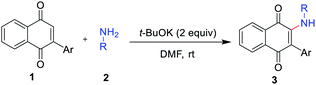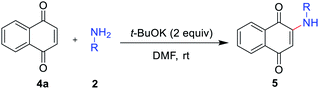Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
t-BuOK mediated oxidative coupling amination of 1,4-naphthoquinone and related 3-indolylnaphthoquinones with amines†
Yu Dong a,
Ting Meia,
Qi-Qi Luoa,
Qiang Fenga,
Bo Changa,
Fan Yanga,
Hong-wei Zhoua,
Zhi-Chuan Shib,
Ji-Yu Wang
a,
Ting Meia,
Qi-Qi Luoa,
Qiang Fenga,
Bo Changa,
Fan Yanga,
Hong-wei Zhoua,
Zhi-Chuan Shib,
Ji-Yu Wang *c and
Bing He*a
*c and
Bing He*a
aCollege of Chemistry and Life Science, Institute of Functional Molecules, Chengdu Normal University, Chengdu, 611130, P. R. China
bSouthwest Minzu University, Chengdu 610041, P. R. China
cChengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu 610041, P. R. China
First published on 8th February 2021
Abstract
The transition-metal free amination of 1,4-naphthoquinone and related 3-indolylnaphthoquinones with amines, such as various (hetero)aromatic amine and aliphatic amine via t-BuOK-mediated oxidative coupling at room temperature has been developed. This reaction provides efficient access to the biologically important and synthetically useful 2-amino-1,4-naphthoquinones and 2-amino-3-indolylnaphthoquinones with good yields under mild conditions. The present protocol is simple, practical and shows good functional group tolerance. In addition, the obtained 2-amino-3-indolylnaphthoquinones were further transformed to synthesize polycyclic N-heterocycles.
Introduction
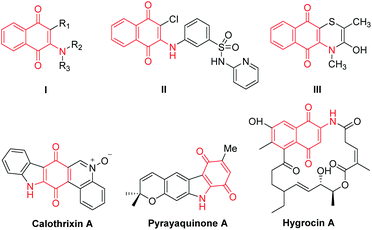
The quinone scaffold can be found not only in various natural products and pharmaceutical compounds1 but it is also well-known as a versatile building block extensively applied in organic synthesis and functional materials.2 Among the derivatives of quinone, the 2-amino-1,4-napthoquinone (I) moiety is found in a considerable number of natural product antibiotics (Fig. 1).3 The representative compounds include the quinone-fused polycyclic N-heterocycles calothrixin A (antiproliferative and potent antimalarial)4 and hygrocins A, isolated during the purification of the immunosuppressive agent rapamycin from Streptomyces hygroscopicus ATC25293.5 Additionally, 2-amino-1,4-napthoquinone is also important as an intermediate for the synthesis of biologically active compounds.62-Amino-1,4-napthoquinone has demonstrated that the amino group in the naphthoquinone structure can change the electron-accepting capacity and therefore result in increased biological activities.7 What's more, this type of compound possesses several interesting biological properties such as antibacterial,8 antifungal,9 and anticancer activities.10 Therefore, much effort has been devoted to developing synthetic methods for the construction of 2-amino-1,4-napthoquinone derivatives. The reaction of amines with 1,4-naphthoquinone derivatives to give 2-amino-1,4-naphthoquinone have been developed with two general methods. On the one hand, 2-amino-1,4-naphthoquinones are prepared by oxidative addition coupling of amines to naphthoquinones in the presence of catalysts such as CeCl3·7H2O,11 FeCl3,12 Cu(OAc)2,13 I2,14 Au15 and HClO4–SiO2.16 Wang reported that 2-amino-1,4-naphthoquinones were obtained by combine the nitro reduction with the 1,4-nucleophilic addition of amines to 1,4-naphthoquinones.17 On the other hand, nucleophilic substitution reactions of 2-halonaphthoquinones,18 or 2-methoxynaphthoquinone derivatives19 also can afford 2-amino-1,4-naphthoquinones. The studies reported that the use of water was beneficial, resulting in nucleophilic substitution and addition reactions with quinones.20 The use of a bentonitic clay and ultrasonic irradiation were reported to give moderate to excellent yields of 2-amino-1,4-naphthoquinones.21
With the objective of studying concise routes into natural products, their analogues, and polyheteroaromatic systems with the 2-amino-1,4-naphthoquinone moiety, we initially investigated a synthetic protocol. To the best of our knowledge, an efficient synthesis of 2-amino-1,4-naphthoquinones via a t-BuOK mediated direct amination has not yet been reported. As always, we have been interested in the synthesis of indolylnaphthoquinones and related derivatives. In consideration of the important pharmaceutical applications of the unique 2-amino-1,4-naphthoquinones structural motif, we report herein a simple and practical method for the synthesis of 2-amino-1,4-naphthoquinones and 2-amino-3-indolylnaphthoquinones by the t-BuOK mediated oxidative coupling amination of 1,4-naphthoquinone and related 3-indolylnaphthoquinones with amines.
Results and discussion
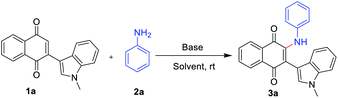
Our investigation to explore amination began with the reaction of indolylnaphthoquinone (1a) and aniline (2a) (see Table 1, as well as Tables S1–S4 in the ESI†). The reaction of t-BuOK (1.5 equiv.), and DMF (2 mL), at room temperature under air atmosphere, for 6 h (Table 1, entry 1), afforded the desired product 3a in 53% isolated yield. Encouraged by this result, subsequently, variation of K2CO3 to NaHCO3, KOH, NaOH, CH3ONa, Cs2CO3, Et3N, or DMAP did not show any improvement (entries 2–7; see ESI†). The use of DMF as a solvent was crucial, as the reaction gave poor results in other solvents such as DMAC, HFIP, dioxane, DCE, PhCF3 or DMSO, (entries 8–11; see ESI†). The reaction performed under an air atmosphere in the absence of base afforded no product (entry 12), which showed that the base played a pivotal role in obtaining the desired product. Afterward, the amount of t-BuOK or 2a were further screened (entries 13–14; see ESI†). It was found that the yields of the product increased with the improved in the amount of t-BuOK or 2a. Therefore, the optimal conditions for the preparation of 2-amino-1,4-naphthoquinones and 2-amino-3-indolylnaphthoquinones were obtained: 2a (2 equiv.), t-BuOK (2 equiv.), in DMF (2 mL) at room temperature for 2 h (Table 1, entry 15).| Entry | Base (equiv.) | Solvent (2 mL) | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol), 2a (0.45 mmol, 1.5 equiv.), base (1.5–2.0 mmol), solvent (2.0 mL), 2–6 h, air, at room temperature.b Isolated yield.c 2a (0.6 mmol, 2 equiv.), DMF = N,N-dimethylformamide; DMAP = 4-dimethylaminopyridine; DMSO = dimethyl sulfoxide; HFIP = 1,1,1,3,3,3-hexafluoroisopropanol; NR = no reaction. | ||||
| 1 | t-BuOK (1.5) | DMF | 6 | 53 |
| 2 | K2CO3 (1.5) | DMF | 6 | NR |
| 3 | NaOH (1.5) | DMF | 6 | 33 |
| 4 | CH3ONa (1.5) | DMF | 6 | NR |
| 5 | Cs2CO3 (1.5) | DMF | 6 | NR |
| 6 | Et3N (1.5) | DMF | 6 | NR |
| 7 | DMAP (1.5) | DMF | 6 | NR |
| 8 | t-BuOK (1.5) | DMAC | 6 | 41 |
| 9 | t-BuOK (1.5) | HFIP | 6 | NR |
| 10 | t-BuOK (1.5) | Dioxane | 6 | NR |
| 11 | t-BuOK (1.5) | DMSO | 6 | 35 |
| 12 | No | DMF | 6 | NR |
| 13 | t-BuOK (2) | DMF | 6 | 71 |
| 14c | t-BuOK (2) | DMF | 6 | 78 |
| 15c | t-BuOK (2) | DMF | 2 | 86 |
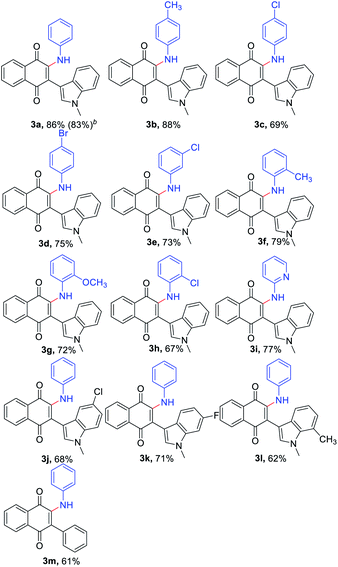
With the optimized reaction conditions in hand, subsequently, a series of substituted indolylnaphthoquinones and amines were tested for the amination (Table 2). To find the substrate scope leading to 3, a variety of substituted anilines were sequentially coupled with 3-indolylnaphthoquinone (1a) afforded the corresponding products 3 in good yields in spite of the electronic nature of aniline (3a–h, Table 2). In a 5 mmol scale reaction, 3a could be obtained in 83% yield, which indicates this transformation could be conducted in a larger scale. It is noteworthy that the valuable groups (F, Cl, Br) could be readily tolerated, which provides an opportunity for further elaboration. Even heteroanilines are well tolerated in this reaction. The use of aminopyridine provides moderate yields of the desired products (3i). The strongly coordinating groups (pyridine), which were employed as reagents for direct C–H functionalization, were fully tolerated with high chemoselectivity and regioselectivity. Next, a variety of indolylnaphthoquinones were also examined as substrates for the reaction strategy. The reaction conditions are mild and notably compatible with chloro, fluoro, and methyl on the aryl ring (3j–l, Table 2). In addition, phenylnaphthoquinone was treated with 2a under the optimized reaction conditions for the synthesis of 3m with moderate yields.
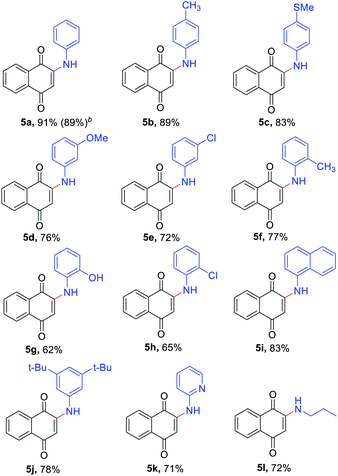
The reaction scope was next examined by using different substituted anilines with 1,4-naphthoquinone 4a as a model substrate (Table 3). Various anilines with different electronic and steric nature were tolerated under the reaction conditions to afford 2-amino-1,4-naphthoquinones 5a–5h in moderate to high yields. In a 5 mmol scale reaction, 5a could be obtained in 89% yield, which indicates this transformation could be conducted in a larger scale. Naphthylamine and disubstituted aniline were also suitable reaction partners to give corresponding products 5i and 5j. Especially, heteroanilines and aliphatic amine were amenable under our reaction conditions and provided the expected coupling product 5k and 5l.
Polycyclic N-heterocycles are the key structural element of natural products, drugs and functional materials.22 Therefore, a Co-catalyzed intramolecular cyclization reaction of some of the 2-amino-3-indolylnaphthoquinones derivatives 3 allowed the generation of polycyclic N-heterocycles derivatives 6 in moderate yields (Scheme 1).
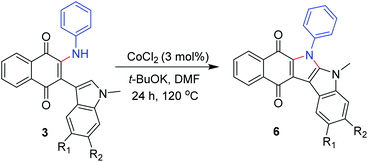 | ||
| Scheme 1 R1 and R2 = H unless otherwise stated. 6a R1 and R2 = H, 81%; 6b R1 = Cl, 62%; 6c R2 = F, 56%. | ||
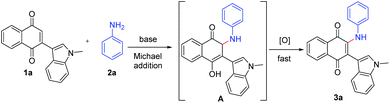
On the basis of our and previous reports,23 a possible reaction mechanism was proposed (Scheme 2). Initially, the Michael addition of indolylnaphthoquinone (1a) and aniline (2a) in the presence of base gave the intermediate A, which was immediately oxidized to the product 3a by O2 or the oxidative naphthoquinone.
Conclusions
In conclusion, we have developed a practical and efficient strategy for t-BuOK-mediated oxidative coupling amination of 1,4-naphthoquinone and related 3-indolylnaphthoquinones with amines at room temperature. A series of 2-amino-1,4-naphthoquinones and 2-amino-3-indolylnaphthoquinones were conveniently synthesized in good yields under air conditions. The reaction took place under mild conditions, displayed excellent functional group compatibility, and did not use metals. In addition, the obtained 2-amino-3-indolylnaphthoquinones derivatives were conducted further transformation to synthesize polycyclic N-heterocycles.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from the Foundation of Applied Basic Research Project of Sichuan Provincial Science and Technology Department (2018JY0262 and 2017JY0177).Notes and references
- (a) C. Puder, K. Wagner, R. Vettermann, R. Hauptmann and O. Potterat, Terphenylquinone Inhibitors of the Src Protein Tyrosine Kinase from Stilbella sp, J. Nat. Prod., 2005, 68, 323–326 CrossRef CAS; (b) J.-K. Liu, Natural Terphenyls: Developments since 1877, Chem. Rev., 2006, 106, 2209–2223 CrossRef CAS; (c) C. Asche, Antitumour quinones, Mini-Rev. Med. Chem., 2005, 5, 449–467 CrossRef CAS.
- (a) A. Skrzyńska, M. Romaniszyn, D. Pomikło and Ł. Albrecht, The Application of 2-Benzyl-1,4-naphthoquinones as Pronucleophiles in Aminocatalytic Synthesis of Tricyclic Derivatives, J. Org. Chem., 2018, 83, 5019–5026 CrossRef; (b) J. Blom, T. K. Johansen, F. Jensen and K. A. Jørgensen, Dynamic resolution of 2-cyclohexylidene acetaldehydes through organocatalytic dienamine [4+2] cycloaddition, Chem. Commun., 2016, 52, 7153–7156 RSC.
- (a) A. K. Jordão, J. Novais, B. Leal, A. C. Escobar, H. M. dos Santos Júnior, H. C. Castro and V. F. Ferreira, Synthesis using microwave irradiation and antibacterial evaluation of new N,O-acetals and N,S-acetals derived from 2-amino-1,4-naphthoquinones, Eur. J. Med. Chem., 2013, 63, 196–201 CrossRef; (b) K. W. Wellington and N. I. Kolesnikova, A laccase-catalysed one-pot synthesis of aminonaphthoquinones and their anticancer activity, Bioorg. Med. Chem., 2012, 20, 4472–4481 CrossRef CAS.
- P. H. Bernardo, C. L. L. Chai, G. A. Heath, P. J. Mahon, G. D. Smith, P. Waring and B. A. Wilkes, Synthesis, electrochemistry, and bioactivity of the cyanobacterial calothrixins and related quinones, J. Med. Chem., 2004, 47, 4958–4963 CrossRef CAS.
- P. Cai, F. Kong, M. E. Ruppen, G. Glasier and G. T. Carter, Hygrocins A and B, Naphthoquinone Macrolides from Streptomyces hygroscopicus, J. Nat. Prod., 2005, 68, 1736–1742 CrossRef CAS.
- P. A. Aristoff and P. D. Johnson, Synthesis of CBI-PDE-I-dimer, the benzannelated analog of CC-1065, J. Org. Chem., 1992, 57, 6234–6239 CrossRef CAS.
- M. Aguilar-Martínez, G. Cuevas, M. Jiménez-Estrada, I. González, B. Lotina-Hennsen and N. Macías-Ruvalcaba, An Experimental and Theoretical Study of the Substituent Effects on the Redox Properties of 2-[(R-phenyl)amine]-1,4-naphthalenediones in Acetonitrile, J. Org. Chem., 1999, 64, 3684–3694 CrossRef.
- A. P. Neves, C. C. Barbosa, S. J. Greco, M. D. Vargas, L. C. Visentin, C. B. Pinheiro, A. S. Mangrich, J. P. Barbosa and G. L. d. Costa, Novel aminonaphthoquinone Mannich bases derived from lawsone and their copper (II) complexes: synthesis, characterization and antibacterial activity, J. Braz. Chem. Soc., 2009, 20, 712–727 CrossRef CAS.
- A. P. Neves, C. C. Barbosa, S. J. Greco, M. D. Vargas, L. C. Visentin, C. B. Pinheiro, A. S. Mangrich, J. P. Barbosa and G. L. d. Costa, Novel aminonaphthoquinone Mannich bases derived from lawsone and their copper (II) complexes: synthesis, characterization and antibacterial activity, J. Braz. Chem. Soc., 2009, 20, 712–727 CrossRef CAS.
- A. I. Francisco, A. Casellato, A. P. Neves, J. W. de Mesquita Carneiro, M. D. Vargas, L. do Canto Visentin, A. Magalhães, C. A. Câmara, C. Pessoa, L. V. Costa-Lotufo, J. D. B. Marinho-Filho and M. O. De Moraes, Novel 2-(R-phenyl)amino-3-(2-methylpropenyl)-[1,4]-naphthoquinones: Synthesis, Characterization, Electrochemical Behavior and Antitumor Activity, J. Braz. Chem. Soc., 2010, 21, 169–178 CrossRef CAS.
- E. Leyva, L. I. López, S. E. Loredo-Carrillo, M. Rodríguez-Kessler and A. Montes-Rojas, Synthesis, spectral and electrochemical characterization of novel 2-(fluoroanilino)-1,4-naphthoquinones, J. Fluorine Chem., 2011, 132, 94–101 CrossRef CAS.
- E. Leyva, K. M. Baines, C. G. Espinosa-González, D. A. Magaldi-Lara, S. E. Loredo-Carrillo, T. A. De Luna-Méndez and L. I. López, 2-(Fluoro-) and 2-(methoxyanilino)-1,4-naphthoquinones. 2-(Fluoro-) and 2-(methoxyanilino)-1,4-naphthoquinones. Synthesis and mechanism and effect of fluorine substitution on redox reactivity and NMR, J. Fluorine Chem., 2015, 180, 152–160 CrossRef CAS.
- C. d. S. Lisboa, V. G. Santos, B. G. Vaz, N. C. de Lucas, M. N. Eberlin and S. J. Garden, C–H Functionalization of 1,4-Naphthoquinone by Oxidative Coupling with Anilines in the Presence of a Catalytic Quantity of Copper(II) Acetate, J. Org. Chem., 2011, 76, 5264–5273 CrossRef CAS.
- B. Liu and S. J. Ji, Facile Synthesis of 2-Amino-1,4-naphthoquinones Catalyzed by Molecular Iodine Under Ultrasonic Irradiation, Synth. Commun., 2008, 38, 1201–1211 CrossRef CAS.
- C. Jiang and S. Wang, Gold (III)-catalyzed 1,4-nucleophilic addition: facile approach to prepare 2-amino-1,4-naphthalenedione and 6-amino-5,8-quinolinedione derivatives, Synlett, 2009, 1099–1102 CAS.
- U. Sharma, D. Katoch, S. Sood, N. Kumar, B. Singh, A. Thakur and A. Gulati, Synthesis, antibacterial and antifungal activity of 2-amino-1,4-naphthoquinones using silica-supported perchloric acid (HClO4-SiO2) as a mild, recyclable and highly efficient heterogeneous catalyst, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2013, 52, 1431–1440 Search PubMed.
- X.-L. Chen, Y. Dong, S. He, R. Zhang, H. Zhang, L. Tang, X.-M. Zhang and J.-Y. Wang, A one-pot approach to 2-(N-substituted amino)-1,4-naphthoquinones with use of nitro compounds and 1,4-naphthoquinones in water, Synlett, 2019, 30, 615–619 CrossRef CAS.
- (a) S. N. Falling and H. Rapoport, Application of iminium salts to the synthesis of naphthoquinone mitosene analogs, J. Org. Chem., 1980, 45, 1260–1270 CrossRef CAS; (b) N. L. Agarwal and W. Schaefer, Quinone chemistry, reaction of 2,3-dichloro-1,4-naphthoquinone with arylamines in pyridine, J. Org. Chem., 1980, 45, 5139–5143 CrossRef CAS; (c) E. A. Couladouros, Z. F. Plyta and V. P. Papageorgiou, A general procedure for the efficient synthesis of (alkylamino) naphthoquinones, J. Org. Chem., 1996, 61, 3031–3033 CrossRef CAS; (d) T. Win and S. Bittner, Novel 2-amino-3-(2,4-dinitrophenylamino) derivatives of 1,4-naphthoquinone, Tetrahedron Lett., 2005, 46, 3229–3231 CrossRef CAS.
- (a) C. A. Camara, A. C. Pinto, M. A. Rosa and M. D. Vargas, Secondary amines and unexpected 1-aza-anthraquinones from 2-methoxylapachol, Tetrahedron, 2001, 57, 9569–9574 CrossRef CAS; (b) A. I. Francisco, M. D. Vargas, J. W. d. M. Carneiro, M. Lanznaster, J. C. Torres, C. A. Camara and A. C. Pinto, General method for the high yield preparation of 2-(4-X-phenylene)amine-1,4-naphthoquinones (X=ferrocenyl, OMe, Me, I, Cl, and NO2) from 2-methoxy-1,4-naphthoquinone and investigation of H+ and Mg2+ catalysts with DFT calculations, J. Mol. Struct., 2008, 891, 228–232 CrossRef CAS.
- J. S. Yadav, B. V. S. Reddy, T. Swamy and K. S. Shankar, Green protocol for conjugate addition of amines to p-quinones accelerated by water, Monatsh. Chem., 2008, 139, 1317 CrossRef CAS.
- E. Leyva, L. I. López, E. Moctezuma and H. de Lasa, A Bentonitic Clay Assisted Method for the Preparation of 2-(R-Anilino)-1, 4-Naphthoquinones, Top. Catal., 2008, 49, 281 CrossRef CAS.
- (a) R. K. Konidena, W. J. Chung and J. Y. Lee, C1-, C2-, and C3-Modified Carbazole Derivatives as Promising Host Materials for Phosphorescent Organic Light-Emitting Diodes, Org. Lett., 2020, 22, 2786–2790 CrossRef CAS; (b) D. Bader, J. Fröhlich and P. Kautny, Thienopyrrolo[3,2,1-jk]carbazoles: Building Blocks for Functional Organic Materials, J. Org. Chem., 2020, 85, 3865–3871 CrossRef CAS.
- (a) Y. Dong, J. Yang, H. Zhang, X.-Y. Zhan, S. He, Z.-C. Shi, X.-M. Zhang and J.-Y. Wang, Cobalt-Catalyzed Cycloamination: Synthesis and Photophysical Properties of Polycyclic N-Heterocycles, Org. Lett., 2020, 22, 5151–5156 CrossRef CAS; (b) H.-B. Zhang, L. Liu, Y.-J. Chen, D. Wang and C.-J. Li, Synthesis of Aryl-Substituted 1,4-Benzoquinone via Water-Promoted and In(OTf)3-Catalyzed in situ Conjugate Addition-Dehydrogenation of Aromatic Compounds to 1,4-Benzoquinone in Water, Adv. Synth. Catal., 2006, 348, 229–235 CrossRef CAS; (c) See ref. 13 and 16.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra00193k |
| This journal is © The Royal Society of Chemistry 2021 |