Open Access Article
Open Access ArticleSurface modification and functionalization of powder materials by atomic layer deposition: a review
Yiyun Hu
 ab,
Jian Lu
ab,
Jian Lu
 c and
Hao Feng
c and
Hao Feng
 *abc
*abc
aScience and Technology on Combustion and Explosion Laboratory, Xi'an Modern Chemistry Research Institute, 168 E. Zhangba Road, Xi'an, 710065, Shanxi, PR China. E-mail: fenghao98@hotmail.com
bLaboratory of Material Surface Engineering and Nanofabrication, Xi'an Modern Chemistry Research Institute, 168 E. Zhangba Road, Xi'an, 710065, Shanxi, PR China
cState Key Laboratory of Fluorine and Nitrogen Chemicals, Xi'an Modern Chemistry Research Institute, 168 E. Zhangba Road, Xi'an, 710065, Shanxi, PR China
First published on 23rd March 2021
Abstract
Powder materials are a class of industrial materials with many important applications. In some circumstances, surface modification and functionalization of these materials are essential for achieving or enhancing their expected performances. However, effective and precise surface modification of powder materials remains a challenge due to a series of problems such as high surface area, diffusion limitation, and particle agglomeration. Atomic layer deposition (ALD) is a cutting-edge thin film coating technology traditionally used in the semiconductor industry. ALD enables layer by layer thin film growth by alternating saturated surface reactions between the gaseous precursors and the substrate. The self-limiting nature of ALD surface reaction offers angstrom level thickness control as well as exceptional film conformality on complex structures. With these advantages, ALD has become a powerful tool to effectively fabricate powder materials for applications in many areas other than microelectronics. This review focuses on the unique capability of ALD in surface engineering of powder materials, including recent advances in the design of ALD reactors for powder fabrication, and applications of ALD in areas such as stabilization of particles, catalysts, energetic materials, batteries, wave absorbing materials and medicine. We intend to show the versatility and efficacy of ALD in fabricating various kinds of powder materials, and help the readers gain insights into the principles, methods, and unique effects of powder fabrication by ALD.
1. Introduction
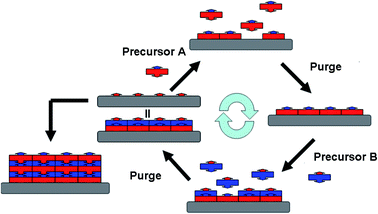
Powder materials are widely used in various industrial areas. In many circumstances, surface modification and functionalization of powder materials are essential for achieving or enhancing the desired performances. Effective and precise surface modification of powder materials has been an everlasting topic for both academia and industry. In order to introduce the desired physical or chemical properties to the powder, many methods, such as sol–gel processing and chemical vapor deposition (CVD), have been developed, to produce functional thin films or discontinuous structures on the surfaces of the prime particles. Although these methods have been successfully applied to many powder systems, most of these methods fail to achieve precise control over the thickness of the surface fabrication layer, which may result in compromised effects and poor consistency or reproducibility. In addition, uncontrolled film thickness may easily cause permanent aggregation of primary particles, which can be detrimental to the performance of the powder.1Unlike many other vapor-phase deposition techniques, atomic layer deposition (ALD) is not a line-of-sight dependent process. ALD processes are based on cyclical self-limiting chemisorptions taking place on the surface of the substrate. In a typical ALD process as depicted in Fig. 1, two precursors are alternately introduced in the vapor form, and each reacts with the surface functional groups generated during the previous reaction step. Each step of the surface reaction is self-limiting and the two reaction steps are separated by inert gas purges which remove the unreacted precursors and reaction by-products. Thus the film thickness is precisely controlled by the number of ALD cycle performed. The typical ALD film growth rate for most inorganic materials is ranged between 0.01 to 0.25 nm per cycle, depending on the specific type of material, precursors, and reaction conditions.2
The special film growth mechanism endows ALD the unique capability of producing conformal coatings on materials with complex structures. A powder bed can be considered a porous system with many inter-connected channels and crevices. In an ALD process, when the gaseous precursor is introduced it can infiltrate the channels and crevices. The self-limiting reactions take place only on the surface and the gas phase over-reactions are avoided. Therefore, continuous exposure to the gaseous precursor until the surface reactions become saturated is the key to obtaining complete and uniform coating on powder materials. This can be achieved by developing advanced ALD reactor and precursor supply systems. Detailed discussions on this topic will be carried out in the next section.
A large number of materials can be made by ALD, including many inorganic materials, a variety of organic polymers, and hybrid materials prepared by alternating inorganic and organic blocks.3 Most types of powder materials, including micrometer or nanometer scale inorganic or organic particles, can be effectively coated. With ALD the structure of the surface fabrication layer can be controlled at atomic level precision, and the surface properties can be precisely tuned. By introducing desired physical or chemical properties to the surface, ALD can provide unlimited possibilities to the design and synthesis of novel functional materials.
This review is intended for introducing recent advances in powder material fabrication by ALD, including reactor and process design for powder fabrication, and applications in many different areas such as stabilization of particles, catalysts, energetic materials, batteries, wave absorbing materials, and medicine. We hope to show the versatility and efficacy of ALD in fabricating various kind of powder materials, and help the readers gain insights into the principles, methods, and effects of powder fabrication by ALD. Some existing reviews on related topics are recommended for a more comprehensive understanding on ALD.1–7
2. ALD reactor and process for powder fabrication
It is generally accepted that ALD was pioneered in Finland in the early 1970s by Suntola and Jorma under the name of “atomic layer epitaxy” (ALE).8 In 2000 Ferguson et al. demonstrated that ALD technique could be used to coat ultrathin films onto the surfaces of primary particles.9 Since then, the great interest in functionalized particles has been driving researchers to develop economically scalable and easy-to-operate ALD reactors for powder fabrication.Currently, powder or particle ALD is mainly carried out in fixed bed, agitated bed, or fluidized bed reactors. Fixed bed ALD reactors are only suitable for processing small amounts of powder samples. When a large amount of powder is loaded, it is difficult for the gas phase precursor to diffuse into the bottom/inside of the powder bed in each precursor injection step, so that saturation of the surface reaction cannot be guaranteed, resulting in nonuniform coating, or more frequently, coating only on the top or external layer of the powder bed. Libera et al. reported an example of depositing ZnO on high surface area silica gel particles using a fixed bed ALD reactor.10 The silica gel powder was held in a container covered by a fine stainless mesh as shown in Fig. 2a. The gap between the powder layer and the cover as well as the thickness of the powder layer should be carefully controlled (Fig. 2b and c) to ensure rapid diffusion of gases between particles. A thick powder bed may lead to non-uniform ZnO coating between particles or inside the particles. Therefore, when using a fixed-bed reactor for powder ALD, it is very important to control the amount of substrate material, uniformly spread the powder in the flow path, and provide sufficient ALD sequence time to ensure the saturated surface reaction. Nevertheless, when large-scale production is needed, the limitations of fixed-bed reactors are difficult to avoid, and other reactor designs must be applied.
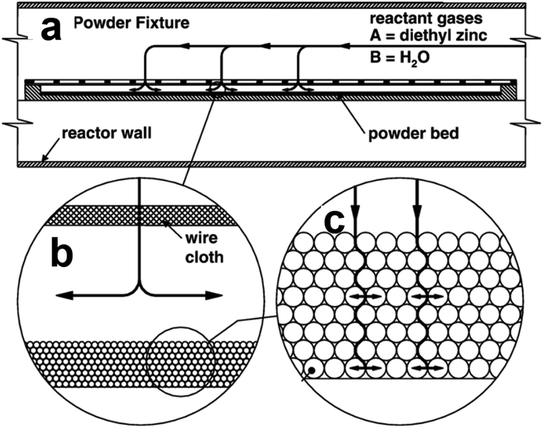 | ||
| Fig. 2 (a) Cross-sectional schematic picture of the powder coating fixture of a fixed bed ALD reactor; detailed structures of the cover (b) and the powder bed (c). Reprinted with permission from ref. 10, Copyright (2008), Elsevier. | ||
Since the introduction of fluidized bed reactors (FBRs) to ALD, a variety of materials have been deposited on different types of powders using FBRs.11–13 Some of the fluidized bed ALD reactors utilize vibration, stirring or other methods of agitation to prevent particles from agglomerating during the coating process, and thus improve the uniformity of coatings. King et al. reported a FBR with mechanical vibration and agitation (Fig. 3a).14 In this reactor inert carrier gas purge was combined with mechanical stirring to effectively fluidize nanometer or micrometer-sized particles. During the fluidization process, agglomerated particles showed a dynamic agglomeration behavior, which means the primary particles will be torn away from some agglomerates and then be absorbed by other agglomerates. In other words, the agglomerates of primary particles continuously break apart and form again. With such mechanism, the surfaces of all primary particles will be exposed to ALD precursors and be uniformly coated.15
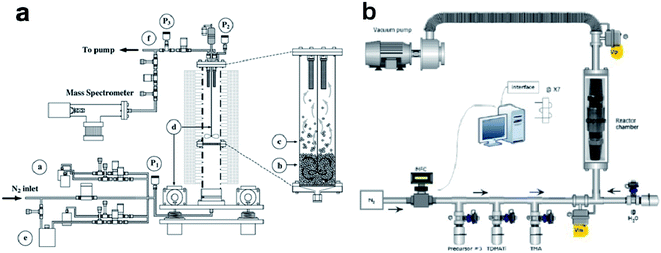 | ||
| Fig. 3 Configurations of ALD reactors for powder fabrication: (a), schematic of the fluidized bed ALD reactor. The flexibility of the dosing zone a) allows for the controlled delivery of a variety of precursors. The particle bed b) is fluidized using either the inert purge gas or the reactive precursor itself. Particles entering the splash zone c) fluidize as soft agglomerates, and operating velocities are controlled to minimize particle elutriation to the filters. Mechanical bed agitation d) facilitates fluidization and precursor bubblers e) can be used to maintain fluidization during dosing. Real-time gas sampling occurs at the outlet of the reactor f) using in situ mass spectrometry. Three capacitance manometers (P1, P2, P3) are employed to monitor fluidization and proper system operation. Reprinted with permission from ref. 14, Copyright (2007), Elsevier. (b) Schematic of the pulsed-bed ALD system. Reprinted with permission from ref. 18, Copyright (2014), Elsevier. | ||
Powder ALD in FBRs can be operated either at reduced pressure or at atmospheric pressure. Beetstra et al. demonstrated that ALD of Al2O3 on LiMn2O4 powder (200–500 nm) is feasible using a fluidized bed reactor under atmospheric pressure.16 One hundred grams of the LiMn2O4 powder was loaded in the FBR and the primary particles were coated individually. Some of the gaps between particles were filled with Al2O3, which might be caused by water build-up due to insufficient inert gas purge. In another case, Pd nanoparticles were deposited onto graphene (GE) nanoplatelets using a FBR at atmospheric pressure.17 The Pd/GE obtained by atmospheric pressure ALD showed lower impurity content, better dispersion and more uniform distribution of Pd nanoparticles. Obviously, it will be much more convenient to scale-up the ALD process under atmospheric pressure, however, efficient supplying and removing of precursors and avoiding side reactions are still challenging under atmospheric pressure.
Despite their efficacy for coating primary particles, FBRs face the problem of low precursor utilization efficiency, because a large fraction of the precursor may be carried away by the fast, continuous gas flow necessary for particle fluidization. A “pulsed-bed” ALD reactor (Fig. 3b) has been proposed to resolve the dilemma.18 When the precursor is introduced, the valve at the suction end is closed simultaneously, and the pressure difference between the two ends of the reactor generates a large pulse of gas flow, which blows up and agitates the powder bed. Compared to conventional fluidized bed reactors and processes, this reactor and process design may significantly reduce the consumption of precursors and carrier gas.
Rotary ALD reactors for fabricating large quantities of powder samples were also reported.19 In a typical design (Fig. 4a), the powder was contained in a porous cylindrical stainless-steel drum inside a vacuum chamber, which was driven through a magnetically coupled rotating engine. The rotation speed was adjusted and a centrifugal acceleration less than 1 G was obtained, thereby the powder bed was agitated by continuous “avalanche”. Compared with FBRs, in the rotary ALD reactor static reactant exposure and agitation of the powder bed are allowed simultaneously, the particle agitation provided by rotation improves contact between the particles and the reaction gases, and does not require continuous flow of gas for fluidization, therefore longer precursor residence time and better utilization of the precursors can be obtained.
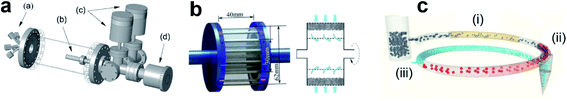 | ||
| Fig. 4 (a) Schematic diagram of the rotary ALD reactor, showing a), multiple input dosing flange, b), small porous metal cylinder, c), 10 and 1000 Torr capacitance manometers, and d), magnetically coupled rotary manipulator. Reprinted with permission from ref. 19. Copyright (2007), American Vacuum Society. (b) Simplified assembly drawing of the rotating fluidized bed ALD reactor and schematic diagram of the particle cartridge. Reprinted with permission from ref. 20. Copyright (2015), with the permission of AIP Publishing. (c) Pneumatic transport line in the spatial ALD reactor made of three segments: preheating zone (i), precursor reaction zone (ii), co-reactant reaction zone (iii), and a collection vessel. Reprinted with permission from ref. 23. Copyright (2015), American Vacuum Society. | ||
A new reactor design named rotating fluidized bed, which combines the advantages of fluidized bed reactor and rotary reactor, has been proposed. Duan et al. demonstrated ALD of alumina on a silica powder using the rotating fluidized bed reactor (Fig. 4b).20 The rotating fluidized bed reactor provides centrifugal force that assists fluidization of particles, and the gasless fluidization may solve the problem of low precursor utilization efficiency.
Recently, the concept of spatial ALD has also been introduced to fabricate powder materials. As a most special feature of spatial ALD, the two ALD half reactions are spatially separated from each other, so that no pumping or purging steps are required.21,22 Van Ommen et al. proposed a novel spatial ALD reactor for catalyst preparation based on pneumatic transportation.23 As shown in Fig. 4c, the spatial ALD reactor includes three parts: (i) the heating zone, (ii) the reaction zone for the first half reaction, and (iii) the reaction zone for the second half reaction. Different reactants were injected at the beginning of each reaction zone. With the N2 flow, particles could be suspended and be transported from one reaction zone to the other. Using the spacial ALD reactor, platinum (Pt) nanoclusters with a diameter of about 1 nm were deposited on P25 titanium dioxide (TiO2) nanoparticles.
Despite the limitations, a lot of lab-scale research on ALD powder fabrication is carried out with fixed bed ALD reactors, due to the fact that most commercially available ALD systems are only equipped with fixed bed reactors primarily designed for coating flat substrates. While for most fundamental research projects only a small amount of sample is needed, to develop practical applications of ALD for powder materials, research on ALD reactors capable of fabricating large quantities of powders and reducing the assumption of precursors is indispensable. To achieve these goals, innovations on reactor designs which can improve the gas–solid mass transfer, and the development of more efficient solid state precursor supply systems, are most important tasks.
3. Applications
3.1 Stabilization and passivation of particles
Owing to their excellent barrier properties, ALD films have been applied to flat substrates for the purpose of surface protection or stabilization. This advantage could also be applied to powder materials. The dense ALD film produced on the surface of particles could provide a unique surface sealing function, which would effectively isolate the underlying material from the environment and thus improve the stability of the particles. Al2O3 was the most widely used ALD coating material for surface passivation against oxidation, high temperature, moisture, corrosion and so on, due to its compactness and mature technology. In the past decade, more and more researchers have tried other oxide materials, such as TiO2, ZrO2, SiO2, and nitride materials such as ZrN, as environmental barrier coatings (EBCs). The uniform surface coverage and precise thickness control of ALD could help minimize the content of surface modification material. This is essential for applications in which bulk properties of the original materials need to be maintained as much as possible.Iron(II,III) oxide (Fe3O4) nanoparticles (NPs) are widely used in magnetism related fields, however, in atmospheric conditions Fe3O4 NPs tend to be spontaneously oxidized to Fe2O3 and lose their magnetization. Using a rotating fluidized bed ALD reactor, Duan et al. deposited uniform and pinhole-free Al2O3 films onto the Fe3O4 nanoparticles (Fig. 5a).24 Thermogravimetric analysis showed that a 5 nm-thick ALD Al2O3 film effectively protected Fe3O4 nanoparticles from spontaneous oxidation, without losing the magnetic properties. Similarly, Cremers et al. applied ALD Al2O3 coatings on micron-scale Fe and Cu powders to improve their oxidation resistance.25 For the Cu powder, an 8 nm ALD Al2O3 film can increase the oxidation temperature by 200 °C, while for the Fe powder, a 25 nm Al2O3 film is required to obtain a similar oxidation resistance effect.
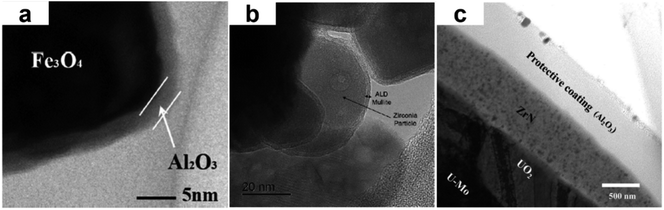 | ||
| Fig. 5 (a) Bright-field TEM image of 50-cycle ALD Al2O3 coated Fe3O4 NPs; Reprinted with permission from ref. 24. Copyright (2016), American Vacuum Society. (b) HRTEM image of ALD mullite film on zirconia NPs; Reprinted with permission from ref. 26. Copyright (2018), John Wiley and Sons. (c) Bright-field TEM image of ALD ZrN film and U–Mo interface. Reprinted with permission from ref. 33. Copyright (2019), Elsevier. | ||
Hoskins et al. reported the synthesis of mullite (Al2O3–SiO2) films on zirconia and silicon carbide powders through ALD for the purpose of high temperature oxidation resistance (Fig. 5b).26 Trimethyl aluminum (TMA), water, tris-dimethylaminosilane (TDMAS), and H2O2 were used as the reactants, and the films were annealed at 1500 °C for 5 hours to be converted to the mullite phase. Reduced oxidation of silicon carbide by up to 62% could be obtained with a 10 nm ALD alumina film. Compared with the ALD alumina film, 1.5 times thicker mullite film is needed to achieve a similar high temperature oxidation resistance effect.
Titanium dioxide (TiO2) is an excellent white pigment. However, the high photocatalytic activity of TiO2 under UV irradiation leads to inevitable degradation of surrounding materials, which seriously affects its application. In order to avoid the degradation caused by the photocatalytic activity of TiO2, many efforts were made to passivate the TiO2 particles through ALD. Alumina films were deposited on the TiO2 nanoparticles by ALD in a fluidized bed reactor.27 The coated TiO2 particles showed reduced photo catalytic activity with the increasing film thickness. Liang et al. deposited aluminum alkoxide (alucone) films on TiO2 particles using TMA and ethylene glycol as the precursors.28 Methylene blue oxidation tests showed that the 20-cycle ALD alucone film could quench the photo catalytic activity of pigment-grade TiO2 particles. With a fluidized bed ALD reactor, King et al. deposited 2 nm thick SiO2 insulating films on TiO2 particles using TDMAS and H2O2 as precursors at 500 °C.29 H2SO4 digest experiment revealed that the ALD process enables high quality, fully dense SiO2 film deposition on the substrates. The photocatalytic activity of pigment-grade anatase TiO2 particles was reduced by 98% after the ALD surface passivation. A recent work by Guo et al. demonstrated that a 1 nm thick ALD Al2O3 layer could effectively inhibit the photo catalytic activity of rutile, anatase and P25 TiO2 nanoparticles, without affecting the optical absorption.30 Ceramic ALD coatings such as Al2O3 and SiO2 can inhibit the photocatalytic activity by preventing the electrons and holes produced by ultraviolet absorption to migrate to the surface, and the large band gap of ceramic oxide materials ensures the optical transparency of the coating and thus retains the bright white color of TiO2. Precise control on the thickness of the coatings allows TiO2 particles to maintain the original gloss and brightness of the pigment while inhibiting the photocatalytic activity.
As a type of potential lighting material, fluoride phosphors have attractive optical properties. However, their sensitivity to moisture may lead to phosphor degradation, and the poor chemical stability limits their applications. ALD Al2O3 and TiO2 layers were deposited onto fluoride phosphor particles to improve their chemical stability.31 Both films could cover the surfaces of the fluoride phosphor particles. The ALD Al2O3 shells suffered from delamination and blistering after deposition, whereas ALD TiO2 layers appeared conformal and uniform. With the ALD TiO2 coating, the fluoride particles obtained enhanced adhesion with the hydrophobic shells and their chemical stability towards moisture was remarkably improved. In addition, for SrAl2O4-based long afterglow phosphor particles, the aqueous durability can be significantly improved by depositing nanoscale ALD Al2O3 or TiO2 layers, as reported by Karacaoglu et al.32
In recent years powder ALD has shown potential in the field of nuclear energy. The precise processing ability and the relatively low deposition temperature are the key advantages of ALD for future applications in nuclear energy field. As an EBC material that could hinder the diffusion between U–Mo fuel and aluminum substrate, a ZrN layer is usually introduced on the surface of nuclear fuel particles by physical vapor deposition. However, due to the limitations of PVD methods, the binding between the ZrN film obtained by PVD and the substrate is relatively weak, and it is easy to delaminate under irradiation. ALD may provide a better solution than PVD. ALD zirconium nitride (ZrN) films were deposited onto natural uranium–molybdenum (U–Mo) based fuel particles (Fig. 5c) to form a diffusion barrier coating that could prevent the harmful interaction between the fuel and the aluminum matrix.33 In situ heavy ion irradiation experiment showed that the ALD ZrN film significantly improved the irradiation stability of the fuel particles. During the extended storage process of spent nuclear fuel, helium gas accumulation from alpha decay could damage the structural integrity of the fuel. ALD Al2O3 layer could serve as a barrier for the retention of helium generated from fuel particles.34 Surrogate micron-scale nickel particles were homogeneously coated with 3–20 nm thick Al2O3 films in a fluidized bed ALD reactor and were then loaded with helium at 800 °C in a tube furnace. Subsequent helium spectroscopy measurements showed that the ALD Al2O3 coating increased the helium storage capacity by up to two orders of magnitude compared to the uncoated nickel particles. Environmental barrier coatings that block hydrogen diffusion are necessary for preventing the embrittlement of uranium fuel substrate and avoiding corrosion. ALD tungsten nitride (WN) films were deposited on zirconia NPs and yttria stabilized zirconia micropowders.35 Subsequent annealing was performed to produce a crystallized tungsten coating. Differential thermal analysis (DTA) test revealed that the reaction of hydrogen with the underlying material was delayed by the ALD film. To completely eliminate the reaction, thicker ALD film or more effective EBC should be selected.
Stabilization and passivation of particles is a universal requirement for a number of different areas. More examples on this topic will be further discussed in the sections of catalyst and energetic material.
3.2 Catalyst
ALD has been widely used in the field of catalysis. ALD enables precise fabrication of nano or sub-nano surface structures, which provides a powerful tool to synthesize model catalysts. For catalysis applications complete encapsulation of particles is usually not required. Clustered or island structures formed at the early stage of film nucleation are the most important reactive sites for catalytic reactions. In most cases only a few cycles of ALD are performed. On the other hand, supports for catalysts may have much more complicated structures as well as much larger surface areas than conventional solid particles. Consequently, in each ALD cycle a very long precursor exposure is usually needed to ensure saturated surface reaction. With the unique capability of ALD in atomic layer by layer fabrication of 3D structures, precisely controlled catalytic materials could be obtained, which are beneficial for establishing the correlation between structural and chemical properties, such as active components, particle size, surface chemical states, and exposed crystal facets and their catalytic performances. Many novel catalytic structures, such as core shell structures, partially embedded structures, single atom catalysts, bimetallic catalysts, ALD overcoatings, controlled micro environments, have been fabricated, for the purpose of improving the catalytic performances in terms of activity, selectivity, and stability.36 In this section, examples will be categorized according to the type of substrate and the catalytic materials.Feng et al. studied the nucleation and growth of ALD Pd films on different oxide substrates using palladium hexafluoroacetylacetonate (Pd(hfac)2) and formalin as precursors.39 Pd nanoparticles were deposited on ZnO and Al2O3 coated mesoporous silica gel and their catalytic performances were evaluated in methanol decomposition. The ALD Pd/Al2O3 catalyst exhibited high activity, while the ALD Pd/ZnO catalyst deactivated rapidly. In situ extended X-ray absorption fine structure (EXAFS) measurements revealed that during methanol decomposition, the Pd species supported on ZnO could “dissolve” into the matrix, leading to gradually diminished activity. An overcoating layer of ALD Al2O3 could bind to Pd species and effectively prohibit the loss of activity. Based on a similar strategy, ultra small Pd particles were synthesized on alumina support by low temperature Pd(hfac)2 exposure followed by depositing ALD Al2O3 protective coatings.40 In the following high temperature reduction step, the size of the Pd particles could remain unchanged. Gong et al. studied the size distribution of Pd particles deposited on an activated carbon (AC) support by ALD.41 The AC support was pre-treated by acid reflux and high temperature calcination to tailor the surface functional groups. The average size of Pd NPs supported on AC was determined by the number of ALD cycle as well as the type and concentration of functional groups on the support (Fig. 6). Pd NPs were also deposited on Fe2O3 modified AC to form a large surface area magnetic Pd/Fe2O3/AC catalyst.42 The AC support was modified by Fe2O3 ALD followed by Pd ALD. In hydrodechlorination of 1,4-dichlorobenzene the Fe2O3 species in close contact with Pd effectively prevented the metal species from being poisoned by chloride ions and thus enhanced the catalytic performance. In another case, Gong et al. deposited TiO2 coatings inside the mesopores of a silica molecular sieve MCM-41.43 Then ALD of Pd was performed to produce a highly dispersed Pd/TiO2/MCM-41 catalyst. In selective hydrogenation of acetylene, the ALD Pd/TiO2/MCM-41 catalyst showed very good conversion and selectivity to ethylene. The spatial confinement effect from internal channels of the molecular sieve leads to a narrow size distribution of Pd nanoparticles and effectively prevents sintering of particles during the high temperature treatment. The small, uniform Pd nanoparticles densely distributed in the meso-pores of MCM-41 significantly enlarged the number of active sites and lowered the reaction temperature.
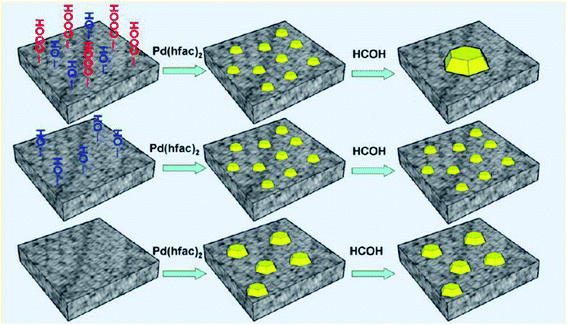 | ||
| Fig. 6 Schematic representation on effects of surface functional groups on Pd particle size synthesized by ALD. Reprinted with permission from ref. 41. Copyright (2015), American Chemical Society. | ||
Platinum (Pt) is also widely used as an industrial catalyst in the areas of fuel cell, automotive and petroleum industries, despite its high price. Lashdaf et al. proposed for the first time that ALD could be used to deposit Pt on silica or alumina support to make a supported catalyst.44 Goulas et al. reported the method to synthesize platinum nanoclusters on TiO2 NPs with an atmospheric pressure fluidized bed ALD reactor using trimethyl methylcyclopentadienyl platinum (MeCpPtMe3) and ozone as reactants.45 Homogeneously distributed and highly dispersed Pt nanoclusters with a narrow size distribution were obtained. Using the same ALD reaction Pt NPs were also uniformly supported on carbon nanotubes (CNT).46 The Pt/CNT catalyst showed excellent catalytic performance in the hydrolysis of ammonia borane. Covering the Pt/CNT with a porous ALD TiO2 layer could further improve the durability of the catalyst. Dendooven et al. reported a strategy to achieve independent control over the Pt particle size and coverage by combining two distinct ALD processes of Pt.47 The particle density was controlled by tuning the ALD cycle using MeCpPtMe3 and oxygen as the precursors, while the particle size was controlled by subsequent growth using the same Pt precursor and nitrogen plasma. X-ray fluorescence (XRF) and grazing incidence small-angle X-ray scattering (GISAXS) measurements confirmed a good control of the loading, density, and average size of Pt particles. This method demonstrates the feasibility of independently controlling the size and coverage of metal particles by combining different ALD processes, which is usually difficult to achieve through a single ALD process. Leus et al. reported the synthesis of ALD Pt NPs confined in a metal organic framework (MOF) material.48 ALD of Pt was conducted at 200 °C using MeCpPtMe3 and O3 as precursors. The utilization of O3, which is a more powerful oxidizer than oxygen, makes it possible to remove the ligand at a lower temperature, which may help prevent coalescence of Pt particles. The structure of the metal organic skeleton was well maintained during the ALD process. By XRF and TEM analysis, it was confirmed that Pt NPs were highly dispersed in the MOF structure. The obtained catalyst exhibited excellent activity in hydrogenation of different linear and cyclic olefins.
High cost is the major drawback of noble metal catalysts. Since only the atoms exposed on the surface of catalyst particles can participate in the catalytic reaction, researchers have been trying to downsize catalyst particles to maximize the utilization of noble metal species. To this end, fabricating single atom noble metal catalyst would be an ideal solution. However, the synthesis and stabilization of single atom catalyst is a big challenge, because metal atoms are extremely mobile and are very easy to sinter under reaction conditions. One possible way is to anchor the single atoms into the surface defects of oxide supports. ALD has been proved to be an important method for the synthesizing and investigating of single atom catalyst.49 Single-atoms and sub-nanometer clusters of Pt were supported on the surface of graphene nanosheets using ALD.50 Very limited anchoring sites for Pt precursor were distributed on the inert surface of graphene, thus effectively prevented agglomeration of Pt atoms. Compared with traditional Pt/C catalysts, these catalysts showed higher activity and excellent CO tolerance in methanol oxidation reaction, which was attributed to low-coordinated and unsaturated 5d orbitals of the single Pt atoms. Using a similar strategy, Cheng et al. synthesized nitrogen-doped graphene nanosheets supported single platinum atoms and clusters for hydrogen evolution reaction (HER) through ALD.51 Compared with commercial Pt/C catalysts, the catalytic activity was significantly improved by up to 37 times. Pt single atoms were also deposited on the MOF-derived N-doped carbon through ALD to produce single-atom catalysts. The catalyst showed 6.5 times higher activity in oxygen reduction reaction (ORR). The electronic structure of Pt single atoms can be tuned by the co-adsorption of hydroxyl and oxygen, which significantly reduces the free energy change of the rate-determining step and thus enhances the ORR activity.52 From the above examples, it can be noticed that the selection of support materials with defect sites, such as nitrogen-doped graphene nanosheets and MOF-derived N-doped porous carbon, may be an effective way to synthesize and stabilize single atoms through strong metal support interaction.
To compare the effects of single atom and nanoparticle catalysts, Pt was loaded onto mesoporous zeolite HZSM-5 with different ratios of Pt single atoms to nanoparticles using ALD at high temperature or solution grafting at room temperature.53 Catalytic performances in CO oxidation and low temperature water–gas shift (WGS) reactions were studied. IR spectroscopy characterization using CO as a probe molecule confirmed that the activity for CO oxidation and low temperature WGS reaction were only observed with Pt nanoparticles but not with Pt single atoms. This result demonstrates that the strategy of using single atoms to improve the catalytic efficiency may not work for all reactions. Yan et al. reported the method of synthesizing Pt2 dimers on graphene using ALD.54 A schematic illustration of the synthetic process is shown in Fig. 7. Pt single-atoms were deposited on the nucleation sites created before, then secondary Pt atoms were attached selectively to the Pt atoms previously deposited. In the reaction of ammonia borane hydrolysis, the Pt2/graphene catalyst showed impressive activity, which was about 17 times higher than graphene supported Pt1 single-atoms and 45 times higher than Pt NPs, respectively. The decreased adsorption energy of ammonia borane and H2 molecules on Pt2 dimers may be the main reason for the promoted activity.
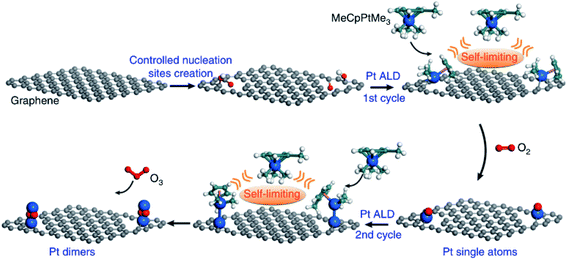 | ||
| Fig. 7 Schematic illustration of the bottom-up synthesis of dimeric Pt2/graphene catalysts. Creation of isolated anchoring sites on pristine graphene; followed by one cycle of Pt ALD at 250 °C for the formation of Pt single atoms; the second cycle of Pt ALD was performed at 150 °C to selectively deposit the secondary Pt atoms on the preliminary ones for the formation Pt2 dimers. The balls in cyan, white, red, and blue represent carbon, hydrogen, oxygen, and platinum while the ball in gray represents carbon atoms in the graphene support. Reprinted with permission from ref. 54. Copyright (2017), Springer Nature. | ||
Single atom Pd catalysts could also be fabricated by ALD. A method of synthesizing single atom Pd1/graphene catalyst (Fig. 8) by performing one cycle of ALD Pd on high temperature annealed graphene was reported.55 In selective hydrogenation of 1,3-butadiene, the Pd1/graphene catalyst showed ∼100% butene selectivity with 95% conversion under a mild reaction condition. The high selectivity may be attributed to the changed adsorption mode of 1,3-butadiene as well as the enhanced steric effect on Pd atoms. The same group also synthesized single atom Pd1/C3N4 catalyst for acetylene hydrogenation reaction, and enhanced selectivity to ethylene and coking resistance were obtained.56
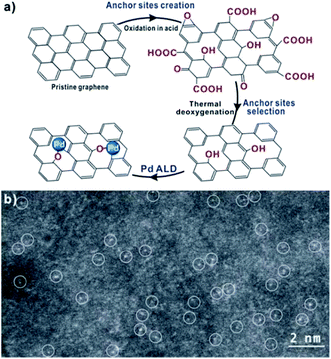 | ||
| Fig. 8 (a) A schematic illustration of single-atom Pd1 catalyst synthesized on high temperature treated graphene through anchoring site creation, selection, and Pd ALD. (b) Representative HAADF-STEM images of Pd1/graphene (atomically dispersed Pd atoms are highlighted by white circles). Reprinted with permission from ref. 55. Copyright (2015), American Chemical Society. | ||
Besides noble metal catalysts, some other active transition metal elements, including Ni, Cu, Fe, and Co, can also be synthesized by ALD. Similar to ALD noble metal catalysts, highly dispersed transition metal NPs or clusters with precisely controlled structures could be generated on a variety of supports, and these highly dispersed metal species can effectively promote the efficiency of active materials. For example, a nickel (Ni) NP catalyst was prepared by performing ALD of Ni on a porous γ-Al2O3 powder using bi(cyclopentadienyl) nickel (NiCp2) and hydrogen as precursors.57 The γ-Al2O3 supported Ni NP catalyst exhibited both high activity and stability in high temperature dry reforming of methane (DRM). The improved performance was attributed to the formation of NiAl2O4 that could be reduced to highly dispersed metallic Ni during the DRM reaction. Through ALD of Cu on a mesoporous silica using Cu(thd)2 (thd = 2,2,6,6-tetramethyl-3,5-heptanedionate) and ozone as precursors, a Cu/SiO2 catalyst was prepared.58 In low temperature WGS reaction, the ALD Cu/SiO2 catalyst exhibited excellent activity, which was related to defect sites on small Cu particles or clusters that may induce strong interactions with the support.
Porous alumina particles were synthesized by depositing thin films of Al2O3 on a highly porous poly styrene-divinylbenzene (PS-DVB) template via ALD, followed by high temperature calcination to remove the organic template.59 The deposition was held at 33 °C with TMA and water as precursors in a fluidized bed reactor. The obtained porous Al2O3 particles had a surface area of 80–100 m2 g−1 and could be used as a template or support material.
VOx were deposited onto an alumina support by ALD, using vanadium oxytriisopropoxide (VOTP) and hydrogen peroxide (H2O2) as precursors.60 A series of VOx/Al2O3 catalysts with precisely controlled structures were synthesized by varying the number of VOx ALD cycle. In the reaction net work of cyclohexane ODH, mono vanadate was found to favor the formation of olefin, while poly vanadates facilitated the direct conversion from cyclohexane to benzene.61 Catalytic performances of the series of ALD VOx/Al2O3 catalysts were also investigated in oxidative dehydrogenation of ethylbenzene with CO2 (CO2-ODEB). In this reaction, isolated VOx monomer was discovered to be more active and stable than the polymeric VOx and crystalline V2O5. V–O–Al bonds were considered as the major active sites for CO2-ODEB.
NiO was deposited on mesoporous SiO2 by means of ALD using NiCp2 and H2O as precursors.62 The NiO NPs were uniformly distributed on the mesoporous SiO2 support. The ALD NiO/SiO2 catalyst was very effective for catalyzing toluene combustion reaction below 200 °C. The high activity was attributed to the large surface area of the mesoporous SiO2 substrate, which could significantly increase the loading of highly dispersed catalytic species and accelerate the oxidation of toluene by enhancing the diffusion of adsorbed toluene from SiO2 to NiO. Similarly, carbon nanotube (CNT) supported NiO NPs were prepared through ALD using NiCp2 and O3 as precursors.63 Due to the lack of defect sites on CNT, it is difficult to directly grow oxides on the surface of CNT. In this study, O3 played both the roles of the defect forming agent and the oxygen source. In the electro-oxidation of methanol, the ALD NiO/CNT catalyst showed significantly improved electrochemical catalytic activity and stability. The enhanced catalytic performance was resulted from the strong interaction between NiO and CNTs, which prevented NiO NPs from dissolution, Ostwald ripening, or aggregation during the reaction.
Singh et al. performed ALD of TiO2 to fabricate the surface of a fibrous nano-silica (KCC) support using titanium tetraisopropoxide (Ti(OiPr)4) and H2O2 as precursors to make an effective photocatalyst.64 Conformal coatings of TiO2 on KCC were obtained. After proper heat treatment uniformly dispersed ultra small TiO2 NPs were formed. In photocatalytic degradation of organic pollutants, activity of the ALD TiO2/KCC catalyst was much higher than many other reported catalysts. The superb catalytic performance was resulted from the combined effects of the unique mesoporous structure of KCC that facilitated diffusion of reactants and the size quantization effect of TiO2 NPs. Chen et al. proposed a strategy to synthesis carbon supported nanoporous nitrogen-doped TiO2 photocatalysts using an ABCB four-step ALD reaction sequence in which titanium tetrachloride (TiCl4, precursor A), ethanolamine (precursor B), and malonyl chloride (precursor C) were used as the precursors.65 After 200 cycles of deposition, the obtained film was annealed at 350 °C and the nanoporous structure was formed. The nanoporous catalyst exhibited excellent photocatalytic activity in the degradation of methylene blue even with visible light radiation. The photocatalytic activity of SnO2 NPs can be enhanced by forming core–shell NPs through ALD, as reported by Podurets et al.66 Ultra thin (<10 nm) ZnO and TiO2 films were deposited onto SnO2 NPs. In photocatalytic degradation of methylene blue under UV-Vis irradiation, full degradation was achieved within 10 min using the core–shell catalysts. The promoted photocatalytic activity was attributed to the core–shell structure which adjusted the band gap value.
Highly dispersed ZnO species were deposited onto ZSM-5 and Y zeolites by ALD using diethyl zinc (DEZ) and H2O as precursors.67 With the incorporation of ZnO, Brønsted acid sites on Y zeolite were completely removed, while those on ZSM-5 were only partially removed. Activities of both types of zeolites were significantly enhanced in the reaction of propane dehydrogenation and aromatization. ZnO species (Lewis acid sites) promoted the dehydrogenation reaction, while subsequent oligomerization and cyclization reactions required participation of Brønsted acid sites.
Iron oxide NP catalyst supported on reduced graphene oxide (rGO) was prepared by ALD using bi(cyclopentadienyl) iron (FeCp2) and oxygen as precursors.68 Fe2O3 NPs with a narrow size distribution were homogenously dispersed on the rGO nanosheets. The ALD Fe2O3/rGO catalyst exhibited high catalytic activity for thermal decomposition of ammonium perchlorate (AP). The enhanced catalytic performance was attributed to the optimized spatial distribution of Fe2O3 NPs supported on the rGO nanosheets and the synergistic effect between them. Porous Fe2O3 nanotubes were prepared by ALD using different kinds of iron containing metal–organic compounds and organic constituents as precursors on a carbon fiber support.69 After proper high temperature annealing porous nanotubes with tunable pore size and α–γ phase junctions were obtained. The porous Fe2O3 nanotubes exhibited superior activity for photo-Fenton reaction. The porous nanotubes produced using iron tert-butoxide (ITBT) and ethylene glycol (EG) as precursors showed the highest photo catalytic activity.
In addition to metal oxides, sulfide materials grown by ALD have aroused attention in the field of catalysis. The ALD processes of metal sulfide materials usually use metal organic compounds and hydrogen sulfide (H2S) as precursors.70 The procedures of metal sulfide ALD are very similar to those of metal oxide ALD. Cobalt sulfide was deposited onto a MOF material named NU-1000 using bis(N,N′-di-i-propylacetamidinato) cobalt(II) (Co(amd)2) and H2S as precursors.71 Cobalt sulfide was uniformly deposited throughout the MOF structure without destroying the crystallinity and porosity of the support. The resulting ALD in MOF (AIM) material exhibited higher catalytic efficiency than cobalt oxide and CoSx reference materials in selective hydrogenation of m-nitrophenol to m-aminophenol, as a result of the high dispersity of CoSx sites. The same group also reported the synthesis of NiS-AIM catalysts.72 Few-atom clusters of nickel sulfide was synthesized on the NU-1000 MOF support by ALD using bis(N,N-di-tert-butylacetamidinato) nickel(II) and H2S as precursors. The NiS-AIM catalyst exhibited excellent activity in a photocatalytic hydrogen evolution reaction (HER). The rate of H2 production reached 4.8 mmol g−1 h−1. A NU-1000 MOF supported iron-thiolate photocatalyst was also synthesized through an MLD process, using iron amidinate coordination complex and 1-dodecanethiol as precursors.73 When exposed to light, the MOF supported iron-thiolate catalyst can convert nitrate into ammonium ions. These results indicate that depositing catalytic components on the active nodes of chemically stable MOF materials using ALD or MLD is a very promising method of synthesizing MOF supported catalysts.
Molybdenum disulfide (MoS2) has been considered an ideal material to replace noble metals as a high-performance electrocatalyst in HER, due to its low cost and high activity. NiSx@MoS2 heterostructure electrocatalysts with controllable interface properties were synthesized by depositing NiSx on MoS2 nanosheets using ALD.74 Compared with single-phase MoS2 and NiSx, NiSx@MoS2 heterojunctions have lower over potential and faster reaction kinetics, which greatly enhanced the HER activity. The improved catalytic performance is attributed to the optimized adsorption of reaction intermediates and the promoted charge transfer near the MoS2/NiSx interface. In addition to the high activity, NiSx@MoS2 heterojunctions also showed high stability in the alkaline medium.
Metal sulfides synthesized by ALD are very promising catalytic materials. However, in most ALD processes of metal sulfides, highly toxic, explosive, and corrosive H2S is used as the sulfur precursor, which seriously limits the large-scale application. In order to solve this problem, an organic sulfur precursor, di-tert-butyl disulfide (TBDS) was developed to replace H2S for metal sulfide ALD.75 It has been proved that the new ALD process using aminated nickel and TBDS precursors has ideal ALD growth behaviors, and high quality NiSx films can be prepared. The new ALD process of metal sulfide that avoids the use of H2S will greatly favor the applications of ALD metal sulfides in catalysis and nanoscience.
In the work of Christensen et al., a method of synthesizing Pt–Ru NPs by sequential ALD of Pt and Ru on an Al2O3 powder was demonstrated.77 The composition of the NPs could be controlled by varying the ratio of Pt to Ru ALD cycles, while the particle size could be controlled by the total number of ALD cycles. These bimetallic catalysts exhibited improved activity for methanol decomposition compared to a mixture of supported Pt and Ru NPs.
Atomic scale controllable synthesis of Pd/Pt and Pt/Pd core–shell NPs via area-selective ALD was reported.78 The method involves pre-treating the substrate using self-assembled monolayers (SAMs) of octadecyl trichlorosilane (ODTS). The formation time of the SAM was controlled to obtain pinholes, in which hydroxyl (–OH) groups were exposed as the intended nucleation sites for metal species. Since the surface ODTS SAMs can effectively prevent the generation of new nucleation sites, the second metal was selectively deposited on the previous nucleation sites to form the Pd/Pt and Pt/Pd NPs with uniform core–shell structures and a narrow size distribution (Fig. 9). The SAMs-assisted area selective ALD is a typical strategy to achieve selective ALD growth. This method can be easily applied to synthesize other core–shell structured NPs with different components, which provides a convenient and flexible way of manufacturing novel nano structures.
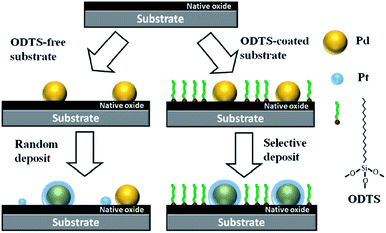 | ||
| Fig. 9 Schematic illustration for fabricating core–shell NPs through area-selective ALD on ODTS modified substrate. Reprinted with permission from ref. 78. Copyright (2015), Springer Nature. | ||
In an ALD process nucleation of metal on the surface of different supports is dependent on reaction conditions such as atmosphere and temperature. Lu et al. reported low temperature selective ALD to synthesis supported bimetallic NPs.79 With careful selection of the co-reactants and deposition temperature, the secondary metal would prefer to grow on the surface of primary metal NPs, rather than on the oxide support (Fig. 10).
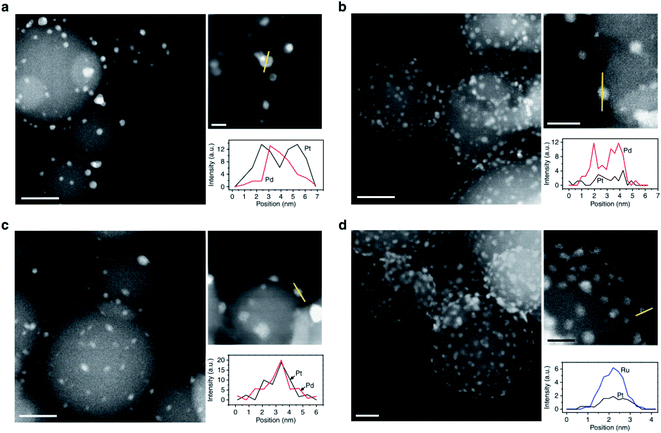 | ||
| Fig. 10 Structures of ALD bimetallic NPs. Aberration-corrected HAADF-STEM images and corresponding EDS line profiles of (a), 5 Pd-core 15 Pt-shell; (b), 12 Pt-core 20 Pd-rich-shell; (c), 12 Pt 10 Pd alloy; and (d), 1 Pt-core 35 Ru-rich-shell bimetallic NPs on spherical alumina support. Reprinted with permission from ref. 79. Copyright (2014), Springer Nature. | ||
ALD can also be combined with other techniques to synthesize supported bimetallic catalysts. In the report of Wang et al., an Au/SiO2 catalyst was first synthesized using a deposition–precipitation method, then Pd was selectively deposited only on the surface of Au NPs by choosing a proper deposition condition.80 Thus Pd@Au core–shell bimetallic NPs were formed on the silica support. Atomic level precise adjustment of the Pd shell thickness could be achieved by changing the Pd ALD cycle. In solventless oxidation of benzyl alcohol, the best catalytic performance was obtained with a Pd shell thickness of 0.6–0.8 nm due to the optimum synergistic effect between the two metals.
Featuring the most typical ALD reaction, Al2O3 is the first reported ALD overcoating material. For example, supported palladium NPs were coated by 45 ALD cycles of Al2O3 at 200 °C using TMA and water as precursors.81 The catalysts were tested in ODH of ethane to ethylene. After reaction at 675 °C for 28 hours, no visible change in the morphology of the catalyst was observed. Thermogravimetric analysis confirmed that coke formation was effectively inhibited. In another report, an effective approach to stabilize Pd/SiO2 catalysts with porous ALD Al2O3 overcoating was demonstrated. Octahedra Pd NPs were supported on SiO2 nanospheres, and then 40 cycles of Al2O3 ALD were performed to coat the Pd/SiO2 catalyst.82 Thermal treatment introduced pores to the Al2O3 overcoating and the embedded Pd particles were partially exposed. The ALD Al2O3 overcoated Pd/SiO2 catalyst can efficiently catalyze methane combustion between 200 and 850 °C without significant deactivation. Applications of ALD Al2O3 overcoated Pd catalysts were also reported in many others catalytic reactions including methanol decomposition,83 hydrogenation of furfural,84 and hydrogenation of 1,3-butadiene.85
ALD coatings of MnOx were also used to decorate Pd catalysts. Al2O3 supported Pd NP catalysts were prepared by wet impregnation, then ALD of manganese oxide was performed using tris(2,2,6,6-tetramethyl-3,5-heptanedionato)manganese(III) and ozone as precursors.86 The ALD MnOx coatings selectively passivated Pd (111) facets, and thus prevented decarbonylation of benzaldehyde and inhibited the formation of toluene byproduct, besides a high yield of benzaldehyde was obtained.
ALD Al2O3 coating was deposited on supported Pt NPs as the stabilizing layer against particle coarsening. In situ synchrotron grazing incidence small angle X-ray scattering measurements revealed that the ALD Al2O3 stabilizing layer successfully prevented Pt particle from coarsening during thermal annealing and improved the Pt surface accessibility.87 A selective ALD method was developed to modify Pt NPs with nickel oxide (NiOx) overcoating to improve the catalytic performance.88 NiOx can be selectively deposited on the low coordination sites of the Pt NPs during the initial growth period. Fourier transform infrared (FTIR) characterization and density functional theory (DFT) simulations proved that the source of selectivity is the intrinsic binding energy discrepancy of the nickel precursor on the platinum site. The ALD NiOx/Pt/Al2O3 catalyst exhibited enhanced catalytic activity for CO oxidation, which could be attributed to the formation of a highly reactive metal oxide interface. Moreover, the sintering resistance ability of the overcoated catalyst was also significantly improved.
It is generally accepted that the catalytic activity of supported gold catalysts is largely dependent on the size of Au NPs, but the cause of this particle size effect is unclear. Yao et al. applied ultra thin TiO2 overcoats of different thicknesses onto Au/TiO2 catalysts with different Au particle size.89 Combining the results of high-resolution TEM and XPS characterization and CO oxidation reaction, they proved that CO adsorption at the low coordination Au site is not the rate-determining step. Furthermore, the particle size effect in CO oxidation actually reflects the effects of perimeter of Au particles at the Au–TiO2 interface. This example demonstrates that, with the help of highly controllable ALD method, many predetermined catalytic structures can be precisely synthesized, which are extremely useful for revealing the mechanism of catalytic reactions.
ALD overcoating can also be applied to non-noble metal catalytic systems. O'Neill et al. reported that copper catalysts can be stabilized by ALD Al2O3 overcoats.90 In liquid-phase hydrogenation of furfural to furfuryl alcohol, the ALD Al2O3 overcoat decreased catalyst deactivation by preventing sintering and leaching of the metal catalyst (Fig. 11a). ALD Al2O3 and TiO2 overcoats were also used to enhance the stability of a Cu based catalyst in furfural hydrogenation, and the effects of different types of overcoats were compared.91 In situ TPR/TPO measurements revealed that TiO2 had less modification effect on Cu than Al2O3. In the furfural hydrogenation reaction, the catalyst overcoated by ALD TiO2 exhibited enhanced stability with unaffected activity.92 After calcination at 500 °C, the interaction between the TiO2 overcoat and the underlying catalyst was strong enough to inhibit the migration and steric hindrance of chromite, but it was much weaker than that between the Al2O3 overcoat and copper chromite, which could reduce the catalytic activity.
 | ||
| Fig. 11 TEM images of (a), AlN and TiO2 bilayer film coated particles, dashed arrows point to the ALD overcoat; Reprinted with permission from ref. 90. Copyright (2013), John Wiley and Sons. (b) The TiO2/Co/TiO2 catalyst calcined at 873 K; (c), the used TiO2/Co/TiO2 catalyst. Reprinted with permission from ref. 93. Copyright (2014), Royal Society of Chemistry. (d) STEM image of TiO2/AlN/Fe NPs. Reprinted with permission from ref. 94. Copyright (2010), American Chemical Society. | ||
Protective ALD TiO2 overcoats were applied to cobalt particles supported on TiO2 (Fig. 11b and c).93 For catalysts with ALD TiO2 overcoats, high catalytic activity was maintained during aqueous-phase hydrogenation (APH) reactions. In contrast, the activity of the uncoated cobalt catalysts dropped quickly due to leaching of the active phase. The ALD TiO2 overcoat stabilized cobalt catalysts were proved to be effective for APH of a series of reactants including xylose, furfuryl alcohol, and furfural, in which the uncoated Co catalysts suffered from severe deactivation.
Iron-based magnetic photo-active NPs were modified by ALD of TiO2 and aluminum nitride (AlN).94 The iron NPs were produced by thermal decomposition of iron oxalate, and then TiO2 films were deposited on the iron NPs in a fluidized bed ALD reactor at 100 °C using TiCl4 and H2O2 as precursors. To prevent oxidation of iron particles, 100 cycles of AlN was performed at 250 °C with TMA and ammonia (NH3) as precursors before the TiO2 deposition (Fig. 11d). The AlN overcoat successfully inhibited iron oxidation while the magnetic moment was not affected. The ALD TiO2 film provided photo catalytic activity for the magnetic NPs, as demonstrated by successful decomposition of a methylene blue (MB) solution under ultraviolet irradiation.
3.3 Energetic material
Energetic materials can store a large amount of chemical energy which can be quickly released upon thermal, mechanical, or electrical actuation. They have found extensive applications in explosives, propellants, and other energy related fields.95 Thermal energy released from exothermic reactions of metal powder fuels has also been widely used. For example, thermite reaction, which is based on the solid state redox reaction between the metal fuel and the oxidizer, can release twice the amount of heat compared to conventional monomolecular energetic materials.96 With the help of ALD, researches have been working on synthesizing new types of nanothermite materials and exploring proper surface modifications of metal fuels to improve their safety, stability, and energy release performances.In general, the diffusion path between oxidant and fuel plays a decisive role in the rate and power of the thermite reaction. In order to obtain faster energy release and higher energy efficiency, oxidants and fuels should be mixed uniformly and the sizes of oxidant/fuel particles should be reduced to nano scale to shorten the diffusion path. Traditional methods of preparing energetic composites, such as sol–gel method, self assembly, and arrested reactive milling, fail to achieve accurate control of the composite structure or uniform mixing of the oxidant and the fuel. Core–shell structured nanothermite materials synthesized by ALD enable uniform combination as well as close contact of the oxidant and the fuel on nanometer scale. Ferguson et al. attempted to deposit oxidizer films on Al NPs to make nanothermites for the first time.97 SnO2 films were deposited on Al NPs using SnCl4 and H2O2 as precursors in a fluidized bed ALD reactor. However, insufficient reactant exposure and unsaturated surface reaction led to a fuel/oxidizer ratio largely deviated from the stoichiometry of the thermite reaction. Nevertheless, SnO2 coated Al NPs reacted much more quickly and violently than the uncoated Al particles. This work was complemented by Qin et al. in 2013.98 By carefully adjusting the ALD pulse sequence of SnCl4 and H2O2, perfect encapsulation of Al NPs by SnO2 layers was achieved and Al@SnO2 core–shell structured nanothermite material was synthesized (Fig. 12a and b). Laser ignition test showed that the resultant nanothermite reacted several times faster than the mechanical mixture of nanopowders. Similarly, Fe2O3 was also deposited onto the surface of Al NPs to produce Al@Fe2O3 core–shell structured nanothermite.99 Compared with mechanically mixed Al–Fe2O3 nanopowders, the Al@Fe2O3 nanothermite had a lower ignition temperature, a larger energy release, and much faster reaction rate. The improved energy performance was attributed to the intimate contact and the uniform distribution of reactants on nanometer scale, which effectively reduced the average mass diffusion distance between the oxidizer and the metal fuel. Yan et al. reported the synthesis of a rGO/Al@Fe2O3 energetic composite by ALD.100 Al NPs were first anchored on reduced graphene oxide (rGO) nanosheets, then Fe2O3 films were deposited onto the rGO/Al substrate by ALD, producing the rGO/Al@Fe2O3 energetic composite. The rGO/Al@Fe2O3 composite containing 4.8 wt% of rGO showed a 50% increase of the energy release compared to the Al@Fe2O3 nanothermite. The further improved energy performance was attributed to the dispersion of Al NPs on the surface of rGO nanosheets, which successfully prevent the agglomeration of Al particles during ALD of Fe2O3.
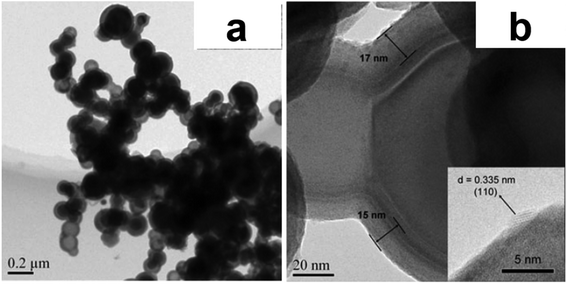 | ||
| Fig. 12 SEM (a) and TEM (b) images of 225-cycle ALD SnO2 coated Al nanopowder. Reprinted with permission from ref. 98, Copyright (2013), Springer Nature. | ||
Surface passivation is important for energetic materials sensitive to air and water. Spontaneous combustion of energetic materials during storage, transportation, or handling are serious safety problems limiting the applications of novel energetic materials. Besides, composition change caused by oxidation or disintegration will reduce the energy density and change the energy release pattern. Consequently, sometimes it is necessary to carry out surface modification of high energy density materials to improve their safety and stability without deteriorating the energy performance. Aluminum hydride (AlH3) is a very promising high energy material. However, its high reactivity with H2O may lead to hydrogen release under ambient conditions. Chen et al. produced conformal passivation layers on α-AlH3 particles by depositing Al2O3 films via ALD.101 In hydrothermal aging tests, the passivated AlH3 powder showed significantly improved stability with the presence of H2O vapor. At elevated temperatures the dehydrogenation rate of the passivated AlH3 is not much affected, indicating that ALD is a viable technique to stabilize AlH3 without affecting its energy release capability.
Al powder is the most widely used metal fuel in many energetic systems, and the stability of Al particles is mainly due to the native oxide (Al2O3) layer. However, under certain conditions the surface native oxide layer is not able to stabilize Al particles. Al NPs were passivated using an ultrathin zirconia (ZrO2) coating by ALD.102 The hydrophobic ALD ZrO2 passivation film successfully prevented the reaction between Al NPs and hot water at 80 °C (Fig. 13). The improved barrier property of ALD Al2O3/ZrO2 laminated film might be a consequence of the ZrAlxOy phase formation at the interface of Al2O3 and ZrO2.
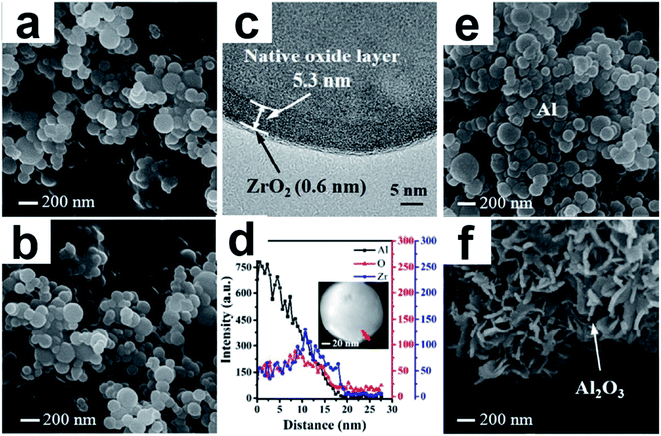 | ||
| Fig. 13 SEM images of Al@Al2O3@ZrO2-8 before (a) and after (b) stability test at 80 °C; TEM image (c) and element line scanning (d) of Al@Al2O3@ZrO2-8 after stability test; SEM images of Al@Al2O3@Al2O3-50 before (e) and after (f) stability test at 60 °C. Reprinted with permission from ref. 102. Copyright (2018), American Chemical Society. | ||
Zr powder is type of metal fuel featuring high volumetric energy density. However, safety issues such as uncontrolled oxidation and ultra-high electrostatic discharge (ESD) ignition sensitivity restrict the its applications. Qin et al.103 applied molecular layer deposition (MLD) of polyimide to encapsulate Zr particles, and the following high temperature annealing converted the polymer films to carbon films. The polymer and carbon coatings greatly reduced the ESD sensitivity of the Zr powder without affecting the energy release. ALD Al2O3 coatings were also deposited on Zr particles. The ALD Al2O3 coating could form a dense gas diffusion barrier, which effectively suppressed oxidation of the Zr powder up to about 700 °C.104 The ignition delay of the Zr powder was extended and the ESD ignition sensitivity was reduced.
So far, reports on surface modification of energetic materials using ALD are only about enhancing their stability and safety. In fact, the energy release processes of energetic materials are also closely related to surface physical and chemical properties, and ALD is an effective approach to regulate these properties. Therefore, ALD may also provide an important tool to control the energy release processes of various energetic materials by precise modifications on surface composition and structure.
3.4 Electrode material
Lithium-ion batteries (LIB) and capacitors (LIC) are among the most popular energy storage devices for applications in portable electronics and electrical vehicles. However, problems such as high cost, volume change during charging, degradation of electrodes, and safety concerns are limiting the applications of LIB and LIC.105–107 Composition and structure of the electrode material, especially the surface chemistry between the electrode and the electrolyte greatly influences the electrochemical properties of batteries and capacitors.108 By applying ultrathin ALD coatings of merely several nanometers, performances of the electrochemical materials can be remarkably improved. Many factors, such as reduced side reactions, promoted ion diffusion, and suppressed volume changes, may be responsible for the improved performances, however the exact mechanism still needs further investigation. In this section some typical applications of ALD for the fabrication of electrode materials used in LIB and LIC are introduced. Several examples of other energy devices, such as sodium ion batteries (SIB), solid oxide fuel cells (SOFC) and solar cells are also included. More detailed studies focusing on applications of ALD in the battery field can be found elsewhere.106,107,109–111Previous studies have shown that the LIB performance can be improved if LiCoO2 cathode material is coated with metal oxides using wet chemical techniques. In the research of Jung et al., the LiCoO2 powder was coated with an ultrathin Al2O3 film by performing only two cycles of ALD.112 The ALD Al2O3 coated LiCoO2 powder exhibited 89% capacity retention after 120 charge–discharge cycles, which is 100% higher than that of the bare LiCoO2 powder. The underlying mechanism may be attributed to the barrier property of ALD Al2O3 film that can minimize Co dissolution or reduce surface electrolyte reactions. Li and co-workers studied the effects of ALD TiO2, ZrO2 and Al2O3 coatings on the electrochemical performance of LiCoO2 electrode.113 It was found that the improvement of battery performance is closely related to the type of coating material and that a dense coating on the LiCoO2 powder may affect the diffusion of Li+ ions and lower the battery performance.
A LIB cathode material Li1.2Ni0.13Mn0.54Co0.13O2 was coated by nanolayers of Al2O3 and TiO2 through ALD.114 The ALD Al2O3 film was smooth and conformal, while the TiO2 layer appeared to be composed of tiny particles. During repeated charging and discharging cycles, the ALD Al2O3 film improved the stability of the cell. The TiO2 layer was more reactive with lithium and a LixTiO2 interface was formed, which slightly increased the cell capacity but the repeated insertion/extraction processes of Li+ ions caused erosion of the surface TiO2 layer and resulted in decreased cell performance.
Lithium-rich layered materials are among the most competitive cathode materials for next-generation electric vehicles. However, a major problem of these materials is the oxygen release during initial charging, which may result in lower initial coulombic efficiency (CE), strong electrolyte oxidation, and thermal instability. According to the report of Xiao et al.,115 the surface of a Li2MnO3 cathode material was protected by an aluminum phosphate (AlPO4) coating synthesized by ALD using TMA, trimethylphosphate ((CH3)3PO4, TMPO), and water as precursors. During the ALD process, part of the C2/m Li2MnO3 phase transformed into a spinel phase which effectively suppressed the release of oxygen, and the initial CE could be significantly enhanced. Compared to the unprotected cathode material, ALD AlPO4 coated cathode material was more stable at high temperatures.
Ultrathin cerium dioxide (CeO2) films were coated on the surface of LiMn2O4 powder by ALD, using tris(i-propylcyclopentadienyl)cerium (Ce(iPrCp)3) and deionized water as precursors.116 The as-prepared cells performed high stability and high initial capacity retention after 1000 charge–discharge cycles. The improved cell performance was explained by suppression of the impedance rise and facile transport of the species.117 ALD of CeO2 was also applied to modify the surface of a Li-rich layered material made from Li1.2Mn0.54Ni0.13Co0.13O2 (LRNMC) particles.118 Compared with the uncoated LRNMC particles, the surface modified samples showed 8% higher capacity and significantly improved capacity retention after 400 charge–discharge cycles. The efficacy of the CeO2 film was interpreted as increasing the substrate conductivity and blocking the dissolution of metal irons.
The most commonly used electrolyte material, lithium hexafluorophosphate (LiPF6), may generate hydrofluoric acid (HF) when it encounters water. Therefore LiPF6 may inevitably contain HF, which might etch metal oxide coatings. Metal fluorides could be more stable against HF corrosion than metal oxides. Jackson et al. developed the ALD process of AlF3 in which TMA and TaF5 were used as precursors.119 Conformal AlF3 films were deposited onto a LiNi0.5Mn0.3Co0.2O2 (NMC) Li-ion battery cathode material. The coin cell with coated cathode particles exhibited significantly improved charge capacity at high discharge rates.
Besides LIBs, cathode materials of other batteries or capacitors were also modified with ALD protective layers. For example, ALD Al2O3 layers were reported to be effective for enhancing the cyclic stability of a Na2/3(Mn0.54Ni0.13Co0.13)O2 (MNC) cathode for sodium ion batteries (SIB).120 The cathode coated with 2 ALD cycles of Al2O3 exhibited the best electrochemical stability and rate performance, while the one coated with 10-cycle Al2O3 showed the highest coulombic efficiency. The enhanced electrochemical stability may be a consequence of the protective effect and the high bandgap energy offered by the ALD Al2O3 coating. Furthermore, the oxide coating could provide structural stability against mechanical stresses during cycling. ALD Al2O3 coatings were also applied to LiNi0.5Co0.2Mn0.3O2 (NCM) particles as the cathode material for lithium ion capacitor (LIC).121 By inhibiting the dissolution of transition metal in NCM and releasing internal stress change during cycling, the ALD Al2O3 coatings significantly improved the cycling capability and rate performance of the capacitor.
A method to stabilize nanostructured solid oxide fuel cell (SOFC) cathode through ALD was proposed.122 By covering the highly active but unstable cathode La0.6Sr0.4CoO3−δ (LSCo) with a thin ALD ZrO2 layer, the fuel cell maintained a high oxygen reduction reactivity (ORR) at 700 °C for 4000 hours (Fig. 14). With the ALD ZrO2 coating, the polarization area specific resistance and the degradation rate were reduced by a factor of 19 and 18 respectively. The ALD ZrO2 coating provided the porosity for O2 to access to LSCo, conducting electrons and oxide ions, limited the thermal growth of LSCo NPs, and inhibited surface segregation of Sr.
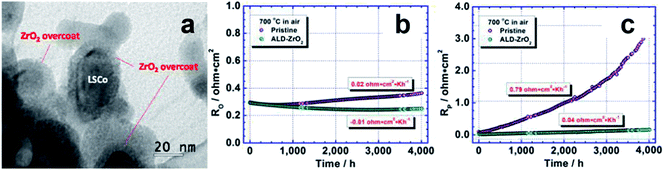 | ||
| Fig. 14 (a) TEM image showing nanoscale ALD ZrO2 coating on LSCo NPs; and comparison on long-term stability of pristine and ALD–ZrO2 coated LSCo cathodes: (b), R0 (overall area-specific resistance); (c), Rp (polarization area-specific resistance). Reprinted with permission from ref. 122. Copyright (2013), American Chemical Society. | ||
Doping electrode particles with ions has been proved an effective way to achieve enhanced electrochemical performances. Metal ions could enter the lattice structure of cathode particles during ALD processes.123,124 The ALD deposition process may begin at the structural defects, after the defects were saturated, the ions began to participate in the formation of oxide films on the surface of the cathode particle. Patel et al. reported that ALD Fe2O3 coating provided significant performance improvement of the LMNO cathode.123 Compared with other doping methods, slight doping using ALD would enhance the cycle stability of LIB cathode, while excessive doping may cause distorted lattice structure or impeded Li+ diffusion channels, which could reduce the capacity.
ALD surface modification layers have shown remarkable effects on improving the stability of cathode materials. Some results on capacity retention of ALD modified LIB cathode materials are summarized in Table 1. In order to protect the electrode material from side reactions and increase the cycle life, thicker protective coatings are preferred. Materials with limited electronic and ionic conductivity, such as alumina, may not be the best choice for cathode coating, because the increased film thickness may affect species transport and damage the capacity. Therefore, an ideal ALD coating material should be able to combine the protective effect and high electronic and ion conductivity. Accurate thickness control of the surface coating is also essential for reaching the optimal balance between high cycle life and high capacity.
| ALD material | Cathode material | Thickness/nm | Capacity retention/% | Ref. | ||
|---|---|---|---|---|---|---|
| Cycling number | w/o ALD | ALD | ||||
| Al2O3 | LiCoO2 | 0.3–0.4 | 120 | 45 | 89 | 112 |
| TiO2 | Li1.2Ni0.13Mn0.54Co0.13O2 | 1 | 100 | 68.2 | 70 | 114 |
| AlPO4 | Li1.2Mn0.54Co0.13Ni0.13O2 | 1 | 40 | Drop rapidly | Stay stable | 115 |
| CeO2 | LiMn2O4 | 3 | 1000 | 0 | 62 | 116 |
| CeO2 | Li1.2Mn0.54Ni0.13Co0.13O2 | 2.5 | 400 | 22 | 62 | 118 |
Thin films of metal oxides are preferred for anode modification. Wang et al. coated Al2O3 films onto SnO2 anode particles by ALD.126 They found that the ALD Al2O3 film with a proper thickness suppressed the volume changes of SnO2 particles, and thus significantly improved the electrochemical behavior of the battery, especially the cycling performance. Kang et al. reported Fe3O4 nanocrystals confined in a meso cellular carbon foam (MSU-F-C) used as an LIB anode material.127 An ultrathin ALD Al2O3 layer deposited on the composite anode could significantly improve the rate capability and durability, and reduce undesirable side reactions. Ultrathin (about 1 nm) ALD Al2O3 films were also coated on the surface of TiO2 nanotubes used as the anode material.128 Compared to the uncoated nanotube, a better electrochemical performance was achieved as a result of the increased mechanical stability and enhanced lithium ion diffusion. A composite LIB anode material was prepared by interwoven Mn3O4 NPs with carbon nanotube (CNT) network through a self-assembly strategy.129 Then an ultra-thin uniform ALD TiO2 coating was deposited on the anode material to stabilize the SEI. The obtained anode material showed a high capacity, stable cycle stability, excellent rate performance, and a commercial grade area capacity. The ALD TiO2 coating stabilized SEI provides high electronic conductivity and lithium ion diffusion ability, and improves the reaction kinetics of Mn3O4 through an “electron density enhancement effect”.
Silicon nanostructures have shown improved capacity and cyclability in LIBs as anodes. Silicon nanowires (SiNWs) were coated with TiN films using TiCl4 and NH3 as precursors at 120 °C and the coated SiNWs were used as the anode material for LIBs.130 The conformal and uniform TiN films limited the growth of SEI and improved the mechanical stability of the anode. In addition, delamination of the nanowire assemblies from the underlying current collector were also reduced. In another work, silicon nanotubes (SiNTs) coated with ALD films of TiO2, TiN, or Al2O3 on the inside, outside, or both surfaces of the nanotubes were used as the anode material for LIBs.131 Enhanced cycling performances were obtained with almost all kinds of coatings on all surfaces. The anode material using SiNTs coated with 1.5 nm TiO2 on both the internal and external surfaces showed the best performance.
ALD coatings have also been applied to enhance the performance of dye sensitized solar cells (DSC). In a recent work published by Li et al.,132 an ultrathin TiO2 binding layer was deposited onto TiO2 host-particles by low temperature ALD, to enhance the interconnection between the host NPs of the photoanode, and to elevate the adhesion between the photoanode and the substrate. With the ALD coating enhanced power conversion was achieved and the ALD TiO2 coating resulted in lower internal resistance and longer electron lifetime of the cell.
Carbon based materials are a typical class of electrode materials for their good electrical conductivity and high surface area. Construction of composite structures integrating carbon and high capacity/capacitance materials such as transition metal oxides and metal phosphates is a common strategy to take the advantages of each component and thus improve electrochemical performances.133 A high specific capacity material LiFePO4 was deposited on CNTs using a quaternary ALD process in which ferrocene (FeCp2), ozone (O3), trimethylphosphate (TMPO), water (H2O), and lithium t-butoxide (LiOtBu) were used as the precursors.134 The surfaces of CNTs were uniformly covered with LiFePO4 layers. Due to the high electric conductivity and high surface area of the CNTs, the LiFePO4/CNT anode remedied the default of low rate performance of LiFePO4. A high power density and ultra-long cycling lifetime were also achieved. Similarly, ultrathin Nb2O5 films were deposited on the surfaces of CNTs by ALD, using niobium ethoxide (Nb(OEt)5) and deionized water as precursors.135 Subsequent high temperature annealing turned the amorphous films into the hexagonal phase. The ALD Nb2O5@CNT anode exhibited high-rate and long-life lithium ion storage, which may be attributed to the synergistic effects of carbon nanotubes, hexagonal Nb2O5, and the ultrathin film structure. A novel nanostructured 3D anode material was fabricated by depositing polyimide films through MLD onto Ti2Nb10O29 (TNO) microspheres, followed by high temperature annealing to convert the polyimide films into carbon layers.136 The resultant TNO@C composite possessed uniformly connected conductive networks offered by MLD carbon, and the anode showed significantly improved electron/ion transport, remarkable high-rate capacity and a high capacity retention after 500 cycles of charge and discharge.
For LIB electrode materials, amorphous coatings would suppress the increasing impedance and favor lithium diffusion due to their soft structures. For instance, amorphous or crystalline ALD SnO2 films were homogeneously deposited on both sides of graphene (GE) nanosheets to form SnO2-GE–SnO2 composites for LIB anodes.137 The crystal structure of SnO2 films could be controlled by changing the reaction conditions of ALD. Both the amorphous and crystalline SnO2-GE anodes exhibited good electrochemical performances, but higher coulombic efficiency and better cycling stability were achieved with the amorphous SnO2-GE anode. The buffer effect of amorphous SnO2 could mitigate the huge volume expansion and reduce the shrinkage of SnO2 lattice, while crystalline SnO2 may pulverize during the charge and discharge processes.
Interstitial metal carbides have high electrical conductivity, high hardness and excellent chemical stability, which make them competitive electrode materials. An ALD assisted template synthesis strategy was reported, for the preparation of single-layer titanium carbide (TiC) hollow sphere arrays on conductive substrates.138 In this method, polystyrene spheres (PS) were used as the template, and an ALD TiO2 layer was uniformly coated on the PS and was then converted into TiC arrays by subsequent carbothermal reaction (Fig. 15). These TiC arrays showed exceptional long-term cycle life and excellent high-rate ability when tested as high temperature supercapacitors. Bi(1,4-di-tert-butyl-1,3-diazabutadienyl) nickel(II) and H2 plasma were used to coat smooth, pure, and ideally crystallized Ni3C films on CNTs, producing a core–shell nanostructured Ni3C/CNT composite.139 The ALD Ni3C/CNT composite exhibited excellent performances as supercapacitors and for electrocatalytic hydrogen evolution.
 | ||
| Fig. 15 (a, d and g) Fabrication process of monolayer TiC hollow sphere arrays on a graphite paper substrate; (b and c), SEM and TEM images of the PS sphere template; (e and f), SEM and TEM images of the TiO2 hollow sphere arrays; (h and i), SEM and TEM images of the TiC hollow sphere arrays. Reprinted with permission from ref. 138. Copyright (2013), Royal Society of Chemistry. | ||
Metal sulfides have shown great potential as the anode for LIB. For instance, the theoretical capacity of Al2S3 is more than 1400 mA h g−1, about four times the capacity of commercial graphite anode. Aluminum sulfide (AlSx) thin film was synthesized by ALD using tris(dimethylamido) aluminum and H2S as precursors.140 The ALD AlSx film was deposited onto nitrogen-doped graphene nanosheets (NGNS) to form an anode of LIB. The anode material exhibited a high capacity of 640 mA h g−1 at a current density of 100 mA g−1 in the voltage window of 0.6–3.5 V. A reliable cyclability over 60 cycles was demonstrated.
Pt-based catalysts are widely used in the electrodes of proton exchange membrane fuel cells (PEMFCs) because of their excellent catalytic performances in both hydrogen oxidation and oxygen reduction reactions (ORR). However, conventional Pt/C catalysts exhibit poor durability due to the detachment and agglomeration of Pt NPs during fuel cell operation. Lee et al. reported a method of synthesizing high-performance Pt/carbon catalyst for PEMFCs using FBR ALD.141 Two types of carbon supports were tested. Uniformly dispersed and highly dense Pt NPs obtained by ALD with an optimized ionomer content exhibited high fuel cell performance and excellent stability. WN was deposited on the ALD Pt/C catalyst using bis(tert-butylimido)bis(dimethylamido) tungsten and ammonia as precursors, producing a conformal coating on the surface of the catalyst particles.142 The subsequent thermal treatment led to a reduced amount of nitride and separated Pt and W domains. The surface modified Pt/C catalyst showed an ORR activity (465 mA mg−1) much higher than that of the bare Pt/C catalyst (277 mA mg−1). After accelerated durability test, the surface modified catalyst exhibited better retention of electrochemical properties and higher mechanical stability. A porous organic–inorganic hybrid inter-layer was deposited on a CNT support through MLD using sequential exposures of TMA and glycerol at 150 °C, followed by high temperature annealing.143 Pt NPs were subsequently deposited onto the MLD-modified CNT surface by ALD. The MLD-modified CNT surface with enriched pores helped anchoring the Pt species and avoided agglomeration or detachment of the NPs. Besides, the inter-layer induced electron transfer from Pt to the substrate. Therefore the as-prepared Pt catalysts showed significantly enhanced ORR activity and durability compared to the catalysts grown on the bare CNT.
3.5 Wave absorbing material
In recent years, wave-absorbing materials are more and more widely used in military and civilian fields. The common wave absorbing agents are powders of carbonyl iron, ferrite, barium titanite, and so on. Carbonyl iron powder has high magnetic permeability, good absorbing ability, and wide absorbing frequency bandwidth, therefore it has become one of the most widely used absorbing materials. However, corrosion and oxidation limit its applications under certain conditions. To improve the corrosion resistance and electromagnetic performance of carbonyl iron, Al2O3 thin films were coated on the carbonyl iron powder by ALD.144 The ALD Al2O3 films conformally covered the surfaces of carbonyl iron particles. Thermal stability and corrosion resistance of the surface modified carbonyl iron powder were significantly improved. Besides, electromagnetic parameters and wave absorbing properties were also enhanced. Under the same experiment conditions, a 40.9% lower reflection loss (RL) can be obtained.For practical application of microwave absorbing materials (MAMs), effective electromagnetic (EM) impedance matching between the permeability and the relative permittivity is crucial to achieve strong absorption. An efficient and lightweight EM absorbing material was prepared by Wang et al. through mixing ALD Fe3O4/graphene and Ni/graphene composites. The ALD Fe3O4 and Ni NPs had narrow size distributions, and were uniformly dispersed throughout the entire graphene surface. The minimum optical reflection loss was −46.4 dB at 15.6 GHz, with a coating thickness of only 1.4 mm. The promoted EM absorption property of the composite material was ascribed to the effective impedance matching, multiple interfacial polarization and increased magnetic loss from the added magnetic constituents.145 The same group also proposed a method to produce ZnO supported Ni NPs with enhanced microwave absorption.146 NiO films were uniformly deposited on ZnO particles by ALD, followed by high temperature reduction to synthesize flower-like ZnO coated with Ni NPs. The ZnO@Ni composite exhibited significantly improved EM absorption properties compared to ZnO (Fig. 16). The enhanced absorption capacity was explained by the combination of various dielectric–magnetic loss mechanisms.
 | ||
| Fig. 16 (a–c) TEM images of ZnO@Ni; (d), size distribution of Ni NPs; (e), HRTEM image of ZnO@Ni; RL curves of ZnO@Ni composites with a thickness of 1.5 mm (f) and 2 mm (g); 3D representations of RL of ZnO (h) and ZnO@Ni. (i) Reprinted with permission from ref. 146. Copyright (2013), Royal Society of Chemistry. | ||
A new type of microwave absorbing material was synthesized by coating carbon nanofibers with multilayer gradient films through ALD.147 The gradient nanofilm was composed of ZnO with different Al2O3 content, which was prepared by alternating ALD ZnO and ALD Al2O3 cycles at different ratios. The multilayer gradient film acted as intermediate layers to tune the impedance matching between air and the nanofiber surface, and the microwave absorption performance was remarkably enhanced. A reflection loss of −58.5 dB was achieved at 16.2 GHz with a composite thickness of only 1.8 mm. In Table 2, the wave absorption performances of different materials fabricated by ALD are listed and compared.
| ALD material | Original wave absorbing material | Frequency for RL ≤ −10 dB/GHz | RL min/dB | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Thickness/mm | w/o ALD | ALD | Thickness/mm | w/o ALD | ALD | |||
| Al2O3 | Carbonyl iron powder | 2.5 | 2.42–3.87 | 2.76–4.65 | 2.5 | −14.5 | −20.3 | 144 |
| Fe3O4 | Graphene | 1.5 | — | 12.4–16.9 | 1.4 | −3.3 | −46.4 | 145 |
| Ni | Flower-like ZnO | 1.5 | — | 12.1–17.4 | 2 | −9.3 | −48 | 146 |
| ZnO/Al2O3 | Carbon nanofibers | 1.9 | — | 12.7–18 | 1.8 | −9.2 | −58.5 | 147 |
3.6 Medicine
There is a great demand for surface modification and engineering in the medical field. Through proper surface modification, the following goals may be achieved: protection and stabilization of pharmaceutical ingredients; controlled release of drugs; enhanced penetration of medicine into human tissues; improved biocompatibility of surgical devices, implants, and artificial tissues.148 ALD perfectly meet the needs of surface engineering in the medical field, and many opportunities are related to powder fabrication.Active pharmaceutical ingredients (APIs) are mainly organic solid powders. In order to be prepared in a variety of dosage forms, many APIs require processing. Powder flow improvement and APIs protection are often desirable. Due to the inhomogeneous nature and irregular shapes of drug particles, conventional coating techniques can hardly achieve conformal coating around the particles. ALD is a potential way to encapsulate drug particles with high quality coatings. Micrometer scale acetaminophen particles were coated with ALD Al2O3, TiO2 and ZnO in a rotary reactor.149 The main polymorph remained unchanged during the ALD process. HPLC analysis showed that no degradation product was produced, which confirmed the stability of ALD coated drug samples. ALD metal oxide coated acetaminophen showed slowed drug release. ALD Al2O3 and TiO2 coated acetaminophen particles exhibited good cytocompatibility. Evaluation in vitro on intestinal Caco-2 cells indicated that thick ZnO coatings showed the greatest cytotoxicity among the materials studied.
ALD coatings were also used for improving the stability of drug particles. Hellrup et al.150 investigated the feasibility of applying ALD Al2O3 coatings to prevent moisture absorption. Dynamic moisture absorption experiments showed that the ALD Al2O3 nanoshells almost completely inhibited the adsorption of water. These results have demonstrated the potential of ALD coatings in improving the long-term stability of drug particles.
Vitamin C is a very important and commonly used medicine, but it suffers from rapid oxidation/degradation in light and humidity. Haghshenas-Lari et al. applied TiO2 coatings to protect the surface of vitamin C particles by ALD, and studied the effects of ALD experiment parameters on the structure and quality of TiO2 coating.151 It was demonstrated that the ALD TiO2 coating can successfully slow down the degradation and improve the medical stability of vitamin C.
ALD protective coatings can also be applied to enhance the medical stability of vaccine.152 Affected by its instability, vaccines usually require refrigerated storage and multiple vaccinations, and thus the practical impacts of the vaccine are often compromised. Garcea et al. prepared thermostabilized powders containing HPV16L1 ingredient using spray drying, and coated these powders with ALD alumina layers to protect the inside antigens and modulate the release. With the ALD alumina protective coating, the antibody titers were unaffected by incubation at 50 °C for one month. Antibody response test demonstrated that a single dose of the ALD-coated vaccine produced neutralizing responses and antibody titers were equivalent or superior to conventional alum-adsorbed two-dose immunization of the L1 protein.
Zhang et al. reported ALD modification of budesonide and lactose organic particles with atomic scale Al2O3 films.153 A vibration-assisted fluidized bed ALD reactor was used, and the coating experiments were carried out under near ambient conditions (<40 °C, 1 bar). Complete and conformal coating on the drug particles (Fig. 17a) and the feasibility to tailor heat transfer, dissolution rate and dispersibility were demonstrated. With the increasing ALD Al2O3 film thickness, the dissolution rates of both budesonide and lactose particles were reduced, which indicated the potential application of ALD coatings for controlled drug release. The coated drug powders were less cohesive, which implied improved powder processability after the ALD surface modification.
 | ||
| Fig. 17 (a) TEM images of ALD Al2O3 coated budesonide (left) and lactose (right) particles; Reprinted with permission from ref. 153. Copyright (2017), Royal Society of Chemistry. (b) Plasma concentration curves for indomethacin measured on blood samples from test animals at different times. Reprinted from ref. 154. Copyright (2019), with permission from Elsevier. | ||
Hellrup et al. also performed pharmacokinetic studies showing that ALD Al2O3 nanoshells coated on the APIs can be used to achieve long sustained drug release.154 30 nm-thick ALD Al2O3 coating was deposited onto micro particles of indomethacin, and the drug level in blood samples were tested in rats for 12 weeks (Fig. 17b). The uncoated indomethacin was eliminated with a half-life of 15 h, and the coated samples showed dramatically slower release rate which sustained for more than 12 weeks.
4. Conclusion and perspective
The growing demands for controllable surface modification of powder materials and precise manufacture of nanostructures have stimulated the development of ALD technology for powder fabrication. This article starts with a brief introduction to the principles of ALD, followed by a retrospection on the development of ALD reactors designed for powder fabrication, and then illustrates typical examples of applications of ALD for fabricating powder materials in various fields. The advantages of ALD for powder fabrication include the diversity of selectable ALD and substrate materials; the conformality of the produced films on 3D structures; the uniformity and high dispersion of the obtained discontinuous structures; and most importantly, the ability to precisely control the dimension as a result of the self-limiting ALD surface reaction. These features make ALD a very unique and effective tool for powder fabrication.The strong potential of ALD for powder fabrication has been proved in many areas, and ALD technology may be applied to even more areas in the future, to produce novel materials or structures through precise surface engineering of powders. For existing applications, ALD powder fabrication may move towards the goal of mass production and cost reduction. Whereas there are still many problems that need to be solved:
(1) The research on ALD reactors and processes for industrial scale powder fabrication should be advanced. Existing fluidized bed reactors, rotating reactors and spatial reactors have demonstrated the potential for mass production of powder materials. The existing reactor designs need to be improved and new reactor designs should be investigated to enhance the mass and heat transfer, reduce the particle agglomeration, and increase the utilization of precursors.
(2) The development of economical precursors and corresponding surface reactions are also necessary for the large scale application of ALD, especially for scalable fabrication of powder materials with huge surface areas.
(3) Emerging applications of ALD powder fabrication should be further investigated. More surface modification/functionalization materials should be tested and novel structures should be designed and synthesized to optimize their performances. Evaluation on the merit or importance of the technology (for instance, significance in improving scientific understanding or potential for industrial application) should be made.
The potential applications for ALD are virtually endless. Surface engineering of powder materials at atomic level precision will enable introduction of the desired structural, physical, or chemical properties to the surface of the powder, which will significantly enrich and promote the performances and/or functions of the materials. The future research of ALD for powder material fabrication may be full of challenges, but it is certainly rewarding.
Abbreviates
| AC | Activated carbon |
| ALD | Atomic layer deposition |
| AP | Ammonium perchlorate |
| APH | Aqueous-phase hydrogenation |
| APIs | Active pharmaceutical ingredients |
| CNT | Carbon nanotubes |
| CVD | Chemical vapor deposition |
| DEZ | Diethyl zinc |
| DFT | Density functional theory |
| DRM | Dry reforming of methane |
| DSC | Dye sensitized solar cells |
| DTA | Differential thermal analysis |
| EBC | Environmental barrier coating |
| EM | Effective electromagnetic |
| ESD | Electrostatic discharge |
| EXAFS | Extended X-ray absorption fine structure |
| FBRs | Fluidized bed reactors |
| FTIR | Fourier transform infrared |
| GE | Graphene |
| GISAXS | Grazing incidence small-angle X-ray scattering |
| HAADF-STEM | High angle annular dark field scanning transmission electron microscopy |
| HER | Hydrogen evolution reaction |
| LIB | Lithium-ion batteries |
| LIC | Lithium-ion capacitors |
| MAMs | Microwave absorbing materials |
| MLD | Molecular layer deposition |
| MO | Metal oxide |
| MOFs | Metal organic frameworks |
| NMR | Nuclear magnetic resonance |
| NPs | Nanoparticles |
| NTs | Nanotubes |
| NWs | Nanowires |
| ODH | Oxidative dehydrogenation |
| OER | Oxygen evolution reaction |
| ORR | Oxygen reduction reaction |
| PEMFCs | Proton exchange membrane fuel cells |
| PS | Polystyrene spheres |
| PS-DVB | Poly styrene-divinylbenzene |
| rGO | Reduced graphene oxide |
| SAMS | Self-assembled monolayers |
| SEI | Solid electrolyte interphase |
| SEM | Scanning electron microscope |
| SERS | Surface enhanced Raman scattering |
| SIB | Sodium ion batteries |
| SOFC | Solid oxide fuel cells |
| TEM | Transmission electron microscope |
| TMA | Trimethyl aluminum |
| WGS | Water-gas shift |
| XAFS | X-ray absorption fine structure spectroscopy |
| XANES | X-ray absorption near edge structure |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by National Science Foundation of China (grant number: 21975200).References
- S. Adhikari, S. Selvaraj and D.-H. Kim, Adv. Mater. Interfaces, 2018, 5, 1800581 CrossRef.
