Open Access Article
Open Access ArticleNative fluorescence of tear fluid as a tool for diagnostics of glaucoma
Kristína Krajčíková a,
Miriama Skirkováb,
Monika Moravskáb,
Anna Birkováa and
Vladimíra Tomečková
a,
Miriama Skirkováb,
Monika Moravskáb,
Anna Birkováa and
Vladimíra Tomečková *a
*a
aDepartment of Medical and Clinical Biochemistry, Faculty of Medicine, Pavol Jozef Šafárik University in Košice, Trieda SNP 1, Košice, 040 11, Slovakia. E-mail: vladimira.tomeckova@upjs.sk
bDepartment of Ophthalmology, University Hospital Louis Pasteur, Pavol Jozef Šafárik University in Košice, Trieda SNP 1, Košice, 040 11, Slovakia
First published on 15th March 2021
Abstract
Glaucoma is one of the leading causes of irreversible vision loss worldwide. There is an enormous need for the detection of its early stages and also speeding up and simplifying regular examinations. Among the new diagnostic approaches, the use of tear fluid has been intensively investigated in recent years. For this purpose, we analyzed the tear fluid of patients with glaucoma and related diseases. To sensitively capture the subtle ocular abnormalities related to glaucoma and manifested in tear fluid, we used synchronous fluorescence spectroscopy. In this observational case–control study, we detected significant differences in the intensity of tear fluid fluorescence located at λex/Δλ = 280/70 nm between the groups of primary open-angle glaucoma (p < 0.01), suspected glaucoma (p < 0.0001), and ocular hypertension (p < 0.05), when compared to the healthy control group. The signal was not significantly higher in women than in men (p = 0.05), and no correlation was found with age (r = −0.05, p > 0.05), nor treatment (p > 0.05). Taken together, tear fluid fluorescence could serve as a discriminative parameter between patients with glaucoma, related diseases, and healthy control subjects and might contribute to the improvement of diagnostics of these diseases.
Introduction
Glaucoma is a group of eye diseases with multifactorial etiology that cause progressive damage to the optic nerve. Intraocular pressure is considered today as a major risk factor for glaucoma, together with other factors such as racial ancestry, family history, high myopia, and age. If left untreated, most types of glaucoma progress towards gradually worsening visual damage and may lead to blindness. Vision loss is irreversible. The World Health Organization states that 6.9 million people globally are blind due to glaucoma and it is estimated that glaucoma prevalence will rise due to the aging of the world's population to 11.8 million by 2040.1 With a rising number of patients, speeding up and simplifying the routine ophthalmological examinations of glaucomatous patients is an absolute necessity.In the last two decades, tear fluid has started to gain attention as a sampling biological material providing the advantages such as simple and non-invasive collection, and the content that is rich in the candidates on biomarkers that could be used to diagnose ocular diseases.2 These diagnostically relevant compounds are various types of proteins, lipids, nucleic acids, vitamins, hormones, and other substances.3–7
In glaucoma, the changes of tear fluid composition have been described in several studies yet,8–10 and they might be revealed by the sensitive spectroscopic methods. These methods have been constantly and consistently used for the study of tear fluid in various diseases and were helpful in better understanding the disease molecular mechanisms.11–13 Moreover, they have the potential not only to make the regular examinations faster and easier but might capture the initial pathological changes in glaucomatous patients.
For this purpose, we studied tear fluid native fluorescence changes of the subjects with glaucoma and related conditions using synchronous fluorescence spectroscopy (SFS). SFS is 3-D fluorescence spectroscopy that measures a native endogenous fluorescence of biological samples after irradiation of a sample with a suitable excitation wavelength. The endogenous fluorophores include proteins, NAD(P)H, flavins, lipofuscins, and related lipopigments, retinoids, porphyrins, bilirubin, and fluorescent fatty acids.14 In the spectrum of tear fluid, three amino acid residues of proteins with aromatic side chain are present, where π → π* electron transitions occur when excited with light: tryptophan (Trp), tyrosine (Tyr), and phenylalanine (Phe). These amino acids in phosphate buffer solution show strong fluorescence peaks located at λex/Δλ ≈ 230/120 nm, 280/70 nm for Trp, 200/100 nm, 220/80 nm, 275/30 nm for Tyr, and 210/70 nm, 260/20 nm for Phe.15 The analysis of these tear fluid spectral signals could suit the need for innovative monitoring of glaucoma patients.
Experimental
Study design
This was an observational case–control pilot study.Subjects
All participants were informed about their rights, the study objectives, and the risks after which they signed informed consent and were treated following the Declaration of Helsinki. The study was approved by Pavol Jozef Šafárik University in Košice ethical committee. The participants were randomly chosen, and the samples were taken at the Glaucoma Clinic of Department of Ophthalmology, University Hospital Louis Pasteur and Pavol Jozef Šafárik University in Košice by the trained specialist. The diagnosis, management, and treatment of glaucoma patients were based on the guidelines from the European Glaucoma Society (European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th). All patients who underwent periodical examination were included in the study and the tear fluid was collected before the examinations. The patients were excluded from the study if they produced the amount of tear fluid insufficient for the analysis. This study had a case–control design. The case samples consisted of 3 groups: primary open-angle glaucoma (POAG) (n = 15), suspected glaucoma (SUSP GLAU) (n = 4), and ocular hypertension (OHT) (n = 8). The control samples (CTR) (n = 8) consisted of volunteers from Pavol Jozef Šafárik University in Košice with no ophthalmological findings.Sample collection
The samples were continuously taken at 9.00–12.00 to avoid the diurnal rhythm changes by glass microcapillary (Sigma-Aldrich, Steinheim, Germany) with attention not to touch the cornea. The samples were transferred on ice and stored at −80 °C until analysis.Synchronous fluorescence spectroscopy
The synchronous fluorescence spectra of tear fluid were carried out in a quartz cuvette using a spectrofluorimeter (PerkinElmer Luminescence Spectrophotometer LS 55, Buckinghamshire, United Kingdom). The 10 simple synchronous spectra were measured from Δλ = 10 nm in the wavelength range λex = 200–600 nm. The distance between the simple spectra was Δ = 20 nm, the scan rate was 1200 nm min−1 with the excitation and emission slits 5 nm. The results were processed in WinLab Software.Statistical evaluation
The data were analyzed using Microsoft Excel software and expressed as mean ± SD. Demographic and clinical characteristics of probands were also expressed in percentage. Equality of variances testing was performed by F-test. An unpaired student's T-test was used to statistically describe significant variations between groups, assuming equal or unequal variations based on the F-test. The results of the correlation analysis are expressed as Pearson's r. p < 0.05 was considered statistically significant.Results and discussion
Demographic and clinical data
Demographic and clinical data for the study subjects are shown in Table 1.| Description | POAG, n = 15 | SUSP GLAU, n = 4 | OHT, n = 8 | CTR, n = 8 |
|---|---|---|---|---|
| a Data are shown as a percentage of the subjects per group. Age is shown as mean (±SD). POAG – primary open-angled glaucoma, SUS GLAU – suspected glaucoma, OHT – ocular hypertension, CTR – controls. | ||||
| Sex | ||||
| Women | 73% (11) | 75% (3) | 50% (4) | 38% (3) |
| Men | 27% (4) | 25% (1) | 50% (4) | 62% (5) |
| Age | ||||
| Mean | 57 (±13) | 42 (±11) | 38 (±19) | 38 (±11) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Treatment | ||||
| Prostamides | 0% (0) | 0% (0) | 25% (2) | — |
| β-Blockers | 67% (10) | 0% (0) | 25% (2) | — |
| Topical carbonic anhydrase inhibitors | 40% (6) | 0% (0) | 13% (1) | — |
| Prostaglandin derivates | 60% (9) | 0% (0) | 38% (3) | — |
| Alpha-2 selective adrenergic agonists | 27% (4) | 0% (0) | 0% (0) | — |
Characterization of tear fluid 3-D fluorescence spectrum
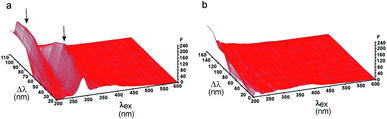
In our study, we firstly characterized a 3-D fluorescence spectrum of tear fluid measuring control tear fluid (Fig. 1a). Two well-defined peaks were detected at the excitation wavelength λex ≈ 230 and 280 nm resulting from the presence of Tyr, Trp residues in proteins, and some Tyr and Trp derivates. Furthermore, the light scattering contributes to the spectral signal at λex/Δλ ≈ 200/80–160 nm that is present also in a normal saline solution without fluorophores (Fig. 1b). These findings are in concordance with the results of Glinská et al.11Fluorescence intensity differences between patients and healthy controls
We next analyzed the synchronous fluorescence spectral changes of tear fluid and their relation to glaucoma pathology. For more detailed analysis, we examined the main fluorescence peak and chose the one simple synchronous fluorescence spectrum at Δλ = 70 nm assessing the Trp-related signal at λex = 280 nm. Based on the average values, tear fluid fluorescence of POAG (p < 0.01), OHT (p < 0.05), and SUSP GLAU (p < 0.0001) significantly rose in comparison with CTR (Table 2 and Fig. 2).| Diagnosis | Fluorescence intensity mean | Standard deviation | Significance vs. CTR |
|---|---|---|---|
| a CTR – controls, POAG – primary open-angled glaucoma, OHT – ocular hypertension, SUS GLAU – suspected glaucoma. | |||
| CTR | 219.84 | 74.05 | — |
| POAG | 312.06 | 81.89 | 0.00742 |
| OHT | 312.53 | 68.01 | 0.01034 |
| SUS GLAU | 457.95 | 47.63 | 0.00009 |
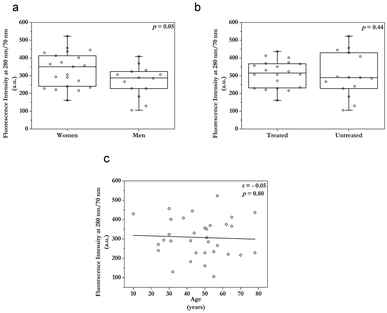
Fluorescence intensity dependence on demographic characteristics
When it comes to demographic characteristics, tear fluid fluorescence intensity at λex/Δλ = 280/70 nm was not significantly higher in women (p = 0.05, Fig. 3a) than in men. Besides, regression analysis showed no correlation of fluorescence intensity with age (Fig. 3b). Similarly, the fluorescent signal did not show dependence on the treatment (p > 0.05, Fig. 3c).Discussion
Fluorescence analyses are widely used in probing cell events, diagnostics, or pharmacokinetics.17–19 Fluorescence can be intrinsic – in the case when an analyte provides a fluorescent signal and extrinsic – when an analyte is probed by the reaction with a specific fluorophore. In our study, we used the native fluorescence of tear fluid that consists of several intrinsic fluorophores.The most prominent peak of tear fluid fluorophores was located at λex/Δλ ≈ 280/70 nm in SFS 3-D spectra. This signal is caused by Tyr, Trp, and some of their derivates. When they are bounded in a protein, the polarity of their microenvironment alters and Trp is highly sensitive for such changes resulting in the spectral shifts16 which were detected also in the tear fluid spectrum. It should be noted that even though Tyr and Trp have very similar quantum yields, Förster resonance energy transfer occurs from Tyr to Trp when they are in close vicinity causing a lower contribution of Tyr to protein fluorescence in comparison with Trp. Furthermore, some of the Tyr and Trp derivates provide signal located similarly to Trp, e.g. epinephrine, norepinephrine, dopamine (all three located at λex/Δλ ≈ 280/50 nm), or serotonin (λex/Δλ ≈ 270/70 nm),20 all present in tear fluid.21,22 These compounds altogether contribute to the shape and fluorescence intensity of the main peak in the tear fluid 3-D fluorescence spectrum.
We showed an increase in the fluorescence intensity of the peak at λex/Δλ ≈ 280/70 nm in patients when compared to control subjects that might be caused by the concentration of Tyr, Trp, and their derivates, and the changes of Trp environment. We assume that this signal was not proportional to the concentration of mentioned fluorophores for two reasons: (i) the strongest contributor to the signal is Trp23 which is predominantly bound in proteins and has been directly used for fluorescent protein concentration assays.24 However, the total protein concentration is not likely to differ between glaucoma patients and healthy controls.25 (ii) Trp is highly sensitive to the protein conformation, the quenchers, and other factors related to its environment.26 Furthermore, the studies which demonstrated qualitative protein differences between glaucoma patients and healthy controls27–29 strengthen our assumption that the increase is rather related to the tear fluid protein composition providing diagnostically relevant information.
Another key finding is the highest increase in fluorescent intensity in the SUSP GLAU group. Several studies are showing the sharp increase of a specific biomarker in the early stages of neurodegenerative diseases revealing the acute pathological alterations at their very beginning.30,31 In the light of our finding that indicates SFS as a tool for detection of the initial changes in the optic nerve, prospective studies monitoring the kinetics of tear fluid fluorescence intensity of these patients are necessary.
To date, although many studies demonstrated the diagnostic potential of SFS,32–35 tear fluid was analyzed by this method only in two studies. Glinská et al.11 studied tear fluid of diabetic patients with different treatments and length of the disease. They analyzed the shifts and fluorescence intensity of synchronous fluorescent spectral signal located at λex/Δλ ≈ 280/50 nm between patients with different treatment and length of the disease. These changes were attributed to protein changes and further confirmed by other spectroscopic methods. On the other hand, Azharuddin et al.36 studied tear fluid of aqueous-deficient dry eye analyzing the simple synchronous fluorescent spectral signal located at λex/Δλ ≈ 280/80 nm. They assigned the signal to Trp that decreased in dry eye patients due to lesser Trp residues' accessibility resulting from the formation of aggregates. These data support our theoretical postulations that by analyzing the Trp signal at λex/Δλ ≈ 280/70 nm we can monitor the tear fluid protein changes.
Apart from this, tear fluid contains sex hormones37,38 from which estrogens possess the native fluorescence located similarly to Trp in aqueous solutions.39 We speculated whether estrogens contributed to the overall λex/Δλ = 280/70 nm signal, however, such a hypothesis has to be further tested also because the median age was 50 for the women group.
Limitations of this study should be noted. Firstly, the study contained a small sample size for the groups. Secondly, there was an uneven distribution of women and men in the groups. Thirdly, there was an uneven age distribution among the groups. We detected no statistically significant dependence of the fluorescence intensity with sex or age, but follow-up studies are necessary to confirm our findings. Nevertheless, our data provide a valuable base for future evaluations, in which the bigger data set and better-matched groups should be tested.
Conclusion
Monitoring of patients with glaucoma and related disorders is a complete necessity in ophthalmology. Growing demand for rapid and precise screening tests has brought focus towards the use of tear fluid. Our preliminary findings present the synchronous fluorescence spectral analysis of human tear fluid as a potential method for discrimination between patients with POAG, OHT, SUSP GLAU, and healthy subjects. Equally important is the prognostic potential of SFS to detect initial pathological changes in SUSP GLAU. The advantage of synchronous fluorescence spectroscopy is its speed, simplicity, environmental friendliness (without the need to use additional reagents), and economy. It could become a valuable clinical tool that could be further developed for screening testing or might help to speed up and simplify the regular ophthalmological examinations. Eventually, our study highlights the use of tear fluid for clinical diagnostics in ophthalmology.Author contributions
K. K – conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing – original draft. M. S. – resources, writing – review & editing. M. M. – resources, writing – review & editing. A. B. – formal analysis, investigation, methodology, validation, writing – review & editing. V. T. – conceptualization, funding, methodology, project administration, supervision, writing – review & editing.Conflicts of interest
There is no conflicting interest for any author.Acknowledgements
This work was supported by The Ministry of Education, Science, Research and Sport of the Slovak Republic under Grant VEGA 1/0333/20.References
- Y.-C. Tham, X. Li, T. Y. Wong, H. A. Quigley, T. Aung and C.-Y. Cheng, Ophthalmology, 2014, 121, 2081–2090 CrossRef PubMed
.
- S. Hagan, E. Martin and A. Enríquez-de-Salamanca, EPMA J., 2016, 7, 15 CrossRef PubMed
.
- C. Aass, I. Norheim, E. F. Eriksen, P. M. Thorsby and M. Pepaj, Anal. Biochem., 2015, 480, 1–5 CrossRef CAS PubMed
.
- S. M. Lam, L. Tong, B. Reux, X. Duan, A. Petznick, S. S. Yong, C. B. S. Khee, M. J. Lear, M. R. Wenk and G. Shui, J. Lipid Res., 2014, 55, 299–306 CrossRef CAS PubMed
.
- J. A. Weber, D. H. Baxter, S. Zhang, D. Y. Huang, K. H. Huang, M. J. Lee, D. J. Galas and K. Wang, Clin. Chem., 2010, 56, 1733–1741 CrossRef CAS PubMed
.
- X. Lu, R. A. Elizondo, R. Nielsen, E. I. Christensen, J. Yang, B. D. Hammock and M. A. Watsky, Invest. Ophthalmol. Visual Sci., 2015, 56, 5880–5887 CrossRef CAS PubMed
.
- R. Susarla, L. Liu, E. A. Walker, I. J. Bujalska, J. Alsalem, G. P. Williams, S. Sreekantam, A. E. Taylor, M. Tallouzi, H. S. Southworth, P. I. Murray, G. R. Wallace and S. Rauz, PLoS One, 2017, 9, e94913 CrossRef PubMed
.
- B. C. Demirdöğen, C. K. Akçin, G. Özge and T. Mumcuoğlu, Exp. Eye Res., 2019, 189, 107837 CrossRef PubMed
.
- Y. Y. Openkova, E. N. Korobeiynikova, V. S. Rykin and G. A. Vinkova, Klin. Lab. Diagn., 2013, 5, 8–11 Search PubMed
.
- J. B. Roedl, S. Bleich, U. Schlötzer-Schrehardt, N. von Ahsen, J. Kornhuber, G. O. H. Naumann, F. E. Kruse and A. G. M. Jünemann, Ophthalmic Res., 2008, 40, 249–256 CrossRef CAS PubMed
.
- G. Glinská, K. Krajčíková, K. Zakutanská, O. Shylenko, D. Kondrakhova, N. Tomašovičová, V. Komanický, J. Mašlanková and V. Tomečková, RSC Adv., 2019, 9, 18050–18059 RSC
.
- Z. Hetman and D. Borchman, Biochem. Biophys. Rep., 2020, 21, 100732 Search PubMed
.
- J. Ruiz-Ederra, M. Levin and A. Verkman, Invest. Ophthalmol. Visual Sci., 2009, 50, 2132–2138 CrossRef PubMed
.
- A. C. Croce, A. Ferrigno, G. Bottiroli and M. Vairetti, Liver Int., 2018, 38, 1160–1174 CrossRef CAS PubMed
.
- H. Yang, X. Xiao, X. Zhao and Y. Wu, 5th International Conference on Advanced Design and Manufacturing Engineering, Zhengzhou, 2016 Search PubMed
.
- A. Bortolotti, Y. H. Wong, S. S. Korsholm, N. H. B. Bahring, S. Bobone, S. Tayyab, M. van de Weert and L. Stella, RSC Adv., 2016, 6, 112870 RSC
.
- X. Han, R. Wang, X. Song, F. Yu, C. Lv and L. Chen, Biomaterials, 2018, 156, 134–146 CrossRef CAS PubMed
.
- A. Sieroń, K. Sieroń-Stołtny, A. Kawczyk-Krupka, W. Latos, S. Kwiatek, D. Straszak and A. M. Bugaj, OncoTargets Ther., 2013, 6, 977–982 Search PubMed
.
- M. Gao, F. Yu, C. Lv, J. Choo and L. Chen, Chem. Soc. Rev., 2017, 46, 2237–3371 RSC
.
- A. Birková, Diagnostická aplikácia fluorescenčných spektrálnych matríc biologických tekutín, Pavol Jozef Šafárik University in Košice, 2013 Search PubMed
.
- A. Kim, M. R. Nodel, T. A. Pavlenko, N. B. Chesnokova, N. N. Yakhno and M. V. Ugrumov, Acta Naturae, 2019, 11, 99–103 CrossRef CAS PubMed
.
- P. Chhadva, T. Lee, C. D. Sarantopoulos, A. S. Hackam, A. L. McClellan, E. R. Felix, R. C. Levitt and A. Galor, Ophthalmology, 2015, 122, 1675–1680 CrossRef PubMed
.
- A. Ghisaidoobe and S. Chung, Int. J. Mol. Sci., 2014, 15, 22518–22538 CrossRef CAS PubMed
.
- J. Wisniewski and F. Gaugaz, Anal. Chem., 2015, 87, 4110–4116 CrossRef CAS PubMed
.
- A. Naik, S. Shrivastava, N. Abidi, R. Yadav, P. Shah and Y. Gala, J. Clin. Ophthalmol. Res., 2018, 6, 66–70 Search PubMed
.
- J. R. Lakowicz, Protein Fluorescence, in Principles of Fluorescence Spectroscopy, Springer, Boston, 3rd edn, 2006, pp. 529–575 Search PubMed
.
- D. Gupta, J. C. Wen, J. L. Huebner, S. Stinnett, V. B. Kraus, H. C. Tseng and M. Walsh, Clin. Ophthalmol., 2017, 11, 411–416 CrossRef CAS PubMed
.
- A. Ghaffariyeh, N. Honarpisheh, Y. Shakiba, S. Puyan, T. Chamacham, F. Zahedi and M. Zarrineghbal, Optometry, 2009, 80, 635–638 CrossRef PubMed
.
- D. Pieragostino, L. Agnifili, V. Fasanella, S. D'Aguanno, R. Mastropasqua, C. Di Ilio, P. Sacchetta, A. Urbani and P. Del Boccio, Mol. BioSyst., 2013, 9, 1108–1116 RSC
.
- M. Suárez-Calvet, G. Kleinberger, M. Á. A. Caballero, M. Brendel, A. Rominger, D. Alcolea, J. Fortea, A. Lleó, R. Blesa, J. D. Gispert, R. Sánchez-Valle, A. Antonell, L. Rami, J. L. Molinuevo, F. Brosseron, A. Traschütz, M. T. Heneka, H. Struyfs, S. Engelborghs, K. Sleegers, C. Van Broeckhoven, H. Zetterberg, B. Nellgård, K. Blennow, A. Crispin, M. Ewers and C. Haass, EMBO Mol. Med., 2016, 8, 466–476 CrossRef PubMed
.
- K. Sheinerman, V. G. Tsivinsky, F. Crawford, M. J. Mullan, L. Abdullah and S. R. Umansky, Aging, 2012, 4, 590–605 CrossRef CAS PubMed
.
- A. Birková, A. Gresova, Z. Steffekova, V. Kraus, A. Ostro, R. Toth and M. Marekova, Neoplasma, 2014, 61, 724–731 CrossRef PubMed
.
- I. Spakova, M. Ferencakova, M. Rabajdova, V. Tomeckova, V. Komanicky and M. Marekova, Curr. Metabolomics, 2018, 6, 2–9 CAS
.
- Z. Guľašová, V. Tomečková, M. Bilecová-Rabajdová, B. Veliká, P. Artemiou, V. Komanický and M. Mareková, Am. J. Med. Biol. Res., 2015, 3, 128–132 CrossRef
.
- K. Dubayová, J. Kušnír and L. Podracká, J. Biochem. Biophys. Methods, 2003, 55, 111–119 CrossRef
.
- M. Azharuddin, J. Khandelwal, H. Datta, A. Kr. Dasgupta and S. O. Raja, Biochem. Mol. Biol. Educ., 2015, 56, 1423–1429 CAS
.
- D. Pieragostino, L. Agnifili, I. Cicalini, R. Calienno, M. Zucchelli, L. Mastropasqua, P. Sacchetta, P. Del Boccio and C. RossI, Int. J. Mol. Sci., 2017, 18, 1349 CrossRef PubMed
.
- R. Bian, J. Lu and W. Zhang, Chin. Ophthalmic Res., 2009, 27, 1031–1034 Search PubMed
.
- M. V. de Liz, B. do Amaral, S. Stets, N. Nagata and P. Peralta-Zamora, J. Braz. Chem. Soc., 2017, 28, 1453–1460 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2021 |