Open Access Article
Open Access ArticleNatural products in Cyperus rotundus L. (Cyperaceae): an update of the chemistry and pharmacological activities
Smith B. Babiaka
 *a,
Aurélien F. A. Moumbock
*a,
Aurélien F. A. Moumbock
 b,
Stefan Günther
b,
Stefan Günther
 b and
Fidele Ntie-Kang
b and
Fidele Ntie-Kang
 *acd
*acd
aDepartment of Chemistry, University of Buea, P. O. Box 63, Buea, Cameroon. E-mail: babiaka.smith@ubuea.cm; fidele.ntie-kang@ubuea.cm
bInstitute of Pharmaceutical Sciences, Albert-Ludwigs-Universitӓt Freiburg, Hermann-Herder-Straße 9, D-79104 Freiburg, Germany
cInstitute of Pharmacy, Martin-Luther University Halle-Wittenberg, Halle (Saale), Germany
dInstitute of Botany, Technical University of Dresden, Dresden, Germany
First published on 23rd April 2021
Abstract
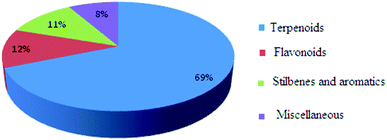
Cyperus rotundus L. (Nutgrass, family Cyperaceae) is a notorious weed which is widespread in temperate tropical and subtropical regions of the world. Owing to its richness and potent pharmacological activities, efforts have been devoted to identify its bioactive constituents. Since 1965, a total of about 192 compounds including terpenoids, flavonoids, stilbenes, aromatics and aliphatic fatty acids have been characterized. This review summarizes the bioactivities and mechanism of action of some of the compounds from C. rotundus L.
1 Introduction
Cyperus rotundus (CR) L. (Nutgrass, family: Cyperaceae) popularly called “the world's worst weed” is widely distributed in subtropical and tropical regions of the world.1–3 It is a notorious weed and has a destructive effect on agricultural yields after it invades the crop fields.4,5 It is a smooth, erect, glabrous, grasslike, fibrous rooted, perennial herb that grows up to 15–60 cm height (Fig. 1) and reproduces widely through rhizomes and tubers.6 In Chinese traditional medicine, the rhizomes are used for the treatment of liver diseases, stomach ache, inflammatory diseases, bowel and menstrual disorders.7–13 They are also recommended in India for the treatment of diabetes, arthritis, diarrhoea, dysentery, leprosy, bronchitis, amenorrhea, dysmenorrhea, fever, arthritis and blood disorders.14–16 In West Asia, the roots are applied in traditional medicine for the treatment of leprosy, thirst, fever, and blood diseases.17,18 In Egyptian folk medicine, the tubers are used as an antihelmintic, aphrodisiac, diuretic, sedative, carminative, stimulant and tonic, and for treating renal colic and stomach ache.19 This perennial herb has recently received much attention due to its broad range of pharmacological and biological activities.5,20–75 Several reports have stated the presence of terpenoids, flavonoids, stilbene derivatives and other classes of compounds.8,11,17,41,76–78 To the best of our knowledge, a total of about 192 NPs with structural diversity have been isolated from this medicinal weed.8,12,13,17,41,79–94 Previous reviews have focused on ethnobotanical uses, and pharmacological activities.6,71,95,96 Also, a majority of researchers have reported in vitro bioactivities and GC-MS analysis of crude extracts of this weed.2,14,41,97–111 The objective of the present review was to provide an update of NPs derived from this plant species, their bioactivity and the mechanism of actions of some of the compounds from published data in literature.2 Data source and preparation
In this review article, a comprehensive search was performed in the following databases: PubMed, SciFinder, Science Direct, Web of Science, Wiley Online, ResearchGate, Google scholar and other search engines were explored for studies published from 1965–2020. Keywords such as: “C. rotundus”, ‘bioactive compounds’, and “pharmacological activities” were used. We removed duplicated papers, then screened the data, ruled out irrelevant publications. The focus was on research or review articles, work on NPs isolated from this weed. Many of the publications were focused on isolation, structure elucidation and pharmacological activities.3 Natural products derived from C. rotundus L. species
After decades of detailed phytochemical investigation, it is evident that this plant species contains two major classes of secondary metabolites, namely, terpenoids and flavonoids mostly harvested from Asia and Africa (Fig. 2).8,12,13,17,40,79–87,89–94,112–1193.1 Terpenoids
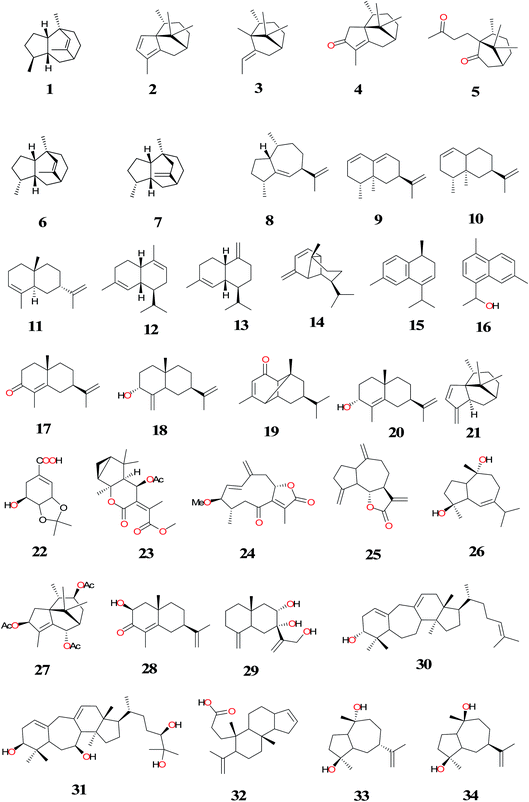
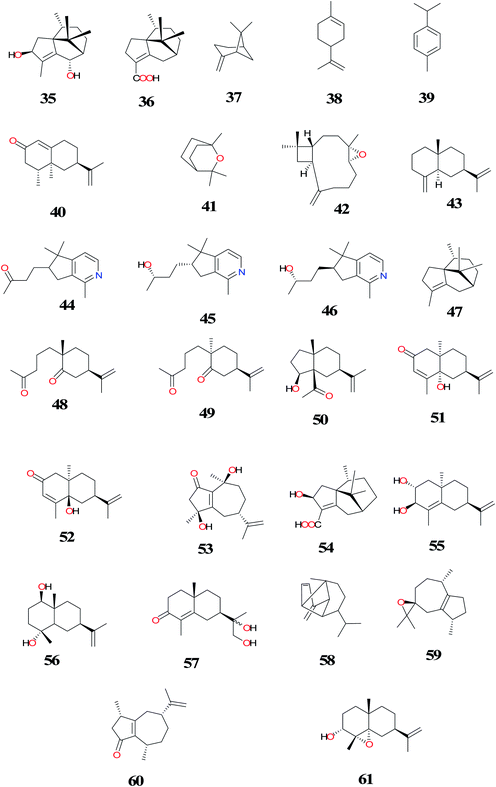
To date, a total of about 131 terpenoids (1–131) have been isolated and identified from C. rotundus (CR) (Fig. 3–7). The summaries of the most interesting results for terpenoids isolated from CR have been shown in Table 1. Sesquiterpenes are the major subclass of NPs isolated from this herb. A majority of the secondary metabolites were isolated from the rhizomes/tubers of the plant and they are structurally related, for example, (1–117).83,120 α-Cyperone (17) isolated from the n-hexane fraction significantly inhibited prostaglandin E2 (PGE2) production by suppressing lipopolysaccharide (LPS)-induced expression of inducible cyclooxygenase-2 (COX-2) at both RNA and the protein levels.121 Compound 17 obtained from the tubers of this plant species also showed insecticidal activity.122 Isocyperol (18) has been found to significantly inhibit LPS-induced production of nitrite oxide (NO),PGE2 and suppressed LPS-induced expression of inducible nitric oxide synthase (iNOS) and COX-2 at the mRNA and protein levels in RAW 264.7 macrophages.123 Extraction of air-dried and chopped rhizomes of CR with hot 70% EtOH followed by purification using GC-MS afforded monoterpenes, sesquiterpenes and aromatic compounds (10, 17, 37–43). The sesquiterpenes; valencene (10) and (+)-nootkatone (40) significantly inhibited inducible nitric oxide (iNOS) expression and nitric oxide (NO) production in LPS-simulated RAW264.7 cells. The anti-inflammatory mechanism of CR is due to heme oxygenase-1 (HO-1) induction by compounds 10 and 40.17 While (+)-nootkatone (40) has been found to have potent inhibitory effect on collagen-, thrombin-, and AA-induced platelet aggregation. Compound 40 was treated with mice and it exhibited significant prolonged bleeding times. It has also shown significant inhibitory effect on rat platelet aggregation ex vivo.77| Compounds | Part (s)/place of harvest of plant studied (VS≠) | Measured activity | References |
|---|---|---|---|
| a VS≠: Voucher specimen number, NM: not mention, ND: not done. | |||
| Rotundene (1) | NM/China | ND | Paknikar et al.,120 |
| 1, (−)-cypera-2,4-diene (2), cyprotene (3), cyperotundone (4), (+)-cyperadione (5), (−)-norrotundene (6), (−)-isorotundene (7), γ-gurjunene (8), nootkatene (9), valencene (10), epi-α-selinene (11), α-muurolene (12), γ-muurolene (13), ylanga-2,4-diene (14), γ-calacorene (15), cadalene (16), α-cyperone (17), isocyperol (18), mustakone (19) and cyperol (20) and (−)-cypera-2,4(15)-diene (21) | NM/gift of K.-D. Protzen, Paul Kadersd GmbH, Hamburg (NM) | ND | Sonwa et al.,83 |
| 3,4-O-isopropylideneshikimic acid (22), rotundusolide A (23), rotundusolide B (24), dehydrocostuslactone (25), (+)-alismoxide (26), sugetriol triacetate (27), 2β-hydroxy-α-cyperone (28), eudesma-4(14),11(13)-diene-7α,8α,12-triol (29), rotundusolide C (30), secomacrogenin B (31), and 3,4-seco-mansumbinoic acid (32) | Rhizomes/Purchased from Lanzhou Traditional Chinese Medicine Market (no. ZY2009C002) | ND | Yang et al.,89 |
| 4, 27, Epi-guaidiol A (33), guaidiol A (34), sugebiol (35) and cyperenoic acid (36) | Rhizomes/Da-Bie-Shan Mountains, Anhui province, China. (No: 20060825) | ND | Xu et al.,85 |
| 10, 17, β-pinene (37), limonene (38), 4-cymene (39), (+)-nootkatone (40), 1, 8-cineole (41), caryophyllene oxide (42), β-selinene (43) | Rhizomes/Purchased from Kyung Dong market place in Seoul, South Korea. (DKH-02561) | Anti-inflammatory activity | Tsoyi et al.,17 |
| 10,17, 37- 43 | Rhizomes/NM | Antiplatelet effects | Seo et al.,77 |
| 18 | Rhizomes/Kyung Dong Crude Drugs Market, Seoul, South Korea. (CYRO1-2011) | Anti-inflammatory activity | Seo et al.,123 |
| 17 | Rhizomes/Kyung Dong Crude Drugs Market, Seoul, South Korea. (CYRO1-2011) | Anti-inflammatory activity | Jung et al.,121 |
| Rotundine A (44), rotundine B (45), and rotundine C (46) | Rhizomes/Purchased from Uchida Wakanyaku Co., Ltd. (Tokyo, Japan) (lot. 242![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 118) 118) |
ND | Jeong et al.,15 |
| α-Cyperene (47) | NM/China (NM) | ND | Komai et al.,80 |
| 17, 19, 40, 2α-(5-oxopentyl)-2β-methyl-5β-isopropenylcyclohexanone (48), 2β-(5-oxopentyl)-2β-methyl-5β-isopropenylcyclohexanone (49), cyperolone (50), α-rutunol (51) and β-rutunol (52) | Roots/Yorishima, Okayama, Japan | Antibacterial activity | Ohira et al.,82 |
| Cyperusol A3 (53), 3β-hydroxycyperenoic acid (54), britanlin E (55), 1β, 4α-dihydroxyeudesm-11-ene (56) and 11,12-dihydroxyeudesm-4-en-3-one (57). | Rhizomes/Kyung Dong Crude Drugs Market, Seoul, South Korea. (CYRO1–2011) | Antitumor activity | Ryu et al.,12 |
| 10, 37–42 | Rhizomes/Kyung Dong Crude Drugs Market, Seoul, South Korea. (NM) | Anti-allergic activity | Jin et al.,10 |
| Copadiene (58), epoxyguaien (59), and rotundone (60) | NM/Japan (NM) | ND | Kapadia et al.,79 |
| 19 | NM/India (NM) | ND | Kapadia et al.,124 |
| 50 | Rhizomes/Japan (NM) | ND | Hikino et al.,125 |
| 4α,5β-oxidoeudesm-11-en-3α-ol (61) | Rhizomes/Japan (NM) | ND | Hikino et al.,127 |
| 51 and 52 | Rhizomes | ND | Hikino et al.,126 |
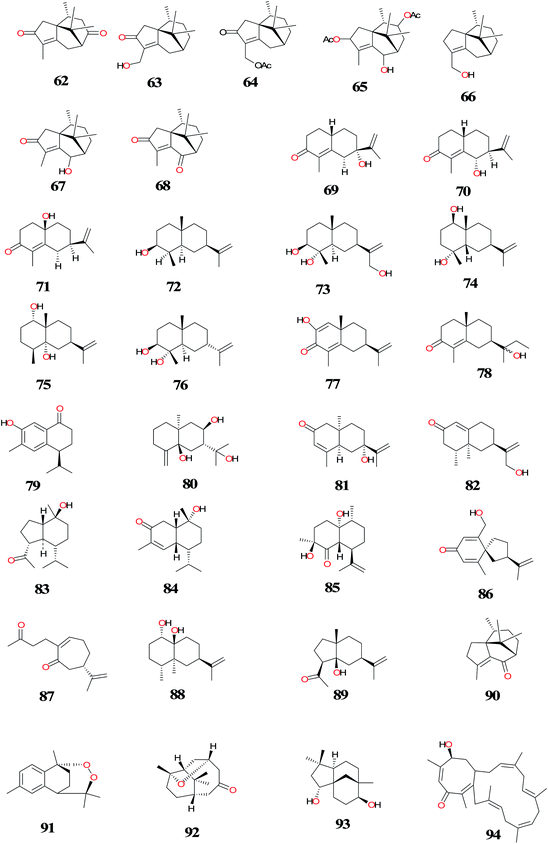
| 4, 17, 20, 27, 36, 51, 54, cyperene-3,8-dione (62), 14-hydroxycyperotundone (63), 14-acetoxycyperotundone (64), sugetriol-3,9-diacetate (65), cyperenol (66), sugeonol (67), scariodione (68), (4aS, 7S)-7-hydroxy-1,4a-dimethyl-7-(prop-1-en-2-yl)-4,4a,5,6,7,8-hexahydronaphthalen-2(3H)-one (69), (4aS, 7S, 8R)-8-hydroxy-1,4a-dimethyl-7-(prop-1-en-2-yl)-4,4a,5,6,7, 8-hexahydronaphtha-len-2(3H)-one (70), 1β-hydroxy-α-cyperone (71), 10-epieudesm-11-ene-3β, 5α-diol (72), 3β-hydroxyilicic alcohol (11(13)-eudesmene-3,4,12-triol) (73), cyperusol C (74), α-corymbolol (75), 3β, 4α-dihydroxy-7-epi-eudesm-11(13)-ene (76), 2-oxo-α-cyperone (77), 7α (H), 10β-eudesm-4-en-3-one-11,12-diol (78), 2-hydroxy-14-calamenenone (79), rhombitriol (80), 7-epi-teucrenone (81), 12-hydroxynootkatone (82), oplopanone (83), 10-hydroxyamorph-4-en-3-one (84), cyperusol D (85), argutosine D (86) and 4,5-seco-guaia-1 (10),11-diene-4,5-dioxo (87), oxyphyllol C (88) and 5-hydroxylucinone (89) | Rhizomes/Purchased from Juhuacun (Kunming, China) (no. 2011041101) | Anti-hepatitis B virus activity | Xu et al.,13 |
| 42, patchoulenone (90) and 10,12-peroxycalamenene (91) | Tubers/Purchased from a Thai traditional dispensary, Bangkok, Thailand | Antimalarial activity | Thebtaranonth et al.,81 |
| Norcyperone (92) and (−)-clovane-2,9-diol (93) | Rhizomes/Dabieshan Mountains of Anhui Province, P.R. China (no: 20060825) | ND | Xu et al.,84 |
| 27 and cyperalin A (94) | Rhizomes/King Abdulaziz University campus, Jeddah, Saudi Arabia (2014-CR110) | Anti-inflammatory activity | Ibrahim et al.,93 |
| 4, 17, 18, 47, 4α,5α-oxidoeudesm-11-en-3-one (95) and cyper-11-ene-3,4-dione (96) | Rhizomes/purchased from Kyungdong-Yakryongsi traditional medicine market in Seoul, Korea (SKKU-PH-12-50) | Estrogenic activity | Park et al.,94 |
| 40, solavetivone (97) and aristolone (98) | Rhizomes/obtained from a registered medicinal plant vendor in Trivandrum (no. 034/2011) | Antioxidant activity | Rani et al.,87 |
| 17 | Tubers/Bogor-Indonesia/NM | Insecticidal activity | Dadang et al.,122 |
| 41, 42, zerumbone (99), α-humulene (100), (+)-dihydrocarvone (101), (R)-(+)-limonene (102), (1S)-(−)-verbenone (103), (S)-(−)-limonene (104), β-caryophyllene (105), (−)-(E)-pinocarveol (106), (E)-carveol (107), (−)-α-copaene (108), (1R)-(−)-myrtenal (109) and (1R)-(−)-myrtenol (110) | Rhizomes/Purchased from the Boeun medicinal herb shop (Seoul Yangnyeongsi, Seoul, South Korea) (CR-01) | Repellent activity | Chang et al.,92 |
| 4 and 17 | Rhizomes/supplied by Morihiro Kinoshita (Nihon Funmatsu Yakuhin Company, Japan) (NM) | Phytotoxicity | Morimoto et al.,128 |
| 4, 17, 20, 43, 47, 101, 109, β-elemene (111), caryophyllene (112), δ-cadinene (113), calamenene (114), patchoulenyl acetate (115) and 6-acetoxy-patchoul-4-en-3-one (116) | Tubers/Islands of Oahu, Maui, Kauai and Hawaii (NM) | ND | Komai et al.,80 |
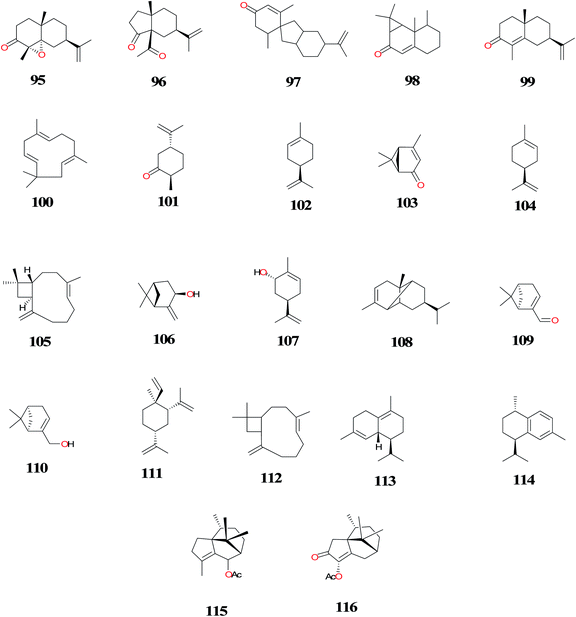
| Cyprotuside A (117) and cyprotuside B (118) | Rhizomes/Zhanjiang, Guangdong Province of China (No.20090903) | Antidepressant activity | Zhou et al.,90 |
| 18-Epi-α-amyrin glucuronoside (119), oleanolic acid arabinoside (120), α-amyrin glucopyranoside (121) and β-amyrin glucopyranoside (122) | Tubers/West Champaran, Bihar, India (no. NISCAIR/RHMD/Consult/-2008-09/1114/145) | ND | Alam et al.,86 |
| Oleanolic acid (123), oleanolic acid-3-O-neohesperidoside (124) and β-sitosterol (125) | Tubers/University of Allahabad campus, Allahabad, India (NM) | ND | Singh et al.,114 |
| 12-Methyl cyprot-3-en-2-one-13-oic acid (126), stigmasterol-n-dodecanoate (127), stigmasterol-n-tetradecanoate (128), β-sitosterol glucoside (129) and lupenyl arabinopyranosyl oleate (130) | Tubers/Purchased from a Delhi market | ND | Sultana et al.,91 |
| Sitosteryl (131) | Aerial parts/El-Safa and El-Marwa, Faculty of Agriculture, Al-Azhar University, Assiut, Egypt. | Antifeedant and Cytotoxic activity | Sayed et al.,33 |
Three novel sesquiterpene alkaloids; rotundines A (44), B (45), and C (46) were isolated from the MeOH extract using standard methods of extraction of alkaloids. The structures of the compounds were determined by comprehensive spectroscopic analyses and chemical methods.15
Ohira et al.82 isolated the new sesquiterpenoids; 2α-(5-oxopentyl)-2β-methyl-5β isopro-penylcyclohexanone (48), 2β-(5-oxopentyl)-2β-methyl-5β-isopropenylcyclohexanone (49), cyperolone (50) together with the known compounds 17, 19, 40, 51 and 52 from the roots of CR. The antibacterial activities of the new hits were screened against Escherichia coli and Bacillus subtilis using the paper disk method. Cyperolone (50) possessed moderate activity against B. suhtilis at a concentration of 0.5 mg per disk; the other compounds did not show notable activities.82
The new sesquiterpenes, cyperusol A3 (53), 3β-hydroxycyperenoic acid (54), along with three known compounds (55–57) were isolated from the ethyl acetate soluble fraction of the rhizomes using a series of column chromatography. The compounds were submitted for their cytotoxic activities against human ovarian cancer cells (A2780) and endometrial adenocarcinoma cells (Ishikawa) using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assays and 11,12-dihydroxyeudesm-4-en-3-one (57) showed the most potent cytotoxic activity with observed IC50 values of 11.06 and 6.46 mm, respectively.12
Jin et al.10 isolated the known compounds; valencene (10), α-cyperone (17), β-pinene (37), limonene (38), 4-cymene (39), (+)-nootkatone (40), 1, 8-cineole (41), caryophyllene oxide (42), and β-selinene (43) and evaluated them for their anti-allergic activity in vitro and in vivo. In rat basophilic leukemia (RBL)-1 cells, the sesquiterpenes (10, 40, 42) were reported to strongly inhibit 5-lipoxygenase-catalyzed leukotrienes production. In addition, they inhibited β-hexosaminidase release by antigen-stimulated RBL-2H3 cells, with valencene having the highest inhibitory effect. Authors also found that the most active sesquiterpene (10) inhibited β-hexosaminidase degranulation by inhibiting the initial activation reaction, Lyn phosphorylation, in IgE-stimulated RBL-2H3 cells. Moreover compounds (10, 40), significantly inhibited the delayed-type hypersensitivity reaction in mice when administered orally at 50–300 mg kg−1.11
The isolation of cyperolone (50) from the essential oil of CR, rekindled the interest of NP Chemists to revisit this plant species. Investigation of the constituents from Chinese origin, led to the isolation of known compounds (19, 50) and new sesquiterpenes; copadiene (58), epoxyguaien (59), rotundone (60) and 4α,5β-oxidoeudesm-11-en-3α-ol (61).79,124–127
Bioactivity and liquid chromatography-mass spectrometry (LC-MS) guided fractionation of 90% EtOH extract using open-column, Sephadex LH-20 and semi-preparative high performance liquid chromatography (HPLC) led to the isolation and identification of thirty-seven sesquiterpenoids.13 The compounds include, five new patchoulane-type sesquiterpenoids, 3β-hydroxycyperenoic acid (54), cyperene-3,8-dione (62), 14-hydroxycyperotundone (63), 14-acetoxycyperotundone (64) and sugetriol-3,9-diacetate (65) along with the known NPs 4, 17, 20, 27, 36, 51 and 66–89. Nine eudesmane-type sesquiterpenoids (20, 71–77 and 78–80) significantly inhibited the hepatitis B virus (HBV) DNA replication with IC50 values of 42.7 ± 75.9, 22.5 ± 71.9, 13.2 ± 71.2, 10.1 ± 70.7, 14.1 ± 71.1, 15.3 ± 72.7, 13.8 ± 70.9, 19.7 ± 72.1 and 11.9 ± 70.6 μM, respectively, of which, compounds 72, 76, 78 and 80 possessed high selectivity index (SI) values of 250.4, 125.5, 259.6 and 127.5, respectively. Two patchoulane-type sesquiterpenoids (54 and 36) effectively suppressed the secretion of HBsAg in a dose-dependent manner with IC50 values of 46.6 ± 714.3 (SI = 31.0) and 77.2 ± 713.0 (SI = 1.7) μM, respectively. Compounds 63, 4, 17, 20, 72 and 81 possessed moderate activities against HBeAg secretion with IC50 values of 162.5 ± 718.9 (SI = 13.3), 399.2 ± 790.0 (SI = 10.6), 274.7 ± 770.8 (SI = 5.2), 313.9 ± 787.5 (SI = 7.2), 334.0 ± 770.4 (SI = 9.9) and 285.3 ± 720.9 (SI = 15.5) μM, respectively.13
Antimalarial activity-guided investigation and HPLC separation of the crude hexane extract of CR tubers led to the isolation of the sesquiterpenes; 42, patchoulenone (90) and 10,12-peroxycalamenene (91). The antimalarial activities of these compounds were determined from their effective concentrations (EC50) values against Plasmodium falciparum. The in vitro activity against P. falciparum (EC50) of compounds 90 and 91 were in the order of 10−4 M (1.08 × 10−4 and 3.45 × 10−4 M respectively).81
Chemical investigation of the rhizomes of CR led to the isolation of a new norsesquiterpenes, named norcyperone (92), (−)-clovane-2,9-diol (93) and other known compounds. The structure of the novel norsesquiterpene (92) with a tetrahydrofuran ring structure was established on the basis of extensive spectroscopic analyses, including 1D- and 2D-NMR, MS experiments, and single-crystal X-ray diffraction.84
A novel norsesquiterpenoid named cyperalin A (94) with the known compound (27) were isolated from freshly-cut rhizomes of CR defatted with n-hexane followed by extraction with methanol and silica gel chromatography. The compounds were screened for their anti-inflammatory activity. Cyperalin A (94) displayed the highest inhibitory activity of PGE2, COX-2, and LOX-5 with IC50s 0.22, 1.03, and 1.37 μM, respectively compared to indomethacin (IC50s 0.15, 0.69, and 0.81 μM, respectively). Compound 27, showed significant activity with IC50s 0.57 (PGE2), 1.74 (COX-2) and 2.03 (LOX-5) μM.93
The new sesquiterpenes, 4α,5α-oxidoeudesm-11-en-3-one (95) and cyper-11-ene-3,4-dione (96) together with the known compounds 4, 17, 18 and 47 were obtained from the hexane and dichloromethane fractions of CR. The compounds were examined for their estrogenic activity by E-screen assay on MCF-7 BUS cells. Compound 2, exhibited the most potent estrogenic activity by increasing transcriptional activities in an estrogen sensitive reporter gene assay. The authors showed that compound 2, has biphasic activities on estrogen receptors which could be useful as an alternative hormone replacement therapy.94 Compounds 4 and 17 isolated from the tubers of CR have showed growth inhibitory effects to both shoots and roots on the lettuce seedlings.128
Rani et al.87 isolated the sesquiterpenes 40; solavetivone (97) and aristolone (98) from the acetone extract of CR by silica gel column chromatography and determine their radical scavenging potential compared with standard gallic acid. Among the three sesquiterpenoids isolated, compound 40 possessed the highest radical scavenging potential (IC50 4.81 g mL−1) followed by 98 (IC50 5.28 g mL−1) and the new compound 97 (IC50 6.82 g mL−1) by DPPH radical scavenging assay.87
Bioassay-guided fractionation of the methanol extracts of the rhizomes of CR led to the isolation of 41, 42, zerumbone (99), α-humulene (100), (+)-dihydrocarvone (101), (R)-(+)-limonene (102), (1S)-(−)-verbenone (103), (S)-(−)-limonene (104), β-caryophyllene (105), (−)-(E)-pinocarveol (106), (E)-carveol (107), (−)-α-copaene (108), (1R)-(−)-myrtenal (109) and (1R)-(−)-myrtenol (110). The compounds were tested for repellency to male Blattella germanica and the results were compared to N,N-diethyl-3-methylbenzamide (deet). In filter-paper choice tests, 99 was the most repellent compound, and 100 was ineffective, which shows that α,β-unsaturated carbonyl group of 99 contributes to repellency. The article reported that at 81.5 μg cm−2, enhanced repellency was produced by binary mixtures of 99 and 41, 101 or 102 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, 50
30, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 and 30
50 and 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 ratios by weight). In Ebeling choice box tests at 652.4 μg cm−2, these compounds and deet resulted in complete repellency to intact male B. germanica, while they exhibited 35–47% repellency to antennectomized male one. Mixtures of active compounds from this plant species could serve as potential repellents for controlling B. germanica.92
70 ratios by weight). In Ebeling choice box tests at 652.4 μg cm−2, these compounds and deet resulted in complete repellency to intact male B. germanica, while they exhibited 35–47% repellency to antennectomized male one. Mixtures of active compounds from this plant species could serve as potential repellents for controlling B. germanica.92
A series of mono and sesquiterpenoids, 4, 17, 20, 43, 47, 101, 109, β-elemene (111), caryophyllene (112), δ-cadinene (113), calamenene (114), petchoulenyl acetate (115) and sugeonyl acetate (116) were isolated from the mature tubers of CR using gas chromatography-mass spectrometry (GC-MS). A new chemotype of CR was found in Hawaii based on the sesquiterpene composition of the mature tubers. The K-type has higher concentrations of 115 and 116 than the three known Asian chemotypes. Information on the distribution of chemotypes could also offer clues to the history of spreading CR weed species.80
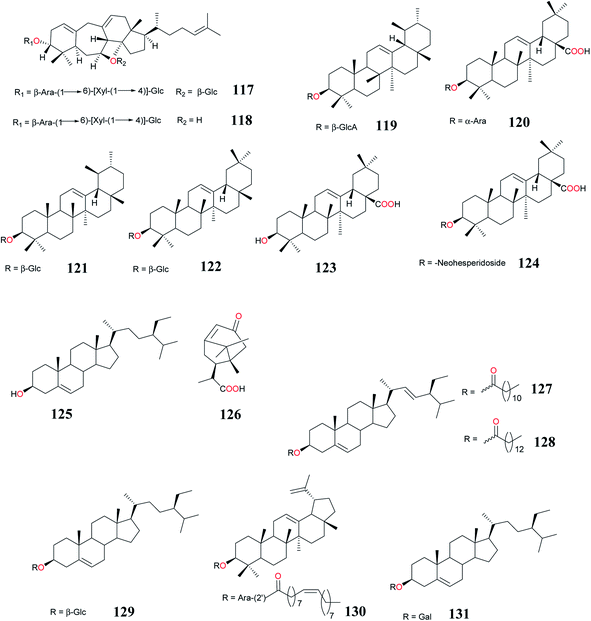
Zhou et al.90 isolated the two novel cycloartane glycosides, cyprotuside A (117) and cyprotuside B (118) from the rhizomes of CR and evaluated their antidepressant activity by forced swimming test (FST) and tail suspension test (TST) in mice. The preliminary in vivo evaluation showed that compounds 117 and 118 exhibited remarkable antidepressant activity in the despair mice models.
Exhaustive extraction of air-dried powdered tubers of CR with methanol followed by chromatography over silica gel column and elution with a gradient of chloroform and methanol afforded two new triterpenic glucosides; 18-epi-α-amyrin glucuronoside (119), oleanolic acid arabinoside (120), and α-amyrin glucopyranoside (121) and β-amyrin glucopyranoside (122).86
The sesquiterpenoid, 12-methyl cyprot-3-en-2-one-13-oic acid (126), steroidal esters; stigmasterol-n-dodecanoate (127), stigmasterol-n-tetradecanoate (128), β-sitosterol glucoside (129) and triterpenoid glycosides; lupenyl arabinopyranosyl oleate (130) were isolated for the first time and they could serve as chromatographic markers for standardization of the tubers of CR.91 A new steroid glycoside; sitosteryl (131) and other compounds were also isolated from the aerial parts of this plant species harvested in Egypt.33
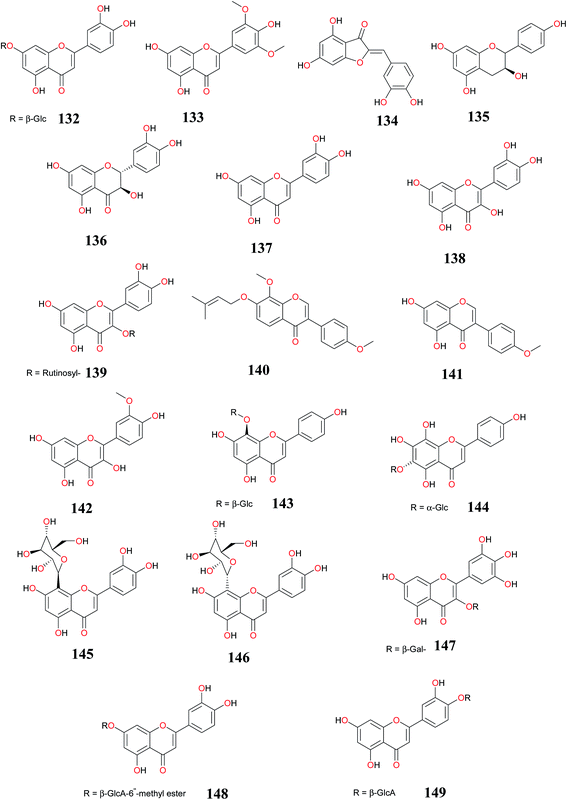
3.2 Flavonoids
Flavonoids continue to attract attention as potentially useful compounds because of their broad spectrum of biological activities.129–140 In this report, summaries of the most interesting results for flavonoids (132–153) isolated from CR have been shown in Table 2, while the chemical structures of the isolated compounds are shown in Fig. 8 and 9. In Table 2, the biological activities of the compounds and the organism studied have been provided.| Compounds | Part (s)/place of harvest of plant studied (VS≠) | Measured activity | References |
|---|---|---|---|
| a VS≠: Voucher specimen number, NM: not mention, ND: not done. | |||
| Luteolin-7-O-glucoside (132), tricin (133) and aureusidin (134) | Leaves/stem, Australia (K. L. Wilson 3309) (Denistone, N.S.W) | ND | Harborne et al.,141 |
| Afzelechin (135), (+)-catechin (136), luteolin (137), and quercetin (138) | Aerial parts/Monastir region in the Center of Tunisia (Cp.10.04) | Antioxidant and antitumor activities | Kilani-Jaziri et al.,40 |
| Rutin (139) | Rhizomes/Al-Azhar University campus, Assiut Branch, Egypt (2009-CR110) | Hepatoprotective activity | Mohamed,78 |
| Pongamone A (140) and biochanin A (141) | Rhizomes/Collected in Zhanjiang, Guangdong Province of China (No.20090903) | ND | Zhou et al.,11 |
| 137 | Rhizomes/Purchased from Matsuura-Yakugyo Co. Ltd. (Nagoya, Japan) CP-0901 | Antiproliferative activity | Ito et al.,18 |
| 133, isorhamnetin (142), vitexin (143), isovitexin (144), oreintin (145), epiorientin (146), myricetin-3-O-β-D-galactopyranoside (147), luteolin-7-O-β-D-glucuronopyranoside-6′′-methyl ester (148), luteolin-4′-O-β-D-glucuronopyranoside (149) and Luteolin-7-O-β-D-glucuronopyranoside (132) | Aerial parts/Experimental Station of El-Safa and El-Marwa, Faculty of Agriculture, Al-Azhar University, Assiut, Egypt | Antioxidant and α-amylase inhibitory activities | Sayed et al.,8 |
| 133 and 143 | Aerial parts/Experimental Station of El-Safa and El-Marwa, Faculty of Agriculture, Al-Azhar University, Assiut, Egypt (NM) | Antifeedant and Cytotoxic activity | Sayed et al.,33 |
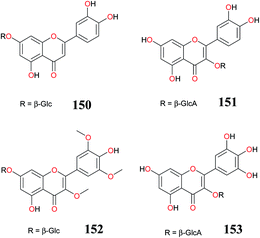
| 143, 145, cinaroside (150), quercetin-O-β-D-glucuronopyranoside (151), cyperaflavoside (152) and myricetin-3-O-β-D-glucuronopyranoside (153) | Aerial parts/King Abdulaziz University campus, Jeddah, Saudi Arabia (2014-CR110) | 5-Lipoxygenase inhibitory activity | Ibrahim et al.,93 |
Harborne et al.141 isolated luteolin-7-O-glucoside (132), tricin (133) and aureusidin (134) from the leaves/stems of CR by paper electrophoresis. The structures of the compounds were identified by standard procedures and co-chromatography with authentic samples carried out in at least 4 solvents.
In an effort to search for novel antioxidant and antiproliferative hits, four flavonoids; afzelechin (135), (+)-catechin (136), luteolin (137), and quercetin (138) and other compounds were isolated from the total oligomers flavonoids and ethyl acetate extracts of CR. Compound 137 was the most active in reducing the production of thiobarbituric acid reactive substances (malondialdehyde = 1.5 nM), inhibiting significantly the proliferation of K562 cells (IC50 = 25 g mL−1) and protecting against H2O2/UV-photolysis induced DNA damage.40 Rutin (139), pongamone A (140) and biochanin A (141) were isolated from the rhizomes of this plant species and the structure was established on the basis of 1D and 2D NMR spectroscopic analyses.11,78 Luteolin (137) has been isolated from the rhizomes of this plant species.18
Chemical investigation of the aerial parts of CR led to the isolation 133, isorhamnetin (142), vitexin (143), isovitexin (144), oreintin (145), epiorientin (146), myricetin-3-O-β-D-galactopyranoside (147), luteolin-7-O-β-D-glucuronopyranoside-6′′-methyl ester (148), luteolin-4′-O-β-D-glucuronopyranoside (149) and luteolin-7-O-β-D-glucuronopyranoside (132). Compounds (146–148) having an ortho-dihydroxyl groups at ring B showed strong antioxidant activity. The presence of a hydroxyl group at position-3 increased the antioxidant activity in 143. Compounds (133, 143, 144, 146, 148) demonstrated strong α-amylase inhibitory activity > 50% influenced by the presence of free hydroxyl groups at 7, 3′ and 4′ positions.8,33 The flavonoids 144, 146, cinaroside (150), quercetin-O-β-D-glucuronopyranoside (151), cyperaflavoside (152) and myricetin-3-O-β-D-glucuronopyranoside (153) were also isolated from the aerial parts and screened for their 5-lipoxygenase inhibitory potential. All compounds demonstrated 5-lipoxygenase inhibitory potentials with IC50s 5.1 (152), 4.5 (143), 5.9 (145), 4.0 (150), 3.7 (151), and 2.3 μM (153), respectively, in comparison to indomethacin (IC50 = 0.98 M).93
3.3 Stilbenes and derivatives
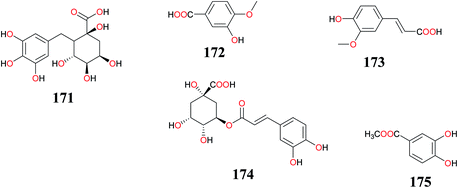
Stilbenes are polyphenols containing resveratrol as a basic subunit. These compounds have received much attention because of their cardioprotective effects, but they also display anti-inflammatory, antioxidative, and antimicrobial activities. They are also known as anticancer and cancer-chemopreventive agents.142 The stilbenes and derivates (154–176) isolated from CR by several NP research groups have shown interesting biological activities during in vitro screening exercise (Table 3).11,17,40,77,78,110 The chemical structures of those isolated from this herb are shown in Fig. 10 and 11.| Compounds | Part (s)/place of harvest of plant studied (VS≠) | Measured activity | References |
|---|---|---|---|
| a VS≠: Voucher specimen number, NM: not mention, ND: not done. | |||
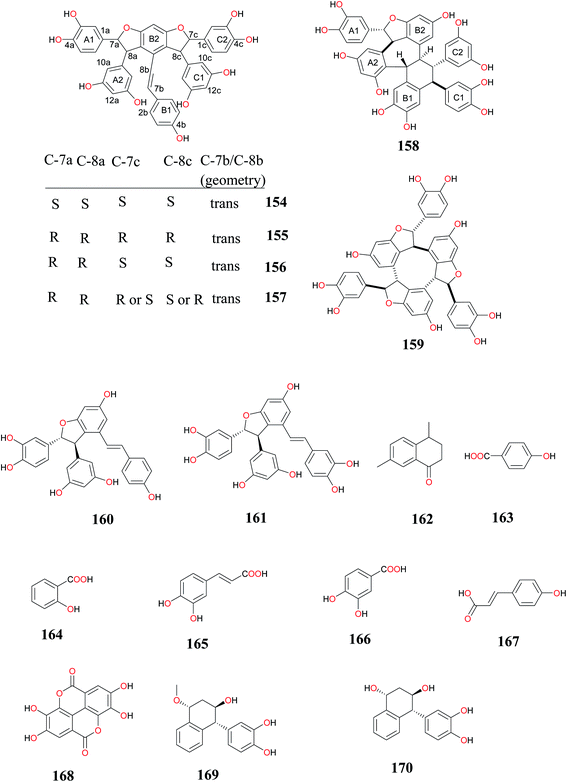
| (+)-Cyperusphenol A (154), (−)-(E)-cyperusphenol A (155), (E)-mesocyperusphenol A (156), cyperusphenol C (157), cyperusphenol B (158), cyperusphenol D (159), trans-scirpusin A (160) and scirpusin B (161) | Rhizomes/Purchased from Matsuura-yakugyo Co. Ltd. (Nagoya, Japan) (CP-0901) | Antiproliferative activity | Ito et al.,18 |
| 4,7-Dimethyl-1-tetralone (162) | Tubers/Purchased from a Thai traditional dispensary, Bangkok, Thailand | Antimalarial activity | Thebtaranonth et al.,81 |
| p-Hydroxybenzoic acid (163) | Rhizomes/Purchased from Uchida Wakanyaku Co., Ltd. (Tokyo, Japan) (lot. 242118) | ND | Jeong et al.,15 |
| Salicylic acid (164), caffeic acid (165), protocatechuic acid (166) and p-coumaric acid (167) | Aerial parts/El-Safa and El-marwa, Faculty of Agriculture, Al-Azhar University, Assiut, Egypt | Antifeedant and Cytotoxic activity | Sayed et al.,33 |
| 167, and ellagic acid (168) | Rhizomes/Purchased from Kyung Dong market place in Seoul, South Korea. (DKH-02561) | Anti-inflammatory activity | Tsoyi et al.,17 |
| 164–167, methoxycyperotundol (169) and cyperotundol (170) | Rhizomes/Collected in Zhanjiang, Guangdong Province of China (no. 20090903) | ND | Zhou et al.,11 |
| Galloylquinic acid (171), 3-hydroxy-4-methoxybenzoic acid (172) and ferulic acid (173) | Aerial parts/Monastir region in the Center of Tunisia (Cp.10.04) | Antioxidant and antitumor activities | Kilani-Jaziri et al.,40 |
| Chlorogenic acid (174) | Rhizomes/obtained as Organic Musta Powder (Khandige Organic Health Product, Bangalore, India) (NM) | Anti-inflammatory activity | Rocha et al.,10 |
| Methyl-3,4-dihydroxybenzoate (175) | Rhizomes/Al-Azhar University campus, Assiut Branch, Egypt (2009-CR110) | Hepatoprotective activity | Mohamed,78 |
 | ||
| Fig. 10 Structures of stilbenes, ellagic acid derivatives and other compounds from Cyperus rotundus (154 to 170). | ||
The novel enantiomeric and meso-stilbene trimers; (+)-cyperusphenol A (154), (−)-(E)-cyperusphenol A (155), (E)-mesocyperusphenol A (156), a trimer bearing a novel hexacyclic ring system, cyperusphenol B (158), together with the known stilbenoids, cyperusphenol C (157), cyperusphenol D (159), trans-scirpusin A (160) and scirpusin B (161) were isolated from the rhizomes of CR. The hits were evaluated for their antiproliferative activity employing the Jurkat cell line (human T-cell leukemia cells), while the IC50 potencies of a racemate of compounds 154–156, 158, and 159 were estimated as 27.4, 40.5, 26.4, and 26.3 μM, respectively. The article reported that the suppression of cell growth by compound 159 was due to the induction of apoptosis, which was characterized by nuclear changes and PARP-1 cleavage determined by western blotting.18 Salicylic acid (164), caffeic acid (165), protocatechuic acid (166) and p-coumaric acid (167) isolated from this weed showed significant antioxidant activity.8 The insect antifeedant activity demonstrated by the crude extracts is due to the presence of furochromones having methoxyl group at positions C-5 and/or C-8 positions.33
3.4 Miscellaneous compounds
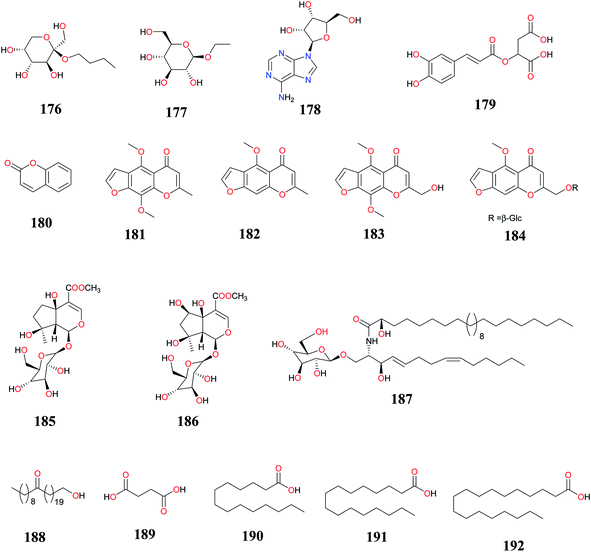
Steroidal glycosides, furochromones and aromatics (177–185) isolated from the aerial parts of CR collected from Egypt demonstrated antioxidant, α-amylase inhibitory and antifeedant activities.8,33 A summary of these bioactive compounds isolated from CR is provided in (Table 4) and their chemical structures in Fig. 12. New iridoids, cerebroside, known aliphatic fatty acids and coumarin (186–192) from this plant species have also shown hepatoprotective, anti-proliferation, and growth inhibitory properties respectively.78,86,143,144| Compounds | Part (s)/place of harvest of plant studied (VS≠) | Measured activity | References |
|---|---|---|---|
| a VS≠: Voucher specimen number, NM: Not mention, ND: Not done. | |||
| n-Butyl-β-D-fructopyranoside (176), ethyl-α-D-glucopyranoside (177), adenosine (178) and (−)-(E)-caffeoylmalic acid (179) | Aerial parts/Experimental Station of El-Safa and El-Marwa, Faculty of Agriculture, Al-Azhar University, Assiut, Egypt | Antioxidant and α-amylase inhibitory activities | Sayed et al.,8 |
| Benzo-α-pyrone (180), khellin (181), visnagin (182), ammiol (183) and khellol-β-D-glucopyranoside (184) | Aerial parts/El-Safa and El-Marwa, Faculty of Agriculture, Al-Azhar University, Assiut, Egypt | Antifeedant and Cytotoxic activity | Sayed et al.,33 |
| Ipolamiide (185) and 6β-hydroxyipolamiide (186) | Rhizomes/Al-Azhar University campus, Assiut Branch, Egypt (2009-CR110) | Hepatoprotective activity | Mohamed,78 |
| 1-O-(β-D-glucopyranosyloxy)-(2S,3R,4E,8Z)-2-[(2′R)-2′-hydroxylignoceranoylamino]-4,8-tetradecene-3-diol (187) | Radix/Shandong, China | Anti-proliferation activity | Liu et al.,143 |
| n-Tricont-1-ol-21-one (188) | Tubers/West Champaran, Bihar, India (No.NISCAIR/RHMD/Consult/-2008-09/1114/145) | ND | Alam et al.,86 |
| Succinic acid (189), myristic acid (190), palmitic acid (191) and stearic acid (192) | Tubers/upland rice field (sandy loam soil) of Manikganj district, Bangladesh (NM) | Growth inhibitory effects | Quayyum et al.,144 |
3.5 Bioactivities and proposed mechanisms of C. rotundus compounds
The pharmacological activities and mechanisms of action of some compounds isolated from C. rotundushave been reported extensively.8,11,17,41,76–78 The summaries of the most interesting results for some NPs isolated from this weed have been shown in Fig. 12.Conclusions
Cyperus rotundus L. (Nutgrass, family Cyperaceae) popularly called “the world's worst weed” has attracted particular attention as a medicinal plant, due its broad spectrum of pharmacological activities. In the past six decades, about 192 NPs have been isolated and characterized from this plant species. Among them, terpenoids and flavonoids are the major bioactive constituents mostly harvested from Asia and Africa. The chemical structures of pure compounds were retrieved from literature sources comprising data collected from articles from major peer-reviewed journals, from all over the world spanning the period 1965 to 2020. The collected data includes region of collection of plant material, voucher specimen number, isolated metabolites and class, and measured biological activities of isolated compounds. The study has provided a survey of the biological activities of 192 NPs and the mechanism of action of some of the compounds isolated from C. rotundus. It is worth mentioning that C. rotundus and its NPs have shown good safety in vitro and in vivo studies. Thus it would be interesting in future to evaluate the toxicities of the NPs from this weed using in silico approaches.Conflicts of interest
There are no conflicts to declare.Acknowledgements
SBB acknowledges the African-German Network of Excellent in Science (AGNES) junior researcher grant supported by Alexander von Humboldt and sponsored by the Federal Ministry of Education and Research. SBB would like to thank the AgroEcoHealth Unit, International Institute of Tropical Agriculture (IITA), Cotonou, Benin fortraining in HPLC. FNK would also like to acknowledge funding from the German Academic Exchange Services (DAAD) for a guest professorship at TU Dresden. AFAM was supported by a doctoral research grant from the German Academic Exchange Service (DAAD, Award No. 91653768).References
- R. Sharma and R. Gupta, Life Sci., 2007, 80, 2389–2392 CrossRef CAS.
- K. H. Kumar, S. Razack, I. Nallamuthu and F. Khanum, Ind. Crops Prod., 2014, 52, 815–826 CrossRef.
- S. B. Badgujar and A. H. Bandivdekar, J. Ethnopharmacol., 2015, 163, 39–42 CrossRef PubMed.
- A. Stierle, R. Upadhyay and G. Strobel, Phytochemistry, 1991, 30, 2191–2192 CrossRef CAS.
- Y. Jaiswal, Z. Liang, P. Guo, H. M. Ho, H. Chen and Z. Zhao, J. Agric. Food Chem., 2014, 62, 7302–7316 CrossRef CAS PubMed.
- T. K. Lim, Cyperus rotundus. Edible medicinal and non-medicinal plants, Springer Science + Business Media Dordrecht, 2016, vol. 10, pp.178–208 Search PubMed.
- W. G. Seo, H. O. Pae, G. S. Oh, K. Y. Chai, T. O. Kwon, Y. G. Yun, N. Y. Kim and H. T. Chung, J. Ethnopharmacol., 2001, 76, 59–64 CrossRef CAS PubMed.
- H. M. Sayed, M. H. Mohamed, S. F. Farag, G. A. Mohamed, O. R. M. Omobuwajo and P. Proksch, Nat. Prod. Res., 2008, 22, 1487–1497 CrossRef CAS PubMed.
- M. Kumar, M. Rani and B. Meher, Curr. Res. Pharm. Sci., 2017, 07, 11–15 CrossRef CAS.
- J. H. Jin, D. U. Lee, Y. S. Kim and H. P. Kim, Arch. Pharm. Res., 2011, 34, 223–228 CrossRef CAS PubMed.
- Z. Zhou and W. Yin, Molecules, 2012, 17, 12636–12641 CrossRef CAS PubMed.
- B. Ryu, H. M. Kim, J. S. Lee, Y. J. Cho, M. S. Oh, J. H. Choi and D. S. Jang, HeIv. Chim. Acta, 2015, 98, 1372–1379 CrossRef CAS.
- H. B. Xu, Y. B. Ma, X. Y. Huang, C. A. Geng, H. Wang, Y. Zhao, T. H. Yang, X. L. Chen, C. Y. Yang, X. M. Zhang and J. J. Chen, J. Ethnopharmacol., 2015, 171, 131–140 CrossRef CAS PubMed.
- N. A. Raut and N. J. Gaikwad, Fitoterapia, 2006, 77, 585–588 CrossRef PubMed.
- S. J. Jeong, T. Miyamoto, M. Inagaki, Y. C. Kim and R. Higuchi, J. Nat. Prod., 2000, 63, 673–675 CrossRef CAS PubMed.
- J. H. Nam, D. Y. Nam and D. U. Lee, J. Nat. Prod., 2016, 79, 1091–1096 CrossRef CAS PubMed.
- K. Tsoyi, H. J. Jang, Y. S. Lee, Y. M. Kim, H. J. Kim, H. G. Seo, J. H. Lee, J. H. Kwak, D. U. Lee and K. C. Chang, J. Ethnopharmacol., 2011, 137, 1311–1317 CrossRef CAS PubMed.
- T. Ito, H. Endo, H. Shinohara, M. Oyama, Y. Akao and M. Iinuma, Fitoterapia, 2012, 83, 1420–1429 CrossRef CAS PubMed.
- L. Boulos and M. N. El-Hadidi, The weed flora of Egypt, The American University in Cairo Press, Cairo, 1984, p. 58 Search PubMed.
- N. Singh, V. K. Kulshrestha, M. B. Gupta and K. P. Bhargava, Indian J. Med. Res., 1970, 58, 103–109 CAS.
- M. B. Gupta, T. K. Palit, N. Singh and K. P. Bhargava, Indian J. Med. Res., 1971, 59, 76–82 CAS.
- K. Komai and K. Ueki, Weed Res. Japan, 1980, 25, 42–47 CAS.
- K. Komai, C. S. Tang and R. K. Nishimoto, J. Chem. Ecol., 1991, 17, 1–8 CrossRef CAS PubMed.
- P. Kalsi, A. Sharma, A. Singh, I. P. Singh and B. R. Chhabra, Fitoterapia, 1995, 66, 94 CAS.
- M. Zhu, H. H. Luk, H. S. Fung and C. T. Luk, Phytother. Res., 1997, 11, 392–395 CrossRef.
- S. C. Umeric and H. O. Ezeuzo, Bioresour. Technol., 2000, 72, 193–196 CrossRef.
- J. H. Ha, K. Y. Lee, H. C. Choi, J. Cho, B. S. Kang, J. C. Lim and D. U. Lee, Biol. Pharm. Bull., 2002, 25, 128–130 CrossRef CAS PubMed.
- M. Zhu, H. H. Luk, H. S. Fung and C. T. Luk, Phytother. Res., 1998, 11, 392–394 CrossRef.
- S. J. Uddin, K. Mondal, J. A. Shilpi and M. T. Rahman, Fitoterapia, 2006, 77, 134–136 CrossRef CAS PubMed.
- D. K. Pal and S. Dutta, Indian J. Pahrm. Sci., 2006, 68, 256–258 CrossRef.
- A. Puratchikody, C. N. Devi and G. Nagalakshmi, Indian J. Pharm. Sci., 2006, 68, 97–101 CrossRef.
- B. Lemaure, A. Touché, I. Zbinden, J. Moulin, D. Courtois, K. Macé and C. Darimont, Phytother. Res., 2007, 21, 724–730 CrossRef PubMed.
- H. M. Sayed, M. H. Mohamed, S. F. Farag, G. A. Mohamed and P. Proksch, Nat. Prod. Res., 2007, 21, 343–350 CrossRef CAS PubMed.
- A. Ardestani and R. Yazdanparast, Int. J. Biol. Macromol., 2007, 41, 572–578 CrossRef CAS PubMed.
- Z. A. Nima, M. S. Jabier, R. I. Wagi and H. A. Hussain, Eng. Technol., 2008, 26, 1156–1159 Search PubMed.
- V. Kempraj and S. K. Bhat, Nat. Prod. Radiance, 2008, 7, 416–419 Search PubMed.
- M. S. Sundaram, T. Sivakumar and G. Balamurugan, Biomedicine, 2008, 28, 302–304 Search PubMed.
- S. I. Shivakumar, H. M. Suresh, C. S. Hallikeri, B. C. Hatapakki, J. S. Handiganur, K. Sankh and B. Shivakumar, J. Nat. Remed., 2009, 9, 92–196 Search PubMed.
- F. Mojab, H. Vahidi, B. Nickavar and M. Kamali-Nejad, J. Med. Plant, 2009, 8, 91–97 Search PubMed.
- S. Kilani-Jaziri, A. Neffati, I. Limem, J. Boubaker, I. Skandrani, M. B. Sghair, I. Bouhlel, W. Bhouri, A. M. Mariotte, K. Ghedira, M. G. Dijoux-Franca and L. Chekir-Ghedira, Chem. Biol. Interact., 2009, 181, 85–94 CrossRef CAS PubMed.
- D. Pal, S. Dutta and A. Sarkar, Acta Pol. Pharm., 2009, 66, 535–541 Search PubMed.
- M. E. Güldür, A. Ozgónül, I. H. Kilic, O. Sögüt, M. Ozaslan, M. Bitiren, M. Yalcin and D. Musa, Int. J. Pharmacol., 2010, 6, 104–110 Search PubMed.
- A. Bisht, G. R. S. Bisht, M. Singh, R. Gupta and V. Singh, Int. J. Res. Pharm. Biomed. Sci., 2011, 2, 661–665 Search PubMed.
- A. Singh, S. Maurya, R. Singh and U. P. Singh, Arch. Phytopathol. Plant Prot., 2011, 44, 2004–2011 CrossRef.
- A. G. Sunil, K. S. Kesavanarayanan, P. Kalaivani, S. Sathiya, V. Ranju, R. J. Priya, B. Pramila, F. D. S. Paul, J. Venkhatesh and C. S. Babu, Brain Res. Bull., 2011, 84, 394–405 CrossRef CAS PubMed.
- P. G. Daswani, S. Brijesh, P. Tetali and T. J. Birdi, Indian J. Pharmacol., 2011, 43, 340–344 CrossRef PubMed.
- G. K. Dang, R. R. Parekar, S. K. Kamat, A. M. Scindia and N. N. Rege, Phytother. Res., 2011, 25, 904–908 CrossRef CAS PubMed.
- S. M. Khan and D. U. Lee, Nat. Prod. Sci., 2011, 17, 250–255 CAS.
- M. Khalili, Z. Kiasalari, M. Roghani and Y. Azizi, J. Med. Plant. Res., 2011, 5, 1140–1146 Search PubMed.
- N. Singh, B. R. Pandey, P. Verma, M. Bhalla and M. Gilca, Indian J. Nat. Prod. Res., 2012, 3, 467–476 CAS.
- D. Jebasingh, S. Venkataraman, D. D. Jackson and B. S. Emerald, Int. J. Appl. Res. Nat. Prod., 2012, 5, 1–8 Search PubMed.
- G. F. A. El-Kaream, J. Intercult. Ethnopharmacol., 2012, 1, 111–118 CrossRef.
- R. S. Chandratre, S. Chandarana and S. A. Mengi, Int. J. Pharm. Biol. Arch., 2012, 3, 598–600 Search PubMed.
- A. Chithran, B. T. Ramesh and N. Himaja, Int. J. Phytopharmacol., 2012, 3, 130–134 Search PubMed.
- K. H. Kumar and F. Khanum, Cell Mol. Neurobiol., 2013, 33, 5–17 CrossRef PubMed.
- H. Weenen, M. H. H. Nkunya, D. H. Bray, L. B. Mwasumbi, L. S. Kinabo, V. A. E. B. Kilimali and J. B. P. A. Wijnberg, Planta Med., 1990, 56, 371 CrossRef CAS PubMed.
- M. Ahmad, M. Mahayrookh, B. R. Asif and N. Jahan, Pak. J. Pharmacol., 2012, 29, 7–13 Search PubMed.
- K. H. Kumar and F. Khanum, Cell Mol. Neurobio., 2013, 33, 5–17 CrossRef PubMed.
- S. Krishna and S. Renu, J. Drug Del. Ther., 2013, 3, 109–113 Search PubMed.
- K. H. Kumar, A. Tamatam, A. Pal and F. Khanum, Neurotoxicol, 2013, 34, 150–159 CrossRef PubMed.
- Z. Rabiei, M. Hojjati, M. Rafieian-Kopaeia and Z. Alibabaei, Biomed. Aging Pathol., 2013, 3, 185–191 CrossRef.
- H. Imam, Z. Riaz and G. Sofi G, Int. J. Green Pharm., 2013, 7, 37–40 CrossRef.
- G. F. Mohammed, Aesthet. Surg. J., 2014, 34, 298–305 CrossRef PubMed.
- S. E. Park, W. T. Shin, C. Park, S. H. Hong, G. Y. Kim, S. O. Kim, C. H. Ryu, S. H. Hong and Y. H. Choi, Oncol. Rep., 2014, 32, 2461–2470 CrossRef PubMed.
- D. Jebasingh, D. D. Jackson, S. Venkataraman, E. Adeghate and B. S. Emerald, Pharm. Biol., 2014, 15, 1–12 Search PubMed.
- M. Z. Imam and C. D. Sumi, BMC Complement. Altern. Med., 2014, 14, 83 CrossRef PubMed.
- H. Imam, S. Zarnigar, A. Ghulamuddin and L. A. Seikh, Int. J. Nutr. Pharmacol. Nuerol. Dis., 2014, 4, 23–27 CrossRef.
- H. H. T. Tran, M. C. Nguyen, H. T. Le, T. L. Nguyen, T. B. Pham and V. M. Chau, J. Pharm. Biol., 2014, 52, 74–77 CrossRef CAS PubMed.
- K. Athesh, M. Divakar and P. Brindha, Asian J. Pharm. Clin. Res., 2014, 7, 88–92 Search PubMed.
- A. M. Peerzada, H. H. Ali, M. Naeem, M. Latif, A. H. Bukhari and A. Tanveer, J. Ethnopharmacol., 2015, 174, 540–560 CrossRef PubMed.
- S. Kasala, K. Ramanjaneyulu, J. Himabindhu, R. Alluri and R. R. Babu, J. Pharmacogn. Phytochem., 2016, 5, 407–409 Search PubMed.
- S. Suryavanshi, A. Choudhari, P. Raina and R. Kaul-Ghanekar, J. Ethnopharmacol., 2019, 242, 10 CrossRef PubMed.
- F. Wang, X. Song, S. Ma, C. Liu, X. Sun, X. Wang, Z. Liu, D. Liang and Z. Yu, Biosci. Rep., 2019, 39, BSR20190502 CrossRef CAS PubMed.
- S. Ma, F. Wang, C. Zhang, X. Wang, X. Wang and Z. Yu, BMC Complementary Med. Therapies, 2020, 20, 262 CrossRef CAS PubMed.
- Z. Shakerin, E. Esfandiari, S. Razavi, H. Alaei, M. Ghanadian and G. Dashti, Adv. Biomed. Res., 2020, 9, 17 CrossRef CAS PubMed.
- R. S. Dhillon, S. Singh, S. Kundra and A. S. Basra, Plant Growth Regul., 1993, 13, 89–93 CrossRef CAS.
- E. J. Seo, D. J. Lee, J. H. Kwak, S. M. Lee, Y. S. Kim and Y. S. Jung, J. Ethnopharmacol., 2011, 135, 48–54 CrossRef CAS PubMed.
- G. A. Mohamed, Bull. Fac. Pharm. Cairo Univ., 2015, 53, 5–9 Search PubMed.
- V. H. Kapadia, V. G. Naik, M. S. Wadia and S. Dev, Tetrahedron Lett., 1967, 47, 4661–4667 CrossRef.
- K. Komai and C. S. Tang, Phytochemistry, 1989, 28, 1883–1886 CrossRef CAS.
- C. Thebtaranonth, Y. Thebtaranonth, S. Wanauppathamkul and Y. Yuthavong, Phytochemistry, 1995, 40, 125–128 CrossRef CAS PubMed.
- S. Ohira, T. Hasegawa, K. I. Hayashi, T. Hoshino, D. Takaoka and H. Nozaki, Phytochemistry, 1998, 47, 1577–1581 CrossRef CAS.
- M. M. Sonwa and W. A. Kӧnig, Phytochemistry, 2001, 58, 799–810 CrossRef CAS PubMed.
- Y. Xu, H. W. Zhang, C. Y. Yu, Y. Lu, Y. Chang and Z. M. Zou, Molecules, 2008, 13, 2474–2481 CrossRef CAS PubMed.
- Y. Xu, H. W. Zhang, X. C. Wan and Z. M. Zoua, Magn. Reson. Chem., 2009, 47, 527–531 CrossRef CAS PubMed.
- P. Alam, M. Ali and V. Aeri, J. Nat. Prod. Plant Resour., 2012, 2, 272–280 CAS.
- M. P. Rani and K. P. Padmakumari, J. Chromatogr. B, 2012, 904, 22–28 CrossRef PubMed.
- G. F. Mohammed, Aesthet. Surg. J., 2014, 34, 298–305 CrossRef PubMed.
- J. L. Yang and Y. P. Shi, Planta Med., 2012, 78, 59–64 CrossRef CAS PubMed.
- Z. L. Zhou, S. Q. Lin and W. Q. Yin, J. Asian Nat. Prod. Res., 2016, 18, 662–668 CrossRef CAS PubMed.
- S. Sultana, M. Ali and S. R. Mir, Open Plant Sci. J., 2017, 10, 82–91 CrossRef CAS.
- K. S. Chang, J. H. Jeon, G. H. Kim, G. W. Jang, S. J. Jeong, Y. R. Ju and Y. J. Ahn, Sci. Rep., 2017, 7, 16643 CrossRef PubMed.
- S. R. M. Ibrahim, G. A. Mohamed, K. Z. Alshali, R. A. Al Haidari, A. A. El-Kholy and M. F. Zayed, Braz. J. Pharmacog., 2018, 28, 320–324 CrossRef CAS.
- Y. J. Park, H. Zheng, J. H. Kwak and K. H. Chung, Biomed. Pharmacother., 2019, 109, 1313–1318 CrossRef CAS PubMed.
- M. A. Gamal, K. M. K. Hani and I. R. M. Sabrin, Int. J. Pharmacogn. Phytochem. Res., 2015, 7, 83–99 Search PubMed.
- A. Kamala, S. K. Middha and C. S. Karigar, 3 Biotech, 2018, 8, 309 CrossRef PubMed.
- L. Jirovetz, A. Wobus, G. Buchbauer, M. P. Shafi and P. T. Thampi, Plant, 2004, 7, 100–106 CAS.
- S. Kilani, A. Abdelwahed, I. Chraief, R. B. Ammar, N. Hayder, M. Hammami, K. Ghedira and L. Chekir-Ghedira, J. Essent. Oil, 2005, 17, 695–700 CrossRef CAS.
- S. Kilani, I. Bouhlel, R. Ben, A. Abdelwahed, N. Hayder, A. Mahmoud, K. Ghedira and L. Chekir-Ghedira, Toxicol. Environ. Chem., 2005, 87, 415–425 CrossRef CAS.
- R. Yazdanparast and A. Ardestani, J. Med. Food, 2007, 10, 667–674 CrossRef CAS PubMed.
- S. Kilani, S. M. Ben, I. Limem, I. Bouhlel, J. Boubaker, W. Bhouri, I. Skandrani, A. Neffatti, R. Ben-Ammar, M. G. Dijoux-Franca, K. Ghedira and L. Chekir-Ghedira, Bioresour. Technol., 2008, 99, 9004–9008 CrossRef CAS PubMed.
- S. Kilani, J. Ledauphin, I. Bouhlel, M. Ben-Sghaier, J. Boubaker, I. Skandrani, R. Mosrati, K. Ghedira, D. Barillier and L. Chekir-Ghedira, Chem. Biodivers., 2008, 5, 729–742 CrossRef CAS PubMed.
- O. A. Lawal and A. O. Oyedeji, Molecules, 2009, 14, 2909–2917 CrossRef CAS PubMed.
- S. Kilani-Jaziri, W. Bhouri, I. Skandrani, I. Limem, L. Chekir-Ghedira and K. Ghedira, S. Afr. J. Bot., 2011, 77, 767–776 CrossRef CAS.
- S. Kilani-Jaziri, D. Mhalla, F. Châbane, Z. Ghedira, I. Limem, K. Ghedira and L. Chekir-Ghedira, BMC Complement. Alternat. Med., 2013, 13, 28 CrossRef PubMed.
- A. Sharma, R. Verma and P. Ramteke, J. Herbal Med., 2014, 4, 74–82 CrossRef.
- P. Mannarreddy, M. Denis, D. Munireddy, R. Pandurangan, K. P. Thangavelu and K. Venkatesan, Biomed. Pharmacother., 2017, 95, 1375–1387 CrossRef PubMed.
- Q. P. Hu, X. M. Cao, D. L. Hao and L. L. Zhang, Sci. Rep., 2017, 7, 45231 CrossRef CAS PubMed.
- R. A. Abo-Altemen, A. M. Al-Shammari and M. S. Shawkat, Energy Procedia, 2019, 157, 1462–1474 CrossRef CAS.
- F. G. Rocha, M. D. M. Brandenburg, P. L. Pawloski, B. D. S. Soley, S. C. A. Costa, C. C. Meinerz, I. P. Baretta, M. F. Otuki and D. A. Cabrini, J. Ethnopharmacol., 2020, 254, 112709 CrossRef CAS PubMed.
- B. Huang, J. Liu, S. Fu, Y. Zhang, Y. Li, D. He, X. Ran, X. Yan, J. Du, T. Meng, X. Gao and D. Liu, Front. Pharmacol., 2020, 11, 281 CrossRef CAS PubMed.
- K. Komai and K. Ueki K, J. Weed Sci. Technol., 1975, 20, 66–71 CrossRef CAS.
- K. Komai, J. Iwamura and K. Ueki, J. Weed Sci. Technol., 1977, 22, 14–18 CrossRef CAS.
- P. N. Singh and S. B. Singh, Phytochemistry, 1980, 19, 2056 CrossRef CAS.
- S. K. Kim, B. Y. Hwang, S. J. Kang, J. J. Lee, J. S. Ro and K. Lee, Koran J. Pharmacogn., 2000, 31, 1–6 CAS.
- S. J. Kim, H. J. Kim, Y. P. Jang, M. S. Oh and D. S. Jang, Bull. Korean Chem., 2012, 33, 3115–3118 CrossRef CAS.
- H. G. Kim, J. Hong, Y. Huh, C. Park, D. S. Hwang, J. H. Choi and M. S. Oh, J. Ethnopharmacol., 2013, 148, 322–328 CrossRef CAS PubMed.
- S. J. Kim, B. Ryu, H. Y. Kim, Y. I. Yang, J. Ham, J. H. Choi and D. S. Jang, Bull. Korean Chem. Soc., 2013, 34, 2207–2210 CrossRef CAS.
- Y. X. Li, J. Chem. Pharm. Res., 2014, 6, 1496–1500 CAS.
- S. K. Paknikar, O. Motl and K. K. Chakravarti, Tetrahedron Lett., 1977, 24, 2121–2124 CrossRef.
- S. H. Jung, S. J. Kim, B. G. Jun, K. T. Lee, S. P. Hong, M. S. Oha, D. S. Jang and J. H. Choi, J. Ethnopharmacol., 2013, 147, 208–214 CrossRef CAS PubMed.
- A. Dadang, K. Ohsawa, S. Kato and I. Yamamoto, J. Pesticide Sci., 1996, 21, 444–446 CrossRef.
- Y. J. Seo, M. Jeong, K. T. Lee, D. S. Jang and J. H. Choi, Int. Immunopharmacol., 2016, 38, 61–69 CrossRef CAS.
- V. H. Kapadia, B. A. Nagasampagi, V. G. Naik and S. Dev, Tetrahedron, 1965, 21, 607–618 CrossRef CAS.
- H. Hikino, K. Aota and T. Takemoto, Tetrahedron, 1967, 23, 2169–2172 CrossRef CAS.
- H. Hikino, K. Aota, D. Kuwano and T. Takemoto, Tetrahedron, 1971, 27, 4831–4836 CrossRef CAS.
- H. Hikino and K. Aota, Phytochemistry, 1976, 16, 1265–1266 CrossRef.
- M. Morimoto and K. Komai, Weed Biol. Manage., 2005, 5, 203–209 CrossRef CAS.
- S. Rune, F. Torgils and M. V. Ingunn, J. Agric. Food Chem., 2007, 55, 10067 CrossRef.
- P. R. Jensen, K. M. Jenkins, D. Porter and W. Fenical, Appl. Environ. Microbiol., 1998, 64, 1490 CrossRef CAS.
- C. N. Liu, S. H. Kuo and M. C. Chung, J. Nat. Prod., 1997, 60, 851 CrossRef PubMed.
- H. W. Chu, H. T. Wu and Y. J. Lee, Tetrahedron, 2004, 60, 2647 CrossRef CAS.
- S. Li, C. Y. Lo and C. T. Ho, J. Agric. Food Chem., 2006, 54, 4176 CrossRef CAS PubMed.
- J. H. Yoon, T. G. Lim, K. M. Lee, A. J. Jeon, S. Y. Kim and K. W. Lee, J. Agric. Food Chem., 2011, 59, 222 CrossRef CAS PubMed.
- S. E. Nielsen, V. Breinholt, C. Cornett and L. O. Dragsted, Food Chem. Toxicol., 2000, 38, 739 CrossRef CAS PubMed.
- Z. Cheng, S. Surichan, K. Ruparelia, R. Arroo and M. R. Boarder, Br. J. Pharmacol., 2011, 162, 1781 CrossRef CAS PubMed.
- S. Li, M. H. Pan, C. S. Lai, C. Y. Lo, S. Dushenkov and C. T. Ho, Bioorg. Med. Chem., 2007, 15, 3381 CrossRef CAS PubMed.
- G. Casano, A. Dumetre, C. Pannecouque, S. Hutter, N. Azas and M. Robin, Bioorg. Med. Chem., 2010, 18, 6012 CrossRef CAS PubMed.
- N. Beldjoudi, L. Mambu, M. Labbaied, P. Grellier, R. David, R. Philippe, T. M. Mare and F. Frappier, J. Nat. Prod., 2003, 66, 1447 CrossRef CAS PubMed.
- D. Paola, B. Ivana, R. Carla, R. Marina, D. Paola, S. Luca and M. Luigi, Flavour Fragrance J, 2010, 26, 34 Search PubMed.
- J. B. Harborne, C. A. Williams and K. L. Wilson, Phytochemistry, 1982, 21, 2491–2507 CrossRef CAS.
- K. Arraki, P. Totoson, A. Decendit, A. Badoc, A. Zedet, J. Jolibois, M. Pudlo, C. Demougeot and C. Girard-Thernier, J. Nat. Prod., 2017, 80, 2432–2438 CrossRef CAS PubMed.
- P. Liu, L. Liu, Y. P. Tang, J. A. Duan and N. Y. Yang, Chin. Chem. Lett., 2010, 21, 606–609 CrossRef CAS.
- H. A. Quayyum, A. U. Mallik, D. M. Leach and C. Gottardo, J. Chem. Ecol., 2000, 26, 2221–2231 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |