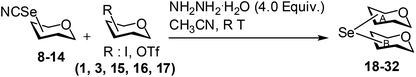Open Access Article
Open Access ArticleOn-water synthesis of glycosyl selenocyanate derivatives and their application in the metal free organocatalytic preparation of nonglycosidic selenium linked pseudodisaccharide derivatives†
Tapasi Manna and
Anup Kumar Misra *
*
Division of Molecular Medicine, Bose Institute, P-1/12, C.I.T. Scheme VII M, Kolkata 700054, India. E-mail: akmisra69@gmail.com; Fax: +91-33-2355-3886
First published on 15th March 2021
Abstract
Glycosyl selenocyanate derivatives were prepared in very good yield by the treatment of glycosyl halide or triflate derivatives with potassium selenocyanate in water. A variety of selenium linked pseudodisaccharide derivatives were prepared in excellent yield using glycosyl selenocyanates as stable building blocks in the presence of hydrazine hydrate under metal-free organocatalytic reaction conditions.
Introduction
In order to support the increasing interest in glycobiology research for the development of novel carbohydrate based therapeutics, a large number of oligo- and polysaccharides as well as a diverse range of glycomimetics have been synthesized in the recent past.1–6 Pseudosugar derivatives (e.g. pseudodisaccharides) have been considered as useful glycomimetics for their use as stable enzyme inhibitors.7 One of the important techniques for the preparation of glycomimetics is to exchange the interglycosidic oxygen atom with other heteroatoms such as nitrogen, sulphur, selenium, etc.8–10 Due to their increased stability towards hydrolysis, a variety of thiosugars and thioglycosides have been prepared for their use in the biochemical investigations of the carbohydrate processing enzymes.11–15 Organoselenium chemistry has become an important topic of research due to the unique chemical behaviour of Se-containing compounds and their pharmacological potential.16–20 Although, selenium has been incorporated in different classes of molecules to improve their therapeutic index,21 the introduction of selenium within the carbohydrate framework has received less attention except for a few reports which include the synthesis of anomeric selenoglycosides22–27 and their application in the preparation of oligosaccharides,28–30 glycal derivatives,31 glycosyl fluorides,32 C-glycosides33 and medicinally useful compounds.34 The synthesis of some cyclic sugar intermediates containing selenium in the ring has also been pursued.35 In addition, some reports also appeared on the synthesis of non-glycosidically selenium linked pseudodisaccharide derivatives.18a,21c,36,37 In most of the cases, elemental selenium, selenium oxide, selenourea or aryl selenol has been used as the source of selenium for its incorporation in the carbohydrate intermediates.22–37 Dialkyl or diaryldiselenides have also been used under reductive reaction conditions to furnish selenoglycosides.26,29 Obviously, there are several shortcomings associated with the above-mentioned reaction conditions such as use of obnoxious reagents, incompatibility of the base labile protecting groups, hazardous and special reaction conditions etc. Therefore, it is quite pertinent to develop reaction conditions avoiding earlier mentioned drawbacks. In the recent past, potassium p-methylselenobenzoate (KSeBz) has been successfully used as the selenium source in the preparation of several selenium containing carbohydrate derivatives.22,27,37 KSeBz has been prepared using a multistep reaction sequence starting from elemental selenium.22a As an alternative, a straightforward reaction condition has been reported for the synthesis of selenium linked glycosides using glycosyl selenoacetate as stable building blocks, prepared by the treatment of glycosyl halides with commercially available potassium selenocyanate (KSeCN) in acetonitrile avoiding the prerequisite preparation of the selenating agent.38 In a separate report, KSeCN has also been used as selenating agent for the preparation of unsymmetrical organoselenide and selenoglycosides.27b KSeCN has also been used as the selenating agent for the aqueous medium preparation di-alkyldiselenides.39 In order to extend the use of KSeCN in the preparation of selenium incorporated carbohydrate derivatives, attempts were made to prepare stable glycosyl selenocyanate derivatives and their utilization in the preparation of selenium linked pseudodisaccharides. Cumpstey and co-worker reported the synthesis of several non-glycosidically selenoether linked pseudodisaccharide derivatives using KSeBz as selenating agent, involving multistep reaction sequence via the formation of diselenide derivatives.37 Lüdtke and co-workers synthesized selenium linked neoglycoconjugates and pseudodisaccharides using lithium diselenide using multistep reaction sequence.36a,b Expensive selenourea has been used as selenating agent in some studies.27b,36d In this context, it would be beneficial to develop an environmentally benign aqueous reaction condition in the “Green chemistry” perspective for the preparation of glycosyl selenocyanate derivatives and their direct use in the synthesis of non-glycosidically selenoether linked pseudodisaccharide derivatives by the treatment of suitable glycosyl electrophiles. Inspired by the earlier studies38,39 on the use of KSeCN as efficient selenylating agent, a straightforward on-water synthesis of stable glycosyl selenocyanate derivatives and their application in the selenoether linked pseudodisaccharide derivatives under a metal-free organocatalytic reaction condition is reported herein (Scheme 1).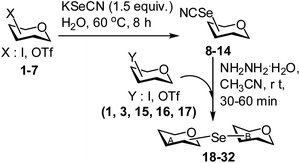 | ||
| Scheme 1 Synthesis of glycosyl selenocyanate derivatives in aqueous medium and their utilization in the organocatalytic preparation of selenium linked pseudodisaccharide derivatives. | ||
Results and discussion
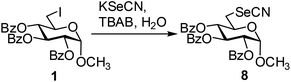
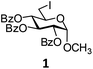
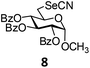
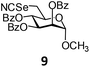
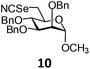
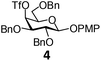
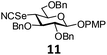
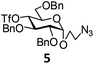
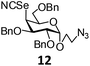
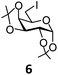
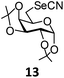
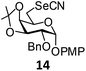
In order to optimize the reaction condition for the preparation of glycosyl selenocyanate derivative, methyl 2,3,4-tri-O-benzoyl-6-deoxy-6-iodo-α-D-glucopyranoside (1) was treated with a varied quantity of KSeCN in the presence of tetrabutylammonium bromide (TBAB), a phase transfer catalyst (PTC) in water. The best yield of methyl 2,3,4-tri-O-benzoyl-6-deoxy-6-selenato-α-D-glucopyranoside (8) was obtained in 76% by using 1.5 equiv. KSeCN and 0.1 equiv. TBAB in H2O at 60 °C in 8 h. Reduction of the quantity of KSeCN and TBAB led to the incomplete reaction with low yield of product formation (Table 1). The reaction became sluggish at room temperature. The reaction did not proceed well in absence of TBAB and starting material decomposed under the reaction condition. Following the similar reaction condition, a series of glycosyl selenocyanate derivatives (8–14) has been prepared in satisfactory yield under aqueous reaction conditions (Table 2). The products were characterized using NMR and mass spectral analysis.Having a set of stable glycosyl selenocyanate derivatives, it was decided to develop a reaction condition for the preparation of nonglycosidically selenoether linked pseudodisaccharide derivatives by the reaction of glycosyl selenocyanate derivatives with appropriate electrophiles in the presence of an organocatalyst. For this purpose, a panel of sugar electrophiles have been selected, which are presented in Fig. 1.
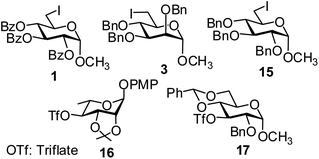 | ||
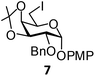
| Fig. 1 Glycosyl iodide and triflate derivatives used as electrophiles for the preparation of non-glycosidically selenium linked pseudodisaccharide derivatives. | ||
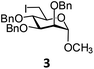
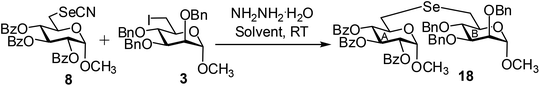
Compound 8 was allowed to react with compound 3 in the presence of a number of base such as NaBH4, K2CO3, hydrazine monohydrate, diethyl amine, piperidine in several organic solvents such as DMF, CH3CN, THF, CH3OH, CH2Cl2. After a number of experimentation it was observed that required pseudodisaccharide derivative 18 was formed in 77% yield using 1.0 equiv. of compound 8 and 1.2 equiv. of compound 3 in the presence of hydrazine monohydrate (4.0 equiv.) in CH3CN at room temperature (Table 3). Use of less quantity of electrophile and hydrazine monohydrate resulted in the incomplete consumption of the selenocyanate derivative. Although the use of DMF and CH3CN as reaction solvents comparable yields of the product 18 was obtained, CH3CN has been chosen as the solvent in the generalized reaction condition. Among several bases used in the reaction, best yield of the product was obtained in the presence of hydrazine monohydrate as an organic base. Earlier, NaBH4 has been used for the hydrolysis of the in situ generated organic selenocyanate derivatives followed by reduction of the diselenide derivatives for the preparation of unsymmetrical selenides.40 Very recently, hydrazine hydrate has been used as a base cum reducing agent in the preparation unsymmetrical glycosyl disulfide derivatives.41 In this study, similar reaction condition has been applied for the preparation of a series of nonglycosidically selenoether linked pseudodisaccharide derivatives (18–32) in excellent yield (Table 4). The products were unambiguously characterized using NMR and mass spectral analysis.