- S. M. George, Chem. Rev., 2010, 110, 111–131 CrossRef CAS PubMed.
- D. M. King, X. H. Liang and A. W. Weimer, Powder Technol., 2012, 221, 13–25 CrossRef CAS.
- C. Marichy, M. Bechelany and N. Pinna, Adv. Mater., 2012, 24, 1017–1032 CrossRef CAS PubMed.
- M. Leskelä and M. Ritala, Thin Solid Films, 2002, 409, 138–146 CrossRef.
- R. W. Johnson, A. Hultqvist and S. F. Bent, Mater. Today, 2014, 17, 236–246 CrossRef CAS.
- A. W. Weimer, J. Nanopart. Res., 2019, 21, 42 CrossRef PubMed.
- T. Suntola, Mater. Sci. Rep., 1985, 4, 261–312 CrossRef.
- J. D. Ferguson, A. W. Weimer and S. M. George, Thin Solid Films, 2000, 371, 95–104 CrossRef CAS.
- J. Libera, J. Elam and M. Pellin, Thin Solid Films, 2008, 516, 6158–6166 CrossRef CAS.
- J. R. Wank, S. M. George and A. W. Weimer, Powder Technol., 2001, 121, 195–204 CrossRef CAS.
- J. R. Wank, S. M. George and A. W. Weimer, J. Am. Ceram. Soc., 2004, 87, 762–765 CrossRef CAS.
- J. R. Wank, S. M. George and A. W. Weimer, Powder Technol., 2004, 142, 59–69 CrossRef CAS.
- D. M. King, J. A. Spencer, X. Liang, L. F. Hakim and A. W. Weimer, Surf. Coat. Technol., 2007, 201, 9163–9171 CrossRef CAS.
- L. F. Hakim, J. L. Portman, M. D. Casper and A. W. Weimer, Powder Technol., 2005, 160, 149–160 CrossRef CAS.
- R. Beetstra, U. Lafont, J. Nijenhuis, E. M. Kelder and J. R. van Ommen, Chem. Vap. Deposition, 2009, 15, 227–233 CrossRef CAS.
- H. Van Bui, F. Grillo, R. Helmer, A. Goulas and J. R. van Ommen, J. Phys. Chem. C, 2016, 120, 8832–8840 CrossRef CAS.
- H. Tiznado, D. Domínguez, F. Muñoz-Muñoz, J. Romo-Herrera, R. Machorro, O. E. Contreras and G. Soto, Powder Technol., 2014, 267, 201–207 CrossRef CAS.
- J. A. McCormick, B. L. Cloutier, A. W. Weimer and S. M. George, J. Vac. Sci. Technol., A, 2007, 25, 67–74 CrossRef CAS.
- C. L. Duan, X. Liu, B. Shan and R. Chen, Rev. Sci. Instrum., 2015, 86, 3727 CrossRef PubMed.
- P. Poodt, A. Lankhorst, F. Roozeboom, K. Spee, D. Maas and A. Vermeer, Adv. Mater., 2010, 22, 3564–3567 CrossRef CAS PubMed.
- P. Poodt, D. C. Cameron, E. Dickey, S. M. George, V. Kuznetsov, G. N. Parsons, F. Roozeboom, G. Sundaram and A. Vermeer, J. Vac. Sci. Technol., A, 2012, 30, 11 CrossRef.
- J. R. van Ommen, D. Kooijman, M. de Niet, M. Talebi and A. Goulas, J. Vac. Sci. Technol., A, 2015, 33, 5 CrossRef.
- C. L. Duan, Z. Deng, K. Cao, H. F. Yin, B. Shan and R. Chen, J. Vac. Sci. Technol., A, 2016, 34, 8 CrossRef.
- V. Cremers, G. Rampelberg, A. Barhoum, P. Walters, N. Claes, T. M. de Oliveira, G. Van Assche, S. Bals, J. Dendooven and C. Detavernier, Surf. Coat. Technol., 2018, 349, 1032–1041 CrossRef CAS.
- A. L. Hoskins, A. H. Coffey, C. B. Musgrave and A. W. Weimer, J. Am. Ceram. Soc., 2018, 101, 2493–2505 CrossRef CAS.
- H. Azizpour, M. Talebi, F. D. Tichelaar, R. Sotudeh-Gharebagh, J. Guo, J. R. van Ommen and N. Mostoufi, Appl. Surf. Sci., 2017, 426, 480–496 CrossRef CAS.
- X. Liang and A. W. Weimer, J. Nanopart. Res., 2010, 12, 135–142 CrossRef CAS.
- D. M. King, X. H. Liang, B. B. Burton, M. K. Akhtar and A. W. Weimer, Nanotechnology, 2008, 19, 8 Search PubMed.
- J. Guo, H. V. Bui, D. Valdesueiro, S. J. Yuan, B. Liang and J. R. van Ommen, Nanomaterials, 2018, 8, 19 CrossRef PubMed.
- R. Verstraete, G. Rampelberg, H. Rijckaert, I. Van Driessche, E. Coetsee, M. M. Duvenhage, P. F. Smet, C. Detavernier, H. Swart and D. Poelman, Chem. Mater., 2019, 31, 7192–7202 CrossRef CAS.
- E. Karacaoglu, E. Ozturk, M. Uyaner and M. D. Losego, J. Am. Ceram. Soc., 2020, 103, 3706–3715 CrossRef CAS.
- S. Bhattacharya, L. Jamison, D. N. Seidman, W. Mohamed, Y. Bei, M. J. Pellin and A. M. Yacout, J. Nucl. Mater., 2019, 526, 9 CrossRef.
- S. L. Zhang, E. Yu, S. Gates, W. S. Cassata, J. Makel, A. M. Thron, C. Bartel, A. W. Weimer, R. Faller, P. Stroeve and J. W. Tringe, J. Nucl. Mater., 2018, 499, 301–311 CrossRef CAS.
- S. K. Bull, W. W. McNeary, C. A. Adkins, T. A. Champ, C. A. Hill, R. C. O'Brien, C. B. Musgrave and A. W. Weimer, Appl. Surf. Sci., 2020, 507, 9 CrossRef.
- B. J. O'Neill, D. H. K. Jackson, J. Lee, C. Canlas, P. C. Stair, C. L. Marshall, J. W. Elam, T. F. Kuech, J. A. Dumesic and G. W. Huber, ACS Catal., 2015, 5, 1804–1825 CrossRef.
- R. L. Puurunen and W. Vandervorst, J. Appl. Phys., 2004, 96, 7686–7695 CrossRef CAS.
- J. Hämäläinen, M. Ritala and M. Leskelä, Chem. Mater., 2014, 26, 786–801 CrossRef.
- H. Feng, J. W. Elam, J. A. Libera, W. Setthapun and P. C. Stair, Chem. Mater., 2010, 22, 3133–3142 CrossRef CAS.
- H. Feng, J. A. Libera, P. C. Stair, J. T. Miller and J. W. Elam, ACS Catal., 2011, 1, 665–673 CrossRef CAS.
- T. Gong, L. Qin, W. Zhang, H. Wan, J. Lu and H. Feng, J. Phys. Chem. C, 2015, 119, 11544–11556 CrossRef CAS.
- W. Zhang, Y. Huang, T. Gong and H. Feng, Catal. Commun., 2017, 93, 47–52 CrossRef CAS.
- T. Gong, Y. Huang, L. J. Qin, W. L. Zhang, J. G. Li, L. F. Hui and H. Feng, Appl. Surf. Sci., 2019, 495, 12 CrossRef.
- M. Lashdaf, J. Lahtinen, M. Lindblad, T. Venäläinen and A. O. I. Krause, Appl. Catal., A, 2004, 276, 129–137 CrossRef CAS.
- A. Goulas and J. R. van Ommen, J. Mater. Chem. A, 2013, 1, 4647–4650 RSC.
- J. K. Zhang, C. Q. Chen, S. Chen, Q. M. Hu, Z. Gao, Y. Q. Li and Y. Qin, Catal. Sci. Technol., 2017, 7, 322–329 RSC.
- J. Dendooven, R. K. Ramachandran, E. Solano, M. Kurttepeli, L. Geerts, G. Heremans, J. Ronge, M. M. Minjauw, T. Dobbelaere, K. Devloo-Casier, J. A. Martens, A. Vantomme, S. Bals, G. Portale, A. Coati and C. Detavernier, Nat. Commun., 2017, 8, 12 CrossRef PubMed.
- K. Leus, J. Dendooven, N. Tahir, R. K. Ramachandran, M. Meledina, S. Turner, G. Van Tendeloo, J. L. Goeman, J. Van der Eycken, C. Detavernier and P. Van Der Voort, Nanomaterials, 2016, 6, 11 CrossRef PubMed.
- B. Qiao, A. Wang, X. Yang, L. F. Allard, Z. Jiang, Y. Cui, J. Liu, J. Li and T. Zhang, Nat. Chem., 2011, 3, 634 CrossRef CAS.
- S. H. Sun, G. X. Zhang, N. Gauquelin, N. Chen, J. G. Zhou, S. L. Yang, W. F. Chen, X. B. Meng, D. S. Geng, M. N. Banis, R. Y. Li, S. Y. Ye, S. Knights, G. A. Botton, T. K. Sham and X. L. Sun, Sci. Rep., 2013, 3, 9 Search PubMed.
- N. C. Cheng, S. Stambula, D. Wang, M. N. Banis, J. Liu, A. Riese, B. W. Xiao, R. Y. Li, T. K. Sham, L. M. Liu, G. A. Botton and X. L. Sun, Nat. Commun., 2016, 7, 9 Search PubMed.
- Z. X. Song, Y. N. Zhu, H. S. Liu, M. N. Banis, L. Zhang, J. J. Li, K. Doyle-Davis, R. Y. Li, T. K. Sham, L. J. Yang, A. Young, G. A. Botton, L. M. Liu and X. L. Sun, Small, 2020, 16, 12 Search PubMed.
- K. Ding, A. Gulec, A. M. Johnson, N. M. Schweitzer, G. D. Stucky, L. D. Marks and P. C. Stair, Science, 2015, 350, 189–192 CrossRef CAS PubMed.
- H. Yan, Y. Lin, H. Wu, W. Zhang, Z. Sun, H. Cheng, W. Liu, C. Wang, J. Li and X. Huang, Nat. Commun., 2017, 8, 1–11 CrossRef.
- H. Yan, H. Cheng, H. Yi, Y. Lin, T. Yao, C. Wang, J. Li, S. Wei and J. Lu, J. Am. Chem. Soc., 2015, 137, 10484–10487 CrossRef CAS PubMed.
- X. H. Huang, Y. J. Xia, Y. J. Cao, X. S. Zheng, H. B. Pan, J. F. Zhu, C. Ma, H. W. Wang, J. J. Li, R. You, S. Q. Wei, W. X. Huang and J. L. Lu, Nano Res., 2017, 10, 1302–1312 CrossRef CAS.
- Z. Y. Shang, S. G. Li, L. Li, G. Z. Liu and X. H. Liang, Appl. Catal., B, 2017, 201, 302–309 CrossRef CAS.
- C. S. Chen, J. H. Lin, T. W. Lai and B. H. Li, J. Catal., 2009, 263, 155–166 CrossRef CAS.
- X. Liang, N.-H. Li and A. W. Weimer, Microporous Mesoporous Mater., 2012, 149, 106–110 CrossRef CAS.
- H. Feng, J. W. Elam, J. A. Libera, M. J. Pellin and P. C. Stair, J. Catal., 2010, 269, 421–431 CrossRef CAS.
- G.-Q. Yang, H. Wang, T. Gong, Y.-H. Song, H. Feng, H.-Q. Ge, H.-b. Ge, Z.-T. Liu and Z.-W. Liu, J. Catal., 2019, 380, 195–203 CrossRef CAS.
- M. G. Jeong, E. J. Park, B. Jeong, D. H. Kim and Y. D. Kim, Chem. Eng. J., 2014, 237, 62–69 CrossRef CAS.
- X. L. Tong, Y. Qin, X. Y. Guo, O. Moutanabbir, X. Y. Ao, E. Pippel, L. B. Zhang and M. Knez, Small, 2012, 8, 3390–3395 CrossRef CAS PubMed.
- R. Singh, R. Bapat, L. Qin, H. Feng and V. Polshettiwar, ACS Catal., 2016, 6, 2770–2784 CrossRef CAS.
- C. Q. Chen, P. Li, G. Z. Wang, Y. Yu, F. F. Duan, C. Y. Chen, W. G. Song, Y. Qin and M. Knez, Angew. Chem., Int. Ed., 2013, 52, 9196–9200 CrossRef CAS.
- A. Podurets, D. Kolokolov, M. K. S. Barr, E. Ubyivovk, M. Osmolowsky, N. Bobrysheva, J. Bachmann and O. Osmolovskaya, Appl. Surf. Sci., 2020, 533, 9 CrossRef.
- T. Gong, L. Qin, J. Lu and H. Feng, Phys. Chem. Chem. Phys., 2016, 18, 601–614 RSC.
- N. Yan, L. Qin, J. Li, F. Zhao and H. Feng, Appl. Surf. Sci., 2018, 451, 155–161 CrossRef CAS.
- C. Q. Chen, F. F. Duan, S. C. Zhao, W. K. Wang, F. Yang, W. Nuansing, B. Y. Zhang, Y. Qin and M. Knez, Appl. Catal., B, 2019, 248, 218–225 CrossRef CAS.
- N. P. Dasgupta, X. B. Meng, J. W. Elam and A. B. F. Martinson, Acc. Chem. Res., 2015, 48, 341–348 CrossRef CAS PubMed.
- A. W. Peters, Z. Li, O. K. Farha and J. T. Hupp, ACS Nano, 2015, 9(8), 8484–8490 CrossRef CAS PubMed.
- H. Li, Y. D. Shao, Y. T. Su, Y. H. Gao and X. W. Wang, Chem. Mater., 2016, 28, 1155–1164 CrossRef CAS.
- H. Choi, A. W. Peters, H. Noh, L. C. Gallington, A. E. Platero-Prats, M. R. DeStefano, M. Rimoldi, S. Goswami, K. W. Chapman, O. K. Farha and J. T. Hupp, ACS Appl. Energy Mater., 2019, 2, 8695–8700 CrossRef CAS.
- Z. Y. He, Z. Guo, Q. B. Wa, X. Zhong, X. W. Wang and Y. Chen, J. Mater. Res., 2020, 35, 822–830 CrossRef CAS.
- H. Li, R. Zhao, J. H. Zhu, Z. Guo, W. Xiong and X. W. Wang, Chem. Mater., 2020, 32, 8885–8894 CrossRef CAS.
- J. Lu, J. W. Elam and P. C. Stair, Surf. Sci. Rep., 2016, 71, 410–472 CrossRef CAS.
- S. T. Christensen, F. Hao, J. L. Libera, N. Guo, J. T. Miller, P. C. Stair and J. W. Elam, Nano Lett., 2010, 10, 3047 CrossRef CAS.
- K. Cao, Q. Q. Zhu, B. Shan and R. Chen, Sci. Rep., 2015, 5, 7 Search PubMed.
- J. L. Lu, K. B. Low, Y. Lei, J. A. Libera, A. Nicholls, P. C. Stair and J. W. Elam, Nat. Commun., 2014, 5, 9 Search PubMed.
- H. Wang, C. Wang, H. Yan, H. Yi and J. Lu, J. Catal., 2015, 324, 59–68 CrossRef CAS.
- J. L. Lu, B. S. Fu, M. C. Kung, G. M. Xiao, J. W. Elam, H. H. Kung and P. C. Stair, Science, 2012, 335, 1205–1208 CrossRef CAS PubMed.
- H. M. Duan, R. You, S. T. Xu, Z. R. Li, K. Qian, T. Cao, W. X. Huang and X. H. Bao, Angew. Chem., Int. Ed., 2019, 58, 12043–12048 CrossRef CAS PubMed.
- H. Feng, J. L. Lu, P. C. Stair and J. W. Elam, Catal. Lett., 2011, 141, 512–517 CrossRef CAS.
- H. B. Zhang, X. K. Gu, C. Canlas, A. J. Kropf, P. Aich, J. P. Greeley, J. W. Elam, R. J. Meyers, J. A. Dumesic, P. C. Stair and C. L. Marshall, Angew. Chem., Int. Ed., 2014, 53, 12132–12136 CrossRef CAS PubMed.
- H. Yi, H. Y. Du, Y. L. Hu, H. Yan, H. L. Jiang and J. L. Lu, ACS Catal., 2015, 5, 2735–2739 CrossRef CAS.
- J. F. Yang, K. Cao, M. Gong, B. Shan and R. Chen, J. Catal., 2020, 386, 60–69 CrossRef CAS.
- E. Solano, J. Dendooven, J. Y. Feng, P. Bruner, M. M. Minjauw, R. K. Ramachandran, M. Van Daele, K. Van de Kerckhove, T. Dobbelaere, A. Coati, D. Hermida-Merino and C. Detavernier, Nanoscale, 2020, 12, 11684–11693 RSC.
- J. M. Cai, J. Zhang, K. Cao, M. Gong, Y. Lang, X. Liu, S. Q. Chu, B. Shan and R. Chen, ACS Appl. Nano Mater., 2018, 1, 522–530 CrossRef CAS.
- Q. Yao, C. L. Wang, H. W. Wang, H. Yan and J. L. Lu, J. Phys. Chem. C, 2016, 120, 9174–9183 CrossRef CAS.
- B. J. O'Neill, D. H. K. Jackson, A. J. Crisci, C. A. Farberow, F. Shi, A. C. Alba-Rubio, J. Lu, P. J. Dietrich, X. Gu, C. L. Marshall, P. C. Stair, J. W. Elam, J. T. Miller, F. H. Ribeiro, P. M. Voyles, J. Greeley, M. Mavrikakis, S. L. Scott, T. F. Kuech and J. A. Dumesic, Angew. Chem., Int. Ed., 2013, 52, 13808–13812 CrossRef PubMed.
- H. B. Zhang, Y. Lei, A. J. Kropf, G. H. Zhang, J. W. Elam, J. T. Miller, F. Sollberger, F. Ribeiro, M. C. Akatay, E. A. Stach, J. A. Dumesic and C. L. Marshall, J. Catal., 2014, 317, 284–292 CrossRef CAS.
- H. B. Zhang, C. Canlas, A. J. Kropf, J. W. Elam, J. A. Dumesic and C. L. Marshall, J. Catal., 2015, 326, 172–181 CrossRef CAS.
- J. C. Lee, D. H. K. Jackson, T. Li, R. E. Winans, J. A. Dumesic, T. F. Kuech and G. W. Huber, Energy Environ. Sci., 2014, 7, 1657–1660 RSC.
- Y. Zhou, D. M. King, J. H. Li, K. S. Barrett, R. B. Goldfarb and A. W. Weimer, Ind. Eng. Chem. Res., 2010, 49, 6964–6971 CrossRef CAS.
- R. Kumar, P. F. Siril and P. Soni, Def. Technol., 2015, 11, 382–389 CrossRef.
- D. Wen, Energy Environ. Sci., 2010, 3, 591 RSC.
- J. D. Ferguson, K. J. Buechler, A. W. Weimer and S. M. George, Powder Technol., 2005, 156, 154–163 CrossRef CAS.
- L. Qin, T. Gong, H. Hao, K. Wang and H. Feng, J. Nanopart. Res., 2013, 15, 2150 CrossRef.
- L. Qin, N. Yan, J. Li, H. Hao, F. Zhao and H. Feng, RSC Adv., 2017, 7, 7188–7197 RSC.
- N. Yan, L. J. Qin, H. X. Hao, L. F. Hui, F. Q. Zhao and H. Feng, Appl. Surf. Sci., 2017, 408, 51–59 CrossRef CAS.
- R. Chen, C.-L. Duan, X. Liu, K. Qu, G. Tang, X.-X. Xu and B. Shan, J. Vac. Sci. Technol., A, 2017, 35, 03E111 CrossRef.
- R. Chen, K. Qu, J. Li, P. Zhu, C. Duan, J. Zhang, X. Li, X. Liu and Z. Yang, ACS Appl. Nano Mater., 2018, 1, 5500–5506 CrossRef CAS.
- L. Qin, Y. Ning, H. Hao, T. An, F. Zhao and F. Hao, Appl. Surf. Sci., 2018, 436, 548–555 CrossRef CAS.
- L. J. Qin, T. Gong, J. G. Li, N. Yan, L. F. Hui and H. Feng, J. Hazard. Mater., 2019, 378, 8 CrossRef PubMed.
- B. Scrosati and J. Garche, J. Power Sources, 2010, 195, 2419–2430 CrossRef CAS.
- X. B. Meng, X. Q. Yang and X. L. Sun, Adv. Mater., 2012, 24, 3589–3615 CrossRef CAS.
- C. Guan and J. Wang, Adv. Sci., 2016, 3, 23 Search PubMed.
- M. Lu, R. B. Nuwayhid, T. Wu, L. Yu and J. Lu, Adv. Mater. Interfaces, 2016, 3, 1600564 CrossRef.
- H. C. M. Knoops, M. E. Donders, M. C. M. van de Sanden, P. H. L. Notten and W. M. M. Kessels, J. Vac. Sci. Technol., A, 2012, 30, 010801 CrossRef.
- B. Ahmed, C. Xia and H. N. Alshareef, Nano Today, 2016, 11, 250–271 CrossRef CAS.
- B. Yan, X. F. Li, Z. M. Bai, X. S. Song, D. B. Xiong, M. L. Zhao, D. J. Li and S. G. Lu, J. Power Sources, 2017, 338, 34–48 CrossRef CAS.
- Y. S. Jung, A. S. Cavanagh, A. C. Dillon, M. D. Groner, S. M. George and S.-H. Lee, J. Electrochem. Soc., 2010, 157, A75–A81 CrossRef CAS.
- X. F. Li, J. Liu, X. B. Meng, Y. J. Tang, M. N. Banis, J. L. Yang, Y. H. Hu, R. Y. Li, M. Cai and X. L. Sun, J. Power Sources, 2014, 247, 57–69 CrossRef CAS.
- X. Zhang, I. Belharouak, L. Li, Y. Lei, J. W. Elam, A. Nie, X. Chen, R. S. Yassar and R. L. Axelbaum, Adv. Energy Mater., 2013, 3, 1299–1307 CrossRef CAS.
- B. Xiao, B. Wang, J. Liu, K. Kaliyappan, Q. Sun, Y. Liu, G. Dadheech, M. P. Balogh, L. Yang and T.-K. Sham, Nano Energy, 2017, 34, 120–130 CrossRef CAS.
- R. L. Patel, H. Xie, J. Park, H. Y. Asl, A. Choudhury and X. Liang, Adv. Mater. Interfaces, 2015, 2, 1500046 CrossRef.
- S. Sarkar, R. L. Patel, X. Liang and J. Park, ACS Appl. Mater. Interfaces, 2017, 9, 30599–30607 CrossRef CAS.
- Y. Gao, R. L. Patel, K. Y. Shen, X. Wang, R. L. Axelbaum and X. Liang, ACS Omega, 2018, 3, 906–916 CrossRef CAS PubMed.
- D. H. K. Jackson, M. R. Laskar, S. Fang, S. Xu, R. G. Ellis, X. Li, M. Dreibelbis, S. E. Babcock, M. K. Mahanthappa, D. Morgan, R. J. Hamers and T. F. Kuech, J. Vac. Sci. Technol., A, 2016, 34, 031503 CrossRef.
- K. Kaliyappan, J. Liu, A. Lushington, R. Li and X. Sun, ChemSusChem, 2015, 8, 2537–2543 CrossRef CAS.
- L. N. Zhao, G. R. Chen, Y. H. Weng, T. T. Yan, L. Y. Shi, Z. X. An and D. S. Zhang, Chem. Eng. J., 2020, 401, 10 Search PubMed.
- Y. Gong, D. Palacio, X. Song, R. L. Patel, X. Liang, X. Zhao, J. B. Goodenough and K. Huang, Nano Lett., 2013, 13, 4340–4345 CrossRef CAS PubMed.
- R. L. Patel, Y. B. Jiang, A. Choudhury and X. Liang, Sci. Rep., 2016, 6, 25293 CrossRef CAS.
- B. Xiao, H. Liu, J. Liu, Q. Sun, B. Wang, K. Kaliyappan, Y. Zhao, M. N. Banis, Y. Liu, R. Li, T. K. Sham, G. A. Botton, M. Cai and X. Sun, Adv. Mater., 2017, 29, 1703764 CrossRef PubMed.
- W. Lu, L. W. Liang, X. Sun, X. F. Sun, C. Wu, L. R. Hou, J. F. Sun and C. Z. Yuan, Nanomaterials, 2017, 7, 325 CrossRef PubMed.
- D. N. Wang, J. L. Yang, J. Liu, X. F. Li, R. Y. Li, M. Cai, T. K. Sham and X. L. Sun, J. Mater. Chem. A, 2014, 2, 2306–2312 RSC.
- E. Kang, Y. S. Jung, A. S. Cavanagh, G.-H. Kim, S. M. George, A. C. Dillon, J. K. Kim and J. Lee, Adv. Funct. Mater., 2011, 21, 2430–2438 CrossRef CAS.
- H. Sopha, G. D. Salian, R. Zazpe, J. Prikryl, L. Hromadko, T. Djenizian and J. M. Macak, ACS Omega, 2017, 2, 2749–2756 CrossRef CAS PubMed.
- W. F. Mao, W. Yue, Z. J. Xu, J. Wang, J. B. Zhang, D. J. Li, B. Zhang, S. H. Yang, K. H. Dai, G. Liu and G. Ai, ACS Appl. Mater. Interfaces, 2020, 12, 39282–39292 CrossRef CAS PubMed.
- A. Kohandehghan, P. Kalisvaart, K. Cui, M. Kupsta, E. Memarzadeh and D. Mitlin, J. Mater. Chem. A, 2013, 1, 12850–12861 RSC.
- E. M. Lotfabad, P. Kalisvaart, A. Kohandehghan, K. Cui, M. Kupsta, B. Farbod and D. Mitlin, J. Mater. Chem. A, 2014, 2, 2504–2516 RSC.
- Y. L. Li, L. C. Ma, Y. Yoo, G. C. Wang, X. D. Zhang and M. J. Ko, J. Ind. Eng. Chem., 2019, 73, 351–356 CrossRef CAS.
- S. Bose, T. Kuila, A. K. Mishra, R. Rajasekar, N. H. Kim and J. H. Lee, J. Mater. Chem., 2012, 22, 767–784 RSC.
- J. Liu, M. N. Banis, Q. Sun, A. Lushington, R. Y. Li, T. K. Sham and X. L. Sun, Adv. Mater., 2014, 26, 6472–6477 CrossRef CAS PubMed.
- S. Y. Zhu, P. H. Xu, J. Q. Liu and J. M. Sun, Electrochim. Acta, 2020, 331, 135268 CrossRef CAS.
- G. Wan, L. Yang, S. Shi, Y. Tang, X. Xu and G. Wang, Chem. Commun., 2019, 55, 517–520 RSC.
- X. F. Li, X. B. Meng, J. Liu, D. S. Geng, Y. Zhang, M. N. Banis, Y. L. Li, J. L. Yang, R. Y. Li, X. L. Sun, M. Cai and M. W. Verbrugge, Adv. Funct. Mater., 2012, 22, 1647–1654 CrossRef CAS.
- Y. Zhong, X. H. Xia, J. Y. Zhan, Y. D. Wang, X. L. Wang and J. P. Tu, J. Mater. Chem. A, 2016, 4, 18717–18722 RSC.
- W. Xiong, Q. Guo, Z. Guo, H. Li, R. Zhao, Q. Chen, Z. W. Liu and X. W. Wang, J. Mater. Chem. A, 2018, 6, 4297–4304 RSC.
- X. B. Meng, Y. Q. Cao, J. A. Libera and J. W. Elam, Chem. Mater., 2017, 29, 9043–9052 CrossRef CAS.
- W. J. Lee, S. Bera, C. M. Kim, E. K. Koh, W. P. Hong, S. J. Oh, E. Cho and S. H. Kwon, NPG Asia Mater., 2020, 12, 13 CrossRef.
- W. W. McNeary, S. F. Zaccarine, A. Lai, A. E. Linico, S. Pylypenko and A. W. Weimer, Appl. Catal., B, 2019, 254, 587–593 CrossRef CAS.
- L. Zhang, Y. Zhao, M. N. Banis, K. Adair, Z. X. Song, L. J. Yang, M. Markiewicz, J. J. Li, S. Z. Wang, R. Y. Li, S. Y. Ye and X. L. Sun, Nano Energy, 2019, 60, 111–118 CrossRef CAS.
- Y. F. Liu, L. X. Li, Y. Y. Wang and C. F. Li, J. Inorg. Mater., 2017, 32, 751–757 CrossRef.
- G. Z. Wang, Z. Gao, G. P. Wan, S. W. Lin, P. Yang and Y. Qin, Nano Res., 2014, 7, 704–716 CrossRef CAS.
- G. Wang, X. Peng, L. Yu, G. Wan, S. Lin and Y. Qin, J. Mater. Chem. A, 2015, 3, 2734–2740 RSC.
- S. C. Zhao, L. L. Yan, X. D. Tian, Y. Q. Liu, C. Q. Chen, Y. Q. Li, J. K. Zhang, Y. Song and Y. Qin, Nano Res., 2018, 11, 530–541 CrossRef CAS.
- S. A. Skoog, J. W. Elam and R. J. Narayan, Int. Mater. Rev., 2013, 58, 113–129 CrossRef CAS.
- T. O. Kaariainen, M. Kemell, M. Vehkamaki, M. L. Kaariainen, A. Correia, H. A. Santos, L. M. Bimbo, J. Hirvonen, P. Hoppu, S. M. George, D. C. Cameron, M. Ritala and M. Leskela, Int. J. Pharm., 2017, 525, 160–174 CrossRef CAS PubMed.
- J. Hellrup, M. Rooth, A. Johansson and D. Mahlin, Int. J. Pharm., 2017, 529, 116–122 CrossRef CAS PubMed.
- M. J. Haghshenas-Lari, N. Mostoufi and R. Sotudeh-Gharebagh, Part. Sci. Technol., 2017, 36, 727–733 CrossRef.
- R. L. Garcea, N. M. Meinerz, M. Dong, H. Funke, S. Ghazvini and T. W. Randolph, npj Vaccines, 2020, 5, 45 CrossRef CAS PubMed.
- D. Zhang, M. J. Quayle, G. Petersson, J. R. van Ommen and S. Folestad, Nanoscale, 2017, 9, 11410–11417 RSC.
- J. Hellrup, M. Rooth, E. Martensson, K. Sigfridsson and A. Johansson, Eur. J. Pharm. Biopharm., 2019, 140, 60–66 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |