materials due to its unique properties. The favorable stability and rich porous structure of silica gel can promote the diffusion of solvents and template, thus helping the removal of the template. In addition, most of the template molecules are situated at the surface of polymers, which will increase the molecular recognition ability between polymers and target compounds and improve the mass transfer kinetics of SMIP.21 Therefore, this strategy can avoid the disadvantages of traditional MIP to some extent. The SMIP prepared with silica gel microspheres as the carrier had excellent properties and was used as an adsorbent for solid-phase extraction (SPE),22 matrix solid-phase dispersion (MSPD)23 and dispersive solid-phase extraction (dSPE)24 for the selective separation, cleanup and enrichment of target compounds from animal-derived food and environmental samples. Cheng et al.25 synthesized a melamine-SMIP on silica gel carrier by surface molecular imprinting technique and then used it to selectively extract melamine in milk as an SPE adsorbent. The results showed that the recovery of the spiked sample was between 75.6% and 96.8%, and the relative standard deviation (RSD) was less than 10%. Anene et al.26 prepared SMIP for patulin in acetonitrile for the selective extraction of patulin from apple juice samples. In the range of 0.1–10 mg L−1, the recovery of patulin was from 82% to 98%, and the RSD was less than 3.83%. The SMIP was also tested for its reusability and the results showed that the average recovery was more than 80% after 6 reuse cycles. Ren et al.27 prepared SMIP for bisphenol A with a sol–gel process on the surface of silica nanoparticles, which could serve as an efficient selective material for the determination of bisphenol A in the water environment. Zhu et al.28 synthesized a magnetic SMIP by microwave-assisted surface-imprinting technology, and SMIP could effectively adsorb and extract 4-nitrophenol from water. In addition, the imprinted polymer was also widely used in the removal of metal ions such as Cd(II)29 and Pb(II)30 from wastewater. There are few reports on the separation and purification of GPA with surface-imprinting technique. Therefore, the establishment of a highly selective pretreatment method based on SMIP has great significance for rapid and reliable monitoring of TEC residues in complex matrices.
Herein, this work represents the first attempt to prepare surface-imprinted polymer for TEC based on modified silica gel. The synthesis conditions of SMIP, including the type of porogenic solvent, molar ratios of the monomer and cross-linker were optimized. The morphology and structure of the prepared SMIP were studied, and the selectivity of SMIP related to the characteristics of monomers and cross-linkers were inferred. The adsorption experiments showed that the obtained SMIP had good selective recognition ability to TEC. Finally, the prepared SMIP was used as a dSPE adsorbent and successfully applied to enrich TEC from real water samples.
2. Materials and methods
2.1. Reagents and materials
MMA, methacrylic acid (MAA), acrylic acid (AA), hydroxyethyl methacrylate (HEMA), 2-hydroxypropyl methacrylate (HPMA), hydroxyethyl acrylate (HEA), hydroxybutyl acrylate (HBA), ethyl methacrylate (EMA), n-propyl methacrylate (PMA), butyl methacrylate (BMA), ethylene glycol diglycidyl ether (EGDE), trimethylolpropane triacrylate (TMPTA), glycidyl methacrylate (GMA) and N,N-methylenebisacrylamide (MBA) were purchased from J&K scientific. 4-Vinyl pyridine (4-VP), 2-vinyl pyridine (2-VP), acrylamide (AM), trimethylolpropane trimethacrylate (TMPTMA), ethylene glycol dimethacrylate (EGDMA), 2,2-azobisisobutyronitrile (AIBN), γ-methacryloxypropyltrimethoxysilane (γ-MPS) and 3-aminopropyltriethoxysilane (APTES) were purchased from Sigma Aldrich. HPLC grade acetonitrile (ACN), formic acid (FA) acetic acid (AA) and methanol (MeOH) were purchased from Fisher Scientific (Fairlawn, NJ, USA). Other analytical-grade solvents including N,N-dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) were brought from Guangzhou Chemical Reagent Factory (Guangzhou, China). Ultrapure water was produced using the Milli-Q water system (Molsheim, France). High purity silica gel (SPS100-5), carboxyl-modified (COOH SPS100-5) and sulfo-modified silica gel (SO3H SPS100-5) were provided by FUJI Corporation (Japan).TEC and VCM hydrochloride were purchased from Shanghai Haoyun Chemical Technology Co., Ltd. (Shanghai, China). Bacitracin (BAT) and colistin (CS) were purchased from Dr Ehrenstrofer (Germany). Spiramycin (SPM) and timicox (TIM) were provided by Sigma Aldrich (USA). Sulfamethazine (SM2), enrofloxacin (EN) and virginiamycin M1 (VGM) were all purchased from J&K scientific (China).
2.2. Apparatus and analytical conditions
High-performance liquid chromatography (HPLC) separations were performed on Agilent 1260 HPLC system equipped with a quaternary pump, an autosampler and an ultraviolet detector. The analytes were separated using an Agilent Extend-C18 column (250 mm × 4.6 mm i.d., 5.0 μm). The mobile phase consisted of (A) MeOH and (B) water containing 0.1% FA and 5 mmol L−1 of ammonium acetate. The gradient elution procedure was as follows: 0–1 min 40% A, 1–4 min 70% A, 4–8 min 70% A, 8–8.1 min 40% A and 8.1–10 min 40% A. The flow rate was 1.0 mL min−1, the injection volume was 10 μL and the UV monitoring was at 254 nm.2.3. Preparation of SMIP
Anhydrous toluene (100 mL), silica gel (5 g, 5 μm) and modifier (APTES, 5 mL) were added to a 150 mL round bottom flask, then triethylamine (1 mL) was added as a catalyst, and the reaction was stirred at 120 °C for 12 h under the argon atmosphere. The product was washed with MeOH and water and dried in a vacuum at 60 °C to obtain amino-modified silica gel particles. Under the same conditions, γ-MPS was used as the modifier to prepare vinyl-modified silica gel.The SMIP was prepared based on surface-modified silica gel. TEC (0.235 g) was dissolved in 30 mL of water. The functional monomer (HPMA, 0.263 mL) and carrier (amino-modified silica gel, 0.5 g) were added in the above solution. The mixture was vortexed and sonicated for 10 minutes and pre-polymerized at 4 °C for 6 hours. Subsequently, the cross-linker (TMPTMA, 1.6 mL) and initiator (AIBN, 10 mg) were added. The solvent was vortexed and sonicated to obtain a homogeneous solution and then argon was purged for 10 minutes to remove oxygen. The polymerization was carried out in an oil bath at 60 °C for 24 hours. After polymerization, the template was extracted with AA/MeOH solvent (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) using the Soxhlet extractor until TEC could not be detected by HPLC. As a control, non-imprinted polymer (NIP) was also prepared without TEC in the same way.
5, v/v) using the Soxhlet extractor until TEC could not be detected by HPLC. As a control, non-imprinted polymer (NIP) was also prepared without TEC in the same way.
2.4. Morphological characterization of the polymer
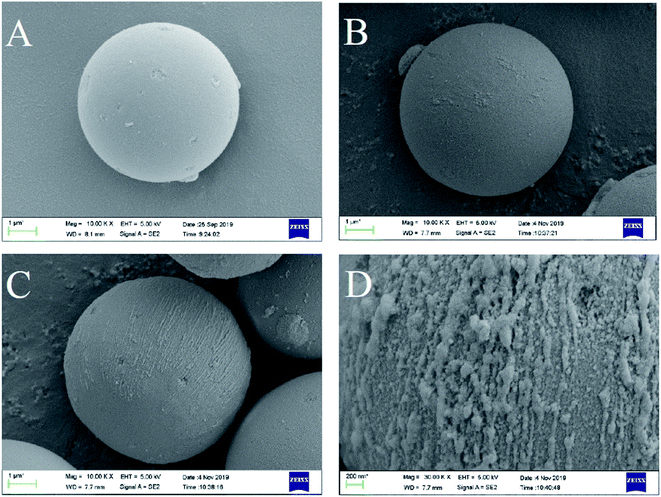
The morphology of SMIP and NIP was studied using scanning electron microscopy (ZEISS EVO18). The polymer microspheres were dried and sprayed with gold before the observation. The average particle size was analyzed using a laser particle size analyzer (Coulter LS230, Coulter Co., USA). The infrared spectrum was recorded from 4000 cm−1 to 500 cm−1 using a Fourier transform infrared spectrometer (Nicolet 6700) with potassium bromide as a blank background. The thermostability of SMIP and NIP was measured using a thermogravimetric analyzer (NETZSCH TG 209 F1 Libra) in the range of 100 °C to 900 °C.2.5. Adsorption and selectivity experiments
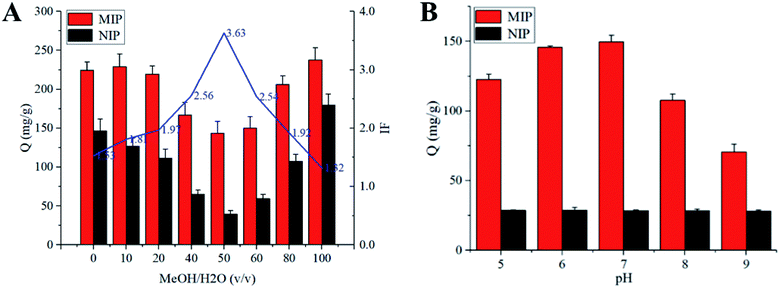
The adsorption experiments were carried out using a constant temperature oscillator (SHA-C, China). The influence of different factors on the adsorption capacity of SMIP and NIP were evaluated, such as solvent, pH, adsorption time and concentration of the substrate. 20 mg of polymers were dispersed in 5 mL of different proportions of MeOH–water (0, 10%, 20%, 40%, 50%, 60%, 80% and 100%) solutions with the initial concentration of 1 mg mL−1. The series of mixtures were shaken for 24 h at room temperature. After centrifugation, the equilibrium concentrations of the TEC were measured using HPLC. The adsorption capacity (Q, mg g−1) was calculated from the following equation:where C0 (mg L−1) and Ce (mg L−1) are the initial and equilibrium concentrations of TEC, respectively; V (L) is the volume of the solution, and W (g) is the weight of the polymer. The imprinting factor (IF) was calculated using the following equation:
| IF = QSMIP/QNIP |
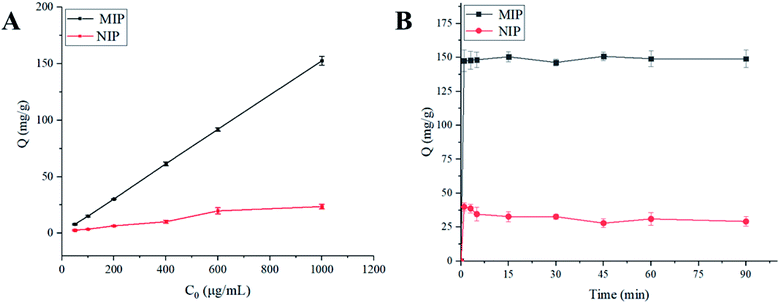
The influence of pH on the adsorption of polymer to TEC was studied in phosphate buffers with different pH values. Under the optimum conditions of solvent and pH, the static adsorption isotherm was performed at the initial concentrations ranging from 0.05 to 1.0 mg mL−1. Similarly, for the kinetic experiments, 20 mg of the polymer was dispersed in 5 mL of 50% MeOH–H2O with the TEC concentration of 1.0 mg mL−1 and then the mixtures were continuously shaken at certain intervals (1, 3, 5, 15, 30, 45, 60 and 90 min). Selective experiments were performed using structural analogs (such as VCM, CS, BAT, VGM) and other commonly used antimicrobials including EN, SPM, TIM and SM2. 20 mg of the polymer was added to 5 mL of each drug solution (200 μg mL−1). After incubation for 30 minutes at room temperature, the adsorption capacity and imprint factor were calculated.
2.6. Sample preparation
Lake, spring and Pearl River water were collected from the surrounding areas of Guangzhou. After sedimentation, they were filtered with a 0.25 μm microporous membrane to remove fine particles. The real water samples were stored in brown glass bottles at 4 °C. 100 mg of the polymer was dispersed in 250 mL of environmental water samples and shook for 20 minutes at room temperature. The samples were filtered and the polymer particles were collected, then the polymer was washed twice with 3 mL water and eluted with 5 mL of 10% ammonia in methanol. The eluate was evaporated to dryness under a gentle stream of nitrogen and the residue was reconstituted with 0.5 mL mobile phase solution before HPLC analysis.3. Results and discussion
3.1. Optimization of surface molecularly imprinted polymer
Due to the absence of specific purification approaches, most available methods for the analysis of GPAs had low selectivity and poor sensitivity. The purpose of this study was to synthesize highly selective imprinted polymer, which can selectively bind TEC and its structural analogues. Therefore, the effects of polymerization factors including carrier, monomer, cross-linker and porogen on the performance of surface-imprinted polymer were evaluated.Compared with the acid-modified silica gel, polymers with higher specificity were obtained using both amino- and vinyl-modified silica gel (synthesized in our laboratory) as carriers. Generally, vinyl groups on the surface of the silica gel could participate in the free radical reaction of the imprinted polymer. However, the adsorption capacity and specificity of the polymer prepared with vinyl-modified silica gel were far lower than those prepared with amino-modified silica gel. It could be explained by the fact that there are a large number of alcohol hydroxyl and phenolic hydroxyl groups in the TEC molecule, which could form abundant hydrogen bonds with amino groups on the surface of the carrier. The SMIP with many specific imprinting sites that are complementary in size and shape to the template was produced. The aminopropyls on the surface of silica gel particles would initiate polycondensation during surface imprinting process. In addition, it could also act as an assistant group to drive template molecules into the formed imprinting polymer during the imprinting polymerization.36 The reactive amino groups on the surface of silica gel continuously capture the newly formed oligomers in the solution through the hydrogen bond interaction with TEC and the monomer, and finally, the imprinted polymer was embedded on the surface of silica gel.
As shown in Table 1, when HPMA (hydroxypropylated MAA) was used as a monomer, the adsorption capacity and selectivity (IF > 2.0) of SMIP were slightly higher than those of HEMA. It was supposed that hydrogen bonds between the template and monomer are the most important for the specificity of SMIP. To prove this view, the derivatives obtained by esterifying MAA with an alkyl side chain (methyl-, ethyl-, propyl- and butyl-) were tested as monomers (Fig. S1†), the adsorptive amounts of all SMIPs increased (possibly due to the increase of hydrophobicity), while the selectivity decreased (IF < 1.4). It was further indicated that the side chain hydroxyl group providing hydrogen bond acceptor (donor) in the series of esterified MAAs had a great influence on the selectivity. Interestingly, the adsorption capacity and selectivity of SMIPs synthesized with AA and its hydroxyalkyl esters as monomers were significantly lower than those of MAA esters, indicating that the existence of α-methyl (steric hindrances) in esterified MAA monomers was very important. Finally, HPMA was selected as the functional monomer in this experiment.
| No. | Monomers | Q (mg g−1) | IF | |
|---|---|---|---|---|
| SMIP | NIP | |||
| a SMIP, surface molecularly imprinted polymer; NIP, non-imprinted polymer; IF, imprinting factor. | ||||
| 1 | Methacrylic acid | 38.9 | 27.0 | 1.44 |
| 2 | Hydroxyethyl methacrylate | 42.7 | 22.0 | 1.94 |
| 3 | 2-Hydroxypropyl methacrylate | 44.8 | 21.9 | 2.05 |
| 4 | Methyl methacrylate | 48.0 | 45.9 | 1.04 |
| 5 | Ethyl methacrylate | 48.1 | 36.4 | 1.32 |
| 6 | n-Propyl methacrylate | 48.6 | 43.9 | 1.11 |
| 7 | Butyl methacrylate | 47.8 | 34.9 | 1.37 |
| 8 | Acrylic acid | 42.7 | 31.4 | 1.36 |
| 9 | Hydroxyethyl acrylate | 18.0 | 18.8 | 0.96 |
| 10 | Hydroxybutyl acrylate | 20.2 | 19.8 | 1.02 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, respectively.
40, respectively.3.2. Characterization
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration and aliphatic C–H stretching, respectively, which confirmed that HPMA and TMPTMA were successfully grafted on the surface of amino-modified silica gel.
O stretching vibration and aliphatic C–H stretching, respectively, which confirmed that HPMA and TMPTMA were successfully grafted on the surface of amino-modified silica gel.3.3. Adsorption and selectivity characteristics
3.4. Determination of TEC in real water samples
The feasibility of SMIP was investigated by enriching TEC from real water. The TEC was extracted and enriched from real water samples by dispersive solid-phase extraction and TEC was not detected in all blank water samples. The typical chromatograms obtained before and after the selective extraction are shown in Fig. S5.† It could be seen that there was no peak of TEC in the blank real water sample, and there was an obvious peak after spiking it with 10 μg L−1 of TEC. The peak of TEC was weak after selective extraction by SMIP, which meant that the templates had been selectively captured. For the proposed HPLC method based on SMIP-dSPE, the average recoveries of TEC in real water samples ranged from 81.4% to 94.6% and the RSD was less than 10.4%. The recoveries were determined based on real water samples spiked at three concentration levels (10, 20 and 50 μg L−1), and the linear range was from 5 μg L−1 to 100 μg L−1. The limit of detection for the TEC was 5 μg L−1, and the enrichment factor was more than 500. The results showed that SMIP could be used for selective extraction, enrichment and detection of TECs in real water samples.4. Conclusions
In this study, a selective surface SMIP was synthesized with TEC as a template and amino-modified silica gel as supporting material. Under the optimized conditions, the SMIP showed good adsorption performance for TEC. The SMIP as a dSPE material could selectively adsorb TEC from environmental water, and recovery and precision met the requirements for trace analysis. This study can provide a reference for the application of surface imprinting technique to glycopeptide antibiotics, also it will help in further development and application of SMIP.Conflicts of interest
The other co-authors declare that they have no conflict of interest.Acknowledgements
This research was funded by the National Science Foundation of China (31572562), Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2019BT02N054) and the Key Program of Guangzhou Science and Technology Plan (201804020019).References
- E. Binda, P. Cappelletti, F. Marinelli and G. L. Marcone, Antibiotics, 2018, 7(2), 36 CrossRef PubMed.
- D. J. Newman, ACS Med. Chem. Lett., 2018, 9, 66–67 CrossRef CAS PubMed.
- P. Sarkar and J. Haldar, Glycopeptide Antibiotics Mechanism of Action and Recent Developments, 2020 Search PubMed.
- Y. Peng, X. Ye, Y. Li, T. Bu, X. Chen, J. Bi, J. Zhou and Z. Yao, PLoS One, 2013, 8(11), e79782 CrossRef PubMed.
- S. Svetitsky, L. Leibovici and M. Paul, Antimicrob. Agents Chemother., 2009, 53, 4069–4079 CrossRef CAS PubMed.
- S. Schwarz, C. Kehrenberg and T. R. Walsh, Int. J. Antimicrob. Agents, 2001, 17, 431–437 CrossRef CAS.
- Ministry of Agriculture and Rural Affairs No. 250 Bulletin of the People's Republic of China, 2020, http://www.moa.gov.cn/gk/tzgg_1/gg/202001/t20200106_6334375.htm.
- X. Song, T. Zhou, J. Zhang, Y. Su, H. Zhou and L. He, Polymers, 2019, 11(7), 1109 CrossRef CAS PubMed.
- Y. Liu, Z. Li and L. Jia, J. Chromatogr. A, 2020, 1622, 461147 CrossRef CAS PubMed.
- B. Babamiri, A. Salimi and R. Hallaj, Biosens. Bioelectron., 2018, 117, 332–339 CrossRef CAS PubMed.
- C. C. de Escobar, Y. P. Moreno Ruiz, J. H. Z. dos Santos and L. Ye, Colloids Surf., A, 2018, 538, 729–738 CrossRef CAS.
- K. Ji, X. Luo, L. He, S. Liao, L. Hu, J. Han, C. Chen, Y. Liu and N. Tan, J. Pharm. Biomed. Anal., 2020, 180, 113036 CrossRef CAS PubMed.
- L. Tan, Y. Li, X. Pan, M. L. Marina and Z. Jiang, J. Chromatogr. A, 2020, 1615, 460776 CrossRef CAS PubMed.
- R. Gao, S. Zhao, Y. Hao, L. Zhang, X. Cui, D. Liu and Y. Tang, RSC Adv., 2015, 5, 88436–88444 RSC.
- K. Bashir, P. Guo, G. Chen, Y. Li, Y. Ge, H. Shu and Q. Fu, Arabian J. Chem., 2020, 13, 4082–4091 CrossRef CAS.
- X. Kong, F. Li, Y. Li, X. He, L. Chen and Y. Zhang, J. Sep. Sci., 2020, 43, 808–817 CrossRef CAS.
- X. Wang, H. Ding, X. Yu, X. Shi, A. Sun, D. Li and J. Zhao, Talanta, 2019, 197, 98–104 CrossRef CAS PubMed.
- J. Li, X. Hu, P. Guan, X. Zhang, L. Qian, R. Song, C. Du and C. Wang, RSC Adv., 2015, 5, 62697–62705 RSC.
- M. Amatatongchai, W. Sroysee, P. Sodkrathok, N. Kesangam, S. Chairam and P. Jarujamrus, Anal. Chim. Acta, 2019, 1076, 64–72 CrossRef CAS PubMed.
- L. Sun, J. Guan, Q. Xu, X. Yang, J. Wang and X. Hu, Polymers, 2018, 10(11), 1248 CrossRef PubMed.
- N. Casado, S. Morante-Zarcero, D. Perez-Quintanilla, J. S. Camara and I. Sierra, Trends Food Sci. Technol., 2020, 98, 167–180 CrossRef CAS.
- X. Hou, W. Huang, F. Zhu, F. Geng and M. Tian, Anal. Methods, 2019, 11, 317–326 RSC.
- S. Shi, D. Fan, H. Xiang and H. Li, Food Chem., 2017, 237, 198–204 CrossRef CAS.
- D. Fan, H. Li, S. Shi and X. Chen, J. Chromatogr. A, 2016, 1470, 27–32 CrossRef CAS.
- W. Cheng, Z. Liu and Y. Wang, Talanta, 2013, 116, 396–402 CrossRef CAS.
- A. Anene, K. Hosni, Y. Chevalier, R. Kalfat and S. Hbaieb, Food Control, 2016, 70, 90–95 CrossRef CAS.
- Y. Ren, W. Ma, J. Ma, Q. Wen, J. Wang and F. Zhao, J. Colloid Interface Sci., 2012, 367, 355–361 CrossRef CAS.
- W. Liang, Y. Lu, N. Li, H. Li and F. Zhu, Microchem. J., 2020, 159, 105316 CrossRef CAS.
- F. Zhu, L. Li and J. Xing, J. Hazard. Mater., 2017, 321, 103–110 CrossRef CAS PubMed.
- F. Zhu, Y. Lu and L. Li, RSC Adv., 2016, 6, 111120–111128 RSC.
- Z. Zhang, L. Chen, F. Yang and J. Li, RSC Adv., 2014, 4, 31507–31514 RSC.
- G. Guan, R. Liu, Q. Mei and Z. Zhang, Chem.–Eur. J., 2012, 18, 4692–4698 CrossRef CAS.
- Z. Zhang, J. Li, J. Fu and L. Chen, RSC Adv., 2014, 4, 20677 RSC.
- X. Li, X. Ma, R. Huang, X. Xie, L. Guo and M. Zhang, J. Sep. Sci., 2018, 41, 2837–2845 CrossRef CAS PubMed.
- W. Huang, P. Xu, W. Yang and W. Xu, RSC Adv., 2016, 6, 74734–74741 RSC.
- L. Tan, W. Li, H. Li and Y. Tang, J. Chromatogr. A, 2014, 1336, 59–66 CrossRef CAS PubMed.
- L. Chen, X. Wang, W. Lu, X. Wu and J. Li, Chem. Soc. Rev., 2016, 45, 2137–2211 RSC.
- E. Verheyen, J. P. Schillemans, M. van Wijk, M.-A. Demeniex, W. E. Hennink and C. F. van Nostrum, Biomaterials, 2011, 32, 3008–3020 CrossRef CAS.
- D. De Smet, V. Kodeck, P. Dubruel, C. Van Peteghem, E. Schacht and S. De Saeger, J. Chromatogr. A, 2011, 1218, 1122–1130 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra00913c |
| This journal is © The Royal Society of Chemistry 2021 |