Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ring opening polymerization of D,L-lactide and ε-caprolactone catalysed by (pyrazol-1-yl)copper(II) carboxylate complexes†
Divambal Appavooa,
Lara C. Spencerb,
Ilia A. Guzeiab,
Carlos J. Gómez-García c,
Juanita L. van Wyka and
James Darkwa
c,
Juanita L. van Wyka and
James Darkwa *a
*a
aDepartment of Chemical Sciences, University of Johannesburg, P.O. Box X524, Auckland Park, 2006, South Africa. E-mail: jdarkwa@gmail.com
bDepartment of Chemistry, University of Wisconsin-Madison, Madison, WI 53706, USA
cDepartment of Inorganic Chemistry and ICMol, C/ Catedrático José Beltrán, 2. University of Valencia, 46980 Paterna, Valencia, Spain
First published on 12th April 2021
Abstract
1,2-Bis{(3,5-dimethylpyrazol-1-yl)methyl}benzene (L) reacts with [Cu(OAc)2] and C6H5COOH, 4-OH-C6H4COOH, 2-Cl-C6H4COOH and (3,5-NO2)2-C6H3COOH to afford the copper complexes [Cu2(C6H5COO)4(L)2] (1), [Cu2(4-OH-C6H4COO)4(L)2] (2), [Cu2(2-Cl-C6H4COO)4(L)2]n (3) and [Cu{(3,5-NO2)2-C6H3COO}2L]n (4) which are characterised by IR, mass spectrometry, elemental analyses, and X-ray crystallography. The structural data revealed two geometries that are adopted by the complexes: (i) paddle wheel in 1, 2·7H2O, 3 and (ii) regular chains in 3 and 4. Magnetic studies show strong antiferromagnetic couplings in the paddle wheel complexes and a weak antiferromagnetic coupling in the monometallic chain one. Catalysis studies performed with these complexes (1–4) showed that they initiate ring opening polymerization (ROP) of ε-caprolactone (ε-CL) under solvent-free conditions and D,L-lactide in toluene at elevated temperatures. Polycaprolactone (PCL) and poly(D,L-lactide) (PLA) obtained from the polymerization reactions are of low molecular weights (858 for PCL and 602 Da for PLA for initiator 1) and polydispersity indices (typically 2.16 for PCL and 1.64 for PLA with 1 as the initiator). End group analysis of the polymers, determined by MALDI-ToF MS, indicates that the polymers have benzoate, hydroxyl, methoxy and cyclic end groups.
Introduction
Aliphatic polyesters continue to receive much interest due to their unique biodegradability, biocompatibility and permeable properties that make them very useful in the medical, pharmaceutical and environmental fields.1–5 The most convenient method for preparing these polyesters is via ring-opening polymerization (ROP) of the respective cyclic esters. The key to achieving controlled ROP, in terms of molecular weight distribution and stereochemistry, lies in the performance of the initiator or catalyst. Different types of catalysts have been studied for this purpose, including organometallic compounds, organocatalysts6,7 as well as enzymes.8 Some common initiators or catalysts reported are [Al(OiPr)3]9,10 and [Sn(Oct)2].1,11 However, due to drawbacks associated with these aluminium and tin catalysts, such as low conversions, poor selectivity and backbiting reactions, the search for better catalysts continues.Several metal-based catalysts have been studied, ranging from main group elements as Ca,12 Al,13 Li,14–16 Mg,17–20 Sn21 to transition metals such as Fe,22–24 Zn,25 Zr,26–28 and lanthanoids.29 One metal of growing interest is copper, which although has not been investigated as much as metals like zinc, shows interesting properties such as biocompatibility and good electron transfer ability that make Cu-based complexes good candidates for ROP catalysis.30–32
The catalytic activity of organometallic complexes is largely determined by the ligands attached to the metal centre. For instance, backbiting reactions can be eliminated by using a suitable sterically bulky ligand that provides some amount of steric barrier around the metal centre. Several ligands have been explored to obtain the optimum catalytic performance of metal complexes; including amino-phenolate,33–35 salicylaldimine,32 formamide,36,37 pyrazolyl,30,38–41 and benzoimidazolyl.42–44 Pyrazole and its derivatives constitute an important group of N-donor ligands that have been used because of their attractive coordination chemistry.45 The structural flexibility of pyrazole and pyrazolyl ligands constitute an interesting advantage. Furthermore, the denticity of pyrazolyl ligands can be greater or equal to one, and the steric hindrance of a pyrazolyl ligand can be controlled by the appropriate choice of groups on the pyrazole ring.40,46–48
We previously reported the ROP of ε-caprolactone (ε-CL) and D,L-lactide using bis(3,5-dimethylpyrazole)–Zn(II) and Cu(II) complexes as initiators.41 The current report further explores the initiator systems that consist of the bidentate ligand, 1,2-bis((3,5-dimethylpyrazol-1-yl)methyl)-benzene (L) coordinated to copper through the pyrazolyl nitrogen atoms, and ancillary ligands derived from a range of benzoic acids, also coordinated to the copper through carboxylate oxygen atoms. Such copper complexes usually adopt a paddle-wheel arrangement, with four carboxylates in equatorial positions that bridge two copper centres and the N-donor ligand occupying the axial positions. Depending on the carboxylate, the coordination geometry around the copper centre can vary, hence impacting the magnetic and electronic properties of the complex. We describe herein the coordination chemistry of four pyrazolyl copper carboxylate complexes formulated as [Cu2(C6H5COO)4(L)2] (1), [Cu2(4-OH-C6H4COO)4(L)2] (2), [Cu2(2-Cl-C6H4COO)4(L)2]n (3) and [Cu{(3,5-NO2)2-C6H4COO}2L]n (4). Three of them show the classical paddle-wheel structure (1–3) and the fourth one is monometallic but polymeric with monodentate L linkages (4). We report their magnetic properties and their structure–activity relationship in initiating the ROP of D,L-lactide and ε-caprolactone.
Results and discussion
Synthesis and spectral characterization of complexes
Complexes 1–4 were synthesized from one-pot reactions of copper(II) acetate [Cu(OAc)2] with different benzoic acids, namely (C6H5COOH (benzoic acid), 4-OH-C6H4COOH (4-hydroxybenzoic acid), (3,5-NO2)2-C6H3COOH (3,5-dinitrobenzoic acid) and 2-Cl-C6H4COOH (2-chlorobenzoic acid) and 1,2-bis((3,5-dimethylpyrazol-1-yl)methyl)benzene (L) (Scheme 1). The reactions proceeded via an initial substitution of the acetate in the copper starting complex by the benzoate group of the respective benzoic acid, accompanied by the release of acetic acid. The appropriate copper benzoate then reacted with L and produced the corresponding pyrazolyl copper benzoate complexes (1–4) in moderate yields (57–78%). Complexes 1–3 were obtained as green solids, while complex 4 was isolated as a purple solid.Complexes 1–4 were characterized by IR spectroscopy, mass spectroscopy and elemental analysis. Formation of the complexes was supported by IR data that showed shifts in the νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band when compared to the corresponding unbound benzoic acid. For example, for complex 3, the IR spectrum shows the carbonyl peak at 1631 cm−1, a significant shift from 1591 cm−1 in the free benzoic acid. This shift is accompanied by the disappearance of the νO–H band of the benzoic acid in the region of 2900 cm−1, further supporting complexation of the benzoate to the metal.
O band when compared to the corresponding unbound benzoic acid. For example, for complex 3, the IR spectrum shows the carbonyl peak at 1631 cm−1, a significant shift from 1591 cm−1 in the free benzoic acid. This shift is accompanied by the disappearance of the νO–H band of the benzoic acid in the region of 2900 cm−1, further supporting complexation of the benzoate to the metal.
Mass spectra data provide additional evidence of the formation of the complexes. For instance, 2 showed a peak at m/z = 494 corresponding to the fragment of the molecular ion after loss of one benzoate group. Similar mass spectra were obtained for the other complexes, with peaks associated with the parent compound without one benzoate. The purities of the complexes were determined by elemental analysis and good correlations between the calculated and found values were obtained.
X-ray structures of complexes
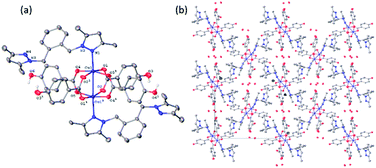
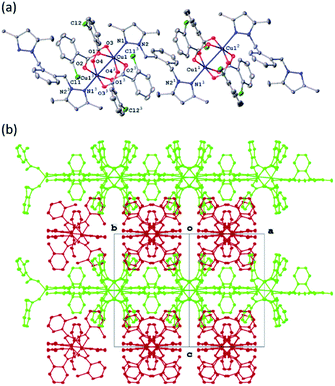
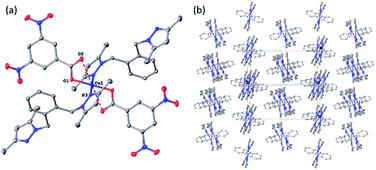
All four copper complexes were characterized by single-crystal X-ray diffraction; their molecular geometries are presented in Fig. 1–4 and Table S1.† The four complexes show structural diversity in the solid state: compounds 1–3 are dimeric paddle-wheel complexes49–52 with weak cuprophilic interactions between octahedral copper centres. Compound 1 crystallizes with discreet dimeric complexes (Fig. 1). Complex 2 co-crystallizes with ∼7 water molecules per dimer (Fig. 2a) and all entities form hydrogen bonds resulting in a three-dimensional hydrogen-bonded framework (Fig. 2b). Complex 3 is a one-dimensional pyrazole-carboxylate coordination polymer connecting dimeric Cu complexes (Fig. 3). Compound 4 is also polymeric (Fig. 4), however the metal core is mononuclear and the Cu centre is square bipyramidal. All bidentate pyrazole-containing linkers have one pyrazole coordinated to a Cu centre whereas the unsubstituted N atom of the pyrazole unit on the other end of the ligand can either form no bonds (complex 1), form a hydrogen bond to a solvent molecule (complex 2), or participate in a polymeric chain formation by binding to a Cu atom from an adjacent complex (compounds 3 and 4). The dinuclear cores in 1–3 have similar metric parameters: the Cu⋯Cu separations are 2.6845(15), 2.6955(5), and 2.6997(11) Å, respectively; the Cu–O distances in 1–3 average 1.969(11), 1.964(9), and 1.965(3) Å, whereas the Cu–N distances are longer and measure 2.1919(16), 2.1917(16), and 2.210(4) Å respectively.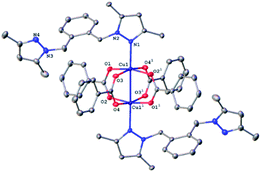 | ||
| Fig. 1 A molecular drawing of complex 1 shown with 50% probability ellipsoids. All H atoms are omitted. Symmetry code: (1) −x, −y, −z. | ||
Dinuclear complex 1 resides on a crystallographic inversion centre, thus only one half of it is symmetry-independent (Fig. 1). The Cu atom coordination environment is octahedral: four oxygen atoms from four bridging carboxylates form the equatorial plane, a pyrazole N atom occupies one apical position, and the other Cu atom resides at the other. The Cu atom is displaced from the equatorial plane toward the nitrogen by 0.2201(5) Å. The Cu⋯Cu separation in the complex (2.6845(15) Å) corresponds to a weak cuprophilic interaction and falls within the distance range observed for similar complexes. A Cambridge Structural Database (CSD) search for relevant structures returned the range of 2.565–2.707 Å for such interactions.
Similarly, to 1, the dinuclear compound 2·7H2O crystallizes with discrete complexes containing four bridging carboxylates, and two terminal pyrazoles. But unlike compound 1, there are ca. seven molecules of solvent water per dinuclear complex 2. These seven water molecules are distributed over eight crystallographic positions. Complex 2 resides on a crystallographic inversion centre, thus only one half of it is symmetry independent. The coordination environment of the Cu centre consists of four oxygens from four carboxylates in the equatorial plane and a N atom and the other Cu atom in the axial positions (Fig. 2a). The Cu atom is displaced by 0.2316(8) Å from the equatorial plane toward the nitrogen. The Cu⋯Cu distance of 2.6955(4) Å is statistically significantly longer than the corresponding distance in 1, and is also at the higher end of the typical range for such interactions in these compounds. The pyrazole linker has two N atoms, one from each pyrazole, that can ligate a metal centre. One of these nitrogen coordinates to the metal centre whereas the other forms a strong hydrogen bond of the type O–H⋯N (O⋯N separation = 2.663(2) Å, the O–H⋯N angle = 178(3)°) with a hydroxyl group from a neighbouring complex. These hydroxyl–pyrazole hydrogen-bonding interactions connect the complexes into two-dimensional networks parallel to the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) plane. These networks stack in such a way that there are solvent-accessible voids in the crystallographic [100] direction. These voids are populated by the solvent water molecules that form hydrogen bonds among themselves and with the complexes, thus all entities are hydrogen-bonded into a three-dimensional framework (Fig. 2b).
01) plane. These networks stack in such a way that there are solvent-accessible voids in the crystallographic [100] direction. These voids are populated by the solvent water molecules that form hydrogen bonds among themselves and with the complexes, thus all entities are hydrogen-bonded into a three-dimensional framework (Fig. 2b).
Complex 3 crystallizes as a merohedral twin with a 0.463(2) second twin component contribution. There is also extensive positional disorder in each ligand with an 85.04(15)% of the major component contribution. In the following discussion the major disorder component only will be considered. The pyrazolate-carboxylate coordination polymer 3 contains dimeric Cu units similar to those in 1 and 2. The dinuclear core in 3 resides on a crystallographic inversion centre and is similar in its geometry to the cores in 1 and 2·7H2O. Only one half of the complex is symmetry-independent. The coordination sphere of each Cu atom consists of four equatorial oxygen atoms from four carboxylates with a pyrazole N atom and second Cu atom in the apical positions (Fig. 3a). The Cu atom is displaced from the equatorial plane toward the nitrogen by 0.2356(17) Å. In contrast to 1 and 2, complex 3 is polymeric. The bidentate linker L bridges adjacent dinuclear Cu units by coordinating with one unsubstituted pyrazole.
N atom to one Cu atom and with the other unsubstituted pyrazole nitrogen to another Cu atom. These one-dimensional polymeric chains propagate in the crystallographically equivalent [110] and [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 10] directions (Fig. 3b). The Cu⋯Cu separation of 2.6997(11) Å is the longest among 1–3 and the difference is statistically significant. There are 13 coordination polymers of this type reported to the CSD with the Cu⋯Cu cuprophilic interactions ranging between 2.565–2.691 Å thus the separation in 3 slightly exceeds this range.
10] directions (Fig. 3b). The Cu⋯Cu separation of 2.6997(11) Å is the longest among 1–3 and the difference is statistically significant. There are 13 coordination polymers of this type reported to the CSD with the Cu⋯Cu cuprophilic interactions ranging between 2.565–2.691 Å thus the separation in 3 slightly exceeds this range.
In the pyrazole-carboxylate coordination polymer 4 there are two symmetry-independent Cu centres (Fig. 4a) that are bridged by the ligand L. The one-dimensional polymer propagates in the crystallographic direction [101], Fig. 4b. Each Cu atom resides on a crystallographic inversion centre, thus there is only one symmetry-independent pyrazole and benzoate coordinated to it; their symmetry-related mates complete the coordination environment about each Cu atom. In contrast to metal centres in 1–3, the Cu atoms in 4 possess distorted trans-square-planar environments comprised of two pyrazole nitrogens and two carboxylate oxygen atoms from two benzoate ligands. The two remaining carboxylate oxygen atoms from each benzoate form weak contacts with the Cu centres to complete their square bipyramidal coordination environment. For the rest of this paragraph we will list metric parameters averaged over the two metal centres. The average distances in the complexes are Cu–O (eq) 1.95(2) Å, Cu–O (axial) 2.65(10), Cu–N 1.9947(13) Å; the Cu–O distances between the two Cu centres are dissimilar whereas the Cu–N distances are very close in length. Apical atom O2 of one benzoate is 0.78 Å further away from atom Cu1 than its equatorial partner O1; similarly, atom O8 of the other benzoate is 0.62 Å is more remote from atom Cu2 than atom O7. The weak Cu1⋯O2 (2.7178(9) Å) and Cu2⋯O8 (2.5817(8) Å) interactions fall in the typical range observed for asymmetrically coordinated carboxylates, numerous examples of which have been structurally characterized and reported to the CSD.
Magnetic measurements
The thermal variation of the product of the molar magnetic susceptibility per Cu(II) ion times the temperature (χmT) for complexes 1–3 show, as expected, very similar behaviors: they show at room temperature a χmT value of ca. 0.4 cm3 K mol−1, slightly higher than the expected one for a magnetically isolated S = 1/2 Cu(II) ion (0.375 cm3 K mol−1) but within the normal range observed for other Cu(II) dimers. When the samples are cooled, χmT decreases and reach a plateau with an almost zero value below ca. 70 K (Fig. 5).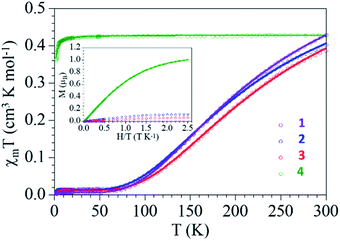 | ||
| Fig. 5 Thermal dependence of χmT per Cu(II) ion and isothermal magnetization at 2 K (inset) for compounds 1–4. Solid lines are the best fit to the models (see text). | ||
This behaviour clearly indicates the presence of a strong antiferromagnetic Cu(II)–Cu(II) coupling inside the dimer and a very small paramagnetic monomeric impurity (since the χmT value at low temperatures is not zero). Accordingly, we have fit the magnetic properties to a simple Bleaney–Bowers dimer model plus a S = 1/2 paramagnetic impurity (c).53 This simple model reproduces very satisfactorily the magnetic properties of compounds 1–3 in the whole temperature range with the following parameters: g = 2.124, J = −298 cm−1 and c = 0.8% for 1, g = 2.054, J = −291 cm−1 and c = 1.9% for 2 and g = 2.053, J = −320 cm−1 and c = 1.6% for 3 (solid lines in Fig. 5, the Hamiltonian is written as H = −JS1S2). The coupling constant values are in good agreement with reported49,50 and calculated54 values for dimeric carboxylate-bridged copper systems of general formula [Cu2(μ-RCOO)4L2]. For instance, the coupling constant for dimeric copper(II) benzoate with pyridine has been reported to be −328 cm−1; a value that corresponds well with that for complex 3 (−320 cm−1).50 The presence of the monomeric impurity is very common and is simply due to a partial decomposition of the dimers.
Compound 4 also shows a room temperature value of ca. 0.42 cm3 K mol−1 (slightly higher than the spin only value for a Cu(II) ion) but a different thermal behaviour: χmT remains constant down to ca. 10 K, where it shows a decrease to reach a value of 0.36 cm3 K mol−1 at 2 K (Fig. 5). This behavior indicates that compound 4 behaves essentially as a paramagnet with a very weak antiferromagnetic Cu⋯Cu coupling (mediated through the L bridge). Since the structure of 4 shows the presence of Cu(II) chains, the magnetic properties were fitted with a simple model for regular S = 1/2 chains.55 This model reproduces very satisfactorily the magnetic properties in the whole temperature range with g = 2.139 and an exchange parameter, J = −0.35 cm−1 (Fig. 5, the hamiltonian is written as H = −JSiSi+1). This low J value confirms the presence of a very weak antiferromagnetic interaction between the Cu(II) ions in the chain.
The isothermal magnetization of the complexes (inset in Fig. 5) shows that compounds 1–3 are essentially diamagnetic, due to the strong antiferromagnetic coupling, and present at low temperatures a residual magnetization arising from the small monomer fraction. In contrast, compound 4 is essentially paramagnetic and reaches a saturation value close to 1.0 μB, the expected one for a S = 1/2. In fact, the magnetization plot of compound 4 can be well reproduced with a Brillouin function for S = 1/2 with a g value of 2.116, in agreement with the result obtained in the fit of the thermal variation of χmT.
Polymerization reactions
The initiator performance of complexes 1–4 was investigated on the ROP of D,L-lactide and ε-caprolactone. The complexes showed good stability in air and were all soluble in hot toluene (above 70 °C). ε-CL polymerization was performed at 110 °C in solvent-free medium and at different [monomer]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [initiator] ratios ([M]
[initiator] ratios ([M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] = 50
[I] = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1500
1, 1500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3333
1 and 3333![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Polymerization of D,L-lactide was first attempted in a toluene solution at 70 °C but no opening of the ester ring was observed after 24 h. Increasing the temperature to 110 °C resulted in successful ring opening of the monomer at [M]/[I] ratio of 100
1). Polymerization of D,L-lactide was first attempted in a toluene solution at 70 °C but no opening of the ester ring was observed after 24 h. Increasing the temperature to 110 °C resulted in successful ring opening of the monomer at [M]/[I] ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The effect of alcohol on the polymerization rate was also studied using methanol as an additive in the polymerization of D,L-lactide.
1. The effect of alcohol on the polymerization rate was also studied using methanol as an additive in the polymerization of D,L-lactide.
Kinetics studies were carried out for the polymerization of ε-CL and D,L-lactide using complexes 1–4 to investigate the effect of initiator structure on the polymerization reactions and polymer properties. The kinetics study was followed by 1H NMR spectroscopy, aliquots were taken at regular time intervals and their 1H NMR spectra were recorded to determine the percentage conversions (from monomer to polymer).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] ratio of 50
[I] ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 110 °C in a solvent-free medium. This study shows that all the reactions were complete within 48 h, except for initiator 1 (Fig. S1†). The performance of the initiators decreased in the order: 4 > 2 > 3 > 1. The best performing initiator for this polymerization is polymeric complex 4, [Cu2{(3,5-NO2)2-C6H3COO}4L2]n, while the slowest initiation was shown by complex 1, [Cu2(C6H5COO)4(L)2].
1 at 110 °C in a solvent-free medium. This study shows that all the reactions were complete within 48 h, except for initiator 1 (Fig. S1†). The performance of the initiators decreased in the order: 4 > 2 > 3 > 1. The best performing initiator for this polymerization is polymeric complex 4, [Cu2{(3,5-NO2)2-C6H3COO}4L2]n, while the slowest initiation was shown by complex 1, [Cu2(C6H5COO)4(L)2].Fig. 6 shows that the variation of ln{[M]o/[M]t} with time follows a linear relationship, indicating a pseudo-first order kinetics of ε-CL for the different initiator systems studied. The apparent rate constant (kapp), obtained from the slope of the graph of ln{[M]o/[M]t} versus time, was found to vary in the range of 0.039–0.073 h−1 (Table 1) for the ε-CL polymerization (see eqn (1) and (2) in ESI†). These results can be compared to similar initiator systems for bis(pyrazole)copper benzoate where the rate of polymerization with the bis(pyrazole)copper benzoate initiators are much faster than observed for 1–4.41 Nevertheless, in both studies the best performance was shown by the nitro-benzoate derivatives, indicating that catalytic performance of initiators is influenced by the substitution on the benzoate ligands.
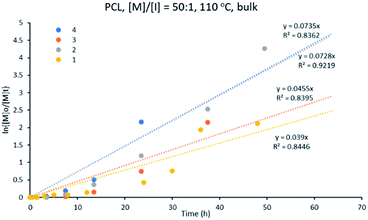 | ||
Fig. 6 Kinetic plot for ε-CL polymerization with [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] of 50 [I] of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 110 °C, using initiators 1–4, in bulk. 1 at 110 °C, using initiators 1–4, in bulk. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] = 50
[I] = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for ε-CL and 100
1 for ε-CL and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for D,L-lactide
1 for D,L-lactide
| Entry | Complex | PCL | PLA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conversion (%) | kapp (h−1) | Mn (1H NMR) | Mn (SEC)a | PDI | Conversion (%) | kapp (h−1) | Mn (1H NMR) | Mn (SEC)b | PDI | ||
| a Using a correcting factor 0.56 for Mn.b Using a correcting factor 0.58 for Mn.c Reaction was carried out using methanol as additive. | |||||||||||
| 1 | 1 | 98 | 0.039 | 4065 | 858 | 2.16 | 97 | 0.048 | 1695 | 602 | 1.64 |
| 2 | 2 | 92 | 0.072 | 3160 | — | — | 100 | 0.053 | 2953 | — | — |
| 3 | 3 | 93 | 0.045 | 3900 | — | — | 91 | 0.003 | 1877 | — | — |
| 4 | 3c | — | — | — | — | — | 91 | 0.030 | — | — | — |
| 5 | 4 | 100 | 0.073 | 5250 | — | — | 96 | 0.012 | 1812 | — | — |
The effect of changing the [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] ratio was studied for initiator system 4 by running the polymerization using [M]
[I] ratio was studied for initiator system 4 by running the polymerization using [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] ratios of 1500
[I] ratios of 1500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3333
1 and 3333![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S2†). With a [M]
1 (Fig. S2†). With a [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] ratio of 1500
[I] ratio of 1500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 we observe a significant drop in the polymerization rate from 0.073 to 0.013 h−1. The decrease in the number of active sites available for polymerization may account for the slower polymerization. Further lowering of the initiator concentration to [M]
1 we observe a significant drop in the polymerization rate from 0.073 to 0.013 h−1. The decrease in the number of active sites available for polymerization may account for the slower polymerization. Further lowering of the initiator concentration to [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] = 3333
[I] = 3333![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 only caused an insignificant change in rate (kapp = 0.012 h−1) compared to [M]
1 only caused an insignificant change in rate (kapp = 0.012 h−1) compared to [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] of 1500
[I] of 1500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This may be due to steric hindrance between the growing polymer chains in the 1500
1. This may be due to steric hindrance between the growing polymer chains in the 1500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio system.
1 molar ratio system.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] of 100
[I] of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 110 °C in toluene. All complexes initiated the polymerization of D,L-lactide at varying rates (Fig. S3†). The initiator performance was in decreasing order of 2 > 1 > 4 > 3, showing that for the ε-CL polymerization, the trend in initiator activity is different than for the ROP of D,L-lactide for L-based complexes. The best performing initiator is 2 while the least activity is showed by complex 3 (Fig. 7a).
1 at 110 °C in toluene. All complexes initiated the polymerization of D,L-lactide at varying rates (Fig. S3†). The initiator performance was in decreasing order of 2 > 1 > 4 > 3, showing that for the ε-CL polymerization, the trend in initiator activity is different than for the ROP of D,L-lactide for L-based complexes. The best performing initiator is 2 while the least activity is showed by complex 3 (Fig. 7a).
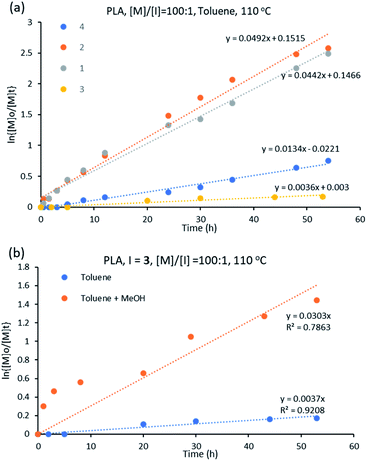 | ||
Fig. 7 (a) Kinetic plot for D,L-lactide using initiators 1–4 in toluene at 110 °C with [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] = 100 [I] = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (b) ROP of D,L-lactide using 3 at [M]/[I] = 100 1 (b) ROP of D,L-lactide using 3 at [M]/[I] = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and at 110 °C in different solvents. 1 and at 110 °C in different solvents. | ||
The constant rates for D,L-lactide ROP using initiators 1–4 varied from 0.003 to 0.053 h−1 (Table 1), indicating better catalytic performance of the initiators in the ROP of ε-CL than of D,L-lactide, which is in agreement with literature.38
Cyclic ester polymerization using [Sn(Oct)2] shows optimum performance when butanol is present in the reaction.56 In this respect, the polymerization of D,L-lactide was repeated for complex 3 with methanol as co-initiator (with a volume ratio of toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 50
methanol = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The polymerization rate increased when methanol was added, with the rate constant increasing from 0.003 to 0.030 h−1, values extracted from the plot of ln{[M]o/[M]t} versus time (Fig. 7b). The influence of the alcohol in the polymerization process agrees with the literature.57
1). The polymerization rate increased when methanol was added, with the rate constant increasing from 0.003 to 0.030 h−1, values extracted from the plot of ln{[M]o/[M]t} versus time (Fig. 7b). The influence of the alcohol in the polymerization process agrees with the literature.57
Although we have not studied the mechanism of the ROP for initiators 1–4, given their similarity with the [Al(OiPr)3] system,58 both with M–O bonds, the ROP mechanism in 1–4 is expected to occur via coordination-insertion.
Characterization of polymers
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 and 5700 Da, respectively.60 This may be explained in terms of accessibility of the metal centre since mononuclear [Al(OiPr)3] and [Sn(Oct)2] have less bulky ligands and less encumbered metal centres than paddle-wheel copper benzoates and, therefore, show a better monomer-metal interaction than in complexes 1–4.
800 and 5700 Da, respectively.60 This may be explained in terms of accessibility of the metal centre since mononuclear [Al(OiPr)3] and [Sn(Oct)2] have less bulky ligands and less encumbered metal centres than paddle-wheel copper benzoates and, therefore, show a better monomer-metal interaction than in complexes 1–4.In general, two reasons account for the low molecular weight of the polymers: inefficient catalyst and trans-esterification reactions.61,62 The latter can either be inter- or intramolecular, leading to lower molecular weight and/or broader PDI. Hence, the lower molecular weights and wider PDI of polymers derived from complexes 1–4 in comparison to their mononuclear analogues indicates that the paddle-wheel structures may be the cause for the drop in the initiator performance. Moreover, the benzoate bridging ligands together with the rigidity of the structure and the bulkiness around the metal centre, hinders the polymer chain growth resulting in short chains, low molecular weight and lower catalytic activity for 1–4. This idea is supported by the better performance of similar copper paddle wheel structures bearing acetate bridging carboxylates, as reported by Ojwach and co-workers.38
The SEC of most PLA and PCL show more than one peak: a main peak and other much smaller peaks. The latter are attributed to the formation of cyclic structures, a result of intramolecular transesterification reactions, involving backbiting. Comparing these two polymers, we can observed: (i) a more controlled polymerization for lactide ROP than for ε-CL, as depicted by the narrower molecular weight distribution, (measured as the polydispersity index, PDI) and (ii) a lesser intermolecular transesterification for PLA (PDI = 1.64) versus PCL (PDI = 2.16). In order to study the polymer structure of the produced PCL and PLA with initiators 1–4, we have used MALDI-ToF mass spectrometry.
The MALDI ToF spectrum of PCL synthesized using complex 1 shows an envelope-shaped spectrum (Fig. S5a†).65 All peaks are associated with either Na+ or K+ ion from the matrix. When the region m/z = 1150–1550 is expanded, a pattern is revealed that repeats itself several times (Fig. 8a). The most intense peaks correspond to a polymer chain that has hydroxyl end groups (type A). Peaks associated to cyclic PCL of type C were also observed; but type B PCL were not identified in the system, indicating that the polymer chains hydrolyzed during the workup process.
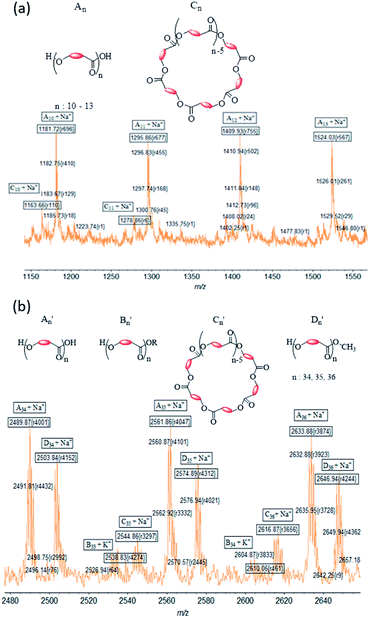 | ||
| Fig. 8 Expansion of MALDI-TOF mass spectrum of (a) PCL in the region m/z = 1150–1550 and (b) PLA in the region m/z = 2480–2660 made using 1. | ||
Molecular weights (m/z values) for PLA obtained with initiator 1 (Fig. S5b†) are much higher than those for PCL. An expansion of the PLA spectrum reveals the presence of more peaks in the repeat segment of the spectrum than was observed for PCL (Fig. 8). Using Na+ in calculating m/z results in a good correlation between the found and calculated values for the most intense peaks. These peaks are assigned to type A′ PLA, whereas lower intensity peaks are assigned to type C′ PLA. Type B′ PLA were also identified in the spectrum when the doping ion is considered to be K+. Surprisingly we identified an additional set of peaks (Fig. 8b), separated by 72 Da, which we assigned to a new polymer described as type D′ PLA that has a methoxy end group. PLA with –OCH3 groups are likely to be formed during the quenching of the reaction with methanolic–HCl solution.
Table 2 is a summary of the types of PCL and PLA that were produced with all four initiators and shows the significant role that an initiator plays in the polymerization reaction. It is clear from the intensity peaks that type A polymer with hydroxyl end groups, resulting from breaking of the metal–polymer bond, is the predominant polymer type. The same analysis shows that there is a very low amount of type B polymer that bears the initiator ligand as end group. This result indicates that the process leading to the ligand at the end of the polymerization process is not significant in the reaction.
| I | PCL | PLA | ||||
|---|---|---|---|---|---|---|
| m/z (obs) | m/z (calc) | Assignment | m/z (obs) | m/z (calc) | Assignment | |
| 1 | 1163 | 1164 | C10 + Na+ | 2489 | 2489 | A′34 + Na+ |
| 1 | 1181 | 1182 | A10 + Na+ | 2503 | 2503 | D′34 + Na+ |
| 1 | 1278 | 1278 | C11 + Na+ | 2538 | 2537 | B′33 + K+ |
| 1 | 1295 | 1286 | A11 + Na+ | 2544 | 2543 | C′35 + Na+ |
| 2 | 1867 | 1867 | A16 + Na+ | 1351 | 1353 | A′18 + K+ |
| 2 | 1981 | 1981 | A17 + Na+ | 1390 | 1391 | A′19 + Na+ |
| 3 | 953 | 954 | A8 + Na+ | 1422 | 1423 | D′19 + Na+ |
| 3 | 995 | 992 | B7 + K+ | 1464 | 1463 | C′20 + Na+ |
| 4 | 3008 | 3008 | A26 + Na+ | 2129 | 2129 | A′29 + Na+ |
| 4 | 3034 | 3038 | D26 + K+ | 2143 | 2143 | D′29 + Na+ |
| 4 | 3122 | 3122 | A27 + Na+ | 2184 | 2183 | C′30 + Na+ |
Conclusions
Four pyrazolyl copper(II) benzoates have been synthesized via one-pot reactions and while the pyrazolyl ligand does not influence the structure of the complexes formed, the ancillary benzoate ligands do. Three of the copper complexes (1–3) with less bulky benzoate ligands (4-OH-C6H4COO−, 2-Cl-C6H4COO− and C6H5COO−) have paddle-wheel bimetallic structures, whereas the complex formed by the bulky benzoate (3,5-NO2)2-C6H3COO− ligand, 4, does not. The different structural types give rise to very different magnetic behaviours. Complex 4, that presents a linear Cu(II) chain, behaves as an antiferromagnetically coupled S = 1/2 regular chain with a very weak antiferromagnetic coupling (J = −0.35 cm−1). In contrast, complexes 1–3, that present Cu(II) paddle wheel dimeric structure with four carboxylate bridging ligands, behave as antiferromagnetic S = 1/2 dimers with strong antiferromagnetic coupling constants (J = −291, −320 and −298 cm−1 in 1–3, respectively). All four complexes are able to initiate ring opening polymerization of both ε-caprolactone and D,L-lactide at high temperatures. The ability of these complexes in initiating the polymerization reaction strongly depends on the structure of the complexes. Polymeric initiator 4 exhibits the best performance in the ROP of ε-CL, while initiator 2 is the best initiator for D,L-lactide ROP. All the copper complexes produced polymer of low molecular weight as determined by NMR spectroscopy and SEC. MALDI-ToF MS shows that the type A polymers, bearing hydroxy end groups, are the major products. Transesterification leading to cyclization of the polymer chains may account for the low molecular weight and broad PDI, suggesting that paddle-wheel copper benzoates may not be the preferred type of structure to obtain monodispersed polymers of high molecular weight.Experimental
General procedures
Syntheses were performed under a dry nitrogen atmosphere using standard Schlenk techniques. All solvents were of analytical grade and were dried and distilled prior to use. Toluene and dichloromethane were dried and distilled from sodium/benzophenone and P2O5 respectively. [Cu(OAc)2], C6H5COOH, 4-OH-C6H4COOH, (3,5-NO2)2-C6H3COOH, 2-Cl-C6H4COOH, magnesium sulphate, tetrabutylammonium bromide and ε-caprolactone (98%) were obtained from Sigma-Aldrich and used as received. D,L-Lactide was obtained from Purac biochem and used as received. The ligand L was synthesized following literature procedure.66The NMR spectra were recorded in chloroform-d (CDCl3) on an Oxford Gemini 2000 instrument (300 MHz for 1H NMR and 75 MHz for 13C{1H} NMR) and a Bruker Ultrashield 400 instrument (400 MHz for 1H NMR and 100 MHz for 13C{1H} NMR) at room temperature. 1H and 13C{1H} NMR chemical shifts were referenced to the residual signals of the protons or carbons of the NMR solvents and are quoted in δ (ppm): CDCl3 at 7.24 and 77.0 ppm for 1H and 13C{1H} NMR spectra, respectively. Infrared spectra were recorded on a Bruker FR-IR Tensor27 spectrometer fitted with an ATP-IR probe. Elemental analyses were performed on a Vario Elementar microcube CHNS analyzer at Rhodes University, South Africa. ESI-MS spectra were recorded on a Waters API Quattro Micro spectrophotometry at the University of Stellenbosch, South Africa. Magnetic susceptibility measurements were performed with an applied magnetic field of 0.1 Tesla in the temperature range 2–300 K on polycrystalline samples of compounds 1–4 (with masses of 36.57, 38.30, 32.95 and 34.94 mg, respectively) with a Quantum Design MPMS-XL-5 SQUID susceptometer. The isothermal magnetization measurements were performed on the same samples at 2 K with fields from −5 to 5 Tesla. Susceptibility data were corrected for the sample holder and the diamagnetic contribution of the salts using Pascal's constants.66 Molecular weight and molecular weight polydispersity of polymers were determined by size exclusion chromatography (SEC) and by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight mass spectrometry (MALDI-ToF MS). SEC analysis was performed at the University of Mauritius using a WGE Dr Bures Q1000 Gel Permeation Chromatogram and at Kyoto University, Japan, using a JASCO GULLIVER system (PU-980, CO-965, RI-930, and UV-1570) equipped with polystyrene gel columns (Shodex columns K803, K804, K805), using THF as an eluent at a flow rate of 1.0 mL min−1, calibrated by polystyrene standards at 40 °C. MALDI-ToF MS measurements were made at Kyoto University and University of Tokyo, Japan using a Shimadzu Biotech Axima CFRplus. Thermal analysis of the polymers was carried out using a Mettler-Toledo DSC 822e and Perkin Elmer STA 6000 Simultaneous Thermal Analyzer.
X-ray crystallography
Crystal evaluation and data collection were performed on a Bruker Quazar SMART APEXII diffractometer with Cu for 3 and Mo for all the other complexes (1, 2 and 4). The crystals were attached to the tip of a MiTeGen MicroMount©. Each crystal was mounted in a stream of cold nitrogen at 100(1) K and centered in the X-ray beam by using a video camera. The initial cell constants were obtained from three series of ω scans at different starting angles. The reflections were successfully indexed by an automated indexing routine built in the APEXII program suite. The absorption correction was based on fitting a function to the empirical transmission surface as sampled by multiple equivalent measurements.67,68 A successful solution by the direct methods provided most non-hydrogen atoms from the E-map. The remaining non-hydrogen atoms were located in an alternating series of least-squares cycles and difference Fourier maps. All non-hydrogen atoms were refined with anisotropic displacement coefficients. All hydrogen atoms were included in the structure factor calculation at idealized positions and were allowed to ride on the neighbouring atoms with relative isotropic displacement coefficients. CCDC 2051015–2051018 contain the supplementary crystal data for complexes 1–4.Synthesis of the copper complexes 1–4
Two methods were used for the complexation reactions: method A and method B.Method A involves a one pot, overnight reaction between copper(II) acetate, benzoic acid and the pyrazolyl ligand, L in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio in methanol (50 mL). Copper(II) acetate and benzoic acid were first refluxed in methanol for 5 h and a solution of L in methanol (2 mL) is then added dropwise with constant stirring and heating. After 16 h of refluxing, the solution is allowed to cool to room temperature and slow evaporation of the solvent results in the formation of crystals. The copper products were characterized by FTIR, elemental analysis, mass spectrometry and magnetic measurements. Complexes 2–4 were synthesized by this method.
1 mole ratio in methanol (50 mL). Copper(II) acetate and benzoic acid were first refluxed in methanol for 5 h and a solution of L in methanol (2 mL) is then added dropwise with constant stirring and heating. After 16 h of refluxing, the solution is allowed to cool to room temperature and slow evaporation of the solvent results in the formation of crystals. The copper products were characterized by FTIR, elemental analysis, mass spectrometry and magnetic measurements. Complexes 2–4 were synthesized by this method.
Method B is an adaptation of the procedure used by Baruah69 to synthesize metal carboxylate complexes with pyrazoles. The method of preparation is a one pot, room temperature reaction between copper(II) acetate, benzoic acid and L in the same mole ratio as method A. Copper(II) acetate is stirred with benzoic acid in methanol (15 mL) at room temperature for 30 min and then a solution of L in toluene (5 mL) is added to the mixture and stirred for a further 30 min. The product of the reaction is isolated by evaporation of the solvent. Complex 1 was prepared using method B.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol mixture (50 mL of 1
methanol mixture (50 mL of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio). During the reaction, a green solid precipitated from the reaction mixture. Compound 1 was then isolated by filtration and dried under vacuum. The solid was re-dissolved in DMSO to obtain green single crystals upon slow evaporation of the solvent. Yield: 0.34 g (57%). FTIR: 1627 cm−1 (νC
1 volume ratio). During the reaction, a green solid precipitated from the reaction mixture. Compound 1 was then isolated by filtration and dried under vacuum. The solid was re-dissolved in DMSO to obtain green single crystals upon slow evaporation of the solvent. Yield: 0.34 g (57%). FTIR: 1627 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). μeff: 1.938 BM. Anal. Calc. for C46H42Cu2N4O8·CH3OH: C, 60.18%; H, 4.94% and N, 5.97%. Found: C, 59.20%; H, 4.709% and N, 6.08%.
O). μeff: 1.938 BM. Anal. Calc. for C46H42Cu2N4O8·CH3OH: C, 60.18%; H, 4.94% and N, 5.97%. Found: C, 59.20%; H, 4.709% and N, 6.08%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). μeff: 1.897 BM. Anal. Calc. for C32H32CuN4O6·0.75CH2Cl2: C, 56.53%; H, 4.85% and N, 8.05%. Found: C, 56.98%; H, 5.36% and N, 7.56%.
O). μeff: 1.897 BM. Anal. Calc. for C32H32CuN4O6·0.75CH2Cl2: C, 56.53%; H, 4.85% and N, 8.05%. Found: C, 56.98%; H, 5.36% and N, 7.56%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). μeff: 1.810 BM. ES-MS: m/z (%); 692 [M + Na]+, 20; 512 [M-2-Cl-C6H4COO]+, 100. Anal. Calc. for C32H30Cl2CuN4O4·0.5C7H8: C, 59.62%; H, 4.79% and N, 7.83%. Found: C, 59.72%; H, 4.65% and N, 8.07%.
O). μeff: 1.810 BM. ES-MS: m/z (%); 692 [M + Na]+, 20; 512 [M-2-Cl-C6H4COO]+, 100. Anal. Calc. for C32H30Cl2CuN4O4·0.5C7H8: C, 59.62%; H, 4.79% and N, 7.83%. Found: C, 59.72%; H, 4.65% and N, 8.07%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). Anal. Calc. for C32H28CuN8O12·CH3OH: C, 48.80%; H, 3.97%; N, 13.80%. Found: C, 48.64%; H, 3.37%; N, 14.02%.
O). Anal. Calc. for C32H28CuN8O12·CH3OH: C, 48.80%; H, 3.97%; N, 13.80%. Found: C, 48.64%; H, 3.37%; N, 14.02%.Polymerization study
The catalytic activities of 1–4 towards ε-caprolactone (ε-CL) and D,L-lactide polymerizations have been evaluated under different conditions. Polymerization of ε-caprolactone was carried out in bulk using a [monomer]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [initiator] ([M]
[initiator] ([M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I]) ratio of 50
[I]) ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and ratios 1500
1 and ratios 1500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3333
1 and 3333![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in some cases. For the polymerization of D,L-lactide, the reaction was performed in toluene using a [M]
1 in some cases. For the polymerization of D,L-lactide, the reaction was performed in toluene using a [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] ratio of 100
[I] ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The polymerization reactions were performed in a carousel reaction station, fitted with 12 tubes, a gas distribution system and a reflux unit.
1. The polymerization reactions were performed in a carousel reaction station, fitted with 12 tubes, a gas distribution system and a reflux unit.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] ratio used, were weighed in a reactor tube and stirred at 110 °C. The polymerization of ε-CL was carried out in bulk with [M]
[I] ratio used, were weighed in a reactor tube and stirred at 110 °C. The polymerization of ε-CL was carried out in bulk with [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] ratios of 50
[I] ratios of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1500
1, 1500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3333
1 and 3333![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After the required reaction time, the reaction mixture was quenched by rapid cooling to room temperature and the crude product was analyzed by 1H NMR spectroscopy in CDCl3. The polymers were cleaned by first dissolving the crude product in CH2Cl2, followed by the addition of cold methanol. The white precipitate formed was filtered and dried in vacuo.
1. After the required reaction time, the reaction mixture was quenched by rapid cooling to room temperature and the crude product was analyzed by 1H NMR spectroscopy in CDCl3. The polymers were cleaned by first dissolving the crude product in CH2Cl2, followed by the addition of cold methanol. The white precipitate formed was filtered and dried in vacuo.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] ratio at 100
[I] ratio at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were dissolved in toluene (2.5 mL) in a reactor tube and stirred at 110 °C. After specified time intervals, each reaction was quenched by rapid cooling to room temperature. The solvent was removed in vacuo and the percentage conversion was determined from the 1H NMR spectrum. The polymer was cleaned by first dissolving the crude product in dichloromethane (15 mL) and adding a hydrochloric acid solution in methanol (0.5 M, 15 mL) to the mixture. The solution was shaken vigorously and allowed to stand to allow the layers to separate. The aqueous layer was then washed with dichloromethane (3 × 10 mL). The combined organic layer was then washed with distilled water (15 mL), followed by sodium hydroxide solution (1.0 M, 15 mL). The basic aqueous layer was washed with CH2Cl2 (3 × 10 mL). The organic phase was dried over anhydrous magnesium sulphate, filtered and concentrated in vacuo, yielding a white fluffy solid. In some cases, the D,L-lactide polymerization was repeated using methanol (0.05 mL) as an additive to the reaction.
1 were dissolved in toluene (2.5 mL) in a reactor tube and stirred at 110 °C. After specified time intervals, each reaction was quenched by rapid cooling to room temperature. The solvent was removed in vacuo and the percentage conversion was determined from the 1H NMR spectrum. The polymer was cleaned by first dissolving the crude product in dichloromethane (15 mL) and adding a hydrochloric acid solution in methanol (0.5 M, 15 mL) to the mixture. The solution was shaken vigorously and allowed to stand to allow the layers to separate. The aqueous layer was then washed with dichloromethane (3 × 10 mL). The combined organic layer was then washed with distilled water (15 mL), followed by sodium hydroxide solution (1.0 M, 15 mL). The basic aqueous layer was washed with CH2Cl2 (3 × 10 mL). The organic phase was dried over anhydrous magnesium sulphate, filtered and concentrated in vacuo, yielding a white fluffy solid. In some cases, the D,L-lactide polymerization was repeated using methanol (0.05 mL) as an additive to the reaction.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was made possible through financial support from the University of Johannesburg and the National Research Foundation (South Africa). We also thank the Spanish MINECO (project CTQ2017-87201-P AEI/FEDER, UE) and the Generalidad Valenciana (Prometeo/2019/076) for financial support.Notes and references
- A. Kowalski, A. Duda and S. Penczek, Macromol. Rapid Commun., 1998, 19, 567–572 CAS.
- Y. Zhao, D. Keroack and R. Prud‘homme, Macromolecules, 1999, 32, 1218–1225 CrossRef CAS.
- B. M. Chamberlain, Y. Sun, J. R. Hagadorn, E. W. Hemmesch, V. G. Young, M. Pink, M. A. Hillmyer and W. B. Tolman, Macromolecules, 1999, 32, 2400–2402 CrossRef CAS.
- T. M. Ovitt and G. W. Coates, J. Am. Chem. Soc., 1999, 121, 4072–4073 CrossRef CAS.
- X. Chen and R. A. Gross, Macromolecules, 1999, 32, 308–314 CrossRef CAS.
- L. Mezzasalma, A. P. Dove and O. Coulembier, Eur. Polym. J., 2017, 95, 628–634 CrossRef CAS.
- S. Liu, C. Ren, N. Zhao, Y. Shen and Z. Li, Macromol. Rapid Commun., 2018, 39, 1800485 CrossRef PubMed.
- C. Bao, X. Xu, J. Chen and Q. Zhang, Polym. Chem., 2020, 11, 682–686 RSC.
- N. Ropson, P. Dubois, R. Jerome and P. Teyssie, Macromolecules, 1994, 27, 5950–5956 CrossRef CAS.
- P. Dubois, N. Ropson, R. Jérôme and P. Teyssié, Macromolecules, 1996, 29, 1965–1975 CrossRef CAS.
- A. Bhaw-Luximon, D. Jhurry, S. Motala-Timol and Y. Lochee, Macromol. Symp., 2005, 231, 60–68 CrossRef.
- Z. Zhong, P. J. Dijkstra, C. Birg, M. Westerhausen and J. Feijen, Macromolecules, 2001, 34, 3863–3868 CrossRef CAS.
- A. Duda, Z. Florjanczyk, A. Hofman, S. Slomkowski and S. Penczek, Macromolecules, 1990, 23, 1640–1646 CrossRef CAS.
- H. R. Kricheldorf and C. Boettcher, Die Makromolekulare Chemie, 1993, 194, 1665–1669 CrossRef CAS.
- B.-T. Ko and C.-C. Lin, J. Am. Chem. Soc., 2001, 123, 7973–7977 CrossRef CAS PubMed.
- M. H. Chisholm, C.-C. Lin, J. C. Gallucci and B.-T. Ko, Dalton Trans., 2003, 3, 406–412 RSC.
- H. R. Kricheldorf, M. Berl and N. Scharnagl, Macromolecules, 1988, 21, 286–293 CrossRef CAS.
- M. H. Chisholm, N. W. Eilerts, J. C. Huffman, S. S. Iyer, M. Pacold and K. Phomphrai, J. Am. Chem. Soc., 2000, 122, 11845–11854 CrossRef CAS.
- M. H. Chisholm, J. Gallucci and K. Phomphrai, Inorg. Chem., 2002, 41, 2785–2794 CrossRef CAS PubMed.
- M. H. Chisholm and K. Phomphrai, Inorg. Chim. Acta, 2003, 350, 121–125 CrossRef CAS.
- H. R. Kricheldorf, M. V. Sumbel and I. Kreiser-Saunders, Macromolecules, 1991, 24, 1944–1949 CrossRef CAS.
- B. J. O'Keefe, L. E. Breyfogle, M. A. Hillmyer and W. B. Tolman, J. Am. Chem. Soc., 2002, 124, 4384–4393 CrossRef PubMed.
- P. Dobrzynski, J. Kasperczyk, H. Janeczek and M. Bero, Polymer, 2002, 43, 2595–2601 CrossRef CAS.
- A. Duda and S. Penczek, in Polymers from Renewable Resources, ed. C. Scholz and R. A. Gross, American Chemical Society, 2001, Wahington DC, vol. 764, ch. 13, pp. 160–198 Search PubMed.
- R. H. Platel, L. M. Hodgson and C. K. Williams, Polym. Rev., 2008, 48, 11–63 CrossRef CAS.
- G. Schwach, J. Coudane, R. Engel and M. Vert, Polym. Int., 1998, 46, 177–182 CrossRef CAS.
- M. Bero, J. Kasperczyk and Z. J. Jedlinski, Die Makromolekulare Chemie, 1990, 191, 2287–2296 CrossRef CAS.
- M. Bero, J. Kasperczyk and G. Adamus, Die Makromolekulare Chemie, 1993, 194, 907–912 CrossRef CAS.
- S. Agarwal, C. Mast, K. Dehnicke and A. Greiner, Macromol. Rapid Commun., 2000, 21, 195–212 CrossRef CAS.
- A. F. Posada, M. A. Macías, S. Movilla, G. P. Miscione, L. D. Pérez and J. J. Hurtado, Polymers, 2018, 10, 1239 CrossRef PubMed.
- R. R. Gowda and D. Chakraborty, J. Mol. Catal. A: Chem., 2011, 349, 86–93 CrossRef CAS.
- S. Bhunora, J. Mugo, A. Bhaw-Luximon, S. Mapolie, J. Van Wyk, J. Darkwa and E. Nordlander, Appl. Organomet. Chem., 2011, 25, 133–145 CrossRef CAS.
- Y. Yang, H. Wang and H. Ma, Inorg. Chem., 2015, 54, 5839–5854 CrossRef CAS PubMed.
- C. K. Williams, L. E. Breyfogle, S. K. Choi, W. Nam, V. G. Young, M. A. Hillmyer and W. B. Tolman, J. Am. Chem. Soc., 2003, 125, 11350–11359 CrossRef CAS PubMed.
- T. Ebrahimi, E. Mamleeva, I. Yu, S. G. Hatzikiriakos and P. Mehrkhodavandi, Inorg. Chem., 2016, 55, 9445–9453 CrossRef CAS PubMed.
- E. D. Akpan, S. O. Ojwach, B. Omondi and V. O. Nyamori, Polyhedron, 2016, 110, 63–72 CrossRef CAS.
- E. D. Akpan, S. O. Ojwach, B. Omondi and V. O. Nyamori, New J. Chem., 2016, 40, 3499–3510 RSC.
- S. O. Ojwach, T. T. Okemwa, N. W. Attandoh and B. Omondi, Dalton Trans., 2013, 42, 10735–10745 RSC.
- S. Ojwach and T. Zaca, S. Afr. J. Chem., 2015, 68, 7–14 CrossRef CAS.
- M. Zikode, S. Ojwach and M. Akerman, J. Mol. Catal. A: Chem., 2015, 413, 24–31 CrossRef.
- D. Appavoo, B. Omondi, I. A. Guzei, J. L. van Wyk, O. Zinyemba and J. Darkwa, Polyhedron, 2014, 69, 55–60 CrossRef CAS.
- N. Attandoh, S. Ojwach and O. Munro, Eur. J. Inorg. Chem., 2014, 2014, 3053–3064 CrossRef CAS.
- N. Attandoh and S. Ojwach, Polym., 2016, 40, 347–354 CAS.
- E. Akpan, B. Omondi and S. Ojwach, Polym. Bull., 2018, 75, 5179–5195 CrossRef CAS.
- S. O. Ojwach and J. Darkwa, Inorg. Chim. Acta, 2010, 363, 1947–1964 CrossRef CAS.
- C. A. Wheaton and P. G. Hayes, Catal. Sci. Technol., 2012, 2, 125–138 RSC.
- P. Hormnirun, E. L. Marshall, V. C. Gibson, R. I. Pugh and A. J. P. White, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15343–15348 CrossRef CAS PubMed.
- S. Tabthong, T. Nanok, P. Kongsaeree, S. Prabpai and P. Hormnirun, Dalton Trans., 2014, 43, 1348–1359 RSC.
- M. A. Malik, P. O'Brien, F. Tuna, R. Pritchard, J. Buchweishaija, E. Kimambo and E. B. Mubofu, Dalton Trans., 2013, 42, 14438–14444 RSC.
- T. Kawata, H. Uekusa, S. Ohba, T. Furukawa, T. Tokii, Y. Muto and M. Kato, Acta Crystallogr., Sect. B: Struct. Sci., 1992, 48, 253–261 CrossRef.
- S. J. Jenniefer and P. T. Muthiah, Chem. Cent. J., 2013, 7, 35 CrossRef PubMed.
- V. Paredes-García, R. C. Santana, R. Madrid, A. Vega, E. Spodine and D. Venegas-Yazigi, Inorg. Chem., 2013, 52, 8369–8377 CrossRef PubMed.
- B. Bleaney and K. D. Bowers, Anomalous Paramagnetism of Copper Acetate, Proc. R. Soc. London, Ser. A, 1952, 214(1119), 451–465 CAS.
- A. Rodríguez-Fortea, P. Alemany, S. Alvarez and E. Ruiz, Chem.–Eur. J., 2001, 7, 627–637 CrossRef.
- D. B. Brown, J. A. Donner, J. W. Hall, S. R. Wilson, R. B. Wilson, D. J. Hodgson and W. E. Hatfield, Inorg. Chem., 1979, 18, 2635–2641 CrossRef CAS.
- A. Duda, S. Penczek, A. Kowalski and J. Libiszowski, Macromol. Symp., 2000, 153, 41–53 CrossRef CAS.
- X. Zhang, D. A. MacDonald, M. F. A. Goosen and K. B. McAuley, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 2965–2970 CrossRef CAS.
- A. Kowalski, A. Duda and S. Penczek, Macromolecules, 1998, 31, 2114–2122 CrossRef CAS.
- J. Baran, A. Duda, A. Kowalski, R. Szymanski and S. Penczek, Macromol. Symp., 1997, 123, 93–101 CrossRef CAS.
- A.-F. Mingotaud, F. Dargelas and F. Cansell, Macromol. Symp., 2000, 153, 77–86 CrossRef CAS.
- P. Dubois, C. Jacobs, R. Jerome and P. Teyssie, Macromolecules, 1991, 24, 2266–2270 CrossRef CAS.
- L. E. N. Allan, G. G. Briand, A. Decken, J. D. Marks, M. P. Shaver and R. G. Wareham, J. Organomet. Chem., 2013, 736, 55–62 CrossRef CAS.
- H. Ma and J. Okuda, Macromolecules, 2005, 38, 2665–2673 CrossRef CAS.
- K. Phomphrai, C. Pongchan-o, W. Thumrongpatanaraks, P. Sangtrirutnugul, P. Kongsaeree and M. Pohmakotr, Dalton Trans., 2011, 40, 2157–2159 RSC.
- G. Montaudo, F. Samperi and M. S. Montaudo, Prog. Polym. Sci., 2006, 31, 277–357 CrossRef CAS.
- C. M. Hartshorn and P. J. Steel, Aust. J. Chem., 1995, 48, 1587–1599 CrossRef CAS.
- G. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- S. J. M. Bruker, SAINT, Software Reference Manual Bruker, AXS Inc, Wisconsin, USA, 2000 Search PubMed.
- R. Sarma, D. Kalita and J. B. Baruah, Dalton Trans., 2009, 36, 7428–7436 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary Fig. S1–S5 and supplementary Table S1 are available. Fig. S1, S2 and S3 show the graphs of conversion versus time for CL using 1–4 initiators, different [M]/[I] ratios and D,L-lactide using 1–4 initiators, respectively. Fig. S4 shows the types of polymer from MALDI-ToF and Fig. S5 is MALDI-ToF of PCL and PLA formed using 1. Table S1 shows X-ray structures of 1–4. CCDC 2051015–2051018. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra00339a |
| This journal is © The Royal Society of Chemistry 2021 |