| Sl no. | Comp. 8 (equiv.) | Comp. 3 (equiv.) | Base (equiv.) | Solvent | Time (min) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 1.0 | 1.2 | NH2NH2·H2O (4.0) | DMF | 45 | 74 |
| 2 | 1.0 | 1.2 | NH2NH2·H2O (4.0) | DMF | 60 | 75 |
| 3 | 1.0 | 1.2 | NH2NH2·H2O (4.0) | CH3CN | 60 | 77 |
| 4 | 1.0 | 1.2 | NH2NH2·H2O (4.0) | CH3CN | 45 | 70 |
| 5 | 1.0 | 1.2 | NH2NH2·H2O (3.0) | CH3CN | 60 | 70 |
| 6 | 1.0 | 1.2 | NH2NH2·H2O (2.0) | CH3CN | 60 | 68 |
| 7 | 1.0 | 1.2 | NH2NH2·H2O (4.0) | THF | 60 | 45 |
| 8 | 1.0 | 1.2 | NH2NH2·H2O (4.0) | CH3OH | 60 | 25 |
| 9 | 1.0 | 1.2 | NH2NH2·H2O (4.0) | CH2Cl2 | 60 | 20 |
| 10 | 1.0 | 1.2 | NaBH4 (2.0) | CH3CN | 60 | 72 |
| 11 | 1.0 | 1.2 | K2CO3 (2.0) | CH3CN | 60 | — |
| 12 | 1.0 | 1.2 | Pyrrolidine (4.0) | CH3CN | 120 | 20 |
| 13 | 1.0 | 1.2 | Pyrrolidine (4.0) | DMF | 120 | 20 |
| 14 | 1.0 | 1.2 | Diethylamine (4.0) | CH3CN | 120 | 10 |
Experimental
General methods
All reactions were monitored by thin layer chromatography over silica gel coated TLC plates. The spots on TLC were visualized by warming ceric sulphate (2% Ce(SO4)2 in 2 N H2SO4) sprayed plates on a hot plate. Silica gel 230–400 mesh was used for column chromatography. 1H and 13C NMR spectra were recorded on Bruker Avance 500 MHz spectrometer using CDCl3 as solvent and TMS as internal reference unless stated otherwise. Chemical shift values are expressed in δ ppm. HR-MS were recorded on a Micromass mass spectrometer.NMR spectral data of glycosyl selenocyanate derivatives (8–14):
Methyl 2,3,4-tri-O-benzoyl-6-deoxy-6-selenocyanato-α-D-glucopyranoside (8). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 7.98–7.21 (m, 15H, Ar-H), 6.18 (t, J = 9.5 Hz, 1H, H-2), 5.41 (t, J = 10.0 Hz, 1H, H-3), 5.30–5.26 (m, 2H, H-1, H-4), 4.41–4.37 (m, 1H, H-5), 3.57 (s, 3H, OCH3), 3.42 (dd, J = 12.5 Hz, 3.0 Hz, 1H, H-6a), 3.31 (dd, J = 12.5 Hz, 8.0 Hz, 1H, H-6b); 13C NMR (125 MHz, CDCl3): δ 165.6, 165.5 (3 PhCO), 133.8–128.3 (Ar-C), 101.3 (SeCN), 97.2 (C-1), 72.5 (C-5), 71.7 (C-2), 69.5 (C-3), 68.2 (C-4), 56.0 (OCH3), 31.0 (C-6); HRMS for C29H25NO8Se [M + H]+: calcd 596.0823; found: 596.0802.
Methyl 2,3,4-tri-O-benzoyl-6-deoxy-6-selenocyanato-α-D-mannopyranoside (9). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 8.04–7.16 (m, 15H, Ar-H), 5.85 (dd, J = 10.0 Hz, 3.0 Hz, 1H, H-3), 5.68 (t, J = 10.0 Hz, 1H, H-4), 5.58 (br s, 1H, H-2), 4.91 (s, 1H, H-1), 4.32–4.29 (m, 1H, H-5), 3.51 (s, 3H, OCH3), 3.40 (dd, J = 12.5 Hz, 5.0 Hz, 1H, H-6a), 3.28 (dd, J = 12.5 Hz, 8.0 Hz, 1H, H-6b); 13C NMR (125 MHz, CDCl3): δ 166.0, 165.2, 165.1 (3 PhCO), 133.8–128.3 (Ar-C), 101.4 (SeCN), 98.8 (C-1), 70.3 (C-2), 70.2 (C-5), 69.1 (C-3), 69.0 (C-4), 55.9 (OCH3), 31.4 (C-6); HRMS for C29H25NO8Se [M + H]+: calcd 596.0823; found: 596.0805.
Methyl 2,3,4-tri-O-benzyl-6-deoxy-6-selenocyanato-α-D-mannopyranoside (10). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 7.23–7.14 (m, 15H, Ar-H), 4.85 (d, J = 11.5 Hz, 1H, PhCH), 4.60 (d, J = 12.0 Hz, 1H, PhCH), 4.54–4.50 (m, 3H, H-1, 2 PhCH), 4.46 (br s, 2H, 2 PhCH), 3.75 (dd, J = 8.5 Hz, 2.5 Hz, 1H, H-3), 3.70–3.66 (m, 2H, H-4, H-5), 3.64 (br s, 1H, H-2), 3.33 (dd, J = 11.5 Hz, 3.0 Hz, 1H, H-6a), 3.19 (s, 3H, OCH3), 3.03 (dd, J = 11.5 Hz, 7.0 Hz, 1H, H-6b); 13C NMR (125 MHz, CDCl3): δ 138.2–127.7 (Ar-C), 102.1 (SeCN), 99.3 (C-1), 80.0 (C-5), 77.4 (C-2), 75.2 (PhCH2), 74.6 (C-3), 73.0, 72.1 (2 PhCH2), 70.5 (C-4), 55.2 (OCH3), 31.9 (C-6); HRMS for C29H31NO5Se [M + H]+: calcd 554.1445; found: 554.1430.
p-Methoxyphenyl-2,3,6-tri-O-benzyl-4-deoxy-4-selenocyanato-β-D-glucopyranoside (11). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 7.38–7.31 (m, 15H, Ar-H), 7.04 (d, J = 9.0 Hz, 2H, Ar-H), 6.85 (d, J = 9.0 Hz, 2H, Ar-H), 5.13 (d, J = 11.0 Hz, 1H, PhCH), 5.04 (d, J = 10.0 Hz, 1H, PhCH), 4.95 (d, J = 8.0 Hz, 1H, H-1), 4.87–4.84 (m, 2H, 2 PhCH), 4.69 (d, J = 12.0 Hz, 1H, PhCH), 4.53 (d, J = 12.0 Hz, 1H, PhCH), 4.03 (dd, J = 11.5 Hz, 4.0 Hz, 1H, H-6a), 3.92 (dd, J = 11.5 Hz, 7.0 Hz, 1H, H-6b), 3.87–3.84 (m, 2H, H-2, H-5), 3.81 (s, 3H, OCH3), 3.78 (t, J = 8.0 Hz, 1H, H-3), 3.41 (t, J = 10.5 Hz, 1H, H-4); 13C NMR (125 MHz, CDCl3): δ 155.6–114.6 (Ar-C), 102.7 (C-1), 99.1 (SeCN), 83.4 (C-3), 81.2 (C-5), 76.4, 75.1 (2 PhCH2), 75.0 (C-2), 73.6 (PhCH2), 69.0 (C-6), 55.5 (OCH3), 46.3 (C-4); HRMS for C35H35NO6Se [M + H]+: calcd 646.1708; found: 646.1690.
2-Azidoethyl-2,3,6-tri-O-benzyl-4-deoxy-4-selenocyanato-β-D-galactopyranoside (12). 1H NMR (500 MHz, CDCl3): δ 7.41–7.24 (m, 15H, Ar-H), 4.79 (d, J = 12.0 Hz, 2H, 2 PhCH), 4.65 (d, J = 4.0 Hz, 1H, H-1), 4.61 (d, J = 12.0 Hz, 1H, PhCH), 4.59 (d, J = 12.0 Hz, 1H, PhCH), 4.56 (d, J = 12.0 Hz, 1H, PhCH), 4.51 (d, J = 12.0 Hz, 1H, PhCH), 4.27–4.23 (m, 2H, H-4, H-5), 4.04 (dd, J = 9.5 Hz, 4.0 Hz, 1H, H-2), 3.75–3.70 (m, 1H, OCH), 3.61 (d, J = 6.0 Hz, 2H, H-6ab), 3.57 (dd, J = 9.5 Hz, 3.0 Hz, 1H, H-3), 3.52–3.48 (m, 1H, OCH), 3.45–3.41 (m, 1H, NCH), 3.34–3.32 (m, 1H, NCH); 13C NMR (125 MHz, CDCl3): δ 138.1–127.9 (Ar-C), 101.5 (SeCN), 98.0 (C-1), 76.8 (C-3), 76.1 (C-2), 73.7, 73.6, 72.4 (3 PhCH2), 70.5 (C-6), 67.8 (C-5), 67.2 (OCH2), 54.8 (C-4), 50.5 (NCH2); HRMS for C30H32N4O5Se [M + H]+: calcd 609.1616; found: 609.1597.
1,2:3,4-Di-O-isopropylidene-6-deoxy-6-selenocyanato-α-D-galactopyranose (13). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 5.44 (d, J = 5.0 Hz, 1H, H-1), 4.58 (dd, J = 8.0 Hz, 2.5 Hz, 1H, H-3), 4.26–4.25 (m, 1H, H-4), 4.20 (dd, J = 8.0 Hz, 1.5 Hz, 1H, H-2), 4.01–3.99 (m, 1H, H-5), 3.30 (dd, J = 12.0 Hz, 8.0 Hz, 1H, H-6a), 3.17 (dd, J = 12.0 Hz, 5.0 Hz, 1H, H-6b), 1.15 (s, 3H, CH3), 1.37 (s, 3H, CH3), 1.27 (s, 6H, 2 CH3); 13C NMR (125 MHz, CDCl3): δ 109 8, 109.1 (2 C(CH3)2), 102 0 (SeCN), 96.4 (C-1), 72.0 (C-3), 70.9 (C-4), 70.3 (C-2), 66.6 (C-5), 29.2 (C-6), 26.0, 25.9, 24.9, 24.3 (4 CH3); HRMS for C13H19NO5Se [M + H]+: calcd 350.0506; found: 350.0487.
p-Methoxyphenyl-2-O-benzyl-3,4-O-isopropylidene-6-deoxy-6-selenocyanato-α-D-galactopyranoside (14). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 7.25–7.15 (m, 5H, Ar-H), 6.91 (d, J = 8.5 Hz, 2H, Ar-H), 6.73 (d, J = 8.5 Hz, 2H, Ar-H), 5.26 (d, J = 3.5 Hz, 1H, H-1), 4.70 (d, J = 10 Hz, 1H, PhCH), 4.66 (d, J = 12.5 Hz, 1H, PhCH), 4.43 (t, J = 6.0 Hz, 1H, H-2), 4.29–4.28 (m, 1H, H-5), 4.15–4.14 (m, 1H, H-4), 3.65 (s, 3H, OCH3), 3.54–3.52 (m, 1H, H-3), 3.36 (dd, J = 12.0 Hz, 8.5 Hz, 1H, H-6a), 3.14 (dd, J = 12.5 Hz, 5.0 Hz, 1H, H-6b), 1.31 (s, 3H, CH3), 1.25 (s, 3H, CH3); 13C NMR (125 MHz, CDCl3): δ 155.3–114.6 (Ar-C), 109 7 (C(CH3)2), 102 0 (SeCN), 96.5 (C-1), 75.7 (C-3), 75.6 (C-4), 74.2 (C-5), 72.5 (PhCH2), 67.2 (C-2), 55.5 (OCH3), 29.6 (C-6), 27.9, 26.3 (2 CH3); HRMS for C24H27NO6Se [M + H]+: calcd 506.1082; found: 506.1065.
NMR spectral data of Se-linked pseudodisaccharide derivatives (18–32):
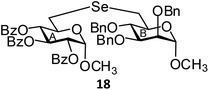
(Methyl 2,3,4-tri-O-benzoyl-6-deoxy-α-D-glucopyranosid-6-yl)-(methyl 2,3,4-tri-O-benzyl-6-deoxy-α-D-mannopyranosid-6-yl)selenide (18). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 8.03–7.28 (m, 30H, Ar-H), 6.16 (t, J = 10 Hz, 1H, H-4A), 5.48 (t, J = 10.0 Hz, 1H, H-3A), 5.28 (dd, J = 10.5 Hz, 3.5 Hz, 1H, H-2A), 5.17 (d, J = 3.5 Hz, 1H, H-1A), 4.99 (d, J = 11.0 Hz, 1H, PhCH), 4.68–4.65 (m, 3H, 3 PhCH), 4.61 (s, 2H, 2 PhCH), 4.59 (br s, 1H, H-1B), 4.30–4.26 (m, 1H, H-5A), 3.83–3.75 (m, 3H, H-3B, H-4B, H-5B), 3.57 (d, J = 5.0 Hz, 1H, H-2B), 3.50 (s, 3H, OCH3), 3.32 (s, 3H, OCH3), 3.11 (d, J = 11.5 Hz, 1H, H-6aA), 2.98–2.91 (m, 2H, H-6bA, H-6aB), 2.89–2.82 (m, 1H, H-6bB); 13C NMR (125 MHz, CDCl3): δ 165.7, 165.6, 165.3 (3 PhCO), 138.5–127.5 (Ar-C), 98.7 (C-1B), 96.7 (C-1A), 80.1 (C-5B), 78.5 (C-2B), 75.2 (PhCH2), 74.5 (C-5A), 73.1 (C-2A), 73.0 (C-3A), 72.5 (PhCH2), 72.2 (C-3B), 72.0 (PhCH2), 70.5 (C-4A), 70.3 (C-4B), 55.5, 54.6 (2 OCH3), 26.8 (C-6B), 25.4 (C-6A); HRMS for C56H56O13Se [M + H]+: calcd 1017.2964; found: 1017.2946.
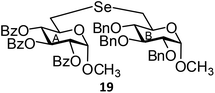
(Methyl 2,3,4-tri-O-benzoyl-6-deoxy-α-D-glucopyranosid-6-yl)-(methyl 2,3,4-tri-O-benzyl-6-deoxy-α-D-glucopyranosid-6-yl)selenide (19). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 8.00–7.25 (m, 30H, Ar-H), 6.13 (t, J = 10.0 Hz, 1H, H-4A), 5.45 (t, J = 10.0 Hz, 1H, H-3A), 5.27 (dd, J = 10.5 Hz, 3.5 Hz, 1H, H-2A), 5.14 (d, J = 3.5 Hz, 1H, H-1A), 4.98 (d, J = 11.0 Hz, 1H, PhCH), 4.92 (d, J = 11.0 Hz, 1H, PhCH), 4.81 (d, J = 11.0 Hz, 1H, PhCH), 4.73 (d, J = 12.0 Hz, 1H, PhCH), 4.64 (d, J = 12.5 Hz, 2H, 2 PhCH), 4.48 (d, J = 3.5 Hz, 1H, H-1B), 4.26–4.22 (m, 1H, H-5A), 3.95 (t, J = 9.0 Hz, 1H, H-4B), 3.83–3.80 (m, 1H, H-5B), 3.49 (s, 3H, OCH3), 3.47–3.45 (m, 1H, H-2B), 3.36 (s, 3H, OCH3), 3.34 (t, J = 9.0 Hz, H-3B), 3.03 (d, J = 12.5 Hz, 1H, H-6aA), 2.86 (d, J = 5.5 Hz, 2H, H-6abB), 2.73 (dd, J = 12.5 Hz, 5.0 Hz, 1H, H-6bA); 13C NMR (125 MHz, CDCl3): δ 165.7, 165.6, 165.3 (3 PhCO), 138.7–127.5 (Ar-C), 97.6 (C-1A), 96.8 (C-1B), 81.8 (C-3A), 81.4 (C-4A), 80.1 (C-2B), 75.6, 75.1, 73.1 (3 PhCH2), 72.9 (C-5B), 72.1 (C-2A), 71.5 (C-5A), 70.6 (C-3B), 70.2 (C-4B), 55.5, 55.0 (2 OCH3), 26.8 (C-6A), 25.6 (C-6B); HRMS for C56H56O13Se [M + H]+: calcd 1017.2964; found: 1017.2945.
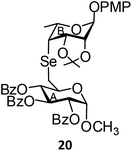
(Methyl 2,3,4-tri-O-benzoyl-6-deoxy-α-D-glucopyranosid-6-yl)-(p-methoxyphenyl 2,3-O-isopropylidene-4,6-di-deoxy-α-L-talopyranosid-4-yl)selenide (20). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 7.89–7.11 (m, 15H, Ar-H), 6.87 (d, J = 6.5 Hz, 2H, Ar-H), 6.79 (d, J = 6.5 Hz, 2H, Ar-H), 5.93 (t, J = 7.0 Hz, 1H, H-3B), 5.55 (s, 1H, H-1A), 5.09 (t, J = 7.0 Hz, 1H, H-4B), 5.03 (d, J = 1.5 Hz, 1H, H-1B), 5.01 (d, J = 2.5 Hz, 1H, H-2B), 4.71 (d, J = 4.0 Hz, 1H, H-2A), 4.69 (dd, J = 4.0 Hz, 1.0 Hz, 1H, H-3A), 4.21 (dd, J = 6.0 Hz, 1.5 Hz, 1H, H-4A), 4.04–4.00 (m, 1H, H-5B), 3.70 (s, 3H, OCH3), 3.37 (s, 3H, OCH3), 3.18–3.16 (m, 1H, H-5A), 2.78 (dd, J = 9.5 Hz, 6.5 Hz, 1H, H-6aB), 2.63 (d, J = 9.5 Hz, 1.5 Hz, 1H, H-6bB), 1.46 (s, 3H, CH3), 1.34 (d, J = 7.0 Hz, 3H, CCH3), 1.29 (s, 3H, CH3); 13C NMR (125 MHz, CDCl3): δ 165.6, 165.2 (3 PhCO), 154.7–112.9 (Ar-C), 106.5 (C(CH3)2), 96.6 (C-1B), 93.1 (C-1A), 85.7 (C-3B, C-5A), 81.8 (C-5B), 72.9 (C-2B), 72.1 (C-4B), 70.9 (C-2A), 70.2 (C-3A), 55.4, 55.3 (2 OCH3), 38.0 (C-4A), 29.7 (C-6B), 26.8, 25.3 (2 CH3), 17.9 (CCH3); HRMS for C44H46O13Se [M + H]+: calcd 863.2182; found: 863.2165.
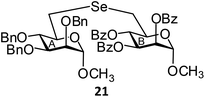
(Methyl 2,3,4-tri-O-benzyl-6-deoxy-α-D-mannopyranosid-6-yl)-(methyl 2,3,4-tri-O-benzoyl-6-deoxy-α-D-mannopyranosid-6-yl)selenide (21). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 7.85–7.11 (m, 30H, Ar-H), 5.74–5.68 (m, 2H, H-3B, H-4B), 5.54 (s, 1H, H-2B), 4.81 (d, J = 11.0 Hz, 1H, PhCH), 4.76 (s, 1H, H-1B), 4.53 (s, 2H, 2 PhCH), 4.50 (d, J = 11.0 Hz, 1H, PhCH), 4.45 (s, 2H, 2 PhCH), 4.44 (s, 1H, H-1A), 4.17–4.14 (m, 1H, H-5A), 3.68–3.61 (m, 4H, H-2A, H-3A, H-4A, H-5B), 3.40 (s, 3H, OCH3), 3.17 (s, 3H, OCH3), 3.00 (d, J = 7.5 Hz, 1H, H-6aA), 2.91–2.81 (m, 2H, H-6aB, H-6bA), 2.70–2.66 (m, 1H, H-6bB); 13C NMR (125 MHz, CDCl3): δ 165.5, 165.4, 165.3 (3 PhCO), 138.5–127.5 (Ar-C), 98.7 (C-1A), 98.5 (C-1B), 80.1 (C-5A), 78.5 (C-2A), 75.1 (PhCH2), 74.6 (C-3A), 73.0 (C-2B), 72.5, 72.0 (2 PhCH2), 71.4 (C-5B), 70.8 (C-3B), 70.6 (C-4B), 69.9 (C-4A), 55.4, 54.6 (2 OCH3), 26.7 (C-6A), 25.7 (C-6B); HRMS for C56H56O13Se [M + H]+: calcd 1017.2964; found: 1017.2945.
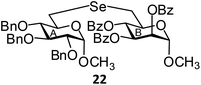
(Methyl 2,3,4-tri-O-benzyl-6-deoxy-α-D-glucopyranosid-6-yl)-(methyl 2,3,4-tri-O-benzoyl-6-deoxy-α-D-mannopyranosid-6-yl)selenide (22). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 8.03–7.10 (m, 30H, Ar-H), 5.72–5.67 (m, 2H, H-3B, H-4B), 5.54 (s, 1H, H-2B), 4.86 (d, J = 11.0 Hz, 1H, PhCH), 4.76–4.74 (m, 2H, H-1B, PhCH), 4.68 (d, J = 11.0 Hz, 1H, PhCH), 4.61 (d, J = 11.0 Hz, 1H, PhCH), 4.51 (t, J = 11.5 Hz, 2H, 2 PhCH), 4.36 (d, J = 3.0 Hz, 1H, H-1A), 4.16–4.13 (m, 1H, H-5B), 3.83 (t, J = 9.0 Hz, 1H, H-4A), 3.71–3.68 (m, 1H, H-5A), 3.41 (s, 3H, OCH3), 3.35 (dd, J = 9.5 Hz, 3.0 Hz, 1H, H-2A), 3.21 (s, 3H, OCH3), 3.19 (t, J = 9.0 Hz, 1H, H-3A), 2.94 (dd, J = 12.5 Hz, 2.0 Hz, 1H, H-6aB), 2.82–2.80 (m, 2H, H-6abA), 2.59 (dd, J = 12.5 Hz, 8.5 Hz, 1H, H-6bB); 13C NMR (125 MHz, CDCl3): δ 165.5, 165.4, 165.3 (3 PhCO), 138.7–127.5 (Ar-C), 98.4 (C-1A), 97.6 (C-1B), 81.8 (C-3A), 81.4 (C-5A), 80.1 (C-2A), 75.6, 75.0, 73.2 (3 PhCH2), 71.4 (C-2B), 71.3 (C-5B), 70.7 (C-3B), 70.5 (C-4B), 69.8 (C-4A), 55.4, 55.1 (2 OCH3), 26.7 (C-6B), 26.0 (C-6A); HRMS for C56H56O13Se [M + H]+: calcd 1017.2964; found: 1017.2946.
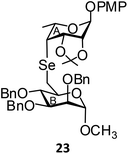
(Methyl 2,3,4-tri-O-benzyl-6-deoxy-α-D-mannopyranosid-6-yl)-(p-methoxyphenyl 2,3-O-isopropylidene-4,6-di-deoxy-α-L-talopyranosid-4-yl)selenide (23). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 7.31–7.19 (m, 15H, Ar-H), 6.91 (d, J = 9.0 Hz, 2H, Ar-H), 6.70 (d, J = 9.0 Hz, 2H, Ar-H), 5.57 (s, 1H, H-1A), 4.75–4.72 (m, 2H, 2 PhCH), 4.69–4.63 (m, 2H, 2 PhCH), 4.54–4.52 (m, 3H, H-1B, 2 PhCH), 3.79–3.69 (m, 5H, H-3A, H-4A, H-3B, H-4B, H-5B), 3.62 (s, 3H, OCH3), 3.60–3.58 (m, 2H, H-2A, H-2B), 3.23 (s, 3H, OCH3), 3.15–3.13 (m, 1H, H-5A), 2.87 (d, J = 13.0 Hz, 1H, H-6aB), 2.64–2.60 (m, 1H, H-6bB), 1.47 (s, 3H, CH3), 1.38 (d, J = 7.0 Hz, 3H, CCH3), 1.30 (s, 3H, CH3); 13C NMR (125 MHz, CDCl3): δ 154.6–112.8 (Ar-C), 107.0 (C(CH3)2), 98.8 (C-1B), 92.1 (C-1A), 86.0 (C-3B), 81.9 (C-5B), 80.2 (C-2B, C-5A), 78.7 (C-4B), 75.1 (PhCH2), 74.8 (C-2A), 72.9 (C-3A), 72.7 (PhCH2), 72.0 (PhCH2), 55.4, 54.7 (2 OCH3), 37.8 (C-4A), 26.9, 25.4 (2 CH3), 25.3 (C-6B), 18.4 (CCH3); HRMS for C44H52O10Se [M + H]+: calcd 821.2804; found: 821.2785.
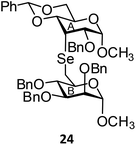
(Methyl 2,3,4-tri-O-benzyl-6-deoxy-α-D-mannopyranosid-6-yl)-(methyl 2-O-benzyl-4,6-O-benzylidene-3-deoxy-α-D-allopyranosid-3-yl)selenide (24). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 7.40–7.10 (m, 25H, Ar-H), 5.43 (s, 1H, PhCH), 4.75 (d, J = 8.0 Hz, 1H, PhCH), 4.69 (d, J = 8.0 Hz, 1H, PhCH), 4.65 (d, J = 8.0 Hz, 1H, PhCH), 4.60 (d, J = 8.0 Hz, 1H, PhCH), 4.54 (s, 1H, H-1B), 4.54 (d, J = 8.0 Hz, 1H, PhCH), 4.50 (d, J = 2.0 Hz, 1H, H-1A), 4.46 (s, 2H, 2 PhCH), 4.27 (d, J = 8.0 Hz, 1H, PhCH), 4.19–4.15 (m, 2H, H-4A, H-5B), 3.83–3.82 (m, 1H, H-5A), 3.66–3.57 (m, 5H, H-2A, H-2B, H-3B, H-6abA), 3.51–3.46 (m, 2H, H-3A, H-4B), 3.31 (s, 3H, OCH3), 3.18 (s, 3H, OCH3), 2.99 (dd, J = 9.0 Hz, 1.5 Hz, 1H, H-6aB), 2.82–2.75 (dd, J = 9.0 Hz, 3.5 Hz, 1H, H-6bB); 13C NMR (125 MHz, CDCl3): δ 138.8–126.3 (Ar-C), 101.4 (PhCH), 98.8 (C-1A), 98.3 (C-1B), 80.0 (C-5B), 79.7 (C-2B), 79.4 (C-2A), 75.1 (PhCH2), 75.0 (C-4A), 74.1 (C-4B), 72.8 (PhCH2), 72.7 (C-3B), 72.1 (PhCH2), 70.1 (PhCH2), 69.0 (C-6A), 59.9 (C-5A), 55.0, 54.8 (2 OCH3), 43.6 (C-3A), 28.6 (C-6B); HRMS for C49H54O10Se [M + H]+: calcd 883.2960; found: 883.2943.
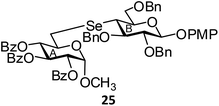
(Methyl 2,3,4-tri-O-benzoyl-6-deoxy-α-D-glucopyranosid-6-yl)-(p-methoxyphenyl 2,3,6-tri-O-benzyl-4-deoxy-β-D-glucopyranosid-4-yl)selenide (25). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 8.00–6.79 (m, 34H, Ar-H), 6.12 (t, J = 10.0 Hz, 1H, H-4B), 5.43 (t, J = 10.0 Hz, 1H, H-3B), 5.21 (dd, J = 10.0 Hz, 3.5 Hz, 1H, H-2B), 5.16 (d, J = 3.0 Hz, 1H, H-1B), 5.05 (d, J = 10.5 Hz, 1H, PhCH), 4.94 (d, J = 10.5 Hz, 1H, PhCH), 4.89 (d, J = 10.5 Hz, 1H, PhCH), 4.81 (d, J = 7.0 Hz, 1H, H-1A), 4.78 (d, J = 10.5 Hz, 1H, PhCH), 4.62 (s, 2H, 2 PhCH), 4.25–4.23 (m, 1H, H-5A), 4.20 (d, J = 10.5 Hz, 1H, H-6aA), 3.92–3.89 (m, 1H, H-6bA), 3.79 (s, 3H, OCH3), 3.65–3.59 (m, 3H, H-2A, H-3A, H-5B), 3.42 (s, 3H, OCH3), 3.10 (t, J = 10.5 Hz, 1H, H-4A), 2.98 (d, J = 5.5 Hz, 2H, H-6abB); 13C NMR (125 MHz, CDCl3): δ 165.6, 165.2 (3 PhCO), 155.2–114.5 (Ar-C), 102.6 (C-1A), 96.9 (C-1B), 84.0 (C-3A), 83.3 (C-5A), 76.1 (PhCH2), 76.0 (C-2A), 74.9, 73.4 (2 PhCH2), 72.7 (C-2B), 72.1 (C-3B), 70.7 (C-6A), 70.1 (C-4B), 69.9 (C-5B), 55.7, 55.5 (2 OCH3), 43.3 (C-4A), 26.0 (C-6B); HRMS for C63H62O13Se [M + H]+: calcd 1107.3434; found: 1107.3420.
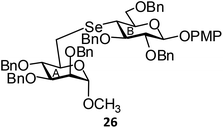
(Methyl 2,3,4-tri-O-benzyl-6-deoxy-α-D-mannopyranosid-6-yl)-(p-methoxyphenyl 2,3,6-tri-O-benzyl-4-deoxy-β-D-glucopyranosid-4-yl)selenide (26). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 7.38–6.78 (m, 34H, Ar-H), 5.10 (d, J = 11.0 Hz, 1H, PhCH), 4.95 (d, J = 11.0 Hz, 1H, PhCH), 4.91 (d, J = 10.0 Hz, 1H, PhCH), 4.86–4.82 (m, 3H, H-1A, 2 PhCH), 4.72 (s, 2H, 2 PhCH), 4.66 (s, 1H, H-1B), 4.59 (s, 2H, 2 PhCH), 4.59–4.51 (m, 2H, 2 PhCH), 4.48 (d, J = 11.0 Hz, 1H, PhCH), 4.20 (d, J = 9.5 Hz, 1H, H-6aA), 3.82–3.79 (m, 5H, H-3A, H-6bA, OCH3), 3.76–3.72 (m, 3H, H-3A, H-5A, H-2B), 3.65–3.63 (m, 2H, H-2A, H-5B), 3.28 (s, 3H, OCH3), 3.09 (dd, J = 12.5 Hz, 2.0 Hz, 1H, H-6aB), 2.97–2.93 (m, 2H, H-4A, H-61); 13C NMR (125 MHz, CDCl3): δ 155.2–114.5 (Ar-C), 102.6 (C-1A), 99.0 (C-1B), 84.0 (C-5B), 83.3 (C-2B), 80.1 (C-5A), 78.4 (C-2A), 76.3 (PhCH2), 75.9 (C-3B), 75.1, 75.0 (2 PhCH2), 74.7 (C-3A), 73.3, 72.7 (2 PhCH2), 72.2 (C-4B), 72.1 (PhCH2), 71.0 (C-6A), 55.5, 54.8 (2 OCH3), 42.7 (C-4A), 26.6 (C-6B); HRMS for C63H68O10Se [M + H]+: calcd 1065.4056; found: 1065.4040.
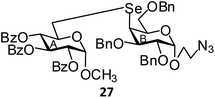
(Methyl 2,3,4-tri-O-benzoyl-6-deoxy-α-D-glucopyranosid-6-yl)-(2-azidoethyl 2,3,6-tri-O-benzyl-4-deoxy-α-D-galactopyranosid-4-yl)selenide (27). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 8.47–7.15 (m, 30H, Ar-H), 6.00 (t, J = 10 Hz, 1H, H-4B), 5.28 (t, J = 10.0 Hz, 1H, H-3B), 5.12 (dd, J = 10.0 Hz, 3.5 Hz, 1H, H-2B), 5.01 (d, J = 2.5 Hz, 1H, H-1B), 4.71 (d, J = 12.0 Hz, 1H, PhCH), 4.65–4.62 (m, 2H, H-1A, PhCH), 4.58 (d, J = 12.0 Hz, 1H, PhCH), 4.55 (d, J = 12.0 Hz, 1H, PhCH), 4.45 (d, J = 12.0 Hz, 1H, PhCH), 4.00 (d, J = 12.0 Hz, 1H, PhCH), 4.17–4.16 (m, 1H, H-5B), 4.07–4.05 (m, 1H, H-5A), 3.91 (dd, J = 9.5 Hz, 3.5 Hz, 1H, H-2A), 3.85 (dd, J = 9.5 Hz, 3.5 Hz, 1H, H-3A), 3.71–3.69 (m, 1H, OCH), 3.62 (d, J = 6.0 Hz, 2H, H-6abA), 3.46–3.42 (m, 2H, OCH, NCH), 3.35–3.32 (m, 4H, H-4A, OCH3), 3.30–3.28 (m, 1H, NCH), 2.88–2.80 (m, 2H, H-6abB); 13C NMR (125 MHz, CDCl3): δ 165.7, 165.6, 165.3 (3 PhCO), 147.4–125.2 (Ar-C), 98.0 (C-1B), 96.8 (C-1A), 78.5 (C-3A), 77.7 (C-2A), 73.3, 73.3, 73.2 (3 PhCH2), 73.0 (C-5A), 72.3 (C-2B), 72.2 (C-6A), 70.3 (C-3B), 70.2 (C-5B), 69.9 (C-4B), 66.7 (OCH2), 55.5 (OCH3), 50.6 (NCH2), 48.4 (C-4A), 26.3 (C-6B); HRMS for C57H57N3O13Se [M + H]+: calcd 1072.3135; found: 1072.3118.
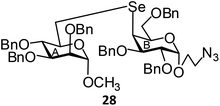
(Methyl 2,3,4-tri-O-benzyl-6-deoxy-α-D-mannopyranosid-6-yl)-(2-azidoethyl 2,3,6-tri-O-benzyl-4-deoxy-α-D-galactopyranosid-4-yl)selenide (28). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 7.32–7.13 (m, 30H, Ar-H), 4.81 (d, J = 2.5 Hz, 1H, H-1A), 4.79 (s, 1H, H-1B), 4.70–4.36 (m, 12H, 12 PhCH), 4.17–4.12 (m, 1H, H-5A), 3.93–3.92 (m, 2H, H-6abA), 3.77–3.75 (m, 1H, OCH), 3.73–3.64 (m, 5H, H-2A, H-2B, H-3A, H-4B, H-5B), 3.58–3.54 (m, 2H, H-3B, H-4A), 3.53–3.49 (m, 1H, OCH), 3.44–3.41 (m, 1H, NCH), 3.37–3.35 (m, 1H, NCH), 3.18 (s, 3H, OCH3), 3.00 (dd, J = 12.5 Hz, 1.5 Hz, 1H, H-6aB), 2.89 (dd, J = 12.5 Hz, 3.0 Hz, 1H, H-6bB); 13C NMR (125 MHz, CDCl3): δ 138.7–127.4 (Ar-C), 98.8 (C-1B), 98.2 (C-1A), 80.1 (C-5B), 79.2 (C-2B), 78.3 (C-3A), 78.2 (C-2A), 75.2 (PhCH2), 74.9 (C-5A), 73.5 (PhCH2), 73.3 (C-3B), 73.2, 72.8, 72.7, 72.5 (4 PhCH2), 72.1 (OCH2), 70.2 (C-4B), 66.7 (C-6A), 54.7 (OCH3), 50.6 (NCH2), 47.2 (C-4A), 27.0 (C-6B); HRMS for C57H63N3O10Se [M + H]+: calcd 1030.3757; found: 1030.3739.
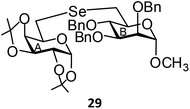
(1,2:3,4-Di-O-isopropylidene-6-deoxy-α-D-galactopyranosid-6-yl)-(methyl 2,3,4-tri-O-benzyl-6-deoxy-α-D-mannopyranosid-6-yl)selenide (29). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 7.27–7.18 (m, 15H, Ar-H), 5.42 (d, J = 5.0 Hz, 1H, H-1A), 4.87 (d, J = 11.0 Hz, 1H, PhCH), 4.67–4.62 (m, 2H, 2 PhCH), 4.59 (s, 1H, H-1B), 4.57 (d, J = 11.0 Hz, 1H, PhCH), 4.51 (s, 2H, 2 PhCH), 4.50 (dd, J = 13.0 Hz, 5.5 Hz 1H, H-3A), 4.26 (d, J = 7.5 Hz, 1H, H-2A), 4.19–4.18 (m, 1H, H-4A), 3.85–3.81 (m, 1H, H-5A), 3.75–3.74 (m, 1H, H-5B), 3.71–3.67 (m, 3H, H-2B, H-3B, H-4B), 3.27 (s, 3H, OCH3), 2.96 (d, J = 12.5 Hz, 1H, H-6aA), 2.76–2.75 (m, 3H, H-6bA, H-6abB), 1.41, 1.33, 1.23, 1.22 (s, 12H, 4 CH3); 13C NMR (125 MHz, CDCl3): δ 138.5–127.5 (Ar-C), 109.0, 108.4 (2 C(CH3)2), 98.8 (C-1B), 96.6 (C-1A), 80.1 (C-5B), 78.7 (C-2B), 75.1 (PhCH2), 74.7 (C-3B), 72.7 (C-4B), 72.7, 72.0 (2 PhCH2), 71.8 (C-3A), 71.0 (C-4A), 70.5 (C-2A), 68.3 (C-5A), 54.7 (OCH3), 26.6 (C-6A), 26.1, 26.0, 24.9, 24.5 (4 CH3), 24.0 (C-6B); HRMS for C40H50O10Se [M + H]+: calcd 771.2647; found: 771.2629.
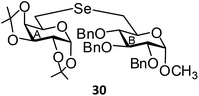
(1,2:3,4-Di-O-isopropylidene-6-deoxy-α-D-galactopyranosid-6-yl)-(methyl 2,3,4-tri-O-benzyl-6-deoxy-α-D-glucopyranosid-6-yl)selenide (30). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 7.25–7.17 (m, 15H, Ar-H), 5.40 (d, J = 4.5 Hz, 1H, H-1A), 4.89 (d, J = 11.0 Hz, 1H, PhCH), 4.81 (d, J = 11.0 Hz, 1H, PhCH), 4.71 (d, J = 11.0 Hz, 1H, PhCH), 4.69 (d, J = 11.0 Hz, 1H, PhCH), 4.58 (t, J = 11.5 Hz, 2H, 2 PhCH), 4.47 (d, J = 2.5 Hz, 2H, H-1B, H-3A), 4.22 (d, J = 7.5 Hz, 1H, H-2A), 4.18–4.16 (m, 1H, H-4A), 3.88 (t, J = 9.5 Hz, 1H, H-3B), 3.80–3.73 (m, 2H, H-5A, H-5B), 3.42 (dd, J = 9.5 Hz, 3.5 Hz, 1H, H-2B), 3.33 (s, 3H, OCH3), 3.26 (t, J = 9.5 Hz, 1H, H-4B), 2.91 (dd, J = 12.5 Hz, 2.0 Hz, 1H, H-6aA), 2.72–2.69 (m, 2H, H-6abB), 2.65 (dd, J = 12.5 Hz, 8.5 Hz, 1H, H-6bA); 13C NMR (125 MHz, CDCl3): δ 138.7–127.5 (Ar-C), 109.1, 108.4 (2 C(CH3)2), 97.8 (C-1B), 96.6 (C-1A), 81.8 (C-5B), 81.6 (C-3B), 80.1 (C-4B), 75.6, 75.1, 73.2 (3 PhCH2), 71.7 (C-3A), 71.1 (C-2B), 71.0 (C-4A), 70.5 (C-2A), 68.4 (C-5A), 55.2 (OCH3), 26.5 (C-6A), 26.1, 26.0, 24.9, 24.5 (4 CH3), 24.0 (C-6B); HRMS for C40H50O10Se [M + H]+: calcd 771.2647; found: 771.2629.
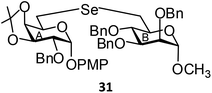
(p-Methoxyphenyl-2-O-benzyl-3,4-O-isopropylidene-6-deoxy-α-D-galactopyranosid-6-yl)-(methyl 2,3,4-tri-O-benzyl-6-deoxy-α-D-mannopyranosid-6-yl)selenide (31). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 7.27–7.13 (m, 20H, Ar-H), 6.96 (d, J = 8.5 Hz, 2H, Ar-H), 6.70 (d, J = 8.5 Hz, 2H, Ar-H), 5.18 (d, J = 3.0 Hz, 1H, H-1A), 4.83 (d, J = 11.0 Hz, 1H, PhCH), 4.69 (d, J = 12.5 Hz, 1H, PhCH), 4.63 (d, J = 12.5 Hz, 1H, PhCH), 4.60–4.59 (m, 2H, 2 PhCH), 4.47–4.44 (m, 4H, H-1B, 3 PhCH), 4.32 (t, J = 7.0 Hz, 1H, H-4B), 4.23–4.18 (m, 2H, H-2B, H-5B), 3.69–3.68 (m, 2H, H-4A, H-5A), 3.62–3.58 (m, 5H, H-3A, H-3B, OCH3), 3.50 (dd, J = 8.0 Hz, 3.5 Hz, 1H, H-2A), 3.12 (s, 3H, OCH3), 2.83–2.75 (m, 3H, H-6abA, H-6aB), 2.70 (dd, J = 12.5 Hz, 8.0 Hz, 1H, H-6bB), 1.29, 1.24 (s, 6H, 2 CH3); 13C NMR (125 MHz, CDCl3): δ 155.0–114.5 (Ar-C), 108.9 (C(CH3)2), 98.8 (C-1B), 96.8 (C-1A), 80.1 (C-5B), 78.2 (C-2B), 76.2 (C-3B), 75.9 (C-4B), 75.1 (PhCH2), 74.6 (C-4A), 74.3 (C-3A), 72.6 (PhCH2), 72.3 (C-5A), 72.1, 72.0 (2 PhCH2), 68.6 (C-2A), 55.4, 54.6 (2 OCH3), 28.2 (CH3), 27.1 (C-6B), 26.4 (CH3), 24.8 (C-6A); HRMS for C51H58O11Se [M + H]+: calcd 927.3222; found: 927.3205.
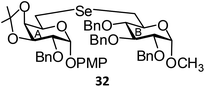
(p-Methoxyphenyl-2-O-benzyl-3,4-O-isopropylidene-6-deoxy-α-D-galactopyranosid-6-yl)-(methyl 2,3,4-tri-O-benzyl-6-deoxy-α-D-glucopyranosid-6-yl)selenide (32). Colorless oil; 1H NMR (500 MHz, CDCl3): δ 7.27–7.12 (m, 20H, Ar-H), 6.95 (d, J = 8.5 Hz, 2H, Ar-H), 6.71 (d, J = 8.5 Hz, 2H, Ar-H), 5.19 (d, J = 3.0 Hz, 1H, H-1A), 4.87 (d, J = 11.0 Hz, 1H, PhCH), 4.79 (d, J = 11.0 Hz, 1H, PhCH), 4.71–4.63 (m, 4H, H-1B, 3 PhCH), 4.55 (d, J = 12.0 Hz, 1H, PhCH), 4.46 (d, J = 11.0 Hz, 1H, PhCH), 4.39–4.36 (m, 2H, H-5A, PhCH), 4.21–4.20 (m, 1H, H-4A), 4.12–4.10 (m, 1H, H-5B), 3.85 (t, J = 9.0 Hz, 1H, H-3B), 3.65 (s, 3H, OCH3), 3.52 (dd, J = 7.5 Hz, 3.5 Hz, 1H, H-3A), 3.38 (dd, J = 9.5 Hz, 3.5 Hz, 1H, H-2B), 3.30 (d, J = 7.5 Hz, 1H, H-2A), 3.24 (s, 3H, OCH3), 3.21 (t, J = 9.0 Hz, 1H, H-4B), 2.80 (dd, J = 12.5 Hz, 2.0 Hz, 1H, H-6aA), 2.74 (d, J = 7.5 Hz, 2H, H-6abB), 2.54 (dd, J = 12.5 Hz, 8.0 Hz, 1H, H-6bA), 1.30, 1.26 (s, 6H, 2 CH3); 13C NMR (125 MHz, CDCl3): δ 155.0–114.5 (Ar-C), 109.0 (C(CH3)2), 97.7 (C-1B), 96.7 (C-1A), 81.8 (C-5B), 81.3 (C-2B), 80.1 (C-3B), 76.0 (C-3A), 75.8 (C-4A), 75.6, 75.0 (2 PhCH2), 74.2 (C-4B), 73.2, 72.2 (2 PhCH2), 70.8 (C-5A), 68.5 (C-2A), 55.4, 55.0 (2 OCH3), 28.1 (CH3), 26.7 (C-6B), 26.4 (CH3), 24.6 (C-6A); HRMS for C51H58O11Se [M + H]+: calcd 927.3222; found: 927.3205.
Conclusions
In summary, an “on-water” reaction condition has been developed for the preparation of stable glycosyl selenocyanate derivatives using readily available KSeCN as selenium source under eco-friendly reaction condition. Furthermore, the glycosyl selenocyanate derivatives have been used as stable building blocks for the stereoselective preparation of nonglycosidically selenoether linked pseudodisaccharide derivatives in excellent yield under a meta-free organocatalytic reaction condition. This approach may be considered as a better alternative to those reported earlier for the preparation of selenoether linked sugars because of its simplicity and yield efficiency. To the best of our knowledge this is the maiden report on the synthesis and characterization of glycosyl selenocyanate derivatives and their application in the metal-free organocatalytic synthesis of selenoether linked disaccharide derivatives.Author contributions
AKM conceived and designed the experiments; TM performed the experiments; AKM and TM analyzed the data; AKM and TM wrote the paper. All authors read and approved the final manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Tapasi Manna thanks UGC, New Delhi for providing senior research fellowship. This work was supported by SERB, New Delhi (Project No. CRG/2019/000352 dated 23.01.2020) (AKM).Notes and references
- A. Tamburrini, C. Colombo and A. Bernardi, Med. Res. Rev., 2019, 1–37 Search PubMed.
- R. Hevey, Pharmaceuticals, 2019, 12, 55 CrossRef CAS PubMed.
- M. Panza, S. G. Pistorio, K. J. Stine and A. Demchenko, Chem. Rev., 2018, 118, 8105–8150 CrossRef CAS PubMed.
- J. L. Magnani and B. Ernst, Discov. Med., 2009, 8, 247–252 Search PubMed.
- N. Parshi, D. Pan, V. Dhavle, B. Jana, S. Maity and J. Ganguly, Int. J. Biol. Macromol., 2019, 141, 626–635 CrossRef CAS PubMed.
- C.-H. Hsu, S.-C. Hung, C.-Y. Wu and C.-H. Wong, Angew. Chem., Int. Ed., 2011, 50, 11872–11923 CrossRef CAS PubMed.
- R. Hevey, Biomimetics, 2019, 4, 53, DOI:10.3390/biomimetics4030053.
- H. Driguez, ChemBioChem, 2001, 2, 311–318 CrossRef CAS PubMed.
- V. Fournière and I. Cumpstey, Tetrahedron Lett., 2010, 51, 2127–2129 CrossRef.
- H. C. Braga, A. D. Wouters, F. B. Zerillo and D. S. Lüdtke, Carbohydr. Res., 2010, 345, 2328–2333 CrossRef CAS PubMed.
- P. Compain, Molecules, 2018, 23, 1658, DOI:10.3390/molecules23071658.
- A. Steiner, A. Stütz and T. Wrodnigg, Sulfur-Containing Glycomimetics, in Glycoscience, ed. B. O. Fraser-Reid, K. Tatsuta and J. Thiem, Springer, Berlin, Heidelberg, 2008, DOI:10.1007/978-3-540-30429-6_50.
- Z. J. Witczak, Phosphorus, Sulfur Silicon Relat. Elem., 2013, 188, 413–417 CrossRef CAS.
- S. André, Z. Pei, H.-C. Siebert, O. Ramström and H.-J. Gabius, Bioorg. Med. Chem., 2006, 14, 6314–6326 CrossRef PubMed.
- L. Szilagyi, T. Z. Illyes and P. Herczegh, Tetrahedron Lett., 2001, 42, 3901–3903 CrossRef CAS.
- (a) T. Wirth, Organoselenium Chemistry: Modern Developments in Organic Synthesis, Top. Curr. Chem., 2020, 4 DOI:10.1007/3-540-48171-0; (b) C. Narajji, M. D. Karvekar and A. K. Das, Indian J. Pharm. Sci., 2007, 69, 344–352 CrossRef CAS; (c) M. J. Davies and C. H. Schiesser, New J. Chem., 2019, 43, 9759–9765 RSC.
- (a) Y. Kobayashi, Y. Ogra, K. Ishiwata, H. Takayama, N. Aimi and K. Sizuki, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 15932–15936 CrossRef CAS PubMed; (b) M. J. Jackson, K. Lunøe, C. Gabel-Jensen, B. Gammelgaard and G. F. Combs Jr, J. Nutr. Biochem., 2013, 24, 2023–2030 CrossRef CAS PubMed.
- (a) I. Cumpstey, C. R. Chimie, 2011, 14, 274–285 CrossRef CAS; (b) K. Sidoryk, L. Rarová, J. Okleštková, Z. Pakulski, M. Strnad, P. Cmoch and R. Luboradzki, Org. Biomol. Chem., 2016, 14, 10238–10248 RSC.
- F. Mangiavacchi, I. F. C. Dias, I. Di Lorenzo, P. Gizes, M. Palomba, O. Rosati, L. Bagnoli, F. Marini, C. Santi, E. J. Lenardao and L. Sancineto, Pharmaceuticals, 2020, 13, 211, DOI:10.3390/ph13090211.
- G. Mugesh, W.-W. du Mont and H. Sies, Chem. Rev., 2001, 101, 2125–2179 CrossRef CAS PubMed.
- (a) T. Zacharias, K. Flouda, T. A. Jepps, B. Gammelgaard, C. H. Schiesser and M. J. Davies, Biochem. Pharmacol., 2020, 173, 113631 CrossRef CAS PubMed; (b) Z. Kónya, B. Bécsi, A. Kiss, I. Tamás, B. Lontay, L. Szilágyi, K. E. Kövér and F. Erdödi, Bioorg. Med. Chem., 2018, 26, 1875–1884 CrossRef PubMed; (c) S. J. Ahn, M. Koketsu, H. Ishihara, S. M. Lee, S. K. Ha, K. H. Lee, T. H. Kang and S. Y. Kim, Chem. Pharm. Bull., 2006, 54, 281–286 CrossRef CAS PubMed; (d) P. Merino-Montiel, S. Maza, S. Martos, Ó. López, I. Maya and J. G. Fernández-Bolaños, Eur. J. Pharm. Sci., 2013, 48, 582–592 CrossRef CAS PubMed.
- (a) Y. Kawai, H. Ando, H. Ozeki, M. Koketsu and H. Ishihara, Org. Lett., 2005, 7, 4653–4656 CrossRef CAS PubMed; (b) M. Nanami, H. Ando, Y. Kawai, M. Koketsu and H. Ishihara, Tetrahedron Lett., 2007, 48, 1113–1116 CrossRef CAS; (c) T. Suzuki, C. Hayashi, N. Komura, R. Tamai, J. Uzawa, J. Ogawa, H.-N. Tanaka, A. Imamura, H. Ishida, M. Kiso, Y. Yamaguchi and H. Ando, Org. Lett., 2019, 21, 6393–6396 CrossRef CAS PubMed.
- S. Valerio, A. Iadonisi, M. Adinolfi and A. Ravidà, J. Org. Chem., 2007, 72, 6097–6106 CrossRef CAS PubMed.
- (a) S. Czernecki and D. Randriamandimby, J. Carbohydr. Chem., 1996, 15, 183–190 CrossRef CAS; (b) P. Chen, Y. Ding, S. Guo and X. Zhang, Tetrahedron Lett., 2019, 60, 469–474 CrossRef CAS.
- (a) F. Santoyo-González, F. G. Calvo-Flores, P. García-Mendoza, F. Hernández-Mateo, J. Isac-García and R. Robles-Díaz, J. Org. Chem., 1993, 58, 6122–6125 CrossRef; (b) E. D. Kazakova, D. V. Yashunsky, E. A. Khatuntseva and N. E. Nifantiev, Pure Appl. Chem., 2020, 92, 1047–1056 CrossRef CAS; (c) V. Di Bussolo, A. Fiasella, F. Balzano, G. U. Barretta and P. Crotti, J. Org. Chem., 2010, 75, 4284–4287 CrossRef CAS PubMed.
- (a) A. Sau and A. K. Misra, Synlett, 2011, 1905–1911 CAS; (b) C. Mukherjee, P. Tiwari and A. K. Misra, Tetrahedron Lett., 2006, 47, 441–445 CrossRef CAS; (c) P. Tiwari and A. K. Misra, Tetrahedron Lett., 2006, 47, 2345–2348 CrossRef CAS; (d) T. Ma, C. Li, H. Liang, Z. Wang, L. Yu and W. Xue, Synlett, 2017, 2311–2314 CAS; (e) C. Venkateswarlu, V. Gautam and S. Chandrasekaran, Carbohydr. Res., 2014, 396, 48–53 CrossRef CAS PubMed.
- (a) F. Zhu, S. O'Neill, J. Rodriguez and M. A. Walczak, Angew. Chem., Int. Ed., 2018, 57, 7091–7095 CrossRef CAS PubMed; (b) Y. Guan and S. D. Townsend, Org. Lett., 2017, 19, 5252–5255 CrossRef CAS PubMed; (c) A. A. Kumar, T. Z. Illyés, K. E. Kövér and L. Szilágyi, Carbohydr. Res., 2012, 360, 8–18 CrossRef CAS PubMed; (d) R. Benhaddou, S. Czernecki and D. Randriamandimby, Synlett, 1992, 967–968 CrossRef CAS; (e) T. Suzuki, N. Komura, A. Imamura, H. Ando, H. Ishida and M. Kiso, Tetrahedron Lett., 2014, 55, 1920–1923 CrossRef CAS; (f) M. Zhu, M. Alami and S. Messaoudi, Org. Lett., 2020, 22, 6584–6589 CrossRef CAS PubMed.
- (a) R. M. van Well, T. S. Kärkkäinen, K. P. R. Kartha and R. A. Field, Carbohydr. Res., 2006, 341, 1391–1397 CrossRef CAS PubMed; (b) R. R. France, R. G. Compton, B. G. Davis, A. J. Fairbanks, N. V. Rees and J. D. Wadhawan, Org. Biomol. Chem., 2004, 2, 2195–2202 RSC; (c) S. Yamago, K. Kokubo, O. Hara, S. Masuda and J.-i. Yoshida, J. Org. Chem., 2002, 67, 8584–8592 CrossRef CAS PubMed; (d) T. Furuta, K. Takeuchi and M. Iwamura, Chem. Commun., 1996, 157–158 RSC.
- (a) S. Mehta and B. M. Pinto, J. Org. Chem., 1993, 58, 3269–3276 CrossRef CAS; (b) S. Mehta and B. M. Pinto, Tetrahedron Lett., 1991, 32, 4435–4438 CrossRef CAS; (c) B. D. Johnston and B. M. Pinto, Carbohydr. Res., 1997, 305, 189–292 CrossRef; (d) K. D. Randell, B. D. Johnston, E. E. Lee and B. M. Pinto, Tetrahedron: Asymmetry, 2000, 11, 207–222 CrossRef CAS; (e) K. Ikeda, Y. Sugiyama, K. Tanaka and M. Sato, Bioorg. Med. Chem. Lett., 2002, 12, 2309–2311 CrossRef CAS PubMed; (f) S. Yamago, T. Yamada, O. Hara, Y. Mino and J.-i. Yoshida, Org. Lett., 2001, 3, 3867–3870 CrossRef CAS PubMed; (g) W.-T. Jiaang, M.-Y. Chang, P.-H. Tseng and S.-T. Chen, Tetrahedron Lett., 2000, 41, 3127–3130 CrossRef CAS.
- (a) M. Spell, X. Wang, A. E. Wahba, E. Conner and J. Ragains, Carbohydr. Res., 2013, 369, 42–47 CrossRef CAS PubMed; (b) I. Cumpstey and D. Crich, J. Carbohydr. Chem., 2011, 30, 469–485 CrossRef CAS.
- (a) D. J. Chambers, G. R. Evans and A. J. Fairbanks, Tetrahedron, 2004, 60, 8411–8419 CrossRef CAS; (b) D. J. Chambers, G. R. Evans and A. J. Fairbanks, Tetrahedron Lett., 2003, 44, 5221–5223 CrossRef CAS; (c) F. Bravo, M. Kassou, Y. Díaz and S. Castillón, Carbohydr. Res., 2001, 336, 83–97 CrossRef CAS.
- (a) S. Tsegay, R. J. Williams and S. J. Williams, Carbohydr. Res., 2012, 357, 16–22 CrossRef CAS PubMed; (b) G. Horne and W. Mackie, Tetrahedron Lett., 1999, 40, 8697–8700 CrossRef CAS.
- (a) Y. Yang and B. Yu, Chem. Rev., 2017, 117, 12281–12356 CrossRef CAS PubMed; (b) L. Grant, Y. Liu, K. E. Walsh, D. S. Walter and T. Gallagher, Org. Lett., 2002, 4, 4623–4625 CrossRef CAS PubMed.
- (a) X. Mao, P. Li, T. Li, M. Zhao, C. Chen, J. Liu, Z. Wang and L. Yu, Chin. Chem. Lett., 2020, 31, 3276–3278 CrossRef CAS; (b) H. Cao, Y. Yang, X. Chen, J. Liu, C. Chen, S. Yuan and L. Yu, Chin. Chem. Lett., 2020, 31, 1887–1889 CrossRef CAS; (c) L. Yu, H. Cao, X. Zhang, C. Yang and L. Yu, Sustainable Energy Fuels, 2020, 4, 730–736 RSC; (d) F. Wang, J. Huang, Y. Yang, L. Xu and L. Yu, Ind. Eng. Chem. Res., 2020, 59, 10763–10767 CrossRef.
- (a) C. H. Schiesser, C. Storkey and M. J. Davies, US Pat. Appl. Publ., US 20140206658 A1 20140724, 2014 Search PubMed; (b) C. H. Schiesser, C. Storkey and M. J. Davies, PCT Int. Appl., WO 2012054988 A1 20120503, 2012 Search PubMed; (c) J. Y. See, H. Yang, Y. Zhao, M. W. Wong, Z. Ke and Y.-Y. Yeung, ACS Catal., 2018, 8, 850–858 CrossRef CAS; (d) F. Chen, C. K. Tan and Y.-Y. Yeung, J. Am. Chem. Soc., 2013, 135, 1232–1235 CrossRef CAS PubMed.
- (a) H. C. Braga, A. D. Wouters, F. B. Zerillo and D. S. Lüdtke, Carbohydr. Res., 2010, 345, 2328–2333 CrossRef CAS PubMed; (b) R. F. Affeldt, H. C. Braga, L. L. Baldassari and D. S. Lüdtke, Tetrahedron, 2012, 68, 10470–10475 CrossRef CAS; (c) P. R. Sridhar, V. Saravanan and S. Chandrasekaran, Pure Appl. Chem., 2005, 77, 145–153 CAS; (d) T.-Z. Illyés, S. Balla, A. Bényei, A. A. Kumar, I. Timári, K. E. Kövér and L. Szilágyi, ChemistrySelect, 2016, 1, 2383–2388 CrossRef.
- (a) V. Fourniére and I. Cumpstey, Tetrahedron Lett., 2010, 51, 2127–2129 CrossRef; (b) I. Cumpstey, C. Ramstadius, T. Akhtar, I. J. Goldstein and H. C. Winter, Eur. J. Org. Chem., 2010, 1951–1970 CrossRef CAS.
- T. Manna and A. K. Misra, Org. Biomol. Chem., 2019, 17, 8902–8912 RSC.
- T. Manna and A. K. Misra, SynOpen, 2018, 2, 229–233 CrossRef CAS.
- (a) A. Krief, W. Dumont and C. Delmotte, Angew. Chem., Int. Ed., 2000, 39, 1669–1672 CrossRef CAS; (b) A. A. Heredia and A. B. Peñéñory, RSC Adv., 2015, 5, 105699–105706 RSC.
- G. R. Morais, B. R. Springett, M. Pauze, L. Schröder, M. Northrop and R. A. Falconer, Org. Biomol. Chem., 2016, 14, 2749–2754 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed analytical data of compounds 8–14 and 18–32, copies of the NMR spectra. See DOI: 10.1039/d1ra00711d |
| This journal is © The Royal Society of Chemistry 2021 |